Abstract
Early recognition of Crohn’s disease (CD) with initiation of disease modifying therapy has emerged as a prominent inflammatory bowel disease (IBD) management strategy. . Clinical practice and trials have often focused on patient symptoms, and more recently, serologic tests, stool inflammatory markers, and/or endoscopic inflammatory features for study entry criteria, treatment targets, disease activity monitoring, and to assess therapeutic response Unfortunately, patient symptoms do not correlate well with biologic disease activity, and endoscopy potentially misses or underestimates disease extent and severity in small bowel CD. Computed tomography enterography (CTE) and magnetic resonance enterography (MRE) are potential tools to identify and quantify transmural structural damage and disease activity in the small bowel. In this review, we discuss the role of CTE and MRE in disease management algorithms in clinical practice. We also compare the currently developed MRE based scoring systems, their strengths and pitfalls, as well as the role for MRE in clinical trials for CD.
Keywords: computed tomography, Crohn’s disease, enterography, magnetic resonance imaging, natural history, radiological response
Background
Crohn’s disease (CD) is a chronic immune mediated transmural inflammatory disorder of the gastrointestinal tract. It has a natural history characterized by a progressive and destructive course, leading to irreversible structural bowel damage. Nearly half of CD patients require surgery within 10 years of the diagnosis.(1, 2) Medical management should be focused on preventing loss of intestinal function and the need for surgery, and preserving function through interventions (e.g., endoscopic dilation, strictureplasty, resection) when medical therapies are unlikely to benefit the patient.(3)
Immunomodulators (azathioprine, 6-mercaptopurine, and methotrexate) and biologic agents (infliximab, adalimumab, certolizumab pegol, and vedolizumab) may have the ability to act as disease modifying anti-CD (DMACD) drugs. This has been a difficult concept to confirm with previous studies that have largely lacked computed tomography (CT) enterography or magnetic resonance (MR) enterography as part of trial entry criteria or radiologic endpoints. Clinical trials have focused on symptoms and mucosal inflammation measured by serologic tests, stool inflammatory markers, and/or endoscopic findings.(3) It has long been realized that in Crohn’s disease, patient symptoms do not correlate with biologic activity(4), and that prospective, randomized studies in Crohn’s often have very high placebo response rates.(5) In population based cohorts, however, up to one-third of CD patients have evidence of stricturing or penetrating complications at diagnosis, findings which are underdiagnosed without the aid of cross-sectional imaging.(1) Hence, patients with varying amount of structural damage and fibrosis are being treated in clinical practice and trials, some with a disease state that is unlikely to respond to medical therapy. A major challenge is to identify patients with ‘early inflammatory CD’ prior to the development of irreversible bowel damage since current medications have not been show to reverse fibrosis.(6, 7) Use of CT enterography (CTE) or MR enterography (MRE) criteria could be used to identify patients unlikely to respond to some medical therapies (stricturing disease or penetrating complications),and enrich the study populations, perhaps limiting sample size. In addition, non-invasive trial endpoints are needed to better reflect transmural disease response and predict outcomes.
Cross-sectional imaging technology using enterography protocols with either computed tomography enterography (CTE) or magnetic resonance enterography (MRE) are potential tools to identify and quantify transmural structural damage and disease activity.(8) Sensitivity and specificity of CTE and MRE for active intestinal inflammation has been reported to be over 90%.(9–11) Both modalities can detect inflammation in regions inaccessible to standard endoscopy (proximal small bowel or stricturing phenotype), and those CD patients with isolated intramural disease.(8, 9) Prior studies have shown that CTE can demonstrate penetrating disease in up to 20% of CD patients, a new findings in 50% of the cohort.(12) CTE has also been shown to alter management decisions in nearly half of CD patients after subspecialist clinical assessment.(13) MRE may have the ability to accurately detect fibrosis in CD small bowel lesions.(14, 15) Robust data now supports CTE and MRE as important tools in the IBD diagnostic armamentarium.
Clinically meaningful CD clinical criteria and treatment endpoints include patient reported symptoms, maintenance of clinical remission without corticosteroids, avoidance of hospitalizations, and the ‘hard’ endpoint of avoiding IBD surgery. While these remain relevant in clinical practice, surrogate endpoints that reflect inflammatory severity are required in clinical practice and trials.(16) We will review the current non-radiological disease assessment tools, the available radiological scoring systems, and the potential role for CTE and MRE in clinical practice and trials.
Biomarkers and Surrogate End-Points
Biomarkers Definitions Working Group of the US National Institutes of Health defines a biomarker as ‘a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention’.(17) Surrogate endpoints are a subset of biomarkers ‘that is intended to substitute for a clinical endpoint, expected to predict clinical benefit (or harm or lack of benefit or harm) based on epidemiologic, therapeutic, pathophysiologic, or other scientific evidence.(17) While surrogate endpoint applies primarily to endpoints in therapeutic intervention trials, their use may be extended to natural history or epidemiologic studies and then to clinical practice, where disease responses are similarly measured.
Non-Radiologic Criteria and End-points
Clinical
Clinical status and disease activity have been assessed in practice and trials using indices such as the Crohn’s Disease Activity Index (CDAI) and the Harvey Bradshaw Index (HBI). The CDAI entails information from the previous 1 week regarding the number of liquid stools, abdominal pain, general well-being, symptoms or findings presumed related to CD (extraintestinal, perianal or fever), use of anti-diarrheals, abdominal mass on examination, and hematocrit. Using the CDAI, clinical remission is defined as a score below 150, and clinical response is defined as a decrease of at least 70 points.(18, 19) The HBI was developed as a “simplified” CDAI. It requires similar information (except for the use of anti-diarrheals and hematocrit), limited to the previous day. The HBI defines clinical remission as a score of ≤4 points and response as a 3-point decrease.(20) These clinical indices, however, have been shown to have poor correlation with objective markers of inflammation, such as CRP, endoscopy, or radiological disease activity parameters.(21, 22)
Despite these difficulties, a simple clinical instrument to assess symptoms from the patient’s perspective is important. Patient-reported outcomes (PROs) have been developed based on how a patient functions or feels in relation to a health condition and/or its therapy.(16, 23) A 2-item (abdominal pain, stool frequency) and 3-item (abdominal pain, stool frequency, and general well-being) PRO have been evaluated using data from a trial of methotrexate in CD.(24) The effect estimates and responsiveness were similar using PRO-2 and 3 with scores corresponding to remission (CDAI< 150) being 8 and 13 respectively, while scores of 5 and 9 correlated with a CDAI change of 70 points. Unlike CDAI and HBI indices, PROs were created according to the FDA guidance and likely to play a role as part of composite end points in CD registration drug trials.(16)
Serology
C-reactive protein (CRP) is an inflammatory biomarker that is easily measured across diagnostic laboratories. It has a short plasma half-life of approximately19 hours determined mainly by synthesis rather than degradation.(25) CRP elevation at baseline has been shown to predict response to anti-TNF therapy.(26) Elevated CRP is also a predictor of relapse in CD patients in whom withdrawal of medical therapy is being considered.(27) Low CRP levels, however, have been reported in clinically active CD patients with ileal disease distribution and a low BMI.(22, 28). The correlation between CRP and endoscopic activity is only modest (Spearman rank correlation, 0.46) with not all patients with elevated CRP having active disease.(22) Additionally, CRP has been shown to be significantly elevated in CD patients with severe inflammation causing increased perienteric fat density on CTE, but CRP was not significantly different between patients with vs. without enteric inflammation confined to only the bowel wall.(29) Thus, CRP must be interpreted with caution and continues to have a limited role in clinical practice and trials.
Fecal Markers
Fecal calprotectin is calcium- and zinc-binding protein belonging to the S100 family that is derived predominantly from neutrophils and, to a lesser extent, from monocytes and reactive macrophages.(30) It is released with cell death or activation, resistant to bacterial degradation, and it is stable in feces for several days, making it a sensitive marker of colonic inflammation.(31) It has been shown to be useful as a biomarker to detect response to therapy in Crohn’s colitis and to predict recurrence after ileocecectomy in CD.(25, 32) Poor correlation, however, has been noted between endoscopic disease activity in the ileum (measured using simple endoscopic score for Crohn’s disease (SES-CD)) and fecal calprotectin levels (33). In contrast, when CT enterography was used to supplement ileoscopic findings, ileal inflammation by CT and endoscopy was highly correlated with fecal calprotectin.(34) Fecal calprotectin has a good positive predictive value but a low negative predictive value for predicting CD relapse on maintenance therapy (25, 35, 36), often used to predict relapse in colonic CD.(37) Questions remain about fecal calprotectin as a reliable disease activity marker in isolated small bowel Crohn’s disease CD.(16)
Fecal lactoferrin is an iron binding glycoprotein secreted by activated neutrophils.(31) It is resistant to proteolysis and unaffected by multiple freeze thaws, providing a potential marker in feces of intestinal inflammation.(38) Fewer studies have evaluated fecal lactoferrin (compared to fecal calprotectin), but it has also been shown to be useful in detecting response to therapy in Crohn’s colitis and to detect postoperative recurrence.(39, 40) Similar to fecal calprotectin, concerns remain regarding poor correlation between endoscopic disease activity in the ileum (SES-CD) and fecal lactoferrin levels.(33)
Histology
Potential histological indices that could serve as criteria for therapy or treatment endpoints include the Global Histological Activity Score and the Naini Cortina score.(41, 42) The Global Histological Activity Score (Supplementary Table 1) includes features of acute and chronic inflammatory changes, epithelial damage, and the extent of inflammation (proportion of biopsy specimens affected).(41) The Naini Cortina score (Supplementary Table 2) includes separate scoring for ileitis and colitis, with points assigned for acute and chronic inflammatory changes, granulomas, pyloric metaplasia, and changes in muscularis mucosa.(42) Further research is needed to determine the predictive value of treating to an endpoint of healing using these indices on long-term outcomes such as disease progression, hospitalization, and surgery.(16, 23)
Endoscopy
The recent Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) program initiated by the International Organization for the Study of Inflammatory Bowel Diseases (IOIBD) identified, using an evidence-based expert consensus process, the absence of ulceration on endoscopic examination as an appropriate CD treatment target.(16) Validated endoscopic scoring systems include the CD Endoscopic Index of Severity (CDEIS) and the Simple Endoscopic Score for CD (SES-CD).(4, 43, 44) The CDEIS consists of recording 9 descriptors across 5 segments including the ileum. The weaknesses of the CDEIS include its complex requirement of data recording, possible underestimation of the severity of disease when only one out of five segments is involved, and the absence of validated score thresholds associated with specific prognostic outcomes.(43) Endoscopic remission is defined as a CDEIS of 0–3, while mild disease corresponds to a score of 3–9, and a score greater than 9 corresponds to a moderate-severe disease.(45) The SES-CD was developed as a simplified version of the CDEIS using 4 variables: ulcers, proportion of the surface covered by ulcers, proportion of the surface with any other lesions, and stenosis. The main limitation of the SES-CD is that it does not take into account the number of explored segments and it assumes that unexplored segments (which may be due to a non-passable stenosis) do not contain lesions.(43) Using a Delphi method, endoscopic response was defined as >50% decrease in SES-CD or CDEIS while an SES-CD 0–2 was accepted as the definition of endoscopic remission in CD.(43)
The CDEIS and SES-CD have been shown to be reproducible, including centralized reading of endoscopy videos (relevant to clinical trials). A lower inter- and intra-observer variability for SES-CD scores than for CDEIS has been reported.(46) It remains unclear, however, regarding the degree of score reduction that is associated with clinical benefit. The only data identifying a prognostic threshold (50% reduction in SES-CD or CDEIS) in a clinical trial setting is restricted to post-hoc analysis of data from the Study of Biologic and Immunomodulator Naive Patients in Crohn’s Disease (SONIC) trial involving 172 participants using SES-CD and CDEIS with centralized reading of endoscopy.(47) A 50% reduction in SES-CD at week 26 of the SONIC trial predicted corticosteroid-free clinical remission (CFREM) at week 50 with a sensitivity of 74% (95% CI, 66%–83%),specificity of 48% (95% CI, 36%–60%), positive likelihood ratio (PLR) of 1.42 (95% CI, 1.11–1.83), and a negative likelihood ratio (NLR) of 0.54 (95% CI, 0.36–0.81). Similar reduction in CDEIS at week 26 predicted CFREM with a 73% (95% CI, 65%–82%) sensitivity, 46% (95% CI, 35%–58%) specificity, a PLR of 1.37 (95% CI, 1.07–1.75), and an NLR of 0.58 (95% CI, 0.38–0.86). The application of these scoring systems in clinical practice has been limited thus far due to both the complexity of using the scoring systems as well as the aforementioned absence of validated prognostic thresholds.(48)
In the postoperative setting, the Rutgeerts score has been used to assess and quantify endoscopic CD recurrence in the neo-terminal ileum after ileal or ileocolonic resection.(49) The Rutgeerts score in an asymptomatic CD patient within 12 months of the ileocolonic resection has been shown to predict the risk of clinical recurrence. A score of i0 (no distal ileal lesions) or i1 (≤5 aphthous lesions in distal ileum) is associated with a very low risk of clinical recurrence (80–85 % asymptomatic at 3 years follow-up) compared to those with i3 (diffuse aphthous ileitis with diffusely inflamed mucosa) or i4 (diffuse inflammation with already large ulcers, nodules and/or narrowing) (<10 % asymptomatic at 3 years follow-up). It parallels MR enteroclysis imaging findings at the neoterminal ileum and anastomosis in predicting postoperative recurrence.(50) The Rutgeerts score, however, has not been validated and further research is needed to differentiate the i2 grade based on the relevance of anastomotic lesions (i2a) versus neoterminal lesions (i2b).(51)
Radiological Criteria and Endpoints
Feasibility
Cross-sectional imaging with either CTE or MRE has become the standard for small bowel CD activity assessments, and to detect obstructive and penetrating complications at many IBD centers.(8) These imaging modalities can be used for the assessment of inflammatory severity and burden (i.e., multifocality, length) for target directed medical therapy and surgical planning. MR scoring systems have been developed that could facilitate the use of MRE in clinical trials. Imaging applications include the identification of patients with appropriate (and similar) disease severity for inclusion, exclusion of patients with penetrating complications or obstructive disease, and longitudinal interrogations for transmural response and healing.(52) There are currently several MR based scoring systems that have been developed in comparison to an external reference standard (Tables 1 and 2).(53–55) Studies are underway to create similar validated CT scoring systems for Crohn’s disease.
Table 1.
MRE scores for Crohn’s disease based on independent predictors of activity
| Derivation (patients/segments) | Validation (patients/segments) | Therapeutic response assessment (patient/segments) | Gold standard | |
|---|---|---|---|---|
| MaRIA(53, 56) | 50/213 | 48/258a | Yes | Ileocolonoscopy (CDEIS) |
| CDMI(54) | 16/44 | 26/26a | No | Surgical specimen (AIS) |
| MEGS(58, 59) | 71/639 | 36/801 | Yes | Extension of London score and correlated to clinical indices (HBI, fecal calprotectin, CRP and CD activity score) |
| Nancy(55) | 40/211 | – | No | Ileocolonoscopy (SES-CD) |
Foot notes:
An additional cohort of 30 patients also validated these indexes(75); CDMI – Crohn’s disease MRI index; CD – Crohn’s disease; CRP – C-reactive protein, HBI – Harvey Bradshaw Index; MaRIA, Magnetic Resonance Index of Activity; MEGS - Magnetic resonance enterography global score (MEGS)
Figure adapted from Bruining et al., Abdom Imaging. 2015 Jun;40(5):965–74, with permission from Springer publishing Group. ©2015
Table 2.
MRE independent predictors for activity on Crohn’s disease according to MaRIA, London, MEGS and Nancy scores
| MaRIA(53, 56) | CDMI(54) | MEGS(58, 59) | Nancy(55) | |
|---|---|---|---|---|
| Wall thickening | Yes | Yes | Yes | Yes |
| Enhancement | Yes (quantification of relative contrast enhancement) | Qualitative (4 different categories) | Qualitative (4 different categories) | Yes (qualitative evaluation) |
| High signal on T2 | Yes (qualitative evaluative) | Yes (4 different categories) | Yes (4 different categories) | Yes (qualitative evaluation) |
| Ulcerations | Yes | – | – | Yes |
| T2 perimural signal | – | Yes (4 different categories) | Yes (4 different categories) | – |
| Mural stratification | – | – | – | Yes |
| Length of disease segment | – | – | Yes | – |
| Diffusion weighting imaging hyperintensity | – | – | – | Yes |
CDMI – Crohn’s disease MRI index; MaRIA, Magnetic Resonance Index of Activity; MEGS - Magnetic resonance enterography global score (MEGS)
Figure adapted from Bruining et al., Abdom Imaging. 2015 Jun;40(5):965–74, with permission from Springer publishing Group. ©2015
Scoring Systems
Magnetic Resonance Index of Activity (MaRIA) Score
The MaRIA score (Supplementary Table 3) was developed in an initial derivation study of 50 patients with established CD who underwent colonoscopy and 3-Tesla MRE performed after concurrent saline rectal enema within a 2 day period.(53) MR features of active inflammation were correlated with CDEIS, where independent predictors for segmental CDEIS were wall thickness (p=0.007), relative contrast enhancement (RCE) (p=0.01), presence of edema (p=0.02) and presence of ulcers at MR (p=0.003). Using a regression model, the MaRIA score per segment was proposed as:
RCE involves the measurement of pre and post-contrast wall signal intensity in the bowel wall by the manual placement of regions of interest as well as the manual measurement of wall thickness at each location of severity assessment, in addition to the standard deviation of pre and post-contrast signal intensity noise measured outside the body. These quantitative measurements are supplemented by visual assessments of intramural edema and luminal ulceration, with the four variables measured for each of four colonic segments and one ileal segment. In this study, segmental MaRIA score was shown to have good correlation with the segmental CDEIS (r = 0.82, p < 0.001). The overall MaRIA score calculated by adding individual segmental scores was also shown to be correlated with total CDEIS (r = 0.78), CRP concentration (r = 0.53), and HBI (r = 0.56). This score has been subsequently validated and found to be reliable and responsive in assessing the response to medical therapy in a prospective, multicenter trial in patients with CD.(56, 57) Segmental MaRIA ≥11 provides a cutoff point for defining severe inflammatory lesions (accuracy 96%) while MaRIA <7 detects segmental mucosal healing (MH) with reasonable accuracy (83%), sensitivity (85%), and specificity (78%).(56) A potential limitation of the MaRIA as currently designed is that it does not take into account the overall length of inflamed segments, even in the small bowel, where extensive disease can occur.
Crohn’s disease MRI Index (CDMI) Score
The CDMI (Supplementary Table 4) was developed by correlating findings on MRE with transmural histopathology at the time of elective small bowel surgical resection in CD patients.(54) A simplified model was developed using the MRE parameters that best predicted transmural inflammation:
The score was then validated in 26 CD patients and correlated to terminal ileal biopsy scores of acute inflammation (“eAIS” score 1–6).(54) Advantages of the CDMI (compared to MaRIA) score is the quicker calculation requiring only a measurement of bowel wall thickness along with visual estimation of T2 signal. The CDMI has similar limitations of not describing the length of bowel involved by CD inflammation.
Magnetic resonance enterography global score (MEGS)
MEGS (Supplementary Table 5) was developed as a modified CDMI to include segmental disease length, evaluation of colonic haustral loss, and evaluation of extra-enteric complications, such as enlarged mesenteric lymph nodes, abscesses and fistulae. It is believed to better capture the full disease burden in CD patients (58):
MEGS has been validated in 36 CD patients where it demonstrated response to anti-tumor necrosis factor-alpha (TNF-α) therapy with good inter-observer agreement.(59)
Nancy Score
The Nancy score (Supplementary Table 6) was developed in 40 CD patients using a combination of MR colonography (MRC) and diffusion weighted imaging (DWI) against a reference standard of SES-CD on colonoscopy.(55) Six different radiological signs were recorded in 5 colonic segments and the ileum, with total MR-score (MR-score-T) calculated by adding the segmental scores (MR-score-S), with values ranging from 0 to 36. The presence of bowel wall thickening (OR=10.03, p<0.0001) and DWI hyperintensity (OR=2.67, p=0.01) independently predicted the presence of endoscopic inflammation in the colon while MR-score-S and MR-score-T were both correlated with the SES-CD (r=0.57, p<0.001 and r=0.54, p=0.001; respectively). A representative example is depicted in FIGURE 1 with MaRIA, CDMI, MEGS and Nancy scores with values before and after initiation of medical therapy for Crohn’s disease.
Figure 1.
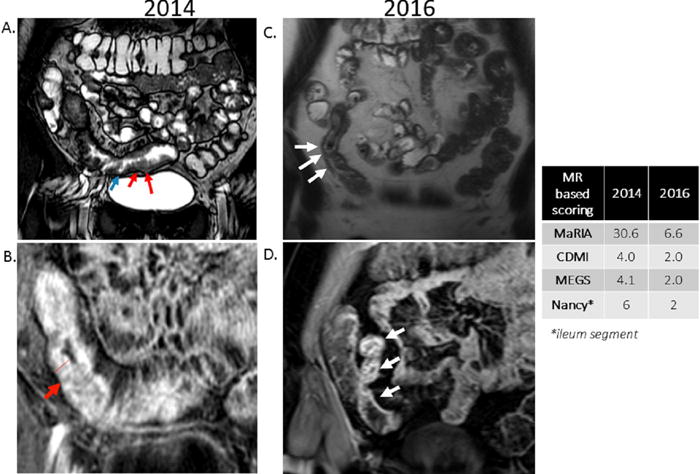
29 y/o female with CD, with MRE performed in 2014 and then subsequently in 2016 (after treatment with adalimumab 40 mg subcutaneously every 2 weeks).
A. 2014 Coronal Fast Imaging Employing Steady-state Acquisition sequence demonstrating distal ileum including terminal ileum with moderate wall thickening (red arrows) and ulcerations (blue arrows).
B. 2014 Coronal post-contrast LAVA sequence demonstrating distal ileum including terminal ileum with moderate wall thickening (10.4mm), and mucosal hyperenhancement (red arrow) consistent with active Crohn’s disease.
C. 2016 Coronal T2 Half Fourier Acquisition Single Shot Turbo Spin Echo sequence demonstrating thickening (4.4mm) of a segment of ileum (white arrows), less than previous MRE in 2014.
D. 2016 Coronal T1 volumetric interpolated breath-hold fat saturated post gadolinium sequence demonstrating bowel wall hyperenhancement (white arrows) much less pronounced than previous MRE in 2014.
Lémann Index
The Lémann index (Supplementary Table 7) is a scoring system that was developed in a prospective, multicenter cross-sectional study involving 38 CD patients across 24 centers in 5 countries.(60, 61) It utilizes clinical, endoscopic, and MRE information to assess the burden of disease. The entire digestive tract was divided into 4 organs and subsequently into segments with disease damage (previous operations, predefined strictures, and/or penetrating lesions of maximal severity) graded per segment and overall damage averaged over all the segments. A global damage evaluation is then calculated (from 0.0 to 10.0) from the organ damage evaluations.(61) The main use of this index is to assess changes in intestinal damage in the context of natural history studies.
MRE Scoring Limitations
Several significant limitations with MRE scoring systems remain. One of the benefits of CT or MR enterography is the identification of proximal and intramural small bowel inflammation not identified at ileocolonoscopy.(34, 62) Most of the current MRI based scoring systems, however, have been modeled and validated against endoscopic scoring systems. If the scoring is calculated using four colonic segments and one ileal segment, MR scoring systems will perpetuate a relative underestimation of small bowel inflammation when there is extensive small bowel disease. Another potential concern is the reproducibility and practicality of these scoring systems outside of academic centers. The MR scores can be time-consuming and some require matched region of interest (ROI) placement on multiple bowel segments in every patient, for example, with the ROI’s placed on pre- and post-contrast scans. Lastly, studies are needed to demonstrate outcomes while treating to a radiological target using these scoring systems.
Justification for Radiologic Assessments
In clinical practice, the benefit of small bowel examinations using an enterography technique has been explored. The largest study included 357 consecutive CD patients who underwent a CTE between August 2004 and October 2005.(12) Nearly 1 in 5 (20.7%) had penetrating disease, representing a new finding in more than half (58.1%) of the patients. CTE also detected extraintestinal IBD in 18.8% of the patients (new finding in 67.2%) and non-IBD significant findings in 45.1% including 2 malignancies. The measurable effect of incorporating CTE into a CD management algorithm was demonstrated in a prospective study involving 273 patients with established or suspected CD undergoing a clinically indicated CTE.(13) In this study, management plans were recorded after history and physical examination by an IBD specialist, with subsequent unblinding of CT results. CTE altered management plans in 1 of every 2 cases independent of clinical, serologic, and histologic findings (P < 0.0001). This was seen in both those with established disease as well as those with suspected disease. In another prospective study of 67 CD patients, CTE data changed clinicians’ perceptions of the likelihood of corticosteroid benefit in more than 60% of the cases(63)
Since CD is a transmural disease, CTE or MRE have the potential to assess transmural response to CD medications. This was analyzed in 63 CD patients undergoing serial imaging while receiving infliximab therapy.(64) Individual lesions were defined as improved if their enhancement or length decreased without worsening of other parameters. Patients were grouped as responders (all lesions improved), partial responders (some lesions improved), and non-responders (worsening or no changes in all lesions). In this study 28 (44.4%) were classified as responders, 12 (19.0%) were partial responders, and 23 (36.5%) were non-responders. Radiological response on CTE had had poor-to-fair agreement with symptoms, endoscopic appearance, and levels of C-reactive protein at time of second CTE (κ = 0.26, 0.07, and 0.30 respectively). CTE and MRE are now considered complementary tests to endoscopy in the clinical care of CD patients.(65)
The strongest justification for treating CD patients to a radiological endpoint would be if radiologic healing/response can alter the natural history of a patient’s disease in terms of rescue corticosteroid usage, hospitalizations for active disease, and/or CD-related surgeries. In a study of 150 patients with small bowel CD undergoing serial CTE/MRE, radiological response with medical therapy was evaluated.(66) Fifty-five patients (37%) were complete radiologic responders; while 39 patients were partial responders (26%), and 56 patients were non-responders (37%). Achievement of complete or partial radiologic response at the 1st follow-up CTE/MRE decreased risk for steroid usage by over 50% (HR’s: 0.37 [95% CI, 0.21–0.64] and 0.45 [95% CI 0.26–0.79], respectively). Complete response decreased risk of subsequent hospitalizations and surgery by over two-thirds (HR’s: 0.28 [95% CI 0.15 – 0.50] and 0.34 [95% CI, 0.18–0.63], respectively). While these findings are encouraging, confirmation in a prospective setting is needed.
Applicability
The applicability of utilizing MRE scoring for entry into research trials was recently explored in a prospective multi-center study. Twenty CD patients across a spectrum of CDAI scores underwent ileocolonoscopy and two MREs (with and without rectal contrast) within a 14-day period.(52) The key findings in this study were that MRE identified 3 patients with intra-abdominal complications, who would otherwise have been included in research trials based on clinical and endoscopic findings. MRE also identified active intestinal inflammation in 2 patients with CDAI <150, and no active luminal disease in 1 patient with CDAI > 220.
Imaging Biomarker-Based Trial Design
The use of radiologic biomarkers to identify CD patients who can benefit from treatment with specific CD therapies has the potential to both improve patient care and accelerate drug development. The greatest utility of biomarkers are as surrogate endpoints in place of clinical efficacy measures in definitive trials, or the use to achieve enrichment when one expects greater effects with interventions in specific groups of subjects (i.e., effect modification).(67) An ideal biomarker is one that is accurate, repeatable, reproducible, sensitive, unique, prognostically important and proven interventions lead to change in the surrogate endpoint which translates into improved prognosis.(68) The role of imaging studies in CD in clinical practice can be to identify patients who are at the highest risk of disease progression and complication especially those with penetrating disease of the small bowel or with long small bowel segments with severe inflammation. (52) The role of imaging studies in CD in clinical trials would be to include a similar cohort of patients in the drug and placebo arms allowing reliable comparison between groups. Baseline measurements of disease activity at trial inclusion would also allow response assessment at pre-defined interim time points, potentially allowing early trial termination and with resultant cost savings, limiting side effects and lowering placebo response rates.
Future directions
The role of CTE and MRE will likely continue to expand in IBD diagnostic and management algorithms. Perfusion imaging with dynamic contrast enhanced-MR imaging is being examined in CD imaging with quantitative parameters to identify responders to anti-TNF-α medications.(69) Recent advances in MR imaging techniques may allow identification of a spectrum of inflammation versus fibrosis in CD lesions.(14) In a study MR scans of 41 patients who underwent elective bowel resection within 4 months before surgery,(14) using percentage of enhancement gain, MR was able to discriminate between mild-moderate and severe fibrosis deposition with a sensitivity of 0.94 and a specificity of 0.89. This is relevant again in the setting of potential anti-fibrotic drugs that are currently in pre-clinical phases of development.(70) Additionally, magnetization transfer MR (MT-MR) is an MRI sequence sensitive to the changes in collagen content, potentially correlating with fibrosis, and enabling the differentiation from edematous or inflamed bowel. There is emerging pilot data utilizing MT-MR along with routine MRE that needs validation in larger prospective studies.(71) Recently concerns regarding intravenous gadolinium contrast accumulation (related to MR imaging) in the brain have been raised. (72–74) Diffusion weighted imaginge (DWI) protocols that derive image contrast from the differences in the diffusion of water molecules between tissues without intravenous contrast, has been shown to have reliable sensitivity in detecting small bowel inflammation.(11) Despite the widespread applicability of MR imaging, access and cost continue to be limiting factors outside major IBD centers. Hence, the development of a CT score validated against existing MR scoring systems is greatly needed.
Conclusion
CTE and MRE have revolutionized the assessment and monitoring plans of CD patients. Imaging based scoring systems have been developed for potential us in clinical trial and practice. The role of enterography for selecting patients and assessing therapeutic efficacy in clinical trials, and treating-to-target of radiological response in clinical practice is evolving. The ability to develop novel biomarkers reflecting inflammation and/or fibrosis on CT and MR imaging is a promising field requiring further research.
Supplementary Material
Acknowledgments
Financial support: This publication was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Abbreviations
- CT
computed tomography
- CTE
computed tomography enterography
- CD
Crohn’s disease
- MR
magnetic resonance
- MRE
magnetic resonance enterography
Footnotes
Guarantor of the article: David H. Bruining, MD.
Specific author contributions:
Dr. Deepak was involved in the conception and design, collection, analysis and interpretation of the data, drafting of the first draft of the article, and final approval of the article.
Dr. Fletcher was involved in conception and design, collection, analysis and interpretation of the data, initial drafting and critical revision of the article for important intellectual content and final approval of the article.
Dr. Fidler was involved in conception and design, collection and interpretation of the data, critical revision of the article for important intellectual content and final approval of the article.
Dr. Bruining was involved in conception and design, study supervision, analysis and interpretation of the data, initial drafting of the article, critical revision of the article for important intellectual content and final approval of the article.
Potential competing interests:
Dr. Parakkal Deepak: None
Dr. Fletcher: None
Dr. Fidler: None
Dr. Bruining: Research support from Genentech.
References
- 1.Peyrin-Biroulet L, Loftus EV, Jr, Colombel JF, et al. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105:289–297. doi: 10.1038/ajg.2009.579. [DOI] [PubMed] [Google Scholar]
- 2.Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590–1605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 3.Van Assche G, Vermeire S, Rutgeerts P. The potential for disease modification in Crohn’s disease. Nat Rev Gastroenterol Hepatol. 2010;7:79–85. doi: 10.1038/nrgastro.2009.220. [DOI] [PubMed] [Google Scholar]
- 4.Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn’s disease: a prospective multicentre study. Groupe d’Etudes Therapeutiques des Affections Inflammatoires du Tube Digestif (GETAID) Gut. 1989;30:983–989. doi: 10.1136/gut.30.7.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su C, Lichtenstein GR, Krok K, et al. A meta-analysis of the placebo rates of remission and response in clinical trials of active Crohn’s disease. Gastroenterology. 2004;126:1257–1269. doi: 10.1053/j.gastro.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 6.Peyrin-Biroulet L, Loftus EV, Jr, Colombel JF, et al. Early Crohn disease: a proposed definition for use in disease-modification trials. Gut. 2010;59:141–147. doi: 10.1136/gut.2009.187120. [DOI] [PubMed] [Google Scholar]
- 7.Peyrin-Biroulet L, Billioud V, D’Haens G, et al. Development of the Paris definition of early Crohn’s disease for disease-modification trials: results of an international expert opinion process. Am J Gastroenterol. 2012;107:1770–1776. doi: 10.1038/ajg.2012.117. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher JG, Fidler JL, Bruining DH, et al. New concepts in intestinal imaging for inflammatory bowel diseases. Gastroenterology. 2011;140:1795–1806. doi: 10.1053/j.gastro.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Siddiki HA, Fidler JL, Fletcher JG, et al. Prospective comparison of state-of-the-art MR enterography and CT enterography in small-bowel Crohn’s disease. AJR Am J Roentgenol. 2009;193:113–121. doi: 10.2214/AJR.08.2027. [DOI] [PubMed] [Google Scholar]
- 10.Siddiki H, Fletcher JG, Hara AK, et al. Validation of a lower radiation computed tomography enterography imaging protocol to detect Crohn’s disease in the small bowel. Inflamm Bowel Dis. 2011;17:778–786. doi: 10.1002/ibd.21364. [DOI] [PubMed] [Google Scholar]
- 11.Seo N, Park SH, Kim KJ, et al. MR Enterography for the Evaluation of Small-Bowel Inflammation in Crohn Disease by Using Diffusion-weighted Imaging without Intravenous Contrast Material: A Prospective Noninferiority Study. Radiology. 2016;278:762–772. doi: 10.1148/radiol.2015150809. [DOI] [PubMed] [Google Scholar]
- 12.Bruining DH, Siddiki HA, Fletcher JG, et al. Prevalence of penetrating disease and extraintestinal manifestations of Crohn’s disease detected with CT enterography. Inflamm Bowel Dis. 2008;14:1701–1706. doi: 10.1002/ibd.20529. [DOI] [PubMed] [Google Scholar]
- 13.Bruining DH, Siddiki HA, Fletcher JG, et al. Benefit of computed tomography enterography in Crohn’s disease: effects on patient management and physician level of confidence. Inflamm Bowel Dis. 2012;18:219–225. doi: 10.1002/ibd.21683. [DOI] [PubMed] [Google Scholar]
- 14.Rimola J, Planell N, Rodriguez S, et al. Characterization of inflammation and fibrosis in Crohn’s disease lesions by magnetic resonance imaging. Am J Gastroenterol. 2015;110:432–440. doi: 10.1038/ajg.2014.424. [DOI] [PubMed] [Google Scholar]
- 15.Higgins PD, Fletcher JG. Characterization of inflammation and fibrosis in Crohn’s disease lesions by magnetic resonance imaging. Am J Gastroenterol. 2015;110:441–443. doi: 10.1038/ajg.2015.26. [DOI] [PubMed] [Google Scholar]
- 16.Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol. 2015;110:1324–1338. doi: 10.1038/ajg.2015.233. [DOI] [PubMed] [Google Scholar]
- 17.Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 18.Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439–444. [PubMed] [Google Scholar]
- 19.Thia KT, Sandborn WJ, Lewis JD, et al. Defining the optimal response criteria for the Crohn’s disease activity index for induction studies in patients with mildly to moderately active Crohn’s disease. Am J Gastroenterol. 2008;103:3123–3131. doi: 10.1111/j.1572-0241.2008.02176.x. [DOI] [PubMed] [Google Scholar]
- 20.Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 21.Peyrin-Biroulet L, Reinisch W, Colombel JF, et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut. 2014;63:88–95. doi: 10.1136/gutjnl-2013-304984. [DOI] [PubMed] [Google Scholar]
- 22.Jones J, Loftus EV, Jr, Panaccione R, et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2008;6:1218–1224. doi: 10.1016/j.cgh.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Levesque BG, Sandborn WJ, Ruel J, et al. Converging goals of treatment of inflammatory bowel disease from clinical trials and practice. Gastroenterology. 2015;148:37–51 e31. doi: 10.1053/j.gastro.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Khanna R, Zou G, D’Haens G, et al. A retrospective analysis: the development of patient reported outcome measures for the assessment of Crohn’s disease activity. Aliment Pharmacol Ther. 2015;41:77–86. doi: 10.1111/apt.13001. [DOI] [PubMed] [Google Scholar]
- 25.Iskandar HN, Ciorba MA. Biomarkers in inflammatory bowel disease: current practices and recent advances. Transl Res. 2012;159:313–325. doi: 10.1016/j.trsl.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reinisch W, Wang Y, Oddens BJ, et al. C-reactive protein, an indicator for maintained response or remission to infliximab in patients with Crohn’s disease: a post-hoc analysis from ACCENT I. Aliment Pharmacol Ther. 2012;35:568–576. doi: 10.1111/j.1365-2036.2011.04987.x. [DOI] [PubMed] [Google Scholar]
- 27.Louis E, Mary JY, Vernier-Massouille G, et al. Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology. 2012;142:63–70 e65. doi: 10.1053/j.gastro.2011.09.034. quiz e31. [DOI] [PubMed] [Google Scholar]
- 28.Florin TH, Paterson EW, Fowler EV, et al. Clinically active Crohn’s disease in the presence of a low C-reactive protein. Scand J Gastroenterol. 2006;41:306–311. doi: 10.1080/00365520500217118. [DOI] [PubMed] [Google Scholar]
- 29.Colombel JF, Solem CA, Sandborn WJ, et al. Quantitative measurement and visual assessment of ileal Crohn’s disease activity by computed tomography enterography: correlation with endoscopic severity and C reactive protein. Gut. 2006;55:1561–1567. doi: 10.1136/gut.2005.084301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konikoff MR, Denson LA. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:524–534. doi: 10.1097/00054725-200606000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Desai D, Faubion WA, Sandborn WJ. Review article: biological activity markers in inflammatory bowel disease. Aliment Pharmacol Ther. 2007;25:247–255. doi: 10.1111/j.1365-2036.2006.03184.x. [DOI] [PubMed] [Google Scholar]
- 32.De Cruz P, Kamm MA, Hamilton AL, et al. Crohn’s disease management after intestinal resection: a randomised trial. Lancet. 2015;385:1406–1417. doi: 10.1016/S0140-6736(14)61908-5. [DOI] [PubMed] [Google Scholar]
- 33.Sipponen T, Karkkainen P, Savilahti E, et al. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment Pharmacol Ther. 2008;28:1221–1229. doi: 10.1111/j.1365-2036.2008.03835.x. [DOI] [PubMed] [Google Scholar]
- 34.Faubion WA, Jr, Fletcher JG, O’Byrne S, et al. EMerging BiomARKers in Inflammatory Bowel Disease (EMBARK) study identifies fecal calprotectin, serum MMP9, and serum IL-22 as a novel combination of biomarkers for Crohn’s disease activity: role of cross-sectional imaging. Am J Gastroenterol. 2013;108:1891–1900. doi: 10.1038/ajg.2013.354. [DOI] [PubMed] [Google Scholar]
- 35.Costa F, Mumolo MG, Ceccarelli L, et al. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut. 2005;54:364–368. doi: 10.1136/gut.2004.043406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology. 2011;140:1817–1826 e1812. doi: 10.1053/j.gastro.2010.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D’Inca R, Dal Pont E, Di Leo V, et al. Can calprotectin predict relapse risk in inflammatory bowel disease? Am J Gastroenterol. 2008;103:2007–2014. doi: 10.1111/j.1572-0241.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 38.Kane SV, Sandborn WJ, Rufo PA, et al. Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Am J Gastroenterol. 2003;98:1309–1314. doi: 10.1111/j.1572-0241.2003.07458.x. [DOI] [PubMed] [Google Scholar]
- 39.Lamb CA, Mohiuddin MK, Gicquel J, et al. Faecal calprotectin or lactoferrin can identify postoperative recurrence in Crohn’s disease. Br J Surg. 2009;96:663–674. doi: 10.1002/bjs.6593. [DOI] [PubMed] [Google Scholar]
- 40.Sipponen T, Bjorkesten CG, Farkkila M, et al. Faecal calprotectin and lactoferrin are reliable surrogate markers of endoscopic response during Crohn’s disease treatment. Scand J Gastroenterol. 2010;45:325–331. doi: 10.3109/00365520903483650. [DOI] [PubMed] [Google Scholar]
- 41.D’Haens GR, Geboes K, Peeters M, et al. Early lesions of recurrent Crohn’s disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology. 1998;114:262–267. doi: 10.1016/s0016-5085(98)70476-7. [DOI] [PubMed] [Google Scholar]
- 42.Naini BV, Cortina G. A histopathologic scoring system as a tool for standardized reporting of chronic (ileo)colitis and independent risk assessment for inflammatory bowel disease. Hum Pathol. 2012;43:2187–2196. doi: 10.1016/j.humpath.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Vuitton L, Marteau P, Sandborn WJ, et al. IOIBD technical review on endoscopic indices for Crohn’s disease clinical trials. Gut. 2015 doi: 10.1136/gutjnl-2015-309903. [DOI] [PubMed] [Google Scholar]
- 44.Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505–512. doi: 10.1016/s0016-5107(04)01878-4. [DOI] [PubMed] [Google Scholar]
- 45.Peyrin-Biroulet L, Panes J, Sandborn WJ, et al. Defining Disease Severity in Inflammatory Bowel Diseases: Current and Future Directions. Clin Gastroenterol Hepatol. 2016;14:348–354 e317. doi: 10.1016/j.cgh.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 46.Khanna R, Zou G, D’Haens G, et al. Reliability among central readers in the evaluation of endoscopic findings from patients with Crohn’s disease. Gut. 2015 doi: 10.1136/gutjnl-2014-308973. [DOI] [PubMed] [Google Scholar]
- 47.Ferrante M, Colombel JF, Sandborn WJ, et al. Validation of endoscopic activity scores in patients with Crohn’s disease based on a post hoc analysis of data from SONIC. Gastroenterology. 2013;145:978–986 e975. doi: 10.1053/j.gastro.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 48.Christensen B, Rubin DT. Understanding Endoscopic Disease Activity in IBD: How to Incorporate It into Practice. Curr Gastroenterol Rep. 2016;18:5. doi: 10.1007/s11894-015-0477-6. [DOI] [PubMed] [Google Scholar]
- 49.Rutgeerts P, Geboes K, Vantrappen G, et al. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990;99:956–963. doi: 10.1016/0016-5085(90)90613-6. [DOI] [PubMed] [Google Scholar]
- 50.Koilakou S, Sailer J, Peloschek P, et al. Endoscopy and MR enteroclysis: equivalent tools in predicting clinical recurrence in patients with Crohn’s disease after ileocolic resection. Inflamm Bowel Dis. 2010;16:198–203. doi: 10.1002/ibd.21003. [DOI] [PubMed] [Google Scholar]
- 51.Gecse K, Lowenberg M, Bossuyt P, et al. Sa1198 Agreement Among Experts in the Endoscopic Evaluation of Postoperative Recurrence in Crohn’s Disease Using the Rutgeerts Score. Gastroenterology. 2014;146:S-227. [Google Scholar]
- 52.Coimbra AJ, Rimola J, O’Byrne S, et al. Magnetic resonance enterography is feasible and reliable in multicenter clinical trials in patients with Crohn’s disease, and may help select subjects with active inflammation. Aliment Pharmacol Ther. 2016;43:61–72. doi: 10.1111/apt.13453. [DOI] [PubMed] [Google Scholar]
- 53.Rimola J, Rodriguez S, Garcia-Bosch O, et al. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut. 2009;58:1113–1120. doi: 10.1136/gut.2008.167957. [DOI] [PubMed] [Google Scholar]
- 54.Steward MJ, Punwani S, Proctor I, et al. Non-perforating small bowel Crohn’s disease assessed by MRI enterography: derivation and histopathological validation of an MR-based activity index. Eur J Radiol. 2012;81:2080–2088. doi: 10.1016/j.ejrad.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 55.Oussalah A, Laurent V, Bruot O, et al. Diffusion-weighted magnetic resonance without bowel preparation for detecting colonic inflammation in inflammatory bowel disease. Gut. 2010;59:1056–1065. doi: 10.1136/gut.2009.197665. [DOI] [PubMed] [Google Scholar]
- 56.Rimola J, Ordas I, Rodriguez S, et al. Magnetic resonance imaging for evaluation of Crohn’s disease: validation of parameters of severity and quantitative index of activity. Inflamm Bowel Dis. 2011;17:1759–1768. doi: 10.1002/ibd.21551. [DOI] [PubMed] [Google Scholar]
- 57.Ordas I, Rimola J, Rodriguez S, et al. Accuracy of magnetic resonance enterography in assessing response to therapy and mucosal healing in patients with Crohn’s disease. Gastroenterology. 2014;146:374–382 e371. doi: 10.1053/j.gastro.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 58.Makanyanga JC, Pendse D, Dikaios N, et al. Evaluation of Crohn’s disease activity: initial validation of a magnetic resonance enterography global score (MEGS) against faecal calprotectin. Eur Radiol. 2014;24:277–287. doi: 10.1007/s00330-013-3010-z. [DOI] [PubMed] [Google Scholar]
- 59.Prezzi D, Bhatnagar G, Vega R, et al. Monitoring Crohn’s disease during anti-TNF-alpha therapy: validation of the magnetic resonance enterography global score (MEGS) against a combined clinical reference standard. Eur Radiol. 2015 doi: 10.1007/s00330-015-4036-1. [DOI] [PubMed] [Google Scholar]
- 60.Pariente B, Cosnes J, Danese S, et al. Development of the Crohn’s disease digestive damage score, the Lemann score. Inflamm Bowel Dis. 2011;17:1415–1422. doi: 10.1002/ibd.21506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pariente B, Mary JY, Danese S, et al. Development of the Lemann index to assess digestive tract damage in patients with Crohn’s disease. Gastroenterology. 2015;148:52–63 e53. doi: 10.1053/j.gastro.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 62.Samuel S, Bruining DH, Loftus EV, Jr, et al. Endoscopic skipping of the distal terminal ileum in Crohn’s disease can lead to negative results from ileocolonoscopy. Clin Gastroenterol Hepatol. 2012;10:1253–1259. doi: 10.1016/j.cgh.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 63.Higgins PD, Caoili E, Zimmermann M, et al. Computed tomographic enterography adds information to clinical management in small bowel Crohn’s disease. Inflamm Bowel Dis. 2007;13:262–268. doi: 10.1002/ibd.20013. [DOI] [PubMed] [Google Scholar]
- 64.Bruining DH, Loftus EV, Jr, Ehman EC, et al. Computed tomography enterography detects intestinal wall changes and effects of treatment in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2011;9:679–683 e671. doi: 10.1016/j.cgh.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 65.Panes J, Bouhnik Y, Reinisch W, et al. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis. 2013;7:556–585. doi: 10.1016/j.crohns.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 66.Deepak P, Fidler JF, et al. Radiologic Response is Associated with Better Long-Term Outcomes and is a Potential Treatment Target in Patients with Small Bowel Crohn’s Disease. Am J Gastroenterol. 2015;110:S786. doi: 10.1038/ajg.2016.177. [DOI] [PubMed] [Google Scholar]
- 67.Fleming TR, Powers JH. Biomarkers and surrogate endpoints in clinical trials. Stat Med. 2012;31:2973–2984. doi: 10.1002/sim.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pitcher A, Ashby D, Elliott P, et al. Cardiovascular MRI in clinical trials: expanded applications through novel surrogate endpoints. Heart. 2011;97:1286–1292. doi: 10.1136/hrt.2011.225904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bhatnagar G, Dikaios N, Prezzi D, et al. Changes in dynamic contrast-enhanced pharmacokinetic and diffusion-weighted imaging parameters reflect response to anti-TNF therapy in Crohn’s disease. Br J Radiol. 2015;88:20150547. doi: 10.1259/bjr.20150547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bettenworth D, Rieder F. Medical therapy of stricturing Crohn’s disease: what the gut can learn from other organs - a systematic review. Fibrogenesis Tissue Repair. 2014;7:5. doi: 10.1186/1755-1536-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pazahr S, Blume I, Frei P, et al. Magnetization transfer for the assessment of bowel fibrosis in patients with Crohn’s disease: initial experience. MAGMA. 2013;26:291–301. doi: 10.1007/s10334-012-0355-2. [DOI] [PubMed] [Google Scholar]
- 72.Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based Contrast Agent Accumulates in the Brain Even in Subjects without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy. Radiology. 2015;276:228–232. doi: 10.1148/radiol.2015142690. [DOI] [PubMed] [Google Scholar]
- 73.McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology. 2015;275:772–782. doi: 10.1148/radiol.15150025. [DOI] [PubMed] [Google Scholar]
- 74.Radbruch A, Weberling LD, Kieslich PJ, et al. Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology. 2015;275:783–791. doi: 10.1148/radiol.2015150337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


