Abstract
Walled-off pancreatic necrosis is a known complication of acute pancreatitis and requires intervention if symptomatic or complicated. Laparoscopic cystogastrostomy as a minimally invasive surgical intervention has been well-described in surgical literature but data on a robotic approach is limited. Here we report a case of robotic cystogastrostomy and debridement of walled-off pancreatic necrosis in a patient with a history of severe biliary pancreatitis.
Introduction
A walled-off pancreatic fluid collection is usually a complication of acute pancreatitis. It can also be the consequence of chronic pancreatitis, pancreatic trauma, or pancreatic duct obstruction. According to the revised Atlanta classification, early (<4 weeks since the acute episode of pancreatitis) peripancreatic fluid collections are divided into two categories: acute peripancreatic fluid collections (APFCs) or acute necrotic fluid collections (ANCs). APFCs are fluid collections that lack a wall and develop in the setting of interstitial edematous acute pancreatitis. They are usually sterile, self-limited, and not associated with necrotizing pancreatitis. ANCs contain necrotic debris due to pancreatic and/or peripancreatic necrosis. On the other hand, late (>4 weeks) peripancreatic fluid collections are divided into pancreatic pseudocysts or walled-off necrosis (WON). A pancreatic pseudocyst is a fluid collection with a well-defined non-epithelized wall that doesn’t contain any solid debris and usually develops as a sequela of interstitial edematous acute pancreatitis. WON is the mature form of ANC and consists of an encapsulated collection of fluid and necrotic tissue [1].
Pancreatic fluid collections often resolve spontaneously with time. Procedures to address these collections are indicated if the patient is symptomatic. In addition, an intervention is often warranted in complicated walled-off pancreatic necrosis. Possible complications warranting intervention include infection, pseudo-aneurysm, obstruction (gastric, duodenal, mesenteric venous or biliary), fistula formation (enteric, pleuropancreatic), and pancreatic ascites. The previously established dogma that a walled-off pancreatic fluid collection measuring 6 cm for more than 6 weeks is an indication for intervention has fallen away. Vitas et al. showed that 38 % of the pseudocysts greater than 10 cm in size treated conservatively resolved more than 6 months after the diagnosis with no serious complications [2]. Other series have shown similar results with successful conservative management in 39–48 % of asymptomatic pancreatic pseudocysts regardless of the size [3, 4].
There are different approaches to debride and drain WON: the classic open necrosectomy consists of entering the lesser sac through the gastrocolic ligament or transverse mesocolon and gently debriding the pancreatic necrosum. The transgastric approach can be performed open or laparoscopically. Finally, a retroperitoneal approach (including open or videoscopic techniques) has been described. A non-surgical approach includes percutaneous or endoscopic debridement. Percutaneous drainage is performed by an interventional radiologist via a CT-guided approach. Endoscopic transmural drainage can be performed by an interventional endoscopist in carefully selected patients but often requires multiple sessions. We will present the case of a patient who developed a symptomatic WON and was debrided and internally drained into the stomach through a robotic approach.
Case presentation
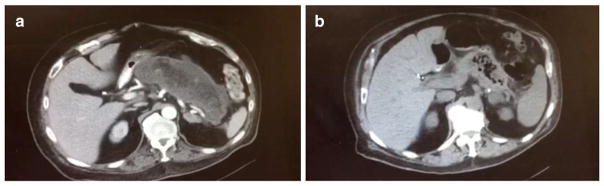
A sixty-four-year-old male with a past medical history of severe depression and hypothyroidism presented with abdominal pain, PO intolerance, and unintentional 60-pound weight loss over the last 4 months. His surgical history was notable for laparoscopic cholecystectomy at an outside hospital following an episode of biliary pancreatitis 5 months earlier. On physical exam, the patient was cachectic and frail. He had no evidence of jaundice. On his abdominal examination, there was obvious epigastric fullness with mild tenderness. Labs were unremarkable except for an albumin level of 2.9 g/dl and pre-albumin level of 15 mg/dl. A CT scan showed that the pancreas was replaced by a large complex fluid collection consistent with walled-off necrosis (Fig. 1a). The collection measured 18 cm transverse × 7 cm AP. Endoscopic ultrasound (EUS) showed a large complex WON not amenable to endoscopic drainage. A common bile duct stone was noted on EUS which was removed by endoscopic retrograde cholangiopancreatography without immediate or delayed post-procedure complications. After careful review of all possible treatment options, a decision was made to take the patient to the operating room for robotic cystogastrostomy and possible debridement of walled-off pancreatic necrosis.
Fig. 1.
Preoperative CT showing large complex fluid collection measuring 18 cm transverse × 7 cm AP consistent with walled-off necrosis (a). Four-month postoperative CT scan showing the resolution of WON (b)
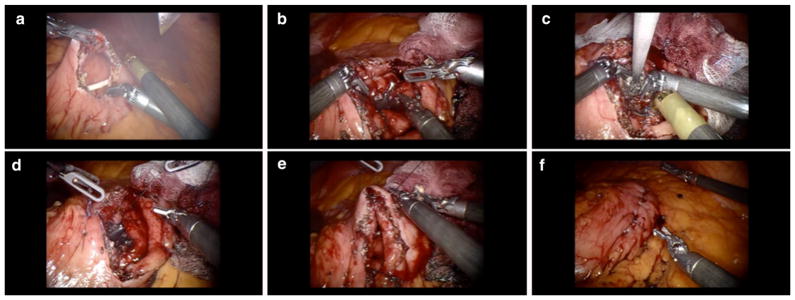
The patient was taken to the operating room and placed in a supine position under general anesthesia. After pneumoperitoneum creation, three 8-mm robotic ports, one 11-mm camera port, and one 11-mm assistant port were introduced. Upon inspection of the abdomen, the stomach was displaced anteriorly toward the abdominal wall by the WON. In the area of maximum bulge, an anterior gastrotomy was performed. The location of the pseudocyst was identified by needle aspiration, which also helped to exclude the possibility of injuring a major vessel. A posterior gastrotomy was performed with monopolar cautery, thereby exposing the WON wall which was then entered. Pancreatic necrotic tissue was debrided and fluid aspirated. The cyst was sewn in a single layer to the posterior gastric wall using two running 3–0 absorbable braided sutures. The cystogastrostomy was made to a diameter of approximately 3 cm to easily accommodate an endoscope in the future should it be necessary. The anterior gastrotomy was closed in a running fashion with a V-LOC device (Fig. 2). A leak test was performed and was negative.
Fig. 2.
Images showing the steps of robotic cystogastrostomy and debridement of WON. Anterior gastrotomy (a), posterior gastrotomy (b), debridement of WON (c), WON wall sutured to the posterior gastric wall (d), anterior gastric wall sutured with a V-LOC device (e), and leak test performed (f)
Postoperatively, the patient started to eat gradually. His weight improved over the next 3 months and his albumin normalized to 3.9 g/dl. A CT scan at 4 months showed the resolution of his WON (Fig. 1b).
Discussion
Open surgical debridement and drainage of WON was the traditional intervention of choice, but the morbidity of this operation is high [2]. Thus, minimally invasive methods have been adopted and preferred over the last few decades. Laparoscopic, endoscopic, percutaneous, and retroperitoneal approaches are other alternatives.
Endoscopic drainage became the first-line therapy in many tertiary care centers compared to surgical or percutaneous approaches [5, 6]. EUS-guided drainage of walled-off pancreatic fluid collections consists of the formation of a tract between the fluid collection and the stomach/duodenum. The tract is generally maintained patent by placing one or more plastic stents [7, 8]. While endoscopic treatment is largely successful for the drainage of a simple pancreatic pseudocyst, it is less so for the treatment of WON. Small plastic stents can become occluded, leading to infection and requiring repeated endoscopic interventions, especially in WON. In this context and in institutions lacking expertise in the field of advanced endoscopy or interventional radiology, surgical therapy is the ultimate treatment of choice in the appropriate clinical scenario. While laparoscopic cystogastrostomy is well-described in the literature, the robotic approach is still not well-established and only few case reports have been published [9, 10].
Minimally invasive surgery is the standard of care for many surgical abdominal pathologies. While laparoscopy has been adopted in many surgical procedures, robotic surgery is still in the developmental and experimental phase for many procedures [11]. A large body of evidence has shown that minimally invasive surgery is superior to an open approach as it is associated with less postoperative pain, a shorter hospital stay, and equivalent oncological outcomes in malignant diseases [11]. During the last decade, minimally invasive pancreatic surgery has been slowly adopted in large tertiary centers for both benign and malignant pathologies [12].
Laparoscopic management of benign pancreatic disease has been established for over a decade now, and it has been shown to be as effective as the open technique, with less morbidity [4]. On the other hand, the role, safety, and efficacy of robotic surgery in the treatment of WON has not been well-studied. We describe here a case of robotic anterior transgastric cystogastrostomy and demonstrate its feasibility and safety.
The robot can overcome some of the laparoscopic limitations in the management of WON, which is usually located in the lesser sac, a challenging location to access. In fact, the robot improves the surgical ergonomics as it increases the degrees of freedom to seven compared to four in laparoscopy. Furthermore, it eliminates the fulcrum effect created by the rigid laparoscopic instruments. This mirrors the human hand dexterity and allows better tissue handling. It facilitates operating in narrow spaces and angles not possible in laparoscopy and allows more precise suturing. In fact, the robot facilitates suturing the WON wall to the posterior gastric wall, which is technically more challenging with the laparoscopic approach and is usually substituted by the use of a staple device. In the case presented here, the WON wall was quite thick and may not have appropriately accommodated a stapler, making a hand-sewn technique more appropriate. Additionally, the cyst wall is often friable and crushes under compression, making stapler height selection somewhat difficult. Moreover, the robotic platform provides a 3-dimensional magnified image and computerized console that allows image-guided surgery. Ultrasound can be integrated and used to locate the WON and decide which approach is more suitable. Depending on the position of the WON and its adherence to the stomach, an anterior or posterior approach can be undertaken to perform a cystogastrostomy. While the robot can provide some technical advantages compared to laparoscopy, there is not enough data on the short- and long-term postoperative outcomes of this procedure for the treatment of WON. Lastly, minimally invasive cystogastrostomy does not preclude subsequent endoscopic debridement as the necrosum continues to evolve.
Conclusion
Robotic cystogastrostomy and debridement of WON is a feasible and safe operation and can be an alternative for the laparoscopic approach when the latter is indicated. There is a need for cost-benefit analysis and comparative studies to determine its efficacy and its long-term outcomes compared to the laparoscopic approach.
Acknowledgments
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award Number UL1TR001105. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Compliance with ethical standards
Consent section All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the editor-in-chief of this journal.
Conflict of interest Ibrahim Nassour, MD, Zeeshan Ramzan, MD, and Sachin Kukreja, MD, declare that they have no conflict of interest.
References
- 1.Sarr MG. 2012 revision of the Atlanta classification of acute pancreatitis. Pol Arch Med Wewn. 2013;123(3):118–124. doi: 10.20452/pamw.1627. [DOI] [PubMed] [Google Scholar]
- 2.Vitas GJ, Sarr MG. Selected management of pancreatic pseudocysts: operative versus expectant management. Surgery. 1992;111:123–130. [PubMed] [Google Scholar]
- 3.Cheruvu CV, Clarke MG, Prentice M, Eyre-Brook IA. Conservative treatment as an option in the management of pancreatic pseudocyst. Ann R Coll Surg Engl. 2003;85(5):313–316. doi: 10.1308/003588403769162413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergman S, Melvin WS. Operative and nonoperative management of pancreatic pseudocysts. Surg Clin North Am. 2007;87:1447–1460. doi: 10.1016/j.suc.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Baron TH, Harewood GC, Morgan DE, Yates MR. Outcome differences after endoscopic drainage of pancreatic necrosis, acute pancreatic pseudocysts, and chronic pancreatic pseudocysts. Gastrointest Endosc. 2002;56(1):7–17. doi: 10.1067/mge.2002.125106. [DOI] [PubMed] [Google Scholar]
- 6.Samuelson AL, Shah RJ. Endoscopic management of pancreatic pseudocysts. Gastroenterol Clin North Am. 2012;41:47–62. doi: 10.1016/j.gtc.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Cahen D, Rauws E, Fockens P, et al. Endoscopic drainage of pancreatic pseudocysts: long-term outcome and procedural factors associated with safe and successful treatment. Endoscopy. 2005;37:977–983. doi: 10.1055/s-2005-870336. [DOI] [PubMed] [Google Scholar]
- 8.Kahaleh M, Shami VM, Conaway MR, et al. Endoscopic ultrasound drainage of pancreatic pseudocyst: a prospective comparison with conventional endoscopic drainage. Endoscopy. 2006;38:355–359. doi: 10.1055/s-2006-925249. [DOI] [PubMed] [Google Scholar]
- 9.Cardenas A, Abrams A, Ong E, Jie T. Robotic-assisted cystogastrostomy for a patient with a pancreatic pseudocyst. J Robot Surg. 2014;8:181–184. doi: 10.1007/s11701-013-0428-x. [DOI] [PubMed] [Google Scholar]
- 10.Calin ML, Rahnemai-Azar AA, Anusak Y. Successful robotic cystogastrostomy after failed endoscopic drainage for infected pancreatic fluid collection post distal pancreatectomy. Chirurgia (Bucur) 2015;110:375–378. [PubMed] [Google Scholar]
- 11.Stafford AT, Walsh RM. Robotic surgery of the pancreas: the current state of the art. J Surg Oncol. 2015;112:289–294. doi: 10.1002/jso.23952. [DOI] [PubMed] [Google Scholar]
- 12.Del Chiaro M, Segersvärd R. The state of the art of robotic pancreatectomy. Biomed Res Int. 2014;2014:920492–920495. doi: 10.1155/2014/920492. [DOI] [PMC free article] [PubMed] [Google Scholar]