Abstract
Background
Previous studies have suggested that preoperative chemoradiation is associated with an improved margin negative resection rate among patients who undergo pancreatoduodenectomy for pancreatic ductal adenocarcinoma (PDAC). However, the optimal preoperative regimen has not been established.
Methods
All patients with PDAC who received chemotherapy and/or chemoradiation followed by pancreatoduodenectomy between 1999-2014 were retrospectively reviewed. The effects of two external-beam radiation regimens – a standard course of 50.4Gy/28 fractions and a hypofractionated course of 30Gy/10 fractions – were compared. Differences in clinicopathologic characteristics, locoregional recurrence (LR), and overall survival (OS) were assessed.
Results
Among 472 patients who received preoperative therapy, 224 (47.5%) received 30Gy, 221 (46.8%) received 50.4Gy and 27 (5.7%) received chemotherapy alone. Patients who received 50.4Gy were more likely to have advanced stage disease and to have received induction and postoperative chemotherapy, but there was no difference in R1 margin status, treatment effect, LR or OS between the two radiation groups (all p>0.05). Patients who received preoperative chemoradiation had a lower rate of LR compared to preoperative chemotherapy alone (p<0.01). On multivariate Cox proportional hazards analysis, 50.4Gy was associated with similar OS and LR compared to 30Gy while the absence of preoperative radiation was associated with a higher rate of LR (OR 2.21, 95% CI 1.04-4.70) and similar OS.
Conclusion
Preoperative hypofractionated chemoradiation was associated with similar local control and overall survival compared to standard CRT in patients undergoing pancreatoduodenectomy for PDAC. The use of chemotherapy alone without chemoradiation was associated with improved local control but similar survival.
Keywords: neoadjuvant therapy, pancreatic cancer, chemotherapy, chemoradiation, local recurrence, gemcitabine, FOLFIRINOX, pancreatectomy, whipple
Introduction
Cancer cells are identified at the resection margins following as many as 90% of operations performed with curative intent for localized pancreatic ductal adenocarcinoma (PDAC)1. Irradiation of the surgical field following pancreatectomy has been advocated by many investigators in an attempt to reduce residual microscopic disease, which has been associated with both locoregional recurrence (LR) and a short duration of overall survival (OS)2,3. However, randomized phase III trials of postoperative chemoradiation (CRT) have not conclusively established its efficacy, so its use remains controversial4–6.
Our group and others have postulated that CRT may be administered most effectively prior to, instead of following, surgical resection, as is true for several other gastrointestinal tumors7,8. Potential advantages to the delivery of CRT preoperatively include the opportunity to select patients with appropriate physiology and tumor biology for major surgery, a reduction in toxicity to adjacent structures, an ability to sterilize tissues at oncologically critical margins, and a possible reduction in the size or anatomic extent of disease prior to subsequent resection. We have previously shown that preoperative CRT, combined with meticulous dissection of the tissue between the pancreatic head and superior mesenteric artery (SMA)9, maximizes clearance of cancer cells from the retroperitoneum10 and is associated with favorable long-term survival11.
Conventional postoperative CRT for PDAC consists of 5.5 weeks of standard fractionated external beam radiotherapy (EBRT) with concurrent sensitizing chemotherapy12. A similar regimen has also been delivered prior to surgery by clinicians who favor a preoperative treatment strategy13–15. However, prolonged local irradiation of the primary tumor and regional lymph nodes has raised concerns for both treatment-related toxicity and systemic disease progression that might preclude subsequent resection. These concerns have led to interest in the use of hypofractionated regimens that allow therapeutic doses of radiation to be delivered in as few as 5 days. Several studies have shown that hypofractionated CRT can be delivered safely and effectively16–18. However, the effects of radiation dose upon the primary tumor and regional lymph nodes have been incompletely characterized, and the influence of these effects upon oncologic outcomes remains unclear.
At the University of Texas MD Anderson Cancer Center, most patients with resectable or borderline resectable cancers of the pancreatic head receive gemcitabine- or 5-fluorouracil-based CRT and/or systemic chemotherapy prior to planned pancreatoduodenectomy (PD). EBRT has historically consisted of either a standard regimen (1.8Gy, 28 fractions, total 50.4Gy) over 5.5 weeks or a hypofractionated regimen (3Gy, 10 fractions, total 30Gy) over 2 weeks. Using this experience, we sought to determine the potential influence of both preoperative radiation and radiation dose on margin status, local control, and overall survival of patients following PD.
Methods
The MD Anderson Cancer Center Institutional Review Board approved this retrospective study. We used a prospectively maintained institutional pancreatic tumor database to identify all patients who received preoperative chemotherapy and/or chemoradiation prior to PD for PDAC between 1999 and 201419.
Staging
Prior to the initiation of preoperative therapy, all patients underwent a comprehensive staging evaluation, including a pancreatic-protocol CT scan. Tumors were classified as potentially resectable (PR), borderline resectable (BR), or locally advanced (LA) using previously published clinical criteria20.
Neoadjuvant Regimens
Two primary chemoradiation regimens were used: hypofractionated EBRT (30 Gy/10 fractions) or standard EBRT (50.4 Gy/28 fractions) with concurrent 5-fluorouracil, capecitabine, or gemcitabine. EBRT was delivered 5 days per week (Monday-Friday). CT-based three-dimensional conformal treatment planning was routinely used. The primary tumor, pancreatoduodenal, portahepatic, superior mesenteric, and celiac axis lymph nodes were typically included in the treatment volume with a 1-cm margin for microscopic extension, 5-mm cranial and caudal margin for respiratory motion, and 5-mm margin for setup error. If the likelihood of resection was estimated as low, the portahepatic nodes were not treated in the patients receiving 50.4Gy to reduce duodenal toxicity. Systemic chemotherapy was delivered prior to CRT selectively.
Surgical Technique
After completion of preoperative therapy, all patients underwent a comprehensive restaging evaluation. Those with optimized physiology and absence of radiographic or intraoperative findings of disease progression were selected for surgical resection20. Radiographic downstaging was not required. PD was performed using a standardized technique9,10. Dissection of the uncinate process was performed by skeletonizing the right lateral aspect of the SMA from its origin to the level of the first jejunal branch of the SMV10.
Histopathologic Analysis
All surgical specimens were evaluated using a standardized protocol21. Specifically, the surgeon and pathologist inked the pancreatic neck, bile duct margin, and SMA margin immediately following removal of the specimen. The pancreatic and bile duct margins were inked en face and were considered positive if tumor cells were present at the ink. In contrast, the entire inked SMA margin was sectioned perpendicularly for microscopic evaluation. The SMA margin distance was prospectively measured as the closest microscopic distance, to the nearest millimeter, between cancer cells and the inked SMA margin. A positive SMA margin was defined as tumor cells at or within 1 mm of the ink. An R1 margin status was assigned to the resection if any margin was defined as positive. Treatment effect was measured histologically as the percentage of residual viable cancer cells22.
Postoperative Therapy and Follow-up
Following PD, postoperative therapy was administered selectively. Patients were evaluated every 4-6 months with cross-sectional imaging, physical examination, and CA 19-9 analysis according to a standardized algorithm23. The development of a new low-density mass in the region of the pancreatic remnant or new lymphadenopathy at the root of the mesentery was considered evidence of LR. Distant recurrence was defined as new lesions in the lung, liver, peritoneum, or elsewhere with imaging characteristics consistent with metastasis.
Statistical Analysis
Clinicopathological variables associated with patients who received 30Gy and 50.4Gy regimens were compared. The same variables were compared between patients who received CRT and those who did not. Categorical variables were compared using the Fisher exact test while continuous variables were compared using the two-tailed student’s t-test. OS, recurrence-free survival (RFS), and LR (occurring as a component of first failure) were compared among the three groups using the Kaplan-Meier method. Statistical significance was assessed using the Mantel-Cox log rank test. Univariate and multivariate Cox proportional hazards regression models were created to identify factors associated with OS and LR. The radiation dose and non-collinear variables with p<0.2 on univariate analysis were included in the multivariate model. Statistical analysis was performed using SPSS 22.0 software (SPSS Inc., Chicago, IL, USA) with significance established at p<0.05.
Results
A total of 483 consecutive patients with localized PDAC received chemotherapy and/or CRT prior to PD between 1999 and 2014. Eleven patients who received an unknown radiation regimen at the referring facility were excluded from further analysis. Of the remaining 472, 221 (46.8%) received 50.4Gy EBRT with or without systemic chemotherapy, 224 patients (47.5%) received 30Gy EBRT with or without systemic chemotherapy, and 27 (5.7%) received systemic chemotherapy alone. The baseline clinical profile of these 472 patients who comprised the study population is presented in Table 1. Patients who received 50.4Gy were more likely to have been staged at presentation with advanced disease, to have received induction and postoperative systemic chemotherapy, and to have been treated in recent years (all p <0.05) compared to patients who received 30Gy.
Table 1.
Clinical, operative, and histopathologic characteristics of patients included in this study (N = 472).
| 50.4Gy (n = 221) |
30Gy (n = 224) |
p* | No XRT (n = 27) |
p# | |
|---|---|---|---|---|---|
| Clinical | |||||
| Age, years, median (range) | 63.9 (34-85) | 64.7 (38-85) | 0.30 | 59.6 (40-75) | 0.25 |
| Sex, n (%) | 0.21 | 0.42 | |||
| Male | 120 (54.3) | 136 (60.7) | 14 (51.9) | ||
| Female | 101 (45.7) | 88 (39.3) | 13 (48.1) | ||
| Radiographic Staging, n (%) | <0.001 | 0.30 | |||
| Potentially Resectable | 125 (56.6) | 202 (90.2) | 24 (88.9) | ||
| Borderline Resectable | 71 (32.1) | 17 (7.6) | 2 (7.4) | ||
| Locally Advanced | 25 (11.3) | 5 (2.2) | 1 (3.7) | ||
| Year of Surgery, n (%) | <0.0001 | 0.01 | |||
| 1999-2004 | 17 (7.7) | 139 (62.1) | 6 (22.2) | ||
| 2005-2009 | 91 (41.2) | 46 (20.5) | 4 (14.8) | ||
| 2010-2014 | 113 (51.1) | 39 (17.4) | 17 (63.0) | ||
| Pre-Treatment CA 19-9, median (range) | 135.5 (1-11482) | 124.6 (1-5830) | 0.31 | 125.4 | 0.94 |
| Preoperative Chemotherapy, n (%) | 139 (62.9) | 97 (43.3) | <0.001 | 27 (100.0) | <0.0001 |
| Sensitizing Chemotherapy, n (%) | <0.001 | <0.0001 | |||
| Gemcitabine | 93 (42.1) | 133 (59.4) | 0 | ||
| Capecitabine/5-Fluorouracil | 124 (56.1) | 91 (40.6) | 0 | ||
| Postoperative Chemotherapy, n (%) | 99 (44.8) | 48 (21.4) | <0.0001 | 21 (77.8) | <0.0001 |
|
| |||||
| Surgical | |||||
| EBL, mL, median (range) | 600 (125-6000) | 650 (100-5100) | 0.21 | 625 (200-1750) | 0.32 |
| Vascular Resection, n (%) | 101 (45.7) | 76 (33.9) | 0.01 | 11 (40.7) | 0.84 |
| Lymph Nodes Excised, median (range) | 23.0 (6-61) | 22.5 (5-60) | 0.06 | 27 (10-68) | 0.001 |
|
| |||||
| Pathology | |||||
| Tumor Size, cm, median (StDev) | 2.4 (0-8.5) | 2.5 (0-8.0) | 0.94 | 2.8 (0.5-5.7) | 0.10 |
| Differentiation, n (%) | 0.05 | 0.48 | |||
| Well | 2 (0.9) | 5 (2.2) | 0 | ||
| Moderate | 129 (58.3) | 151 (67.4) | 21 (77.8) | ||
| Poor | 83 (37.6) | 58 (25.9) | 6 (22.2) | ||
| Unknown | 7 (3.2) | 10 (4.5) | 0 | ||
| SMA Margin Length, mm, median (range) | 5.0 (0-30) | 5.0 (0-40) | 0.30 | 4.0 (0-17) | 0.09 |
| SMA Margin Length, n (%) | 0.96 | 0.36 | |||
| 0-1.0 mm | 49 (22.2) | 52 (23.2) | 6 (22.2) | ||
| >1.0-5.0 mm | 71 (32.1) | 75 (33.5) | 12 (44.4) | ||
| >5.0 mm | 94 (42.5) | 94 (42.0) | 8 (18.5) | ||
| Margin Status†, n (%) | |||||
| R0 | 171 (77.4) | 167 (74.6) | 0.60 | 18 (66.7) | 0.31 |
| R1 | 50 (22.6) | 57 (25.4) | 9 (33.3) | ||
| Lymph Node Status, n (%) | |||||
| Positive (N1) | 102 (46.2) | 132 (58.9) | <0.01 | 22 (81.5) | <0.01 |
| Negative (N0) | 119 (53.8) | 92 (41.1) | 5 (18.5) | ||
| Lymph Node Ratio, mean (StDev) | 0.06 (0.1) | 0.09 (0.12) | <0.01 | 0.16 (0.15) | <0.0001 |
| Percentage of Viable Cells, n (%) | |||||
| 0 | 8 (3.6) | 7 (3.1) | 0.84 | 0 | <0.001 |
| >0-5% | 25 (11.3) | 25 (11.2) | 0 | ||
| >5-10% | 31 (14.0) | 24 (10.7) | 0 | ||
| >10-30% | 66 (29.9) | 69 (30.8) | 3 (11.1) | ||
| >30% | 89 (40.3) | 96 (42.9) | 21 (77.8) | ||
| Lymphovascular Invasion, n (%) | <0.0001 | 0.02 | |||
| Positive | 95 (43.0) | 85 (37.9) | 18 (66.7) | ||
| Negative | 120 (54.3) | 107 (47.8) | 9 (33.3) | ||
| Unknown | 6 (2.7) | 32 (14.3) | 0 | ||
| Perineural Invasion, n (%) | <0.001 | 0.22 | |||
| Positive | 163 (73.8) | 164 (73.2) | 24 (88.9) | ||
| Negative | 53 (24.0) | 37 (16.5) | 3 (11.1) | ||
| Unknown | 5 (2.2) | 23 (10.3) | 0 | ||
Abbreviations: XRT, radiation therapy; EBL, estimated blood loss; StDev, standard deviation; SMA, superior mesenteric artery.
p-Values compare 50.4Gy vs 30Gy;
p-Values compare XRT (50.4Gy or 30Gy) with No XRT;
See methods for definition
Surgical and histopathologic variables are also presented in Table 1. Tumors treated to 50.4Gy were more likely to be poorly differentiated and to be associated with lymphovascular invasion than those treated to 30Gy, but the 50.4Gy regimen was associated with a higher rate of negative lymph nodes and a lower mean LN ratio than 30Gy (all <0.05). The median SMA margin length, SMA length distribution,rate of R1 margin status, and treatment effect scores of tumors treated to 50.4Gy and 30Gy were similar. Furthermore, there was no significant difference in LR, RFS, or OS between the two groups of patients (Table 2 and Figure).
Table 2.
Outcomes of patients who received neoadjuvant therapy prior to pancreaticoduodenectomy
| 50.4Gy (n = 221) |
30Gy (n = 224) |
p-Value* | No XRT (n = 27) |
p-Value# | |
|---|---|---|---|---|---|
| Locoregional Recurrence, n (%) | 48 (21.7) | 49 (21.9) | 0.73 | 9 (33.3) | <0.01 |
| Any Recurrence | 0.54 | <0.05 | |||
| Recurrence-Free Survival, Months, Median | 14.7 | 16.0 | 9.9 | ||
| 5-year Recurrence-Free Survival | 28.1% | 31.4% | 14.2% | ||
| Overall Survival | 0.41 | 0.33 | |||
| Overall Survival, Months, Median | 38.2 | 33.7 | 28.4 | ||
| 5-year Overall Survival | 36.1% | 28.9% | 27.2% | ||
| Follow-up, Months, Mean (StDev) | 36.3 (23.1) | 49.1 (45.0) | <0.001 | 30.8 (20.9) | 0.08 |
p-Values compare 30Gy vs 50.4Gy;
p-Values compare XRT (30Gy or 50.4Gy) vs No XRT
Figure.
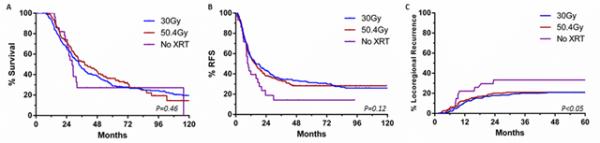
Kaplan-Meier curves of A) overall survival, B) recurrence-free survival, and C) locoregional recurrence based on preoperative radiation regimens for patients with ductal adenocarcinoma who underwent pancreaticoduodenectomy
Patients who did not receive preoperative CRT were clinically similar at baseline to patients who did. Although there was no difference in margin status or median SMA margin length, the absence of radiation was associated with a higher rate of positive lymph nodes, a higher LN ratio, and a poorer treatment effect score (all p<0.05, Table 1). The absence of preoperative CRT was associated with a higher rate of LR but no significant difference in OS (Table 2 and Figure).
Results of a multivariate Cox proportional hazards regression analysis for OS and LR are reported in Table 3 and Table 4, respectively. Compared to the 30Gy regimen, 50.4Gy was associated with similar OS (OR 0.93, 95% CI 0.71-1.22) and LR (OR 1.30, 95% CI 0.81-2.08). The absence of CRT was associated with a higher rate of LR (OR 2.21, 95% CI 1.04-4.70) but no difference in OS (OR 1.24, 95% CI 0.16-9.50).
Table 3.
Results of univariate and multivariate Cox regression analysis for overall survival
| Odds Ratio (95% Confidence Interval), p | ||
|---|---|---|
| Univariate | Multivariate | |
| Clinical | ||
| Age | ||
| <50 years | - | |
| 50-70 years | 0.89 (0.59-1.34), 0.59 | |
| >70 years | 1.09 (0.70-1.70), 0.70 | |
| Male Sex | 1.22 (0.97-1.53), 0.09 | 1.09 (0.82-1.45), 0.54 |
| Radiographic Staging | ||
| Potentially Resectable | - | |
| Borderline Resectable | 0.86 (0.63-1.17), 0.34 | |
| Locally Advanced | 0.76 (0.48-1.20), 0.23 | |
| Year of Surgery | ||
| 1999-2004 | - | |
| 2005-2009 | 0.90 (0.70-1.17), 0.45 | |
| 2010-2015 | 0.84 (0.62-1.14), 0.25 | |
| Pre-Treatment CA 19-9 | 1.29 (1.03-1.62), 0.03 | 1.04 (0.79-1.37), 0.78 |
| Neoadjuvant Chemotherapy | 1.15 (0.92-1.45), 0.22 | |
| Sensitizing Chemotherapy | ||
| Gemcitabine | - | |
| Capecitabine/5-Fluorouracil | 1.28 (1.01-1.62), 0.04 | 1.31 (1.0-1.73), 0.053 |
| XRT | ||
| 30Gy | - | |
| 50.4Gy | 0.91 (0.72-1.15), 0.42 | 0.93 (0.71-1.22), 0.60 |
| None | 1.21 (0.74-1.97), 0.45 | 1.24 (0.16-9.50), 0.84 |
| Adjuvant Chemotherapy | 0.94 (0.73-1.20), 0.60 | |
|
| ||
| Surgical | ||
| EBL | ||
| ≤500 mL | - | - |
| >500-1000 mL | 1.20 (0.93-1.55), 0.16 | 1.11 (0.81-1.52), 0.51 |
| >1000 mL | 1.61 (1.19-2.18), 0.002 | 1.42 (0.98-2.07), 0.07 |
| Vascular Resection | 1.55 (1.24-1.95), <0.001 | 1.46 (1.09-1.94), 0.01 |
| Lymph Nodes Excised | ||
| <15 | - | |
| 15-30 | 1.02 (0.72-1.43), 0.93 | |
| >30 | 1.00 (0.67-1.49), 1.0 | |
|
| ||
| Pathology | ||
| Tumor Size | 1.15 (1.07-1.25), <0.001 | 0.95 (0.86-1.07), 0.40 |
| Differentiation | ||
| Well/Moderate | - | - |
| Poor | 1.34 (1.05-1.71), 0.02 | 1.37 (1.02-1.83), 0.03 |
| Margin Status | ||
| R0 | - | - |
| R1 | 2.07 (1.62-2.65), <0.001 | 1.37 (1.01-1.86), 0.04 |
| SMA Margin Length | ||
| 0 mm | - | |
| >0-1.0 mm | 1.08 (0.65-1.80), 0.77 | |
| >1.0-5.0 mm | 0.67 (0.41-1.09), 0.11 | |
| >5.0 mm | 0.43 (0.26-0.71), 0.001 | |
| Positive Lymph Nodes | 1.87 (1.48-2.35), <0.001 | 1.40 (1.04-1.90), 0.03 |
| Percentage of Viable Cells | ||
| 0 | - | - |
| >0-5% | 1.62 (0.63-4.2), 0.32 | 1.62 (0.21-12.52), 0.65 |
| >5-10% | 2.38 (0.94-6.04), 0.07 | 1.89 (0.25-14.45), 0.54 |
| >10-30% | 2.85 (1.16-7.0), 0.02 | 1.91 (0.25-14.49), 0.53 |
| >30% | 2.73 (1.12-6.66), 0.03 | 1.69 (0.22-12.99), 0.62 |
| Lymph Node Ratio | ||
| 0 | - | |
| 0-0.2 | 1.61 (1.25-2.06), <0.001 | |
| ≥0.2 | 3.36 (2.42-4.65), <0.001 | |
| Lymphovascular Invasion | 1.75 (1.38-2.22), <0.001 | 1.08 (0.79-1.46), 0.63 |
| Perineural Invasion | 2.50 (1.80-3.49), <0.001 | 1.84 (1.21-2.80), 0.004 |
Table 4.
Results of univariate and multivariate Cox regression analysis for local recurrence
| Odds Ratio (95% Confidence Interval), p | ||
|---|---|---|
| Univariate | Multivariate | |
| Clinical | ||
| Age | ||
| <50 years | - | |
| 50-70 years | 0.77 (0.40-1.48), 0.43 | |
| >70 years | 0.61 (0.29-1.30), 0.20 | |
| Male Sex | 0.81 (0.55-1.18), 0.27 | |
| Radiographic Staging | ||
| Potentially Resectable | - | |
| Borderline Resectable | 1.03 (0.62-1.72), 0.92 | |
| Locally Advanced | 1.16 (0.58-2.32), 0.67 | |
| Year of Surgery | ||
| 1999-2004 | - | |
| 2005-2009 | 0.77 (0.48-1.23), 0.27 | |
| 2010-2015 | 0.95 (0.60-1.50), 0.83 | |
| Pre-Treatment CA 19-9 | 1.57 (1.07-2.31), 0.02 | 1.39 (0.89-2.17), 0.15 |
| Neoadjuvant Chemotherapy | 1.92 (1.29-2.88), 0.001 | 1.50 (0.92-2.47), 0.11 |
| Sensitizing Chemotherapy | ||
| Gemcitabine | - | |
| Capecitabine/5-Fluorouracil | 1.18 (0.79-1.76), 0.43 | |
| XRT | ||
| 30Gy | - | - |
| 50.4Gy | 1.07 (0.72-1.61), 0.73 | 1.30 (0.81-2.08), 0.28 |
| None | 2.41 (1.25-4.64), <0.01 | 2.21 (1.04-4.70), 0.04 |
| Adjuvant Chemotherapy | 1.29 (0.87-1.90), 0.20 | |
|
| ||
| Surgical | ||
| EBL | ||
| ≤500 mL | - | - |
| >500-1000 mL | 1.04 (0.67-1.62), 0.87 | 1.05 (0.64-1.72), 0.86 |
| >1000 mL | 1.84 (1.13-3.0), 0.015 | 1.69 (0.97-2.94), 0.06 |
| Vascular Resection | 1.70 (1.16-2.50), 0.007 | 1.21 (0.77-1.91), 0.41 |
| Lymph Nodes Excised | ||
| <15 | - | |
| 15-30 | 1.45 (0.75-2.80), 0.27 | |
| >30 | 1.02 (0.48-2.19), 0.95 | |
|
| ||
| Pathology | ||
| Tumor Size | 1.20 (1.06-1.35), 0.004 | 1.05 (0.88-1.24), 0.61 |
| Differentiation | ||
| Well/Moderate | - | |
| Poor | 0.89 (0.57-1.38), 0.60 | |
| Margin Status | ||
| R0 | - | - |
| R1 | 1.94 (1.27-2.95), 0.002 | 1.27 (0.78-2.05), 0.33 |
| SMA Margin Length | ||
| 0mm | - | |
| >0-1.0mm | 1.06 (0.43-2.63), 0.90 | |
| >1.0-5.0mm | 0.78 (0.33-1.85), 0.58 | |
| >5.0mm | 0.44 (0.18-1.04), 0.06 | |
| Positive Lymph Nodes | 2.06 (1.39-3.06), <0.0001 | 1.63 (1.01-2.62), 0.05 |
| Lymph Node Ratio | ||
| 0 | - | |
| 0-0.2 | 1.70 (1.11-2.61), 0.01 | |
| ≥0.2 | 4.22 (2.43-7.33), <0.0001 | |
| Lymphovascular Invasion | 1.67 (1.12-2.50), 0.01 | 0.85 (0.52-1.38), 0.50 |
| Perineural Invasion | 3.72 (1.93-7.16), <0.0001 | 2.60 (1.26-5.36), 0.01 |
Discussion
In this retrospective review of almost 500 patients with localized PDAC who underwent PD following preoperative therapy, we found no significant difference in the rate of positive surgical margins, the rate of LR, or the duration of OS among patients who received hypofractionated CRT to 30Gy over 2 weeks versus those who received standard CRT to 50.4Gy over 5.5 weeks. Compared to patients who received systemic chemotherapy alone, the receipt of either radiation regimen prior to pancreatectomy was associated with improvement in locoregional control but not survival.
The role of perioperative radiation therapy in the management of patients with localized PDAC remains controversial. The results of early randomized trials evaluating the efficacy of postoperative CRT were inconsistent: a Gastrointestinal Tumor Study Group study concluded postoperative CRT was beneficial6; a European Organization for Research and Treatment of Cancer study showed no benefit4, and a European Study Group for Pancreatic Cancer trial (ESPAC-1) concluded that it adversely effects survival5. Subsequent large, albeit retrospective, analyses have suggested a survival advantage with postoperative CRT, so its use remains common in the United States 24,25. The RTOG-0848 study, which is actively randomizing patients following pancreatectomy to either gemcitabine or gemcitabine followed by conventional CRT, should clarify the role of postoperative CRT26.
In the preoperative setting, existing data have been generated in small, phase I/II trials of hypofractionated16,17, standard fractionated14,15, and several novel radiotherapy regimens27–30. Although these studies have suggested that CRT can be administered safely prior to PD, the efficacy of radiation in this setting remains unclear because few comparative studies have been performed. Golcher et al performed the first randomized controlled trial to compare preoperative CRT to surgery alone in resectable PDAC. Although no significant differences were seen in survival, the study was terminated early after enrolling only 73 of the anticipated 254 patients31. In an attempt to provide further insight, the NEOPA study will randomize patients with resectable PDAC to preoperative, standard fractionated gemcitabine-based EBRT or immediate surgery. The study is expected to conclude in 202032. In the BR disease setting, in which preoperative therapy is often recommended33, the Alliance for Clinical Trials in Oncology Study A021501, a phase II trial set to activate in May 2016, will randomize patients to either FOLFIRINOX or FOLFIRINOX followed by hypofractionated CRT prior to planned PD34.
The preoperative period provides a critical opportunity to select patients with favorable tumor biology and appropriate physiology for subsequent surgery, and to leverage the cytotoxic effects of anticancer therapies. An advantage specifically ascribed to radiation treatments in this setting is its purported ability to sterilize surgical margins. Although we could demonstrate no difference between the status of surgical margins of patients who received preoperative CRT and those who received systemic chemotherapy alone, the results herein suggest that PD may be associated with improved locoregional cancer control when preceded by CRT. To the extent that this benefit truly exists, hypofractionated regimens might be preferable over conventional CRT for several reasons. First, conventional CRT is generally delivered over a duration of 5.5 weeks followed by a 6-week treatment break. Because radiotherapy acts only upon the primary tumor and regional lymph nodes, the systemic micrometastatic disease that is presumed to exist in all patients with PDAC may be suboptimally treated for as many as 3-4 months prior to resection. Hypofractionated regimens, delivered over a duration as short as 5 days, may reduce this window substantially and help alleviate concern for tumor progression during radiation therapy. Furthermore, hypofractionated regimens may be better tolerated and associated with lower toxicity than the conventional approach—an important consideration in the preoperative setting35,36.
It should be emphasized that hypofractionated regimens treat the primary tumor and regional lymph nodes to a lower total dose than standard fractionated CRT: using the linear-quadratic formula, early and late biologically equivalent doses (BED) for the 50.4Gy and 30Gy regimens described here are approximately 59.5Gy10 and 80.6Gy3 vs 39Gy10 and 60Gy3, respectively. Nonetheless, a short course, hypofractionated regimen appears to be sufficient for many patients with localized disease, in whom tumor resection is the ultimate therapeutic objective. As suggested by the data herein, treatment to a higher total dose would seem appropriate for patients with regionally advanced disease and known lymphadenopathy. A conventional regimen similar to that used in the locally advanced setting should also be strongly considered for patients unlikely to ever undergo pancreatectomy on the basis of their personal physiology, tumor anatomy, or the anticipated behavior of their cancer.
The results of this study should be interpreted within the context of its limitations, foremost among them being the use of a single-institution, retrospective dataset of patients for whom treatment decisions were not randomly made. The differences observed in disease stage of the 30Gy and 50.4Gy groups likely reflect, at least in part, provider selection bias to treat more advanced tumors with higher doses of radiation. Therefore, although the data herein could be interpreted as indicating that the two radiation regimens were equally effective, an alternative interpretation might be that the higher dose of radiation is necessary to achieve equivalent clinical results in advanced disease settings. A related limitation is the inclusion of only patients that successfully underwent resection; it is possible that the longer duration over which treatment was administered in the 50.4Gy group allowed more patients with aggressive disease to develop systemic metastases, eliminating them from analysis. For this reason, we were also unable to evaluate the effects of CRT on the anatomic extent of the primary tumor. However, we previously demonstrated that a minority of patients experience a partial Response Evaluation Criteria in Solid Tumors (RECIST) response to preoperative therapy, and that such a response does not correlate with survival37. Finally, while no toxicity data were evaluated in this study, we have shown in prospective trials that both of these radiation regimens are associated with minimal toxicities that do not preclude subsequent surgery16,35.
The strengths of this study must also be acknowledged. This is the largest study that has evaluated the relative efficacy of two different preoperative radiation regimens in the setting of PD for PDAC. Furthermore, the translational database we use is prospectively maintained and managed by trained, full-time personnel using abstracted data and standardized algorithms similar to those used in the management of large, national data sets. Finally, despite the baseline differences among the treatment groups, a multivariate analysis was constructed to control for these differences and their potential influence upon oncologic outcomes.
In conclusion, in this study designed to investigate the impact of preoperative radiation regimens on outcomes of patients undergoing PD for PDAC, we found that patients who received any form of preoperative CRT had better locoregional control than those receiving chemotherapy alone. Furthermore, hypofractionated CRT was associated with similar margin-negative resection rates, treatment effect, LR, and OS compared to standard fractionated CRT.
Precis.
In this single institution review of patients who received chemotherapy and/or chemoradiation prior to pancreatoduodenectomy for pancreatic ductal adenocarcinoma, the administration of hypofractionated chemoradiation was associated with similar margin negative resection rates, treatment effect, locoregional recurrence and overall survival compared to standard chemoradiation. The use of either chemoradiation regimen was associated with improved locoregional control, but not overall survival, compared to preoperative systemic chemotherapy alone.
Acknowledgments
Funding Sources: Supported by the Khalifa Bin Zayed Al Nahyan Foundation, the Various Donor Pancreatic Research Fund at the University of Texas MD Anderson Cancer Center, and by NIH/NCI under award number P30CA016672.
Footnotes
Conflicts of Interest: None
Author Contributions:
Cloyd: conceptualization, methodology, formal analysis, investigation, writing-original draft,writing-review and editing
Das: conceptualization, writing-review and editing
Krishnan: conceptualization, writing-review and editing
Prakash: writing-review and editing, methodology
Snyder: writing-review and editing
Varadhachary: conceptualization, writing-review and editing
Wolff: conceptualization, writing-review and editing, supervision
Javle: conceptualization, writing-review and editing
Shroff: conceptualization, writing-review and editing
Fogelman: conceptualization, writing-review and editing
Overman: conceptualization, writing-review and editing
Wang: conceptualization, writing-review and editing, methodology, validation, investigation
Maitra: conceptualization, writing-review and editing
Lee: conceptualization, writing-review and editing, funding acquisition, methodology, resources
Fleming: conceptualization, writing-review and editing, supervision
Katz: conceptualization, writing-review and editing, methodology, validation, resources, writing-original draft, visualization, supervision, project administration, funding acquisition
References
- 1.Verbeke CS, Gladhaug IP. Resection margin involvement and tumour origin in pancreatic head cancer. Br. J. Surg. 2012;99:1036–1049. doi: 10.1002/bjs.8734. [DOI] [PubMed] [Google Scholar]
- 2.Neoptolemos JP, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann. Surg. 2001;234:758–768. doi: 10.1097/00000658-200112000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gnerlich JL, et al. Microscopic margins and patterns of treatment failure in resected pancreatic adenocarcinoma. Arch. Surg. Chic. Ill 1960. 2012;147:753–760. doi: 10.1001/archsurg.2012.1126. [DOI] [PubMed] [Google Scholar]
- 4.Klinkenbijl JH, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann. Surg. 1999;230:776–782. doi: 10.1097/00000658-199912000-00006. discussion 782–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neoptolemos JP, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N. Engl. J. Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 6.Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch. Surg. Chic. Ill 1960. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 7.van Hagen P, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 8.Improved survival with preoperative radiotherapy in resectable rectal cancer Swedish Rectal Cancer Trial. N. Engl. J. Med. 1997;336:980–987. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 9.Katz MHG, et al. Retroperitoneal Dissection in Patients with Borderline Resectable Pancreatic Cancer: Operative Principles and Techniques. J. Am. Coll. Surg. 2012;215:e11–e18. doi: 10.1016/j.jamcollsurg.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz MHG, et al. Effect of neoadjuvant chemoradiation and surgical technique on recurrence of localized pancreatic cancer. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract. 2012;16:68–78. doi: 10.1007/s11605-011-1748-7. discussion 78–79. [DOI] [PubMed] [Google Scholar]
- 11.Katz MHG, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann. Surg. Oncol. 2009;16:836–847. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regine WF, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi H, et al. Preoperative gemcitabine-based chemoradiation therapy for resectable and borderline resectable pancreatic cancer. Ann. Surg. 2013;258:1040–1050. doi: 10.1097/SLA.0b013e31829b3ce4. [DOI] [PubMed] [Google Scholar]
- 14.Katz MHG, et al. Preoperative modified FOLFIRINOX (mFOLFIRINOX) followed by chemoradiation (CRT) for borderline resectable (BLR) pancreatic cancer (PDAC): Initial results from Alliance Trial A021101. ASCO Meet. Abstr. 2015;33:4008. [Google Scholar]
- 15.Mehta VK, et al. Preoperative chemoradiation for marginally resectable adenocarcinoma of the pancreas. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract. 2001;5:27–35. doi: 10.1016/s1091-255x(01)80010-x. [DOI] [PubMed] [Google Scholar]
- 16.Evans DB, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008;26:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 17.Varadhachary GR, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008;26:3487–3495. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 18.Kim EJ, et al. A multi-institutional phase 2 study of neoadjuvant gemcitabine and oxaliplatin with radiation therapy in patients with pancreatic cancer. Cancer. 2013;119:2692–2700. doi: 10.1002/cncr.28117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang RF, et al. Development of an integrated biospecimen bank and multidisciplinary clinical database for pancreatic cancer. Ann. Surg. Oncol. 2008;15:1356–1366. doi: 10.1245/s10434-008-9833-1. [DOI] [PubMed] [Google Scholar]
- 20.Tzeng C-WD, et al. Defined clinical classifications are associated with outcome of patients with anatomically resectable pancreatic adenocarcinoma treated with neoadjuvant therapy. Ann. Surg. Oncol. 2012;19:2045–2053. doi: 10.1245/s10434-011-2211-4. [DOI] [PubMed] [Google Scholar]
- 21.Liu L, et al. Superior Mesenteric Artery Margin of Posttherapy Pancreaticoduodenectomy and Prognosis in Patients With Pancreatic Ductal Adenocarcinoma. Am. J. Surg. Pathol. 2015;39:1395–1403. doi: 10.1097/PAS.0000000000000491. [DOI] [PubMed] [Google Scholar]
- 22.Chatterjee D, et al. Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: a predictor for patient outcome. Cancer. 2012;118:3182–3190. doi: 10.1002/cncr.26651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tzeng C-WD, et al. Yield of clinical and radiographic surveillance in patients with resected pancreatic adenocarcinoma following multimodal therapy. HPB. 2012;14:365–372. doi: 10.1111/j.1477-2574.2012.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu CC, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Ann. Surg. Oncol. 2010;17:981–990. doi: 10.1245/s10434-009-0743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDade TP, et al. A national propensity-adjusted analysis of adjuvant radiotherapy in the treatment of resected pancreatic adenocarcinoma. Cancer. 2010;116:3257–3266. doi: 10.1002/cncr.25069. [DOI] [PubMed] [Google Scholar]
- 26.Chuong MD, Boggs DH, Patel KN, Regine WF. Adjuvant chemoradiation for pancreatic cancer: what does the evidence tell us? J. Gastrointest. Oncol. 2014;5:166–177. doi: 10.3978/j.issn.2078-6891.2014.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moningi S, et al. The Role of Stereotactic Body Radiation Therapy for Pancreatic Cancer: A Single-Institution Experience. Ann. Surg. Oncol. 2015;22:2352–2358. doi: 10.1245/s10434-014-4274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kharofa J, et al. Neoadjuvant chemoradiation with IMRT in resectable and borderline resectable pancreatic cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2014;113:41–46. doi: 10.1016/j.radonc.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong TS, et al. A phase 1/2 and biomarker study of preoperative short course chemoradiation with proton beam therapy and capecitabine followed by early surgery for resectable pancreatic ductal adenocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2014;89:830–838. doi: 10.1016/j.ijrobp.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chakraborty S, et al. Accelerated fraction radiotherapy with capecitabine as neoadjuvant therapy for borderline resectable pancreatic cancer. Gastrointest. Cancer Res. GCR. 2014;7:15–22. [PMC free article] [PubMed] [Google Scholar]
- 31.Golcher H, et al. Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer: results of the first prospective randomized phase II trial. Strahlenther. Onkol. Organ Dtsch. Röntgenges. Al. 2015;191:7–16. doi: 10.1007/s00066-014-0737-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tachezy M, et al. Sequential neoadjuvant chemoradiotherapy (CRT) followed by curative surgery vs. primary surgery alone for resectable, non-metastasized pancreatic adenocarcinoma: NEOPA- a randomized multicenter phase III study ( NCT01900327, DRKS00003893, ISRCTN82191749) BMC Cancer. 2014;14:411. doi: 10.1186/1471-2407-14-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Comprehensive Cancer Network NCCN practice guidelines for pancreatic cancer. Available at: http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. (Accessed: 1st March 2016)
- 34.Katz MHG, Ahmad S, Boughey JC. Improving resection rates in borderline resectable pancreatic cancer: Pilot study shows favorable results. Bulletin of the American College of Surgeons. [serial online]. 2015. Available from http://bulletin.facs.org/2015/10/improving-resection-rates-in-borderline-resectable-pancreatic-cancer-pilot-study-shows-favorable-results/ [accessed February 9, 2016]. [PubMed]
- 35.Evans DB, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch. Surg. Chic. Ill 1960. 1992;127:1335–1339. doi: 10.1001/archsurg.1992.01420110083017. [DOI] [PubMed] [Google Scholar]
- 36.Spitz FR, et al. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1997;15:928–937. doi: 10.1200/JCO.1997.15.3.928. [DOI] [PubMed] [Google Scholar]
- 37.Katz MHG, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;118:5749–5756. doi: 10.1002/cncr.27636. [DOI] [PubMed] [Google Scholar]


