Abstract
MicroRNAs (miRNAs), an important component of epigenetic mechanisms of carcinogenesis, have been shown to play crucial roles in cancer initiation, metastasis, prognosis and responses to drug treatment and may serve as biomarkers for early diagnosis of cancer and tools for cancer therapy. Metal carcinogens, such as arsenic, cadmium, hexavalent chromium and nickel, are well-established human carcinogens causing various cancers upon long term exposure. However, the mechanism of metal carcinogenesis has not been well understood, which limits our capability to effectively diagnose and treat human cancers resulting from chronic metal carcinogen exposure. Over recent years, the role of miRNAs in metal carcinogenesis has been actively explored and a growing body of evidence indicates the critical involvement of miRNAs in metal carcinogenesis. This review aims to discuss recent studies showing that miRNAs play important roles in metal carcinogen-induced cell malignant transformation and tumorigenesis. Some thoughts for future further studies in this field are also presented.
Keywords: MicroRNA (miRNA), epigenetics, metal, metal carcinogenesis, arsenic, cadmium, chromium, nickel
1. Introduction
1.1. miRNA biogenesis, function and regulation
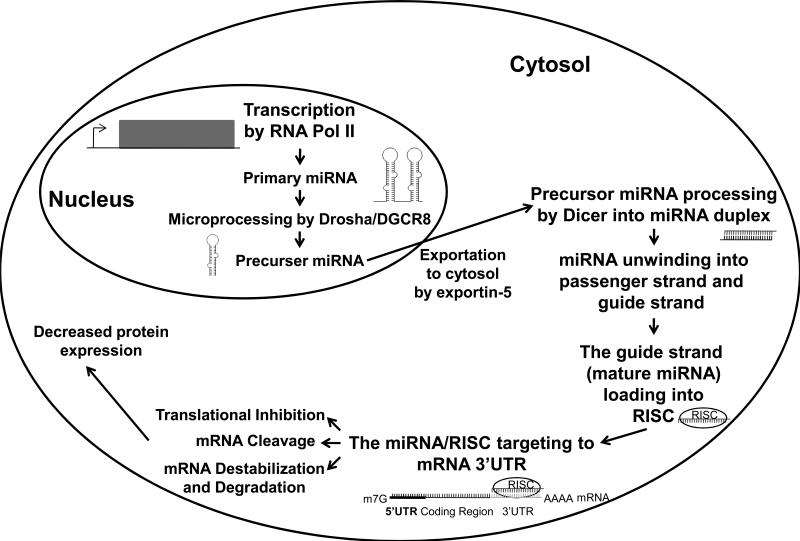
MicroRNAs (miRNAs or miRs), are a large family of small non-coding RNA molecules that negatively regulate protein-coding gene expression post-transcriptionally (Bartel, 2004; Hobert, 2008). miRNAs are initially transcribed either mono- or polycistronically by RNA polymerase II (RNA Pol II) in the nucleus to yield primary transcripts (termed primary miRNAs, or pri-miRNAs) that are hundreds to thousands of nucleotides long (Lee et al., 2004; Cai et al., 2004). Pri-miRNAs are then polyadenylated, capped, and subjected to a microprocessing event carried out by the type III RNase Drosha with its cofactor DiGeorge Syndrome Critical Region 8 (DGCR8) to reduce them to ~70 nucleotide precursor miRNAs (pre-miRNAs) (Lee et al., 2003; Han et al., 2004). The pre-miRNA then forms a complex with exportin-5 and Ran-GTP, a GTP-binding nuclear protein, which then exports the pre-miRNA into the cytosol. Once in the cytosol the pre-miRNA is then subjected to another processing event by another type III RNase Dicer resulting in a ~21-22 nucleotide miRNA duplex (Hutvágner et al., 2001; Grishok et al., 2001). Following miRNA duplex unwinding, one strand (the passenger strand or the star strand) is usually degraded, while the other strand (the guide strand or the mature miRNA) is associated with the Argonaute protein and subsequently incorporated into the RNA-induced silencing complex (RISC) (Meister, 2013). The mature miRNA/RISC complex is then able to regulate the expression of target genes.
Typically, miRNAs elicit their regulatory function by base pairing with the 3’ untranslated regions (3’ UTR) of their target messenger RNAs (mRNAs) through their seed sequences. However instances of miRNAs interacting with other parts of the mRNA have also been reported (Zhou and Rigoutsos, 2014; Qin et al., 2010). The seed sequence is the second to eighth nucleotide of the 5’ end of the mature miRNA that gives the miRNA specificity towards their target mRNAs. The binding of a miRNA to its target mRNA can result in mRNA destabilization and degradation, translational inhibition or direct cleavage (Pillai et al., 2007; Bartel, 2009; Karginov et al., 2010; Bracken et al., 2011). Destabilization and degradation of mRNA is a common form of protein-coding gene expression regulation, which usually is mediated by the same imperfect miRNA:mRNA base pairing that causes translational inhibition (Eichhorn et al., 2014; Baek et al., 2008; Selbach et al., 2008). However, direct cleavage of the target mRNA typically requires more extensive base pairing (Karginov et al., 2010; Bracken et al., 2011). Even though the seed sequence of a miRNA is the most prominent characteristic that regulates miRNA:mRNA interactions, there are examples of miRNAs having weak seed sequence binding and better overall complementarity to the mRNA which can lead to the inhibition of gene expression (Bartel, 2009; Brennecke et al., 2005; Carroll et al., 2014). A brief schematic description of miRNA biogenesis and mechanism of down-regulating gene expression is presented in Figure 1.
Figure 1. A brief schematic description of miRNA biogenesis and the mechanism of miRNA down-regulating protein-coding gene expression.
MiRNAs are transcribed in the nucleus by RNA Polymerase II (RNA Pol II) into primary-miRNA (pri-miRNA). The pri-miRNA is polyadenylated and capped, and then processed by Drosha and its binding partner DiGeorge syndrome critical region 8 (DGCR8) to generate the precursor-miRNA (pre-miRNA). This premiRNA is then exported out of the nucleus by exportin-5. In the cytosol, the pre-miRNA is processed via Dicer into a miRNA duplex. The miRNA duplex is then unwound into its guide strand (mature miRNA) and passenger strand (star strand). While the passenger strand is usually degraded, the mature miRNA then associates with the Argonaute protein and is subsequently loaded into the RNA-induced silencing complex (RISC). The mature miRNA/RISC complex goes to find its target mRNA based upon the miRNA seed sequence. The interaction of miRNA/RISC complex with the target mRNA can cause mRNA destabilization and degradation, cleavage, or inhibition of translation of the mRNA.
Since the seed sequence of a miRNA can be the same for multiple miRNAs and that an individual miRNA can target many genes, miRNAs have been thought to regulate at least two-thirds of all protein coding genes in humans (Friedman et al., 2009). It is not surprising that experimental evidence has shown that miRNAs are involved in almost all aspects of cellular functions and many important biological processes. For example, numerous studies have demonstrated that miRNAs play crucial roles in cell proliferation (Piccoli et al., 2015) and differentiation (Lazare et al., 2014), cell death (Su et al., 2015), immunological functions (Chen et al., 2013), stem cell functions (Shenoy and Blelloch, 2014), angiogenesis (Santulli, 2015), and cancer development and progression (Frixa et al., 2015; Humphries and Yang, 2015; Oom et al., 2014; Iorio and Croce, 2012). The importance of miRNAs in many biological processes is further evidenced by the high evolutionary conservation of individual miRNA sequences and the process of miRNA biogenesis among many different species of organisms (Mattick, 2003; Taft et al., 2007).
Similar to protein-coding genes, the expression of miRNAs can also be regulated through genetic and epigenetic mechanisms. Studies indicate that modifications to the promoter regions of miRNAs can alter their expression. Hypermethylation of CpG islands and acetylation of promoter regions have been shown to decrease and increase the expression of miRNAs, respectively (Hou et al., 2011). Furthermore, the promoter regions of miRNAs can also be bound by certain transcription factors to regulate their expression. In addition to promoter modifications and interactions that regulate miRNA expression levels, the mature miRNAs can also be sequestered in the cytosol by long intergenic noncoding RNAs (lincRNAs). These lincRNAs that sequester mature miRNAs and reduce their activity are also referred to as miRNA sponges or competing endogenous RNAs (ceRNAs). More information about ceRNAs can be found in recent excellent reviews (Kartha and Subramanian, 2014; Sanchez-Mejias and Tay, 2015).
1.2. miRNAs and cancer
Calin et al. (2002) were the first to show that miRNAs are dysregulated in cancer. Since this discovery many studies have shown that miRNA expression is dysregulated in cancer through many different mechanisms, such as amplifications or deletions (Croce, 2009; Iorio and Croce, 2012). Further studies demonstrated that miRNAs are involved in cancer initiation and metastasis and dysregulated miRNAs may act as either oncogenes or tumor suppressors in cancer and are often referred to as oncomirs (Esquela-Kerscher and Slack, 2006). In addition, numerous studies also showed that circulating miRNAs in the blood are stable and may function as potential biomarkers for cancer diagnosis and prediction of cancer prognosis (Mitchell et al., 2008; Chim et al., 2008; Lawrie et al., 2008; Chen et al., 2008)..
Given the important roles of miRNAs in almost all aspects of cell functions and variety of biological processes, it is likely that miRNAs may play crucial roles in regulating cellular responses to chemical carcinogen exposure and thus are critically involved in chemical carcinogen-induced cell malignant transformation and tumorigenic process. Many studies have shown that the expression and function of cellular miRNAs are deregulated upon chronic exposure to chemical carcinogens (Izzotti and Pulliero, 2014; Pogribny et al., 2015). This review aims to discuss the growing body of evidence showing that the dysregulated miRNAs play important roles in a particular class of chemical carcinogens, metal carcinogen-induced cell malignant transformation and tumorigenesis.
2. The role of miRNAs in metal carcinogen-induced cell malignant transformation and tumorigenesis
Metal carcinogens such as arsenic, cadmium, hexavalent chromium, and nickel, are usually atomic dense metallic elements that can be toxic or poisonous even at low concentrations (Duffus, 2002). Exposure to metal carcinogens through air, soil, water, and food has been shown to increase the risk of cancer (Hu, 2002; Langie et al., 2015), however, the underlying mechanisms of metal carcinogenesis have not been well understood. An increasing body of evidence has shown that miRNA dysregulation plays an important role in metal carcinogen-induced cell transformation and tumorigenesis.
2.1. Arsenic
Arsenic is one of the most common environmental pollutants and one of the most well-known human carcinogens. General population exposure to arsenic is mainly through consuming contaminated drinking water, and long term arsenic exposure significantly increases the risk of developing lung, skin, bladder and other cancers (Smith et al., 1992; Frumkin and Thun, 2001; IARC, 2004; Tapio and Grosche, 2006; Celik et al., 2008; Tokar et al., 2010a). Unlike many typical carcinogens, arsenic is not considered as a strong genotoxic carcinogen and it is generally accepted that the epigenetic mechanism and other non-genotoxic mechanisms may play crucial roles in arsenic carcinogenesis (Yang and Frenkel, 2002; Ren et al., 2011). Dysregulation of miRNAs, an important component of epigenetic mechanism of carcinogenesis, has been shown over recent years to be critically involved in arsenic-induced cell malignant transformation and tumorigenesis.
2.1.1. miR-200 family
The miR-200 family consists of five members located on two different chromosomes, miR-200b, −200a, −429 located on chromosome 1 and miR-200c and −141 on chromosome 12 in humans. The miR-200 family has been the focus of much research over the years because of their capability to inhibit epithelial-to-mesenchymal transition (EMT) (Bracken et al., 2008; Burk et al., 2008; Gregory et al., 2008; Korpal et al., 2008; Park et al., 2008) and the potential of the miR-200 members to suppress cancer metastasis (Gibbons et al., 2009; Li et al., 2014; Humphries et al., 2014; Humphries and Yang, 2015;).
Research in our lab has been the first to show the critical role of miRNA dysregulation in the effects of chronic arsenic exposure on cells (Wang et al., 2011). A 16-week continuous exposure of immortalized human bronchial epithelial cells (HBECs), that either had normal p53 expression or p53 expression stably knocked down (p53lowHBECs), with a low dose of arsenite resulted in drastic changes only in p53lowHBECs. These changes include a morphological shift from epithelial to a spindle-like mesenchymal morphology, a loss of the epithelial marker gene E-cadherin expression, and increases of the mesenchymal markers vimentin, zinc-finger E-box-binding homeobox factor 1 (ZEB1) and 2 (ZEB2) expression indicating the occurrence of epithelial-to-mesenchymal transition. Furthermore, a significant increase of colony formation in soft agar was only observed in arsenite-exposed p53lowHBECs (As-p53lowHBECs), and subcutaneous injection of As-p53lowHBECs into nude mice formed tumors whereas passage-matched control p53lowHBECs and arsenite-exposed p53-normal HBECs did not. These findings demonstrate that chronic arsenite exposure causes malignant transformation of p53lowHBECs.
To determine if miRNAs play a role in arsenite-induced cell malignant transformation, a miRNA microarray was performed (Wang et al., 2011). Results from the microarray showed that six miRNAs (the five miR-200 family members and miR-205) were downregulated more than two-fold in As-p53lowHBECs compared to passage-matched control p53lowHBECs. Further QPCR analysis confirmed the significant down-regulation of miR-200 family in As-p53lowHBECs. To determine if the loss of miR-200 plays a role in arsenite-induced cell transformation and tumor formation, miR-200b was stably re-expressed in As-p53lowHBECs. Stable expression of miR-200b in As-p53lowHBECs (As-p53lowHBECs-GFP-200b) resulted in restored E-cadherin expression, an epithelial-like morphology, and a decreased expression of ZEB1 and ZEB2. Furthermore, miR-200b stable expression in As-p53lowHBECs significantly decreased their soft agar colony formation, and completely blocked subcutaneous tumor formation when inoculated into nude mice. To further determine whether stably expressing miR-200 in parental p53lowHBECs is capable of reducing or preventing cell transformation by chronic arsenic exposure, miR-200b was stably expressed in parental p53lowHBECs and subjected the cells to a similar chronic low dose of arsenite exposure. It was found that stable expression of miR-200b prevented cellular transformation by chronic arsenite exposure (Wang et al., 2011). Taken together, these results indicate that miR-200b down-regulation plays a causal role in arsenite-induced cell malignant transformation and tumorigenesis. In addition, our further follow-up studies also demonstrated that down-regulation of miR-200b plays critical roles in promoting arsenic-transformed cell migration, invasion and tumor angiogenesis (Wang et al., 2012b, 2013, 2014b). Moreover, significant down-regulation of miR-200c level in arsenic-transformed human bronchial epithelial (HBE) cells was recently also observed by other group (Xu et al., 2015a).
Since arsenic exposure has previously been shown to cause DNA methylation (Cui et al., 2006; Arita and Costa, 2009; Hernandez et al., 2009; Reichard and Puga, 2010), we looked at promoter hypermethylation as a possible mechanism for miR-200 down-regulation by chronic arsenic exposure. A melt curve PCR analysis found that both the miR-200b/200a/429 and miR-200c/141 cluster promoter regions were highly methylated in arsenite-transformed cells (Asp53lowHBECs) (Wang et al., 2011). Furthermore, treatment of As-p53lowHBECs with the DNA methyltransferase inhibitor 5-aza-2’-deoxycytidine (5Aza) increased the expression of miR-200b and −200c, as well as E-cadherin (Wang et al., 2011). This data suggests that increased DNA methylation plays an important role in chronic arsenic exposure-caused down-regulation of miR-200 family expression.
Similarly, another recent study reported an important role of miR-200 down-regulation in arsenic-induced human urothelial cell transformation. Michailidi et al. (2015) found that chronic treatment of immortalized human urothelial cells (HUC1) with arsenic resulted in an EMT-like change in morphology at 4, 6, 8, and 10 months. Concurrent with these morphological changes, arsenic-treated cells also had a dramatic increase in the PI3K-Akt signaling pathway, as well as a decrease in E-cadherin at 6 and 10 months and an increase of vimentin at 10 months. Due to the EMT-like morphological changes, Michailidi et al. (2015) analyzed the expression of the EMT-regulating miR-200 family as well as miR-205. It was found that miR-200a, −200b, and −200c were significantly reduced at months 6, 8 and 10 in the arsenic-treated HUC1 cells compared to untreated HUC1 cells.
Moreover, Michailidi et al. (2015) further determined whether this phenotype also occurred in an arsenic-exposed human population. It was found that urine levels of miR-200a, −200b, −200c, and −205 was reduced in the arsenic-exposed population. Further analysis on urine levels of miR-200 in humans exposed to different levels of arsenic in water revealed that the level of miR-200c was inversely associated with the levels of arsenic exposure (Michailidi et al., 2015). These findings suggest that down-regulation of miR-200 family members may also play an important role in arsenic exposure-caused human cancers.
2.1.2 miR-21
miR-21 has been reported to be one of the most commonly up-regulated miRNAs in various human cancers functioning as a key regulator of carcinogenic process, and has been considered as a novel target in cancer therapeutics (Selcuklu et al., 2009; Krichevsky and Gabriely, 2009; Pan et al., 2010; Kumarswamy et al., 2011; Wang et al., 2014a). It is interesting that recent studies also show that miR-21 is critically involved in arsenic-induced cell malignant transformation and tumorigenesis.
Ling et al. (2012) first reported the involvement of miR-21 in arsenic-induced transformation of human embryo lung fibroblast cells (HELFs). After continuously treating HELF cells with arsenite for 30 passages (15 weeks), Ling et al. found HELF cells were transformed as evidenced by increased soft agar colony formation by the arsenite-treated cells. The expression levels of miR-21 were assessed at 10, 20, and 30 passages of arsenite treatment. At each of the time points, the miR-21 expression level was significantly higher in cells treated with arsenite than that in passage-matched control cells. Furthermore, treatment of HELF cells with arsenite resulted in increased miR-21 expression starting at 3 hrs post-treatment. The authors then determined the mechanism by which arsenite treatment up-regulates miR-21 expression. It was found that arsenite treatment increased the formation of reactive oxygen species (ROS), which triggered the extracellular signal-regulated kinase (ERK)-nuclear factor-κB (NF-κB) pathway activation. The activated NF-κB directly binds to the miR-21 promoter region, resulting in increased miR-21 expression.
To determine a potential role of miR-21 up-regulation in arsenite-induced transformation of HELF cells, Ling et al. (2012) further analyzed the expression levels of known targets of miR-21 and found that the level of Spry, a negative regulator of the Ras/MEK/ERK pathway, was decreased in arsenite-treated cells. Given the well-established role of ERK/NF-κB pathway in cancer, these findings suggest that miR-21 up-regulation plays a critical role in arsenite-induced transformation of HELF cells.
Further studies from the same laboratory showed that miR-21 up-regulation also plays a role in arsenite-induced transformation of human lung epithelial cells. Luo et al. (2013) found that the expression level of miR-21 was increased in arsenite-transformed human bronchial epithelial cells (HBECs). Mechanistic studies revealed that arsenite treatment increases the secretion of a pro-inflammatory cytokine interleukin-6 (IL-6), which in turn causes activation of signal transducer and activator of transcription 3 (STAT3), a transcription factor. Activated STAT3 subsequently increased the expression of miR-21 as inhibition of STAT3 blocked the arsenite-induced increase of miR-21 expression (Luo et al., 2013). The important role of miR-21 in arsenic-induced cell transformation and tumorigenesis was further demonstrated by recent studies showing that exosomal miR-21 derived from arsenite-transformed cell promotes cell proliferation (Xu et al., 2015b) and that knockdown of miR-21 expression reduces tumor angiogenesis in xenograft tumors produced by inoculation of arsenic-transformed cells (Zhao et al., 2013). Together, these studies indicate that miR-21 plays a multifaceted role in arsenite-induced cellular transformation and tumorigenesis.
2.1.3 Other miRNAs
In addition to the studies discussed above showing the important roles of miR-200 and miR-21 in arsenic-induced human lung cell transformation and tumorigenesis, a recent study using another human lung epithelial cell line (BEAS-2B) showed that the expression level of miR-199a-5p was down-regulated more than 100-fold in arsenic-transformed cells (He et al., 2014). In contrast, stably expressing miR-199a-5p in arsenic-transformed cells significantly reduced mouse xenograft tumor growth and tumor angiogenesis. Further mechanistic studies revealed that arsenic exposure down-regulates miR-199a-5p expression by generating ROS (He et al., 2014). Another recent study showed that miR-191 was significantly up-regulated in arsenic-transformed human bronchial epithelial (HBE) cells and inhibition of miR-191 significantly reduced their transformed phenotypes (Xu et al., 2015a). Together, these studies suggest that the dysregulation of multiple miRNA expression levels may play important roles in arsenic-caused human lung cell malignant transformation.
In addition to causing lung cancer, arsenic exposure also increases the risk of developing skin cancer (Smith et al., 1992). Chronic arsenic exposure is capable of inducing human skin cell malignant transformation although the underlying mechanism has not been clearly defined (Pi et al., 2008). Jiang et al. (2014) recently reported that the levels of let-7 family miRNAs were significantly reduced in arsenic-transformed human keratinocytes HaCaT cells. Re-expression of let-7c, a member of the let-7 family of miRNAs, significantly reduced the malignant phenotypes of arsenic-transformed HaCaT cells. Mechanistic studies suggest that arsenic treatment down-regulates let-7 expression by increasing DNA methylation as treatment with 5-aza-2-deoxycytidine, an inhibitor of DNA methyltransferases, prevents the arsenic-caused decreases of let-7 levels. Consistent with the fact that oncogenic Ras is the target of the let-7 family (Johnson et al., 2005; Choudhury and Li, 2012), Jiang et al. (2014) observed that the Ras/NF-κB pathway is activated in arsenic-transformed HaCaT cells, and re-expression of let-7c inhibits the Ras/NF-κB pathway. The findings from this study suggest that down-regulation of let-7 family of miRNAs may play an important role in arsenic-caused transformation of human keratinocytes.
Previous studies showed that chronic arsenic exposure causes malignant transformation of human prostate epithelial cells (Achanzar et al., 2002; Tokar et al., 2010b). A recent study analyzed the miRNA expression profiles of transformed and non-transformed human prostate epithelial RWPE-1 cells (Ngalame et al., 2014). Compared to controls, arsenic-transformed RWPE-1 (CAsE-PE) cells had 29 and arsenic-transformed prostate stem (As-CSC) cells had 13 differentially expressed miRNAs with the majority of them being downregulated. Of the downregulated miRNAs, many of them correlated with an increased expression of RAS and RAN oncogenes in CAsE-PE (miR-134, −373, −155, −138, −205, −181d, −181c, let-7b, let-7i, let-7e, let-7c) and As-CSC (miR-143, −34c-5p, and −205) cells, respectively (Ngalame et al., 2014). The authors concluded that arsenic induces malignant cellular transformation of human prostate cells by dysregulating the cellular miRNA profiles, which leads to an increase in the activation of oncogenic pathways such as RAS. Indeed, a recent follow-up study from the same group showed that restoration of miR-143, one of the significantly down-regulated miRNAs in arsenic-transformed prostate stem cells (As-CSC), greatly reduced multiple malignant phenotypes in the As-CSCs (Ngalame et al., 2015).
2.2 Nickel
Nickel is a metal that is commonly used to form alloys with iron, copper, chromium and zinc. Although commonly found in the earth's crust, nickel is released into the environment mainly from the industrial plants that make alloys or from power plants and trash incinerators (ATSDR, 2005). Nickel exposure is most commonly through inhalation of contaminated air or smoking tobacco. Chronic nickel exposure leads to increased lung and nasal cancers and it is generally accepted that epigenetic mechanisms and other non-genotoxic mechanisms may play critical roles in nickel carcinogenesis (Andersen et al., 1996; Grimsrud et al., 2002; Arita and Costa, 2009; Cameron et al., 2011; Sun et al., 2013).
Ji et al. (2008) found that the expression level of DNA methyltransferase 1 (DNMT1) was up-regulated in nickel (NiS)-transformed human bronchial epithelial (16HBE) cells, which is associated with the silenced expression of O6-methylguanine DNA methyltransferase (MGMT), an important DNA damage repair gene. To determine the mechanism by which nickel exposure up-regulates DNMT1 expression level, the authors investigated whether the expression of miRNAs that are capable of down-regulating DNMT1 are reduced in nickel-transformed cells (Ji et al. 2013). By using miRNA target prediction computer program analysis, Ji et al. (2013) identified a number of miRNAs, which show sequence complementarity to the 3′-UTR of DNMT1, including miR-148a, −148b and −152. Further Q-PCR analysis revealed that the expression level of miR-152 was significantly down-regulated in nickel-transformed cells compared to passage-matched control cells. Ectopic expression of miR-152 in nickel-transformed cells greatly reduced the level of DNMT1. Interestingly, treatment with 5-Aza, an inhibitor of DNMT, or knockdown of DNMT1 using shRNA, significantly up-regulated the expression of miR-152 in nickel-transformed cells. These observations indicate that there is a double-negative loop for regulating the expression of miR-152 and DNMT1 in nickel-transformed cells. The authors further determined that re-expression of miR-152 in nickel-transformed cells significantly reduced cell proliferation and colony formation in a 14-day colony formation assay. Collectively, these findings suggest that miR-152 down-regulation may play an important role in nickel-induced human bronchial epithelial cell transformation.
To study the role of miRNA dysregulation in nickel-induced cell transformation and tumorigenesis, Zhang and colleagues (2013a) took a different approach by direct intramuscular injection of Ni3S2 into Wistar rats. Thirty two weeks after injection, muscle tumors formed by nickel treatment were collected for miRNA analysis to establish a miRNA library. From this miRNA library, miR-222 expression level was found to be significantly higher in nickel-induced tumor tissues than normal muscle tissue. Moreover, the authors found that the expression level of miR-222 is also significantly up-regulated in nickel-transformed human bronchial epithelial cells (16HBE), which produced tumors upon inoculation into nude mice. The findings from these in vitro cell culture and in vivo animal model studies suggest that miR-222 up-regulation may play an important role in nickel-induced cell transformation and tumorigenesis.
In addition, Zhang and colleagues (2013b) also found that miR-203 is significantly down-regulated in nickel-transformed 16HBE cells. Overexpressing miR-203 in nickel-transformed 16HBE cells significantly decreased cell proliferation, colony formation and mouse xenograft tumor growth. Mechanistic studies revealed hypermethylation of CpGs in the miR-203 promoter and first exon area as well. Treatment with 5-AzadC, a DNA methyltransferase inhibitor, significantly increased miR-203 levels in nickel-transformed cells. Interestingly, treatment with a histone deacetylase inhibitor (TSA) also significantly up-regulated miR-203 levels in nickel-transformed cells. These findings suggest that chronic nickel exposure decreases miR-203 levels through DNA methylation and other deregulated epigenetic mechanisms; and miR-203 down-regulation could play a critical role in nickel-induced cell transformation and tumorigenesis. Further mechanistic studies showed that miR-203 down-regulation leads to up-regulation of its target gene ABL1, a well-established oncogene, and up-regulation of ABL1 may promote nickel-induced cell transformation and tumorigenesis (Zhang et al., 2013b).
As mentioned above, miR-21 is one of the most commonly up-regulated miRNAs in various human cancers and acts as a key regulator of carcinogenic process (Selcuklu et al., 2009; Krichevsky and Gabriely, 2009; Kumarswamy et al., 2011). A recent study reported that nickel treatment is able to increase miR-21 expression levels in a dose-dependent manner in human lung cancer cells (Chiou et al., 2015), suggesting that miR-21 may play a role in nickel carcinogenesis. Chiou et al. (2015) also observed that patients’ lung cancer tissue nickel levels are associated with miR-21 expression levels. Moreover, Kaplan–Meier plot analysis showed that the high-nickel/high-miR-21 patient subgroup has significantly shorter overall survival and relapse free survival periods than the low-nickel/low-miR-21 patient subgroup. Mechanistically, it was determined that nickel exposure induces miR-21 expression through activation of the EGFR/NF-kB signaling pathway.
2.3 Chromium
Chromium can be found in three different states (0, III, and VI) and it is chromium VI [Cr(VI)] that has carcinogenic effect (ATSDR, 2012b). Human Cr(VI) exposure can be through air, water, food, and skin contact with contaminated soil (ATSDR, 2012b).
Although Cr(VI) is a well-known human carcinogen that increases the risk for lung cancer, the underlying mechanism has not yet been fully elucidated. Since Cr(VI) is generally considered a strong genotoxic carcinogen, much less research has been done on the nongenotoxic mechanism of Cr(VI) carcinogenesis. As a result, the role of miRNA dysregulation in Cr(VI)-induced cell transformation and tumorigenesis has rarely been investigated. One recent study has shown that miR-143 was significantly down-regulated in Cr(VI)-transformed human bronchial epithelial BEAS-2B cells (He et al., 2013). As discussed above, the expression level of miR-143 was also found to be significantly decreased in arsenic-transformed human prostate stem cells and miR-143 re-expression greatly reduced malignant phenotypes of arsenic-transformed cells (Ngalame et al., 2014, 2015). Similarly, re-expression of miR-143 in Cr(VI)-transformed BEAS-2B cells significantly reduced their malignant phenotypes as evidenced by decreased mouse xenograft tumor angiogenesis and tumor growth (He et al., 2013). Moreover, the authors also found that miR-143 levels were significantly lower in human lung cancer A549 and H2195 cells than that in immortalized non-tumorigenic human bronchial epithelial BEAS-2B cells. These findings suggest that down-regulation of miR-143 may play an important role in Cr(VI)-induced cell transformation and lung cancer. A further mechanistic study showed that down-regulation of miR-143 promotes Cr(VI)-induced cell transformation probably through increasing the expression of insulin-like growth factor-1 receptor (IGF-I-R) and insulin receptor substrate-1 (IRS1) expression, which in turn activates ERK/hypoxia-induced factor 1α/NF-κB signaling pathway (He et al., 2013).
2.4 Cadmium
Cadmium is a metal that is usually extracted as a byproduct during the production of other metals such as zinc, lead, or copper. Once extracted and purified, cadmium is mostly used in batteries, in the making of color pigments, and in the coating and plating of goods (ATSDR, 2012a). Cadmium is typically emitted into the soil, water, and air by metal mining and refining as well as through the use of fertilizers and fossil fuel combustion. Therefore, human exposure risk exists through air, food, and water (ATSDR, 2012a), and human cadmium exposure through air causes lung cancer (Waalkes, 2003; Huff et al., 2007; Hartwig, 2013).
While it is generally believed that epigenetic mechanisms play an important role in cadmium carcinogenesis (Arita and Costa, 2009; Wang et al., 2012a), little is known about the role of miRNA deregulation in cadmium-induced cell transformation and tumorigenesis. A recent study showed that a number of miRNAs are differentially expressed in cadmium (CdCl2)-transformed 16HBE cells (Liu et al., 2015). Further bioinformatics analysis suggested that differentially expressed miRNAs are predicted to target genes involved in cell cycle regulation, p53 signaling, Wnt signaling, etc. Although whether miRNA deregulation plays a role in cadmium-induced cell transformation and tumorigenesis was not experimentally investigated, this study identified a few candidate miRNAs to further study their involvement in cadmium carcinogenesis.
3. Conclusions and Future Studies
As an important mechanism of epigenetic regulation of gene expression, miRNAs have been increasingly recognized as an important class of players in cancer initiation, metastasis, and responses to cancer therapeutics. Studies on the dysregulation of a variety of miRNAs during chronic metal carcinogen exposure have increased our understanding of the crucial roles that they may play in metal carcinogenesis. Even though limitations exist, the findings from many studies discussed above clearly show that miRNAs are critically involved in metal carcinogen-induced cell malignant transformation and tumorigenesis. However, further studies are needed for better defining the role of miRNAs in metal carcinogenesis, and translating the knowledge for potential clinical applications to benefit the diagnosis and treatment of human cancers resulting from metal carcinogen exposure.
First, current studies have almost exclusively been carried out in in vitro cell culture and mouse xenograft tumor models, which provide invaluable information for understanding the mechanism of metal carcinogenesis but may not fully reflect changes of miRNAs in metal carcinogen exposure-induced cancers in in vivo situations. Future studies need to further demonstrate the important role of miRNA dysregulation in metal carcinogenesis using animal models that develop various cancers upon metal carcinogen exposure. Second, although the role of miRNAs in metal carcinogenesis has in general been fairly actively explored, not all metal carcinogens are well studied. The majority of current studies have investigated the role of miRNA dysregulation in arsenic- and nickel-induced cell transformation and tumorigenesis. However, very few studies have been done to understand the role of miRNAs in hexavalent chromium- and cadmium-induced cell transformation and tumorigenesis. More studies are needed to determine whether miRNA deregulation is critically involved in chromium and cadmium carcinogenesis. Third, while many studies showed the dysregulation of certain miRNAs during metal carcinogen exposure and that manipulating the level of deregulated miRNAs in cells already transformed by metal carcinogens could change their malignant phenotypes, very few studies have been done to show whether manipulating the levels of deregulated miRNAs in parental non-transformed cells is able to prevent metal-carcinogen-induced cell transformation and tumor formation. More studies are needed to further determine whether miRNA deregulation plays a causal role in metal-carcinogen-induced cell transformation and tumorigenesis. Forth, further studies in animals and humans are needed to explore the potential value of dysregulated miRNAs as biomarkers for early diagnosis of cancers caused by metal carcinogen exposure.
Supplementary Material
Acknowledgments
Funding information: This work was supported by the National Institutes of Health [R01ES017777 to C.Y.]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of conflict of interests: None declared
References
- Achanzar WE, Brambila EM, Diwan BA, Webber MM, Waalkes MP. Inorganic arsenite-induced malignant transformation of human prostate epithelial cells. J. Natl. Cancer Inst. 2002;94:1888–1891. doi: 10.1093/jnci/94.24.1888. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for Nickel. U.S. Department of Health and Human Services, Public Health Service; Atlanta, GA: 2005. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for Cadmium. U.S. Department of Health and Human Services, Public Health Service; Atlanta, GA: 2012a. [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for Chromium. U.S. Department of Health and Human Services, Public Health Service; Atlanta, GA: 2012b. [PubMed] [Google Scholar]
- Andersen A, Berge SR, Engeland A, Norseth T. Exposure to nickel compounds and smoking in relation to incidence of lung and nasal cancer among nickel refinery workers. Occup. Environ. Med. 1996;53:708–713. doi: 10.1136/oem.53.10.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita A, Costa M. Epigenetics in metal carcinogenesis: nickel, arsenic, chromium and cadmium. Metallomics. 2009;1:222–228. doi: 10.1039/b903049b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNA Target Recognition and Regulatory Functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken CP, Gregory PA, Kolesnikoff N, Bert AG, Wang J, Shannon MF, Goodall GJ. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res. 2008;68:7846–7854. doi: 10.1158/0008-5472.CAN-08-1942. [DOI] [PubMed] [Google Scholar]
- Bracken CP, Szubert JM, Mercer TR, Dinger ME, Thomson DW, Mattick JS, Michael MZ, Goodall GJ. Global analysis of the mammalian RNA degradome reveals widespread miRNA-dependent and miRNA-independent endonucleolytic cleavage. Nucleic Acids Res. 2011;39:5658–5668. doi: 10.1093/nar/gkr110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennecke J, Stark A, Russell RB, Cohen SM. Principles of MicroRNA-Target Recognition. PLoS Biol. 2005;3:e85. doi: 10.1371/journal.pbio.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Hagedorn CH, Cullen BR. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10:1957–1966. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. PNAS. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron KS, Buchner V, Tchounwou PB. Exploring the Molecular Mechanisms of Nickel-Induced Genotoxicity and Carcinogenicity: A Literature Review. Rev. Environ. Health. 2011;26:81–92. doi: 10.1515/reveh.2011.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll AP, Goodall GJ, Liu B. Understanding principles of miRNA target recognition and function through integrated biological and bioinformatics approaches. WIREs RNA. 2014;5:361–379. doi: 10.1002/wrna.1217. [DOI] [PubMed] [Google Scholar]
- Celik I, Gallicchio L, Boyd K, Lam TK, Matanoski G, Tao X, Shiels M, Hammond E, Chen L, Robinson KA, Caulfield LE, Herman JG, Guallar E, et al. Arsenic in drinking water and lung cancer: a systematic review. Environ. Res. 2008;108:48–55. doi: 10.1016/j.envres.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Chen CZ, Schaffert S, Fragoso R, Loh C. Regulation of immune responses and tolerance: the microRNA perspective. Immunol. Rev. 2013;253:112–128. doi: 10.1111/imr.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, Wang W, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- Chim SSC, Shing TKF, Hung ECW, Leung TY, Lau TK, Chiu RWK, Lo YMD. Detection and characterization of placental microRNAs in maternal plasma. Clin. Chem. 2008;54:482–490. doi: 10.1373/clinchem.2007.097972. [DOI] [PubMed] [Google Scholar]
- Chiou YH, Liou SH, Wong RH, Chen CY, Lee H. Nickel may contribute to EGFR mutation and synergistically promotes tumor invasion in EGFR-mutated lung cancer via nickel-induced microRNA-21 expression. Toxicol. Lett. 2015;237:46–54. doi: 10.1016/j.toxlet.2015.05.019. [DOI] [PubMed] [Google Scholar]
- Choudhury SN, Li Y. miR-21 and let-7 in the Ras and NF-κB pathways. Microrna. 2012;1:65–69. doi: 10.2174/2211536611201010065. [DOI] [PubMed] [Google Scholar]
- Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat. Rev. Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Wakai T, Shirai Y, Hatakeyamate K, Hirano S. Chronic oral exposure to inorganic arsenate interferes with methylation status of p16(INK4a) and RASSF1 and induces lung cancer in A/J mice. Toxicol. Sci. 2006;91:372–381. doi: 10.1093/toxsci/kfj159. [DOI] [PubMed] [Google Scholar]
- Duffus JH. Heavy metals-a meaningless term? Pure Appl. Chem. 2002;74:793–807. [Google Scholar]
- Eichhorn SW, Guo H, McGeary SE, Rodriquez-Mias RA, Shin C, Baek D, Hsu SH, Ghoshal K, Villén J, Bartel DP. mRNA Destabilization Is the Dominant Effect of Mammalian MicroRNAs by the Time Substantial Repression Ensues. Mol. Cell. 2014;56:104–115. doi: 10.1016/j.molcel.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquela-Kerscher A, Slack FJ. Oncomirs—microRNAs with a role in cancer. Nat. Rev. Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- Friedman RC, Farh KKH, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frixa T, Donzelli S, Blandino G. Oncogenic MicroRNAs: Key Players in Malignant Transformation. Cancers (Basel) 2015;7:2466–2485. doi: 10.3390/cancers7040904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frumkin H, Thun MJ. Arsenic. CA Cancer J. Clin. 2001;51:254–262. doi: 10.3322/canjclin.51.4.254. [DOI] [PubMed] [Google Scholar]
- Gibbons DL, Lin W, Creighton CJ, Rizvi ZH, Gregory PA, Goodall GJ, Thilaganathan N, Du L, Zhang Y, Pertsemlidis A, Kurie JM. Contextual extracellular cues promote tumor cell EMT and metastasis by regulating miR-200 family expression. Genes Dev. 2009;23:2140–2151. doi: 10.1101/gad.1820209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- Grimsrud TK, Berge SR, Haldorsen T, Andersen A. Exposure to Different Forms of Nickel and Risk of Lung Cancer. Am. J. Epidemiol. 2002;156:1123–1132. doi: 10.1093/aje/kwf165. [DOI] [PubMed] [Google Scholar]
- Grishok A, Pasquinelli AE, Conte D, Conte N, Li S, Parrish S, Ha I, Baillie DL, Fire A, Ruvkun G, Mello CC. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell. 2001;106:23–34. doi: 10.1016/s0092-8674(01)00431-7. [DOI] [PubMed] [Google Scholar]
- Han J, Lee Y, Yeom KH, Kim YK, Jin H, Kim VN. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004;18:3016–3027. doi: 10.1101/gad.1262504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig A. Cadmium and cancer. Met. Ions Life Sci. 2013;11:491–507. doi: 10.1007/978-94-007-5179-8_15. [DOI] [PubMed] [Google Scholar]
- He J, Qian X, Carpenter R, Xu Q, Wang L, Qi Y, Wang ZX, Liu LZ, Jiang BH. Repression of miR-143 Mediates Cr (VI)-Induced Tumor Angiogenesis via IGF-IR/IRS1/ERK/IL-8 Pathway. Toxicol. Sci. 2013;134:26–38. doi: 10.1093/toxsci/kft101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Wang M, Jiang Y, Chen Q, Xu S, Xu Q, Jiang BH, Liu LZ. Chronic Arsenic Exposure and Angiogenesis in Human Bronchial Epithelial Cells via the ROS/miR-199a-5p/HIF-1α/COX-2 Pathway. Environ. Health Perspect. 2014;122:255–261. doi: 10.1289/ehp.1307545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez LG, van Steeg H, Luijten M, van Benthem J. Mechanisms of non-genotoxic carcinogens and importance of a weight of evidence approach. Mutat. Res. 2009;682:94–109. doi: 10.1016/j.mrrev.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Hobert O. Gene regulation by transcription factors and microRNAs. Science. 2008;319:1785–1786. doi: 10.1126/science.1151651. [DOI] [PubMed] [Google Scholar]
- Hou L, Zhang X, Wang D, Baccarelli A. Environmental chemical exposures and human epigenetics. Int. J. Epidemiol. 2011;41:79–105. doi: 10.1093/ije/dyr154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H. Human health and heavy metals exposure. In: McCally M, editor. Life Support: the Environment and Human Health. MIT Press; Cambridge: 2002. pp. 65–82. [Google Scholar]
- Huff J, Lunn RM, Waalkes MP, Tomatis L, Infante PF. Cadmium-induced cancers in animals and in humans. Int. J. Occup. Environ. Health. 2007;13:202–212. doi: 10.1179/oeh.2007.13.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries B, Wang Z, Oom AL, Fisher T, Tan D, Cui Y, Jiang Y, Yang C. MicroRNA-200b targets protein kinase Cα and suppresses triple negative breast cancer tumor metastasis. Carcinogenesis. 2014;35:2254–2263. doi: 10.1093/carcin/bgu133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries B, Yang C. The microRNA-200 family: small molecules with novel roles in cancer development, progression and therapy. Oncotarget. 2015;6:6472–6498. doi: 10.18632/oncotarget.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutvágner G, McLachlan J, Pasquinelli AE, Balint E, Tuschl T, Zamore PD. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293:834–838. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- Internation Agency for Research on Cancer (IARC) Arsenic in drinking water. International agency for research on cancer monographs on the evaluation of carcinogenic risk to humans. Some Drinking Water Disinfectants and Contaminants, Including Arsenic. Vol. 84. IARC Press; Lyon: 2004. pp. 269–477. [Google Scholar]
- Iorio MV, Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol. Med. 2012;4:143–159. doi: 10.1002/emmm.201100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzotti A, Pulliero A. The effects of environmental chemical carcinogens on the microRNA machinery. Int. J. Hyg. Environ. Health. 2014;217:601–27. doi: 10.1016/j.ijheh.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Ji W, Yang L, Yu L, Yuan J, Hu D, Zhang W, Yang J, Pang Y, Li W, Lu J, Fu J, Chen J, Lin Z, Chen W, Zhuang Z. Epigenetic silencing of O6-methylguanine DNA methyltransferase gene in NiS-transformed cells. Carcinogenesis. 2008;29:1267–1275. doi: 10.1093/carcin/bgn012. [DOI] [PubMed] [Google Scholar]
- Ji W, Yang L, Yuan J, Yang L, Zhang M, Qi D, Duan X, Xuan A, Zhang W, Lu J, Zhuang Z, Zeng G. MicroRNA-152 targets DNA methyltransferase 1 in NiS-transformed cells via a feedback mechanism. Carcinogenesis. 2013;34:446–453. doi: 10.1093/carcin/bgs343. [DOI] [PubMed] [Google Scholar]
- Jiang R, Li Y, Zhang A, Wang B, Xu Y, Xu W, Zhao Y, Luo F, Liu Q. The acquisition of cancer stem cell-like properties and neoplastic transformation of human keratinocytes induced by arsenite involves epigenetic silencing of let-7c via Ras/NF-κB. Toxicol. Lett. 2014;227:91–98. doi: 10.1016/j.toxlet.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Karginov FV, Cheloufi S, Chong MMW, Stark A, Smith AD, Hannon GJ. Diverse endonucleolytic cleavage sites in the mammalian transcriptome depend upon microRNAs, Drosha, and additional nucleases. Mol. Cell. 2010;38:781–788. doi: 10.1016/j.molcel.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartha RV, Subramanian S. Competing endogenous RNAs (ceRNAs): new entrants to the intricacies of gene regulation. Front. Genet. 2014;5:8. doi: 10.3389/fgene.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpal M, Lee ES, Hu G, Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J. Biol. Chem. 2008;283:14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krichevsky AM, Gabriely G. miR-21: a small multi-faceted RNA. J. Cell Mol. Med. 2009;13:39–53. doi: 10.1111/j.1582-4934.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarswamy R, Volkmann I, Thum T. Regulation and function of miRNA-21 in health and disease. RNA Biol. 2011;8:706–713. doi: 10.4161/rna.8.5.16154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langie SAS, Koppen G, Desaulniers D, Al-Mulla F, Al-Temaimi R, Amedei A, Azqueta A, Bisson WH, Brown D, Brunborg G, Charles AK, Chen T, Colacci A, et al. Causes of genome instability: the effect of low dose chemical exposures in modern society. Carcinogenesis. 2015;36(Suppl 1):S61–S88. doi: 10.1093/carcin/bgv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K, Banham AH, Pezzella F, Boultwood J, Wainscoat JS, Hatton CSR, Harris AL. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Brit. J. Haematol. 2008;141:672–675. doi: 10.1111/j.1365-2141.2008.07077.x. [DOI] [PubMed] [Google Scholar]
- Lazare SS, Wojtowicz EE, Bystrykh LV, de Haan G. microRNAs in hematopoiesis. Exp. Cell Res. 2014;329:234–238. doi: 10.1016/j.yexcr.2014.08.033. [DOI] [PubMed] [Google Scholar]
- Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, Kim VN. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Roslan S, Johnstone CN, Wright JA, Bracken CP, Anderson M, Bert AG, Selth LA, Anderson RL, Goodall GJ, Gregory PA, Khew-Goodall Y. MiR-200 can repress breast cancer metastasis through ZEB1-independent but moesin-dependent pathways. Oncogene. 2014;33:4077–4088. doi: 10.1038/onc.2013.370. [DOI] [PubMed] [Google Scholar]
- Ling M, Li Y, Xu Y, Pang Y, Shen L, Jiang R, Zhao Y, Yang X, Zhang J, Zhou J, Wang X, Liu Q. Regulation of miRNA-21 by reactive oxygen species-activated ERK/NF-κB in arsenite-induced cell transformation. Free Radic. Biol. Med. 2012;52:1508–1518. doi: 10.1016/j.freeradbiomed.2012.02.020. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zheng C, Shen H, Zhou Z, Lei Y. MicroRNAs-mRNAs Expression Profile and Their Potential Role in Malignant Transformation of Human Bronchial Epithelial Cells Induced by Cadmium. Biomed. Res. Int. 2015;2015:902025. doi: 10.1155/2015/902025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Xu Y, Ling M, Zhao Y, Xu W, Liang X, Jiang R, Wang B, Bian Q, Liu Q. Arsenite evokes IL-6 secretion, autocrine regulation of STAT3 signaling, and miR-21 expression, processes involved in the EMT and malignant transformation of human bronchial epithelial cells. Toxicol. Appl. Pharmacol. 2013;273:27–34. doi: 10.1016/j.taap.2013.08.025. [DOI] [PubMed] [Google Scholar]
- Mattick JS. Challenging the dogma: the hidden layer of non-protein-coding RNAs in complex organisms. Bioessays. 2003;25:930–939. doi: 10.1002/bies.10332. [DOI] [PubMed] [Google Scholar]
- Meister G. Argonaute proteins: functional insights and emerging roles. Nat. Rev. Genet. 2013;14:447–459. doi: 10.1038/nrg3462. [DOI] [PubMed] [Google Scholar]
- Michailidi C, Hayashi M, Datta S, Sen T, Zenner K, Oladeru O, Brait M, Izumchenko E, Baras A, VandenBussche C, Argos M, Bivalacqua TJ, Ahsan H, et al. Involvement of epigenetics and EMT related miRNA in arsenic induced neoplastic transformation and their potential clinical use. Cancer Prev. Res. (Phila) 2015;8:208–221. doi: 10.1158/1940-6207.CAPR-14-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A, Lin DW, Urban N, Drescher CW, et al. Circulating microRNAs as stable blood-based markers for cancer detection. PNAS. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngalame NN, Makia NL, Waalkes MP, Tokar EJ. Mitigation of arsenic-induced acquired cancer phenotype in prostate cancer stem cells by miR-143 restoration. Toxicol. Appl. Pharmacol. 2015 doi: 10.1016/j.taap.2015.12.013. 2015 Dec 22. pii: S0041-008X(15)30159-9. doi: 10.1016/j.taap.2015.12.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngalame NNO, Tokar EJ, Person RJ, Xu Y, Waalkes MP. Aberrant microRNA expression likely controls RAS oncogene activation during malignant transformation of human prostate epithelial and stem cells by arsenic. Toxicol. Sci. 2014;138:268–277. doi: 10.1093/toxsci/kfu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oom AL, Humphries BA, Yang C. MicroRNAs: novel players in cancer diagnosis and therapies. Biomed. Res. Int. 2014;2014:959461. doi: 10.1155/2014/959461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Wang ZX, Wang R. MicroRNA-21: a novel therapeutic target in human cancer. Cancer Biol. Ther. 2010;10:1224–1232. doi: 10.4161/cbt.10.12.14252. [DOI] [PubMed] [Google Scholar]
- Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi J, Diwan BA, Sun Y, Liu J, Qu W, He Y, Stýblo M, Waalkes MP. Arsenic-induced malignant transformation of human keratinocytes: Involvement of Nrf2. Free Radic. Biol. Med. 2008;45:651–658. doi: 10.1016/j.freeradbiomed.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli MT, Gupta SK, Thum T. Noncoding RNAs as regulators of cardiomyocyte proliferation and death. J. Mol. Cell Cardiol. 2015;89:59–67. doi: 10.1016/j.yjmcc.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Pillai RS, Bhattacharyya SN, Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? TRENDS Cell Biol. 2007;17:118–126. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Pogribny IP, Beland FA, Rusyn I. The role of microRNAs in the development and progression of chemical-associated cancers. Toxicol. Appl. Pharmacol. 2015 doi: 10.1016/j.taap.2015.11.013. 2015 Nov 24. pii: S0041-008X(15)30140-X. doi: 10.1016/j.taap.2015.11.013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Qin W, Shi Y, Zhao B, Yao C, Jin L, Ma J, Jin Y. miR-24 Regulates Apoptosis by Targeting the Open Reading Frame (ORF) Region of FAF1 in Cancer Cells. PLoS ONE. 2010;5:e9429. doi: 10.1371/journal.pone.0009429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard JF, Puga A. Effects of arsenic exposure on DNA methylation and epigenetic gene regulation. Epigenomics. 2010;2:87–104. doi: 10.2217/epi.09.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, McHale CM, Skibola CF, Smith AH, Smith MT, Zhang L. An Emerging Role for Epigenetic Dysregulation in Arsenic Toxicity and Carcinogenesis. Environ. Health Perspect. 2011;119:11–19. doi: 10.1289/ehp.1002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Mejias A, Tay Y. Competing endogenous RNA networks: tying the essential knots for cancer biology and therapeutics. J. Hematol. Oncol. 2015;8:30. doi: 10.1186/s13045-015-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santulli G. microRNAs and Endothelial (Dys) Function. J. Cell Physiol. 2015 doi: 10.1002/jcp.25276. 2015 Dec 2. doi: 10.1002/jcp.25276. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbach M, Schwanhäusser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- Selcuklu SD, Donoghue MT, Spillane C. miR-21 as a key regulator of oncogenic processes. Biochem. Soc. Trans. 2009;37:918–925. doi: 10.1042/BST0370918. [DOI] [PubMed] [Google Scholar]
- Shenoy A, Blelloch RH. Regulation of microRNA function in somatic stem cell proliferation and differentiation. Nat. Rev. Mol. Cell Biol. 2014;15:565–576. doi: 10.1038/nrm3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Hopenhayn-Rich C, Bates MN, Goeden HM, Hertz-Picciotto I, Duggan HM, Wood R, Kosnett MJ, Smith MT. Cancer Risks from Arsenic in Drinking Water. Environ. Health. Perspect. 1992;97:259–267. doi: 10.1289/ehp.9297259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Yang Z, Xu Y, Chen Y, Yu Q. MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget. 2015;6:8474–8490. doi: 10.18632/oncotarget.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Shamy M, Costa M. Nickel and epigenetic gene silencing. Genes (Basel) 2013;4:583–595. doi: 10.3390/genes4040583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taft RJ, Pheasant M, Mattick JS. The relationship between non-protein-coding DNA and eukaryotic complexity. Bioessays. 2007;29:288–299. doi: 10.1002/bies.20544. [DOI] [PubMed] [Google Scholar]
- Tapio S, Grosche B. Arsenic in the aetiology of cancer. Mutat. Res. 2006;612:215–246. doi: 10.1016/j.mrrev.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Tokar EJ, Benbrahim-Tallaa L, Ward JM, Lunn R, Sams RL, 2nd, Waalkes MP. Cancer in experimental animals exposed to arsenic and arsenic compounds. Crit. Rev. Toxicol. 2010a;40:912–927. doi: 10.3109/10408444.2010.506641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokar EJ, Diwan BA, Waalkes MP. Arsenic exposure transforms human epithelial stem/progenitor cells into a cancer stem-like phenotype. Environ. Health Perspect. 2010b;118:108–115. doi: 10.1289/ehp.0901059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalkes MP. Cadmium carcinogenesis. Mutat. Res. 2003;533:107–120. doi: 10.1016/j.mrfmmm.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Wang B, Li Y, Shao C, Tan Y, Cai L. Cadmium and its epigenetic effects. Curr. Med. Chem. 2012a;19:2611–2620. doi: 10.2174/092986712800492913. [DOI] [PubMed] [Google Scholar]
- Wang W, Li J, Zhu W, Gao C, Jiang R, Li W, Hu Q, Zhang B. MicroRNA-21 and the clinical outcomes of various carcinomas: a systematic review and meta-analysis. BMC Cancer. 2014a;14:819. doi: 10.1186/1471-2407-14-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Humphries B, Xiao H, Jiang Y, Yang C. Epithelial to mesenchymal transition in arsenic-transformed cells promotes angiogenesis through activating β-catenin-vascular endothelial growth factor pathway. Toxicol. Appl. Pharmacol. 2013;271:20–29. doi: 10.1016/j.taap.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Humphries B, Xiao H, Jiang Y, Yang C. MicroRNA-200b Suppresses Arsenic-transformed Cell Migration by Targeting Protein Kinase Cα and Wnt5b-Protein Kinase Cα Positive Feedback Loop and Inhibiting Rac1 Activation. J. Biol. Chem. 2014b;289:18373–18386. doi: 10.1074/jbc.M114.554246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Yang J, Fisher T, Xiao H, Jiang Y, Yang C. Akt Activation is Responsible for Enhanced Migratory and Invasive Behavior of Arsenic-Transformed Human Bronchial Epithelial Cells. Environ. Health Perspect. 2012b;120:92–97. doi: 10.1289/ehp.1104061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhao Y, Smith E, Goodall GJ, Drew PA, Brabletz T, Yang C. Reversal and Prevention of Arsenic-Induced Human Bronchial Epithelial Cell Malignant Transformation by micro-RNA-200b. Toxicol. Sci. 2011;121:110–122. doi: 10.1093/toxsci/kfr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Ji J, Xu Y, Liu Y, Shi L, Liu Y, Lu X, Zhao Y, Luo F, Wang B, Jiang R, Zhang J, Liu Q. MicroRNA-191, by promoting the EMT and increasing CSC-like properties, is involved in neoplastic and metastatic properties of transformed human bronchial epithelial cells. Mol. Carcinog. 2015a;54(Suppl 1):E148–E161. doi: 10.1002/mc.22221. [DOI] [PubMed] [Google Scholar]
- Xu Y, Luo F, Liu Y, Shi L, Lu X, Xu W, Liu Q. Exosomal miR-21 derived from arsenite-transformed human bronchial epithelial cells promotes cell proliferation associated with arsenite carcinogenesis. Arch. Toxicol. 2015b;89:1071–1082. doi: 10.1007/s00204-014-1291-x. [DOI] [PubMed] [Google Scholar]
- Yang C, Frenkel K. Arsenic-mediated cellular signal transduction, transcription factor activation, and aberrant gene expression: implications in carcinogenesis. J. Environ. Pathol. Toxicol. Oncol. 2002;21:331–342. [PubMed] [Google Scholar]
- Zhang J, Zhou Y, Ma L, Huang S, Wang R, Gao R, Wu Y, Shi H, Zhang J. The alteration of miR-222 and its target genes in nickel-induced tumor. Biol. Trace Elem. Res. 2013a;152:267–274. doi: 10.1007/s12011-013-9619-6. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhou Y, Wu YJ, Li MJ, Wang RJ, Huang SQ, Gao RR, Ma L, Shi HJ, Zhang J. Hyper-methylated miR-203 dysregulates ABL1 and contributes to the nickel-induced tumorigenesis. Toxicol. Lett. 2013b;223:42–51. doi: 10.1016/j.toxlet.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Xu Y, Luo F, Xu W, Wang B, Pang Y, Zhou J, Wang X, Liu Q. Angiogenesis, mediated by miR-21, is involved arsenite-induced carcinogenesis. Toxicol. Lett. 2013;223:35–41. doi: 10.1016/j.toxlet.2013.08.020. [DOI] [PubMed] [Google Scholar]
- Zhou H, Rigoutsos I. MiR-103a-3p targets the 5’ UTR of GPRC5A in pancreatic cells. RNA. 2014;20:1431–1439. doi: 10.1261/rna.045757.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.