Abstract
Folic acid is used for preventing and treating multiple diseases and disorders, administered in the form of oral supplements. The present research work was aimed to study the influence of two non-ionic surfactants Poloxamer and Tween 80 (Polysorbate 80) on pectin submicrospheres formulations. Typical natural polymer pectin was used to encapsulate folic acid by cross linking method. The resultant submicrospheres contributed to improve the aqueous solubility to enhance the bioavailability of folic acid. During investigation, it was observed that pectin polymers influenced kinetics of the rate of reaction more intensively than the surfactants. The physical phenomenon caused the change in their size, shape and chemistry of pectin polymers transforming into submicrospheres in aqueous condition. The characteristic differences of submicrospheres were assessed by scanning electron microscopy, differential scanning calorimetry and Fourier-transform infrared spectroscopy. The average diameters of the submicrospheres ranged between 250 and 500 nm. The encapsulation efficiency of submicrospheres ranged between 80 and 96 %. The characteristic swelling behavior of lyophilized submicrospheres was influenced by the ratio of pectin polymers and folic acid used in the formulations. The submicrospheres systems exhibited controlled release of folic acid due to the pH-dependent solubility of pectin polymers in aqueous medium. The submicrospheres showed good haemocompatibility suggesting them to be promising candidates for oral delivery.
Keywords: Pectin, Folicacid, Surfactants, Submicrosphere, Hemocompatibility
Introduction
Folic acid (pteroyl-l-glutamic acid) is a water-soluble vitamin (vitamin B9) found to be essential for numerous bodily functions [1]. As such, folic acid is not biologically active but the biological properties of the molecule relay upon its reduction to tetrahydrofolate and other related forms. It is evident from earlier reports that tetrahydrofolate is converted into dihydrofolic acid in the liver [2]. Both adults and children need folic acid to make normal red blood cells and prevent anemia. Children and adults both require folic acid to produce healthy red blood cells and prevent anemia [3, 4]. Thus folic acid deficiency hinders DNA synthesis and cell division. Because RNA and protein synthesis are not hindered, large red blood cells called megaloblasts are produced, resulting in megaloblastic anemia [5, 6]. Folic acid also helps prevent changes to DNA that may lead to cancer. Also, one of the most extensively studied small molecule targeting moieties for drug delivery is folic acid (folate) [7, 8]. The high-affinity vitamin is a commonly used ligand for cancer targeting because folate receptors are frequently overexpressed in a range of tumor cells [9–12].
It is evident that poor solubility of drugs adversely affects on the bioavailability after oral administration. As the use of microparticles for oral administration is documented and an attempt to enhance the bioavailability of poorly water-soluble drugs has been well thought of in order to increase the intestinal absorption of the drug [13–15]. Pectin is a natural, non-toxic, anionic, soluble and heterogeneous complex polysaccharide composed of linear α-d-galacturonic acid with 1,4-glycosidic linkages [16–19]. In recent days, pectin has also received significant attention as high fibre diet which is beneficial to health. Another important aspect is that it can reduce cholesterol levels, serum glucose levels and may also have anticancer activities [20, 21]. Pectin and pectin oligosaccharides have shown to induce apoptosis in human adenocarcinoma cells [22]. In food industry, pectin is used as a stabilizer for acidified milk drinks and yoghurts [23]. In addition, pectin is capable of reducing the interfacial tension between an oil phase and a water phase, which can be effective in the preparation of emulsions [24, 25].
In comparison, pectin is less susceptible to degradation in the gastrointestinal tract than alginate. The degradation of pectin occurs mainly in the colon by pectinolytic enzymes secreted by microorganisms. As a result pectin has increasingly gained acceptance as the carrier polymer for sustained release and site specific delivery dosage forms, such as beads, pellets, tablets, and films [26, 27]. Upon addition, pectin forms rigid gels with calcium, which cross-links the galacturonic acid chains [28]. Several researchers have successfully incorporated protein or peptide into calcium pectinate beads for a colonic delivery system [29–33]. Cheng et al. [34] recently formulated insulin-loaded calcium pectinate nanoparticles using an ionotropic gelation or cross linking method reported for the manufacture of calcium pectinate beads. This method is advantageous over others because it’s short-cut method which does not involve organic solvent, harsh processing conditions and expensive equipment.
In the present study, we have developed a delivery system for folic acid encapsulated by pectin polymers using non-ionic surfactants Poloxamer/Tween 80, as organic templates. The resultant submicrospheres have been characterized for their physical and morphological properties and encapsulation efficiencies. Further, their toxicity on human red blood cells was also evaluated.
Materials and Methods
Materials
Pectin pure (Himedia Laboratories Pvt. Ltd., Mumbai.), folic acid (Loba Chemical Pvt Ltd), Calcium chloride dihydrate AR grade, Sodium hydroxide pellets pure AR, Sodium chloride, EDTA, Gluteraldehyde (S D fine Chemical Limited, Mumbai), Poloxamer/Tween 80 (Himedia, Mumbai). All the above chemicals were used without further purification.
Preparation of Pectin Submicrospheres
Pectin solution (0.5 %) was prepared by dissolving the high methoxy pectin in distilled water under gentle agitation using magetic stirrer as described by the Atmaram P Pawer [16] with slight modification. Folic acid (5 mg) was disperssed in distilled water (10 ml) under constant stirring for uniform distribution using magnetic stirrer. Then calcium chloride solution (0.5 %) was extruded drop wise through a 1.2 mm diameter needle into pectin solution and added with surfactant (Poloxamer/Tween 80, 1 ml) at room temperature with constant stirring. The addition flow rate (4 ml/min) was maintained and then submicrospheres formed were allowed to remain on the stirrer for 10 min. The compositions are shown in Table 1. The submicrospheres formed were collected, freeze dried overnight and stored in airtight container until further use.
Table 1.
Compositions and % encapsulation efficiency (EE) of folic acid-free and folic acid-loaded pectin submicrospheres formulations (PE1–PE6)
| Formulations | Formulation ingredients | Batch code | Conc. of folic acid (mg) | Conc. of pectin (mg) | Ratio of folic acid and pectin | % EE of submicrospheres with drug at pH 7.0 | % EE of submicrospheres with drug at pH 6.8 | % EE of submicrospheres with drug at pH 7.4 |
|---|---|---|---|---|---|---|---|---|
| Folic acid-free pectin submicrospheres with/without surfactants | Pectin-alone | PE1 | 5 | 20 | 1:4 | – | – | – |
| Pectin-alone + tween 80 | PE3 | 5 | 20 | 1:4 | – | – | – | |
| Pectin-alone + poloxamer | PE5 | 5 | 20 | 1:4 | – | – | – | |
| Folic acid -loaded pectin submicrospheres with/without surfactants | Pectin + folic acid | PE2 | 5 | 20 | 1:4 | 84.38 ± 0.93 | 96.28 ± 0.92 | 96.20 ± 0.19 |
| Pectin + folic acid + tween 80 | PE4 | 5 | 20 | 1:4 | 93.52 ± 0.63 | 96.00 ± 0.36 | 95.34 ± 0.45 | |
| Pectin + folic acid + poloxamer | PE6 | 5 | 20 | 1:4 | 92.72 ± 0.85 | 95.88 ± 0.62 | 95.99 ± 0.21 |
Standardization of Folic Acid
Stock solution was prepared by dissolving folic acid (10 mg) in 0.1 N NaOH (10 ml). Working standard (0.01 N NaOH, 1/10) was prepared from the stock solution (0.1 N NaOH) after appropriate dilution. Working standard solution (0.01 N NaOH, 100–600 µl range) was taken into a series of the test tubes and volumes were adjusted to 3 ml. The absorbance was read at 366 nm using UV–VIS spectrophotometer (Optizen 2120UV Plus, Mecasys, Korea) and a calibration curve was constructed against reagent blank.
Estimation of Encapsulation Efficiency
Efficiency of the encapsulation was assayed by the spectrophotometer method as described in our previous studies [35]. A known amount of pectin submicrospheres was taken to measure the free folic acid content of in the supernatant by dissolving in PBS. The pectin submicrospheres were subjected to centrifugation at 3500 rpm for 20 min. Then 1 ml of the supernatant was taken and read the absorbance of folic acid present in the supernatant using UV–VIS spectrophotometer (Optizen 2120UV Plus, Mecasys co., Ltd) at 366 nm. The amount of folic acid incorporated into the pectin submicrospheres was calculated by subtracting the free drug present in the supernatant from the total drug added before the preparation. The following formula was used for the calculation.
Swelling Capacity
Into a pre-weighed centrifuge tube the freeze dried submicrospheres (100 mg) was taken and resuspended with PBS (20 ml, pH 7.4 and 6.8) and incubated for 12 h at 37 °C. The suspension was centrifuged (1000×g, 20 min) at 37 °C to separate the swollen spheres, decanted the supernatant and then excess buffer remained with spheres removed carefully using the blotting paper at the edge. The centrifuge tube with swollen spheres was cooled to room temperature and re-weighed immediately. The swelling capacity (%) was calculated using the following equation:
where, Mw and M1 are the weight of the submicrospheres taken in into centrifuge tube before and after the centrifugation.
Scanning Electron Microscopy (SEM)
An aliquot of freeze dried submicrospheres (2 mg) were used for shape and size analysis by scanning electron microscopy (JNCASR, Bangalore, India.). The observation was performed using a 20 kV, LaB6 (or tungsten filament) scanning electron microscope equipped with an Everhart–Thornley secondary electron detector and a Cambridge four quadrant backscatter detector LEO 1530 (LEO 1455VP Cambridge, England) operated using 0.5 mA filament current. The submicrosphere size in the dry state was determined by averaging the size of 35 submicrospheres. Sample was prepared as aqueous dispersion of submicrospheres were finely spread over a glass slide and dried under vacuum. The dried slide was placed onto carbon conductive double-side tape (Euromedex, France) and dried further at room temperature. The processed submicrospheres were coated with gold (2 nm) and placed inside the vacuum column of the microscope after pumping the air out of the chamber.
Fourier Transform Infrared Spectroscopy (FTIR)
FTIR is needed to understand the folic acid-pectin polymer interaction in order to determine any chemical interaction between them. It becomes crucial information because of the interactions that affect on the in vitro release behavior and predominant feature to assess its release from the folic acid submicrospheres [36]. The submicrospheres from pectin-alone and folic acid-loaded pectin, and folic acid-alone were allowed to form pellets at pressure of 10.3 × 104 Pa. The FTIR spectrum of the samples was recorded with Nicolet IR 200 (Thermo Electron Corporation, USA). FTIR spectra of the samples were taken in the wavelength region 4000–400 cm−1 at room temperature using potassium bromide pellets (Merck, IR grade).
Differential Scanning Calorimetry (DSC)
In order to evaluate the crystalline state of folic acid in the pectin encapsulated polymeric nanoparticles Differential Scanning Calorimetry is used. The freeze-dried submicrospheres samples (3–6 mg) were weighed into an aluminium pan and their thermal behaviour was characterized by DSC (Mettler-Toledo DSC). Initially, the pectin was heated to 30 °C and reached gradually to 360 °C at a heating rate of 10 °C/min per cycle. Inert atmosphere was maintained throughout by purging nitrogen at the flow rate of 100 ml/min.
In Vitro Release Studies
In vitro release studies were performed by dialysis method [37] at 37 ± 0.5 °C and folic acid release was monitored spectrophotometrically. Folic acid loaded pectin submicrospheres samples (3 mg) were dispersed in 0.1 N NaOH (3 ml) and placed in the dialysis membrane bag (Mw cut-off 10,000; Sigma, USA). The bag tied at both ends by using cotton thread and immersed in phosphate buffer saline (pH 7.4, 25 ml) containing vessel. The dialysis set was placed in a shaking water bath (50 rev min−1) and a constant temperature was maintained at 37 °C throughout the dialysis. An aliquot of folic acid-loaded pectin submicrospheres (0.5 ml) was withdrawn from the dialysate at predetermined time intervals (1, 2, 4, 6, 12 and 24 h) and immediately equal volume of PBS was replaced. The samples (0.5 ml) withdrawn were analyzed for folic acid release by measuring the absorbance at 366 nm using UV–VIS spectrophotometer (Optizen 2120UV Plus, Mecasys, Korea).
Hemocompatibility Study
Fresh blood (20 ml) from a healthy volunteer was withdrawn into centrifuge tubes containing EDTA (2 mg). The tube was centrifuged (117 g, 20 min) in a REMI 24-C centrifuge to separate the erythrocytes. Buffy coat was removed and the packed cells were washed thrice with normal saline (0.9 % NaCl). Equal amount of 0.9 % NaCl was added to the erythrocytes and centrifuged (117 g, 10 min). The supernatant was discarded and the process was repeated thrice to obtain washed erythrocytes. Normal saline was added to the erythrocytes to obtain 50 % hematocrit. The final concentration of erythrocytes was arrived to contain around 2 × 108 cells per ml in normal saline, as counted by hemocytometer. Haemolysis experiments were in accordance with a method used previously in our laboratory with slight modifications [38]. A 0.1 ml samples solution (15 mg/ml) in normal saline was then added to 0.1 ml of erythrocyte solution and the final volume was made 3 ml with normal saline. The contents were incubated at 37 °C for 1 h in a water bath (ILE instrument, Bangalore), at that time blood cells and sample precipitates were sedimented in a centrifuge (10 min at 11,752 g). The reaction was terminated using 50 µl of 2.5 % gluteraldehyde. The experiment was carried out in the triplicate. The samples were then centrifuged (117 g, 10 min) and release of hemoglobin from the erythrocyte cells was measured spectrophotometrically at 540 nm using UV–VIS spectrophotometer Optizen 2120UV Plus, Mecasys co., Ltd, Korea. A spontaneous hemolysis control group was determined by incubating erythrocytes in normal saline at the concentration 1 × 108 cells ml−1.
Statistical Analysis
All the experiments were conducted in triplicate. The Statistical analysis of experimental data utilized the Student’s t test, and the results were given as mean ± standard deviation (SD). Differences were considered significant at a level of p < 0.05 (Fig. 1).
Fig. 1.
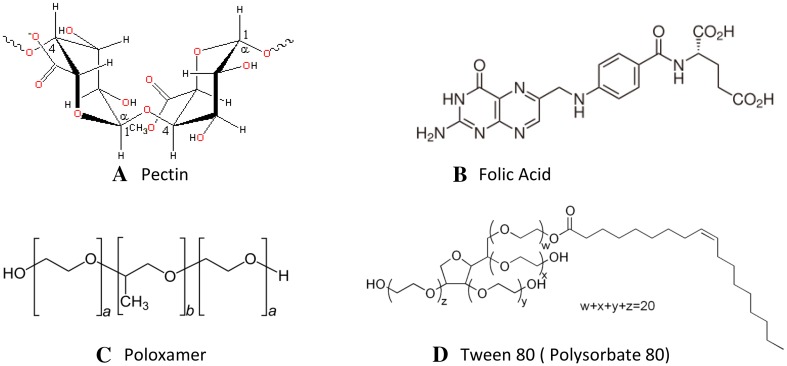
General structures of a pectin, b folic acid, c poloxamer and d Tween 80 (Source Wikipedia)
Result and Discussion
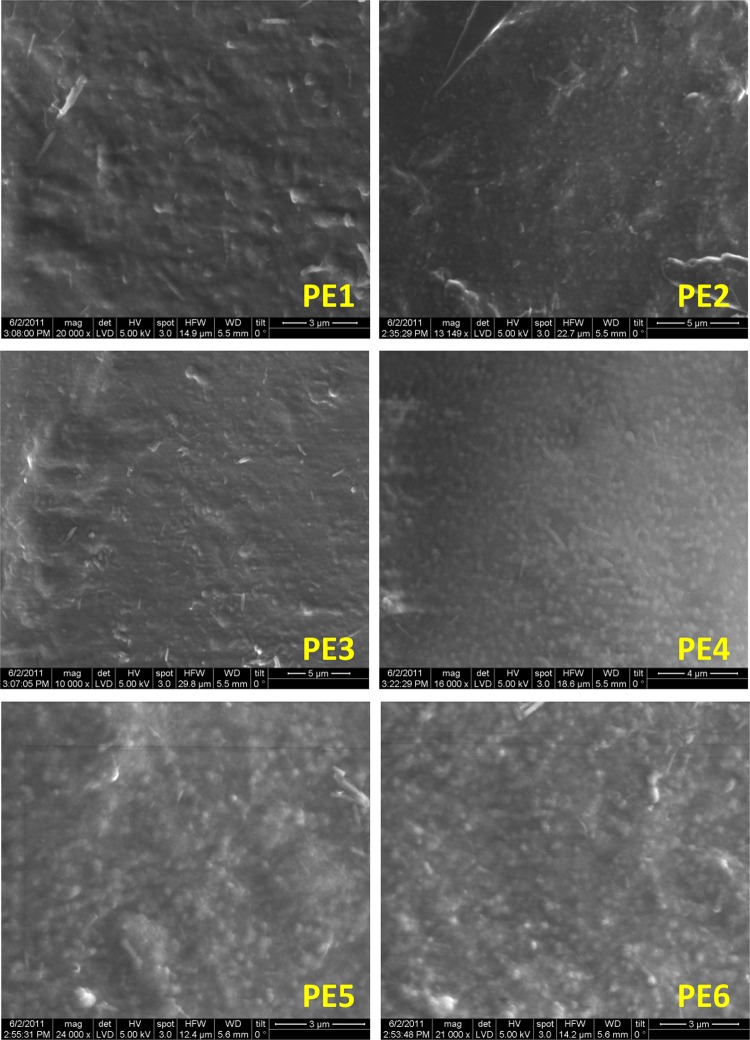
The folic acid and pectin submicrospheres formulations prepared in the presence or absence of surfactants is presented in Table 1. The morphological examinations by SEM of dried folic acid-loaded pectin submicrospheres revealed less clumping and spherical in nature ranging between 250 and 500 nm (Fig. 2PE1–PE6). The use of calcium chloride to assist in cross-link of pectin polymers the addition of either Tween 80 or Poloxamer did not adversely affected on spheres showing uniform size distributions. Upon careful observation, PE2 and PE4 spheres revealed more spherical form, smoother surface and discrete with less clumping than other spheres. Addition of either Tween 80 or Poloxamer did not influence on size and spherical nature of any category of submicrospheres, but kept the spheres away from clumping.
Fig. 2.
Scanning electron micrographs of folic acid-free and folic acid-loaded pectin submicrospheres formulations (PE1–PE6)
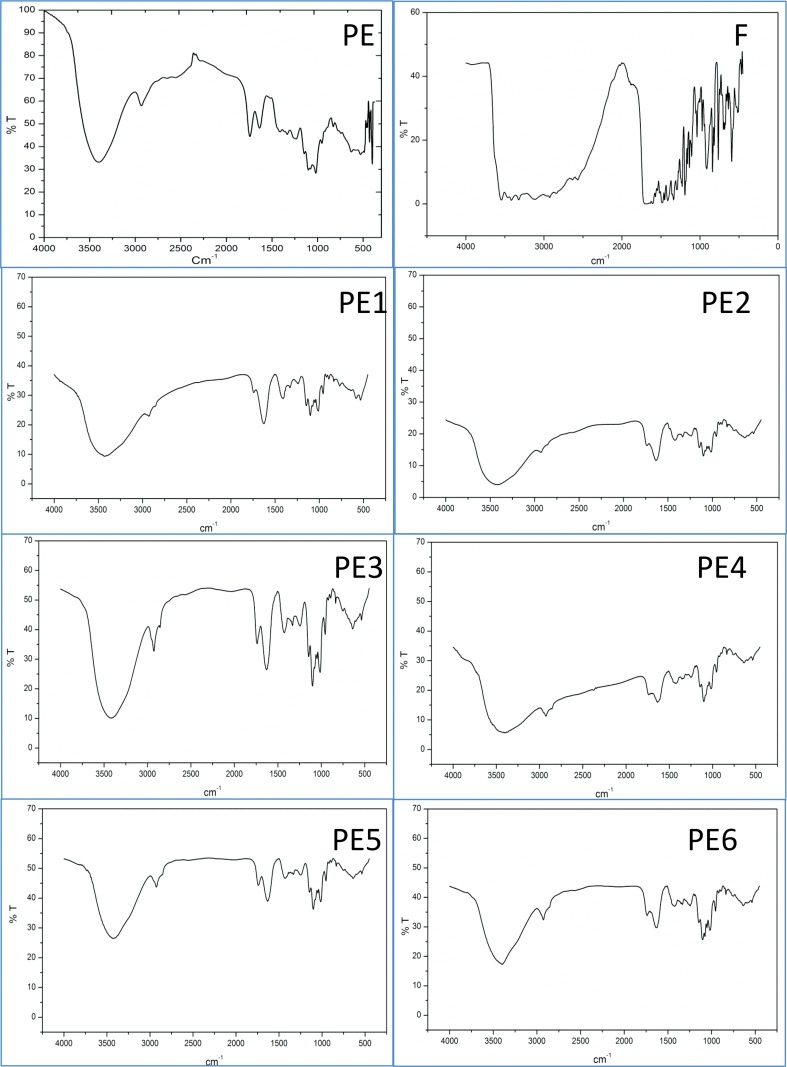
The FTIR spectra taken at the room temperature indicated there was no interaction between the pectin and folic acid (Fig. 3). Many peaks of pectin and folic acid were observed which shows a broad-OH stretching absorption band at 3534.1 cm−1 in folic acid. Another major absorption band at 3417.38 cm−1 corresponded to free amino group (–NH2) a major peak present in pectin. Generally, the spectral analysis measures the selective absorption of light by the vibration modes of specific chemical bonds in the compounds interacting [39]. While evaluating the experiment, any interaction occurring between the folic acid and pectin polymer would produce altered vibrations of their atoms involved in the interaction. Normally, the change in the frequency and intensity of interacted molecules and uninterested (stand-alone) complexes were compared with their respective standard compounds in free form (here folic acid-alone and pectin-alone) [40]. Figure 3 exhibited FT-IR spectra of folic acid-alone (F) and formulations containing folic acid free-pectin submicrospheres with/without surfactants (PE1, PE3 and PE5) and formulations of folic acid-loaded pectin submicrospheres with/without surfactants (PE2, PE4 and PE6), respectively. The pure folic acid compound (F) gave the peaks contributed by an intense and broad band in the region characteristic peaks at 3534.1 cm−1 to the proton vibrations in a medium short hydrogen bond formed between O–H group and the O-atom of the carboxylate group. One sharp peak at 3417.38 cm−1 corresponded to N–H bending. The spectra for pectin of different formulations gave the peaks at 1752 cm−1 which is its characteristic peak due to typical C=O (carbonyl) group, exhibited C–O stretching at 1610 cm−1. Physically, pectin in its free form whether surfactants present or not formulations showed peaks resulting from simple super position of their separated components in the infrared spectra. In the case of folic acid-loaded submicrospheres, the peak of folic acid showing very less intensity as the folic acid concentration in the submicrospheres was low. It is also observed that there were neither shifting of peaks nor disappearance of functional peaks in the pectin and folic acid- loaded pectin formulations. The spectral analysis folic acid and pectin polymer indicated that the specific functional groups such as O–H, C–O, C=O, N–H and others of polymeric material in the submicrospheres surface have almost the same chemical characteristics of the pure polymer and the folic acid entrapped exhibited their main characteristics peaks. The study concluded that there was no occurrence of molecular interactions which may likely to cause altered structure of both folic and pectin in the various submicrospheres formulations.
Fig. 3.
FTIR spectra of folic acid and pectin formulations. PE: pectin blank; F: folic acid-alone; PE1, PE3 and PE5 are the formulations containing folic acid free-pectin submicrospheres with/without surfactants formulations; and folic acid-loaded pectin submicrospheres with/without surfactants formulations (PE2, PE4 and PE6)
Differential scanning calorimetry (DSC) is a thermal analytical technique which provides information about the physical properties of products, i.e. about the crystalline or amorphous nature of the formulations prepared. To determine the state of folic acid in nanoparticle, the samples were subjected to DSC.
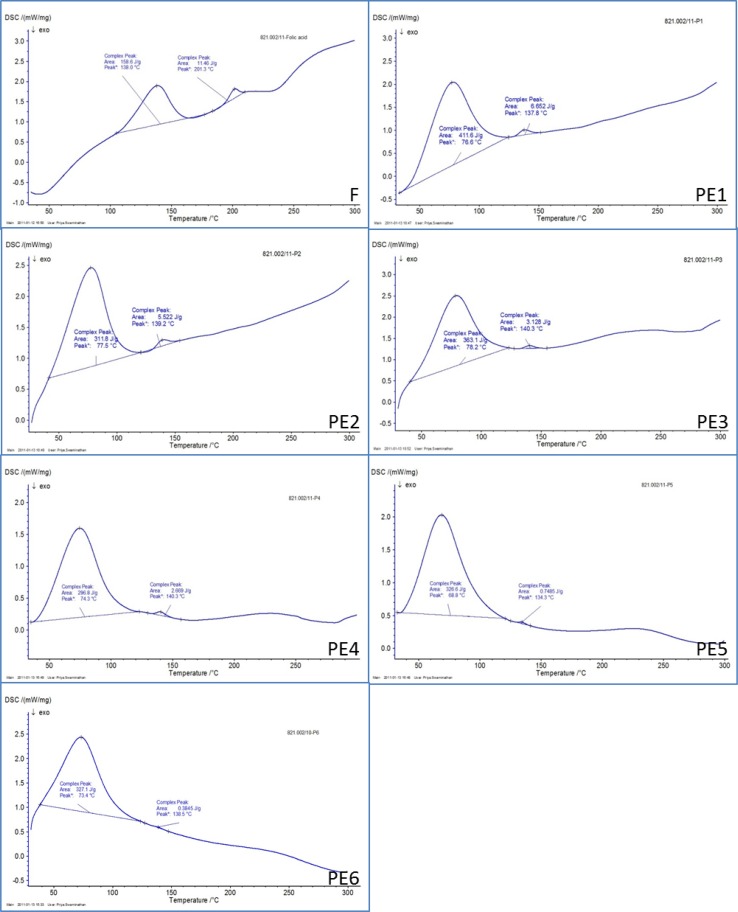
By virtue of their molecular structure, folic acid exhibits two endothermic peaks (138.0 and 201.3 °C) indicating a wide range of crystallinity of the formulations prepared. Whereas, in polymeric formulations like folic acid–loaded pectin system, both encapsulated molecules like folic acid and encapsulating polymer pectin would never counter influence on each other. Therefore, release kinetics of folic acid molecules located at the core in any given polymeric formulations, will not be affected when they exist in crystalline form [36]. In Fig. 4F, PE1–PE6 the DSC thermograms of submicrospheres of pure folic acid (F) and Pectin either individually or loaded with folic acid in the presence or absence of surfactants depicted sharp endothermic peaks at different melting points (Table 2). Figure 4F, PE1, PE3 and PE5 exhibited the DSC thermograms of folic acid-alone (F) exhibited two different melting peak at 138.0 and 201.3 °C, whereas the formulations of spheres containing pectin-alone submicrospheres with/without surfactants, respectively showed nearly the same thermal behavior as the individual components, indicating that there was no interaction between the folic acid and the pectin polymer in the crystalline state (Table 2). Similarly, Fig. 4PE2, PE4 and PE6 were the formulations of folic acid-loaded pectin submicrospheres with/without surfactants, respectively, interesting those exhibited identical peaks resembled the same and appeared to be crystalline (Table 2).
Fig. 4.
DSC thermograms of folic acid-alone (F), pectin submicrospheres containing folic acid (PE2, PE4 and PE6) and pectin submicrospheres without folic acid (PE1, PE3 and PE5) prepared in presence or absence of surfactants
Table 2.
Points of melting in folic acid-alone, pectin-alone and folic acid-loaded pectin submicrospheres
| Formulations | Initial melting point (°C) | Final melting point (°C) |
|---|---|---|
| F | 138.0 | 201.3 |
| PE1 | 76.6 | 137.8 |
| PE2 | 77.5 | 139.2 |
| PE3 | 78.2 | 140.3 |
| PE4 | 74.3 | 140.3 |
| PE5 | 68.8 | 134.3 |
| PE6 | 73.4 | 138.5 |
Swelling Test
Pectin appeared as key formulation parameters in submicrospheres formulation, as it controlled microsphere swelling and degradation with time and pH. In the results a pectin presenting lower swelling properties as the increased amount of drug in the formulations. It exhibited in the range of 44–52 % swelling. It would permit to enhance the pectin mass fraction in the formulations, and thus final macroporosity of the formulations, but would also result in reduced drug release ability and longer microsphere degradation time. The Table 3 showed the formulations with percentage of swelling in folic acid free-pectin submicrospheres with/without surfactants (PE1, PE3 and PE5) and formulations of folic acid-loaded pectin submicrospheres with/without surfactants (PE2, PE4 and PE6), respectively. A compromise must be found to adjust on-demand formulation properties in terms of delivery and final macroporosity.
Table 3.
Percentage swelling of folic acid-free and folic acid-loaded pectin submicrospheres
| Polymer | Code | Folic acidconc. (mg) | Pectin conc. (%) | Percentage of swelling |
|---|---|---|---|---|
| Pectin | PE1 | – | 0.5 | 53.33 ± 1.53 |
| Pectin | PE2 | 5 | 0.5 | 49.33 ± 2.52 |
| Pectin | PE3 | – | 0.5 | 44.67 ± 2.52 |
| Pectin | PE4 | 5 | 0.5 | 44.67 ± 3.06 |
| Pectin | PE5 | – | 0.5 | 42.33 ± 2.52 |
| Pectin | PE6 | 5 | 0.5 | 40.33 ± 3.51 |
Finally, it was observed that submicrospheres formulation of free-pectin submicrospheres with/without surfactants (PE1, PE3 and PE5) and formulations of folic acid-loaded pectin submicrospheres with/without surfactants (PE1, PE3 and PE5) showed moderate swelling ability. Pectin submicrospheres behave as hydrophilic matrices whose release ability is currently related to their swelling ability in dissolution media. This result considered of good prognostic in obtaining further sustained drug delivery and controlled erosion/degradation with time and pH [41].
Encapsulation Efficiency
The spectrophotometric method was used for determination of the encapsulation efficiency of folic acid in pectin submicrospheres. The folic acid-loaded pectin submicrospheres were separated from the aqueous suspension medium by centrifugation at 3500 rpm for 20 min. The amount of free folic acid was measured in the clear supernatant by UV at a wavelength of 366 nm. The folic acid encapsulation efficiency (EE) of the pectin submicrosphere and their % encapsulation efficiency (% EE) were calculated. The amount of the unincorporated folic acid present in the supernatant is presented in Table 4. Submicro-encapsulation efficiency of PE2, PE4 and PE6 formulations was 85.03, 90.35 and 96.11 %, respectively.
Table 4.
Encapsulation efficiency of folic acid-loaded pectin submicrospheres
| Pectin polymer | Code | Conc. of folic acid (mg) | Conc. of pectin (mg) | Ratio of the folic acid:pectin | Encapsulation efficiency (folic acid content) |
|---|---|---|---|---|---|
| Pectin | PE1 | – | 20 | – | – |
| Pectin | PE2 | 5 | 20 | 1:4 | 85.03 ± 0.64 % |
| Pectin | PE3 | – | 20 | – | – |
| Pectin | PE4 | 5 | 20 | 1:4 | 90.35 ± 0.62 % |
| Pectin | PE5 | – | 20 | – | – |
| Pectin | PE6 | 5 | 20 | 1:4 | 96.11 ± 0.63 % |
In Vitro Release of Folic Acid from Pectin Submicrospheres
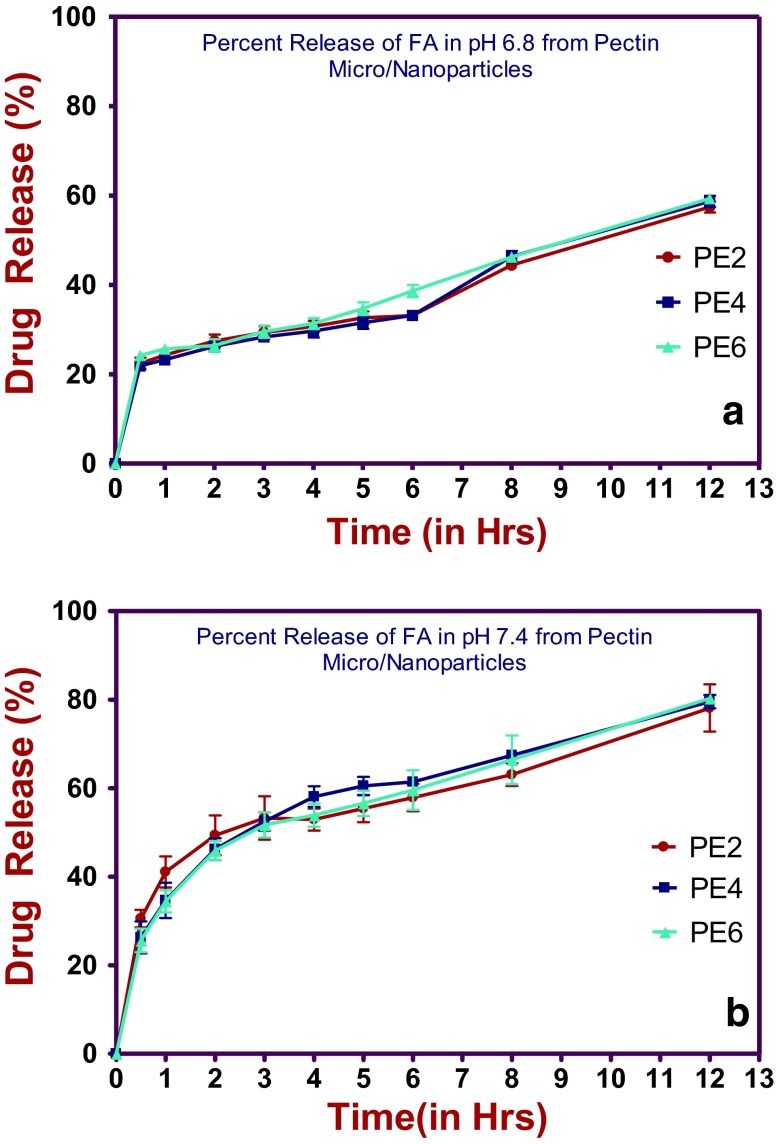
In vitro studies by dialysis were carried out in the phosphate buffer solution at two different pH 7.4 and pH 6.8, at 37 °C for PE2, PE4 and PE6 folic acid-loaded pectin submicrospheres with/without surfactants. The folic acid release may vary from formulation to formulation depending upon the pectin concentration. From Table 5, PE2 formulation showed initial folic acid release of 30.55 % at pH 6.8, and slightly decreased release 22.43 % at pH 7.4 within 30 min this may be due to initial burst effect which is important also to the pharmacological point of view. Whereas PE4 and PE6 formulations showed similar initial burst of folic acid 26.3 and 25.6 % at pH 6.8, and slightly decreased release 21.89 and 24.22 % at pH 7.4 respectively, within 30 min. Interestingly, the PE2, PE4 and PE6 submicrospheres formulations showed the significant increase in folic acid release 80.30 % the most and 78.13 % the least at pH 6.8 after 12 h gave the release pattern in controlled manner, but surprisingly the same formulations showed a significant decrease in folic acid release 59.32 % the most and 57.43 % the least at pH 7.4 after 12 h. The trend continued to follow the same in pH 6.8 from first hour of release and onwards indicating slow and sustained release of folic acid. On comparison of the release profile of the three (PE2, PE4 and PE6) formulations, it was observed that the release pattern had marginal difference between the PE2, PE4 and PE6 submicrospheres formulations, but pH 6.8 was found to be the preferred condition than that of pH 7.4. Release percentage of folic acid within 48 h (80.30 % in pH 6.8 and 59.32 % in pH 7.4), clearly showing that there is no interaction between the polymer pectin and the drug folic acid. The data reveals that slightly acidic pH was appropriate than that of slightly basic pH 7.4, which favored the release with respect to percent encapsulation efficiency (%EE) and bettered folic acid diffusion out of the dialysis bag will fall in the order PE6 > PE4 > PE2 submicrospheres formulations. Such sustained release can be explained by folic acid-loaded pectin submicrospheres sensitivity to ionic conditions. They behave as hydrophilic matrices whose release ability is currently related to their swelling ability and the degree of porosity of the formulations in dissolution media (Fig. 5).
Table 5.
Percentage of folic acid release in different time intervals at pH 6.8 and pH 7.4 from folic acid-loaded pectin submicrospheres
| Time interval (h) | Folic acid-loaded pectin submicrospheres folic acid release (%) at pH 6.8 | Folic acid-loaded pectin submicrospheres folic acid release (%) at pH 7.4 | ||||
|---|---|---|---|---|---|---|
| PE2 | PE4 | PE6 | PE2 | PE4 | PE6 | |
| 0.5 | 30.55 ± 1.99 | 26.30 ± 3.62 | 25.60 ± 2.66 | 22.43 ± 1.28 | 21.89 ± 1.10 | 24.22 ± 1.12 |
| 1.0 | 41.07 ± 3.51 | 34.67 ± 3.99 | 34.46 ± 2.51 | 24.28 ± 1.14 | 23.26 ± 1.12 | 25.68 ± 1.18 |
| 2.0 | 49.37 ± 4.48 | 46.23 ± 2.48 | 45.91 ± 2.13 | 27.46 ± 1.43 | 26.26 ± 1.23 | 26.42 ± 1.39 |
| 3.0 | 53.26 ± 4.90 | 52.48 ± 2.07 | 51.70 ± 2.84 | 29.34 ± 1.46 | 28.35 ± 1.17 | 29.53 ± 1.39 |
| 4.0 | 52.93 ± 2.50 | 58.11 ± 2.34 | 53.90 ± 2.62 | 30.70 ± 1.29 | 29.66 ± 1.43 | 31.36 ± 1.23 |
| 5.0 | 55.40 ± 3.08 | 60.50 ± 2.08 | 56.59 ± 2.85 | 32.65 ± 1.43 | 31.53 ± 1.34 | 34.69 ± 1.41 |
| 6.0 | 57.87 ± 3.09 | 61.44 ± 1.18 | 59.57 ± 4.50 | 33.14 ± 1.05 | 33.19 ± 1.03 | 38.64 ± 1.38 |
| 8.0 | 63.08 ± 2.53 | 67.45 ± 1.05 | 66.43 ± 5.51 | 44.40 ± 1.18 | 46.35 ± 1.22 | 46.23 ± 1.04 |
| 12.0 | 78.13 ± 5.31 | 79.57 ± 1.51 | 80.30 ± 1.06 | 57.43 ± 1.25 | 58.69 ± 1.27 | 59.32 ± 1.14 |
Fig. 5.
In vitro release folic acid (%) with the passage of time at a pH 6.8 and b pH 7.4 from folic acid-loaded pectin submicrospheres (PE2, PE4, PE6)
Hemocompatibility Study
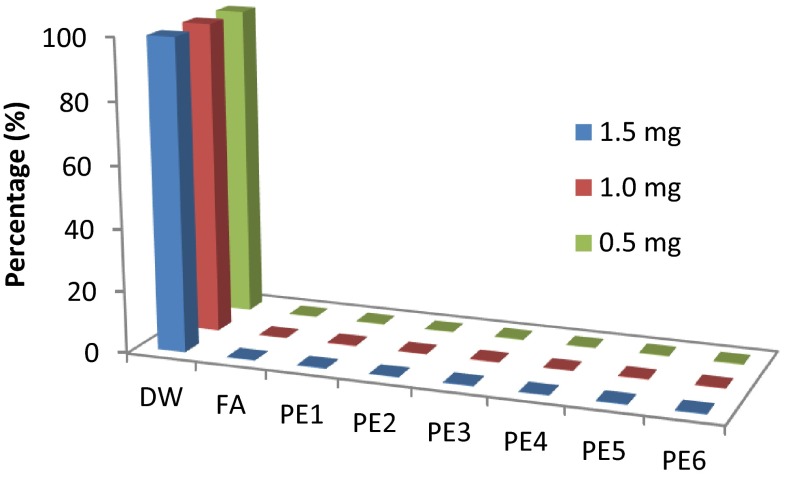
The hemolytic potential of any administered pharmaceuticals must be evaluated to avoid the possibility of red blood cells toxicity. In vitro haemolysis test of folic acid-alone and folic acid-loaded pectin submicrospheres were carried out (Fig. 6). From study, it was evident that folic acid-alone and pectin-alone did not cause any hemolysis at 0.5, 1 and 1.5 mg/ml, whereas folic acid-loaded pectin submicrospheres also showed negligible amount of the hemolysis at different concentrations.
Fig. 6.
Hemolysis of folic acid-alone, pectin-alone and folic acid-loaded pectin submicrospheres (PE1–PE6)
Conclusion
In the present investigation, for the first time folic acid-loaded pectin submicrospheres have been shown to have a novel controlled folic acid delivery which offer several potential benefits. Folic acid-loaded pectin submicrospheres have shown an excellent capacity for their association. It is an anti-anemic drug and was selected as candidate in the present study in order to explore the possibility of whether the polymer pectin could show better encapsulation? If so, does it release the same in a slow and sustained manner that is needed to establish rapid onset and relatively short duration of action? Average submicron size diameter particles, drug-polymer interaction and percentage encapsulation efficiency were found to be the criteria for optimal formulations.
The folic acid-loaded pectin submicrospheres formulations were evaluated with respect to their size, shape, encapsulation efficiency and other physical characteristics. The DSC analysis measurement had suggested that the folic acid in the folic acid-loaded pectin submicrospheres formulations was found in the crystalline form. FT-IR showed that there is no interaction between the folic acid and the pectin polymer. The encapsulation efficiency evaluated by spectrophotometric method exhibited better encapsulation efficiency. The in vitro release profile of folic acid from folic acid-loaded pectin submicrosphere exhibited a typical biphasic release phenomenon, showing initial burst release within 30 min and followed by slow and sustained release in an incremental form until 12 h. The in vitro release also indicated that the release property of folic acid from folic acid-loaded pectin submicrospheres not only depended on adsorption of the folic acid but also on diffusion through the pectin matrix. Folic acid-loaded pectin submicrospheres and pectin-alone submicrospheres show no hemolysis.
Acknowledgments
The authors wish to express their gratitude to the Kuvempu University, Davangere University and Dr. V. Rajendran, Professor, KSRCT, Thiruchengode, TN., for providing laboratory facilities and support. Thanks are due to Dr. G. U Kulkarni, JNCASR, Jakkur, Bangalore, for help with Scanning Electron Microscopy.
References
- 1.Hoffbrand AV, Weir DG. The history of folic acid. Br J Haematol. 2001;113:579–589. doi: 10.1046/j.1365-2141.2001.02822.x. [DOI] [PubMed] [Google Scholar]
- 2.Bailey SW, Ayling JE. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. In: Proceedings of the National Academy of Sciences of the United States of America; 2009. p. 15424–29. [DOI] [PMC free article] [PubMed]
- 3.Yoo HS, Park TG. Folate receptor targeted biodegradable polymeric doxorubicin micelles. J Controlled Release. 2004;96:273–283. doi: 10.1016/j.jconrel.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Stevanovic Magdalena, Radulovic Aleksandra, Jordovic Branka, Uskokovic Dragan. Poly(dl-lactide-co-glycolide) nanospheres for the sustained release of folic acid. J Biomed Nanotechnol. 2008;4:349–358. doi: 10.1166/jbn.2008.321. [DOI] [Google Scholar]
- 5.Shaw GM, Schaffer D, Velie EM, Morland K, Harris JA. Periconceptional vitamin use, dietary folate and the occurrence of neural tube defects. Epidemiology. 1995;6:219–226. doi: 10.1097/00001648-199505000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Goh YI, Koren G. Folic acid in pregnancy and fetal outcomes. J Obstet Gynaecol. 2008;28:3–13. doi: 10.1080/01443610701814195. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Miss Mdel R, Perez-Mutul J, Lopez-Canul B, et al. Folate, homocysteine, interleukin-6, and tumor necrosis factor alfa levels, but not the methylenetetrahydrofolate reductase C677T polymorphism, are risk factors for schizophrenia. J Psychiatr Res. 2010;44:441–446. doi: 10.1016/j.jpsychires.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Kanthamneni N, Prabhu S. Formulation development of targeted nanoparticle-based drug delivery systems for the chemoprevention of colon cancer. AAPS annual meeting exposition; 2006.
- 9.Vollset SE, Clarke R, Lewington S, Ebbing M, Halsey J, Lonn E, Armitage J, Manson JE, Hankey GJ, Spence JD, Galan P, Bønaa KH, Jamison R, Gaziano JM, Guarino P, Baron JA, Logan RF, Giovannucci EL, den Heijer M, Ueland PM, Bennett D, Collins R, Peto R. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50 000 individuals. Lancet. 2013;381:1029–1036. doi: 10.1016/S0140-6736(12)62001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinstein SJ, Hartman TJ, Stolzenberg-Solomon R, et al. Null association between prostate cancer and serum folate, vitamin B(6), vitamin B(12) and homocysteine. Cancer Epidemiol Biomarkers Prev. 2003;12:1271–1272. [PubMed] [Google Scholar]
- 11.Kafrissen ME, Oakley G. Pharmaceutical methods of delivering folic acid. US Patent 6 190 693. 2001.
- 12.Antony AC. The biological chemistry of folate receptors. Blood. 1992;79:2807–2820. [PubMed] [Google Scholar]
- 13.Chaudhary A, Nagaich U, Gulati N, Sharma VK, Khosa RL. Enhancement of solubilization and bioavailability of poorly soluble drugs by physical and chemical modifications: a recent review. J Adv Pharm Educ Res. 2012;2:32–67. [Google Scholar]
- 14.Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev. 2004;56:1649–1659. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Gu FX, Karnik R, Wang AZ, Alexis F, Levy-Nissenbaum E, Hong S, Langer RS, Farokhzad OC. Targeted nanoparticles for cancer therapy. Nano Today. 2007;2:14–21. doi: 10.1016/S1748-0132(07)70083-X. [DOI] [Google Scholar]
- 16.Pawar AP, Gadhe AR, Venkatachalam P, Sher P, Mahadik KR. Effect of core and surface cross-linking on the entrapment of metronidazole in pectin beads. Acta Pharm. 2008;58:78–85. doi: 10.2478/v10007-007-0046-0. [DOI] [PubMed] [Google Scholar]
- 17.Sharma HK, Sarangi B, Pradhan SP. Preparation and in vitro evaluation of mucoadhesive microbeads containing timolol maleate using mucoadhesive substances of Dilleniaindica L. Arch Pharm Sci Res. 2009;1:181–188. [Google Scholar]
- 18.Fattal E, Honnas H, Andremont A, Bourgeois S. Polysaccharide beads for colon delivery of antibiotic degrading enzymes. In: 15th international symposium on MICROENCAPSULATION, Parma, Italy; 2005. p. 18–21.
- 19.Goudanavar PS, Bagali RS, Chandrashekhara S, Patil SM. Design and characterization of diclofenac sodium microbeads by ionotropic gelation technique. Int J Pharma Bio Sci. 2010;2:1–10. doi: 10.4103/0975-7406.62691. [DOI] [Google Scholar]
- 20.Yamada H. Contribution of pectins on health care. In: Visser J, Voragen AGJ, editors. Pectins and pectinases. Amsterdam: Elsevier; 1996. pp. 173–190. [Google Scholar]
- 21.Behall K, Reiser S. Effects of pectin on human metabolism. In: Fishman ML, Ren JJ, editors. Chemistry and functions of pectins. Washington, DC: American Chemical Society; 1986. pp. 248–265. [Google Scholar]
- 22.Olano-Martin E, Rimbach GH, Gibson GR, Rastall RA. Pectin and pectic-oligosaccharides induce apoptosis in in vitro human colonic adenocarcinoma cells. Anticancer Res. 2003;23:341–346. [PubMed] [Google Scholar]
- 23.Willats WGT, Knox JP, Mikkelsen JD. Pectin: new insights into an old polymer are starting to gel. Trends Food Sci Technol. 2006;17:97–104. doi: 10.1016/j.tifs.2005.10.008. [DOI] [Google Scholar]
- 24.Sriamornsak P, Konthong S, Burapapadh K, Sungthongjeen S. Drug-loaded pectin microparticles prepared by emulsion-solvent evaporation. Adv Mater Res. 2012;506:282–285. doi: 10.4028/www.scientific.net/AMR.506.282. [DOI] [Google Scholar]
- 25.Burapapadh K, Kumpugdee-Vollrath M, Chantasart D, Sriamornsak P. Fabrication of pectin-based nanoemulsions loaded with itraconazole for pharmaceutical application. Carbohydr Polym. 2010;82:384–393. doi: 10.1016/j.carbpol.2010.04.071. [DOI] [Google Scholar]
- 26.Rubinstein I, et al. Effect of mouthpiece, nose clips, and head position on airway area measured by acoustic reflections. J Appl Physiol. 1987;63:1469–1474. doi: 10.1152/jappl.1987.63.4.1469. [DOI] [PubMed] [Google Scholar]
- 27.Ashford M, et al. Studies on pectin formulations for colonic drug delivery. J Controlled Release. 1994;30:225–232. doi: 10.1016/0168-3659(94)90028-0. [DOI] [Google Scholar]
- 28.Rolin C. Pectin. In: Whistler RL, Bemiller JN, editors. Industrial gums: polysaccharides and their derivatives. 3. New York: Academic Press; 1993. pp. 257–293. [Google Scholar]
- 29.Sriamornsak P. Effect of calcium concentration, hardening agent and drying condition on release characteristics of oral proteins from calcium pectinate gel beads. Eur J Pharm Sci. 1999;8:221–227. doi: 10.1016/S0928-0987(99)00010-X. [DOI] [PubMed] [Google Scholar]
- 30.Kim TH, Park YH, Kim KJ, Cho CS. Release of albumin from chitosan-coated pectin beads in vitro. Int J Pharm. 2003;250:371–383. doi: 10.1016/S0378-5173(02)00553-7. [DOI] [PubMed] [Google Scholar]
- 31.Atyabi F, Inanloo K, Dinarvand R. Bovine serum albumin loaded pectinate beads as colonic peptide delivery system: preparation and in vitro characterization. Drug Deliv. 2005;12:367–375. doi: 10.1080/10717540590968666. [DOI] [PubMed] [Google Scholar]
- 32.Bourgeois S, Laham A, Besnard M, Andremont A, Fattal E. In vitro and in vivo evaluation of pectin beads for the colon delivery of beta-lactamases. J Drug Target. 2005;13:277–284. doi: 10.1080/10611860500206583. [DOI] [PubMed] [Google Scholar]
- 33.Bourgeois S, Gernet M, Pradeau D, Andremont A, Fattal E. Evaluation of critical formulation parameters influencing the bioactivity of beta-lactamases entrapped in pectin beads. Int J Pharm. 2006;324:2–9. doi: 10.1016/j.ijpharm.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 34.Cheng K, Lim L-Y. Insulin-loaded calcium pectinate nanoparticles: effects of pectin molecular weight and formulation pH. Drug Dev Ind Pharm. 2004;30:359–367. doi: 10.1081/DDC-120030930. [DOI] [PubMed] [Google Scholar]
- 35.Ravikumara NR, Madhusudhan B. Chitosan nanoparticles for tamoxifen delivery and cytotoxicity to MCF-7 and Vero cells. Pure Appl Chem. 2011;83:2027–2040. doi: 10.1351/PAC-CON-11-01-06. [DOI] [Google Scholar]
- 36.Ravikumara NR, Madhusudhan B. Evaluation of anticancer efficacy of daidzein loaded poly(d,l) lactic acid nanoparticles. J Bionanosci. 2011;5:122–129. doi: 10.1166/jbns.2011.1061. [DOI] [PubMed] [Google Scholar]
- 37.Ravikumara NR, Madhusudhan B, Nagaraj TS, Hiremat SR, Raina G. Preparation and evaluation of nimesulide-loaded ethyl cellulose and methylcellulose nanoparticles and microparticles for oral delivery. J Biomater Appl. 2009;24:47–64. doi: 10.1177/0885328209103406. [DOI] [PubMed] [Google Scholar]
- 38.Jenquin MR, McGinity JW. Characterization of acrylic resin matrix films and mechanisms of drug-polymer interactions. Int J Pharm. 1994;101:23–34. doi: 10.1016/0378-5173(94)90072-8. [DOI] [Google Scholar]
- 39.Surolia R, Pachauri M, Ghosh PC. Preparation and characterization of monensin loaded PLGA nanoparticles. In vitro anti-malarial activity against plasmodium falciparum. J Biomed Nanotechnol. 2012;8:172–181. doi: 10.1166/jbn.2012.1366. [DOI] [PubMed] [Google Scholar]
- 40.Mainardes RM, Gremiao MPD, Evangelista RC. Thermoanalytical study of praziquantel-loaded PLGA nanoparticles. Braz J Pharm Sci. 2006;42:523–530. [Google Scholar]
- 41.Matias R, Ribeiro PRS, Sarraguça MC, Lopes JA. A UV spectrophotometric method for the determination of folic acid in pharmaceutical tablets and dissolution tests. Anal Methods. 2014;6:3065–3307. doi: 10.1039/c3ay41874j. [DOI] [Google Scholar]