Abstract
The total proteins in human urine have been compared by sulfosalicylic acid, sulfosalicylic acid with sodium sulphate and trichloroacetic acid methods with pyrogallol red molybdate method as there are no studies found quantifying imprecision and bias components. Fresh urine of 36 patients was analyzed by four methods. Imprecision and inaccuracy were determined by repeated analysis and method comparison studies using correlation plots, Bland and Altman, and Passing and Bablok regression analyses respectively. The coefficient of variation was 5.07 % for pyrogallol red molybdate; 6.84 % for sulfosalicylic acid; 3.97 % for sulfosalicylic acid with sodium sulphate and 5.93 % for trichloroacetic acid methods. Bland and Altman analysis showed a bias of 5.8, 1.7 and −5.4 for pyrogallol red molybdate versus sulfosalicylic acid, sulfosalicylic acid with sodium sulphate and trichloroacetic acid methods respectively. Passing and Bablok regression revealed a constant bias for pyrogallol red molybdate versus all turbidimetric methods but a proportional bias only with trichloroacetic acid method. Sulfosalicylic acid with sodium sulphate method is preferred to sulfosalicylic acid and trichloroacetic acid methods.
Keywords: Urinary protein assays, Turbidimetric methods, Dye-binding methods, Correlation, Bland and Altman analysis, Passing and Bablok regression analysis
Introduction
The measurement of proteins in human urine provides an important tool in the diagnosis of renal diseases. Several methods are available for the estimation of total proteins in urine including turbidimetric methods such as sulfosalicylic acid (SSA) [1], sulfosalicylic acid with sodium sulphate (SSSS) [2, 3], trichloroacetic acid (TCA) [4], or benzethonium chloride (BEC) [5] and the protein dye-binding methods utilizing coomassie brilliant blue (CBB) [6, 7] or pyrogallol red molybdate (PRM) [8]. Among the turbidimetric methods Meulemans [3] found that SSSS method is better than SSA method and TCA method which has greater sensitivity and better reproducibility. Among the dye-binding methods PRM method is commonly used in most of the hospitals because it is more sensitive, precise and practicable [9].
The relative agreements between the different laboratory analytical methods that measure the same chemical substance are assessed by method comparison studies. There is paucity of Indian data on comparison of proteins in human urine by different methods [10]. Also there were no studies found showing the agreement between PRM and the turbidimetric methods in human urine samples. Hence in this study the results of total protein concentration in human urine obtained by SSA, SSSS and TCA methods were compared with the more commonly used PRM method to identify and quantify imprecision and bias components (both constant and proportional bias) using the advanced statistical techniques like Bland and Altman plots and Passing and Bablok regression.
Materials and Methods
Urine Samples
Fresh urine specimens of 36 patients collected from the central laboratory, MediCiti Institute of Medical Sciences (MIMS), Ghanpur, Ranga Reddy district, Telangana, India, between July and October 2014, without preservatives covering a wide range of protein concentrations (urine dipstick: nil, trace, 1+, 2+ and ≥3+) were randomly taken for analysis of total proteins. They were centrifuged (2500×g for 10 min) and then subjected to analysis by the four methods.
Reagents
Micro protein PRM kit and urine dip stick strips were purchased from Euro diagnostic systems, India Pvt. Ltd. Analytical grade reagents for SSA, SSSS and TCA methods were purchased from SD fine company.
Protein Assays
Urine Dipstick
The dipstick test for total proteins is based on the “protein error of indicators” phenomenon in which certain chemical indicators demonstrate one colour in the presence of protein and another in its absence. The reagent is most sensitive to albumin and less sensitive to globulins, Bence–Jones protein, mucoproteins and haemoglobin. The test procedure was performed as described by the manufacturer. The colour was read exactly after 1 min. Colours formed range from yellow for a negative reaction to yellow green and green to blue green for a positive reaction [11].
Pyrogallol Red Molybdate Dye-Binding Assay
The urinary protein reacts with PRM dye reagent to form blue purple coloured complex with maximum absorbance at 600 nm. The assay procedure was performed as described by the manufacturer [12]. 20 µl of urine sample and protein calibrator (BSA, 100 mg/dl) was gently mixed with 1 ml of Microprotein PRM reagent and after 3 min incubation at 37 °C the absorbance of the assay mixture was measured at 600 nm against reagent blank within 30 min using a double beam (UV–vis) spectrophotometer (Systronics 2201, India). The concentration of unknown urinary protein was then determined from a plot of concentration versus absorbance obtained for the standard protein solutions. The method is linear up to 200 mg/dl. The protein concentration above 200 mg/dl was diluted and the value multiplied by the corresponding dilution factor. The reference range in healthy adult males and females is 1–15 mg/dl or 20–140 mg/24 h. This method has been validated in most of the autoanalysers [8].
Turbidimetric Methods: SSA, SSSS and TCA Methods
The total proteins present in urine were measured by precipitating the proteins with precipitating reagents, 3 % sulphosalicylic acid (SSA), 3 % sulphosalicylic acid in 7 % sodium sulphate (SSSS) and 3 % trichloroacetic acid (TCA). The turbidity formed was compared with that of a protein standard at 660 nm [1–4]. 1 ml of urine and bovine serum albumin (BSA, 200 mg/dl) calibrator were gently mixed with 3 ml of the precipitating reagents and after 5 min at 25–35 °C the absorbance of the assay mixture was measured at 660 nm against reagent blank using a double beam (UV–vis) spectrophotometer (Systronics 2201, India). A protein solution of known concentration was used to prepare a standard curve covering the range from 0 to 200 mg/dl. The concentration of unknown urinary protein was then determined from a plot of concentration versus absorbance obtained for the standard protein solutions. The assay is linear up to 200 mg/dl. The protein concentration above 200 mg/dl was diluted and the value multiplied by the corresponding dilution factor.
Statistical Analysis
The data was entered into MS excel database and was imported to MedCalc (MedCalc Software, 12.6.0 version, Belgium) for all analyses. Descriptive statistics were reported as median and range. Imprecision was reported in terms of coefficient of variation. Inaccuracy was assessed by method comparison study using correlation plots, Bland and Altman plots [13] and Passing and Bablok regression analysis [14].
Results
The data for urinary proteins by all the studied methods was not normally distributed. It was assessed based on the Kolmogorov–Smirnov test [16]. The median and range for the different methods is shown in Table 1.
Table 1.
Urinary protein concentration (mg/dl) in different methods
| PRM | SSA | SSSS | TCA | |
|---|---|---|---|---|
| Median | 33.21 | 30.66 | 31.05 | 43.81 |
| Range | 385.57 | 394.59 | 371.11 | 424.77 |
Measurement of Imprecision
Twenty replicate values of standard solution of bovine serum albumin (BSA) (100 mg/dl) [15] were analyzed by SSA, SSSS, TCA and PRM methods on the same day in one run and the mean (SD) and coefficient of variation’s (CV) are shown in Table 2.
Table 2.
Within-batch Imprecision (bovine serum albumin, 100 mg/dl)
| Method | Mean (mg/dl) | SD (mg/dl) | CV % |
|---|---|---|---|
| PRM | 111.44 | 5.65 | 5.07 |
| SSA | 104.76 | 7.17 | 6.84 |
| SSSS | 99.02 | 3.93 | 3.97 |
| TCA | 74.33 | 4.41 | 5.93 |
Measurement of Inaccuracy
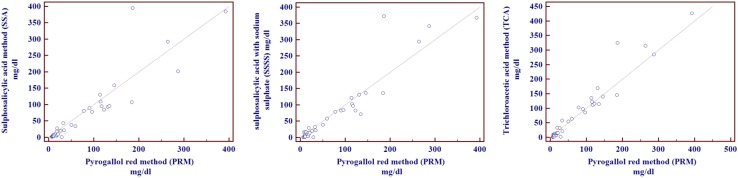
Visual examination of the data patterns was done with scatter diagrams to inspect the distribution of the data and relations between values obtained with PRM versus each of the turbidimetric methods (Fig. 1; Table 3).
Fig. 1.
Comparison of urinary proteins by PRM versus turbidimetric methods: Correlation plots
Table 3.
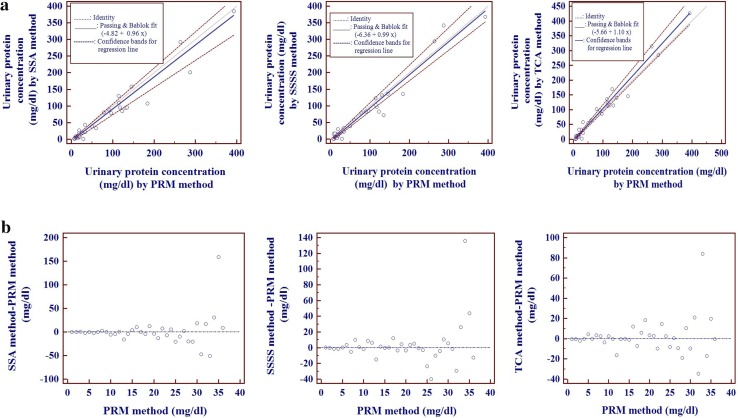
Outcome of Passing and Bablok regression analysis
| Method | r-value | Intercept A | 95 % CI | Fixed bias or constant bias | Slope B | 95 % CI | Proportional bias |
|---|---|---|---|---|---|---|---|
| PRM versus SSA | 0.905* | −4.82 | −8.61 to −2.96 | Yes | 0.96 | 0.82–1.09 | No |
| PRM versus SSSS | 0.936* | −6.36 | −9.43 to −3.83 | Yes | 0.99 | 0.92–1.13 | No |
| PRM versus TCA | 0.968* | −5.66 | −7.56 to −4.01 | Yes | 1.1 | 1.01–1.21 | Yes |
* Statistically significant
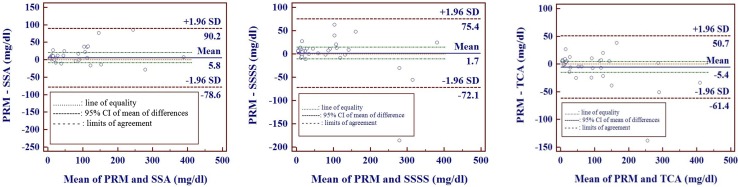
In Bland and Altman analysis we observed a positive bias for PRM versus SSA and PRM versus SSSS methods and a negative bias for PRM versus TCA methods (Fig. 2). Passing and Bablok regression analysis (Table 3; Fig. 3a, b) revealed that there is a constant bias between PRM versus each of the turbidimetric methods (SSA, SSSS, TCA). There is no proportional bias for PRM versus SSA and PRM versus SSSS methods but not for PRM versus TCA methods. Residual plot (Fig. 3b) and cumulative sum linearity test indicates no significant deviation from linearity (p = 0.74). The Residual standard deviation (RSD) ± 1.96 (31.62, −61.98 to 61.98 for PRM vs. SSA; 27.26, −53.42 to 53.42 for PRM vs. SSSS; 18.23, −35.74 to 35.74 for PRM vs. TCA) indicate that 95 % of random differences are within these intervals.
Fig. 2.
Bland and Altman plots of urinary proteins
Fig. 3.
a Passing and Bablok regression analyses of urinary proteins: scatter diagram with regression line and confidence bands for the regression line. b Passing and Bablok regression analyses of urinary proteins: Residual plot of distribution of difference around the fitted regression line
Discussion
In this study the turbidimetric methods were compared with PRM method for the analysis of total proteins in human urine samples. It was observed that SSSS method had good precision when compared to the other methods. The TCA method showed good correlation followed by SSSS and SSA methods with PRM method. However, the TCA method showed both constant and proportional bias in comparison with PRM method.
Several studies have been performed on comparison of urinary total proteins by turbidimetric methods and dye binding methods [1–4] [9] [15]. The turbidimetric methods were most commonly used because they are simple to perform, rapid, familiar to many clinical laboratories but were found to have poor precision and sensitivity, limited linearity, variable response to different proteins [9] and also require a large sample volume [15]. The PRM method which is a dye binding method was found to have better precision, sensitivity and practicability but also is subjected to variations in binding to different proteins [9]. Among the turbidimetric methods Meulemans [3] and Pennock et al. [2] found SSSS method, which is not influenced by albumin-globulin ratio to be better than SSA and TCA methods. The SSA method is affected by the albumin-globulin ratio. In a series of solutions having an equal concentration of total proteins but with increasing concentration of albumin there was an increase in protein concentration by SSA method, whereas a minor change was observed with SSSS and TCA methods [2]. In the present study it was observed that SSSS method had good precision in comparison with SSA, TCA and PRM methods. In Bland and Altman analysis the SSSS method had a least positive bias of 1.7 in comparison with PRM method. In Passing and Bablok regression analysis it was observed that there is constant but not proportional bias for SSA and SSSS methods in comparison with PRM method. However, TCA method had both constant and proportional bias when compared to PRM method. It is likely that TCA method is less sensitive to pure albumin as we observed a mean BSA value of 74.33 mg/dl in the repeatability experiment, but with respect to human urine samples this method showed higher results indicating that this method picked up other proteins also in addition to albumin.
There are limitations to this study. We were not able to study the Between-day precision, linearity and interferences. A large sample size is required for this type of study. Various methods respond differently to different proteins. Hence this preliminary study needs to be extended to address these issues.
In conclusion among the turbidimetric methods, which require a large sample volume it was found that SSSS method was more preferable when compared to SSA and TCA methods. PRM method which is a dye binding method can also be used for routine purpose as it requires less sample volume and is more practicable.
Acknowledgments
We thank the U.S. National Institutes of Health Fogarty-supported (D43 TW009078) SHARE INDIA and University of Pittsburgh team for conducting the research method courses which contributed to the conduct and publication of this research work. We also thank Dr. Sashidar B, professor/HOD and Dr. Tanuja K, Department of Biochemistry, Osmania University, Hyderabad for their valuable suggestions during the study period. Also, we would thank the senior technician Mr. Venkatesh K who had helped us in the sample analysis.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Henry RJ, Sobel CSM. Turbidimetric determination of proteins with sulphosalicylic and trichloroacetic acids. Proc Soc Exp Biol Med. 1956;92:748–751. doi: 10.3181/00379727-92-22601. [DOI] [PubMed] [Google Scholar]
- 2.Pennock Ca, Passant LP, Bolton FG. Estimation of cerebrospinal fluid protein. J Clin Pathol. 1968;21(4):518–520. doi: 10.1136/jcp.21.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meulemans O. Determination of total protein in spinal fluid with sulphosalicylic acid and trichloroacetic acid. Clin Chim Acta. 1960;5:757–761. doi: 10.1016/0009-8981(60)90020-6. [DOI] [PubMed] [Google Scholar]
- 4.Shahangian S, Brown PI, Ash KO. Turbidimetric measurement of total urinary proteins: a revised method. Am J Clin Pathol. 1984;81:651–654. doi: 10.1093/ajcp/81.5.651. [DOI] [PubMed] [Google Scholar]
- 5.Iwata J, Nishikaze O. New micro-turbidimetric method for determination of protein in cerebrospinal fluid and urine. Clin Chem. 1979;25:1317–1319. [PubMed] [Google Scholar]
- 6.McIntosh JC. Application of dye-binding method to the determination of protein in urine and cerebrospinal fluid. Clin Chem. 1977;23:1939–1940. [PubMed] [Google Scholar]
- 7.Schleicher E, Wieland OH. Evaluation of the Bradford method for protein determination in body fluids. J Clin Chem Clin Biochem. 1978;16:533–534. [PubMed] [Google Scholar]
- 8.Watanabe N, Kamei S, Ohkubo A, Yamanaka M, Ohsawa S, Makino K, et al. Urinary protein as measured with a pyrogallol red-molybdate complex, manually and in a Hitachi 726 automated analyzer. Clin Chem. 1986;32:1551–1554. [PubMed] [Google Scholar]
- 9.Orsonneau JL, Douet P, Massoubre C, Lustenberger P, Bernard S. An improved pyrogallol red-molybdate method for determining total urinary protein. Clin Chem. 1989;35:2233–2236. [PubMed] [Google Scholar]
- 10.Chaturvedi S, Namita Jain AB. Evaluation of semi-quantitative methods for protein and suger estimation in urine. Indian J Pathol Microbiol. 2001;44(4):399–401. [PubMed] [Google Scholar]
- 11.Johnson AM, Rohlfs EM, Silverman LM. Analysis of proteins. In: Burtis CAAE, editor. Tietz fundamentals of clinical chemistry. 5. Philadelphia: W.B Saunders Company; 2001. pp. 343–351. [Google Scholar]
- 12.Johnson AM, Rohlfs EM, Silverman LM. Analysis of proteins. In: Burtis CA, editor. Tietz fundamentals of clinical chemistry. 3. Philadelphia: W.B Saunders Company; 1999. pp. 477–540. [Google Scholar]
- 13.Altman D, Bland J. Measurement in medicine: the analysis of method comparison studies. Stat [Internet] 1983; 32:307–17. http://www.jstor.org/stable/2987937.
- 14.Passing H, Bablok W. A new biometrical procedure for testing the equality of measuremments from two different analytical methods: application of linear regression procedures for method comparison studies in clinical chemistry, Part 1. J Clin Chem Clin Biochem. 1983;21:709–720. doi: 10.1515/cclm.1983.21.11.709. [DOI] [PubMed] [Google Scholar]
- 15.Dilena BA, Penberthy LA, Fraser CG. Six methods for determining urinary protein compared. Clin Chem. 1983;29:553–557. [PubMed] [Google Scholar]
- 16.Neter J, Wasserman W, Whitmore GA. Applied statistics. 3. Boston: Allyn and Bacon company; 1988. [Google Scholar]