Abstract
The C677T polymorphism of the methylenetetrahydrofolate reductase (MTHFR) gene was implicated to be associated with thrombophilia due to its role in catalyzing the formation of 5-methylenetetrahydrofolate, a co-substrate for the conversion of homocysteine to methionine. Several case–control studies were investigated MTHFR C677T polymorphism as risk for recurrent pregnancy loss (RPL). These studies rendered contradictory results, some indicating that the polymorphism is associated with the risk of RPL whereas others concluded there is no association. To shed light on these inconclusive findings, a meta-analysis of all available studies published from Asian population relating the C677T polymorphism to the risk of RPL was conducted. The following electronic databases were searched without language restrictions: PubMed, Google Scholars, Elsevier and Springer Link up to December, 2015. Meta-analysis was performed using MetaAnalyst and Mix version 1.7. Meta-analysis results suggested that MTHFR C677T polymorphism contributed to the increased RPL risk in Asian population using all five genetic models (for T vs. C: OR 1.35, 95 % CI 1.09–1.68, p = 0.009; for TT + CT vs. CC: OR 1.44, 95 % CI 1.14–1.82, p = 0.006; for CT vs. CC: OR 1.39, 95 % CI 1.07–1.8, p = 0.01; for TT vs. CC: OR 1.79, 95 % CI 1.23.2.6, p = 0.007; for TT vs. CT + CC: OR 1.61, 95 % CI 1.02–2.56, p = 0.04). In conclusion, this meta-analysis demonstrates a strong association between the MTHFR C677T variant and RPL in Asian population and raising the importance of the use of folate in its treatment and prevention.
Keywords: Recurrent pregnancy loss, Thrombophilic gene, MTHFR, C677T, Meta-analysis, Folate
Introduction
Recurrent pregnancy loss (RPL) or spontaneous abortions (SA) is defined as three or more consecutive miscarriages [1–3]. RPL is a major concern in gynecology, affecting about 1–5 % of couples [2, 4, 5] and frequently accompanied by maternal morbidity as well as a considerable psychological burden. The risk of recurrence increases with the maternal age and number of successive losses [6, 7]. It is a multifactorial disorder caused very often by genetic abnormalities (gene mutations and abnormal embryonic karyotypes), endocrine disorders, uterine anatomy anomalies, infectious or immunologic factors, alcohol use and chemical exposure [8–11]. Despite intense anatomic, endocrinologic, and immunologic screening efforts, up to 50 % of RPL remain unexplained [12].
Published studies showed that the inherited thrombophilic polymorphisms are significant risk factors for obstetric complications, such as pre-eclampsia, placental abruption, stillbirth and fetal growth restriction [13–16]. RPL is also speculated to be associated with inherited thrombophilia that encompass diverse conditions including the thermolabile variant of the methylenetetrahydrofolate reductase (MTHFR) [17, 18]. MTHFR is a key enzyme in folate/homocysteine pathway, which catalyzes the reduction of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate (5-THF), and then methionine synthase catalyzed the conversion of 5-THF and homocysteine to methionine and tetrahydrofolate. Under the condition of folate deficiency and/or hypo functional MTHFR facilitate the conversion of 5,10-methylene THF to less 5-methyl THF, and causes less conversion of homocysteine to methionine, which may result in abnormal DNA methylation and DNA stand breaks etc. The gene encoding MTHFR has been mapped to chromosomal region 1p36.3. There were about 40 different genetic polymorphisms of MTHFR and out of which C677T variant is most studied and clinically important polymorphism. C677T missense mutation (rs 1,801,133; Ala 222 Val) at nucleotide 677 results in an enzyme that is thermolabile and exhibits reduced activity compared with the wild type. This mutation is associated with hyperhomocysteinemia [19–21]. TT MTHFR homozygotes are predisposed to increased plasma homocysteine levels, particularly in individuals with low folate [22, 23]. Hyperhomocysteinemia has been implicated in premature vascular disease [24], venous thrombosis [25] and unexplained early pregnancy loss [23, 26]. Hyperhomocysteinemia caused by the C677T polymorphism has been associated with coronary artery disease, venous thrombosis and complications of pregnancy i.e. RPL.
Numerous studies have focused on the relationship between MTHFR C677T polymorphism and RPL risk [27–30], but the conclusions remain controversial. The discrepancies among studies may be ascribed to the relatively small sample size in each investigation as well as ethnicity difference. Therefore, present meta-analysis was carried out by using genotype data from all eligible investigations to provide a more precise evaluation of the association of MTHFR C677T polymorphisms with RPL susceptibility in Asian population.
Methods
The articles were identified by searching PubMed, Google Scholar, Elsevier and Springer Link databases up to December, 2015 using following terms: ‘‘methylenetetrahydrofolate reductase’’, “MTHFR”, “C677T” and “Recurrent pregnancy loss”, “RPL”. A cited reference search of the retrieved articles was carried out, and publications were also identified by reviewing their bibliographies.
Data Extraction
Following data from each publication were extracted: author name; country of origin; selection and characteristics of cases and controls; demographic information; racial descent of the study population; numbers of eligible and genotyped cases and controls; and numbers of cases and controls for each MTHFR genotype.
Inclusion–Exclusion Criteria
The following criteria were used to include published studies: (a) Studies must have a case–control and must be published as full papers, (b) Authors must investigate RPL patients and healthy control subjects, (c) Authors must provide information on genotype/allele numbers of the MTHFR C677T polymorphism or sufficient data to calculate these. The major reasons for exclusion of studies were (1) only case studied, (2) review papers, editorial, letter to editor and (3) containing overlapping data and (4) no enough data to estimate OR with 95 % CI.
Statistical Analysis
The meta-analysis examined the overall association for the allele contrast (T vs. C), homozygotes (TT vs. CC), heterozygote/co-dominant (CT vs. CC), recessive (TT vs. CT + CC) and dominant (TT + CT vs. CC) models. The effect of association was indicated as odds ratio (OR) with the corresponding 95 % confidence interval (CI). The pooled OR was estimated using fixed effects (FE) [31] and random effects (RE) [32] models [33]. Sensitivity analysis performed by exclusion of the studies in which control population was not in Hardy–Weinberg equilibrium, studies with small sample sizes and higher p value.
For the assessment of publication bias the Begg’s test (funnel plot method) and the Egger regression asymmetry test was used. The significance of the intercept was determined with the t-test suggested by Egger. p < 0.05 was considered representative of statistically significant publication bias [34, 35]. All analyses were performed using the computer program MIX version 1.7 [36]. A p value less than 0.05 was considered statistically significant, and all the p values were two sided.
Results
Characteristics of Included Studies
One hundred two (102) articles were retrieved after search of PubMed, Google Scholar, Elsevier and Springer Link databases. After screening the titles and abstracts of all retrieved articles, 37 articles were excluded. Then 65 full texts were reviewed and 12 articles were further excluded. Another 24 articles from remaining 53 articles were again excluded because studied population was not Asian. Finally, 29 studies were included in present meta-analysis [11, 15, 16, 21, 27–30, 37–57] (Fig. 1; Table 1).
Fig. 1.
Flow chart shows study selection procedure. Twenty-five case–control studies were included in present meta-analysis
Table 1.
Characteristics of twenty-five studies included in the present meta-analysis
| Study | Country | Control | Case | Reference |
|---|---|---|---|---|
| Brener et al. (1999) | Israel | 106 | 76 | Thromb. Haemost. 82, 6–9 |
| Lissak et al. (1999) | Israel | 18 | 41 | Am J Obstet Gynecol 181, 126–130 |
| Wang et al. (2002) | China | 119 | 62 | Lancet 18, 291–293. |
| Kumar et al. (2003) | India | 24 | 24 | J Obstet Gynaecol 23, 55–58 |
| Li et al. (2004) | China | 50 | 57 | Zhonghua Yi Xue Yi Chuan Xue Za Zhi 21, 39–42 |
| Makino et al. (2004) | Japan | 76 | 85 | Am J Reprod Immunol 52, 60–66 |
| Wang et al. (2004) | China | 82 | 147 | Zhonghua Fu Chan Ke Za Zhi 39, 238–241 |
| Guan et al. (2005) | China | 117 | 127 | Zhonghua Yi Xue Yi Chuan Xue Za Zhi 22, 330–333 |
| Kobashi et al. (2005) | Japan | 174 | 38 | Semin Thromb Hemost 31, 266–271 |
| Song et al. (2005) | China | 56 | 50 | Zhonghua Wei Chan Yi Xue Za Zhi 8, 160–164 |
| Mtiraoui et al. (2006) | Behrain | 200 | 200 | Reproduction 131, 395–401 |
| Wang et al. (2006) | China | 82 | 147 | International Journal of Gynecology and Obstetrics (2006) 92, 264–265 |
| Vettriselvi et al. (2008) | India | 120 | 104 | J Obstet Gynaecol Res 34, 301–306 |
| Govindaiah et al. (2009) | India | 140 | 140 | Clin Biochem 42, 380–386 |
| Mukhopadhyay et al. (2009) | India | 80 | 84 | Genet Test Mol Biomarker |
| Abu-Asab et al. (2011) | Palestine | 402 | 329 | Volume 13, Number 6, 2009 |
| Jeddi-Tehrani et al. (2011) | Iran | 100 | 100 | American Journal of Reproductive Immunology 66 (2011) 149–156 |
| Settin et al. (2011) | Egypt | 136 | 70 | Am J Reprod Immunol 67, 251–255 |
| Dissanayke et al. (2012) | Srilanka | 171 | 200 | Genetic testing and Molecular Biomarkers, 15, 887–892 |
| Nair et al. (2012) | India | 140 | 106 | J Obstet Gynaecol Res Vol. 38, No. 9: 1168–1176 |
| Ozdemir et al. (2012) | Turkey | 106 | 327 | Reproductive Sciences, 19(2), 210–215. |
| Torabi et al. (2012) | Iran | 100 | 100 | Genetic testing and Molecular Biomarker, 16, 279–28 |
| Zonouzi et al. (2012) | Iran | 50 | 89 | ISRN Obst Gynec. Article ID 945486, 6 |
| Kaur et al. (2012) | India | 593 | 107 | J Reprod Infertil 13(2), 89–94 |
| Parveen et al. (2013) | India | 300 | 200 | ISRN Obstet Gynecol, 2012;94 |
| Cao et al. (2014) | China | 166 | 82 | Genes Nutr 402–407 |
| Yousefian et al. (2014) | Iran | 204 | 116 | Iran Red Crescent Med J. 16(7), e16763 |
| Farahmand et al. (2015) | Iran | 330 | 350 | J Matern Fetal Neonatal Med. doi:10.3109/14767058.2015.1044431 |
| Vanill et al. (2015) | India | 15 | 15 | J Clin Diagn Res 9(2), 15–18 |
All included studies were published between 1999 and 2013. All these twenty-five studies were performed in different countries like-Behrain [47], China [38–40, 42, 44, 46, 54], Egypt [52], India [11, 21, 29, 30, 41, 48, 49, 57], Iran [28, 51, 53, 55, 56], Israel [15, 37], Japan [43, 45], Palestine [50], SriLanka [16], Turkey [27]. Smallest sample size was 24 [41] and largest sample size was 329 [50]. Seven studies did not show any association between C677T polymorphism and RPL risk [21, 28, 29, 37, 43, 47, 52], remaining twenty-two studies showed significant association. In twenty-nine studies, total cases were 3725 with CC (1971), CT (1325) and TT (429), and controls were 4105 with CC (2545), CT (1218), and TT (342). In controls, genotypes percentage of CC, CT and TT were 61.99, 29.67 and 8.33 % respectively. In total cases, percentage of CC, CT and TT genotypes were 52.91, 35.57 and 11.51 % respectively. Frequencies of CC and CT genotypes were highest in both cases and controls (Table 2). Number of C and T alleles were also calculated and presented in Table 2. Control population of ten studies was not in HWE [15, 28, 29, 38, 39, 44, 46, 47, 51, 52].
Table 2.
The distributions of MTHFR C677T genotypes and allele frequencies of RPL disease cases and controls in Asian studies
| Study ID | Genotype | Alleles | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CC | CT | TT | C | T | ||||||
| Case | Control | Case | Control | Case | Control | Case | Control | Case | Control | |
| Brener et al. (1999) | 54 | 86 | 8 | 9 | 14 | 11 | 116 | 36 | 181 | 31 |
| Lissak et al. (1999) | 17 | 7 | 20 | 7 | 4 | 4 | 54 | 28 | 21 | 15 |
| Wang et al. (2002) | 13 | 43 | 33 | 71 | 16 | 5 | 59 | 65 | 157 | 81 |
| Kumar et al. (2003) | 18 | 22 | 6 | 2 | 0 | 0 | 42 | 6 | 46 | 2 |
| Li et al. (2004) | 16 | 25 | 32 | 20 | 9 | 5 | 64 | 50 | 70 | 30 |
| Makino et al. (2004) | 33 | 29 | 42 | 32 | 10 | 15 | 108 | 62 | 90 | 62 |
| Wang et al. (2004) | 49 | 43 | 78 | 16 | 20 | 23 | 176 | 118 | 102 | 62 |
| Guan et al. (2005) | 13 | 19 | 59 | 73 | 55 | 25 | 85 | 169 | 111 | 123 |
| Kabashi et al. (2005) | 5 | 67 | 30 | 82 | 3 | 25 | 40 | 36 | 216 | 132 |
| Song et al. (2005) | 36 | 40 | 2 | 12 | 12 | 4 | 74 | 26 | 92 | 20 |
| Mtiraoui et al. (2006) | 156 | 92 | 30 | 47 | 14 | 61 | 342 | 58 | 231 | 169 |
| Wang et al. (2006) | 49 | 43 | 78 | 34 | 20 | 5 | 176 | 118 | 120 | 44 |
| Vettriselvi et al. (2008) | 86 | 98 | 15 | 19 | 3 | 3 | 187 | 21 | 215 | 25 |
| Govindaiah et al. (2009) | 111 | 112 | 25 | 28 | 4 | 0 | 247 | 33 | 252 | 28 |
| Mukhopadhyay et al. (2009) | 75 | 78 | 6 | 2 | 3 | 0 | 156 | 12 | 158 | 2 |
| Abu-Asab et al. (2011) | 145 | 182 | 151 | 177 | 33 | 43 | 441 | 217 | 541 | 263 |
| Jeddi-Tehrani et al. (2011) | 43 | 66 | 42 | 25 | 15 | 9 | 128 | 72 | 157 | 43 |
| Settin et al. (2011) | 40 | 67 | 26 | 68 | 4 | 1 | 106 | 34 | 202 | 70 |
| Dissanayke et al. (2012) | 158 | 142 | 39 | 27 | 3 | 2 | 355 | 45 | 311 | 31 |
| Nair et al. (2012) | 75 | 118 | 26 | 21 | 5 | 1 | 176 | 36 | 257 | 23 |
| Ozdemir et al. (2012) | 145 | 79 | 130 | 27 | 52 | 0 | 420 | 234 | 185 | 27 |
| Torabi et al. (2012) | 43 | 66 | 42 | 25 | 15 | 9 | 128 | 72 | 157 | 43 |
| Zonouzi et al. (2012) | 53 | 27 | 30 | 22 | 6 | 1 | 136 | 42 | 76 | 24 |
| Kaur et al. (2013) | 86 | 463 | 16 | 109 | 5 | 21 | 188 | 26 | 1035 | 151 |
| Parveen et al. (2013) | 110 | 196 | 70 | 90 | 20 | 14 | 290 | 110 | 482 | 118 |
| Cao et al. (2014) | 53 | 29 | 83 | 43 | 30 | 10 | 189 | 101 | 143 | 63 |
| Yousefian et al. (2014) | 96 | 63 | 90 | 43 | 18 | 10 | 282 | 169 | 126 | 63 |
| Farahmand et al. (2015) | 180 | 230 | 114 | 85 | 36 | 35 | 474 | 545 | 186 | 155 |
| Vanill et al. (2015) | 13 | 13 | 2 | 2 | 0 | 0 | 28 | 28 | 2 | 2 |
Meta-analysis
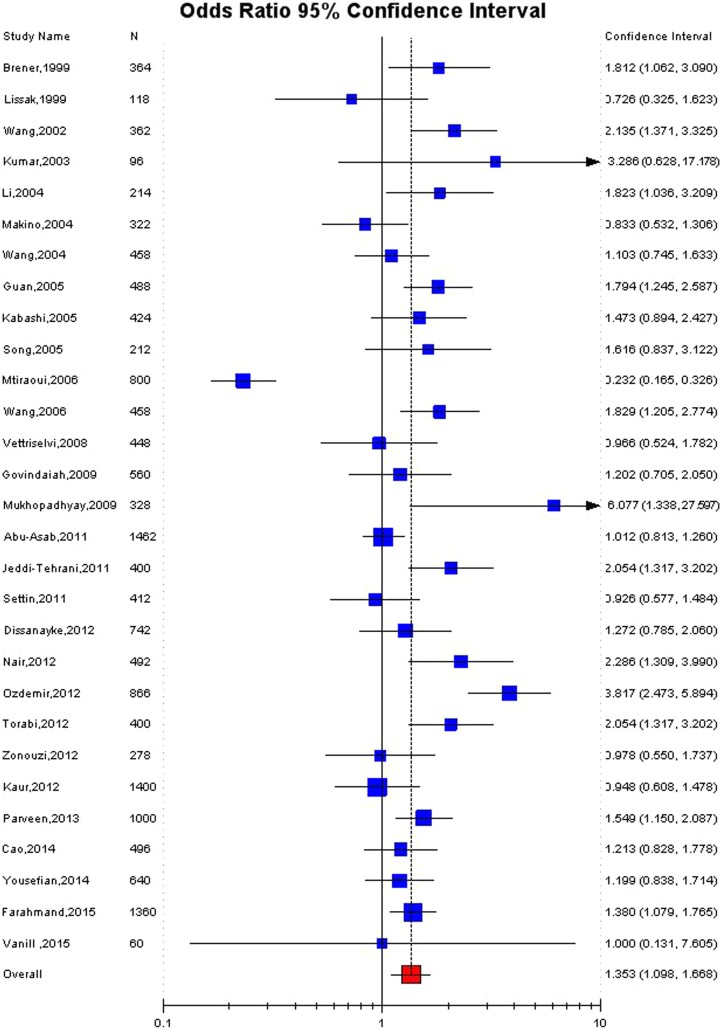
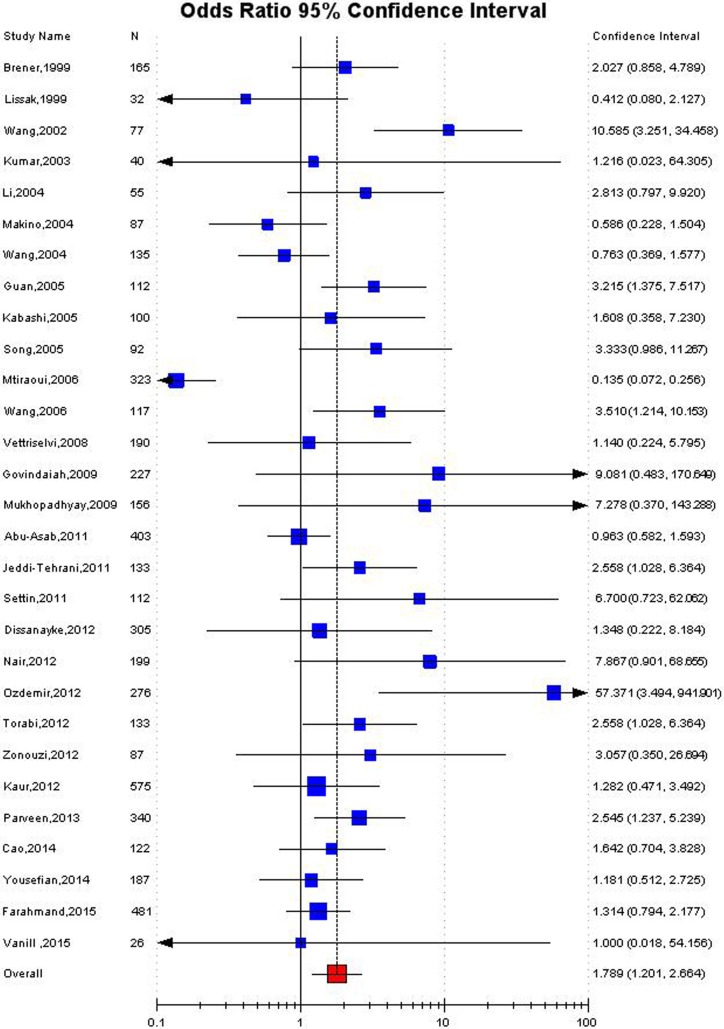
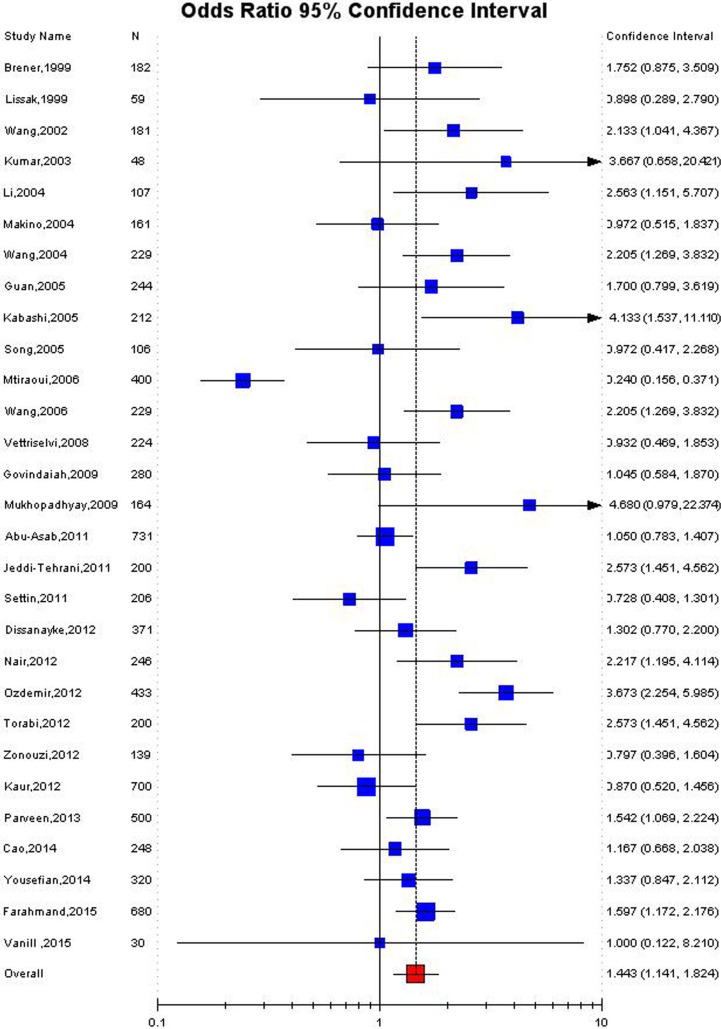
Significant association was detected between the MTHFR C677T polymorphism and the susceptibility to RPL in Asian population in all the genetic models using random effect model (for T vs. C: OR 1.38, 95 % CI 1.08–1.75, p = 0.009; CT vs. CC: OR 1.39, 95 % CI 1.07–1.8, p = 0.01; for TT + CT vs. CC: OR 1.47, 95 % CI 1.10–1.9, p = 0.006; for TT vs. CC: OR 1.95, 95 % CI 1.2–3.2, p = 0.007; for TT vs. CT + CC: OR 1.61, 95 % CI 1.02–2.56, p = 0.04) (Table 3; Figs. 2, 3, 4). Significant association was also found in fixed effect models using all genetic models (for T vs. C: OR 1.28, 95 % CI 1.17–1.4,, p < 0.0001; for TT + CT vs. CC: OR 1.32, 95 % CI 1.18–1.47, p < 0.0001; for TT vs. CC: OR 1.43, 95 % CI 1.18–1.7, p = 0.0002; for TT vs. CT + CC: OR 1.29, 95 % CI 1.08–1.5; for CT vs. CC: OR 1.32, 95 % CI 1.17–1.5, p = 0.004) (Table 3).
Table 3.
Summary estimates for the odds ratio (OR) of MTHFR C677T in various allele/genotype contrasts, the significance level (p value) of heterogeneity test (Q test), and the I2 metric and publication bias p value (Egger Test)
| Genetic Models | Fixed effect OR (95 % CI), p |
Random effect OR (95 % CI), p |
Heterogeneity p value (Q test) | I2 (%) | Publication Bias (p of Egger’s test) |
|---|---|---|---|---|---|
| Allele Contrast (T vs. C) | 1.28 (1.17–1.4), <0.0001 | 1.35 (1.09–1.68), 0.009 | <0.0001 | 85.07 | 0.24 |
| Co-dominant (CT vs. CC) | 1.32 (1.17–1.5), <0.0001 | 1.39 (1.07–1.8), 0.01 | <0.0001 | 72.43 | 0.45 |
| Homozygoote (TT vs. CC) | 1.43 (1.18–1.7), 0.0002 | 1.79 (1.23.2.6), 0.007 | <0.0001 | 77.98 | 0.02 |
| Dominant (TT + CT vs. CC) | 1.32 (1.18–1.47), <0.0001 | 1.44 (1.14–1.82), 0.006 | <0.0001 | 80.8 | 0.16 |
| Recessive (TT vs. CT + CC) | 1.29 (1.08–1.5), 0.004 | 1.61 (1.02–2.56), 0.04 | <0.0001 | 78.1 | 0.06 |
Fig. 2.
Forest plot for the association between MTHFR C677T polymorphism and RPL for allele contrast model (T vs. C) with random effect model in Asian population
Fig. 3.
Forest plot for the association between MTHFR C677T polymorphism and RPL for homozygote model (TT vs. CC) with random effect model in Asian population
Fig. 4.
Forest plot for the association between MTHFR C677T polymorphism and RPL for dominant model (TT + CT vs. CC) with random effect model in Asian population
Heterogeneity and Sensitivity Analysis
A true heterogeneity existed between studies for allele contrast (pheterogeneity < 0.0001, Q = 160.70, I2 = 85.07 %, t2 = 0.30, z = 2.59), genotype homozygote (pheterogeneity < 0.0001, Q = 104.46, I2 = 77.98 %, t2 = 1.0, z = 2.65), dominant (pheterogeneity < 0.0001, Q = 1274.97, I2 = 80.8 %, t2 = 0.337, z = 2.72) and recessive (pheterogeneity < 0.0001, Q = 105.05, I2 = 78.1 %, t2 = 0.87, z = 2.05) comparisons.
Control population of ten studies [15, 28, 29, 38, 39, 44, 46, 47, 51, 52] were not in HW equilibrium and exclusion of these ten studies decreased heterogeneity (p < 0.0001, I2 = 72.84 %) and increased OR (OR 1.54, 95 % CI 1.2–1.96). However, exclusion of three studies with small sample size, less than 50 [37, 41, 45] did not decreased heterogeneity (pheterogeneity < 0.0001, I2 = 86.65 %). Similarly exclusion of seven studies with very high p value [21, 28, 29, 37, 39, 48, 50] did not decrease heterogeneity (pheterogeneity < 0.0001, I2 = 88.38 %) but increased odds ratio (OR 1.59, 95 % CI 1.14–2.22).
Publication Bias
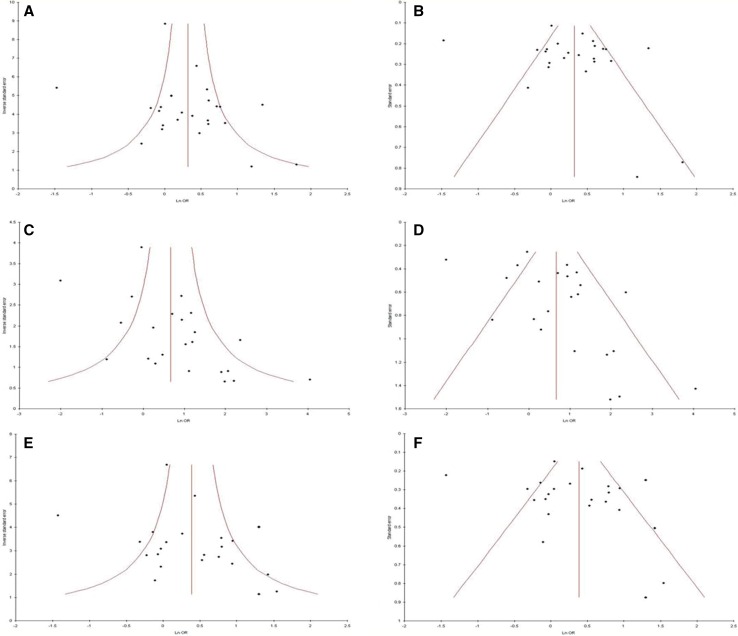
Except homozygote model, p values of Begg’s and Egger’s tests were more than 0.05 (Begg’s p = 0.84, Egger’s p = 0.24 for T vs. C; Begg’s p = 0.02, Egger’s p = 0.02 for TT vs. CC; and Begg’s p = 0.27, Egger’s p = 0.45 for CT vs. CCA; Begg’s p = 0.27, Egger’s p = 0.16 for TT + CT vs. CC; Begg’s p = 0.04, Egger’s p = 0.06 for TT vs. CT + CC) (Table 3). The funnel plots were also symmetrical (Fig. 5).
Fig. 5.
a Forest plot for the association between MTHFR C677T polymorphism and RPL for allele contrast model (T vs. C) with fixed effect model, b funnel plot precision versus OR (T vs. C), c standard error versus OR (T vs. C) in Asian studies
Discussion
Malnutrition and malabsorption of folate and vitamin B12 or inherited MTHFR deficiency, may result in hyperhomocysteinemia. C677T polymorphism in MTHFR gene was associated with elevated plasma homocysteine level, increased risk of arterial stiffness [58] and women with elevated total homocysteine concentrations showed a significant association with defective chorionic villous vascularization [59–61]. In embryonic development during pregnancy, the embryo survives and grows by stimulating its own blood supply through angiogenesis. A good exchange between fetus and mother is necessary to ensure normal fetal growth; therefore, impaired chorionic villous vascularization may result in embryonic death leading to miscarriage [62]. It has been also suggested that independent of homocysteinemia, association between C677T polymorphism and RPL was due to interference with red cell folate metabolism [18, 63].
Hyperhomocysteinemia is known to cause direct endothelial injury through increased oxidative stress to induce increased blood pressure and impairment in endothelial synthesis of vasodilatory substances, to increase the expression of procoagulants and to increase platelet aggregation [51]. This may cause thrombophilia, which is an important factor in increasing the risk of RPL in mothers. Both MTHFR polymorphism and hyperhomocysteinemia have been reported to predispose to placental vasculopathy associated with intrauterine growth retardation, abruption placentae and pre-eclampsia [64].
Meta-analysis is the statistical analysis of a large collection of analysis results for the purpose of integrating the findings and it is a powerful tool for systematic review of a focused topic in the literature that provides a quantitative estimate for the effect of a treatment intervention or exposure [65]. Because of the large sample sizes, meta-analysis has more statistical power than a single study to obtained reliable result. Several large-scale meta-analyses combining data from multiple studies have been published investigating the association between MTHFR C677T polymorphism and various disease/disorders such as—Down syndrome [66], Neural Tube defects [67], cleft lip with/without palate [68], congenital heart defects [69], stroke [70], diabetes mellitus [71], Alzheimers disease [72], schizophrenia [73] and cancer [74].
Three meta-analysis studies have been reported in an effort to draw conclusions on the association of MTHFR C677T polymorphism with RPL [18, 75, 76] but the information is incomplete on the Asian population, hence present meta-analysis was conducted on previously published case–control reports on Asian population. Ren and Wang [75] found in their meta-analysis that MTHFR C677T mutation was not related with RPL, except in Chinese population. They covered 28 studies in their meta-analysis, and most of the studies were conducted in the Caucasian population, among these only five studies conducted in China resulted in positive relation of that mutation with RPL. This large meta-analysis clearly showed the importance of the ethnicity in single nucleotide mutations. Cao et al. [76] conducted a meta-analysis (3559 RPL cases and 5097 healthy controls) and reported overall random-effects odds ratios (ORs) as 1.68 (95 % CI, 1.32–2.13) for TT versus CC genotypes, and 1.35 (95 % CI, 1.04–1.76) for TT + CT genotype combined versus total CC genotypes.
The quality of meta-analysis is compromised by presence of heterogeneity. However to minimize this limitation, author tried to use appropriate inclusion and exclusion criteria, performed sensitivity analysis and included samples only from single ethnic population (Asian) to reduce selection bias and to lower heterogeneity [76, 77] but failed to minimize the heterogeneity. The heterogeneity might be due to different sampling method and variations in genetic background of the subjects etc.
The current meta-analysis has few limitations to be addressed. First, the sample size of cases from some eligible studies is relatively limited (<100). The relative limited cases may have compromised statistical power. Second, the overall results were based on unadjusted ORs; while a more precise evaluation should be adjusted by potentially confounding factors, including age, gender, body mass index, smoking status, drink abuse, and environmental factors. Third, heterogeneity was observed, so the results should be interpreted cautiously. Fourth, only one gene was considered, other genes involved in folate metabolism should be considered for a more comprehensive understanding of the exact role of the folate pathway in RPL susceptibility. Finally, the effect of gene–gene and gene–environment interactions was not fully addressed in the meta-analysis due to the lack of sufficient data. Along with limitations, present meta-analysis had strengths also like-absence of publication bias and inclusion of larger number of studies of single ethnic population.
In conclusion, results of present meta-analysis suggested that the women having MTHFR C677T polymorphism may have an increased risk of RPL. This finding supports the hypothesis that folic acid may play a role in the etiology of RPL. Large and rigorous case–control studies that investigate gene–gene and gene–environment interactions need to be performed before conclusive claims about the genetics of RPL.
Acknowledgments
The author is highly grateful to Leon Bax (Chief Scientific Officer at BiostatXL, UMC Utrecht) for his valuable suggestions, which help her in statistical analysis.
Compliance with Ethical Standards
Conflict of interest
None.
References
- 1.Stirrat GM. Recurrent miscarriage: definition and epidemiology. Lancet. 1990;336:673–675. doi: 10.1016/0140-6736(90)92159-F. [DOI] [PubMed] [Google Scholar]
- 2.Berry CW, Bramabati B, Eskes TKAB, Exalto N, Fox H, Geraedts JP, et al. The Euro-Team Early Pregnancy (ETEP) protocol for recurrent miscarriage. Hum Reprod. 1995;10:1516–1520. doi: 10.1093/HUMREP/10.6.1516. [DOI] [PubMed] [Google Scholar]
- 3.Bricker L, Farquharson RG. Types of pregnancy loss in recurrent miscarriage: implications for research and clinical practice. Hum Reprod. 2002;17:1345–1350. doi: 10.1093/humrep/17.5.1345. [DOI] [PubMed] [Google Scholar]
- 4.Cook CL, Pridham DD. Recurrent pregnancy loss. Curr Opin Obstet Gynecol. 1995;7:357–366. doi: 10.1097/00001703-199510000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Younis JS, Ohel G, Brenner B, Ben-Ami M. Familial thrombophilia—the scientific rationale for thrombophylaxis in recurrent pregnancy loss? Hum Reprod. 1997;12:1389–1390. doi: 10.1093/humrep/12.7.1389. [DOI] [PubMed] [Google Scholar]
- 6.Brigham S, Conlon C, Farquharson RG. A longitudinal study of pregnancy outcome following idiopathic recurrent miscarriage. Hum Reprod. 1999;14:2868–2871. doi: 10.1093/humrep/14.11.2868. [DOI] [PubMed] [Google Scholar]
- 7.Andersen AMN, Wohlfahrt J, Christens P, Melbye M. Maternal age and fetal loss: population based register linkage study. BMJ. 2000;320:1708–1712. doi: 10.1136/bmj.320.7251.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cramer DW, Wise LA. The epidemiology of recurrent pregnancy loss. Semin Reprod Med. 2000;18:331–339. doi: 10.1055/s-2000-13722. [DOI] [PubMed] [Google Scholar]
- 9.Fenster L, Eskenazi B, Windham GC, Swan SH. Caffeine consumption during pregnancy and spontaneous abortion. Epidemiology. 1991;2:168–174. doi: 10.1097/00001648-199105000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Parazzini F, Bocciolone L, Fedele L, Negri E, La Vecchia C, Acaia B. Risk factors for spontaneous abortion. Int J Epidemiol. 1991;20:157–161. doi: 10.1093/ije/20.1.157. [DOI] [PubMed] [Google Scholar]
- 11.Nair RR, Khanna A, Singh K. MTHFR C677T polymorphism and recurrent early pregnancy loss risk in North Indian population. Reprod Sci. 2012;19:210–215. doi: 10.1177/1933719111417888. [DOI] [PubMed] [Google Scholar]
- 12.Regan L, Rai R. Thrombophilia and pregnancy loss. J Reprod Immunol. 2002;55:163–180. doi: 10.1016/S0165-0378(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 13.Kupferminc M, Eldor A, Steinman N, Many A, Bar-Am A, Jaffa A, et al. Increased frequency of genetic thrombophilia in women with complications of pregnancy. N Engl J Med. 1999;340:9–13. doi: 10.1056/NEJM199901073400102. [DOI] [PubMed] [Google Scholar]
- 14.Kupferminc M, Fait G, Many A, Gordon D, Eldor A, Lessing J. Severe preeclampsia and high frequency of genetic thrombophilic mutations. Obstet Gynecol. 2000;96:45–49. doi: 10.1016/s0029-7844(00)00861-9. [DOI] [PubMed] [Google Scholar]
- 15.Brenner B, Sarig G, Weiner Z, Younis J, Blumenfeld Z, Lanir N. Thrombophilic polymorphisms are common in women with fetal loss without apparent cause. Thromb Haemost. 1999;82:6–9. [PubMed] [Google Scholar]
- 16.Dissanayake VHW, Sirisena ND, Weerasekera LY, Gammulla G, Seneviratne HR, Jayasekara RW. Candidate gene study of genetic thrombophilic polymorphisms in pre-eclampsia and recurrent pregnancy loss in Sinhalese women. J Obstet Gynaecol Res. 2012;38:1168–1176. doi: 10.1111/j.1447-0756.2012.01846.x. [DOI] [PubMed] [Google Scholar]
- 17.Jilma B, Kamath S, Lip GY. ABC of antithrombotic therapy: antithrombotic therapy in special circumstances: II—in children, thrombophilia, and miscellaneous conditions. BMJ. 2003;326:93–96. doi: 10.1136/bmj.326.7380.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu X, Zhao L, Zhu H, He D, Tang W, Luo Y. Association between the MTHFR C677T polymorphism and recurrent pregnancy loss: a meta-analysis. Genet Test Mol Biomarker. 2012;16:806–811. doi: 10.1089/gtmb.2011.0318. [DOI] [PubMed] [Google Scholar]
- 19.Arruda VR, von Zuben PM, Chiaparini LC, Annichino-Bizzacchi JM, Costa FF. The mutation Ala6774Val in the methylene tetrahydrofolate reductase gene: a risk factor for arterial disease and venous thrombosis. Thromb Haemost. 1997;77:818–821. [PubMed] [Google Scholar]
- 20.Boers GH. Hyperhomocysteinemia as a risk factor for arterial and venous disease: a review of evidence and relevance. Thromb Haemost. 1997;78:520–522. [PubMed] [Google Scholar]
- 21.Vettriselvi V, Vijayalakshmi K, Paul SF, Venkatachalam P. ACE and MTHFR gene polymorphisms in unexplained recurrent pregnancy loss. J Obstet Gynaecol Res. 2008;34:301–306. doi: 10.1111/j.1447-0756.2008.00792.x. [DOI] [PubMed] [Google Scholar]
- 22.Jacques PF, Bostom AG, Williams RR, Ellison RC, Eckfeldt JH, Rosenberg IH, et al. Relation between folate status, a common mutation in methylene tetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation. 1996;93:7–9. doi: 10.1161/01.CIR.93.1.7. [DOI] [PubMed] [Google Scholar]
- 23.Murphy RP, Donoghue C, Nallen RJ, D’Mello M, Regan C, et al. Prospective evaluation of the risk conferred by factor V Leiden and thermolabile methylenetetrahydrofolate reductase polymorphisms in pregnancy. Arterioscler Thromb Vasc Biol. 2000;20:266–270. doi: 10.1161/01.ATV.20.1.266. [DOI] [PubMed] [Google Scholar]
- 24.Morita H, Taguchi J, Kurihara H, Kitaoka M, Kaneda H, Kurihara Y, et al. Genetic polymorphism of 5,10-methylenetetrahydrofolate reductase (MTHFR) as a risk factor for coronary artery disease. Circulation. 1997;95:2032–2036. doi: 10.1161/01.CIR.95.8.2032. [DOI] [PubMed] [Google Scholar]
- 25.Legnani C, Palareti G, Grauso F, Sassi S, Grossi G, Piazzi S, et al. Hyperhomocyst(e)inemia and a common methylenetetrahydrofolate reductase mutation (Ala223Val MTHFR) in patients with inherited thrombophilic coagulation defects. Arterioscler Thromb Vasc Biol. 1997;17:2924–2929. doi: 10.1161/01.ATV.17.11.2924. [DOI] [PubMed] [Google Scholar]
- 26.Nelen WL, Steegers EA, Eskes TK, Blom HJ. Genetic risk factor for unexplained recurrent early pregnancy loss. Lancet. 1997;350:861. doi: 10.1016/S0140-6736(97)24038-9. [DOI] [PubMed] [Google Scholar]
- 27.Ozdemir O, Yenicesu GI, Silan F, Keoksal B, Atik S, Ozen F, et al. Recurrent pregnancy loss and its relation to combined parental thrombophilic gene mutations. Genet Test Mol Biomarkers. 2012;16:279–286. doi: 10.1089/gtmb.2011.0191. [DOI] [PubMed] [Google Scholar]
- 28.Zonouzi P, Chaparzadeh N, Estiar MA, Sadaghiani MM, Farzadi L, Ghasemzadeh A et al. Methylenetetrahydrofolate reductase C677T and A1298C mutations in women with recurrent spontaneous abortions in the Northwest of Iran. ISRN Obst Gynec. 2012; Article ID 945486, 6. [DOI] [PMC free article] [PubMed]
- 29.Kaur L, Puri M, Kaushik S, Sachdeva MP, Trivedi SS, Saraswathy KN. Genetic thromobophilia in pregnancy: a case–control study among North Indian women. J Thromb Thrombolysis. 2013;35:250–256. doi: 10.1007/s11239-012-0797-4. [DOI] [PubMed] [Google Scholar]
- 30.Parveen F, Tuteja M, Agrawal S. Polymorphisms in MTHFR, MTHFD, and PAI-1 and recurrent miscarriage among North Indian women. Arch Gynecol Obstet. 2013;288:1171–1177. doi: 10.1007/s00404-013-2877-x. [DOI] [PubMed] [Google Scholar]
- 31.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1954;22:719–748. [PubMed] [Google Scholar]
- 32.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 33.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 34.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 35.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1999;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KG. Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC Med Res Methodol. 2006;6:50–53. doi: 10.1186/1471-2288-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lissak A, Sharon A, Fruchter O, Kassel A, Sanderovitz J, Abramovici H. Polymorphism for mutation of cytosine to thymine at location 677 in the methylenetetrahydrofolate reductase gene is associated with recurrent early fetal loss. Am J Obstet Gynecol. 1999;181:126–130. doi: 10.1016/S0002-9378(99)70447-3. [DOI] [PubMed] [Google Scholar]
- 38.Wang YW, Li F, Li YP. The study on the relationship between the methylenetetrahydofolaye reductase 667C–>T mutation and unexpected recurrent pregnancy loss. Zhongguo Shi Yong Fu Ke Yu Chan Ke Za Zhi. 2002;18:291–293. [Google Scholar]
- 39.Wang XP, Lin QD, Ma ZW. C677T and A1298C mutation of the methylenetetrahydrofolate reductase gene in unexplained recurrent spontaneous abortion. Zhonghua Fu Chan Ke Za Zhi. 2004;39:238–241. [PubMed] [Google Scholar]
- 40.Wang X, Ma Z, Lin Q. Inherited thrombophilia in recurrent spontaneous abortion among Chinese women. Int J Gynec Obst. 2006;92:264–265. doi: 10.1016/j.ijgo.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Kumar KS, Govindaiah V, Naushad SE, Devi RR, Jyothy A. Plasma homocysteine levels correlated to interactions between folate status and methylene tetrahydrofolate reductase gene mutation in women with unexplained recurrent pregnancy loss. J Obstet Gynaecol. 2003;23:55–58. doi: 10.1080/0144361021000043263. [DOI] [PubMed] [Google Scholar]
- 42.Li X, Zhang Y, Xu X, Jinag S. Study on the relationship of MTHFR polymorphisms with unexplained recurrent spontaneous abortion. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2004;21:39–42. [PubMed] [Google Scholar]
- 43.Makino A, Nakanishi T, Sugiura-Ogasawara M, Ozaki Y, Suzumori N, Suzumori K. No association of C677T methylenetetrahydrofolate reductase and an endothelial nitric oxide synthase polymorphism with recurrent pregnancy loss. Am J Reprod Immunol. 2004;52:60–66. doi: 10.1111/j.1600-0897.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- 44.Guan LX, Du XY, Wang JX, Gao L, Wang RL, Li HB, et al. Association of genetic polymorphisms in plasminogen activator inhibitor-1 gene and 5,10-methylenetetrahydrofolate reductase gene with recurrent early spontaneous abortion. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2005;22:330–333. [PubMed] [Google Scholar]
- 45.Kobashi G, Kato EH, Morikawa M, Shimada S, Ohta K, Fujimoto S, et al. MTHFR C677T polymorphism and factor V Leiden mutation are not associated with recurrent spontaneous abortion of unexplained etiology in Japanese women. Semin Thromb Hemost. 2005;31:266–271. doi: 10.1055/s-2005-872430. [DOI] [PubMed] [Google Scholar]
- 46.Song LY. Relationship between genetic polymorphism of homocysteine metabolism enzyme and unexplained repeated spontaneous abortion. Zhonghua Wei Chan Yi Xue Za Zhi. 2005;8:160–164. [Google Scholar]
- 47.Mitraoui N, Zammiti W, Ghazouani L, Jmili Braham N, Saidi S, Finan RR, et al. Methylenetetrahydrofolate reductase C677T and A1298C polymorphism and changes in homocysteine concentrations in women with idiopathic recurrent pregnancy loss. Reproduction. 2006;131:395–401. doi: 10.1530/rep.1.00815. [DOI] [PubMed] [Google Scholar]
- 48.Govindaiah V, Naushad SM, Prabhakara K, Krishna PC. Radha Rama Devi A. Association of parental hyperhomocysteinemia and C677T Methylene tetrahydrofolate reductase (MTHFR) polymorphism with recurrent pregnancy loss. Clin Biochem. 2009;42:380–386. doi: 10.1016/j.clinbiochem.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Mukhopadhyay R, Saraswathy KN, Ghosh PK. MTHFR C677T and factor V Leiden in recurrent pregnancy loss: a study among an endogamous group in North India. Genet Test Mol Biomarkers. 2009;13:861–865. doi: 10.1089/gtmb.2009.0063. [DOI] [PubMed] [Google Scholar]
- 50.Abu-Asab NS, Ayesh SK, Ateeq RO, Nassar SM, EL-Sharif WA. Association of Inherited thrombophilia with recurrent pregnancy loss in Palestinian women. Obstet Gynecol International. 2011; ID 689684. [DOI] [PMC free article] [PubMed]
- 51.Jeddi-Tehrani M, Torabi R, Mohammadzadeh A, Arefi S, Keramatipour M, Zeraati H, et al. Investigating association of three polymorphisms of coagulation factor XIII and recurrent pregnancy loss. Am J Reprod Immunol. 2010;64:212–217. doi: 10.1111/j.1600-0897.2010.00838.x. [DOI] [PubMed] [Google Scholar]
- 52.Settin A, Elshazli R, Salama A, ElBaz R. Methylenetetrahydrofolate reductase gene polymorphisms in Egyptian women with unexplained recurrent pregnancy loss. Genet Test Mol Biomarkers. 2011;15:887–892. doi: 10.1089/gtmb.2011.0049. [DOI] [PubMed] [Google Scholar]
- 53.Torabi R, Zarei S, Zeraati H, Zarnani AH, Akhondi MM, Hadavi R, et al. Combination of thrombophilic gene polymorphisms as a cause of increased the risk of recurrent pregnancy loss. J Reprod Infertil. 2012;13(2):89–94. [PMC free article] [PubMed] [Google Scholar]
- 54.Cao Y, Zhang Z, Zheng Y, Yuan W, Wang J, Liang H, Chen J, Du J, Shen Y. The association of idiopathic recurrent early pregnancy loss with polymorphisms in folic acid metabolism-related genes. Genes Nutr. 2014;9:402–407. doi: 10.1007/s12263-014-0402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yousefian E, Kardi MT, Allahveisi A. Methylenetetrahydrofolate Reductase C677T and A1298C Polymorphism in Iranian Women With Idiopathic Recurrent Pregnancy Losses. Iran Red Crescent Med J. 2014;16(7):e16763. doi: 10.5812/ircmj.16763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farahmand K, Totonchi M, Hashemi M, Sabet FR, Kalantari H, Gourabi H, Meybodi AM. Thrombophilic genes alterations as risk factor for recurrent pregnancy loss. J Matern Fetal Neonatal Med. 2015 doi: 10.3109/14767058.2015.1044431. [DOI] [PubMed] [Google Scholar]
- 57.Vanilla S, Dayanand CD, Kotur PF, Kutty AM, Vegi PK. Evidence of paternal N5, N10—methylenetetrahydrofolate reductase (MTHFR) C677T gene polymorphism in couples with recurrent spontaneous abortions (RSAs) in Kolar district—a south west of India. J Clin Diagn Res. 2015;9(2):15–18. doi: 10.7860/JCDR/2015/10856.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trabetti E. Homocysteine, MTHFR gene polymorphisms, and cardio-cerebrovascular risk. J Appl Genet. 2008;49:267–282. doi: 10.1007/BF03195624. [DOI] [PubMed] [Google Scholar]
- 59.Nelen WL, Bulten J, Steegers EA, Blom HJ, Hanselaar AG, Eskes TK. Maternal homocysteine and chorionic vascularization in recurrent early pregnancy loss. Hum Reprod. 2000;15:954–960. doi: 10.1093/humrep/15.4.954. [DOI] [PubMed] [Google Scholar]
- 60.Nelen WL, Blom HJ, Steegers EA, den Heijer M, Eskes TK. Hyperhomocysteinemia and recurrent early pregnancy loss: a meta-analysis. Fertil Steril. 2000;74:1196–1199. doi: 10.1016/S0015-0282(00)01595-8. [DOI] [PubMed] [Google Scholar]
- 61.Nelen WL, Blom HJ, Steegers EA, den Heijer M, Thomas CM. Eskes TK Homocysteine and folate levels as risk factors for recurrent early pregnancy loss. Obstet Gynaecol. 2000;95:519–524. doi: 10.1016/s0029-7844(99)00610-9. [DOI] [PubMed] [Google Scholar]
- 62.Meegdes BH, Ingenhoes R, Peeters LL, Exalto N. Early pregnancy wastage: relationship between chorionic vascularization and embryonic development. Fertil Steril. 1988;49:216–220. doi: 10.1016/S0015-0282(16)59704-0. [DOI] [PubMed] [Google Scholar]
- 63.Golbahar J, Aminzadeh MA, Sharifkazemi MB, Rezaian GR. Association of red blood cell 5-methyltetrahydrofolate and severity of coronary artery disease: a cross-sectional study from Shiraz, southern Iran. Heart Vessels. 2005;20:203–206. doi: 10.1007/s00380-004-0823-3. [DOI] [PubMed] [Google Scholar]
- 64.Molen E, Arends G, Nelen W, Put N, Heil S, Eskes T, et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene as new risk factor for placental vasculopathy. Am J Obstet Gynecol. 2000;182:1258–1263. doi: 10.1067/mob.2000.105199. [DOI] [PubMed] [Google Scholar]
- 65.Munafo MR, Flint J. Meta-analysis of genetic association studies. Trends Genet. 2004;20:439–444. doi: 10.1016/j.tig.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 66.Rai V, Yadav U, Kumar P, Yadav SK, Mishra OP. Maternal methylenetetrahydrofolate reductase C677T polymorphism and down syndrome risk: a meta-analysis from 34 studies. PLoS One. 2014;9:e108552. doi: 10.1371/journal.pone.0108552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yadav U, Kumar P, Yadav SK, Mishra OP, Rai V. Polymorphisms in folate metabolism genes as maternal risk factor for neural tube defects: an updated meta-analysis. Metab Brain Dis. 2015;30(1):7–24. doi: 10.1007/s11011-014-9575-7. [DOI] [PubMed] [Google Scholar]
- 68.Zhao M, Ren Y, Shen L, Zhang Y, Zhou B. Association between MTHFR C677T and A1298C polymorphisms and NSCL/P risk in Asians: a meta-analysis. PLoS One. 2014;9:e88242. doi: 10.1371/journal.pone.0088242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang W, Wang Y, Gong F, Zhu W, Fu S. MTHFR C677T polymorphism and risk of congenital heart defects: evidence from 29 case–control and TDT studies. PLoS One. 2013;8:e58041. doi: 10.1371/journal.pone.0058041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yadav S, Hasan N, Marjot T, Khan MS, Prasad K, Bentley P, et al. Detailed analysis of gene polymorphisms associated with ischemic stroke in south Asians. PLoS One. 2013;8(3):e57305. doi: 10.1371/journal.pone.0057305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu B, Wu X, Zhi X, Liu L, Zheng Q, Sun G. Methylenetetrahydrofolate reductase C677T polymorphism and type 2 diabetes mellitus in Chinese population: a meta-analysis of 29 case–control studies. PLoS One. 2014;9(7):e102443. doi: 10.1371/journal.pone.0102443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang MY, Miaoa L, Li YS, Hub GY. Meta-analysis of the methylenetetrahydrofolate reductase C677T polymorphism and susceptibility to Alzheimer’s disease. Neurosci Res. 2010;68:142–150. doi: 10.1016/j.neures.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 73.Hu CY, Qian ZZ, Gong FF, Lu SS, Feng F, Wu YL, et al. Methylenetetrahydrofolate reductase (MTHFR) polymorphism susceptibility to schizophrenia and bipolar disorder: an updated meta-analysis. J Neural Transm. 2014;122(2):307–320. doi: 10.1007/s00702-014-1261-8. [DOI] [PubMed] [Google Scholar]
- 74.Rai V. The methylenetetrahydrofolate reductase C677T polymorphism and breast cancer risk in Asian populations. Asian Pac J Cancer Prev. 2014;15:5853–5860. doi: 10.7314/APJCP.2014.15.14.5853. [DOI] [PubMed] [Google Scholar]
- 75.Ren A, Wang J. Methylenetetrahydrofolate reductase C677T polymorphism and the risk of unexplained recurrent pregnancy loss: a meta-analysis. Fertil Steril. 2006;86(6):1716–1722. doi: 10.1016/j.fertnstert.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 76.Cao Y, Zhang Z, Zheng Y, Yuan W, Wang J, Liang H, et al. The association of idiopathic recurrent early pregnancy loss with polymorphisms in folic acid metabolism-related genes. Genes Nutr. 2014;9(3):402. doi: 10.1007/s12263-014-0402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang P, Gao X, Zhang Y, Hu Y, Ma H, Wang W, et al. Association between MTHFR C677T polymorphism and venous thromboembolism risk in the Chinese population: a meta-analysis of 24 case–control studies. Angiology. 2014;66(5):422–432. doi: 10.1177/0003319714546368. [DOI] [PubMed] [Google Scholar]