Abstract
Lymphatic filariasis is a mosquito borne parasitic infection and can severely affect the normal working ability of an individual. Currently there is no vaccine available to prevent this infection and the development of a potential vaccine could effectively support the on-going mass drug administration program by World Health Organization (WHO). Filarial parasites have complex mechanisms to modulate the host immune responses against them. The glutathione-S-transferases (GST) are the important enzymes effectively involved to counteract the oxidative free radicals produced by the host. In the present study, we have shown that the mastomys which are fully permissible rodents for Brugia malayi when immunized with Wuchereria bancrofti recombinant GST (rWbGST) could induce 65.5 % in situ cytotoxicity against B. malayi infective (L3) larvae. There was a balanced Th1/Th2 immune response in the vaccinated animals, characterized by higher levels of WbGST-specific IgG1 and IgG2a antibodies and pronounced IFN-γ, IL-10 and IL-4 cytokines production by the spleen cells.
Electronic supplementary material
The online version of this article (doi:10.1007/s12291-016-0556-y) contains supplementary material, which is available to authorized users.
Keywords: Wuchereria bancrofti, Brugia malayi, Glutathione-S-transferases, Vaccine, Nematodes
Introduction
Lymphatic filariasis is a major mosquito borne disorder, caused by the three lymph dwelling parasites; Wuchereria bancrofti, Brugia malayi and Brugia timori. It is estimated that over one billion people are at a risk of getting infected with this disorders whereas, over 120 million individuals are already infected with the lymphatic filariasis around the world [1]. It inflicts a considerable social and economic burden on many tropical and sub-tropical countries. India alone accounts for over 40 % of the total disease burden and the disease has been recognized as a disease of National importance [2]. World Health Organization has initiated the ‘Global programme to eliminate lymphatic filariasis (GPELF) by 2020’ which targets to restrict the transmission of disease via mass drug administration (MDA) and thus significantly reducing the incidence of lymphatic filariasis [3].
However, there are many logistical problems associated with the MDA, as it requires several rounds of drug administration, additionally, development of drug resistance and non-compliance has been a critical issue severely affecting the complete eradication of the disease from some of the endemic areas [4]. The disadvantages in the current system of drug delivery has been restricting to achieve the significant eradication of transmission in the highly affected and densely populated endemic areas. Therefore, development of vaccines as a preventive tool to control the infection is important. Earlier studies have reported the utilization of recombinant proteins as vaccine candidates for the lymphatic filariasis [5–9].
Filarial parasites are smart modulators of our immune system and this modulatory molecules are secreted by them the moment they enter into the body of host. Thus, the molecules secreted in the infective larval (L3) stage by these parasites such as glutathione-S-transferase (GST), catalase, superoxide dismutase (SOD), peroxiredoxins, glutathione peroxidase, etc. are important for their establishment and survival in the host [10]. Neutralizing these molecules by vaccination could act as a preventive tool to restrict the infection of these parasites within the host. GST has already been successfully tested as a vaccine candidate for other helminth infections such as Seteria cervi or Schistosoma mansoni or S. bovis [11–13]. Earlier studies from our laboratory showed that the W. bancrofti infective larval glutathione-S-transferase (WbGST) to be critical for the survival of the parasite in the host and is the potential vaccine candidate for filariasis [7]. In the present study, we have assessed the immunogenicity and protective efficacy of WbGST in fully permissible filarial animal model Mastomys coucha and further characterized the humoral cellular and cytotoxic responses associated with the induced protection.
Materials and Methods
Experimental Animals and B. malayi Parasites
Female mastomys (Mastomys coucha) (6–8 weeks of age), bred and maintained in the Committee for the Purpose of Control and Supervision of Experiments on Animals, Government of India registered animal house facility of our institute, were used in this study. The animals were maintained under standard laboratory conditions with free access to animal chow and drinking water ad libitum and all the surgical procedures were performed under the strict aseptic conditions. All the experiments were approved by the Institutional Animal Ethics Committee.
Brugia malayi infective stage (L3) larvae used in this study were obtained using Baermann’s technique [14] by the method described previously [4]. Four days old Aedes aegypti mosquitoes were fed with the blood of mastomys infected with B. malayi and dissected after 2 weeks to recover L3 stage larvae.
Wuchereria bancrofti Recombinant GST (rWbGST)
rWbGST was expressed and purified as described previously [7]. The Escherichia coli BL21 bacterial cells containing pRSET-A-WbGST construct was grown at 37 °C to A600, 0.6 in LB medium containing suitable antibiotics. The expression of recombinant protein was induced by the addition of isopropyl β-D-1-thiogalactopyranoside (1 mM; Merck Millipore, Bengaluru, Karnataka, India). The recombinant protein was purified using a nickel affinity chromatography column (Thermo Fisher Scientific, Mumbai, Maharashtra, India) and the protein content was estimated using a bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Mumbai). Endotoxin contamination was checked by a quantitative LAL chromogenic endotoxin quantitation kit and the endotoxin content was found to be within permissible limits (Thermo Fisher Scientific, Mumbai).
Immunization of Mastomys with rWbGST
Five mastomys (n = 5 in each group) each were recruited into two groups as: rWbGST group (mastomys immunized with rWbGST in alum adjuvant) and Alum control group (mastomys administered with alum alone). Immunization of mastomys with rWbGST consisted of three intraperitoneal doses of rWbGST (15 μg/dose in 200 μl of alum adjuvant) administered at 15 days intervals followed by one booster dose. Animals in alum control group received four doses of alum adjuvant only. Ten days after the final dose of immunization, the sera were collected from each mastomys through caudal vein and tested for the presence of anti-WbGST antibody levels.
Analysis of Anti-WbGST Antibody Levels in the Sera of Mastomys
The levels of total anti-WbGST Immunoglobulin (Ig)-G antibody and IgG antibody isotypes (IgG1, IgG2a, IgG3 and IgG4) were determined in the sera samples of mastomys using an indirect ELISA. Immuno plates of 96 wells (Thermo Fisher Scientific, Mumbai) were coated with rWbGST protein (100 ng/100 µl/well) in carbonate-bicarbonate buffer (100 mM, pH 9.5) and incubated overnight at 4 °C. The wells were washed once with PBS/T (0.05 M PBS containing 0.05 % of tween 20, pH 7.2) and blocked by BSA (2 % in PBS, 300 µl/well) for 1 h at 37 °C. After washing thrice, the optimally diluted sera samples (diluted in PBS) were added and incubated for 1 h at 37 °C. After washing the wells for five times, the bounded antibodies were detected by addition of HRP conjugated goat anti-mouse IgG (1:10000) or IgG1 (1:1000) or IgG2a (1:15000) or IgG3 (1:5000) or IgG4 (1:15000) antibodies (diluted in PBS; Thermo Fisher Scientific, Mumbai). After incubation of 45 min at 37 °C, the colour developed was recorded by measuring absorbance at 450 nm using spectrophotometer (Biotek India, Mumbai).
Depletion of Anti WbGST Antibodies from Sera
Anti-WbGST antibodies in the sera of immunized mastomys were depleted by passing the sera over rWbGST coupled to Cobalt IMAC resin (Thermo Fisher Scientific, Mumbai). 1 mg of his-tagged rWbGST was coupled to the resin, washed and incubated overnight at 4 °C with about 200 µl of neat sera. Supernatant was collected by centrifuging the resin mixture for 2 min at 750 rpm. Depletion of anti-WbGST antibodies were confirmed using an ELISA as described above.
In Vitro Antibody Dependent Cellular Cytotoxicity (ADCC) Assay
The cytotoxic effect of anti-rWbGST antibodies against B. malayi L3 larvae was determined by in vitro cytotoxicity assay by the method described previously [7, 15, 16]. Pooled sera samples from immunized mastomys before and after depletion of anti-WbGST antibodies were used in this assay. The ADCC assay was performed by adding about 20 L3 larvae of B. malayi to a suspension of peritoneal exudates cells (PEC; 2 × 105 cells/well in 100 μl of RPMI medium) collected from normal mastomys. Sera samples (50 µl) were added to respective wells and the final volume of each well was adjusted to 200 μl by addition of RPMI medium in 96 well tissue culture plate (Thermo Fisher Scientific, Mumbai). After incubation (48 h at 37 °C in 5 % CO2), larval viability was determined under a light microscope. The larvae were considered as dead if they appeared limpid and straight with no movements. Percentage of cytotoxicity was expressed as the ratio of number of immobile or dead larvae to the number of larvae recovered within the experimental period.
In situ Cytotoxicity Against L3 Larvae in Immunized Mastomys
In situ cytotoxic response against B. malayi L3 in immunized and control groups of mastomys was analysed by micropore chamber technique as described previously [17]. Briefly, micropore chambers (Diffusion chamber with hole; Millipore India, Bangalore, Karnataka, India) containing about 20 live and infective larvae in RPMI-1640 medium were implanted into the peritoneal cavity of experimental mastomys under the effect of anaesthesia (ketamine). After 48 h of implantation, the mastomys were killed and the chambers were taken out from the peritoneum, washed in normal saline and the contents were removed onto a glass slide and examined microscopically for cell adherence and cytotoxicity. The percentage of cytotoxicity was expressed as mentioned above. (Fig. S1 Experimental design).
Assessment of Splenocytes Proliferation and Cytokine Analysis in In Vitro Culture of Splenocytes
The spleens were aseptically removed from the mastomys and minced in RPMI 1640 medium (supplemented with 80 µg/ml gentamicin, 25 mM HEPES, 2 mM glutamine and 10 % foetal calf serum), pelleted and resuspended in erythrocyte lysis buffer (0.1 % ammonium hydrochloride). Cells (0.2 × 106 cells/well in 200 µl of RPMI media) were plated in triplicates in 96 well flat bottom tissue culture plate (Thermo Fisher Scientific, Mumbai). The cells were then stimulated with rWbGST (1 μg/well/200 µl) or Concanavalin A (1 μg/well/200 µl) (Con A; Sigma-Aldrich, Mumbai). Wells with media alone served as un-stimulated controls. After incubation (48 h at 37 °C in 5 % CO2), cell proliferation was measured using Cell Titre 96 Aqueous non-radioactive cell proliferation kit (MTS assay; Promega, New Delhi). Cell proliferation expressed in terms of stimulation index (SI) was calculated by dividing geometric mean, GM absorbance of the cells stimulated by antigen/mitogen by the absorbance (GM) of the unstimulated cells.
Similar sets of cell cultures were placed in 24 well tissue culture plates and after the incubation for 72 h (at 37 °C in 5 % CO2), culture supernatants were collected in separate micro-centrifuge tubes by centrifugation for the estimation of the release of interleukin (IL)-4, IL-10 and interferon (IFN)-γ cytokines using ELISA kits from Invitrogen (Mumbai) as per the manufacturer’s instructions.
Statistical Analysis
The statistical analysis was performed using SPSS 21.0 (IBM, India) software. The data were checked for normality assumptions. Comparison between independent means was analysed by Mann–Whitney U test and the Kruskal–Wallis test followed by Dunn’s post hoc test was used to compare the difference between multiple groups. p values ≤0.05 were considered to be significant.
Results
High Titres of Anti-GST Antibody and Isotype Responses in the Sera of Vaccinated Mastomys
To evaluate the immunoprophylactic effect of rWbGST, sera from mastomys immunized with rWbGST was checked for the total IgG antibody levels by ELISA. rWbGST immunized mastomys showed high levels of anti-WbGST antibodies with mean titre of up to 10,000.
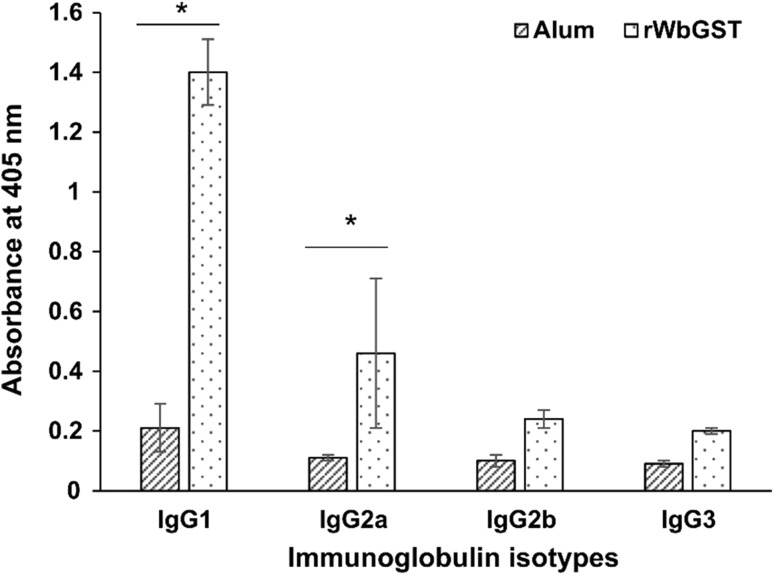
The isotype profile in the sera of mastomys immunized with rWbGST showed significantly elevated levels of IgG1 (p < 0.05) and IgG2a (p < 0.05) isotype of antibodies as compared to the levels in the sera of control group of mastomys (Fig. 1). Whereas, no significant change was observed in the levels of IgG2b and IgG3 antibodies in the vaccinated group of mastomys (Fig. 1).
Fig. 1.
Measurement of anti-WbGST IgG antibody isotypes in the mastomys immunized with rWbGST. The levels of anti-WbGST IgG antibody isotypes in the sera of mastomys immunized with rWbGST were measured by ELISA. Each data point represents Mean ± SD; n = 5 mastomys per group; *p < 0.05 in comparison with Alum group as analysed by Mann–Whitney U test
Antibody Dependent Cellular Cytotoxicity (ADCC) Induced Against Infective Larvae of B. malayi Parasite by the Sera of Vaccinated Mastomys
Sera from the vaccinated mastomys promoted the adherence of PEC to the infective (L3) larvae thereby inducing the cell mediated cytotoxicity of filarial parasite (Fig. 2). Serum from rWbGST immunized mastomys demonstrated significant (p = 0.035) cell mediated cytotoxicity (71.55 %) against L3 compared to the cytotoxicity induced by serum from control group of mastomys administered with alum alone (12.93 %) (Table 1). While, the depletion of antibodies from the sera of immunized animals was found to impart the significant (p = 0.035) reduction in the cytotoxicity (12.04 %) induced by the antibodies (Table 1).
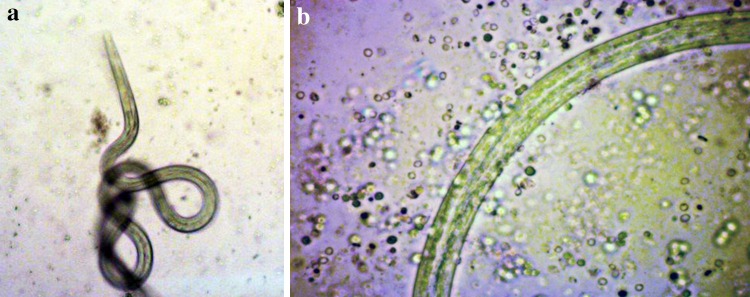
Fig. 2.
Light micrographs of L3 larvae of B. malayi recovered from cultures after in vitro ADCC assay. a L3 incubated with the pooled sera sample from mastomys administered with Alum adjuvant and peritoneal exudate cells (PEC). There are no cells adhered to the larva and the larva was active. b L3 incubated with the pooled sera sample from mastomys immunized with rWbGST and PEC. The cells are observed to be adhered throughout the surface of larvae causing death of the larvae
Table 1.
In vitro antibody dependent cellular cytotoxicity induced against B. malayi L3 by pooled sera of mastomys immunized with rWbGST
| Number of B. malayi L3 recovered followed by treatment with sera of different groups of mastomys immunized with | |||
|---|---|---|---|
| Alum | rWbGST | Anti-WbGST Ab depleted sera | |
| Live L3 larvae | 18 | 7 | 13 |
| 19 | 5 | 16 | |
| 17 | 6 | 16 | |
| Dead L3 larvae | 3 | 16 | 4 |
| 2 | 14 | 3 | |
| 3 | 15 | 5 | |
| % cytotoxicity (Mean ± SD) | 12.93 ± 2.97 | 71.55 ± 2.06* | 21.04 ± 4.55 |
Peritoneal exudate cells (PEC) were incubated with the pooled sera from the mastomys administered with rWbGST/Alum/anti-WbGST Ab depleted sera and L3 larvae in vitro. The total live and dead worms were counted in each well after 48 h. The data shown is the number of larvae recovered from three different sets of experiments
* p = 0.035 in comparison with Alum and anti-WbGST Ab depleted groups as analysed by Kruskal–Wallis test followed by Dunn’s post hoc test
In Situ Cytotoxicity Induced Against Infective Larvae of B. malayi
Micropore chamber method was used to evaluate the immunoprophylactic efficacy of rWbGST. The microscopic observation of chambers implanted in the peritoneum of immunized mastomys showed the migration of hosts’ immune cells into the chambers leading to their adherence and killing of the L3 larvae within 48 h of their implantation. Results showed that, the antibodies in the rWbGST immunized mastomys were capable of inducing the significant (p = 0.016) cytotoxicity (65.5 %) compared to the control group of mastomys (7.79 %) (Table 2).
Table 2.
In situ cytotoxicity assay against B. malayi L3
| Groups of mastomys (n = 5) immunized with | L3 larvae recovered | % cytotoxicity (Mean ± SD) | ||
|---|---|---|---|---|
| Live | Dead | Total | ||
| Alum | 13 | 1 | 14 | 7.14 |
| 15 | 2 | 17 | 11.76 | |
| 15 | 1 | 16 | 6.25 | |
| 13 | 1 | 14 | 7.14 | |
| 14 | 1 | 15 | 6.66 | |
| 7.79 ± 2.25 | ||||
| rWbGST | 5 | 9 | 14 | 64.28 |
| 5 | 11 | 16 | 68.75 | |
| 6 | 11 | 17 | 64.70 | |
| 5 | 9 | 14 | 64.28 | |
| 65.5 ± 2.17* | ||||
Mastomys immunized with rWbGST/Alum were challenged intraperitoneally with B. malayi L3. After 48 h, live L3 were recovered from the micropore chambers implanted into the peritoneal cavity of mastomys immunized with rWbGST
* p = 0.016 in comparison with Alum group as analysed by Mann–Whitney U test
Effect on the Splenocytes Proliferation and Cytokine Analysis
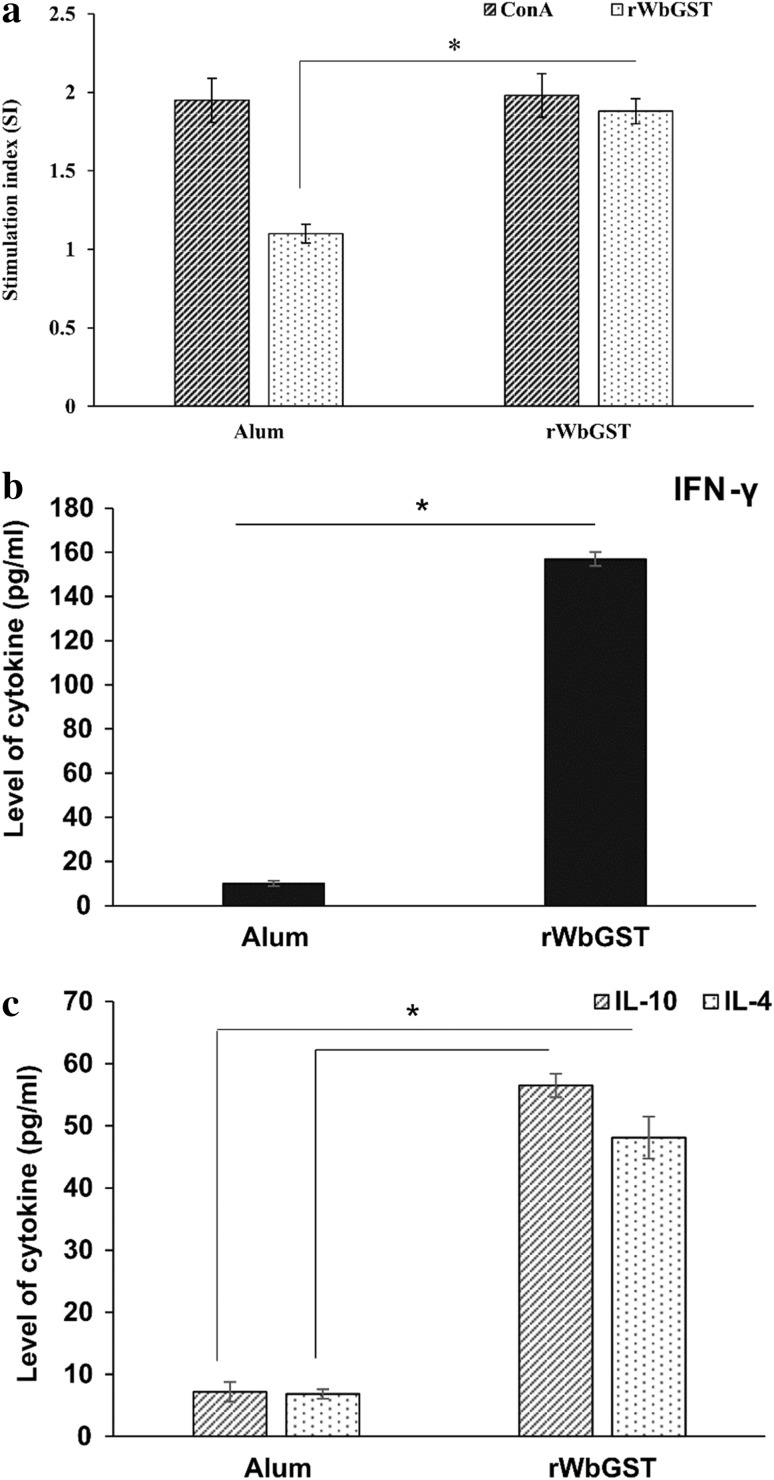
Cellular response was analysed to check the ability of the test protein to stimulate lymphocytes from mastomys immunized with rWbGST after re-stimulation with the same protein. There was significant (p < 0.05) proliferative response of spleen cells from animals immunized with rWbGST (Stimulation index i.e. SI of 1.88 ± 0.08) as compared with the control group of mastomys treated with alum (1.10 ± 0.06) (Fig. 3a).
Fig. 3.
Effect of the immunization of mastomys with rWbGST on splenocytes proliferation and cytokines profile. a Splenocytes from mastomys administered with rWbGST/Alum were cultured in vitro and re-stimulated with either ConA or rWbGST and the effect on the splenocytes proliferation was checked by MTS assay after 48 h. Each bar represents mean stimulation index (SI) ± SD. The levels of cytokines b IFN-γ and c IL-10, IL-4 were measured in the culture supernatants of splenocytes after 72 h using ELISA. Each bar represents Mean ± SD. n = 5 mastomys per group; * p < 0.05 in comparison with Alum group as analyzed by Mann–Whitney U test
The release of Th1-inflammatory cytokine, IFN-γ, was significantly (p = 0.008) high in the culture supernatants of splenocytes from mastomys immunized with rWbGST (Mean ± SD value of 157 ± 3.16 pg/ml) (Fig. 3b). Additionally, the release of anti-inflammatory-Th2 cytokines IL-10 (56.48 ± 1.91 pg/ml) and IL-4 (48.12 ± 3.36 pg/ml) were also increased in response to rWbGST in the mastomys immunized with rWbGST suggesting the rWbGST induced mixed Th1-Th2 type of immune response (Fig. 3c).
Discussion
Glutathione-S-transferases has been presented as a potential vaccine candidate against several parasitic infections [11, 18–20]. In the earlier study, rWbGST was shown as potential vaccine candidate by conducting experiments in BALB/c mice and jirds [7]. In the present study, the vaccine potential of rWbGST was assessed in another rodent model that is fully permissible for B. malayi infection and further we have also characterized the humoral, cellular and cytokine responses associated with the protection induced by rWbGST. The mastomys which are fully permissible for B. malayi when vaccinated with rWbGST developed significant titres of anti-WbGST antibodies. The immunoglobulin antibody isotype profile for the rWbGST-immunized mastomys showed predominance of both IgG1 and IgG2a antibodies which is suggestive of a balanced Th1/Th2 immune response. Increased IgG1 and IgG2a isotypes in rodents have the ability to fix complement and bind to protein antigens and have been shown to participate in ADCC reactions against invading pathogens [21, 22].
ADCC is one of the principal immunological mechanisms working behind the dis-appearance of circulating filarial parasites and their clearance [15, 16, 23]. Here in, we observed that the sera from mastomys immunized with rWbGST promoted adherence of peritoneal exudate cells to L3 larvae and induced significant killing of parasite (71.55 % of cytotoxicity against L3). Whereas, depletion of these antibodies from the sera of immunized mastomys reverted the killing activity of parasite. These results indicate that, anti-WbGST antibodies may be playing a crucial role against the filarial infection.
The results observed in the ADCC experiment were replicated in the in situ micropore chamber experiment. Mastomys vaccinated with rWbGST induced significantly higher cytotoxicity of 65.5 %. The chambers retrieved from the immunized mastomys showed high cellular infiltration. Our results are in agreement with the previous studies in which B. malayi challenge to jird model has achieved nearly 61 % of protection [7]. Sera from mastomys immunized using GST purified through affinity chromatography (SdGST) from cattle filarial parasite Setaria digitate provided 58–82 % cytotoxicity against microfilaria in in vitro ADCC assay [24]. GST isolated from Setaria cervi has been shown to induce 77.8 and 75 % cytotoxicity to B. malayi microfilariae and infective larvae in immunized mastomys [25]. In another study, S. cervi GST showed about 82 % protection in jirds infected with L3 larvae of B. malayi [26]. The enzyme GST has also been explored as vaccine against other parasitic infections [27, 28]. Cattles vaccinated with GST provided 49–69 % protection from Fasciola hepatica [27]. Calves vaccinated with the recombinant Schistosoma bovis-derived GST resulted reduction in S. mattheei worm burden [28]. About 65 % protection from the filarial GST induced in the present study is comparable with the results of these studies and suggest that GST is a promising vaccine candidate against parasitic infections.
T cells are also important in inducing the antibody meditated protection against the parasite. We observed significantly higher levels of WbGST specific Th1 cytokine IFN-γ in the vaccinated animals. The immunized mastomys showed balanced Th1/Th2 type of immune response associated with the significant increase in Th2 cytokines IL-10 and IL-4, which is in correlation with the predominance of IgG1 and IgG2a antibody isotypes as seen in the antibody analysis. Th1 type of immune responses are shown to be important for eliciting the immune protection to filarial infection [29]. Moreover, studies have also reported that Th2 responses are responsible for providing the resistance to intestinal helminths [30]; however, it is difficult to extrapolate such results to tissue-dwelling nematodes such as filariae. The absence of Th2 cells altogether can result in fatal immunopathology [31] which suggests the contribution of balanced Th1/Th2 immune response for a healthy outcome [32].
GSTs are highly expressed gene in the third stage larvae of B. malayi. Because of their abundance in the infective stage larvae, they are more accessible to the immune system to become the potential targets for providing protective immunity. GSTs are postulated to protect the parasite against host-mediated lipid peroxidation of the membrane [33, 34] and involved in the defense against oxidative stress [35, 36]. Rao et al. [37] reported that B. malayi GSTs are the major metabolic enzymes that play an important role in the parasite’s survival. GST from adult female S. cervi has been reported to be able to induce prophylactic effect [20]. W. bancrofti GST has also been previously shown for its significant potential as a vaccine candidate [7].
Taken together, this study shows the protective immune response elicited by the WbGST protein against filarial infection which is found to be associated with the increased IFN-γ production along with the presence of both Th1 and Th2 protective immune responses. The findings suggest that, WbGST could be a promising vaccine candidate against lymphatic filariasis infection.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Experimental design (DOCX 41 kb)
Acknowledgments
The financial support by Department of Biotechnology (DBT) through research project (Project Id. BT/PR/4988/INF/22/155/2012) and FIST grant from Department of Science and Technology (Project Id. SR/FST/LSI-470/2010) both from Ministry of Science & Technology, Government of India is gratefully acknowledged.
Funding
This research work was supported by the research funding received from the Department of Biotechnology (Project Id. BT/PR/4988/INF/22/155/2012) and FIST grant from Department of Science and Technology (Project Id. SR/FST/LSI-470/2010) both from Ministry of Science and Technology, Government of India.
Compliance with Ethical Standards
Conflict of interest
All the authors have declared that they have no conflict of interest.
Ethical approval
This study does not contain any studies with human participants performed by any of the authors. This study was approved by Institutional Animal Ethics Committee and national guidelines as per Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) for the care and use of animals were followed.
References
- 1.World Health Organization. Global Programme to Eliminate Lymphatic Filariasis. Progress report 2000–2009 and Strategic plan 2010–2020: halfway towards eliminating lymphatic filariasis. Geneva: World Health Organization; 2010. Contract No.: WHO/HTM/NTD/PCT/2010.6.
- 2.Ramaiah KD, Das PK, Michael E, Guyatt HL. The economic burden of lymphatic filariasis in India. Parasitol Today. 2000;16(6):251–253. doi: 10.1016/S0169-4758(00)01643-4. [DOI] [PubMed] [Google Scholar]
- 3.Hotez PJ. Mass drug administration and integrated control for the world’s high-prevalence neglected tropical diseases. Clin Pharmacol Ther. 2009;85(6):659–664. doi: 10.1038/clpt.2009.16. [DOI] [PubMed] [Google Scholar]
- 4.Anugraha G, Madhumathi J, Prince PR, Jeya Prita PJ, Khatri VK, Amdare NP, et al. Chimeric epitope vaccine from multistage antigens for lymphatic filariasis. Scand J Immunol. 2015;82(4):380–389. doi: 10.1111/sji.12340. [DOI] [PubMed] [Google Scholar]
- 5.Anand SB, Murugan V, Prabhu PR, Anandharaman V, Reddy MV, Kaliraj P. Comparison of immunogenicity, protective efficacy of single and cocktail DNA vaccine of Brugia malayi abundant larval transcript (ALT-2) and thioredoxin peroxidase (TPX) in mice. Acta Trop. 2008;107(2):106–112. doi: 10.1016/j.actatropica.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Vanam U, Pandey V, Prabhu PR, Dakshinamurthy G, Reddy MV, Kaliraj P. Evaluation of immunoprophylactic efficacy of Brugia malayi transglutaminase (BmTGA) in single and multiple antigen vaccination with BmALT-2 and BmTPX for human lymphatic filariasis. Am J Trop Med Hyg. 2009;80(2):319–324. [PubMed] [Google Scholar]
- 7.Veerapathran A, Dakshinamoorthy G, Gnanasekar M, Reddy MV, Kalyanasundaram R. Evaluation of Wuchereria bancrofti GST as a Vaccine Candidate for Lymphatic Filariasis. PLoS Negl Trop Dis. 2009;3(6):e457. doi: 10.1371/journal.pntd.0000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anand SB, Kodumudi KN, Reddy MV, Kaliraj P. A combination of two Brugia malayi filarial vaccine candidate antigens (BmALT-2 and BmVAH) enhances immune responses and protection in jirds. J Helminthol. 2011;85(04):442–452. doi: 10.1017/S0022149X10000799. [DOI] [PubMed] [Google Scholar]
- 9.Dakshinamoorthy G, Samykutty AK, Munirathinam G, Reddy MV, Kalyanasundaram R. Multivalent fusion protein vaccine for lymphatic filariasis. Vaccine. 2013;31(12):1616–1622. doi: 10.1016/j.vaccine.2012.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dzik JM. Molecules released by helminth parasites involved in host colonization. Acta Biochim Pol. 2006;53(1):33–64. [PubMed] [Google Scholar]
- 11.Grezel D, Capron M, Grzych JM, Fontaine J, Lecocq JP, Capron A. Protective immunity induced in rat schistosomiasis by a single dose of the Sm28GST recombinant antigen: effector mechanisms involving IgE and IgA antibodies. Eur J Immunol. 1993;23(2):454–460. doi: 10.1002/eji.1830230223. [DOI] [PubMed] [Google Scholar]
- 12.Capron AE, Capron M, Dombrowicz D, Riveau G. Vaccine strategies against schistosomiasis: from concepts to clinical trials. Int Arch Allergy Immunol. 2001;124(1–3):9–15. doi: 10.1159/000053656. [DOI] [PubMed] [Google Scholar]
- 13.Bushara HO, Bashir ME, Malik KH, Mukhtar MM, Trottein F, Capron A, et al. Suppression of Schistosoma bovis egg production in cattle by vaccination with either glutathione S-transferase or keyhole limpet haemocyanin. Parasite Immunol. 1993;15(7):383–390. doi: 10.1111/j.1365-3024.1993.tb00623.x. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki T, Seregey IG. Mass dissection technique for determining infectivity rate of filariasis vector. Jpn J Exp Med. 1979;49(2):117–121. [PubMed] [Google Scholar]
- 15.Chandrashekar R, Rao UR, Parab PB, Subrahmanyam D. Brugia malayi: serum dependent cell-mediated reactions to microfilariae. Southeast Asian J Trop Med Public Health. 1985;16(1):15–21. [PubMed] [Google Scholar]
- 16.Chandrashekar R, Rao UR, Subrahmanyam D. Serum dependent cell-mediated immune reactions to Brugia pahangi infective larvae. Parasite Immunol. 1985;7(6):633–641. doi: 10.1111/j.1365-3024.1985.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 17.Weiss N, Tanner M. Studies on Dipetalonema viteae (Filarioidea) 3. Antibody-dependent cell-mediated destruction of microfilariae in vivo. Tropenmed Parasitol. 1979;30(1):73–80. [PubMed] [Google Scholar]
- 18.Morrison CA, Colin T, Sexton JL, Bowen F, Wicker J, Friedel T, et al. Protection of cattle against Fasciola hepatica infection by vaccination with glutathione S-transferase. Vaccine. 1996;14(17):1603–1612. doi: 10.1016/S0264-410X(96)00147-8. [DOI] [PubMed] [Google Scholar]
- 19.Sexton JL, Milner AR, Panaccio M, Waddington J, Wijffels G, Chandler D, et al. Glutathione-S-transferase: novel vaccine against Fasciola hepatica infection in sheep. J Immunol. 1990;145(11):3905–3910. [PubMed] [Google Scholar]
- 20.Gupta S, Bhandari YP, Reddy MV, Harinath BC, Rathaur S. Setaria cervi: immunoprophylactic potential of glutathione-S-transferase against filarial parasite Brugia malayi. Exp Parasitol. 2005;109(4):252–255. doi: 10.1016/j.exppara.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Akiyama Y, Lubeck MD, Steplewski Z, Koprowski H. Induction of mouse IgG2a- and IgG3-dependent cellular cytotoxicity in human monocytic cells (U937) by immune interferon. Cancer Res. 1984;44(11):5127–5131. [PubMed] [Google Scholar]
- 22.Lawrence RA. Immunity to filarial nematodes. Vet Parasitol. 2001;100(1):33–44. doi: 10.1016/S0304-4017(01)00481-2. [DOI] [PubMed] [Google Scholar]
- 23.Mehta K, Subrahmanyam D, Sindhu RK. Immunogenicity of homogenates of the developmental stages of Litomosoides carinii in albino rats. Acta Trop. 1981;38(3):319–324. [PubMed] [Google Scholar]
- 24.Bal M, Mandal N, Achary KG, Das MK, Kar SK. Immunoprophylactic potential of filarial glutathione-S-transferase in lymphatic filariaisis. Asian Pac J Trop Med. 2011;4(3):185–191. doi: 10.1016/S1995-7645(11)60066-7. [DOI] [PubMed] [Google Scholar]
- 25.Gupta S, Bhandari YP, Reddy MV, Harinath BC, Rathaur S. Setaria cervi: immunoprophylactic potential of glutathione-S-transferase against filarial parasite Brugia malayi. Exp Parasitol. 2005;109(4):252–255. doi: 10.1016/j.exppara.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Yadav M, Liebau E, Haldar C, Rathaur S. Identification of major antigenic peptide of filarial glutathione-S-transferase. Vaccine. 2011;29(6):1297–1303. doi: 10.1016/j.vaccine.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 27.Morrison CA, Colin T, Sexton JL, Bowen F, Wicker J, Friedel T, et al. Protection of cattle against Fasciola hepatica infection by vaccination with glutathione S-transferase. Vaccine. 1996;14(17):1603–1612. doi: 10.1016/S0264-410X(96)00147-8. [DOI] [PubMed] [Google Scholar]
- 28.Grzych JM, De Bont J, Liu J, Neyrinck JL, Fontaine J, Vercruysse J, et al. Relationship of impairment of schistosome 28-kilodalton glutathione s-transferase (GST) activity to expression of immunity to Schistosoma mattheei in calves vaccinated with recombinant Schistosoma bovis 28-kilodalton GST. Infect Immun. 1998;66(3):1142–1148. doi: 10.1128/iai.66.3.1142-1148.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin C, Al-Qaoud KM, Ungeheuer MN, Paehle K, Vuong PN, et al. IL-5 is essential for vaccine-induced protection and for resolution of primary infection in murine filariasis. Med Microbiol Immunol. 2000;189(2):67–74. doi: 10.1007/PL00008258. [DOI] [PubMed] [Google Scholar]
- 30.Else KJ, Finkelman FD. Intestinal nematode parasites, cytokines and effector mechanisms. Int J Parasitol. 1998;28(8):1145–1158. doi: 10.1016/S0020-7519(98)00087-3. [DOI] [PubMed] [Google Scholar]
- 31.Brunet LR, Finkelman FD, Cheever AW, Kopf MA, Pearce EJ. IL-4 protects against TNF-alpha-mediated cachexia and death during acute schistosomiasis. J Immunol. 1997;159(2):777–785. [PubMed] [Google Scholar]
- 32.Brunet LR, Dunne DW, Pearce EJ. Cytokine interaction and immune responses during Schistosoma mansoni infection. Parasitol Today. 1998;14(10):422–427. doi: 10.1016/S0169-4758(98)01317-9. [DOI] [PubMed] [Google Scholar]
- 33.Brophy PM, Barrett J. Glutathione-S-transferase in helminths. Parasitology. 1990;100(2):345–349. doi: 10.1017/S0031182000061369. [DOI] [PubMed] [Google Scholar]
- 34.Brophy PM, Pritchard DI. Parasitic helminth Glutathione s transferase: an update of their potential as targets for immuno- and chemotherapy. Exp Parasitol. 1994;79(1):89–96. doi: 10.1006/expr.1994.1067. [DOI] [PubMed] [Google Scholar]
- 35.Liebau E, Wildenburg G, Brophy PM, Walter RD, Henkle-Dührsen K. Biochemical analysis, gene structure and localization of the 24 kDa glutathione S-transferase from Onchocerca volvulus. Mol Biochem Parasitol. 1996;80(1):27–39. doi: 10.1016/0166-6851(96)02660-6. [DOI] [PubMed] [Google Scholar]
- 36.Liebau E, Eschbach ML, Tawe W, Sommer A, Fischer P, Walter RD, et al. Identification of a stress-responsive Onchocerca volvulus glutathione S-transferase (Ov-GST-3) by RT-PCR differential display. Mol Biochem Parasitol. 2000;109(2):101–110. doi: 10.1016/S0166-6851(00)00232-2. [DOI] [PubMed] [Google Scholar]
- 37.Rao UR, Salinas G, Mehta K, Klei TR. Identification & localization of Glutathione-S-transferase as a potential enzyme in Brugia species. Parasitol Res. 2000;86(11):908–915. doi: 10.1007/s004360000255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental design (DOCX 41 kb)