The choroid plexus, which is responsible for the maintenance of the biochemical milieu of the cerebrospinal fluid (CSF), avidly sequesters Pb. In order to test the hypothesis that chronic Pb exposure may impair choroid plexus function, male weanling Sprague–Dawley rats were exposed to Pb in drinking water at doses of 0, 50, or 250 µg Pb/ml (as Pb acetate) for 30, 60, or 90 days. The function of the choroid plexus was assessed as reflected by CSF concentrations of transthyretin (TTR, a major CSF protein manufactured by brain choroid plexus) and CSF essential metal ions (Ca2+, Mg2+, K+, and Na+). TTR concentrations were determined by radioimmunoassay using a monospecific rabbit anti-rat TTR polyclonal antibody, and CSF metal ions analyzed by flame atomic absorption spectrophotometry. Two-way ANOVA of CSF TTR concentrations revealed highly significant dose (p < 0.0001), time (p < 0.0223), and dose-by-time effects (p < 0.0379). Moreover, the percentage of reduction of CSF TTR was directly correlated with Pb concentrations in the choroid plexus (r = 0.703, p < 0.05). Pb exposure significantly increased CSF concentrations of Mg2+, but did not markedly altered CSF concentrations of Ca2+, K+, and Na+. Histopathologic examination under the light microscope did not show distinct alterations of plexus structure in Pb-treated rats. Since TTR is responsible for transport of thyroid hormones to the developing brain, we postulate that the depression of choroid plexus TTR production (and/or secretion) by Pb may impair brain development in young animals by depriving the CNS Of thyroid hormones.
For lead (Pb) to induce neurotoxicity, it must first cross the barriers, i.e., the blood–brain and blood-cerebrospinal fluid (CSF) barriers, that separate the central nervous system (CNS) compartment from blood and other body compartments. Many reports have indicated that Pb damages the blood–brain barrier (Dingwall-Fordyce and Lane, 1963; Goldstein, 1984; Bressler et al., 1994). However, little is known as to whether Pb impairs the blood–CSF barrier during its route to the CNS. This lack of information is particularly glaring in light of the fact that the choroid plexus, where the blood–CSF barrier resides, is an anatomical region which accumulates Pb to an extraordinary degree (Friedheim et al., 1983; Zheng, 1996).
The choroid plexus constitutes the blood–CSF barrier. The structural and functional integrity of this barrier is crucial to the homeostasis of the internal milieu of the CNS (Smith, 1991; Johanson, 1995). The choroid plexus regulates the chemical stability of the CSF by rigorously restricting access of substances from the blood to the CNS compartment, by producing and secreting essential materials toward the CNS, and by transporting some substances bidirectionally between the blood and CSF (Smith, 1991; Nilsson et al., 1992; Johanson, 1995). The impairment of this barrier, has been reportedly associated with certain clinical encephalopathies (Ormerod and Venkatesan, 1970; Rudin, 1981; Levine, 1987; Jorgensen, 1988; Philip et al., 1994).
We have previously reported that the choroid plexus sequesters toxic metals, particularly Pb (Friedheim et al., 1983; Zheng et al., 1991). Using human autopsy material, an increase of Pb in the choroid plexus was found to be significantly associated with age (Friedheim et al., 1983). This observation was subsequently confirmed by Manton et al. (1984). Animal studies using an acute exposure model also demonstrated that the accumulation of Pb in the choroid plexus was dependent upon the dose and time of exposure (Zheng et al., 1991). To date, at least nine different heavy metals or metalloids have been found to accumulate in the choroid plexus (for detailed review, see Zheng, 1996). Nevertheless, little information exists regarding the consequences of accumulation of toxic metals in the choroid plexus. In particular, little attention has been directed to the possibility that the choroid plexus may be the critical target site of Pb neurotoxicity.
The purpose of this study was to test the hypothesis that low-dose, long-term exposure to Pb may alter choroid plexus function. The function of the choroid plexus was assessed as reflected by CSF concentrations of transthyretin (TTR) and CSF essential metal ions (Ca2+, K+, Na+, and Mg2+). TTR is a 55,000-Da protein consisting of four identical sub-units in a tetrahedral symmetry (Ingenbleek and Young, 1994). We chose CSF TTR as a primary parameter to evaluate choroid plexus function for two reasons. First, TTR in CSF is exclusively produced, secreted, and regulated by the choroid plexus in mammalian brain (Herbert et al., 1986; Wade et al., 1988; Nilsson et al., 1992; Aldred et al., 1995). Per unit of weight, rat choroid plexus contains 10 times more TTR mRNA than the liver, the major organ for serum TTR (Dickson et al., 1985), and synthesizes TTR 13 times faster than the liver (Dickson et al., 1986; Schreiber et al., 1990). Thus, alterations of TTR in CSF by Pb exposure, either increase or decrease, may reflect the status of choroid plexus function. Second, CSF TTR, which constitutes 20% of total CSF proteins in humans (Ingenbleek and Young, 1994), carries thyroid hormones to the developing brain. The numerous consequences of thyroid hormone deficiency on brain development and function are well established (Smith et al., 1957; Glorieux et al., 1983; Legrand, 1984). In addition, we determined CSF concentrations of Ca2+, K+, Na+, and Mg2+ since the choroid plexus plays an essential role in regulating the homeostasis of these metal ions in CSF (Johanson, 1995).
MATERIALS AND METHODS
Animals and pretreatment
Male Sprague–Dawley rats (Harlan, Indianapolis, IN) ages 20–22 days, weighing 30–50 g upon arrival, were assigned to three groups such that the group mean body weights were comparable. The animals were housed in a temperature-controlled, 12/12 light/dark room and allowed to have free access to pelleted Purina semipurified rat chow (Purina Mills Test Diet, 5755C, Purina Mills, Richmond, IN) as well as preprepared drinking water. On the third day (age of 22–24 days) after arrival, the animals started to receive Pb in drinking water. An additional group of control, Pb-untreated rats was euthanized at 25–27 days of age and is referred as the “Day 0” group.
The exposure paradigm of Cory-Slechta (Cory-Slechta et al., 1983; Cohn and Cory-Slechta, 1993) was chosen since this was known to be associated with subtle developmental deficits. Thus, drinking water was prepared by dissolving Pb acetate in distilled, deionized water (50 and 250 µg Pb/ml). Pb concentrations were verified by graphite furnace atomic absorption spectrophotometry. For the control group, sodium acetate with an acetate concentration equivalent to the high dose of Pb acetate was prepared in the same manner.
Sample collection
At 30, 60, and 90 days following initiation of exposure, 10 rats from each dose group were anesthetized with pentobarbital (50 mg/kg, ip). CSF samples (about 100–150 µl) were obtained through a butterfly needle (26-gauge) attached to polyethylene tubing. The needle was inserted between the protruberance and the spine of the atlas. CSF samples visibly contaminated with blood were discarded and those free of blood were used for the biochemical analyses. The brain was then removed, the choroid plexus dissected, wet weight recorded, and frozen at −20°C until further analyses. For those animals killed at 90 days, blood samples were collected from the inferior vena cava for determination of blood Pb concentration (BPb).
Radioimmunoassay (RIA) of TTR
The RIA procedure for determining TTR concentrations in serum, CSF, and tissues has been well established in Dr. Blaner’s laboratory and previously described in detail (Navab et al., 1977; Blaner, 1990). The RIA for TTR employs purified rat plasma TTR (both as standard and for use as [125I]TTR) and a monospecific rabbit anti-rat TTR polyclonal antibody. A standard displacement curve was established by plotting the percentage of maximal binding of [125I]TTR with known amounts of homogeneously purified rat plasma TTR for a standard dilution of anti-TTR. The purified rat TTR was iodinated by the lactoperoxidase procedure as described by Blaner (1990). TTR concentrations in CSF were quantitated using this procedure. As little as 2 µl of CSF was usually sufficient for the assay of CSF TTR by this method. The procedure has proven to be sensitive, specific, and reproducible (Navab et al., 1977; Blaner, 1990). Within and between assay coefficients of correlation for the assay of plasma TTR were 4.8 and 6.2%, respectively.
Atomic absorption spectrophotometry (AAS) analysis
Pb concentrations in the CSF and choroid plexus were determined by a flameless graphite furnace AAS. Choroid plexus tissues were combusted in acid-washed crucibles at 800°C for 3–4 hr. The crucibles were then rinsed with 200 µl of 0.5% HNO3/0.2% NH4H2PO4 and transferred to autosampler vials. The CSF samples were diluted 40-fold with 0.5% HNO3/0.2% NH4H2PO4 solution in autosampler vials immediately before AAS to avoid contamination. A Perkin-Elmer Model 3030 Zeeman AAS, equipped with an HGA-600 graphite furnace, was used for quantification. The detection limit for this method was 1.35 ng Pb/ml of assay solution. BPb concentrations were determined using the procedure of Fernandez and Hilligoss (1982); our laboratory is certified for BPb analysis by OSHA. CSF concentrations of Ca2+, Na+, K+, and Mg2+ were determined by the standard flame AAS methods (Perkin-Elmer, 1984). The samples were diluted directly with distilled, deionized water so as to keep the absorbance reading within the linear ranges of the measurement. The following dilution factors were applied: for Ca, 250-fold; Na, 15,000-fold; and K and Mg, 800-fold.
Determination of protein content
CSF total protein content was measured by a Bio-Rad Protein Assay Kit (Bio-Rad Lab, Richmond, CA) using bovine serum albumin as the standard.
Statistics
Concentrations of TTR, Pb, and other essential metal ions in CSF and choroid plexus were analyzed by two-way analysis of variance (ANOVA). When ANOVA revealed an overall treatment effect, contrast analyses were performed at individual time points using Scheffe’s multiple comparison test (Scheffe, 1967).
Materials
Chemicals were obtained from the following sources: Pb acetate from Sigma Chemical Co. (St. Louis, MO); Na acetate from Fisher Scientific Co. (Fair Lawn, NJ); NH4H2PO4 from Aldrich Chemical Co. (Milwaukee, WI); AA standards of Pb, Ca, Mg, K, and Na from Alfa Products (Danvers, MA); 125Iodide (sp act: 17 mCi/µg) from Du Pont (Boston, MA). All reagents were of analytical grade, HPLC grade, or the best available pharmaceutical grade.
RESULTS
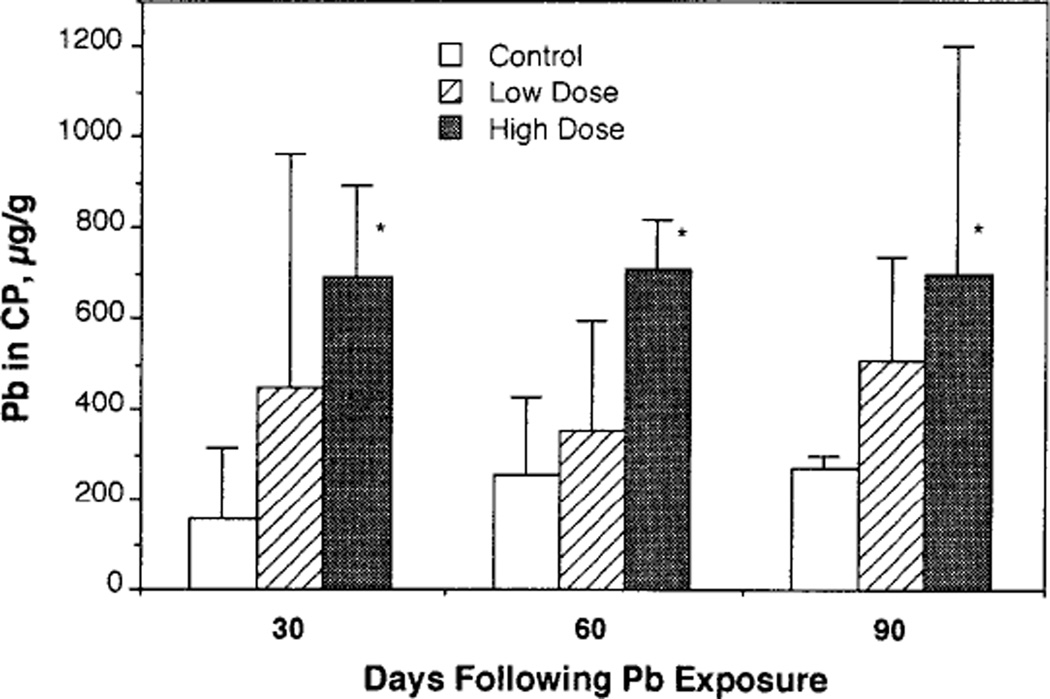
Following chronic Pb exposure, the mean body weights of rats in the high-dose group (254 ± 13.1, 355 ± 19.5, and 421 ± 24.7 g, mean ± SD for Days 30, 60, and 90, respectively, same in the following), but not in the low-dose group (270 ± 15.2, 382 ± 20.2, and 453 ± 31.8 g), were significantly lower than those in the control group (271 ± 12.2, 388 ± 17.5 g, and 469 ± 31.8 g) at all three time points. At Day 90, BPb in control rats was below the detection limit (<1.0 µg/dl), while the mean BPb of rats in the low- and high-dose groups were 18.2 and 48.9 µg/dl, respectively. Rats under this dose regimen showed a significant accumulation of Pb in the choroid plexus (Fig. 1). It is notable that the prolonged exposure for 60 and 90 days did not seem to further increase Pb deposition in the choroid plexus, suggesting a possible saturation with Pb by 30 days.
FIG. 1.
Accumulation of Pb in rat choroid plexus (CP) following chronic Pb exposure. Rats were exposed to Pb in drinking water at the doses of 0, 50, or 250 µg/ml for 30, 60, or 90 days. Choroid plexus tissues were combusted and concentrations of Pb determined by flameless AAS. Data represent mean ± SD (n = 3). *p <0.05 as compared to the control.
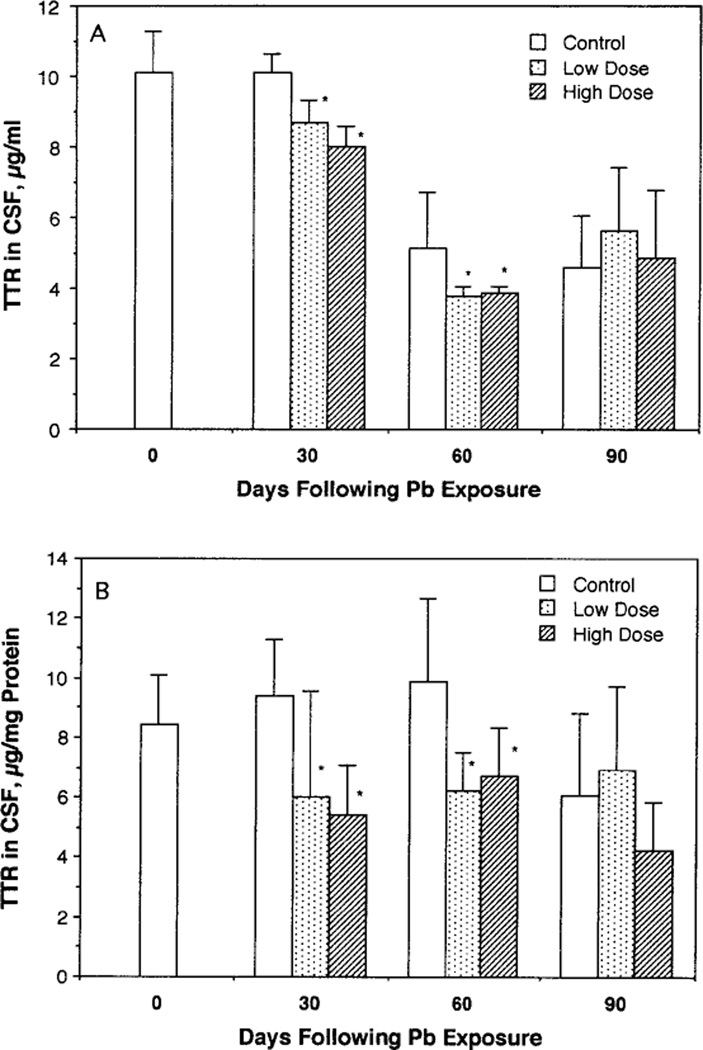
Low-dose, long-term exposure to Pb caused a significant decrease of CSF concentrations of TTR (Fig. 2A). The Pb effect became even more striking when CSF concentrations of TTR were normalized to CSF protein concentration (Fig. 2B). Two-way ANOVA analysis revealed that dose, time, and the dose-by-time interaction were all highly significantly related to CSF TTR (p values: for dose, p < 0.0001; time, p < 0.0223; dose × time, p < 0.0379). The alteration of CSF TTR by Pb was characterized by (1) a rapid decline of CSF TTR and (2) a direct association between the percentage of reduction and choroid plexus Pb concentration (Fig. 3). The decline of CSF TTR occurred early at the first time point, i.e., 30 days, when decreases of approximately 36 and 42% CSF TTR were observed in the low- and high-dose groups, respectively. Exposure to Pb for 60 days did not further depress CSF TTR (about 32–37% decreases). At Day 90, no significant difference could be seen between CSF TTR in Pb-treated animals as compared to the control group. In addition, it is interesting to note that untreated, control neonatal rats (27-day-old) had a higher CSF TTR than adults (Fig. 2A), suggesting an age-related decline in CSF TTR. This is reassuring since human studies report a higher level (~31%) of CSF TTR in neonatal than in young adults (Larsen and DeLallo, 1989).
FIG. 2.
Reduction of CSF TTR following chronic Pb exposure in rats. CSF samples were free of blood. The data represent mean ± SD of 6–10 observations. *p < 0.05 as compared to the control by Scheffe’s method. TTR concentrations in the CSF were expressed as (A) µg of TTR per ml of CSF and (B) µg of TTR per mg of CSF proteins.
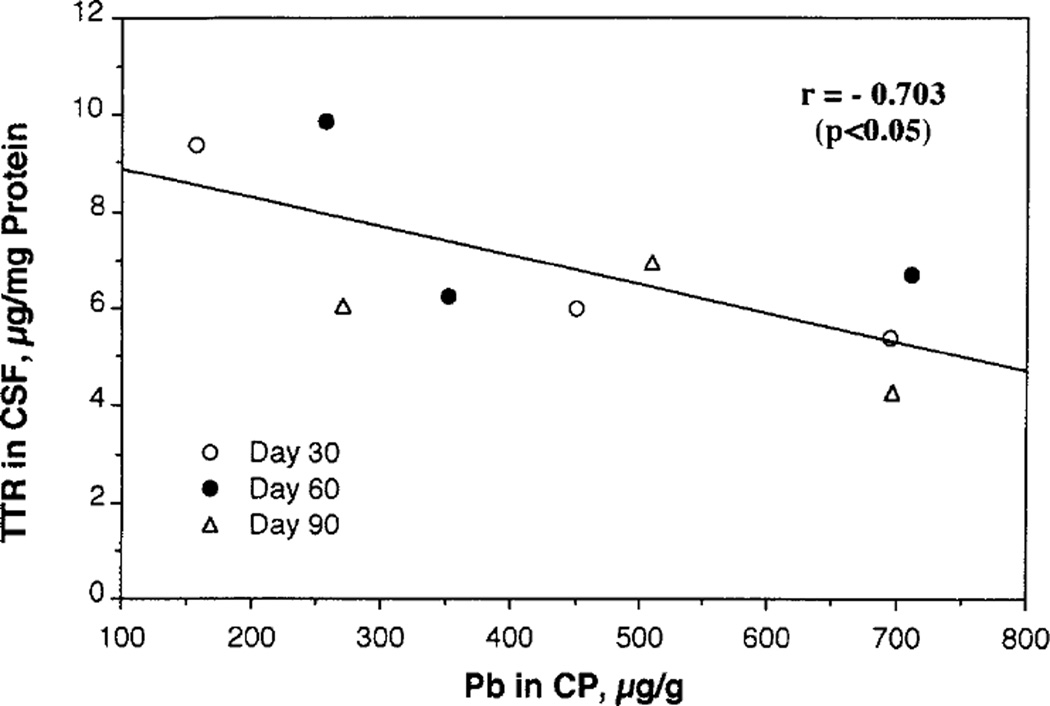
FIG. 3.
Correlation between CSF TTR and Pb deposited in the choroid plexus (CP) following chronic Pb exposure. CSF TTR data were derived from Fig. 2 and CP Pb data from Fig. 1.
By plotting CSF TTR against Pb deposited in the choroid plexus, it became evident that CSF TTR was inversely associated with concentrations of Pb in the choroid plexus (r = 0.703, p < 0.05).
The CSF concentrations of Ca2+, K+, Na+, and Mg2+ obtained from our control rats (Table 1) were comparable to those reported in literature (Davson and Segal, 1996). While the CSF concentrations of Ca2+, Na+, and K+ in Pb-treated rats were not statistically significantly different from those in control rats, a dose-related elevation of CSF Mg2+ at Day 90 was evident (p < 0.05) (Table 1). Also, there seemed to be an age-dependent increase in CSF K+ and Mg2+ in all three tested groups (Table 1), but this did not achieve significance.
TABLE 1.
CSF Concentrations of Essential Metal Ions as Influenced by Pb Chronic Exposure
| Control (0 µg Pb/ml) |
Low dose (50 µg Pb/ml) |
High dose (250 µg Pb/ml) |
|
|---|---|---|---|
| Calcium | |||
| Day 30 | 43.51 ± 1.63 | 44.80 ± 1.87 | 44.03 ± 2.28 |
| Day 60 | 56.96 ± 2.70 | 58.81 ± 2.68 | 59.75 ± 2.46 |
| Day 90 | 37.83 ± 3.62 | 33.26 ± 1.32 | 38.10 ± 15.32 |
| Potassium | |||
| Day 30 | 101.1 ± 4.76 | 103.3 ± 7.64 | 98.30 ± 3.90 |
| Day 60 | 115.7 ± 11.9 | 114.7 ± 12.6 | 106.3 ± 13.3 |
| Day 90 | 116.3 ± 22.6 | 116.9 ± 19.4 | 124.1 ± 15.7 |
| Magnesium | |||
| Day 30 | 22.67 ± 1.24 | 22.71 ± 0.93 | 22.78 ± 1.02 |
| Day 60 | 23.68 ± 2.81 | 23.58 ± 3.40 | 22.73 ± 2.65 |
| Day 90 | 26.09 ± 7.08 | 28.80 ± 5.95* | 30.84 ± 4.41* |
| Sodium | |||
| Day 30 | 2803 ± 173.1 | 2756 ± 218.4 | 2938 ± 173.3 |
| Day 60 | 3530 ± 574.2 | 4353 ± 1025 | 3308 ± 442.3 |
| Day 90 | 2475 ± 185.0 | 2402 ± 33.10 | 2587 ± 230.0 |
Note. The numbers of rats involved in this study: For 30-Day experiment, control, n = 9, low dose, n = 7, high dose, n = 8; for 60-Day experiment, control, n = 8, low dose, n = 10, high dose, n = 8; for 90-Day experiment, control, n = 9, low dose, n = 8, high dose, n = 9.
p < 0.05 as compared to the control.
Histopathologic examination of the choroidal epithelia was conducted in three rats from each group. No distinct structural alteration in choroidal epithelia could be identified under light microscopy (data not shown). However, the current observation does not rule out the possibility of ultra-structural alterations.
DISCUSSION
Our results demonstrate that low-dose, long-term exposure to Pb significantly decreases CSF TTR in rats. The decrease in CSF TTR by Pb likely represents an important adverse effect of Pb on the CNS. As shown in Fig. 3, the decrease in CSF TTR was closely associated with the increase in concentrations of Pb in the choroid plexus. Such a correlation may explain the early decline of CSF TTR but not further decrease at later times following Pb treatment (Fig. 2), the phenomenon probably resulting from the early saturable deposition of Pb in the choroid plexus (Fig. 1). It is remotely possible that the decline of CSF TTR might be attributable to the overall effect of Pb on total TTR production by liver. However, this seems to be rather dubious since TTR in CSF is not derived from the liver, but solely from the choroid plexus (Dickson et al., 1986; Herbert et al., 1986; Aldred et al., 1995). Moreover, the production of TTR by the choroid plexus is entirely independent of that in liver (Dickson et al., 1986; Wade et al., 1988).
Our results raise a number of interesting questions. First, since CSF TTR transports thyroid hormones to and in the brain, does the Pb-induced decrease in CSF TTR in early postnatal life disrupt the homeostasis of thyroid hormones in the CNS and thereby mediate the developmental deficits? In humans, TTR is the major protein in CSF and conveys about 60–80% of CSF thyroxine to the CNS (Hagen and Elliott, 1973; Herbert et al., 1986; Larsen and DeLallo, 1989). Recent evidence has revealed that the choroid plexus transports thyroid hormones from blood to CSF via TTR synthesis in the choroidal epithelia (Schreiber et al., 1990; Dratman et al., 1991; Chanoine et al., 1992; Southwell et al., 1993); a small portion of thyroxine may also enter the brain across the blood-brain barrier (Blay et al., 1993). In the choroid plexus, the TTR gene is expressed early in fetal development, a phenomenon consistent with the importance of the thyroid hormones in embryonic brain development (Thomas et al., 1989; Cavallaro et al., 1993).
Thyroid hormones have striking effects on the CNS, particularly during the developmental period (Dussault and Ruel, 1987). Deprivation of thyroid hormones in children 3d causes irreversible mental retardation (Smith et al., 1957; Glorieux et al., 1983; Legrand, 1984). In the current study, we adapted a Pb-exposure protocol previously used in Pb behavioral toxicity studies (Cory-Slechta et al., 1983). Other rats treated by this protocol reportedly displayed a typical pattern of deficiency in growth, learning, and performance (Cory-Slechta et al., 1983; Cohn and Cory-Slechta, 1993; Hammond et al., 1993). However, it is not known whether thyroid hormone status in brain tissues and CSF in such animals is normal. It is also necessary to point out that the Pb exposure in this study was initiated at the age of 21 days. Since thyroid hormones play a major role in early brain development, the future studies should focus on the consequences of Pb exposure at even younger ages.
Second, how does Pb reduce CSF TTR? If Pb simply disrupted the barrier function of the choroid plexus, namely increasing the barrier permeability, we should have observed an increase in CSF TTR, since the concentration of TTR is higher in serum than in CSF. To the contrary, we observed a decrease. Moreover, our histopathological examination could not detect any distinct structural damage in the choroid plexus of Pb-treated rats. Therefore, the observed decline of CSF TTR by Pb exposure is more likely attributable to impaired processes of production, transportation, and/or secretion of TTR by the choroid plexus. The choroid plexus transports many amino acids, hormones, and proteins including TTR (Smith, 1991; Nilsson et al., 1992). Although the additional effects of Pb on other transport systems in the choroid plexus remain to be explored, one early study did report that Pb selectively altered tyrosine transport in the isolated choroid plexus (Kim and O’Tuama, 1978).
In conclusion, chronic Pb exposure results in Pb deposition in the choroid plexus, which in turn is associated with a significant decrease in CSF TTR. The magnitude of this effect is associated with the concentration of Pb in the choroid plexus. These findings promise to shed light on the mechanism whereby Pb induces neurotoxicity in children. Since TTR is responsible for transport of thyroid hormones to the developing brain, we postulate that the depression of choroid plexus TTR production (and/or secretion) by Pb may impair brain development by depriving the CNS of thyroid hormones.
Acknowledgments
The authors gratefully acknowledge the technical assistance of Vesna Slavkovich. This research was supported in part by Grants P20-ES06831 and RO1-ES03460 and by the Lucille Markey Foundation.
REFERENCES
- Aldred AR, Brack CM, Schreiber G. The cerebral expression of plasma protein genes in different species. Comp. Biochem. Physiol. 1995;111B:1–15. doi: 10.1016/0305-0491(94)00229-n. [DOI] [PubMed] [Google Scholar]
- Blaner WS. Radioimmunoassays for retinol-binding protein, cellular retinol-binding protein, and cellular retinoic acid-binding protein. Methods Enzymol. 1990;189:270–281. doi: 10.1016/0076-6879(90)89298-v. [DOI] [PubMed] [Google Scholar]
- Blay P, Nilsson C, Owman C, Aldred A, Schreiber G. Transthyretin expression in rat brain: Effect of thyroid functional state and role in thyroxine transport. Brain Res. 1993;632:114–120. doi: 10.1016/0006-8993(93)91145-i. [DOI] [PubMed] [Google Scholar]
- Bressler J, Forman S, Goldstein GW. Phospholipid metabolism in neural microvascular endothelial cells after exposure to lead in vitro. Toxicol. Appl. Pharmacol. 1994;126:352–360. doi: 10.1006/taap.1994.1126. [DOI] [PubMed] [Google Scholar]
- Cavallaro T, Martone RL, Stylianopoulou F, Herber J. Differential expression of the insulin-like growth factor-II and transthyretin genes in the developing rat choroid plexus. J. Neuropathol. Exp. Neurol. 1993;52:153–162. doi: 10.1097/00005072-199303000-00008. [DOI] [PubMed] [Google Scholar]
- Chanoine JP, Alex S, Fang SL, Stone S, Leonard JL, Korhle J, Braverman LE. Role of transthyretin in the transport of thyroxine from the blood to the choroid plexus, the cerebrospinal fluid, and the brain. Endocrinology. 1992;130:933–938. doi: 10.1210/endo.130.2.1733735. [DOI] [PubMed] [Google Scholar]
- Cohn J, Cory-Slechta DA. Subsensitivity of lead-exposed rats to the accuracy-impairing and rate-altering effects of MK-801 on a multiple schedule of repeated learning and performance. Brain Res. 1993;600:208–218. doi: 10.1016/0006-8993(93)91375-3. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Weiss B, Cox C. Delayed behavioral toxicity of lead with increasing exposure concentration. Toxicol. Appl. Pharmacol. 1983;71:342–352. doi: 10.1016/0041-008x(83)90021-2. [DOI] [PubMed] [Google Scholar]
- Davson H, Segal MB. Physiology of the CSF and Blood–Brain Barrier. New York: CRC Press; 1996. pp. 15–18. [Google Scholar]
- Dickson PW, Aldred AR, Marley PD, Tu GF, Howlett GJ, Schreiber G. High prealbumin and transferrin mRNA levels in the choroid plexus of rat brain. Biochem. Biophys. Res. Commun. 1985;127:890–895. doi: 10.1016/s0006-291x(85)80027-9. [DOI] [PubMed] [Google Scholar]
- Dickson PW, Aldred AR, Marley PD, Bannister D, Schreiber G. Rat choroid plexus specializes in the synthesis and the secretion of transthyretin (prealbumin). Regulation of transthyretin synthesis in choroid plexus is independent from that of liver. J. Biol. Chem. 1986;261:3475–3478. [PubMed] [Google Scholar]
- Dingwall-Fordyce I, Lane RE. A following up study of lead workers. Br. J. Indust. Med. 1963;20:313. doi: 10.1136/oem.20.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dratman MB, Crutchfield FL, Schoenhoff MB. Transport of iodothyronines from bloodstream to brain: Contributions by blood:brain and choroid plexus:cerebrospinal fluid barrier. Brain Res. 1991;554:229–236. doi: 10.1016/0006-8993(91)90194-z. [DOI] [PubMed] [Google Scholar]
- Dussault JH, Ruel J. Thyroid hormones and brain development. Ann. Rev. Physiol. 1987;49:321–334. doi: 10.1146/annurev.ph.49.030187.001541. [DOI] [PubMed] [Google Scholar]
- Fernandez F, Hilligoss D. An improved graphite furnace method for the determination of lead in blood using matrix modification and the L’vov platform. Atomic Spectr. 1982;3:130–131. [Google Scholar]
- Friedheim E, Corvi C, Graziano J, Donnelli T, Breslin D. Choroid plexus as protective sink for heavy metals? Lancet. 1983;i(8331):981–982. doi: 10.1016/s0140-6736(83)92099-8. [DOI] [PubMed] [Google Scholar]
- Glorieux J, Dussault JH, Letarte J, Guyde H, Morissette J. Preliminary results on the mental development of hypothyroid children detected by the Quebec Screening Program. J. Pediatr. 1983;102:19–22. doi: 10.1016/s0022-3476(83)80279-0. [DOI] [PubMed] [Google Scholar]
- Goldstein GW. Brain capillaries: A target for inorganic lead poisoning. Neurotoxicology. 1984;5:167–176. [PubMed] [Google Scholar]
- Hagen GA, Elliott WJ. Transport of thyroid hormones in serum and cerebral spinal fluid. J. Clin. Endocrinol. Metab. 1973;37:415–422. doi: 10.1210/jcem-37-3-415. [DOI] [PubMed] [Google Scholar]
- Hammond PB, Minnema DJ, Succop PA. Reversibility of lead-induced depression of growth. Toxicol. Appl. Toxicol. 1993;123:9–15. doi: 10.1006/taap.1993.1215. [DOI] [PubMed] [Google Scholar]
- Herbert J, Wilcox JN, Pham KC, Fremeau RT, Zeviani M, Dwork A, Soprano DR, Makover A, Goodman DS, Zimmerman EA, Roberts JL, Schon EA. Transthyretin: A choroid plexus- specific transport protein in human brain. Neurology. 1986;36:900–911. doi: 10.1212/wnl.36.7.900. [DOI] [PubMed] [Google Scholar]
- Ingenbleek Y, Young V. Transthyretin (prealbumin) in health and disease: Nutritional implications. Annu. Rev. Nutr. 1994;14:495–533. doi: 10.1146/annurev.nu.14.070194.002431. [DOI] [PubMed] [Google Scholar]
- Johanson CE. Ventricles and cerebrospinal fluid. In: Conn PM, editor. Neuroscience in Medicine. Philadelphia: Lippincott; 1995. pp. 171–196. [Google Scholar]
- Jorgensen OS. Neural cell adhesion molecule (NCAM) and prealbumin in cerebrospinal fluid from depressed patients. Acta Psychiatr. Scand. 1988;345(Suppl):29–37. doi: 10.1111/j.1600-0447.1988.tb08565.x. [DOI] [PubMed] [Google Scholar]
- Kim CS, O’Tuama LA. Selective changes in tyrosine transport by isolated choroid plexus exposed to inorganic lead. Toxicol. Appl. Pharmacol. 1978;45:213–217. doi: 10.1016/0041-008x(78)90042-x. [DOI] [PubMed] [Google Scholar]
- Larsen PD, DeLallo L. Cerebrospinal fluid transthyretin in the neonate and blood–cerebrospinal fluid barrier permeability. Ann. Neurol. 1989;25:628–630. doi: 10.1002/ana.410250617. [DOI] [PubMed] [Google Scholar]
- Legrand J. Effect of thyroid hormones on central nervous system development. In: Yanai J, editor. Neurobehavioral Teratology. New York: Elsevier; 1984. pp. 331–363. [Google Scholar]
- Levine S. Choroid plexus: Target for systemic disease and pathway to the brain. Lab. Invest. 1987;56:231–233. [PubMed] [Google Scholar]
- Manton WI, Kirkpatrick JB, Cook JD. Does the choroid plexus really protect the brain from lead? Lancet. 1984;ii(8398):351. doi: 10.1016/s0140-6736(84)92719-3. [DOI] [PubMed] [Google Scholar]
- Navab M, Smith JE, Goodman DS. Rat plasma prealbumin. Metabolic studies on effects of vitamin A status and on tissue distribution. J. Biol. Chem. 1977;252:5107–5114. [PubMed] [Google Scholar]
- Nilsson C, Lindvall-Axelsson M, Owman C. Neuroendocrine regulatory mechanisms in the choroid plexus–cerebrospinal fluid system. Brain Res. Rev. 1992;17:109–138. doi: 10.1016/0165-0173(92)90011-a. [DOI] [PubMed] [Google Scholar]
- Ormerod WE, Venkatesan S. The choroid plexus in African sleeping-sickness. Lancet. 1970;2(676):777. doi: 10.1016/s0140-6736(70)90257-6. [DOI] [PubMed] [Google Scholar]
- Perkin-Elmer . Analytical Methods for Atomic Absorption Spectrophotometry. Norwalk; Publication B332, Perkin-Elmer: 1984. [Google Scholar]
- Philip KA, Dascombe MJ, Fraser PA, Pentreath VW. Blood–brain barrier damage in experimental African trypanosomiasis. Ann. Trop. Med. Parasitol. 1994;88:607–616. doi: 10.1080/00034983.1994.11812911. [DOI] [PubMed] [Google Scholar]
- Rudin DO. The choroid plexus and system disease in mental illness. II. Systemic lupus erythematosus: A combined transport dysfunction model for schizophrenia. Biol. Psychiatr. 1981;16:373–397. [PubMed] [Google Scholar]
- Scheffe H. The Analysis of Variance. New York: Wiley; 1967. [Google Scholar]
- Schreiber G, Aldred AR, Jaworowski A, Nilsson C, Achen MG, Segal MB. Thyroxine transport from blood to brain via transthyretin synthesis in choroid plexus. Am. J. Physiol. 1990;258:R338–R345. doi: 10.1152/ajpregu.1990.258.2.R338. [DOI] [PubMed] [Google Scholar]
- Smith DW, Blizzard RM, Wilkins L. The mental prognosis in hypothyroidism of infancy in childhood. Pediatrics. 1957;19:1011–1022. [PubMed] [Google Scholar]
- Smith QR. The blood–brain barrier and the regulation of amino acid uptake and availability to brain. Adv. Exp. Med. Biol. 1991;291:55–71. doi: 10.1007/978-1-4684-5931-9_6. [DOI] [PubMed] [Google Scholar]
- Southwell BR, Duan W, Alcorn D, Brack C, Richardson SJ, Kohrle J, Schreiber G. Thyroxine transport to the brain: Role of protein synthesis by the choroid plexus. Endocrinology. 1993;133:2116–2126. doi: 10.1210/endo.133.5.8404661. [DOI] [PubMed] [Google Scholar]
- Thomas T, Schreiber G, Jaworowski A. Developmental patterns of gene expression of secreted proteins in brain and choroid plexus. Devl. Biol. 1989;134:38–47. doi: 10.1016/0012-1606(89)90076-6. [DOI] [PubMed] [Google Scholar]
- Wade S, Bleiberg-Daniel E, LeMoullac B. Rat transthyretin: the effects of acute short-term food deprivation and refeeding on the serum and cerebrospinal fluid concentration and on the hepatic mRNA level. J. Nutr. 1988;118:199–205. doi: 10.1093/jn/118.2.199. [DOI] [PubMed] [Google Scholar]
- Zheng W, Perry DF, Nelson DL, Aposhian HV. Protection of cerebrospinal fluid against toxic metals by the choroid plexus. FASEB J. 1991;5:2188–2193. doi: 10.1096/fasebj.5.8.1850706. [DOI] [PubMed] [Google Scholar]
- Zheng W. The choroid plexus and metal toxicities. In: Chang LW, editor. Toxicology of Metals. New York: CRC Press; 1996. pp. 605–622. [Google Scholar]