Abstract
Objective
To demonstrate the usefulness of a novel medical device based on Raman spectroscopy for the rapid point-of-care diagnosis of gout and pseudogout.
Methods
A shoebox-sized point-of-care Raman spectroscopy (POCRS) device was developed for use in the diagnosis of gout and pseudogout. The device included a disposable syringe microfiltration kit to collect arthropathic crystals from synovial fluid and a customized automated Raman spectroscopy system to chemically identify crystal species. Diagnosis according to the findings of POCRS was compared with the clinical standard diagnosis based on compensated polarized light microscopy (CPLM) of synovial fluid aspirates collected from symptomatic patients (n = 174). Kappa coefficients were used to measure the agreement between POCRS and CPLM findings.
Results
Overall, POCRS and CPLM results were consistent in 89.7% of samples (156 of 174). For the diagnosis of gout, the kappa coefficient for POCRS and CPLM was 0.84 (95% confidence interval [95% CI] 0.75–0.94). For the diagnosis of pseudogout, the kappa coefficient for POCRS and CPLM was 0.61 (95% CI 0.42–0.81).
Conclusion
Kappa coefficients indicated that there was excellent agreement between POCRS and CPLM for the diagnosis of gout, with good agreement for the diagnosis of pseudogout. The POCRS device holds the potential to standardize and expedite the time to clinical diagnosis of gout and pseudogout, especially in settings where certified operators trained for CPLM analysis are not available.
Crystal-associated arthritis is caused by an inflammatory response to micron-sized crystals deposited in joint spaces and soft tissues. Monosodium urate monohydrate (MSU) crystals (involved in gout) and calcium pyrophosphate dihydrate (CPPD) crystals (involved in pseudogout) are the 2 most frequently observed crystals and were clinically characterized decades ago (1–6). Gout affects 8.3 million Americans (7), and pseudogout affects as much as 3% of the population in the age range of 60–70 years, according to the American College of Rheumatology. The prevalence of chondrocalcinosis (i.e., deposition of CPPD crystals in the soft tissues of the joint) increases exponentially with age (8). The estimated overall cost of gout in the US is in the tens of billions of dollars and is comparable to the cost of other chronic conditions, such as Parkinson’s disease or migraine (9).
Gout and pseudogout usually share common symptoms with other forms of arthritis. Compensated polarized light microscopy (CPLM) of synovial aspirates is the standard clinical method used in the diagnosis of gout and pseudogout. While CPLM is sensitive and specific in the hands of a skilled technician, CPLM may carry an average false-negative rate of as much as 30% depending on the operator (10–12). As such, experienced operators in Clinical Laboratory Improvement Amendments (CLIA)–certified laboratories are essential for the successful diagnosis by CPLM findings. However, many clinical settings may lack such operators in real-time, or the clinical schedules may not allow for the coordination of sample transfer for CPLM. Therefore, many gout patients are diagnosed presumptively, based on symptoms, in the primary care setting (13), where the majority of gout cases are diagnosed and managed (14,15).
In addition to CPLM, other approaches have been used to diagnose the presence of gout and pseudogout. Serum urate analysis is associated with unsatisfactory sensitivity (57–67%) and specificity (78–92%) values (16–18). In comparisons of ultrasound and radiography in the diagnosis of gout, Rettenbacher et al (19) found ultrasound to be more sensitive (96% versus 31%) but less specific (73% versus 93%) than radiography. Filippou et al (20) reported the diagnosis of pseudogout by ultrasound to have a sensitivity of 86.7% and a specificity of 96%. These studies had relatively low number of patients, and CPLM was accepted as the gold standard. As with CPLM, successful diagnosis by ultrasound requires expert technicians.
Raman spectroscopy is a chemical analysis technique that is 100% specific for fingerprinting species based on the identification of chemical bonds unique to each material. Raman spectroscopy has been used in several studies (21–23) to identify crystals in synovial fluid, but costly and bulky laboratory-grade instruments were used, which limits the applicability of this technique in the clinic. Recent work from our laboratory addressed these challenges by developing an automated, shoebox-sized point-of-care Raman spectroscopy (POCRS) device for use in the diagnosis of gout and pseudogout from synovial aspirates (24). The aim of the present study was to demonstrate the usefulness of the POCRS device in the diagnosis of gout and pseudogout, and to investigate the diagnostic agreement between POCRS and CPLM findings in a large clinical sample set.
MATERIALS AND METHODS
Synovial fluid samples
A total of 174 synovial fluid samples were collected: 114 from MetroHealth Medical Center and 60 from Henry Ford Hospital. Samples were obtained from patients who presented with symptomatic arthritis requiring clinically indicated joint aspiration and/or intra-articular joint injection. A recent corticosteroid injection was not an exclusion criterion. Adult patients were included without regard to sex or race. The age range of the patients was 18–88 years. Both sexes were comparably represented, with 48.3% men and 51.7% women in the population.
The study was approved by the Institutional Review Boards of the respective institutions. All patients gave their consent for study.
CPLM and POCRS
Synovial fluid samples were separated into 2 aliquots, one of which was sent to the clinical pathology laboratory for crystal identification by CPLM, and the other was placed in a sealed sterile container. As part of the normal treatment course in these patients, CPLM analyses were conducted by experts (rheumatologists or pathologists with >20 years’ experience) in CLIA-certified laboratories at both institutions. Samples were stored at −80°C until their delivery in dry ice via overnight shipment to Case Western Reserve University (CWRU) for the POCRS analysis. Samples were then stored at −20°C in a freezer at CWRU until they were used for the POCRS analysis. There was a single freeze–thaw cycle prior to the POCRS analysis.
POCRS consisted of 2 major steps (Figure 1). First, a disposable syringe–microfiltration step was performed, which included a brief digestion and dilution to collect and concentrate crystals for Raman spectroscopic analysis. Second, a Raman spectroscopy step was performed, in which a shoebox-sized optoelectromechanical system was customized for conducting an automated Raman spectroscopic analysis to identify crystal species, as detailed in a previous publication from our group (24).
Figure 1.
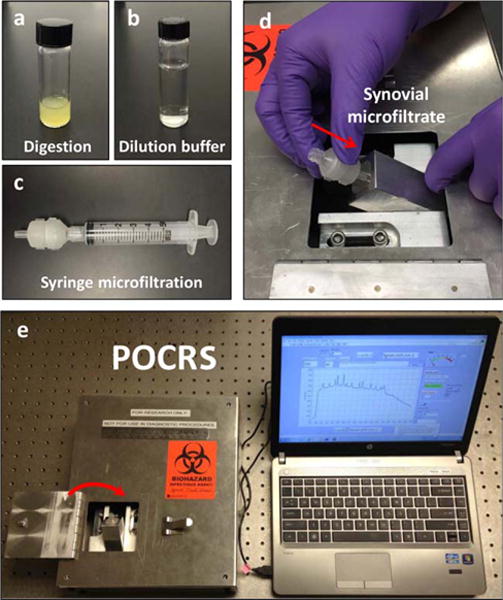
Point-of-care Raman spectroscopy (POCRS). The POCRS system consists of 2 parts: a syringe microfiltration kit for isolating and collecting arthritic crystals from synovial fluid (a–c) and a shoebox-sized optoelectromechanical system for acquiring diagnostic signals (d and e). To use the system, synovial fluid is loaded in a glass vial with digestive enzymes (a). After 30 minutes of digestion at 40°C, the uric acid–supplemented buffer (b) is used to dilute the digested synovial fluid. Following dilution, the synovial fluid is transferred into a standard syringe (c) and pushed through the disposable microfiltration cartridge for crystal collection. After microfiltration, the cartridge is directly inserted into the optoelectromechanical system (d) for diagnostic signal acquisition (e).
Brief digestion of hyaluronic acid and inflammatory organic debris was essential for isolating crystals from synovial fluid via microfiltration. The digestion solution contained 1 mg/ml of hyaluronidase (item no. H3506; Sigma), 2 mg/ml of proteinase K (item no. P2308; Sigma), and 0.5% (volume/weight) of sodium dodecyl sulfate (item L3771; Sigma). Following 30 minutes of incubation at 40°C, the digested fluid was then diluted by adding uric acid–supplemented buffer at a volume ratio of 2-to-1. Uric acid supplement was used to prevent MSU crystals from dissolving, and dilution helped to facilitate the microfiltration process by thinning the digested synovial fluid. Uric acid was added into 1× phosphate buffered saline at a concentration of 60 μg/ml, and the solution was filtered using a 0.2-μm filter. Dissolution of uric acid was confirmed by cross-polarized imaging at high magnification. Previous research from our group (24) demonstrated that this pretreatment process, including the digestion and the uric acid supplementary buffer, preserves the crystals, as confirmed by the lack of significant difference between crystal counts performed before versus after treatment. It was also affirmed that artifactual nucleation of crystals from the buffer does not occur.
The digested synovial fluid was transferred to a standard syringe mounted with a customized disposable microfiltration cartridge (24) and was pushed through the cartridge for crystal collection. Via the microfiltration step, crystals were retained within a round spot of ~900 μm in diameter on a filter membrane in the cartridge. Following microfiltration, the cartridge was directly inserted into the optoelectromechanical system for acquiring Raman signals from 30 sampling points distributed over the filtrate spot. MSU and CPPD crystals were detected based on the presence of signature Raman peaks at 631 cm−1 (25) and 1,050 cm−1 (26), respectively. Signal acquisition was fully automated and was completed within 15 minutes. The crystal concentration was estimated according to our previous strategy, based on the calibration curves established previously (24).
Data management
Study data were collected and managed using REDCap electronic data capture tools hosted at MetroHealth Medical Center (27). REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry, 2) audit trails for tracking data manipulation and export procedures, 3) automated export procedures for seamless data downloads to common statistical packages, and 4) procedures for importing data from external sources.
Statistical analysis
The laboratory personnel at CWRU who were conducting the POCRS were blinded with regard to the outcome of the CPLM findings at the 2 hospitals and vice versa. Both the CPLM and POCRS results were aggregated and analyzed by a biostatistician (SL, a member of the Center for Health Care Research and Policy at Metro-Health Medical Center), who measured the agreement between POCRS and CPLM in the diagnosis of gout and pseudogout, respectively (28). The kappa coefficient is a measure of agreement between 2 methods that assumes values from 0 to 1, where 0 represents agreement just by chance and 1 represents perfect agreement. Generally, kappa coefficients are rated as <0.20, which indicates poor agreement, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 good, and 0.81–1.00 excellent agreement (28). The sample size of 174 subjects provided 0.96 power value when using the kappa coefficient to measure the rate of agreement between POCRS and CPLM.
RESULTS
Typical Raman spectra of affirmed gout and pseudogout samples demonstrated peaks associated with MSU (631 cm−1) and CPPD (1,050 cm−1) crystals, respectively, and samples which did not appear to include crystals were devoid of such peaks (Figure 2). The MSU crystal concentration as measured by POCRS varied from 0.1 μg/ml to 84.3 μg/ml, with an average of 9.0 μg/ml, and the CPPD crystal concentration ranged from 2.5 μg/ml to 109.0 μg/ml, with an average of 19.2 μg/ml (Figure 3).
Figure 2.
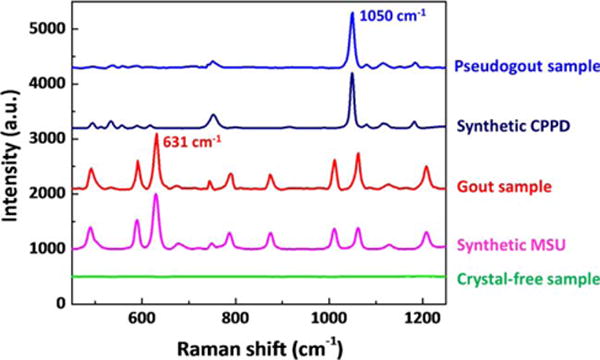
Raman peaks associated with monosodium urate monohydrate (MSU; 631 cm−1) and calcium pyrophosphate dihydrate (CPPD; 1,050 cm−1) crystals. Raman peaks associated with MSU and CPPD crystals are presented in the spectra of samples from patients with confirmed gout and pseudogout, respectively. No crystal-associated peak was observed in the spectra of crystal-free synovial fluid samples. Spectra for synthetic pure MSU and CPPD crystals are included as references. Fluorescence background and filter membrane–associated peaks were removed from these spectra.
Figure 3.
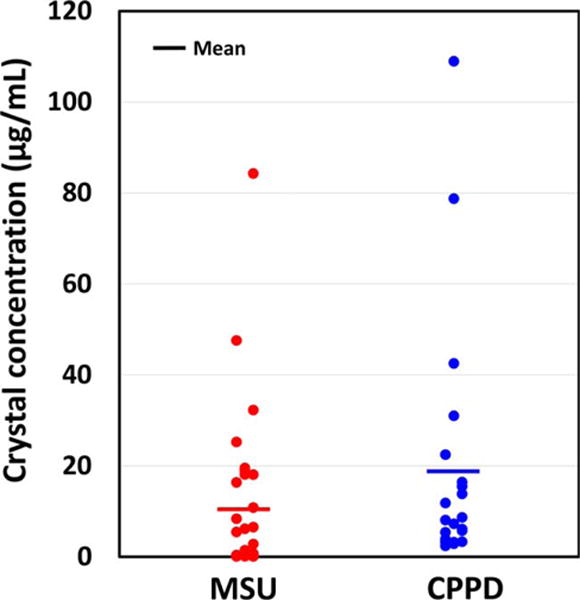
Concentrations of monosodium urate monohydrate (MSU) and calcium pyrophosphate dihydrate (CPPD) crystals, as measured by point-of-care Raman spectroscopy. Each symbol represents a single sample.
The CPLM and POCRS findings were consistent for 89.7% of the samples analyzed (156 of 174). For 18 samples, the diagnosis by POCRS and CPLM findings showed discrepancies (Table 1). By CPLM, 44 samples indicated gout and 12 indicated pseudogout. By POCRS, 36 samples had MSU crystals and 20 samples had CPPD crystals. POCRS, but not CPLM, showed 2 samples with coexistent MSU and CPPD crystals (Table 2).
Table 1.
Diagnostic results of 18 samples with inconsistent findings between CPLM and POCRS*
| ID no. | CPLM result | POCRS result |
|---|---|---|
| 4 | X | CPPD |
| 7 | X | CPPD |
| 9 | X | CPPD |
| 32 | X | MSU |
| 36 | MSU | X |
| 43 | X | CPPD |
| 60 | MSU | X |
| 64 | MSU | CPPD |
| 67 | MSU | CPPD |
| 88 | X | CPPD |
| 102 | MSU | X |
| 118 | MSU | X |
| 122 | X | CPPD |
| 146 | MSU | X |
| 148 | MSU | X |
| 159 | X | CPPD |
| 162 | X | MSU |
| 172 | CPPD | X |
X represents a negative result, and no crystal was detected. CPLM = compensated polarized light microscopy; POCRS = point-of-care Raman spectroscopy; CPPD = calcium pyrophosphate dihydrate; MSU = monosodium urate monohydrate.
Table 2.
Number of samples found to be positive on CPLM and POCRS*
| POCRS result | CPLM result | |
|---|---|---|
| Gout (MSU crystals) | 36 | 44 |
| Pseudogout (CPPD crystals) | 20 | 12 |
| Both gout and pseudogout | 2 | 0 |
| Total | 58 | 56 |
CPLM = compensated polarized light microscopy; POCRS = point-of-care Raman spectroscopy; MSU = monosodium urate monohydrate; CPPD = calcium pyrophosphate dihydrate.
For the diagnosis of gout, both CPLM and POCRS showed that 128 samples lacked MSU crystals and 36 samples contained MSU crystals. However, 2 samples identified by POCRS as being positive for MSU crystals were missed by CPLM, and 8 samples identified by CPLM as being positive for MSU crystals were missed by POCRS (Table 3).
Table 3.
Comparison of CPLM and POCRS results for the diagnosis of gout and pseudogout*
| CPLM negative | CPLM positive | Total | |
|---|---|---|---|
| Gout (MSU crystals) | |||
| POCRS negative | 128 | 8 | 136 |
| POCRS positive | 2 | 36 | 38 |
| Total | 130 | 44 | 174 |
| Pseudogout (CPPD crystals) | |||
| POCRS negative | 151 | 1 | 152 |
| POCRS positive | 11 | 11 | 22 |
| Total | 162 | 12 | 174 |
A negative result means that no monosodium urate monohydrate (MSU) or calcium pyrophosphate dihydrate (CPPD) crystals were detected. A positive result means that MSU and/or CPPD crystals were detected. CPLM = compensated polarized light microscopy; POCRS = point-of-care Raman spectroscopy.
For the diagnosis of pseudogout, CPLM and POCRS showed 151 negative samples and 11 positive samples. However, 11 samples identified by POCRS as being CPPD crystal–positive were missed by CPLM, and 1 sample identified by CPLM as being CPPD crystal–positive was missed by POCRS (Table 3).
In detecting MSU crystals, the kappa coefficient for POCRS and CPLM was 0.84 (95% confidence interval [95% CI] 0.75–0.94). In detecting CPPD crystals, the kappa coefficient for both analyses was 0.61 (95% CI 0.42–0.81). Kappa coefficients indicated that POCRS and CPLM had excellent agreement for the diagnosis of gout, and good agreement for the diagnosis of pseudogout (28).
DISCUSSION
This work demonstrated the usefulness of a novel medical device (POCRS) based on Raman spectroscopy for use in the point-of-care diagnosis of gout and pseudogout. Our clinical study of 174 samples indicated that POCRS was comparable to the clinically accepted method of CPLM in detecting MSU and CPPD crystals. It must be emphasized that the POCRS method identifies the type of crystals, and this information alone would not constitute a conclusive diagnosis of gout or pseudogout. The diagnoses of gout and pseudogout remain clinical diagnoses but are highly contingent on the identification of MSU and CPPD crystals, respectively, in synovial aspirates from inflammatory joints. It must also be emphasized that we do not propose POCRS as a replacement or alternative to CPLM. Rather, POCRS can be used in settings where time and resources are limited or no CLIA-certified staff is available to perform CPLM. In some situations, POCRS can also be used in conjunction with CPLM, as when there is ambiguity in identifying the type of crystal microscopically. Our results and those published in the literature (29–31) indicate such ambiguity to exist for CPPD crystals, which appear to evade detection by CPLM. Therefore, use of methods such as POCRS would help to mitigate this shortcoming.
In the current study, we refrained from deriving a sensitivity value for POCRS or CPLM because there is no gold standard method that gives a 100% accurate diagnosis. Therefore, the focus was placed on reporting the extent of the agreement between the 2 methods by using the kappa coefficient as the measure of agreement. Kappa coefficients indicated excellent agreement and good agreement, respectively, between CPLM and POCRS in identifying the presence of MSU and CPPD crystals, suggesting that most samples for which gout or pseudogout is diagnosed via CPLM can also be diagnosed by POCRS findings.
The use of CPLM to analyze synovial fluid is highly dependent on the skill and experience of the operator in identifying negatively (MSU) and/or positively (CPPD) birefringent crystals, along with the corresponding crystal morphologies (10,11,32–34). Diagnoses of the same sample set by different personnel frequently vary (11). When crystal sizes are small, birefringence is weak or absent, and crystal concentrations are low, it becomes difficult for the operators to make decisive judgments of the crystal species.
Although CPLM is used as the routine approach by rheumatologists to aid in the clinical diagnosis of gout and pseudogout, appropriate personnel and facilities are frequently unavailable in urgent care centers, emergency departments, and local community healthcare settings, where patients usually present with acute symptoms. Doctors may have to turn to symptom-based criteria to reach a presumptive diagnosis, which carries a high false-negative rate (13). Gout is therefore misdiagnosed and undertreated in such settings. Failure to detect and identify MSU and/or CPPD crystals in a timely and correct manner may result in suboptimal management of crystal-induced arthritis, which may include the use of inappropriate medications. Inaccurate diagnosis of gout also results in potentially avoidable hospital admissions due to diagnostic uncertainty. A point-of-care automated device would enable aspirate-based diagnosis in healthcare settings that lack trained operators, and it may also reduce the healthcare costs associated with avoidable hospital admissions.
The coexistence of MSU and CPPD crystals in synovial fluid has previously been reported in studies of cytocentrifugation analyses (35). In our sample set, CPLM was unable to identify any samples with both MSU and CPPD crystals. POCRS indicated the combined presence of MSU and CPPD crystals simultaneously in samples no. 14 and no. 145. Due to its subjectivity, physicians reporting results of CPLM may be reluctant to diagnose both gout and pseudogout unless the sample is laden with both types of crystals. Unlike CPLM, POCRS is objective, and it clearly confirmed the coexistence of MSU and CPPD crystals, which could alter the course of treatment in such patients.
To the best of our knowledge, there is no study that formally provides any applicable quantitative measurement of MSU and CPPD crystal concentrations in the diagnosis of gout and pseudogout. POCRS, as demonstrated in this work, was able to estimate the crystal concentration based on the Raman signal intensity. The crystal concentration in synovial fluid may be an indicator for tracing the efficacy of gout and pseudogout medications.
The limit of detection of POCRS is defined by the amount of crystals retained in the cartridge and less so by the volume of the synovial fluid. An aliquot of 0.1 ml of synovial fluid that is heavily laden with crystals may provide a Raman signal, whereas 10 ml of synovial fluid that is absent of crystals would not. In this study, the smallest volume of clinical synovial fluid in a sample was ~0.5 ml. The estimated concentrations in this study were generally consistent with the anecdotally reported clinical ranges between 10 μg/ml and 100 μg/ml (32); however, our Raman analysis by POCRS also identified patients with crystal concentrations in the range of 0.1–10 μg/ml. The detection limit of POCRS was favorably positioned for identifying most crystal specimens collected in the clinic.
For samples with greater amounts of MSU crystals, POCRS and CPLM had good agreement. However, the 2 methods differed when the samples had lower concentrations of crystals. POCRS was not able to detect MSU crystals if the crystal concentration was below the threshold of 0.1 μg/ml (24). CPLM can identify MSU crystals even when a single crystal is detectable in the sample. Therefore, CPLM may be more sensitive than POCRS in situations when crystal concentration is exceedingly low.
The performance of CPLM in the diagnosis of pseudogout seemed to be unsatisfactory, such that many samples that were positively identified as containing CPPD crystals by POCRS were not so identified by CPLM. The challenges of identifying CPPD crystals microscopically have previously been shown (29): the weak birefringence of CPPD crystals makes it harder to detect them during identification by cross-polarized imaging techniques. Furthermore, CPPD crystals may be much smaller than can be detected at magnifications used by CPLM. However, the strong Raman characteristic peak of CPPD crystals enable their identification by Raman analysis.
In this study, the analyses of clinical synovial samples were conducted after a single freeze–thaw cycle. Several studies demonstrated freezing not to affect crystal morphology or amount (36–38); however, it is unclear whether these reports apply to samples where crystal concentrations are extremely low (e.g., <0.1 μg/ml). This limitation would not be applicable to Raman analysis at the point of care, because it would be applied to synovial fluid freshly collected in a clinical context.
Basic calcium phosphate (BCP) crystals were not observed, either by POCRS or CPLM, in any of the samples included in this study. BCP provides strong Raman signals (39) and would be detectable by POCRS if they had been present in sufficient quantity in the filtrate. There may be various reasons for the lack of BCP crystals. BCP crystals may have dissolved during the sample preparation procedure. They are known to be of small size, and they may also have been lost during the filtration process. The concentrations of BCP crystals in these samples may have been below the detection limit of the current setup. It is also possible that this patient population may not be prone to the development of BCP crystals. Previous studies have shown the abundance of BCP crystals in the joints of patients with advanced osteoarthritis, patients scheduled for joint replacement, and in aged patients (40,41). While osteoarthritis was present in some of the patients in our study, most of the patients underwent aspiration because of suspected gout.
The POCRS concept holds translational promise for the future. The device is a collection of several dozen optical, digital, and mechanical components that can be feasibly integrated using off-the-shelf components. While research-grade Raman systems can cost in excess of $100,000, the cost of components for integrating POCRS was ~$10,000. The aggregate cost of sample preparation reagents and the microfiltration cartridge is in the range of ~$10. In a clinical setting, the clinic would procure 1 device and then 1 sample kit per patient. Sample preparation for POCRS consists of 3 steps: digestion, dilution, and microfiltration, which takes ~1 hour (24). The majority of this time is devoted to the digestion step (<45 minutes). Otherwise, the actual sample-handling time is minimal (<5 minutes).
In summary, an innovative, clinically applicable Raman device was developed for the rapid detection of MSU and CPPD crystals. Analysis of clinically collected samples demonstrated that POCRS findings were in general agreement with CPLM findings over the entire pool of samples. POCRS could be used to help guide the initiation of targeted outpatient therapy and potentially reduce the need for inpatient admission in patients with joint effusion, in whom diagnosis might otherwise be uncertain. This could potentially improve the use of inpatient resources as well as the overall quality of patient care.
Acknowledgments
The authors thank Stanley Ballou, Marina Magrey, Sobia Hassan, Muhammad Khan, Cheung Yue, Ingrid Cobb, Sonia Manocha, Uzma Syeda, Zohair Abbas, Maria Antonelli, and Bassam Alhaddad at MetroHealth Medical Center and Bernard Rubin at Henry Ford Health System for identifying and recruiting the patients and collecting the clinical samples.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, or the National Center for Advancing Translational Sciences.
Supported by the NIH (National Institute of Arthritis and Musculoskeletal and Skin Diseases grant R01-AR-057812 [OA]) and by the Clinical and Translational Science Collaborative of Cleveland and its Clinical Research Unit Core based at MetroHealth Medical Center, which receive support from the National Center for Advancing Translational Sciences (grant UL1-TR-000439) and the NIH Road-map for Medical Research.
Footnotes
Drs. Li and Akkus are coinventors on patent no. WO2014201088 A1 for the technology concerning methods and devices for the diagnosis of particles in biologic fluids.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Akkus had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Li, Singer, Yeni, Haggins, Akkus.
Acquisition of data. Li, Singer, Yeni, Haggins, Barnboym, Oravec, Lewis.
Analysis and interpretation of data. Li, Lewis, Akkus.
References
- 1.Howell RR, Eanes ED, Seegmiller JE. X-ray diffraction studies of the tophaceous deposits in gout. Arthritis Rheum. 1963;6:97–103. doi: 10.1002/art.1780060202. [DOI] [PubMed] [Google Scholar]
- 2.Seegmiller JE, Frazier PD. Biochemical considerations of the renal damage of gout. Ann Rheum Dis. 1966;25:668–72. doi: 10.1136/ard.25.suppl_6.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seegmiller JE. The acute attack of gouty arthritis. Arthritis Rheum. 1965;8:714–25. doi: 10.1002/art.1780080431. [DOI] [PubMed] [Google Scholar]
- 4.O’Duffy JD. Hypophosphatasia associated with calcium pyrophosphate dihydrate deposits in cartilage. Arthritis Rheum. 1970;13:381–8. doi: 10.1002/art.1780130404. [DOI] [PubMed] [Google Scholar]
- 5.Parlee DE, Freundlich IM, McCarty DJ., Jr A comparative study of roentgenographic techniques for detection of calcium pyrophosphate dihydrate deposits (pseudogout) in human cartilage. Am J Roentgenol Radium Ther Nucl Med. 1967;99:688–94. doi: 10.2214/ajr.99.3.688. [DOI] [PubMed] [Google Scholar]
- 6.Skinner M, Cohen AS. Calcium pyrophosphate dihydrate crystal deposition disease. Arch Intern Med. 1969;123:636–44. [PubMed] [Google Scholar]
- 7.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63:3136–41. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 8.Felson DT, Anderson JJ, Naimark A, Kannel W, Meenan RF. The prevalence of chondrocalcinosis in the elderly and its association with knee osteoarthritis: the Framingham Study. J Rheumatol. 1989;16:1241–5. [PubMed] [Google Scholar]
- 9.Wertheimer A, Morlock R, Becker MA. A revised estimate of the burden of illness of gout. Curr Ther Res Clin Exp. 2013;75:1–4. doi: 10.1016/j.curtheres.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dieppe P, Swan A. Identification of crystals in synovial fluid. Ann Rheum Dis. 1999;58:261–3. doi: 10.1136/ard.58.5.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swan A, Amer H, Dieppe P. The value of synovial fluid assays in the diagnosis of joint disease: a literature survey. Ann Rheum Dis. 2002;61:493–8. doi: 10.1136/ard.61.6.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segal JB, Albert D. Diagnosis of crystal-induced arthritis by synovial fluid examination for crystals: lessons from an imperfect test. Arthritis Care Res. 1999;12:376–80. doi: 10.1002/1529-0131(199912)12:6<376::aid-art5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Malik A, Schumacher HR, Dinnella JE, Clayburne GM. Clinical diagnostic criteria for gout: comparison with the gold standard of synovial fluid crystal analysis. J Clin Rheumatol. 2009;15:22–4. doi: 10.1097/RHU.0b013e3181945b79. [DOI] [PubMed] [Google Scholar]
- 14.Harrold LR, Mazor KM, Negron A, Ogarek J, Firneno C, Yood RA. Primary care providers’ knowledge, beliefs and treatment practices for gout: results of a physician questionnaire. Rheumatology (Oxford) 2013;52:1623–9. doi: 10.1093/rheumatology/ket158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pal B, Foxall M, Dysart T, Carey F, Whittaker M. How is gout managed in primary care? A review of current practice and proposed guidelines. Clin Rheumatol. 2000;19:21–5. doi: 10.1007/s100670050005. [DOI] [PubMed] [Google Scholar]
- 16.Schlesinger N, Baker DG, Schumacher HR., Jr Serum urate during bouts of acute gouty arthritis. J Rheumatol. 1997;24:2265–6. [PubMed] [Google Scholar]
- 17.Logan JA, Morrison E, McGill PE. Serum uric acid in acute gout. Ann Rheum Dis. 1997;56:696–7. doi: 10.1136/ard.56.11.696a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rigby AS, Wood PH. Serum uric acid levels and gout: what does this herald for the population? Clin Exp Rheumatol. 1994;12:395–400. [PubMed] [Google Scholar]
- 19.Rettenbacher T, Ennemoser S, Weirich H, Ulmer H, Hartig F, Klotz W, et al. Diagnostic imaging of gout: comparison of high-resolution US versus conventional X-ray. Eur Radiol. 2008;18:621–30. doi: 10.1007/s00330-007-0802-z. [DOI] [PubMed] [Google Scholar]
- 20.Filippou G, Frediani B, Gallo A, Menza L, Falsetti P, Baldi F, et al. A “new” technique for the diagnosis of chondrocalcinosis of the knee: sensitivity and specificity of high-frequency ultrasonography. Ann Rheum Dis. 2007;66:1126–8. doi: 10.1136/ard.2007.069344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng X, Haggins DG, York RH, Yeni YN, Akkus O. Analysis of crystals leading to joint arthropathies by Raman spectroscopy: comparison with compensated polarized imaging. Appl Spectrosc. 2009;63:381–6. doi: 10.1366/000370209787944280. [DOI] [PubMed] [Google Scholar]
- 22.McGill N, Dieppe PA, Bowden M, Gardiner DJ, Hall M. Identification of pathological mineral deposits by Raman microscopy. Lancet. 1991;337:77–8. doi: 10.1016/0140-6736(91)90738-b. [DOI] [PubMed] [Google Scholar]
- 23.Chen KH, Li MJ, Cheng WT, Balic-Zunic T, Lin SY. Identification of monoclinic calcium pyrophosphate dihydrate and hydroxyapatite in human sclera using Raman microspectroscopy. Int J Exp Pathol. 2009;90:74–8. doi: 10.1111/j.1365-2613.2008.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li B, Yang S, Akkus O. A customized Raman system for point-of-care detection of arthropathic crystals in the synovial fluid. Analyst. 2014;139:823–30. doi: 10.1039/c3an02062b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kodati VR, Tu AT, Turumin JL. Raman spectroscopic identification of uric-acid-type kidney stone. Appl Spectrosc. 1990;44:1134–6. [Google Scholar]
- 26.Miura K, Fukuda H, Mineta H, Yamaguchi K, Harada H, Yusa H, et al. Phosphoglyceride crystal deposition disease. Pathol Int. 2000;50:992–8. doi: 10.1046/j.1440-1827.2000.01138.x. [DOI] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleiss JL, Levin B, Paik MC. The measurement of interrater agreement. In: Fleiss JL, Levin B, Paik MC, editors. Statistical methods for rates and proportion. New York: Wiley Interscience; 2003. pp. 603–17. [Google Scholar]
- 29.Ivorra J, Rosas J, Pascual E. Most calcium pyrophosphate crystals appear as non-birefringent. Ann Rheum Dis. 1999;58:582–4. doi: 10.1136/ard.58.9.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swan A, Chapman B, Heap P, Seward H, Dieppe P. Submicroscopic crystals in osteoarthritic synovial fluids. Ann Rheum Dis. 1994;53:467–70. doi: 10.1136/ard.53.7.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bjelle A, Crocker P, Willoughby D. Ultra-microcrystals in pyrophosphate arthropathy. Acta Med Scand. 1980;207:89–92. [PubMed] [Google Scholar]
- 32.Gordon C, Swan A, Dieppe P. Detection of crystals in synovial fluids by light microscopy: sensitivity and reliability. Ann Rheum Dis. 1989;48:737–42. doi: 10.1136/ard.48.9.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasselbacher P. Variation in synovial fluid analysis by hospital laboratories. Arthritis Rheum. 1987;30:637–42. doi: 10.1002/art.1780300606. [DOI] [PubMed] [Google Scholar]
- 34.Lumbreras B, Pascual E, Frasquet J, Gonzalez-Salinas J, Rodriquez E, Hernandez-Aquado I. Analysis for crystals in synovial fluid: training of the analysts results in high consistency. Ann Rheum Dis. 2005;64:612–5. doi: 10.1136/ard.2004.027268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robier C, Neubauer M, Quehenberger F, Rainer F. Coincidence of calcium pyrophosphate and monosodium urate crystals in the synovial fluid of patients with gout determined by the cytocentrifugation technique. Ann Rheum Dis. 2011;70:1163–4. doi: 10.1136/ard.2010.136721. [DOI] [PubMed] [Google Scholar]
- 36.Galvez J, Saiz E, Linares LF, Climent A, Marras C, Pina MF, et al. Delayed examination of synovial fluid by ordinary and polarised light microscopy to detect and identify crystals. Ann Rheum Dis. 2002;61:444–7. doi: 10.1136/ard.61.5.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGill NW, Swan A, Dieppe PA. Survival of calcium pyrophosphate crystals in stored synovial fluids. Ann Rheum Dis. 1991;50:939–41. doi: 10.1136/ard.50.12.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graf SW, Buchbinder R, Zochling J, Whittle SL. The accuracy of methods for urate crystal detection in synovial fluid and the effect of sample handling: a systematic review. Clin Rheumatol. 2013;32:225–32. doi: 10.1007/s10067-012-2107-0. [DOI] [PubMed] [Google Scholar]
- 39.Yavorskyy A, Hernandez-Santana A, McCarthy G, McMahon G. Detection of calcium phosphate crystals in the joint fluid of patients with osteoarthritis: analytical approaches and challenges. Analyst. 2008;133:302–18. doi: 10.1039/b716791a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Derfus BA, Kurian JB, Butler JJ, Daft LJ, Carrera GF, Ryan LM, et al. The high prevalence of pathologic calcium crystals in pre-operative knees. J Rheumatol. 2002;29:570–4. [PubMed] [Google Scholar]
- 41.Schumacher HR., Jr Crystals, inflammation, and osteoarthritis. Am J Med. 1987;83:11–6. doi: 10.1016/0002-9343(87)90845-x. [DOI] [PubMed] [Google Scholar]


