Abstract
Objectives
This study compared the relative prognostic utility of the gross tumor volume (GTV), maximum standardized uptake value (SUVmax), and metabolic tumor volume (MTV) in a uniform cohort of oropharyngeal squamous cell carcinoma (OPSCC) patients treated with platinum-based concurrent chemoradiation therapy (CCRT).
Methods and Materials
One-hundred OPSCC with a pretreatment [18F] fluorodeoxyglucose (FDG) positron emission tomography positron-emission tomography computed-tomography (PET-CT) were treated with CCRT. Kaplan-Meier curves and Cox proportional hazard models were generated.
Results
When dichotomized by the median, a smaller MTV correlated with improved 5-year locoregional control (LRC) (98.0% versus 87.0%, p = .049), freedom from distant metastasis (FDM) (91.7% versus 65.0%, p = .005), progression-free survival (PFS) (80.3% versus 56.7%, p = .015), and overall survival (OS) (84.1% versus 57.8%, p = .008), whereas a smaller GTV correlated with improved PFS (80.3% versus 57.4%, p = .040) and OS (82.1% versus 60.1%, p = .025). SUVmax failed to correlate with any outcome. On multivariate analysis, when adjusted for GTV, T-stage, and N-stage a smaller MTV remained independently correlated with improved FDM, PFS, and OS. GTV failed to reach significance in the multivariate model.
Conclusions
A smaller MTV correlates with improved LRC, FDM, PFS, and OS in OPSCC patients undergoing platinum-based CCRT.
Keywords: Head and Neck Cancer, Oropharyngeal Cancer, Metabolic Tumor Volume (MTV), Gross Tumor Volume (GTV), Standardized Uptake Value (SUV), PET, PET-CT
Introduction
Concomitant chemoradiation for locally advanced oropharyngeal squamous cell carcinoma (OPSCC) offers excellent locoregional control (LRC) rates while allowing organ preservation with acceptable rates of acute and chronic toxicities [1]. Unfortunately, a significant percentage of these patients develop distant metastases (DM) despite exceptional rates of LRC. While patients with human papillomavirus (HPV)-associated oropharyngeal cancers tend to have better LRC, distant metastases remain problematic [2]. Given increased interest in treatment de-intensification, especially within HPV positive patients, improved prognostic indices are needed to risk stratify patients and identify those with the most favorable disease.
Computed tomography (CT), magnetic resonance imaging, and [18F] fluorodeoxyglucose positron emission tomography (FDG-PET) have become increasingly incorporated into the pretreatment workup of patients with OPSCC. While many studies have reported on the correlation of gross tumor volume (GTV), standardized uptake value (SUV), and metabolic tumor volume (MTV) with various clinical endpoints, it remains unclear if these correlations are simply an effect of gross disease (i.e., GTV), metabolic activity (i.e., SUV), or something different altogether, a hybrid, the metabolically active tumor (i.e., MTV).
Therefore the purpose of this study was to compare the relative prognostic utility of GTV, SUVmax, and MTV in a uniform cohort of OPSCC patients treated with definitive platinum-based chemotherapy administered concurrently with intensity-modulated radiation therapy (IMRT).
METHODS
Patient selection
The institutional review board approved this retrospective study with a waiver of informed consent. Between September 1998 and May 2009, 442 consecutive patients with pathologically confirmed non-metastatic and non-recurrent oropharyngeal cancer were treated with concurrent chemoradiation at a single institution. Of these, 266 patients were excluded because the 18F-FDG had been performed as PET only (rather than as PET/CT) and/or the PET/CT had been performed at an outside institution. Additional patients were excluded if they were diagnosed more than 180 days prior to starting treatment (n=1), underwent prior surgical resection (n=11), or did not receive platinum-based chemotherapy concurrent with IMRT (n=22). Of the remaining 142 patients, 100 had restorable treatment plans (required for GTV assessment) and formed the study cohort.
All patients were re-staged according to the 2010 American Joint Committee on Cancer Staging Manual, 7th edition. In addition, all patient had a complete history and physical examination, focused head and neck evaluation, direct flexible fiber-optic endoscopic examination, complete blood counts, liver function tests, chest X-ray, and dental evaluation [3].
FDG PET/CT
FDG PET-CT was obtained on all patients prior to receiving any therapy. In preparation for PET/CT, patients fasted for 6 hours. Blood glucose levels were confirmed to be <200 mg/dL prior to FDG (12–15 mCi) intravenous injection. Patients drank oral contrast during the uptake period (72 ± 17 min). Low-dose CT (120–140 kV, 80 mA) and PET were then obtained for the torso (3 min/bed position, thoracic inlet to upper thigh) with arms up, followed by dedicated images of the head and neck (5 min/bed position), with arms down. Intravenous contrast was administered immediately prior to the radiation simulation scan. Images were reviewed on a workstation integrated with a PACS (Volume Viewer 2, AW Suite version 2.0, GE Healthcare) that allowed multiplanar reformatting of images.
FDG-PET image analysis was performed as previously described [4]. Briefly, a single blinded nuclear medicine radiologist retrospectively analyzed the PET/CT images. Region-of-interest borders were set by manual adjustment in 3 planes to exclude adjacent physiologic FDG-avid structures. The SUVmax was defined as the maximum voxel intensity within the volumetric region of interest. A threshold of 42% of the maximum signal intensity was used to delineate the MTV, defined as the total volume of the primary tumor, Fig. 1 [5].
Figure 1.
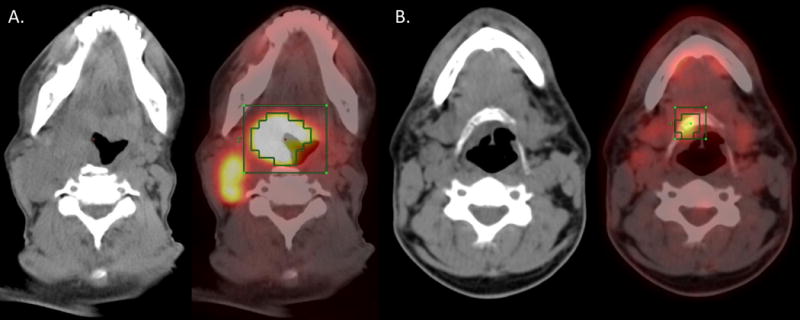
CT (left) and PET-CT fusion (right) in a patient with a large primary tumor MTV (A.) and a small primary tumor MTV (B.).
Gross tumor volume determination
Volumetric data was retrospectively collected from the original physician GTV contour of the primary tumor in restored clinical treatment plans with our in-house treatment-planning system. At the time of contouring, the GTV was based on all available radiographic imaging, clinical examination, and direct endoscopic examination, and did not include any areas of suspected microscopic disease or lymph nodes areas at risk.
Dose-painted IMRT
All patients in this study were treated with dose-painted IMRT. The guidelines for the determination and delineation of the clinical and nodal target volumes along with a detailed description of our dose-painted IMRT technique have been reported previously [6–8]. Briefly, patients received a median prescribed dose of 70 Gy to the planning target volume (PTV70), 59.4 Gy to the high-risk subclinical disease (PTV59.4), and 54 Gy to the lower risk subclinical disease (PTV54). The median dose per fraction was 2.12 Gy to PTV70, 1.8 Gy to PTV59.4, and 1.64 Gy to PTV54.
Chemotherapy
All patients were treated concurrently with platinum-based chemotherapy regimens. Patients received high-dose cisplatin alone, n=61 (61%), consisting of 100 mg/m2 (either as a single dose or split over 2 days) every 3 weeks for a planned 3 cycles; cisplatin and bevacizumab, n=16 (16%), administered at 100 mg/m2 (split over 2 days) and 15 mg/kg, respectively, every 3 weeks for a planned three cycles; cisplatin/paclitaxel, n=1 (1%), administered at 20 mg/m2 and 30 mg/m2, respectively, weekly; carboplatin and 5-flurouracil, n=19 (19%), administered at 70 mg/m2 daily for 4 days and 2400 mg/m2 as a 96-hour infusion, respectively, in three cycles every 3 weeks; or carboplatin and paclitaxel, n=3 (3%), administered at 70 mg/m2 and 50 mg/m2, respectively, weekly.
Follow-up and response assessment
All patients were seen by their treating physicians weekly during the course of treatment and in post-treatment follow-up visits jointly by radiation oncology, medical oncology, and head and neck surgery (planned for 4, 8, and 12 weeks after completion of treatment, then every 3 months for 2 years, followed by every 6 months thereafter). Post-treatment, the patients were evaluated with direct flexible fiber-optic endoscopic examinations along with post-treatment imaging studies (CT, PET/CT, magnetic resonance imaging). Recurrences were all verified with biopsy.
Statistical analysis
Locoregional failure, the development of distant metastasis, and death was recorded from the start of radiotherapy. Disease-free survival was defined as those patients rendered disease free and alive at last follow-up. The LRC, freedom from distant metastasis (FDM), progression-free survival (PFS), and overall survival (OS) rates were calculated by the Kaplan-Meier method by the log-rank test with GTV, SUVmax, and MTV dichotomized by median value [9].
Univariate hazard ratios (HR) with 95% confidence intervals (CI) were computed using Cox proportional hazards were generated [10]. Multivariate analyses were performed using Cox regression modeling. Age at diagnosis (years), Karnofsky Performance Status (KPS), GTV, SUVmax, and MTV were analyzed as continuous variables, whereas gender (male versus female), smoking history (smokers versus nonsmokers), tumor stage (T1 versus T2 versus T3 versus T4), nodal stage (N0 versus N1 versus N2 versus N3), tumor site (tonsil versus base of tongue versus others) were analyzed as categorical variables. HRs reflect a change of 10 for GTV (cm3), SUVmax, and MTV (cm3). A probability value of less than 0.05 was considered statistically significant for all analyses. All analyses were performed in SPSS statistics version 21 (IBM, USA).
Results
Patient and tumor characteristics
One hundred patients met the criteria for study inclusion (Table 1). The median age was 56 years with an overall follow-up of 55.8 months for surviving patients and 49.0 months for all patients. Male patients comprised 86% of the study participants. Tumor sites included tonsil (n=42; 42%), base of tongue (n=53; 53%), soft palate (n=3; 3%), and pharyngeal wall (n=2; 2%). Locally advanced disease comprised 47% of patients, with N2/N3 disease in 84%.
Table 1.
Patient, tumor and treatment characteristics.
| Variable | Median (range) |
|---|---|
| Age (years) | 56.0 (27–81) |
| Overall follow-up (months)a | 55.8 (8.1–95.5) |
| Variable | n (column percent) |
|
| |
| Sex | |
| Male | 86 (86.0%) |
| Female | 14 (14.0%) |
| Tumor site | |
| Tonsil | 42 (42.0%) |
| Base of tongue | 53 (53.0%) |
| Other | 5 (5.0%) |
| Tumor Stage | |
| T1 | 14 (14.0%) |
| T2 | 39 (39.0%) |
| T3 | 23 (23.0%) |
| T4 | 24 (24.0%) |
| Nodal Stage | |
| N0 | 4 (4.0%) |
| N1 | 12 (12.0%) |
| N2 | 80 (80.0%) |
| N3 | 4 (4.0%) |
| KPSb | |
| 100–90 | 79 (84.9%) |
| ≤80 | 14 (15.1%) |
| Smoking history | |
| No | 28 (28.0%) |
| Yes | 72 (72.0%) |
| Chemotherapy regimen | |
| Cisplatin alone | 61 (61.0%) |
| Cisplatin + bevacizumab | 16 (16.0%) |
| Cisplatin + paclitaxel | 1 (1/0%) |
| Carboplatinum + 5-fluorouracil | 19 (19.0%) |
| Carboplatinum + paclitaxel | 3 (3.0%) |
Abbreviation: KPS, Karnofsky Performance Status.
Among living patients.
N=93 patients.
Disease control and patterns of failure
Locoregional recurrence occurred in 7 patients with a median time to failure of 6.5 months. Distant metastases were noted in 19 patients with a median time to progression of 8.8 months. The estimated 5-year actuarial LRC rate was 92.6% and the 5-year FDM rate was 78.6%. Overall, 28 patients died, with a median time to death of 16.4 months. The estimated 5-year actuarial OS rate was 70.9%.
Univariate analyses
Tumor stage
A more advanced tumor stage correlated with an increased risk for distant metastases (HR, 1.68; 95% CI, 1.05–2.68; p = .032), disease progression or death (HR, 1.49; 95% CI, 1.0–2.13; p = .031), and death (HR, 1.66; 95% CI, 1.13–2.45; p = .010), but no correlation was seen with locoregional failure (HR, 1.60; 95% CI, 0.74–3.48; p = .234).
GTV
A larger GTV demonstrated a statistically significant increased risk of distant metastasis (HR, 1.10; 95% CI, 1.01–1.20; p = .027) and death (HR, 1.08; 95% CI, 1.00–1.17; p = .048) (Table 2).
TABLE 2.
Univariate association between gross tumor volume (GTV), maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), and clinical outcomes.
| Variable | Locoregional Failure | Distant Metastases | Disease Progression or Death | Death | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | HR 95% CI | p | HR | HR 95% CI | p | HR | HR 95% CI | P | HR | HR 95% CI | p | |
| Tumor stage | 1.60 | 0.74–3.48 | 0.234 | 1.68 | 1.05–2.68 | 0.032 | 1.49 | 1.04–2.13 | 0.031 | 1.66 | 1.13–2.45 | 0.010 |
| Nodal stage | 0.52 | 0.19–1.44 | 0.209 | 1.99 | 0.75–5.27 | 0.168 | 1.27 | 0.635–2.54 | 0.499 | 1.65 | 0.74–3.70 | 0.225 |
| GTV | 1.12 | 0.97–1.27 | 0.148 | 1.10 | 1.01–1.20 | 0.027 | 1.07 | 1.00–1.16 | 0.056 | 1.08 | 1.00–1.17 | 0.048 |
| SUVmax | 1.07 | 0.33–3.49 | 0.917 | 1.32 | 0.66–2.65 | 0.431 | 0.97 | 0.56–1.68 | 0.908 | 1.14 | 0.64–2.05 | 0.660 |
| MTV | 2.35 | 1.42–3.88 | 0.001 | 1.98 | 1.43–2.74 | <0.001 | 1.75 | 1.33–2.30 | <0.001 | 1.85 | 1.37–2.50 | <0.001 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Tumor and nodal stage were analyzed as categorical variables, while GTV, SUVmax, and MTV were analyzed as continuous variables.
Hazard ratios reflect a change of 10 for GTV (cm3), SUVmax, and MTV (cm3).
When dichotomized by median value (40.7 cm3), patients with a GTV less than the median had significantly improved 5-year actuarial rates of PFS (80.3% versus 57.0%, p = .011) and OS (82.1% versus 60.1%, p = .025) compared with patients with a GTV greater than the median (Fig. 2a–d).
Figure 2.
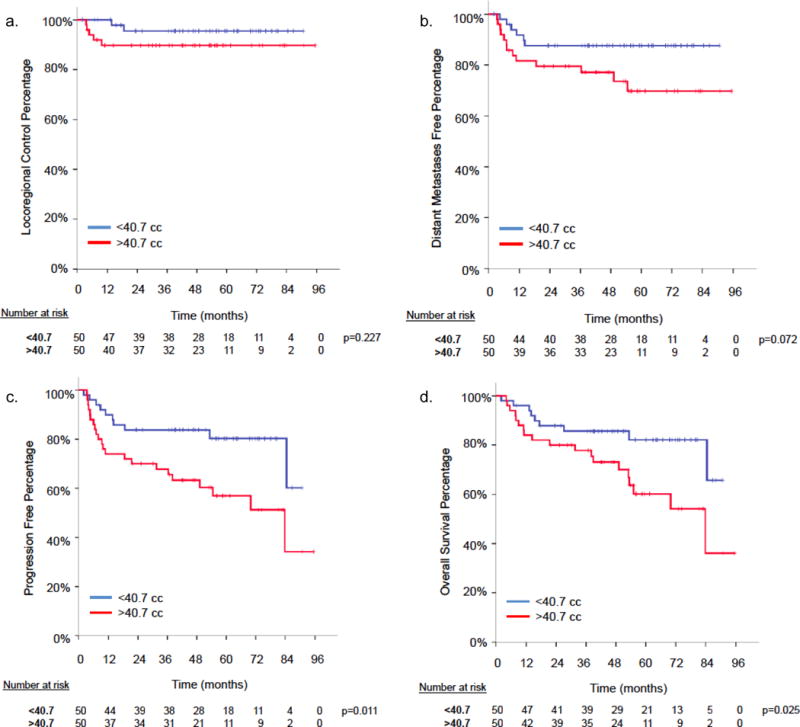
Gross tumor volume (GTV) Kaplan Meir curves for (a) Locoregional control (LRC), (b) freedom from distant metastasis, (c) progression-free survival, (d) overall survival.
SUVmax
On univariate analysis, SUVmax failed to correlate with locoregional failure, DM, DPD, and death as a continuous variable (Table 2). When dichotomized by the median SUVmax (13.1), there was no significant correlation with LRC, FDM, PFS, or OS.
MTV
A larger MTV was associated with a statistically significant increased risk of locoregional failure (HR, 2.35; 95% CI, 1.42–3.88; p = 0.001), distant metastases (HR, 1.98; 95% CI, 1.43–2.74; p < .001), disease progression or death (HR, 1.75; 95% CI, 1.33–2.30; p < .001), and death (HR, 1.85; 95% CI, 1.37–2.50; p < .001) on univariate analysis (Table 2).
When dichotomized by median value (9.7 cm3), patients with a MTV less than the median had significantly improved 5-year actuarial rates of LRC (98.0% versus 87.0%, p = .049), FDM (91.7% versus 65.0%, p = .005), PFS (80.3% versus 56.7%, p = .015), and OS (84.1% versus 57.8%, p = .008) compared with patients with a MTV greater than the median (Fig. 3a–d).
Figure 3.
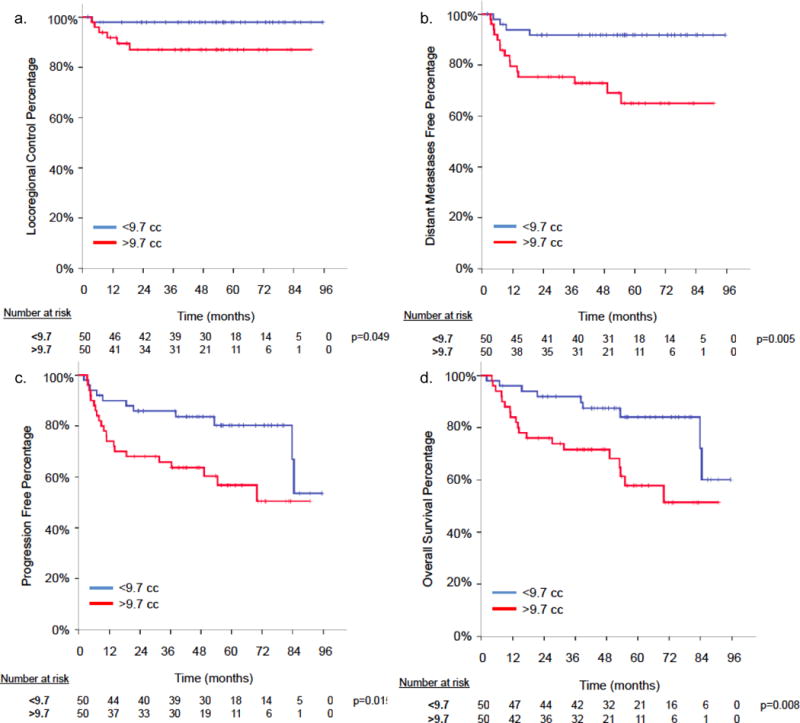
Metabolic tumor volume (MTV) Kaplan Meir curves for (a) locoregional control (LRC), (b) freedom from distant metastasis, (c) progression-free survival, (d) overall survival.
Multivariate analysis
A multivariate analysis was performed to determine if the volumetric indices were independently associated with the development of distant metastases, progression of disease or death, and death (Table 3). Given the low number of events, locoregional failure was not included in this analysis. SUVmax was excluded, as it was deemed non-significant in the univariate model.
Table 3.
Multivariate analysis of the association between tumor stage, gross tumor volume (GTV), and metabolic tumor volume (MTV).
| Variable | Distant Metastases | Disease Progression or Death | Death | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| HR | HR 95% CI | p | HR | HR 95% CI | p | HR | HR 95% CI | p | |
| Tumor stage | 0.99 | 0.52–1.88 | 0.982 | 1.05 | 0.65–1.68 | 0.850 | 1.16 | 0.70–1.94 | 0.565 |
| GTV | 0.95 | 0.81–1.10 | 0.480 | 0.94 | 0.82–1.07 | 0.314 | 0.92 | 0.80–1.06 | 0.241 |
| MTV | 2.47 | 1.46–4.17 | 0.001 | 2.17 | 1.40–3.38 | 0.001 | 2.37 | 1.44–3.89 | 0.001 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
When tumor stage, GTV, and MTV were included in the model, a larger MTV retained a significant correlation with an increased risk for distant metastasis (HR, 2.47; 95% CI, 1.46–4.17; p = .001), disease progression or death (HR, 2.17; 95% CI, 1.40–3.38; p = .001), and death (HR, 2.37; 95% CI, 1.44–3.89; p = .001). Conversely, tumor stage and GTV failed to correlate with DM, DPD, and death in this model.
MTV was confirmed to retain significance when adjusted individually for age at diagnosis, sex, smoking status, KPS, primary tumor site, nodal stage, SUVmax, and GTV for distant metastasis, disease progression or death, and death.
Discussion
This study compared the relative prognostic utility of GTV, SUVmax, and MTV in a uniform cohort of OPSCC patients treated with definitive platinum-based chemotherapy administered concurrently with dose-painted IMRT. MTV demonstrated an increased HR compared with GTV and SUVmax for all outcomes including locoregional failure, distant metastases, disease progression or death, and death. Given the increased utilization of FDG-PET in the pretreatment workup for OPSCC, MTV represents a novel radiobiological marker that can be routinely and systematically calculated, allowing pretreatment stratification.
Previously, we demonstrated in a cohort of 340 OPSCC patients that a larger primary tumor volume (>32.79 cm3) correlated with local failure, development of distant metastases, and death [11]. Similarly, Romesser et al. demonstrated the primary tumor GTV to correlate with local, nodal, distant, and overall control as well as OS when dichotomized by the mean GTV (32.0 cm3) in a 42-patient head and neck cohort [12]. Our findings further corroborate those of others [13–15]. The GTV has not been incorporated into clinical algorithms secondary to difficulty in reproducibility and an absence of prospective data validating these predominately retrospective reports.
Controversy has surrounded SUVmax with multiple series reporting both for and against a correlation with outcomes in head and neck cancer [16]. A prospective trial conducted at MD Anderson Cancer Center evaluated this particular question and failed to demonstrate any significant clinical correlation between pre-radiotherapy PET-CT SUV parameters and treatment outcomes [17]. This is in agreement with our study, as we did not find a correlation with SUVmax and treatment outcomes.
From the onset, we sought to evaluate if MTV is simply a surrogate of GTV and SUVmax or if the metabolically active tumor volume represents a distinct radiobiological variable altogether. Herein we demonstrated that MTV correlated with locoregional and distant control as well as PFS and OS. MTV retained significance when adjusted in a multivariate model for tumor stage and GTV. Conversely, GTV lost significance when tumor stage and/or MTV were included in the model, and similarly tumor stage lost significance when GTV and/or MTV were included in the model. MTV further retained significant utility when individually adjusted for tumor site, KPS, age, gender, and smoking status. These data support the growing body of literature (Table 4) which suggests that MTV is an independent and robust prognostic metric in patients with OPSCC undergoing platinum-based concurrent chemoradiation [4, 16, 18–22].
TABLE 4.
Summary of metabolic tumor volume (MTV) studies in head and neck patients treated with radiotherapy.
| First Author, Year | N | Sites | Stage | % IMRT | % CCRT | MTV Delineation Method | Median MTV (cc) | MTV P value
|
SUVmax | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LRC | FDM | DFS | OS | |||||||||
| Chung, 2009 | 82 | NP, OP, HP | I, II, III, IV | Not stated | 90% | Fixed SUV 2.5 threshold | 39 | 0.03 | – | 0.04 | – | Not Predictive |
| La, 2009 | 85 | NP, OC, OP, HP, L, UP | II, III, IV | 89% | 100% | 50% threshold | 11.2 | – | <0.001 | – | <0.001 | Not Predictive |
| Deron, 2011 | 22a | OC, OP, HP, L | III, IV | 100% | 100% | 50% threshold | 31.3 | – | 0.04 | – | NS | Not Predictive |
| Romesser, 2012 | 41 | NP, OC, OP, HP, L | I, II, III, IV | 100% | 89% | Gradient dependent | 7.2 | 0.0003 | 0.002 | 0.001 | 0.04 | Not Predictive |
| Lim, 2012 | 176 | OP | I, II, III, IV | 100% | 100% | 42% threshold | 9.7 | 0.005 | <0.001 | – | <0.001 | OS |
| Tang, 2012 | 83 | NP, OC, OP, HP, L, UP | I, II, III, IVb | 95% | 96% | 50% threshold | 10.5 | 0.014 | 0.023 | 0.0002 | 0.005 | Distant metastasis |
| Picchio, 2014 | 19 | OP, NP, L | I, II, III, IV | 100% | 84% | 40% threshold | 21.1c | <0.01 | <0.05 | <0.025 | <0.005 | Not Predictive |
| Current study | 100 | OP | I, II, III, IV | 100% | 100% | 42% threshold | 9.7 | 0.049 | 0.005 | 0.015 | 0.008 | Not Predictive |
Abbreviations: CCRT, concurrent chemoradiation therapy; DFS, disease-free survival; FDM, freedom from distant metastasis; IMRT, intensity-modulated radiation therapy; LRC, locoregional control; OS, overall survival; SUV, standardized uptake value.
Reference limited to analysis on 22 non-surgical patients.
Deduced from TNM data.
Mean value.
HPV status has been demonstrated to be prognostic in oropharyngeal cancer and has been incorporated into pre-treatment risk-stratification algorithms [23–25]. The prognostic utility of MTV seems to be independent of HPV. Tang et al. demonstrated MTV’s ability to stratify patients within HPV-positive and HPV-negative cohorts [18]. Unfortunately, HPV status was defined in only 26% of our population (12% HPV negative, 14% HPV positive, 74% status unknown) preventing it from being included in our analysis. In lieu smoking status was used a surrogate. Reassuringly, there was no difference noted in median MTV between HPV-positive tumors, HPV-negative tumors, and HPV-indeterminate tumors (data not reported).
The quintessential radiobiological marker needs to be easily obtainable, generalizable, and widely interpretable. SUV is influenced by multiple factors including differences in imaging technique, injected FDG dose, incubation period, protocol, scanner, and reconstruction algorithm variation [26, 27]. Acknowledgement of the limitations of PET-CT led us to investigate the MTV calculated by utilizing a percentage threshold (in our case a 42% threshold), which allows each individual tumor scan to serve effectively as its own control. We expect this to improve inter-institutional volumetric measurements and overcome the lack of generalizability of SUV-based studies. When appropriately calculated, as in this case with easily adoptable and commercially available software, the metabolic tumor volume represents a reproducible and high-throughput radiobiological metric, which can be rapidly integrated into clinical algorithms. MTV may be able to risk stratify OPSCC patients allowing the identification of low-risk patients eligible for treatment de-escalation.
While this study demonstrated MTV as independently correlated with treatment outcome and patient survival in OPSCC, certain study limitations merit discussion. We attempted to limit retrospective bias by blinding both the nuclear medicine physician evaluating the pretreatment images to clinical outcomes and the radiation oncologist collecting clinical outcomes to radiological metrics until the time of analysis. We focused on a relatively homogeneous population of OPSCC patients who underwent concurrent platinum-based chemoradiation, but tumor site and chemotherapy variability remain. HPV data was only available in 26% of our population, preventing us from appropriately controlling for this known prognostic factor. MTV calculation was based on predefined thresholds; depending on the chosen threshold the actual volume may vary. Moreover, since volumetric calculation is based on the intensity of FDG uptake (only tumor regions with intensity greater than the threshold are included in the MTV), standardization of imaging protocols, in particular uptake time, is necessary. Lastly, limiting the inclusion criteria to patients who underwent a pretreatment PET-CT at our institution and SUV / MTV calculation by a single nuclear medicine physician raises the question of the generalizability of our findings.
Conclusions
Metabolic tumor volume is independently correlated with locoregional control, freedom from distant metastases, progression free survival, and overall survival in oropharyngeal patients undergoing platinum-based concurrent chemoradiation. The increasing utilization of FDG-PET, relative ease in defining MTV, and the robust correlation with treatment outcome is promising and should be validated in a prospective study with a rigorous standardized protocol for PET/CT imaging. It remains to be proven that this observed radiological correlation results from differences in cancer biology and correlative studies needed to explore the molecular mechanisms underlying these correlations.
HIGHLIGHTS.
The prognostic utility of 3 key radiobiological markers (GTV, SUVmax, MTV) in OPC SCC was evaluated
A smaller MTV correlates with improved outcomes and survival in OPC patients
MTV can be routinely and accurately delineated on pre-treatment PET-CT scans
De-intensification strategies can be considered in patients with a smaller MTV
Acknowledgments
Funding: None
Role of the funding source: None
Abbreviations
- OPC
oropharyngeal cancer
- SCC
squamous cell carcinoma
- GTV
gross tumor volume
- SUVmax
maximum standardized uptake value
- MTV
metabolic tumor volume
- PET-CT
positron emission tomography-computed tomography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the 54th Annual Meeting of the American Society for Radiation Oncology, October 28th–31st, 2012, Boston, Massachusetts, USA.
Conflict of interest statement
None declared
References
- 1.Setton J, Caria N, Romanyshyn J, Koutcher L, Wolden SL, Zelefsky MJ, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2012;82:291–8. doi: 10.1016/j.ijrobp.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 2.Rischin D. Oropharyngeal cancer, human papilloma virus, and clinical trials. J Clin Oncol. 2010;28:1–3. doi: 10.1200/JCO.2009.24.9045. [DOI] [PubMed] [Google Scholar]
- 3.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th. Chicago, IL: Springer; 2010. [Google Scholar]
- 4.Lim R, Eaton A, Lee NY, Setton J, Ohri N, Rao S, et al. 18F-FDG PET/CT metabolic tumor volume and total lesion glycolysis predict outcome in oropharyngeal squamous cell carcinoma. J Nucl Med. 2012;53:1506–13. doi: 10.2967/jnumed.111.101402. [DOI] [PubMed] [Google Scholar]
- 5.Erdi YE, Mawlawi O, Larson SM, Imbriaco M, Yeung H, Finn R, et al. Segmentation of lung lesion volume by adaptive positron emission tomography image thresholding. Cancer. 1997;80:2505–9. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2505::aid-cncr24>3.3.co;2-b. [DOI] [PubMed] [Google Scholar]
- 6.Lee N, Mechalakos J, Puri DR, Hunt M. Choosing an intensity-modulated radiation therapy technique in the treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2007;68:1299–309. doi: 10.1016/j.ijrobp.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 7.de Arruda FF, Puri DR, Zhung J, Narayana A, Wolden S, Hunt M, et al. Intensity-modulated radiation therapy for the treatment of oropharyngeal carcinoma: the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2006;64:363–73. doi: 10.1016/j.ijrobp.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Lee N, Lu J. Target Volume Delineation and Field Setup: A Practical Guide for Conformal and Intensity-Modulated Radiation Therapy. New York: Springer; 2013. [Google Scholar]
- 9.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:25. [Google Scholar]
- 10.Cox D. Regression models and life-tables. J Roy Stat Soc. 1972;34:34. [Google Scholar]
- 11.Lok BH, Setton J, Caria N, Romanyshyn J, Wolden SL, Zelefsky MJ, et al. Intensity-modulated radiation therapy in oropharyngeal carcinoma: effect of tumor volume on clinical outcomes. Int J Radiat Oncol Biol Phys. 2012;82:1851–7. doi: 10.1016/j.ijrobp.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romesser PB, Qureshi MM, Subramaniam RM, Sakai O, Jalisi S, Truong MT. A prognostic volumetric threshold of gross tumor volume in head and neck cancer patients treated with radiotherapy. Am J Clin Oncol. 2012 doi: 10.1097/COC.0b013e31826e04d6. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doweck I, Denys D, Robbins KT. Tumor volume predicts outcome for advanced head and neck cancer treated with targeted chemoradiotherapy. Laryngoscope. 2002;112:1742–9. doi: 10.1097/00005537-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Studer G, Lutolf UM, El-Bassiouni M, Rousson V, Glanzmann C. Volumetric staging (VS) is superior to TNM and AJCC staging in predicting outcome of head and neck cancer treated with IMRT. Acta Oncol. 2007;46:386–94. doi: 10.1080/02841860600815407. [DOI] [PubMed] [Google Scholar]
- 15.Strongin A, Yovino S, Taylor R, Wolf J, Cullen K, Zimrin A, et al. Primary tumor volume is an important predictor of clinical outcomes among patients with locally advanced squamous cell cancer of the head and neck treated with definitive chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:1823–30. doi: 10.1016/j.ijrobp.2010.10.053. [DOI] [PubMed] [Google Scholar]
- 16.Romesser PB, Qureshi MM, Shah BA, Chatburn LT, Jalisi S, Devaiah AK, et al. Superior prognostic utility of gross and metabolic tumor volume compared to standardized uptake value using PET/CT in head and neck squamous cell carcinoma patients treated with intensity-modulated radiotherapy. Ann Nucl Med. 2012;26:527–34. doi: 10.1007/s12149-012-0604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moeller BJ, Rana V, Cannon BA, Williams MD, Sturgis EM, Ginsberg LE, et al. Prospective risk-adjusted [18F]Fluorodeoxyglucose positron emission tomography and computed tomography assessment of radiation response in head and neck cancer. J Clin Oncol. 2009;27:2509–15. doi: 10.1200/JCO.2008.19.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang C, Murphy JD, Khong B, La TH, Kong C, Fischbein NJ, et al. Validation that metabolic tumor volume predicts outcome in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2012;83:1514–20. doi: 10.1016/j.ijrobp.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung MK, Jeong HS, Park SG, Jang JY, Son YI, Choi JY, et al. Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin Cancer Res. 2009;15:5861–8. doi: 10.1158/1078-0432.CCR-08-3290. [DOI] [PubMed] [Google Scholar]
- 20.La TH, Filion EJ, Turnbull BB, Chu JN, Lee P, Nguyen K, et al. Metabolic tumor volume predicts for recurrence and death in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2009;74:1335–41. doi: 10.1016/j.ijrobp.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deron P, Mertens K, Goethals I, Rottey S, Duprez F, De Neve W, et al. Metabolic tumour volume. Prognostic value in locally advanced squamous cell carcinoma of the head and neck. Nuklearmedizin. 2011;50:141–6. doi: 10.3413/Nukmed-0367-10-11. [DOI] [PubMed] [Google Scholar]
- 22.Picchio M, Kirienko M, Mapelli P, Dell’Oca I, Villa E, Gallivanone F, et al. Predictive value of pre-therapy (18)F-FDG PET/CT for the outcome of (18)F-FDG PET-guided radiotherapy in patients with head and neck cancer. Eur J Nucl Med Mol Imaging. 2014;41:21–31. doi: 10.1007/s00259-013-2528-2. [DOI] [PubMed] [Google Scholar]
- 23.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Sullivan B, Huang SH, Siu LL, Waldron J, Zhao H, Perez-Ordonez B, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31:543–50. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 25.Network NCC. NCCN Clinical Practice Guidelines in Oncology: Head and Neck Cancers. 2012 [Google Scholar]
- 26.Kinahan PE, Doot RK, Wanner-Roybal M, Bidaut LM, Armato SG, Meyer CR, et al. PET/CT Assessment of Response to Therapy: Tumor Change Measurement, Truth Data, and Error. Transl Oncol. 2009;2:223–30. doi: 10.1593/tlo.09223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fahey FH, Kinahan PE, Doot RK, Kocak M, Thurston H, Poussaint TY. Variability in PET quantitation within a multicenter consortium. Med Phys. 2010;37:3660–6. doi: 10.1118/1.3455705. [DOI] [PMC free article] [PubMed] [Google Scholar]


