Abstract
Transient lymphopenia is one hallmark of sepsis, and emergent data indicates the CD4 T cell compartment in sepsis survivors is numerically and functionally altered (when examined at the Ag-specific level) compared to non-septic controls. Previous data from our laboratory demonstrated Ag-independent, lymphopenia-induced homeostatic proliferation to be a contributing mechanism by which CD4 T cells numerically recover in sepsis survivors. However, we reasoned it is also formally possible that some CD4 T cells respond directly to Ag expressed by gut resident microbes released during polymicrobial sepsis. The effect of gut microbiome leakage on CD4 T cells is currently unknown. In this study, we explored the number and function of endogenous CD4 T cells specific for segmented filamentous bacterium (SFB) after cecal ligation and puncture (CLP)-induced sepsis using mice that either contained or lacked SFB as a normal gut resident microbe. Interestingly, SFB-specific CD4 T cells underwent Ag-driven proliferation in CLP-treated SFB+, but not in SFB−, mice. Moreover, CLP-treated SFB+ mice showed resistance to secondary lethal infection with recombinant SFB Ag-expressing virulent Listeria (but not wildtype virulent Listeria) suggesting the CLP-induced polymicrobial sepsis primed for a protective response by the SFB-specific CD4 T cells. Thus, our data demonstrate that the numerical recovery and functional responsiveness of Ag-specific CD4 T cells in sepsis survivors is, in part, modulated by the intestinal barrier’s “health” discreetly defined by individual bacterial populations of the host’s microbiome.
INTRODUCTION
Sepsis is a poorly understood syndrome of systemic inflammation responsible for thousands of deaths annually (1). One reason for the high mortality rate from sepsis is the profound acute lymphopenia seen in humans and mouse models, such as cecal ligation and puncture (CLP) (2). The numerical reduction of multiple immune cell populations in septic individuals, including naïve and memory T cells, is typically observed as lymphopenia followed by prolonged immune suppression (immunoparalysis (3)) that decreases the ability of the host to respond to secondary infections (4-9). Consequently, understanding the mechanism(s) the lead to the immunoparalysis and susceptibility to pathogens normally cleared by the immune system in healthy individuals (10) are essential in developing future therapies for the medical management of sepsis.
Using the mouse model of CLP-induced polymicrobial sepsis (11), we reported CD4 T cell recovery from CLP-induced lymphopenia occurs, in part, by Ag- and thymus-independent homeostatic proliferation (6). We also posited that some endogenous CD4 T cell populations respond directly to the antigenic epitopes within the proteins expressed by gut symbionts, leading to a difference in functional potential versus those CD4 T cells undergoing Ag-independent homeostatic proliferation. Advancements in the development and use of peptide:MHC II (p:MHC II) tetramers permit detection of endogenous Ag-specific CD4 T cell populations (12). Using p:MHC II tetramers containing an immunodominant epitope from a protein (SFBNYU_003340) present in the segmented filamentous bacteria (SFB) Candidatus arthromitus, we examined the quantitative and qualitative features of endogenous SFB-specific CD4 T cells after CLP in C57BL/6 (I-Ab) mice with (B6 Taconic; B6NTac) and without (B6 Jackson; B6J) SFB as one of the naturally occurring flora constituents. SFB-specific CD4 T cells in B6J mice underwent CLP-induced lymphopenia and eventually recovered, but the same SFB-specific CD4 T cell population displayed Ag-driven proliferation in CLP-treated B6NTac mice. This numerical difference correlated with functional differences after secondary infection with recombinant Listeria expressing the SFB Ag suggesting numerical recovery and functional responsiveness of CD4 T cells in sepsis survivors were, in part, controlled and modulated by the microbes constituting the normal gut flora.
MATERIALS and METHODS
Mice
C57BL/6 mice were purchased from The Jackson Laboratory (C57BL/6J; Bar Harbor, ME), and Taconic Farms (C57BL6/NTac; Hudson, NY). Procedures were approved by the Minneapolis VA Healthcare System IACUC.
Cecal ligation and puncture
Sepsis was induced by CLP (11). Briefly, mice were anesthetized and the abdomen was shaved, disinfected, and a midline incision was made. The distal third of the cecum was ligated and punctured once with a 25-g needle to extrude a small amount of cecal content. The cecum was returned to the abdomen, the peritoneum was closed via continuous suture, and the skin was sealed using surgical glue (Vetbond; 3M, St. Paul, MN). Saline (1 ml) was provided s.c. following the procedure for resuscitation, Bupivicaine was administered at the incision site, and Meloxicam (2 mg/kg i.p.) was administered three times for postoperative analgesia. Sham-treated mice underwent the same procedure excluding ligation and puncture.
LPS endotoxemia
A rodent model of endotoxemia (13) was induced by a single i.p. injection of LPS (10 mg/kg body weight; Sigma, St. Louis, MO). Body weight was measured daily for 7 d post injection to monitor morbidity.
Anti-CD4 mAb-induced depletion of CD4+ T cells
Mice were transiently depleted of CD4+ T cells with a single i.p. injection of the anti-CD4 mAb GK1.5 (25 μg). Control mice received an isotype control mAb. The frequency of CD4+ T cells in the peripheral blood was determined prior to mAb injection, as well as 3 and 33 d after injection by staining for CD45.2+CD3+CD4+ cells in a heparinized blood sample.
Analysis of Ag-specific CD4 T cells
I-Ab–specific tetramers containing SFB 3340-N5 (VSYTESSANPTP), 2W1S (EAWGALANWAVDSA), or L. monocytogenes LLO190-201 (NEKYAQAYPNVS) peptides were used to quantify Ag-specific CD4 T cells (12,14). Biotinylated monomers were made into tetramers with streptavidin (SA)- phycoerythrin (PE) or SA-allophycocyanin (APC; Prozyme, Hayward, CA) as previously described (12,14). The enriched cells were then stained with fluorochrome-labeled mAb to detect additional surface proteins. In some cases, the cells were fixed with PBS containing 2% paraformaldehyde after surface staining to permit intracellular staining. mAb were purchased from: BD Biosciences (San Diego, CA) - Horizon™ V500 Thy1.2 (clone 53-2.1); BioLegend (San Diego, CA) - Brilliant Violet™ (BV) 510 and FITC CD3 (clone 17A2), BV421 and BV605 CD4 (clone GK1.5), BV650 CD8 (clone 53-6.7), AlexaFluor®700 CD44 (clone IM7), and APC and BV650 IFNγ (clone XMG1.2); or eBioscience (San Diego, CA) - PerCP-Cy5.5 B220 (clone RA3-6B2), -CD11b (clone M1/70), -CD11c (clone N418), - F4/80 (clone BM8); FITC FoxP3 (clone FJK-15S), and FITC and PE-Cy7 CD11a (clone M17/4).
Listeria monocytogenes infection and titer
The plasmid containing the full-length SFBNYU_003340 Ag used to generate SFBNYU_003340-expressing, ActAΔ L. monocytogenes was obtained from Dr. Francis Alonzo III (Loyola University) (15). Mice were infected with att. Lm-SFB (107 CFU i.v./mouse (6)). Wild-type and full-length SFBNYU_003340 Ag-expressing virulent Lm strain 10403S-inlAm (5 × 103 CFU i.v./mouse (16)) were obtained from Dr. Dan Littman (New York University). Some mice infected with vir. Lm-SFB were first depleted of CD4+ T cells by injecting the anti-CD4 mAb GK1.5 (3 daily injections of 100 μg; (17)). As a measure of bacterial clearance, livers were removed 5 d post-infection, placed in 0.2% IGEPAL solution (Sigma, St. Louis, MO), and homogenized. Serial dilutions were plated on tryptic soy broth agar containing 50 μg/ml streptomycin. Bacterial colonies were counted after 24 h incubation at 37°C (8).
Co-housing
B6J and B6NTac mice were co-housed for 4 weeks on arrival to permit horizontal transfer of SFB. The presence of SFB was confirmed by PCR (18).
Statistical analyses
Data shown are presented as mean values ± SEM. GraphPad Prism 6 was used for statistical analysis. Statistical tests performed are indicated in figure legends: * p < 0.05, ** p < 0.01, *** p < 0.005, **** p < 0.001.
RESULTS
Host colonization with SFB influences endogenous SFB-specific CD4 T cell response after CLP
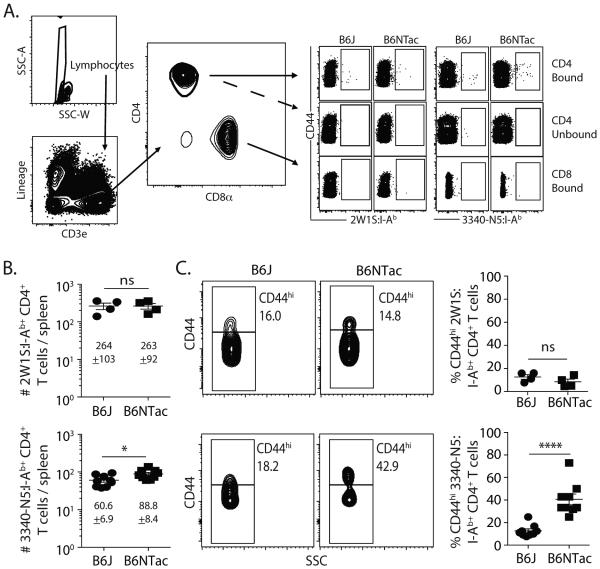
Transient lymphopenia is one hallmark of a septic event (19), and most preclinical and clinical studies have monitored CLP-induced lymphopenia at the level of bulk immune cell populations. We recently showed the importance of examining endogenous Ag-specific T cell populations in septic mice using p:MHC II tetramer enrichment (6). These studies examined different CD4 T cell populations specific for pathogen-derived antigenic epitopes normally absent in conventional “specific pathogen free” housing, such as HSV glycoprotein D (gD)290, influenza nucleoprotein (NP)311, LCMV glycoprotein (gp)66, and L. monocytogenes LLO190. In this study, we combined the use of SFB colonization differences in B6 mice (16,18) with our ability to quantitate total numbers of all CD4 T cells and SFB-specific CD4 T cells after sham or CLP surgery to examine the extent to which an Ag-specific CD4 T cell population specific for a gut resident microbe was affected by CLP-induced sepsis. When analyzing naïve B6J (SFB−) and B6NTac (SFB+) mice (Figure 1A), we saw a small, but significant, difference in the average number of CD4 T cells in the spleen specific for the N5 epitope from SFBNYU_003340 (Figure 1B). The number of CD4 T cells specific for the 2W1S variant of peptide 52-68 from the I-E α chain (14,20) in naïve B6J and B6NTac mice, by comparison, was nearly identical. Moreover, the frequency of CD44hi SFB-specific CD4 T cells in the naïve B6NTac mice was dramatically higher (Figure 1C). The frequency of CD44hi 2W1S-specific CD4 T cells was equivalently low in both B6J and B6NTac mice. These data are consistent with published data showing how the presence of SFB within the gut can influence the “Ag-experienced” state of SFB-specific CD4 T cells in naïve B6 mice obtained from different commercial sources (16,18).
Figure 1.
Naïve B6J and B6NTac mice differ in number of SFB-specific CD4 T cells and frequency of CD44hi SFB-specific CD4 T cells. (A) Representative flow plots show the gating strategy used in tetramer-enriched cell fractions to detect Ag-specific CD4 T cell populations. Shown are examples used to detect 2W1S:I-Ab- and 3340-N5:I-Ab-specific CD4+ T cells. Gating for p:I-Ab-specific cells was determined using CD8+ T cells as an internal negative control for tetramer binding. (B) Number of 2W1S:I-Ab- and 3340-N5:I-Ab-specific CD4 T cells and (C) frequency of CD44hi 2W1S:I-Ab- and 3340-N5:I-Ab-specific CD4 T cells in the spleens of naïve B6J and B6NTac mice. Statistical significance was determined using group-wise, one-way ANOVA with multiple-testing correction using the Holm-Sidak method, and α = 0.05. Data shown are from 9 mice/group.
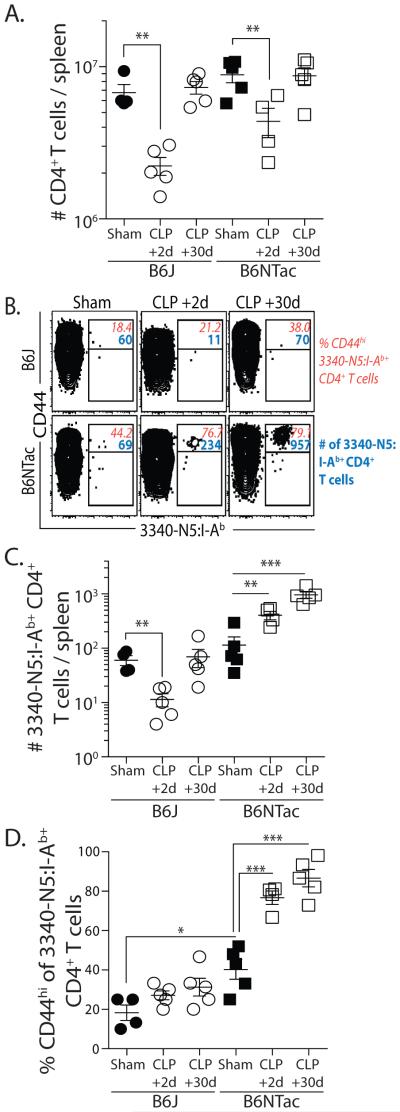
To determine the extent to which SFB gut colonization affected the SFB-specific CD4 T cells after CLP, we determined the number of total and SFB-specific CD4 T cell in the spleens of sham- and CLP-treated B6J and B6NTac mice. There was a similar decrease in total number of CD4 T cells 2 d after CLP surgery that recovered to sham levels by d 30 in both B6J and B6NTac mice (Figure 2A); however, the SFB-specific CD4 T cells showed strikingly different patterns. The SFB-specific CD4 T cells declined in B6J mice 2 d post-CLP, only to recover by d 30 (Figure 2B-C), much like total CD4 T cells. In contrast, there was an immediate increase in SFB-specific CD4 T cells in B6NTac mice 2 d after CLP that remained elevated on d 30. We also determined the frequency of CD44hi SFB-specific CD4 T cells in B6J and B6NTac mice after surgery. There was a marginal, but insignificant, increase in frequency of CD44hi SFB-specific CD4 T cells in CLP-treated B6J mice 30 d after CLP surgery compared to sham-treated mice that can likely be explained by homeostatic proliferation during the post-CLP recovery phase (6), but there was a dramatic increase in frequency of CD44hi SFB-specific CD4 T cells in B6NTac mice as early as d 2 after CLP that remained high after 30 d suggesting that CLP-induced leakage of SFB might lead to Ag-specific recognition and activation of endogenous SFB-specific CD4 T cells.
Figure 2.
Differential response of SFB-specific CD4 T cells in CLP-treated B6J and B6NTac mice. (A) Number of total CD4 T cells in spleens of B6J and B6NTac mice on d 2 and recovery by d 30 after CLP. (B) Representative plots show the number of SFB-specific CD4 T cells and frequency of CD44hi SFB-specific CD4 T cells in sham- and CLP-treated B6J and B6NTac mice. (C-D) Number of SFB-specific CD4 T cells and frequency of CD44hi SFB-specific CD4 T cells in sham- and CLP-treated B6J and B6NTac mice 2 or 30 d after surgery. Statistical significance was determined using group-wise, one-way ANOVA with multiple-testing correction using the Holm-Sidak method, and α = 0.05. Data shown represent 2 independent experiments, with 4-5 mice/group in each experiment.
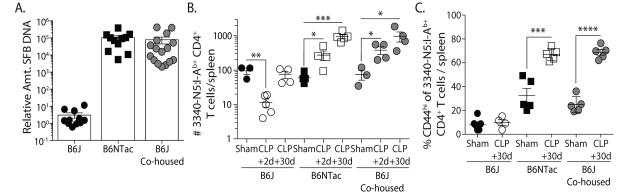
To formally confirm that SFB colonization dictated the behavior of the SFB-specific CD4 T cells after CLP, we co-housed B6J and B6NTac mice for 4 weeks to permit horizontal SFB transfer (18) (Figure 3A). The SFB-specific CD4 T cells in co-housed B6J mice now increased after CLP, following a pattern similar to that seen in SFB+ B6NTac mice (Figure 3B), instead of undergoing the CLP-induced loss and recovery seen in housing-restricted B6J mice. Moreover, the frequency of CD44hi SFB-specific CD4 T cells in sham- and CLP-treated co-housed B6J mice increased as in B6NTac mice (Figure 3C). Together, these data show the profound effect the presence of SFB in the gut has on the number and phenotype of SFB-specific CD4 T cells in CLP-treated mice and suggest that gut resident microbes can influence the numerical recovery of particular Ag-specific CD4 T cells and contribute to the overall changes in composition of the CD4 T cell pool observed in sepsis survivors.
Figure 3.
Horizontal transfer of SFB by co-housing permits the expansion of SFB-specific CD4 T cells in B6J mice after CLP. B6J and B6NTac mice were housed separately or co-housed for 4 weeks. (A) The presence of SFB in fecal pellets in individual mice was determined by PCR prior to sham or CLP surgery. Each symbol represents an individual mouse. (B-C) The number of SFB-specific CD4 T cells and frequency of CD44hi SFB-specific CD4 T cells in the spleens of B6J, B6NTac, and co-housed B6J mice on d 2 and 30 after CLP surgery. Statistical significance was determined using group-wise, one-way ANOVA with multiple-testing correction using the Holm-Sidak method, and α = 0.05. Data shown are the representative from at least 2 independent experiments per population analyzed, with 4-5 mice/group in each experiment.
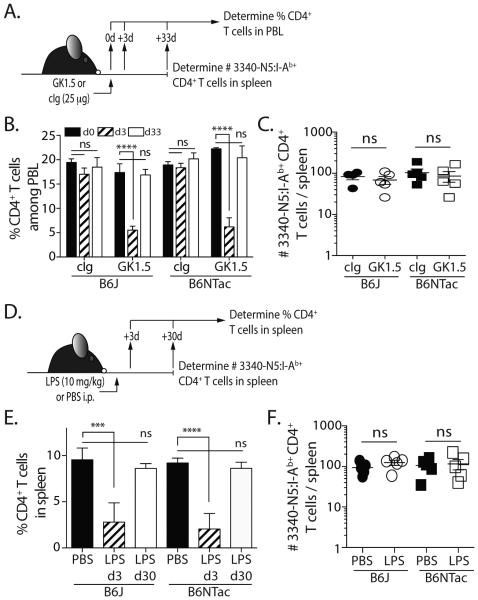
A transient reduction in CD4 T cells in SFB colonized mice is not sufficient to drive enhanced SFB-specific CD4 T cell recovery
Based on our findings, we hypothesized that the release of SFB into the peritoneum from the confined intestinal space during the CLP procedure was required to drive the expansion of the SFB-specific CD4 T cells in the SFB+ B6NTac and co-housed B6J mice. Thus, we tested this hypothesis using two different methods of transiently reducing the number of CD4 T cells without CLP and bacteria release. First, we injected a low dose (25 μg) of the anti-CD4 depleting mAb GK1.5 or control Ig and measured CD4 T cell frequencies in the blood at times similar to those used in the CLP sepsis model (Figure 4A). When looking at the bulk CD4 T cells in the circulation, the kinetics of CD4 T cell loss and recovery after a single GK1.5 injection were similar to that seen during CLP (Figure 4B). However, when we quantitated the number of SFB-specific CD4 T cells in the spleen on d33 we found no difference in the number of cells in control Ig- and GK1.5-treated B6J and B6NTac mice (Figure 4C). Similar data were found in B6J and B6NTac mice in a model of LPS endotoxemia (13). Specifically, both LPS-treated B6J and B6NTac mice experienced transient lymphopenia marked by the reduced frequency of CD4 T cells in the spleen on d 3 that recovered by d 30, but there was no increase in number of SFB-specific CD4 T cells in the B6NTac mice (Figure 4D-F). Together, these data support the conclusion that bacterial release from the perforated cecum during CLP is needed to drive the expansion of SFB-specific CD4 T cells.
Figure 4.
Transient reduction of CD4 T cells without bacteremia is not sufficient to drive SFB-specific CD4 T cell expansion in B6NTac mice. (A) B6J and B6NTac mice were injected with 25 μg of the anti-CD4 depleting mAb GK1.5 or an isotype control mAb. Mice were bled before mAb injection and on d 3 and 33 after injection. (B) The frequency of CD45.2+CD3+CD4+ T cells in the blood was determined. (C) On d 33, the number of SFB-specific CD4 T cells in the spleens was determined by tetramer enrichment and flow cytometry. (D) B6J and B6NTac mice were injected with 10 mg/kg LPS or PBS. (E) Spleens were collected on d3 and 30 to measure the frequency of CD45.2+CD3+CD4+ T cells. (F) On d 30, the number of SFB-specific CD4 T cells in the spleens was determined by tetramer enrichment and flow cytometry.
SFB colonization influences the proliferative and functional capacity of SFB-specific CD4 T cells after sepsis
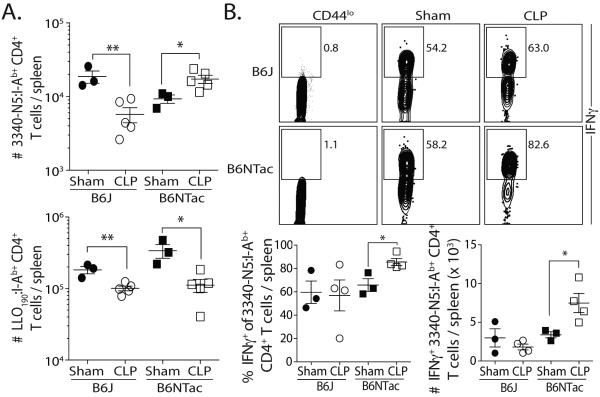
Based on the data showing differential responses of SFB-specific CD4 T cells in CLP-treated mice depending on their SFB colonization status, we examined the extent to which CLP-induced sepsis influenced SFB-specific CD4 T cell function. B6J and B6NTac mice were challenged with attenuated Listeria monocytogenes expressing 3340-N5 from SFB (att. Lm-SFB) 30 d after sham or CLP surgery. We then calculated the number of SFB-specific CD4 T cells 7 d later and found the SFB-specific CD4 T cell response was decreased in CLP-treated B6J mice compared to sham-treated mice, but increased in CLP-treated B6NTac mice (Figure 5A). We simultaneously determined the number of LLO190-specific CD4 T cells from the same infected B6J and B6NTac mice because LLO190-specific CD4 T cells do not numerically recover after CLP (6), LLO190-specific CD4 T cells are not cross-reactive with gut flora, and the number of naïve CD4 T cell precursors closely correlates with the magnitude of the peak CD4 T cell response after priming (14). Interestingly, the LLO190-specific CD4 T cells in CLP-treated B6J and B6NTac mice showed a similar reduction in proliferative capacity versus similarly infected sham-treated mice. These data suggest the importance of the Ag expressed by the gut resident microbes released during CLP on the proliferative capacity of different Ag-specific CD4 T cell populations in response to secondary challenge.
Figure 5.
SFB colonization changes the function of SFB-specific CD4 T cells in sepsis survivors. (A-B) B6J and B6NTac mice were infected with att. Lm-SFB (107 CFU in 0.1 ml i.v.) 30 d after sham or CLP surgery. (A) Proliferative capacity – The number of LLO190- and SFB-specific CD4 T cells was determined in the spleen 7 d after infection. (B) Functional capacity – 7 d after infection, splenocytes were stimulated for 4 h with PMA/ionomycin. The samples were enriched for SFB-specific CD4 T cells and IFNγ production was determined. Representative flow plots show the frequency of SFB-specific CD4 T cells producing IFNγ. The frequency and number of IFNγ+ SFB-specific CD4 T cells are graphed. Statistical significance in A-B was determined using group-wise, one-way ANOVA analyses followed by multiple-testing correction using the Holm-Sidak method, with α = 0.05. Data shown represent 2 independent experiments, with 3-5 mice/group in each experiment.
We next examined the effect of CLP on the functional capacity of the SFB-specific CD4 T cells. Sham- and CLP-treated B6J and B6NTac mice were challenged with att. Lm-SFB 30 d after surgery, and the ability of the SFB-specific CD4 T cells to produce cytokine was defined using in vitro PMA/ionomycin stimulation followed by tetramer enrichment and intracellular cytokine staining (21). IFNγ-producing SFB-specific CD4 T cells were present at a higher frequency and number in CLP-treated B6NTac mice compared to sham-treated mice, but there was no increase in B6J mice (Figure 5B). These data show the increased proliferative capacity and cytokine production of SFB-specific CD4 T cells in CLP-treated B6NTac mice, unlike other Ag-specific populations we have tested in this model (6), suggesting the exposure to SFB Ag during the septic event primes the SFB-specific CD4 T cells to develop into memory CD4 T cells and acquire enhanced function after a second antigenic encounter.
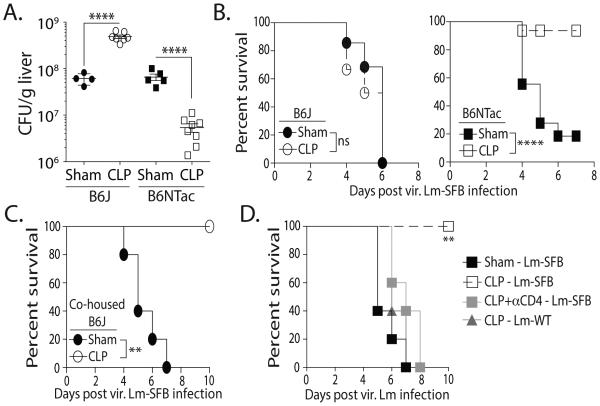
The data presented so far show that gut microbes can influence the quantitative and qualitative attributes of a CD4 T cell response after sepsis. To test if the increase in number and function of SFB-specific CD4 T cells in sepsis survivors can provide a measurable difference in response to pathogen challenge, sham- and CLP-treated B6J and B6NTac mice were infected with virulent L.monocytogenes expressing the 3340-N5 epitope (vir. Lm-SFB) 30 d after surgery. When measured 5 d after infection, the sham-treated B6J and B6NTac mice had similar pathogen burden (CFU) in the liver (Figure 6A). Interestingly, there was an increase in CFU/g liver tissue in CLP-treated B6J mice after vir. Lm-SFB infection, but a decrease in CLP-treated B6NTac mice. These CFU differences correlated with striking differences in survival, as CLP-treated B6NTac mice showed increased survival compared to infected sham-treated mice (Figure 6B). There was no difference in survival in infected sham- and CLP-treated B6J mice. We also challenged B6J mice that had acquired SFB through co-housing with B6NTac mice. Unlike the housing restricted SFB− B6J mice, the CLP-treated SFB+ co-housed B6J mice were now resistant to the vir. Lm-SFB infection (Figure 6C). While the importance of CD8 T cells in controlling and eradicating intracellular pathogens, such as L. monocytogenes, is well documented (22), Listeria-specific CD4 T cells can also provide protection even in the absence of CD8 T cells (23-25). To confirm the SFB-specific CD4 T cells in CLP-treated B6NTac mice were required for the observed survival advantage, CLP-treated B6NTac mice were either depleted of CD4 T cells before vir. Lm-SFB infection or infected with wild-type vir. Lm (Lm-WT) that lacked expression of the SFB Ag. Both of these groups of CLP-treated mice were similarly susceptible to the vir. Lm infection as sham-treated B6NTac mice (Figure 6D). These data further define the influence of the gut microbiota on the response of Ag-specific CD4 T cells in CLP-treated mice. In summary, data presented here suggest the composition of the gut microbiome has the capacity to shape the CD4 T cell compartment by changing the quantity and quality of Ag-specific CD4 T cells, a notion that should be taken into consideration when designing therapies aimed at T cell recovery in sepsis survivors.
Figure 6.
SFB colonization influences the protective capacity of SFB-specific CD4 T cells in sepsis survivors to secondary infection. (A-B) B6J and B6NTac mice were infected with vir. Lm-SFB (5 × 103 CFU in 0.1 ml i.v.) 30 d after sham or CLP surgery. (A) Mice were sacrificed on d 5 post-infection to determine Listeria burden in the liver, while other cohorts (B) were monitored for survival. (C) Additional cohorts of B6J mice co-housed with B6NTac mice for 4 weeks before sham or CLP surgery were similarly infected with vir. Lm-SFB 30 d after surgery. (D) B6NTac mice were infected with vir. wild-type Lm (Lm-WT) or Lm-SFB (5 × 103 CFU in 0.1 ml i.v.) 30 d after sham or CLP surgery. Some of the CLP-treated mice were depleted of CD4 T cells using the GK1.5 mAb (3 daily injection of 100 μg i.p.) immediately before infection. Survival was monitored. Statistics shown were obtained using Mann-Whitney U test statistics (for B) and one-way ANOVA analyses (for A, C, and D) with multiple-testing correction using the Holm-Sidak method, and α = 0.05. Survival data are cumulative from 3 independent experiments with 5 mice/group in each experiment.
DISCUSSION
Advances in our understanding of gut-initiated or -propagated critical illness have led to studies into how microbial membership and gut function may be targeted for therapeutic gain in the ICU (26). However, the interplay between microbiome composition and commensal-specific T cell responses remains relatively unexplored within the context of polymicrobial sepsis. Due to the importance of CD4 T cells in protection and memory against pathogens, understanding the role of microbiota on T cell responses to infection is crucial. It was assumed the intestinal commensals played a detrimental role in promoting systemic inflammation or infection in critically ill septic patients (27), where suppression of T cell immunity (6-9) and apoptosis of epithelial barriers (28) are recognized complications. With this in mind, we performed quantitative and qualitative analyses of CD4 T cell populations specific for Ag expressed by gut resident microbes in septic mice. We show the constituents of the gut flora play an important role in defining the responsiveness of such a CD4 T cell population after CLP-induced sepsis.
T cell responsiveness to Ag expressed by gut commensal bacteria is becoming more appreciated largely through the identification of defined T cell epitopes within the proteins of specific bacterial species. Knowledge that bacteria, as well as other microorganisms, live in symbiotic and (in some cases) pathogenic relationships with humans has been known since the times of Leeuwenhoek, and many normal host functions require the presence of gut microbiota. The development and implementation of germ free mice into a variety of immunological assays, along with studies showing diet and antibiotic usage alter the composition of the gut microbiome, have cemented the concept of commensal bacteria playing a vital role in shaping the immune response to infectious pathogens and tumors (29-31). The data presented herein add to this growing body of research, with the important addition that we included an analysis of CD4 T cells specific for the immunodominant epitope within the SFBNYU_003340 Ag of the segmented filamentous bacteria (SFB) C. arthromitus (16). Inclusion of reagents to identify these SFB-specific CD4 T cells and test their function were essential in revealing the differences in CLP-treated B6J and B6NTac mice. The majority of studies examining sepsis-induced modulation of T cell immunity have done so at the bulk population level, which can make it difficult to see subtle, yet significant, differences that occur at the Ag-specific T cell level. This can be best exemplified by data in Figure 2 showing similar numerical dynamics of bulk CD4 T cells in CLP-treated B6J and B6NTac mice, but the starkly different results when examining the SFB-specific CD4 T cell population. We realize the benefits of examining Ag-specific T cell populations is presently limited by the tools available, and may even be non-existent for some of the clinically relevant pathogens that commonly infect sepsis patients. Regardless, we strongly believe there is tremendous benefit to extending the analyses of immune system fitness, especially T cells, to the level of endogenous Ag-specific T cells, which permit a much higher resolution of specificity and sensitivity that is lost in the “noise” of bulk T cell analysis.
Another important point to stress is that the expansion of the SFB-specific CD4 T cells in SFB+ mice required the release of gut bacteria into the peritoneum. Bacteria in the blood is a common indicator of sepsis (32), and we can detect bacteria in the blood of both B6J and B6NTac mice after CLP (data not shown) – but this technique does not distinguish between bacterial species. SFB is the best-studied (and likely first) example of a commensal bacterial species that modulates host adaptive immune cell homeostasis (33); however, SFB is an anaerobic bacterium with no known culturing methods. Previous studies demonstrating SFB colonization in mice have done so by determining the presence of SFB 16S rRNA in the feces by quantitative PCR (18). Our data in Figure 3 are consistent with published data indicating B6J mice lack SFB, B6NTac mice contain SFB, and SFB can be transferred horizontally between these mice by co-housing. The selective expansion of the SFB-specific CD4 T cells in the CLP-treated B6NTac mice and co-housed B6J mice (but not housing restricted B6J mice) strongly, however, suggest the release from SFB from the punctured cecum. Additional support for the need to have SFB release into the peritoneum to drive the expansion of SFB-specific CD4 T cells was provided by the data generated using mice given anti-CD4 mAb to transiently deplete these cells or LPS to induce endotoxemia (Figure 4). Neither of these experimental settings led to the expansion of SFB-specific CD4 T cells in SFB+ B6NTac mice.
We chose to focus on SFB and SFB-specific CD4 T cells in the present study because of the reagents available that allowed us to track the number, phenotype, and function of SFB-specific CD4 T cells in commercially available B6 mice naturally lacking or are colonized with SFB. However, it is important to note that SFB is not the only difference between B6J and B6NTac mice when it comes to the gut microbiome. Ivanov et al. (18) identified 479 significantly different taxa, but most differences were subtle and only 2 taxa were >25-fold more abundant in B6NTac mice – Lactobacillus murinus ASF361 and the segmented filamentous bacteria Candidatus arthromitus. Importantly, only SFB induced an immune response program in the gut. We cannot rule out the possibility that some other bacterial species that is enriched in the gut microbiome of B6NTac mice (or the converse enrichment in B6J mice) could also induce similar CD4 T cell expansion. We are currently unable to test this hypothesis due to the lack to defined Ag-specific CD4 T cell subsets that would fall into this category.
Human autopsy studies and mouse models of sepsis have shown widespread cell death in the gut lumen of septic hosts (34,35), changes to the microbial composition of the gut (36), and increased permeability of the gut epithelial barrier (37). Co-morbidity between iatrogenic lymphopenia and gut epithelial cell damage is also seen in chemotherapy-induced mucositis and bone marrow transplantation (38). More importantly, opportunistic infection after BMT is the most common cause of mortality not related to transplant relapse, just as in sepsis. Our data also suggest the possibility of deterring or promoting immune function after sepsis by modulation of the bacterial composition in the small intestine of patients. While literature on the subject is scarce, recent clinical case reports of septic patients undergoing fecal microbiota transplantation look promising (39). Sepsis and diarrhea resolution were correlated with increased Firmicutes, the taxonomical phyla under which C. arthromitus is classified. However, more research is needed on this topic before we can confidently define how microbial modulation can be harnessed for clinical benefit against sepsis-induced immune suppression.
In summary, the present study shows that gut resident microbes can modulate the recovery of certain portions of the CD4 T cell repertoire after iatrogenic lymphopenia, as exemplified by septic injury. Our data also shows how a CD4 T cell population with specificity for a gut resident bacterium fares better after sepsis in terms of expansion after heterologous challenge and functional capacity after sepsis when that bacterium is resident in the gut at the time of polymicrobial sepsis induction. The increased proliferation of SFB-specific CD4 T cells, their superior cytokine production, and the survival benefit during secondary virulent pathogen challenge implies these responses are enhanced by SFB colonization in sepsis survivors. It is important to emphasize that these findings were revealed only when we examined the CD4 T cells in an Ag-specific manner, instead of simply monitoring all CD4 T cells. We expect other significant changes within the lymphocyte compartment are present during and after a septic event, but these differences will only be revealed when Ag specificity and gut microbiome differences between individuals are taken into consideration. Lastly, our data may implicate previously underappreciated differences in the composition in the gut microbiome in how some individuals are more susceptible to certain secondary infections after sepsis.
Acknowledgments
Supported by a U.S. Department of Veterans Affairs Merit Review Award (T.S.G.) and National Institutes of Health Grants T32AI007313 (J.C.-P.), T35AI118620 (J.C.B.), GM113961, AI119160 and AI114543 (V.P.B.).
REFERENCES
- 1.Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, Angus DC, Rubenfeld GD, Singer M, F. Sepsis Definitions Task Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:775–787. doi: 10.1001/jama.2016.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vulliamy PE, Perkins ZB, Brohi K, Manson J. Persistent lymphopenia is an independent predictor of mortality in critically ill emergency general surgical patients. Eur. J. Trauma Emerg. Surg. 2015 doi: 10.1007/s00068-015-0585-x. [DOI] [PubMed] [Google Scholar]
- 3.Hamers L, Kox M, Pickkers P. Sepsis-induced immunoparalysis: mechanisms, markers, and treatment options. Minerva Anestesiol. 2015;81:426–439. [PubMed] [Google Scholar]
- 4.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Jr., Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, Karl IE. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J. Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 5.Unsinger J, Kazama H, McDonough JS, Griffith TS, Hotchkiss RS, Ferguson TA. Sepsis-induced apoptosis leads to active suppression of delayed-type hypersensitivity by CD8+ regulatory T cells through a TRAIL-dependent mechanism. J. Immunol. 2010;184:6766–6772. doi: 10.4049/jimmunol.0904054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabrera-Perez J, Condotta SA, James BR, Kashem SW, Brincks EL, Rai D, Kucaba TA, Badovinac VP, Griffith TS. Alterations in antigen-specific naive CD4 T cell precursors after sepsis impairs their responsiveness to pathogen challenge. J. Immunol. 2015;194:1609–1620. doi: 10.4049/jimmunol.1401711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Condotta SA, Rai D, James BR, Griffith TS, Badovinac VP. Sustained and incomplete recovery of naive CD8+ T cell precursors after sepsis contributes to impaired CD8+ T cell responses to infection. J. Immunol. 2013;190:1991–2000. doi: 10.4049/jimmunol.1202379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duong S, Condotta SA, Rai D, Martin MD, Griffith TS, Badovinac VP. Polymicrobial sepsis alters antigen-dependent and -independent memory CD8 T cell functions. J. Immunol. 2014;192:3618–3625. doi: 10.4049/jimmunol.1303460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Condotta SA, Khan SH, Rai D, Griffith TS, Badovinac VP. Polymicrobial sepsis increases susceptibility to chronic viral infection and exacerbates CD8+ T cell exhaustion. J. Immunol. 2015;195:116–125. doi: 10.4049/jimmunol.1402473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 2013;13:260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moon JJ, Chu HH, Hataye J, Pagan AJ, Pepper M, McLachlan JB, Zell T, Jenkins MK. Tracking epitope-specific T cells. Nat. Protoc. 2009;4:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markwart R, Condotta SA, Requardt RP, Borken F, Schubert K, Weigel C, Bauer M, Griffith TS, Forster M, Brunkhorst FM, Badovinac VP, Rubio I. Immunosuppression after sepsis: systemic inflammation and sepsis induce a loss of naive T-cells but no enduring cell-autonomous defects in T-cell function. PLoS One. 2014;9:e115094. doi: 10.1371/journal.pone.0115094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orr MT, Orgun NN, Wilson CB, Way SS. Cutting edge: Recombinant Listeria monocytogenes expressing a single immune-dominant peptide confers protective immunity to herpes simplex virus-1 infection. J Immunol. 2007;178:4731–4735. doi: 10.4049/jimmunol.178.8.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Torchinsky MB, Gobert M, Xiong H, Xu M, Linehan JL, Alonzo F, Ng C, Chen A, Lin X, Sczesnak A, Liao JJ, Torres VJ, Jenkins MK, Lafaille JJ, Littman DR. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature. 2014;510:152–156. doi: 10.1038/nature13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.VanOosten RL, Griffith TS. Activation of tumor-specific CD8+ T Cells after intratumoral Ad5-TRAIL/CpG oligodeoxynucleotide combination therapy. Cancer Res. 2007;67:11980–11990. doi: 10.1158/0008-5472.CAN-07-1526. [DOI] [PubMed] [Google Scholar]
- 18.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drewry AM, Samra N, Skrupky LP, Fuller BM, Compton SM, Hotchkiss RS. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock. 2014;42:383–391. doi: 10.1097/SHK.0000000000000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudensky A, Rath S, Preston-Hurlburt P, Murphy DB, Janeway CA., Jr. On the complexity of self. Nature. 1991;353:660–662. doi: 10.1038/353660a0. [DOI] [PubMed] [Google Scholar]
- 21.Dileepan T, Linehan JL, Moon JJ, Pepper M, Jenkins MK, Cleary PP. Robust antigen specific Th17 T cell response to group A Streptococcus is dependent on IL-6 and intranasal route of infection. PLoS pathogens. 2011;7:e1002252. doi: 10.1371/journal.ppat.1002252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Condotta SA, Richer MJ, Badovinac VP, Harty JT. Probing CD8 T cell responses with Listeria monocytogenes infection. Adv. Immunol. 2012;113:51–80. doi: 10.1016/B978-0-12-394590-7.00005-1. [DOI] [PubMed] [Google Scholar]
- 23.Bhardwaj V, Kanagawa O, Swanson PE, Unanue ER. Chronic Listeria infection in SCID mice: requirements for the carrier state and the dual role of T cells in transferring protection or suppression. J. Immunol. 1998;160:376–384. [PubMed] [Google Scholar]
- 24.Harty JT, Schreiber RD, Bevan MJ. CD8 T cells can protect against an intracellular bacterium in an interferon gamma-independent fashion. Proc. Natl. Acad. Sci. U. S. A. 1992;89:11612–11616. doi: 10.1073/pnas.89.23.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brocke S, Hahn H. Heat-killed Listeria monocytogenes and L. monocytogenes soluble antigen induce clonable CD4+ T lymphocytes with protective and chemotactic activities in vivo. Infect. Immun. 1991;59:4531–4539. doi: 10.1128/iai.59.12.4531-4539.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuijt TJ, van der Poll T, Wiersinga WJ. Gut microbiome and host defense interactions during critical illness. In: Vincent JL, editor. Annual Update in Intensive Care and Emergency Medicine 2012. Springer-Verlag; Berlin, Heidelberg: 2012. pp. 29–42. [Google Scholar]
- 27.Nieuwenhuijzen GA, Goris RJ. The gut: the 'motor' of multiple organ dysfunction syndrome? Curr. Opin. Clin. Nutr. Metab. Care. 1999;2:399–404. doi: 10.1097/00075197-199909000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Sodhi CP, Neal MD, Siggers R, Sho S, Ma C, Branca MF, Prindle T, Jr., Russo AM, Afrazi A, Good M, Brower-Sinning R, Firek B, Morowitz MJ, Ozolek JA, Gittes GK, Billiar TR, Hackam DJ. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology. 2012;143:708–718. doi: 10.1053/j.gastro.2012.05.053. e701-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Althani AA, Marei HE, Hamdi WS, Nasrallah GK, El Zowalaty ME, Al Khodor S, Al-Asmakh M, Abdel-Aziz H, Cenciarelli C. Human microbiome and its association with health and diseases. J. Cell Physiol. 2016;231:1688–1694. doi: 10.1002/jcp.25284. [DOI] [PubMed] [Google Scholar]
- 30.Becattini S, Taur Y, Pamer EG. Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol. Med. 2016 doi: 10.1016/j.molmed.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bultman SJ. The microbiome and its potential as a cancer preventive intervention. Semin. Oncol. 2016;43:97–106. doi: 10.1053/j.seminoncol.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moldawer LL, Efron PA. Cecal ligation and puncture. Curr. Protoc. Immunol. 2010 doi: 10.1002/0471142735.im1913s91. Chapter 19:Unit 19 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ericsson AC, Hagan CE, Davis DJ, Franklin CL. Segmented filamentous bacteria: commensal microbes with potential effects on research. Comp. Med. 2014;64:90–98. [PMC free article] [PubMed] [Google Scholar]
- 34.Coopersmith CM, Stromberg PE, Dunne WM, Davis CG, Amiot DM, 2nd, Buchman TG, Karl IE, Hotchkiss RS. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA. 2002;287:1716–1721. doi: 10.1001/jama.287.13.1716. [DOI] [PubMed] [Google Scholar]
- 35.Turnbull IR, Buchman TG, Javadi P, Woolsey CA, Hotchkiss RS, Karl IE, Coopersmith CM. Age disproportionately increases sepsis-induced apoptosis in the spleen and gut epithelium. Shock. 2004;22:364–368. doi: 10.1097/01.shk.0000142552.77473.7d. [DOI] [PubMed] [Google Scholar]
- 36.Freestone PP, Williams PH, Haigh RD, Maggs AF, Neal CP, Lyte M. Growth stimulation of intestinal commensal Escherichia coli by catecholamines: a possible contributory factor in trauma-induced sepsis. Shock. 2002;18:465–470. doi: 10.1097/00024382-200211000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Fox AC, McConnell KW, Yoseph BP, Breed E, Liang Z, Clark AT, O'Donnell D, Zee-Cheng B, Jung E, Dominguez JA, Dunne WM, Burd EM, Coopersmith CM. The endogenous bacteria alter gut epithelial apoptosis and decrease mortality following Pseudomonas aeruginosa pneumonia. Shock. 2012;38:508–514. doi: 10.1097/SHK.0b013e31826e47e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thorpe DW, Stringer AM, Gibson RJ. Chemotherapy-induced mucositis: the role of the gastrointestinal microbiome and toll-like receptors. Exp. Biol. Med. 2013;238:1–6. doi: 10.1258/ebm.2012.012260. [DOI] [PubMed] [Google Scholar]
- 39.Li Q, Wang C, Tang C, He Q, Zhao X, Li N, Li J. Successful treatment of severe sepsis and diarrhea after vagotomy utilizing fecal microbiota transplantation: A case report. Crit. Care. 2015;19:37. doi: 10.1186/s13054-015-0738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]