Abstract
Objective
Through binding to folate receptor-β (FR-β), the new 99mTc–EC20 (Etarfolatide) imaging technique detects activated but not resting macrophages in vivo. The goal of this study was to investigate macrophage-related inflammation in osteoarthritis (OA).
Methods
Twenty-five individuals (50 knees) with symptomatic OA of at least one knee underwent SPECT-CT imaging of both knees and planar imaging of the whole body after injection of Etarfolatide. Scans and knee radiographs were scored blinded to clinical information including knee and other joint site pain severity. Measures of association controlled for age, gender, BMI and employed repeated measures to adjust for correlation between knees.
Design
Activated macrophages were present in the majority (76%) of knees. The quantity of knee-related macrophages was significantly associated with knee pain severity (R=0.60, p<0.0001) and radiographic knee OA severity including joint space narrowing (R=0.68, p=0.007), and osteophyte (R=0.66, p=0.001). Macrophages were also localized to joints commonly affected by OA including hand finger joints (12%), thumb bases (28%), shoulders (26%), great toes (18%) and ankles (12%). The presence of joint pain at fingers, wrists, ankles and great toes was significantly positively associated with presence of activated macrophages at these sites (p<0.0001–0.04).
Conclusions
This study provides the first direct in vivo evidence for macrophage involvement in OA in a substantial proportion of human knees. The association of quantity of activated macrophages with radiographic knee OA severity and joint symptoms suggests that drugs targeting macrophages and macrophage-associated inflammatory pathways may have the potential to be both symptom and structure modifying.
Keywords: inflammation, osteoarthritis, macrophage, knee, joint pain
Introduction
Although it is well accepted that inflammation and activated synovial macrophages play a key role in rheumatoid arthritis (RA) [1], as evinced by recent reviews, the role of inflammation in osteoarthritis (OA) has been heavily debated [2, 3]. Evidence is mounting that low-grade inflammation induced by the metabolic syndrome, innate immunity and inflammaging all play a role in the pathogenesis of OA [3]. In OA, cartilage destructive responses are mediated by both chondrocyte and synovial macrophage derived inflammation-related degradative proteases, aggrecanases and matrix metalloproteinases [4–6]. Joint inflammation is also believed to play a role in the phasic progression of OA [7, 8].
99mTc–EC20 (Etarfolatide) is a folate receptor-specific molecular imaging agent developed to non-invasively localize sites of activated macrophage accumulation and regions of folate receptor positive tumor growth [9]. The basis for these capabilities lies in the high affinity of 99mTc–EC20 (Kd ~1 nM) for glycosylphosphatidylinositol-anchored folate receptor β (FR-β) on activated, but not resting macrophages or other immune cells [10], and folate receptor α (FR-α) on some tumors [11–15]. The major folate transport pathways for essentially all other cells in the body involve either the reduced folate carrier or proton coupled folate transporter [16], both of which prefer reduced folates over oxidized forms of the vitamin and neither of which can transport folate-linked drugs like 99mTc–EC20 [17]. In a pilot study of 40 patients with rheumatoid arthritis, the number of actively involved joints by Etarfolatide uptake was correlated with inflammatory indicators, erythrocyte sedimentation rate and C reactive protein [18]. Thus, Etarfolatide allows for noninvasive, whole body assessments of high affinity folate receptor expression and provides a means to quantify inflammation related to activated macrophages. The purpose of this study was to investigate the involvement of macrophages in OA through Etarfolatide imaging. Specifically, we evaluated the frequency, localization, and intensity of Etarfolatide uptake, its association with symptoms and radiographic severity of knee OA, and its presence and association with joint symptoms at other sites throughout the body. We hypothesized that a subset of subjects with knee OA would have knee inflammation, as reflected by Etarfolatide uptake. Knowing that inflammation is a major cause of pain in knee OA [19], we further hypothesized that Etarfolatide uptake would correlate with joint symptoms.
Patients and Methods
Study Description
This investigator-initiated single center study was conducted at Duke University Medical Center. Participants ≥18 years old were recruited on the basis of radiographic OA (Kellgren Lawrence grade 1–4 severity) [20] in at least one knee, and knee pain in the index knee on most days of any one-month in the last year [21]. A total of 25 participants were enrolled. Participants were excluded with a history of any of the following conditions: rheumatoid arthritis or other known inflammatory arthropathy; avascular necrosis; periarticular fracture; current anticoagulant therapy; current immune modulator therapy or any such therapy within 4 weeks of study procedures; inability to discontinue use of non-steroidal anti-inflammatory drugs within 3 days prior to study procedures (although low dose aspirin of up to 325 mg per day was permitted); Paget's disease; villonodular synovitis; joint infection; ochronosis; neuropathic arthropathy; acromegaly; hemochromatosis; Wilson's disease; osteochondromatosis; arthroscopic knee surgery within the previous 12 months; intra-articular injection or systemic (oral, intravenous, or intramuscular) steroid within the previous 6 months; any knee replacement; lactation; relationship to personnel directly affiliated with this study or their family members; and current or recent (last 30 days) enrollment in a clinical trial involving an off-label use of an investigational drug or device. All women of childbearing potential (n=7) were screened by serum β-HCG and had a confirmed negative result within the 48 hours prior to any imaging procedures.
Self-reported joint pain intensity was ascertained by the NHANES I criterion [21] and recorded on a 0–3 point scale (none, mild, moderate, and severe) for the knee (as pain, aching or stiffness on most days of any one-month in the last year). "Joints that bothered" the individual were selected and scored (0–3) from a defined list of 21 other joint sites throughout the body.
This clinical investigation was conducted according to Declaration of Helsinki principles, with the approval of the Duke University Medical Center Institutional Review Board and the Food and Drug Administration (IND 108,677). Written informed consent was received from participants prior to inclusion in the study and the study was registered at ClinicalTrials.gov (NCT01237405) prior to enrollment of the first participant.
Imaging
A total of 25 individuals underwent Etarfolatide imaging of both knees and the whole body. Etarfolatide (EC20, provided by Endocyte, Inc) consists of folate, the targeting moiety, linked to a chelating moiety to which 99mTc was non-covalently coupled on the day of the imaging procedure (in the Duke Radiopharmacy) to provide a nuclear medicine imaging agent suitable for localizing activated macrophages throughout the body (Figure 1). One hour after intravenous injection of this radiopharmaceutical, single-photon emission computed tomography combined with high-resolution computed tomography (SPECT/CT) images were acquired for all 50 knees with scan slice thickness 13.25 mm for coronal images and 8.18 mm for the transaxial images. Following the knee scans, anteroposterior, and posteroanterior planar images of the whole body were acquired approximately 80 minutes after Etarfolatide injection. All images were acquired using a GE Infinia Hawkeye detector (GE Healthcare) with resolution of ~8.5 mm for planar and SPECT imaging and ~9.5 mm for whole body scans. Knee SPECT/CT images were scored by the consensus of two readers blinded to the clinical data; several weeks later, 30% of the images were rescored in a blinded fashion by one reader for assessment of intra-rater reliability. Knee images were scored by anatomic location (medial, lateral and patellofemoral compartments), tissue localization (joint capsule, synovium and subchondral bone), and intensity of uptake (0–3: 0=normal, 1=mild, 2=moderate, 3=intense). Overall intensity of Etarfolatide uptake was graded for 7 sites for each knee: medial, lateral and patellofemoral joint capsule; medial and lateral synovium; and medial and lateral subchondral bone. The designation 'capsular' was consistently applied to uptake immediately adjacent to the joint; this could represent uptake in the capsule, underlying synovium or both. The designation 'synovial' was applied to uptake in a pattern consistent with proliferative synovium in any location around the joint including the synovial membrane and membranes of the communicating bursae (such as the suprapatellar and popliteal bursae). It was not possible to identify macrophages in synovial fluid on Etarfolatide SPECT/CT images.
Fig 1. Structure of the EC20 imaging reagent.
Folate, the targeting moiety, is shown on the left; the chelating agent to which 99mTc is conjugated, is shown on the right.
Because 17–94% of solid tumors express an FR isoform (FR-α) that binds folate [17], all whole body images were immediately evaluated qualitatively for any suspicious activity outside of joint tissues; no such lesions were identified. The intensity of joint tissue uptake on whole body planar images was scored semi-quantitatively (0–3: 0=normal, 1=mild, 2=moderate, 3=intense) for 30 sites (17 different joint groups) throughout the body beyond the knees: bilateral glenohumoral and acromioclavicular shoulders, elbows, wrists, hands (finger joints), thumb bases, hips, sacroiliac, medial and lateral ankles, forefoot, big toes, and sternoclavicular joints; and unilateral cervical, thoracic and lumbar spine, and manubriosternal joint.
Knee radiographs were obtained as previously described using an optimal and standardized method with the SynaFlexer™ frame [22]. Radiographs were graded blinded to other imaging and clinical data by the consensus of two experienced readers and scored for global Kellgren Lawrence (KL) grade [20] as well as features of joint space narrowing severity (scored 0–3) and osteophyte size (scored 0–3) using a standardized atlas [23].
Immunohistochemical analysis of synovial fluid cells
A total of 48 synovial fluid samples were obtained, 28 by direct aspiration and 20 by small volume lavage. Samples were spun at 3500 RPM for 10 minutes after which aliquots of the supernatant were frozen at −80°C and the cell pellet preserved in 100 µl of RNAlater and frozen at −80°C. We gained access to a Shandon Cytospin at the end of the study; it was therefore only possible to attempt cytospins of synovial fluid cells of the final 4 samples. Sufficient numbers of cells (≥50,000) were obtained from the index knee of the final two patients (patients 24 and 25) to perform preliminary immunofluorescent analyses for M1 (iNOS) and/or M2 (TGF-β) macrophage markers. These samples were centrifuged at 2500g, 4°C, for 15 minutes; the cell pellet was washed twice with 1ml cold phosphate-buffered saline (PBS), then centrifuged at 1000g, 4°C, for 15 minutes. After the second wash, cells were resuspended in PBS to a concentration of 1000 cells per microliter. A total of 5µl of 10% BSA-PBS was added to a 50µl cell suspension and the samples were spun at maximum speed for 1–3 minutes. Slides were rinsed briefly in cold PBS, fixed in ice-cold 100% acetone for 10 mins, washed twice with ice cold PBS then stored at −80°C until immunostained.
Cytospin preps were fixed with paraformaldehyde and permeabilized with Triton X-100 using BD Cytofix/Cytoperm™ Plus Fixation and Permeabilization Kit (BD Biosciences), followed by incubation with 1% BSA in PBST (PBS with 0.25% Triton X-100) for 30 mins at room temperature to block nonspecific binding of antibodies. The cytospins were pretreated using the Strepavidin/Biotin Blocking Kit (Vector Laboratories) before adding biotinylated antibody to block the enodogenous biotin or biotin-binding proteins. The cytospins were then stained with the following primary antibodies at 1:100 dilution overnight at 4°C: a biotinylated anti-FR-β monoclonal antibody [24] (gift of Dr. Phil Low), rabbit polyclonal anti-human iNOS/NOS II antibody (ab15323, Abcam), and mouse anti-human TGF-β (MCA797, AbD Serotec). The cytospins were incubated in 1% BSA for 1 hr at room temperature in the dark with a mixture of secondary antibodies raised in different species with different fluorochromes: AlexaFluor 635-conjugated Strepavidin (Invitrogen) for FR-β, goat-anti-rabbit FITC (Sigma) for iNOS, and Donkey-anti-mouse DyLight 549 (Jackson ImmunoResearch) for TGF-β. Cytospins were washed extensively in PBS, and mounted with a drop of a glycerol-based, aqueous mounting medium (Vector Laboratories). Cells were counterstained by incubation with 0.1–1 µg/ml Hoechst or DAPI (DNA stain) for 1 min. Cells were analyzed with a V1000 confocal unit and a 60×/1.2 NA oil objective (Olympus). Images were processed using FLUOVIEW software (Olympus).
Statistical Analysis
The abundance of activated macrophages was determined by scoring the intensity of Etarfolatide uptake for each region of interest on knee SPECT/CT images. Intra-rater reliability of knee Etarfolatide SPECT/CT knee scoring was assessed by determination of the weighted kappa statistic (appropriate for ordinal data) for the images scored twice, several weeks apart, blinded to the original scores. Associations of macrophage presence and relative abundance with severity of radiographic knee OA and severity of knee joint symptoms were assessed by repeated measures multivariable regression employing generalized estimating equations (GEE) [25] to account for the correlation between fellow knees as implemented in SAS 9.3 (SAS Institute Inc., SAS Campus Drive, Cary, North Carolina 27513). These analyses controlled for age, gender, and body mass index (BMI). Cytospins of synovial fluid were evaluated qualitatively for expression of the FR-β folate receptor, and macrophage M1 (iNOS) and M2 (TGF-β) markers. The association of site-specific intensity of Etarfolatide uptake and joint symptoms was determined by Pearson correlation. A p value <0.05 was considered significant. Data were analyzed using SAS software.
Results
Participants
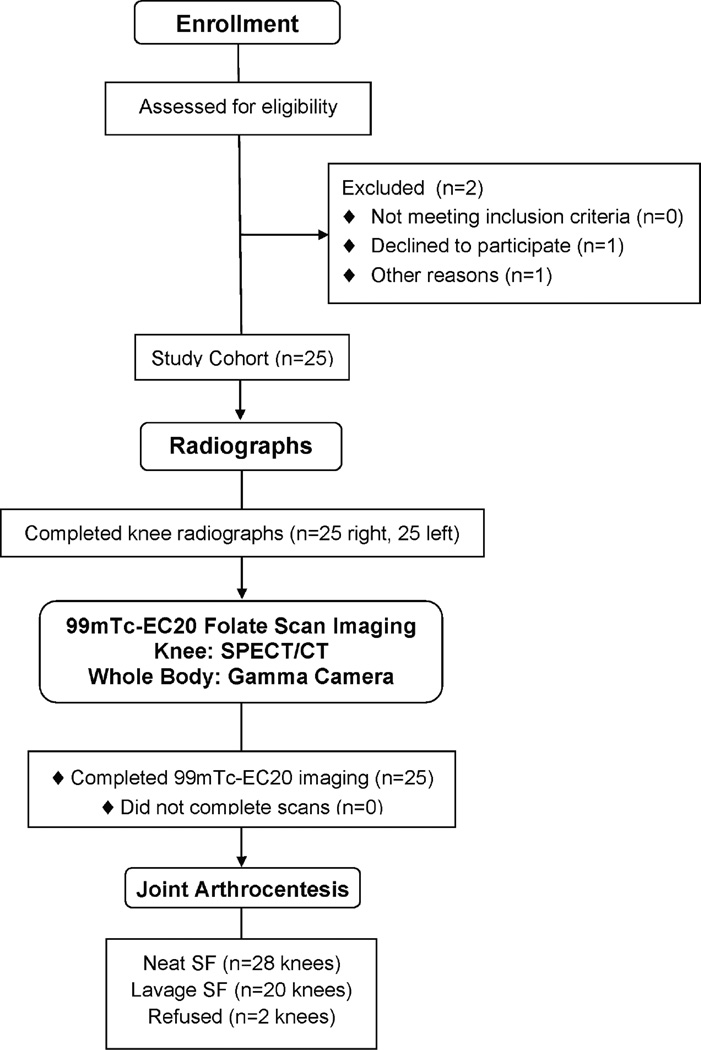
A total of 25 participants with radiographic knee OA completed the full study protocol (Figure 2). The cohort was 72% female, 92% Caucasian, mean (SD) age 62.4 ± 15.8 years (range 30–89), and mean (SD) BMI 29.2 ± 4.8 (range 22.5–38.4) kg/m2. Despite only requiring unilateral knee radiographic OA for study entry, the majority (76%) of participants had moderate to severe bilateral knee radiographic OA with 24% mild (KL grade 1), 58% moderate (KL grade 2–3), and 18% severe (KL grade 4). Immediately upon injection of Etarfolatide, one participant reported dysgeusia (an impairment or dysfunction of the sense of taste), a known side effect of Etarfolatide that lasted for 9 minutes. No other adverse events were observed. There is a potential for Etarfolatide to sensitively identify folate receptor bearing epithelial cancers [15]; however, no findings suggestive of cancer were identified in this study.
Fig 2. Consort Flow Diagram.
Flow of participants through each stage of the observational study.
Frequency of activated macrophages in the knee
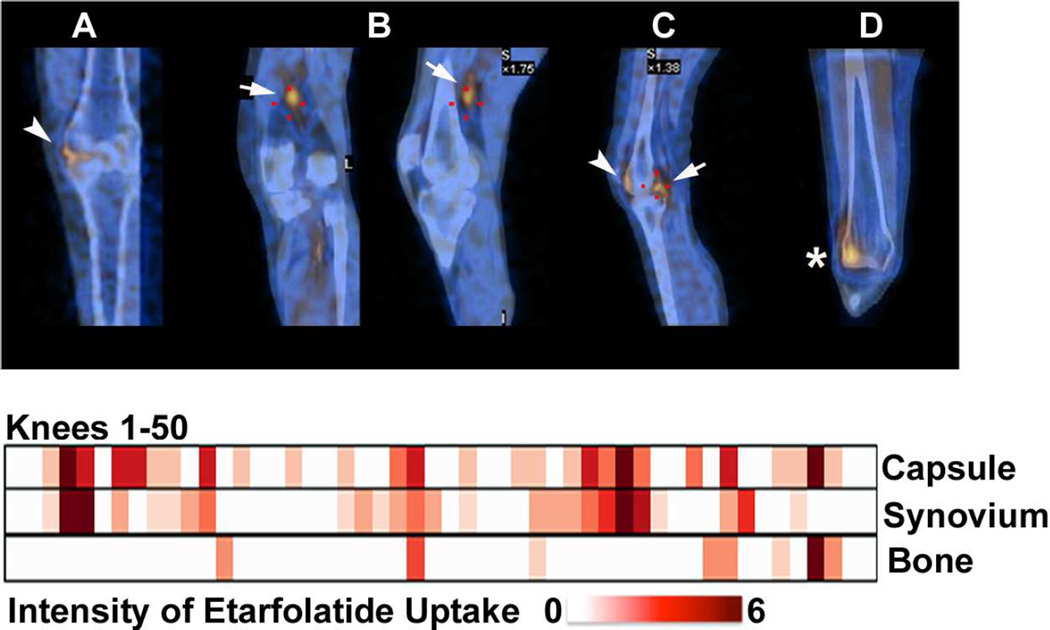
SPECT/CT images were obtained on all 50 knees. The intra-rater reliability of scoring Etarfolatide uptake was substantial to near perfect for all knee regions (Table 1). Representative images for capsular, synovial and subchondral bone Etarfolatide uptake are provided (see Figure 3 A–D). The majority of knees (76%) were positive for activated macrophages in at least one knee region; overall joint capsule uptake was observed in 66% of knees (medial, lateral and/or patellofemoral compartments), synovial uptake in 52% of knees, and subchondral bone uptake in 16% of knees. Joint capsule and synovial uptake were observed together in 38% of knees and joint capsule together with bone uptake in 8% of knees. A few knees (6%) had uptake in all these locations (Figure 3).
Table 1.
Intra-rater reliability of scoring of Etarfolatide SPECT/CT knee images.
| Knee Location | Kappa Statistic |
95% CI | Agreement* | |
|---|---|---|---|---|
| Joint (Capsule) | tibiofemoral | 0.78 | 0.60 – 0.96 | Substantial |
| patellofemoral | 0.64 | 0.25 – 1.00 | Substantial | |
| Synovial | 0.68 | 0.41 – 0.96 | Substantial | |
| Subchondral Bone | 0.90 | 0.72 – 1.00 | Almost perfect | |
CI = confidence interval
Magnitude guidelines reflecting the strength of agreement have been described by Landis and Koch: a value <0 indicates no agreement; a value between 0–0.2 slight agreement; 0.21–0.4 fair agreement; 0.41–0.6 moderate agreement; 0.61–0.8 substantial agreement; and 0.81–1.00 almost perfect agreement [47].
Fig 3. Representative patterns and intensity of Etarfolatide uptake in each of the 50 knees.
Representative images (top row) of Etarfolatide uptake in the: A) joint 'capsule' (showing capsular and/or underlying synovial uptake, indicated in coronal view by arrowhead); B) 'synovium' (showing uptake by proliferative synovium in the posterior synovial recess, indicated in coronal and sagittal views by arrow); C) 'synovium' and joint 'capsule' (indicated in sagittal view by arrow and arrowhead, respectively); and D) bone (indicated in coronal view by asterisk). Heat map graphic (bottom) representing the uptake intensity for each knee as quantified for three regions: joint 'capsule', 'synovium' and subchondral bone; the maximum score for each joint region was 6, 6 and 5, respectively.
Frequency of activated macrophages in other joints
The frequency of uptake of EC20 was also assessed for the whole body outside the knee (Table 2). Not unexpectedly, Etarfolatide uptake captured by planar gamma camera imaging was less intense and localized than results by SPECT/CT for the knees. Nevertheless, whole body imaging and scoring were possible. Several general conclusions could be drawn from the images. Outside the knee, activated macrophages were definitely present in other joints; the sites with the highest frequency of uptake were those often affected by OA, including the finger joints (12%) and thumb bases (28%), shoulders (26%), big toes (18%) and ankles (12%). This is particularly interesting given the fact that these subjects were only recruited for their knee OA and not for OA symptoms at any other joint site.
Table 2.
Correlations of Etarfolatide uptake and presence of joint symptoms in and beyond the knee.
| Joint Site | Positive Etarfolatide scan N (Frequency) |
Correlation of Etarfolatide scan with symptoms at same site |
|
|---|---|---|---|
| R (Pearson)* |
p value (Pearson) |
||
| Knee – tibiofemoral joint capsule | 33 (66%) | 0.46 | 0.004 |
| Knee – synovium | 26 (52%) | 0.47 | <0.0001 |
| Shoulder-glenohumoral | 13 (26%) | 0.35 | 0.13 |
| Wrist | 4 (8%) | 0.35 | 0.010 |
| Hand-total | 15 (30%) | 0.79 | 0.002 |
| Hand-interphalangeal | 6 (12%) | 0.55 | 0.040 |
| Hand-thumb base | 14 (28%) | 0.42 | 0.026 |
| Sternoclavicular | 1 (2%) | NA | NA |
| Hip | 2 (4%) | 0.14 | 0.76 |
| Sacroiliac | 1 (2%) | NA | NA |
| Ankle-total | 6 (12%) | 0.57 | <0.0001 |
| Big Toe | 8 (16%) | 0.74 | <0.0001 |
| Upper Back / thoracic spine | 1 (4%) | 0.40 | 0.003 |
p<0.05 are depicted in bold; No uptake was observed in the acromioclavicular shoulder, elbow, cervical or lumbar spine, manubriosternal, or forefoot joints.
NA=not assessed
Association of activated macrophages and clinical features of joint pain and OA severity
We analyzed the correlation of Etarfolatide uptake with clinical symptoms based on self-reported pain, aching and stiffness (PAS); the mean (SD) knee pain score was 1.6 ± 0.9 (range 0–3). Among the 50 imaged knees, 6% had no symptoms, 48% mild, 28% moderate, and 18% severe knee PAS. A total of 88% of the imaged knees were symptomatic in the last month. Analyzed on a knee basis (n=50), knee symptoms were significantly associated with synovial (R=0.47, p<0.0001, parameter estimate 0.23, 95% CI 0.13, 0.33) and joint (tibiofemoral) capsular (R=0.46, p=0.004, parameter estimate 0.23, 95% CI 0.08, 0.39) Etarfolatide uptake, but not patellofemoral or subchondral bone Etarfolatide uptake (with GEE adjusted for age, gender, BMI) (Table 2). Knee symptoms were even more strongly associated with Etarfolatide uptake combining (as z-scores) synovial and capsular Etarfolatide uptake (R=0.60, p<0.0001). Very interestingly, Etarfolatide uptake was also strongly and significantly associated with presence of joint symptoms at several other joints, including the finger, thumb base, wrist, ankle and great toe joints (Table 2).
Etarfolatide uptake was significantly positively associated with radiographic severity of knee OA. Synovial and joint capsular uptake were associated with severity of joint space narrowing, indicative of cartilage loss and/or meniscal extrusion (R=0.67, p=0.006, parameter estimate 0.16, 95% CI 0.04, 0.27 for synovial uptake; and R=0.68, p=0.007, parameter estimate 0.23, 95% CI 0.06, 0.41 for joint capsular uptake). Synovial and joint capsular uptake were also associated with severity of osteophytes (size and number), indicative of the anabolic joint response to the disease (R=0.60, p=0.01, parameter estimate 0.76, 95% CI 0.17, 1.34 for synovial uptake; and R=0.66, p=0.001, parameter estimate 0.99, 95% CI 0.39, 1.99 for joint capsular uptake) (adjusted for age, gender and BMI by GEE).
It was possible to perform immunohistochemistry on synovial fluid of two patients whose samples yielded sufficient cells (≥50,000) for cytospins. Knee radiographs and matching Etarfolatide SPECT/CT images are shown for these two participants (Figure 4). The left knees were more severely affected radiographically, and had greater Etarfolatide uptake and pain than their contralateral knees. Synovial fluid cytospins were immunostained for FR-β and inducible nitric oxide synthase (iNOS, a pro-inflammatory M1 macrophage marker), while the second sample could be additionally immunostained with antibodies against transforming growth factor-β (TGF-β, an anti-inflammatory M2 macrophage marker) (Figure 4). Immunostains of these two patients revealed that all synovial fluid cells co-expressed FR-β and iNOS. The second synovial fluid sample demonstrated the co-localization of TGF-β, iNOS and FR-β on all the cells, suggesting a potential block in transition of macrophages from a pro-inflammatory (M1) to an anti-inflammatory/repair (M2) state.
Fig 4. Knee radiographs, Etarfolatide scans and synovial fluid cell immunohistochemistry for knees of two study participants.
In both participants, the left knee was the index knee. The panels on the left correspond to one participant and on the right to a second participant). Top: Bilateral anteroposterior knee radiographs show osteoarthritis bilaterally with more severe disease of the left knees for each participant; total osteoarthritis severity scores (joint space narrowing plus osteophyte) were 5, 9, 3 and 11 for right and left knees of participants 24 and 25 respectively. Corresponding pain scores were 1, 3, 1 and 3 respectively. Middle: The total Etarfolatide knee scores were 3, 5, 0 and 9 respectively (the second row shows SPECT images and the third row shows matched fused SPECT-CT images of the knee; coronal and transaxial images include both right and left knees, and the sagittal images include the index left knees of each participant. Synovial fluid cytospins were stained for iNOS, FR-β and TGF-β. Merged images demonstrate co-expression of FR-β (identifying the cells as activated macrophages), an M1 macrophage marker (iNOS) and an M2 macrophage marker (TGF-β). R=right knee; L=left knee.
Discussion
To our knowledge, this study provides the first direct in vivo evidence for macrophage involvement in human OA. Several very important inferences can be made from the results of this study. First, that macrophages and inflammation are strongly associated with joint symptoms and radiographic severity of knee OA. In contrast, radiographic severity of knee OA is itself generally not a strong predictor of symptoms. The fact that the presence of activated macrophages in joints outside the knee was also associated with symptoms suggests that macrophage associated inflammation is a generalizable phenomenon and possibly an etiologic or aggravating factor in OA symptoms at affected joints throughout the body.
SPECT/CT combines the functional imaging data of nuclear medicine co-registered with the preCIe anatomical data of CT, all performed in one imaging session, to yield a 3D representation of the joint. Interestingly, the frequency of inflammation in OA knees as detected by SPECT/CT was remarkably congruent with the frequency of signs of inflammation in symptomatic knee OA detected in ultrasound (70–78% effusions, 34% synovial thickening) or MRI (80–81% effusions, 50% synovial thickening) [26, 27] studies. The frequency of macrophage-related inflammation in the hands of our study subjects (30%) was also congruent with the observed frequency of inflammation in finger joints (up to 21%) from recently published studies [28, 29]. Although inflammation has been associated with knee OA progression [30–32], this study was limited to a cross-sectional analysis and therefore could not directly address the association of Etarfolatide uptake and OA progression risk.
SPECT imaging with Etarfolatide has demonstrated activated macrophages in murine models of rheumatoid arthritis and post-traumatic OA from injury (transection of the anterior cruciate ligament) or chemical insult (intra-articular monoiodoacetate) [33]. To date, proof of concept that macrophage targeting could be anti-arthritic has been demonstrated by folate-targeted therapy for adjuvant-induced arthritis in rats [34–36].
Immunostains of joint fluids from the knees of two subjects showed coexpression of FR-β and an M1 macrophage marker. One of the fluids could be additionally stained for an M2 marker; this sample showed the coexpression of FR-β with both M1 and M2 macrophage markers. These preliminary findings are consistent with a recent study showing that FR-β positive macrophages in OA synovial tissues express a mixed pattern of both M1 (iNOS) and M2 (IL-10 and TGF-β) markers [37]. Macrophages play a dual role with involvement in inflammation as well as regeneration. In fact, salamander limb regeneration is dependent upon a regeneration-permissive environment that is dependent on macrophages [38]. A block in the M1 to M2 transition has been implicated in failure to heal chronic skin wounds [39]. We posit that a similar block in transition of macrophages from a pro-inflammatory (M1) to an anti-inflammatory/repair (M2) state perpetuates ongoing joint degeneration and prevents tissue repair and healing in OA. These preliminary findings require confirmation in a larger sample.
Macrophages comprise a continuum of activation states determined by the local tissue and cytokine microenvironment [40, 41]. Activated macrophages associated with tumors [40], asthma [42] and atherosclerosis [43] all express FR-β and regulatory M2 macrophage phenotypes [44]. M2 macrophages can be sub-divided into several subsets [45]. Although M2 macrophages are generally considered to be involved in immunosuppression and tissue repair, their reduction, reflected in reduction of Etarfolatide uptake in the inflamed lesions of murine models of rheumatoid arthritis, ulcerative colitis, pulmonary fibrosis, and atherosclerosis, predict a beneficial clinical response to treatment [46]. Our preliminary immunohistochemistry results suggested an activated pro-inflammatory state of the FR-β positive synovial fluid macrophages based on iNOS expression (both cases), and an M2 phenotype based on TGF-β expression (one case). Moreover, the intensity of Etarfolatide uptake correlated with radiographic severity of joint space narrowing and osteophyte. Results of our secondary analyses of soluble macrophage-associated markers (CD14 and CD163), reported separately [32], showed that their synovial fluid concentrations correlated with intensity of Etarfolatide imaging scores and radiographic osteophyte and joint space narrowing severity. In addition, these soluble markers in the synovial fluid of a longitudinal cohort predicted OA progression [32]. Soluble CD14 and CD163 are generally considered to reflect an M2 phenotype. Taken together, these results suggest that the activated macrophages identified by Etarfolatide imaging represent disease mediating cells in a variety of chronic diseases, including OA. Neither computed tomography nor radiographs can identify inflammation, so Etarfolatide imaging provides a means of identifying a subgroup with inflammation related to macrophages who are at risk for OA progression. From this perspective, this imaging technology could be very useful for research and clinical trial purposes. However, the radiation exposure attributable to SPECT/CT would likely preclude its routine use. The preCIe magnetic resonance imaging features that correlate with Etarfolatide uptake have not yet been eludicated. Ideally, a soluble or MRI (no radiation exposure involved) biomarker could be identified corresponding to these findings that would facilitate routine identification and monitoring of inflammatory OA subsets in the context of clinical trials and the clinic.
There were several limitations of this study. For one, it represented a small sample size however this was partially mitigated by the analysis of both knees with appropriate control for correlation with GEE. By design, this study was intended to be an initial survey of the potential utility of Etarfolatide imaging in knee OA and did not include a control group. In addition, cytospins could only be attempted on 4 synovial fluids with successful immunohistochemistry on two of these due to sufficiency of cell yield. These results are therefore preliminary and need to be further validated. The whole body Etarfolatide scores were generated from antero-posterior and postero-anterior gamma camera body images but were of lower resolution than the SPECT scans of the knee. It would not be unexpected that deep structures, such as the hip, would be poorly imaged under these circumstances. However, the lack of Etarfolatide uptake in the spine was surprising considering the fact that reporting of some back symptoms was positive in 64%, 24% and 60% of individuals for the neck, upper and lower back, respectively. The reasons for the lack of concordance of Etarfolatide uptake with spine symptoms are unknown but may be technical (higher resolution and longer scan times needed) or biological (the mechanisms of back pain in the majority of these patients are unrelated to activated macrophages). Given the limited number of positive scores for these sites, the results should be interpreted with caution.
In summary, this study is the first direct in vivo indication in humans for activated macrophage involvement in OA. These results provide insights into the pathogenesis and role of inflammation in OA. Etarfolatide imaging demonstrates that macrophage-related inflammation in OA is a common finding. The agent is safe and well tolerated and may have utility in selecting patients for clinical trials who are likely to benefit from macrophage-targeted therapy such as provided by folate receptor targeting. In contrast to radiographs, Etarfolatide imaging provides an objective disease indicator that is significantly associated with severity of joint symptoms. These results also suggest that macrophages are etiologic factors in OA pain at joint sites throughout the body.
Acknowledgments
Funding: in part by Eli Lilly and Company, in part by NIH/NIH P30-AG-028716 (salary support from to VBK and TVS) and donation of Etarfolatide by Endocyte Inc.
We wish to thank Dr. R Edward Coleman (now deceased) who supervised the nuclear medicine scans and scored all the Etarfolatide images. We also wish to thank Erin K. O'Reilly, PhD, RAC, Regulatory Affairs Associate of the Duke Translational Medicine Institute who was instrumental in assisting with the preparation and filing of the FDA IND and who was supported in part by Duke University's CTSA grant 1 UL1 RR024128 from NCRR/NIH. We also wish to thank Steve Conlon, Department of Pathology at Duke University for his expert assistance with production of Figures 3 and 4.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: All authors met all criteria for authorship in the ICMJE Recommendations.
Virginia B Kraus designed the study and supervised all aspects of the study conduct, interpretation and reporting.
Gary McDaniel, PAC served as the study coordinator under the supervision of Dr. Kraus for all aspects of the study.
Janet L Huebner assisted with study logistics and performed, sample handling and study monitoring.
Thomas Stabler coordinated and performed all sample management.
Carl F Pieper provided statistical support for the project.
Steven Shipes served as the study coordinator for all radiological procedures and performed all the Etarfolatide imaging studies.
Neil A. Petry actively participated in the imaging protocol design, IND preparation, study management and manuscript preparation and review process. He was also responsible for the procurement, compounding, quality control and dispensing of 99mTc–Etarfolatide imaging agent administration to subjects enrolled in this study.
Philip S Low assisted with study design and interpretation. Dr. Low is the inventor of EC20 (Etarfolatide).
Jiayin Shen performed the immunohistochemical staining for the synovial fluid samples and was an employee of Endocyte.
Peter Mitchell, contributor to the study concept and design, and Terry A McNearney, contributor to the study design and logistics, are employees of Eli Lilly and Co.
References
- 1.Rollett A, Reiter T, Nogueira P, Cardinale M, Loureiro A, Gomes A, et al. Folic acid-functionalized human serum albumin nanocapsules for targeted drug delivery to chronically activated macrophages. Int J Pharm. 2012;427:460–466. doi: 10.1016/j.ijpharm.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 2.Dequeker J, Luyten FP. The history of osteoarthritis-osteoarthrosis. Ann Rheum Dis. 2008;67:5–10. doi: 10.1136/ard.2007.079764. [DOI] [PubMed] [Google Scholar]
- 3.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!) Osteoarthritis Cartilage. 2013;21:16–21. doi: 10.1016/j.joca.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Bondeson J, Wainwright SD, Lauder S, Amos N, Hughes CE. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther. 2006;8:R187. doi: 10.1186/ar2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bondeson J, Blom AB, Wainwright S, Hughes C, Caterson B, van den Berg WB. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 2010;62:647–657. doi: 10.1002/art.27290. [DOI] [PubMed] [Google Scholar]
- 6.Houard X, Goldring MB, Berenbaum F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr Rheumatol Rep. 2013;15:375. doi: 10.1007/s11926-013-0375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharif M, Kirwan JR, Elson CJ, Granell R, Clarke S. Suggestion of nonlinear or phasic progression of knee osteoarthritis based on measurements of serum cartilage oligomeric matrix protein levels over five years. Arthritis Rheum. 2004;50:2479–2488. doi: 10.1002/art.20365. [DOI] [PubMed] [Google Scholar]
- 8.Roach HI, Aigner T, Soder S, Haag J, Welkerling H. Pathobiology of osteoarthritis: pathomechanisms and potential therapeutic targets. Curr Drug Targets. 2007;8:271–282. doi: 10.2174/138945007779940160. [DOI] [PubMed] [Google Scholar]
- 9.Paulos CM, Turk MJ, Breur GJ, Low PS. Folate receptor-mediated targeting of therapeutic and imaging agents to activated macrophages in rheumatoid arthritis. Adv Drug Deliv Rev. 2004;56:1205–1217. doi: 10.1016/j.addr.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Leamon CP, Parker MA, Vlahov IR, Xu LC, Reddy JA, Vetzel M, et al. Synthesis and biological evaluation of EC20: a new folate-derived, (99m)Tc-based radiopharmaceutical. Bioconjug Chem. 2002;13:1200–1210. doi: 10.1021/bc0200430. [DOI] [PubMed] [Google Scholar]
- 11.Low PS, Henne WA, Doorneweerd DD. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc Chem Res. 2008;41:120–129. doi: 10.1021/ar7000815. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima-Matsushita N, Homma T, Yu S, Matsuda T, Sunahara N, Nakamura T, et al. Selective expression of folate receptor beta and its possible role in methotrexate transport in synovial macrophages from patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:1609–1616. doi: 10.1002/1529-0131(199908)42:8<1609::AID-ANR7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 13.Van Der Heijden JW, Oerlemans R, Dijkmans BA, Qi H, Laken CJ, Lems WF, et al. Folate receptor beta as a potential delivery route for novel folate antagonists to macrophages in the synovial tissue of rheumatoid arthritis patients. Arthritis Rheum. 2008;60:12–21. doi: 10.1002/art.24219. [DOI] [PubMed] [Google Scholar]
- 14.Teng L, Xie J, Teng L, Lee RJ. Clinical translation of folate receptor-targeted therapeutics. Expert Opin Drug Deliv. 2012;9:901–908. doi: 10.1517/17425247.2012.694863. [DOI] [PubMed] [Google Scholar]
- 15.Maurer AH, Elsinga P, Fanti S, Nguyen B, Oyen WJ, Weber WA. Imaging the folate receptor on cancer cells with 99mTc-etarfolatide: properties, clinical use, and future potential of folate receptor imaging. J Nucl Med. 2014;55:701–704. doi: 10.2967/jnumed.113.133074. [DOI] [PubMed] [Google Scholar]
- 16.Hou Z, Matherly LH. Biology of the major facilitative folate transporters SLC19A1 and SLC46A1. Curr Top Membr. 2014;73:175–204. doi: 10.1016/B978-0-12-800223-0.00004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackman AL, Jansen G, Ng M. Folate receptor targeted thymidylate synthase inhibitors. In: Jackman AL, Leamon CP, editors. Targeted Drug Strategies for Cancer and Inflammation. New York: Springer; 2011. pp. 93–118. [Google Scholar]
- 18.Matteson EL, Lowe VJ, Prendergast FG, Crowson CS, Moder KG, Morgenstern DE, et al. Assessment of disease activity in rheumatoid arthritis using a novel folate targeted radiopharmaceutical Folatescan. Clin Exp Rheumatol. 2009;27:253–259. [PMC free article] [PubMed] [Google Scholar]
- 19.Ioan-Facsinay A, Kloppenburg M. An emerging player in knee osteoarthritis: the infrapatellar fat pad. Arthritis Res Ther. 2013;15:225. doi: 10.1186/ar4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis MA, Ettinger WH, Neuhaus JM. Obesity and osteoarthritis of the knee: evidence from the National Health and Nutrition Examination Survey (NHANES I) Seminars in Arthritis & Rheumatism. 1990;20:34–41. doi: 10.1016/0049-0172(90)90045-h. [DOI] [PubMed] [Google Scholar]
- 22.Charles HC, Kraus VB, Ainslie M, Hellio Le Graverand-Gastineau MP. Optimization of the fixed-flexion knee radiograph. Osteoarthritis Cartilage. 2007;15:1221–1224. doi: 10.1016/j.joca.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15(Suppl A):A1–A56. doi: 10.1016/j.joca.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Feng Y, Shen J, Streaker ED, Lockwood M, Zhu Z, Low PS, et al. A folate receptor beta-specific human monoclonal antibody recognizes activated macrophage of rheumatoid patients and mediates antibody-dependent cell-mediated cytotoxicity. Arthritis Res Ther. 2011;13:R59. doi: 10.1186/ar3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Glynn RJ, Felson DT. Musculoskeletal disease research: should we analyze the joint or the person? J Rheumatol. 1996;23:1130–1134. [PubMed] [Google Scholar]
- 26.Tarhan S, Unlu Z. Magnetic resonance imaging and ultrasonographic evaluation of the patients with knee osteoarthritis: a comparative study. Clin Rheumatol. 2003;22:181–188. doi: 10.1007/s10067-002-0694-x. [DOI] [PubMed] [Google Scholar]
- 27.Song IH, Althoff CE, Hermann KG, Scheel AK, Knetsch T, Schoenharting M, et al. Knee osteoarthritis. Efficacy of a new method of contrast-enhanced musculoskeletal ultrasonography in detection of synovitis in patients with knee osteoarthritis in comparison with magnetic resonance imaging. Ann Rheum Dis. 2008;67:19–25. doi: 10.1136/ard.2006.067462. [DOI] [PubMed] [Google Scholar]
- 28.Kortekaas MC, Kwok WY, Reijnierse M, Huizinga TW, Kloppenburg M. Follow-up study of inflammatory ultrasound features in hand osteoarthritis over a period of 3 months: variable as well as constant. Osteoarthritis Cartilage. 2014;22:40–43. doi: 10.1016/j.joca.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 29.Kortekaas MC, Kwok WY, Reijnierse M, Kloppenburg M. Inflammatory ultrasound features show independent associations with progression of structural damage after over 2 years of follow-up in patients with hand osteoarthritis. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-205003. [DOI] [PubMed] [Google Scholar]
- 30.Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis -- results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage. 2005;13:361–367. doi: 10.1016/j.joca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Roemer FW, Guermazi A, Felson DT, Niu J, Nevitt MC, Crema MD, et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheum Dis. 2011;70:1804–1809. doi: 10.1136/ard.2011.150243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daghestani HN, Pieper CF, Kraus VB. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol. 2015;67:956–965. doi: 10.1002/art.39006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piscaer T, Müller C, Mindt T, Lubberts E, Stoop R, Verhaar J, et al. Folate mediated imaging of activated macrophages in experimental osteoarthritis using SPECT/CT. Osteo Cartilage. 2008;16:S27–S28. A33. [Google Scholar]
- 34.Turk MJ, Breur GJ, Widmer WR, Paulos CM, Xu LC, Grote LA, et al. Folate-targeted imaging of activated macrophages in rats with adjuvant-induced arthritis. Arthritis Rheum. 2002;46:1947–1955. doi: 10.1002/art.10405. [DOI] [PubMed] [Google Scholar]
- 35.Yi YS, Ayala-Lopez W, Kularatne SA, Low PS. Folate-targeted hapten immunotherapy of adjuvant-induced arthritis: comparison of hapten potencies. Mol Pharm. 2009;6:1228–1236. doi: 10.1021/mp900070b. [DOI] [PubMed] [Google Scholar]
- 36.Lu Y, Stinnette TW, Westrick E, Klein PJ, Gehrke MA, Cross VA, et al. Treatment of experimental adjuvant arthritis with a novel folate receptor-targeted folic acid-aminopterin conjugate. Arthritis Res Ther. 2011;13:R56. doi: 10.1186/ar3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsuneyoshi Y, Tanaka M, Nagai T, Sunahara N, Matsuda T, Sonoda T, et al. Functional folate receptor beta-expressing macrophages in osteoarthritis synovium and their M1/M2 expression profiles. Scand J Rheumatol. 2012;41:132–140. doi: 10.3109/03009742.2011.605391. [DOI] [PubMed] [Google Scholar]
- 38.Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci U S A. 2013;110:9415–9420. doi: 10.1073/pnas.1300290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puig-Kroger A, Sierra-Filardi E, Dominguez-Soto A, Samaniego R, Corcuera MT, Gomez-Aguado F, et al. Folate receptor beta is expressed by tumor-associated macrophages and constitutes a marker for M2 anti-inflammatory/regulatory macrophages. Cancer Res. 2009;69:9395–9403. doi: 10.1158/0008-5472.CAN-09-2050. [DOI] [PubMed] [Google Scholar]
- 41.Steinman RM, Idoyaga J. Features of the dendritic cell lineage. Immunol Rev. 2010;234:5–17. doi: 10.1111/j.0105-2896.2009.00888.x. [DOI] [PubMed] [Google Scholar]
- 42.Shen J, Chelvam V, Cresswell G, Low PS. Use of folate-conjugated imaging agents to target alternatively activated macrophages in a murine model of asthma. Mol Pharm. 2013;10:1918–1927. doi: 10.1021/mp3006962. [DOI] [PubMed] [Google Scholar]
- 43.Jager NA, Westra J, Golestani R, van Dam GM, Low PS, Tio RA, et al. Folate receptor-beta imaging using 99mTc-folate to explore distribution of polarized macrophage populations in human atherosclerotic plaque. J Nucl Med. 2014;55:1945–1951. doi: 10.2967/jnumed.114.143180. [DOI] [PubMed] [Google Scholar]
- 44.Xia W, Hilgenbrink AR, Matteson EL, Lockwood MB, Cheng JX, Low PS. A functional folate receptor is induced during macrophage activation and can be used to target drugs to activated macrophages. Blood. 2009;113:438–446. doi: 10.1182/blood-2008-04-150789. [DOI] [PubMed] [Google Scholar]
- 45.Sudan B, Wacker MA, Wilson ME, Graff JW. A systematic approach to identify markers of distinctly activated human macrophages. Front Immunol. 2015;6:253. doi: 10.3389/fimmu.2015.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelderhouse LE, Robins MT, Rosenbalm KE, Hoylman EK, Mahalingam S, Low PS. Prediction of Response to Therapy for Autoimmune/Inflammatory Diseases Using an Activated Macrophage-Targeted Radioimaging Agent. Mol Pharm. 2015;12:3547–3555. doi: 10.1021/acs.molpharmaceut.5b00134. [DOI] [PubMed] [Google Scholar]
- 47.Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics. 1977;33:363–374. [PubMed] [Google Scholar]