Abstract
Due to the growing burden of tumors and chronic infections, manipulating CD8 T cell responses for clinical use has become an important goal for immunologists. Here, we show that dendritic cell (DC) immunization coupled with relatively early (days 1–3) or late (days 4–6) administration of enhanced IL-2-signals both increase peak effector CD8 T cell numbers, but only early IL-2 signals enhance memory numbers. IL-2 signals delivered at relatively late time points drive terminal differentiation, marked Bim mediated contraction and do not increase memory T cell numbers. In contrast, early IL-2 signals induce effector cell metabolic profiles more conducive to memory formation. Of note, down-regulation of CD80 and CD86 was observed on DCs in vivo following early IL-2 treatment. Mechanistically, early IL-2 treatment enhanced CTLA-4 expression on regulatory T (Treg) cells, and CTLA-4 blockade alongside IL-2 treatment in vivo prevented the decrease in CD80 and CD86, supporting a cell-extrinsic role of CTLA-4 in down-regulating B7-ligand expression on DCs. Finally, DC immunization followed by early IL-2 treatment and αCTLA-4 blockade resulted in lower memory CD8 T cell numbers compared to the DC + early IL-2 treatment group. These data suggest that curtailed signaling through the B7-CD28 co-stimulatory axis during CD8 T cell activation limits terminal differentiation and preserves memory CD8 T cell formation and thus, should be considered in future T cell vaccination strategies.
Introduction
Upon recognition of cognate peptide presented in the context of peptide-MHC I complex on DCs, one naïve antigen-specific CD8 T cell will give rise to more than 104 daughter cells that have now acquired effector functions (1, 2). The accumulation of these effector CD8 T cells depends on co-stimulation through the CD28 receptor (3), as well as signals from inflammatory cytokines that prolong division (4). Following the peak of expansion, a relatively constant fraction of effector CD8 T cells undergo Bim-mediated apoptotic death while the surviving cells initiate the memory CD8 T cell pool (1). Previously, manipulation of input signals, such as deleting CD28 (3, 5) or quelling inflammatory cytokines during pathogen infection (4, 6–11), yielded proportional numerical decreases in both the effector and memory populations, suggesting that these two phases of the CD8 T cell response are numerically linked. Thus, in the context of T cell vaccination, where activation signals are modifiable, strategies to enhance the initial peak of T cell expansion (12–14), as a means to enhance memory formation have become standard practice.
Due to their unique ability to recognize and protect the host from intracellular pathogens or tumors, CD8 T cells have become the focus of numerous T cell vaccination strategies (15–19). Despite decades of effort, however, prophylactic T cell vaccines developed against both malignancy (20) and chronic viral pathogens (21, 22) have been a costly disappointment. Ongoing T cell vaccination approaches against chronic viral infections are developed empirically, with little focus on the immunological mechanisms that lead to protection or durability of the T cell response (23). Strategies to elicit high antibody titers through vaccination are well established, with many effective Ab-dependent vaccines available (24, 25). Now, basic mechanisms guiding CD8 T cell activation and memory generation must be investigated further to advance current T cell vaccination practices.
Previously, we used ex vivo peptide-pulsed DCs as a tool to study basic mechanisms controlling Ag-specific CD8 T cell responses. DCs offer many advantages, such as precise control over APC number, Ag load, and peptide presentation within the host. Additionally, they express high surface MHC I and co-stimulatory ligands to provide sufficient signal 1 and 2 to CD8 T cells. DC immunization can be administered alongside stimulators of inflammation such as model pathogens (Listeria monocytogenes (Lm) and lymphocytic choriomeningitis virus (LCMV) (26)); adjuvants, like CpG (4); or immunomodulators such as interleukin-2 (IL-2) (27), to elicit environmental signals that alter various phases of the CD8 T cell response. We recently showed that combining DC immunization with enhanced IL-2 signals (IL-2/anti-IL-2 mAb complexes) from D4–6 increased tumor-specific effector CD8 T cell number, function and control of pre-existing malignancy (27). Here, we evaluate if and by what mechanism enhanced IL-2 signals can be harnessed to optimize memory CD8 T cell numbers after DC immunization.
Materials and methods
Mice and Dendritic Cells
C57BL/6 mice were purchased from the National Cancer Institute (Frederick, MD, USA). OT-I cells, TCR-transgenic CD8+ T cells specific for Ova257-264, have been previously described (28). P14 cells, TCR-transgenic T cells specific for LCMV gp33-41, have been previously described (29). Bim−/− OT-I cells were generously provided by Martin Prlic (Fred Hutchinson Cancer Research Center; Seattle, WA). FoxP3-GFP mice were kindly provided by Stanley Perlman (University of Iowa). The University of Iowa Animal Care and Use Committee approved animal experiments. LPS-matured Ova257-264 or gp33-41-peptide-coated DCs were prepared as previously described (30) and were injected i.v. (5 × 105/mouse). Specifically, conventional CD11c+ DCs were enriched in vivo by s.c. injection of B16 tumor cells expressing FMS-like tyrosine kinase ligand (B16-flt3L) for the duration of 3 weeks.
Adoptive Transfer and Immunomodulators
Approximately 3×104 naïve Thy1.1+ OT-I or P14 cells isolated from naïve donors were transferred into each naive Thy1.2+ C57BL/6 recipient mouse i.v at day −1. Mice were injected i.v. with 5×105 matured/peptide-coated DCs at day 0 followed by Rat Ig or murine IL-2/anti-IL-2 S4B6 complexes at the indicated concentrations i.p from either day 1–3 (early) or 4–6 (late). Complexes were made by incubating murine IL-2 (PeproTech) with S4B6 anti–IL-2 mAb at a 2:1 molar ratio (7.5 μg/mL IL-2: 250 μg/mL S4B6) for 15 min at 37 degrees and serially diluted to lower concentrations. For blockade experiments, CTLA-4 mAb (Clone UC10-4F10) was purchased from BioXCell and administered i.p. at 250μg/mouse from D0–3 post-DC immunization.
Tissue Preparation and Flow Cytometry
Single cell suspensions were prepared from spleen, lung, and inguinal lymph nodes at day 7 or day 66 post-DC immunization. Lungs were additionally incubated for 1h at 37C with DNAse (0.1 mg/mL) and collagenase (0.38 mg/mL) followed by a Percoll (Sigma; St. Louis, MO) gradient isolation. Spleens were incubated for 20 minutes at 37C with DNAse/collagenase mixture when harvesting CD45.1+ DCs for DC transfer experiments. Single cell suspensions were diluted and counted using standard methods. Cells were labeled with anti-CD8 (eBiosciences; San Diego, CA), -Thy1.1, CD80 and –CD86 (Biolegend; San Diego, CA), and –STAT5 (pY694; BD Biosciences; San Jose, CA) antibodies using standard protocols. Cells were analyzed on an LSRFortessa flow cytometer (BD Biosciences) and analyzed by Flowjo software (Tree Star; Ashland, OR).
Immunoblot Assay
Spleens from OT-I-transferred, DC±IL-2c-treated mice were harvested on D6, stained with anti-Thy1.1-APC antibody (Clone OX-7, BD Pharmingen) and purified with anti-APC-antibody magnetic beads according to standard AutoMacs protocols. A total of 2 × 106 OT-I cells were washed with cold PBS and lysed in NP40 buffer (20 mM HEPES [pH 7.9], 100 mM NaCl, 5 mM EDTA, 0.5 mM CaCl, 1% Nonidet P-40, 1 mM PMSF, 10 μg/ml leupeptin, 5 μg/ml aprotinin, and 1 mM Na3VO4). Ten micrograms of protein were resolved by SDS-PAGE, transferred to nitrocellulose, and probed with Bim or β-actin antibodies (Cell Signal Technology; Beverly, MA) as indicated. Antibodies were detected with goat anti-rabbit conjugated to horseradish peroxidase (Santa Cruz) and Supersignal (Thermo Scientific). Images were captured with ImageJ software.
Metabolism Assay
Thy1.1+ OT-I CD8+ T cells were purified from the spleens of DC±IL-2c-treated mice at D6 post-DC immunization with anti-Thy1.1+ APC antibody and Anti-APC bead sorting using standard AutoMACS protocols (Miltenyi Biotec; San Diego, CA). After AutoMACS purification, 2×105 OT-I cells were plated in each well of an XF-96 plate (Seahorse Biosciences; MA, USA). Oxygen consumption rates (OCR) and extracellular acidification rates (ECAR) for OT-I CD8+ T cells were measured in XF media (nonbuffered DMEM containing 25 mM glucose, 2 mM L-glutamine, and 1 mM sodium pyruvate) under basal conditions or in response to 1 μM oligomycin, 1.5 μM fluoro-carbonyl cyanide phenylhydrazone (FCCP), and 0.5 μM rotenone + 2 μM antimycin A (Sigma) with XF-96 Extracellular Flux Analyzer (Seahorse Bioscience; Massachusetts, USA).
In vitro DC:Treg Assay
CD4+FoxP3− (non-Treg) or CD4+FoxP3+ (Treg) cells were sort-purified from FoxP3-GFP mice. Magnetic CD11c+ bead-sorted DCs were then plated alone or in a 2:1 ratio with non-Tregs or Tregs. Samples were incubated for a total of 3 hours in the presence of 20nM Bafilomycin A (Invivogen; San Diego, CA) prior to analysis of surface CD80 and CD86 expression.
Statistical Analysis
Significance was calculated by student’s T test using Graphpad Prism 5 for Macintosh in experimental comparisons between two groups, and one-way ANOVA for comparisons among three treatment groups. p-values<0.05 were considered significant.
Results
DC immunization followed by late IL-2 signals fail to enhance memory CD8 T cell numbers
The CD8 T cell field has established a paradigm of fixed contraction, where a relatively constant percentage (90–95%) of effector T cells are lost by apoptotic death following the peak of expansion (1, 31–33). This suggests a direct correlation between peak CD8 T cell numbers and memory numbers, a concept that has since been adopted for T cell vaccine development (15). However, fixed contraction has largely been described in multi-epitope-specific CD8 T cells responding to a common infection, where each responding cell, no matter its specificity experiences a common spectrum of pathogen-induced cytokines. It is unclear whether manipulating CD8 T cell responses through vaccine adjuvants or specific cytokines will uniformly enhance memory numbers. We previously showed that DC immunization, followed 4–6 days later by enhanced IL-2-signals, in the form of IL-2/anti-IL-2 antibody complexes, increased effector CD8 T cell expansion by up to 100-fold (27). Briefly, complexes of IL-2 and the IL-2-specific mAb S4B6 have been shown to enhance the biological properties of soluble IL-2 in vivo by obstructing renal excretion of the cytokine (34) and redirecting it’s bioavailability to CD122+CD25− effector T cells (35). Importantly, stimulating T cell responses with the IL-2/S4B6 complex does not occur through activating Fc receptors, thus limiting the biological effects on T cells to that of IL-2 only (36). To test whether a direct link between expansion and memory numbers exists in this controlled immunization setting, we utilized an adoptive transfer model of OT-I TCR-transgenic CD8 T cells, followed by Ova257 peptide-pulsed DC immunization coupled with Rat Ig or stimulating IL-2c treatment from day 4–6 post DC-immunization (DC±IL-2c4-6). Using this approach, we were able to fix the naïve CD8 T cell precursor number, the duration and amount of antigen presentation, as well as eliminate the complex pathogen-specific cytokine milieu (25) to isolate the effects of enhanced proliferative expansion on memory CD8 T cell numbers.
For our analysis, OT-I cells were adoptively transferred into mice followed by DC-Ova257 immunization and treatment with Rat Ig, 0.2μg or 1.5μg of IL-2c D4–6 post-DC immunization and quantified frequencies and total numbers of OT-I cells in the blood over time. Frequency of OT-I cells increased in a dose-dependent manner by D7 post-DC immunization, encompassing up to 50% of the entire peripheral blood leukocyte population following IL-2c4-6 treatment. However, these peak responses dropped to similar frequencies by D90 (Fig. 1A). In concordance with these results, the number of OT-I cells from DC+IL-2c4-6-treated mice was increased up to 100-fold at D7, but this did not lead to increased memory numbers compared to DC alone immunization (Fig. 1B, C). CD8 T cells contract in a Bim-dependent manner following infection (37); thus, we compared the contraction of WT (Fig. 1B) and Bim−/ − TCR-transgenic OT-I cells following DC±IL-2c4-6 treatment. We observed that DC+IL-2c4-6 -treated Bim−/− OT-I cells also underwent enhanced expansion (Fig. 1D), similar to WT OT-I cells, but exhibited no visible contraction within the first 20 days (Fig. 1E). Additionally, DC+IL-2c4-6 -treated Bim−/ − OT-I cells maintained a statistically significant numerical difference from the DC+Rat Ig control group throughout the experiment by frequency and total number in the blood (Fig. 1D, E), in opposition to the WT OT-I that exhibited indistinguishable memory numbers with or without IL-2c4-6 treatment (Fig. 1A–C). These data demonstrate that enhanced Bim-mediated contraction underlies the failure of IL-2c4-6-treatment to enhance memory CD8 T cell numbers despite the 100-fold increase in effector CD8 T cell numbers. Notably, a physiologic CD8 T cell response following attenuated Listeria monocytogenes-expressing Ova257 peptide (attLM-Ova) and IL-2c from D4–6 post-infection also contracted to the same memory CD8 T cell numbers as control (Fig. S1A), illustrating the same phenomenon outside the DC immunization setting. In addition, transgenic P14 cells, activated by lymphocytic choriomeningitis virus (LCMV)-specific gp33 peptide-pulsed DCs followed by low or high dose of IL-2c4-6 also exhibited enhanced expansion in blood (Fig. S1B, C), but no enhancement in memory numbers in blood (Fig. S1C), spleen (Fig. S1D), inguinal lymph node (Fig. S1E), and lung (Fig. S1F). These data suggest that similar memory CD8 T cell numbers observed following DC±IL-2c4-6 are not due to a difference in trafficking, and can be generalized across CD8 T cell specificities.
Figure 1.
3×104 Thy1.1+ WT or CD45.2+ Bim−/ − OT-I cells were adoptively transferred into naïve Thy1.2+ or CD45.1+ B6 mice, respectively, and immunized with 5×105 DC-Ova followed by Rat Ig, 0.2μg or 1.5μg IL-2c on D4–6 post-DC immunization. (A) Representative flow plots depicting OT-I cell frequencies in the blood at D7 and D90 post-DC immunization. (B) Kinetics graph of total number of WT OT-I cells quantified per mL of blood longitudinally across 90 days in all treatment groups. (C) Summary bar graph (mean ± SEM) of total number of OT-I cells harvested from the spleen at D90 post-DC immunization. (D) Same as (A) except frequency of Bim−/ − OT-I cells. (E) Same as (B) except total number of Bim−/ − OT-I cells in the blood. (F) Immunoblot of BimEL and BimL isoforms in WT OT-I cells harvested from spleen at D6 post-DC immunization across treatment groups (top). Immunoblot of β-actin protein expression as loading control (bottom). Data are representative of two experiment with at least n=5 mice/group/experiment. * = p<0.05; ** = p<0.005; *** = p<0.0005; ns, not significant.
To assess the effect of IL-2 signaling on Bim protein expression in activated OT-I cells, Bim long (BL) and extra long (BEL) isoforms, specific for T cells (37), were visualized by immunoblot assay from protein extracts derived from DC±IL-2c4-6-treated mice at D7 post-DC immunization. The greatest protein expression of both Bim isoforms was exhibited in the OT-I cells from mice treated with1.5μg of IL-2c4-6, with intermediate expression in 0.2μg IL-2c4-6-treated cells and little detectable expression in Rat Ig-treated control OT-I cells (Fig. 1F). These data suggest that IL-2c4-6 treatment enhances the population of terminally differentiated effector CD8 T cells, destined to undergo Bim-mediated apoptosis during contraction, that fail to contribute to the memory population. Hence, immunization strategies that aim to enhance Ag-specific memory CD8 T cell numbers long-term must increase the effector population in a manner that preserves the proportion of memory precursors and maintains a proportional contraction phase following the peak of expansion. In addition, these data demonstrate that memory numbers are not uniformly determined by peak effector CD8 T cells numbers after vaccination, but will depend on the specific signals used to amplify the response.
DC immunization followed by early IL-2 signals enhances memory CD8 T cell numbers
Previous reports indicate that CD11c+ DCs have an in vivo lifespan of 3 days (38) and can present Ag for roughly 48 hours (39). Hence, our DC+IL-2c4-6 immunization would occur after this limited DC priming phase, with IL-2c signaling solely to T cells once Ag presentation is complete. Numerous studies suggest that memory CD8 T cell numbers may be programmed (10, 40) by signals, including endogenous IL-2 (41, 42), received early during the priming phase. Thus, we hypothesized that IL-2c treatment during priming may alter memory CD8 T cell numbers.
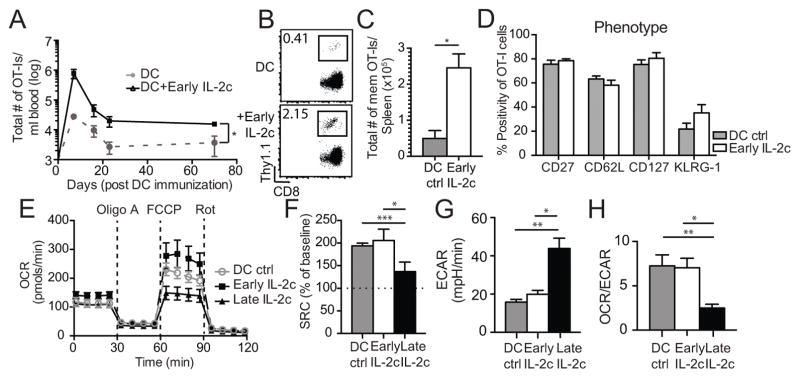
To address whether enhanced IL-2c signals during DC-T cell interaction could increase memory CD8 T cell numbers long-term, we repeated OT-I adoptive transfer experiments followed by DC immunization with the optimal high dose of IL-2c treatment shifted to D1–3 post-DC immunization (DC+IL-2c1-3). When directly monitoring the quantity of memory CD8 T cells after DC+IL-2c1-3 treatment, a 20-fold increase in effector CD8 T cell expansion along with stable enhancement of memory CD8 T cell numbers was observed in the blood after DC+IL-2c1-3 treatment compared to DC+Rat Ig treated mice (Fig. 2A). To ensure this was an overall increase in memory CD8 T cell numbers, and not simply due to higher circulating memory CD8 T cells in the blood, OT-I cells were quantified in the spleen of DC±IL-2c1-3 mice at D70 post-DC immunization. As observed in the blood, DC+IL-2c1-3 treatment resulted in a roughly 5-fold increase in frequency and total numbers of OT-I cells in the spleen (Fig. 2B, C). Importantly, memory OT-I cells derived from DC+IL-2c1-3 treatment displayed similar memory surface markers (Fig. 2D), cytokine expression (IFNγ, TNFα, IL-2; Fig. S2A, B), and cytotoxicity (GrzB, CD107a/b; Fig. S2C) as those from DC+Rat Ig controls. These data support that DC+IL-2c1-3 treatment generates a similar quality of memory CD8 T cells as DC+Rat Ig controls. Thus, enhanced IL-2c signals delivered during the priming phase of the CD8 T cell response increased effector populations with a concomitant increase in memory numbers.
Figure 2.
3×104 Thy1.1+ OT-I cells were adoptively transferred into naïve Thy1.2+ B6 mice and subsequently immunized with 5×105 DC-Ova followed by Rat Ig or IL-2c on D1–3 (Early) post-DC immunization. (A) OT-I cells quantified per mL of blood longitudinally across 70 days in all treatment groups. (B) Representative frequency of OT-I cells in the spleen of Rat Ig or IL-2c-treated mice at D70. (C) Summary (mean ± SEM) of total number of OT-I cells in the spleen of Rat Ig or IL-2c-treated mice on D70 post-DC immunization. (D) Summary (mean ± SEM) of the %OT-I cells expressing CD27, CD62L, CD127, and KLRG-1 in the spleen of Rat Ig or IL-2c-treated mice on D70 post-DC immunization. (E) 3×104 Thy1.1+ OT-I cells were adoptively transferred into naïve Thy1.2+ B6 mice and administered DC only, DC + D1–3 (Early), or DC + D4–6 (Late) IL-2c treatment. OT-I cells were purified from spleens at D6 post-DC immunization and assessed for metabolic function. Time-course of oxygen consumption rate (OCR) in pmols/min from all treatment groups. (F) Same as (E) except summary (mean ± SEM) of spare respiratory capacity (SRC) in all treatment groups normalized to percent of baseline. (G) Same as (E) except basal extracellular acidification rate (ECAR) in mpH/min. (H) Same as (E) except ratio of basal OCR/ECAR in all treatment groups. Data are representative of 2 independent experiments with at least n=3 mice/group/experiment. OCR, oxygen consumption rate; ECAR, extraellular acidification rate; Oligo A, oligomycin A (ATPase inhibitor); FCCP, p-triflouromethoxyphenylhydrazone (mitochondrial uncoupler), and Rot, Rotenone (Complex I inhibitor). * = p<0.05; ** = p<0.005; *** = p<0.0005; ns, not significant.
Several studies report that the memory CD8 T cell population is a result of effector vs. memory fate decisions that occur during the activation and effector phases of the response (43–45). Additionally, it is widely accepted that short-lived effector cells (SLECs) and memory precursor effector cells (MPECs) at these effector timepoints can be identified using IL-7R expression (46), with high IL-7R favoring memory precursor differentiation. Thus, we measured surface expression of IL-7R on OT-I cells from DC, DC+IL-2c1-3, and DC+IL-2c4-6 treatment groups at D7. OT-I cells from DC and IL-2c1-3-treated mice expressed bimodal and high IL-7R expression, respectively (Fig. S2D); however, OT-I cells from IL-2c4-6 treatment groups expressed no detectable surface IL-7R (Fig. S2D, E). While these data are consistent with IL-24-6 treatment preferentially generating terminally differentiated OT-I cells, IL-2 signals have been shown to directly downregulate IL-7R expression on CD8 T cells in a PI3K-Akt-dependent manner (47). Thus, we utilized another method to discriminate SLEC from MPEC populations following DC±IL-2c1-3 immunization.
Pearce et al. previously demonstrated that terminal effector CD8 T cells could be distinguished from memory precursors by their metabolic profiles (48). Predominant usage of aerobic glycolysis is indicative of SLECs, being necessary for optimal translation of IFN-γ(49, 50). In contrast, preferential use of oxidative phosphorylation with a high mitochondrial spare respiratory capacity (SRC) has been previously demonstrated in memory OT-I cells (48). Thus, we investigated the metabolic profiles of DC±IL-2c-primed CD8 T cells. OT-I cells were isolated from the spleen of DC alone and DC + IL-2c1-3 or IL-2c4-6-treated mice at D6 post-DC immunization to measure oxidative phosphorylation (oxygen consumption rate; OCR) and aerobic glycolysis (extracellular acidification rate; ECAR). OT-I cells from DC+IL-2c1-3-treated mice displayed the highest maximum OCR capacity (Fig. 2E) and therefore, high SRC compared to OT-I cells from DC+IL-2c4-6-treated mice (Fig 2F). The low baseline ECAR readings in OT-I cells after IL-2c1-3 treatment also correlated with higher memory potential than IL-2c4-6 (Fig. 2G). The ratio of OCR/ECAR, which provides an assessment of the preferential metabolic state of OT-I cells from all treatment groups illustrated that early IL-2c treatment resulted in approximately 3-fold higher OCR/ECAR ratio than late IL-2c treatment (Fig. 2H). Additionally, OT-I cells from DC controls displayed similar SRC, ECAR, and OCR/ECAR ratios as DC+IL-2c1-3 (Fig. 2F–H), suggesting that a similar metabolic profile and proportion of MPECs are generated by DC+IL-2c1-3 treatment, despite increased effector expansion in the latter group. Hence, these data support the hypothesis that DC+IL-2c1-3 treatment preserves the memory potential of effector CD8 T cell populations with enhanced expansion, in contrast to DC+IL-2c4-6, which drives terminal differentiation without an increase in memory numbers.
Early IL-2c treatment down-regulates B7-ligands on DCs in vivo
Earlier reports suggested CD25 expression on certain CD11c+ DC subsets (51, 52) that may confer IL-2 responsiveness (53). Thus, IL-2c1-3 could have a direct effect on the priming DCs to enhance memory T cell potential. As previous studies indicate the requirement of appropriately timed co-stimulatory signals through the CD28-B7 axis for memory CD8 T cell generation (3, 5), we hypothesized that altered co-stimulatory molecule expression on the surface of DCs following early IL-2c treatment may account for the generation of higher memory CD8 T cell numbers. To test a direct effect of IL-2c on DCs, LPS-matured DCs were plated for 3 days with or without the addition of IL-2c. As expected, mature DCs expressed high CD80 and CD86, but no detectable changes in their expression were observed following IL-2c-treatment (Fig. 3A). Analysis of CD122 and CD25 on our donor DC population with or without IL-2c revealed low expression of both IL-2R chains (Fig. 3B) and pSTAT5 signal, despite an increase in T cell controls 10 minutes following exposure to IL-2c (Fig. 3C). These in vitro data suggest that IL-2c does not have a direct effect on the priming DCs, but does not preclude a possible indirect effect in vivo.
Figure 3.

LPS-matured DCs were isolated via magnetic bead selection for CD11c+ cells from B16-flt3L-inoculated donor B6 mice. 1×105 CD11c+ DCs were plated in 96-well plate with RP10 1640 media control or addition of 50ng/mL IL-2c. (A) Representative histogram plots and summary (mean ± SEM) graphs depicting CD80 and CD86 surface expression on DCs with or without IL-2c. (B) Representative histograms depicting CD25 and CD122 expression on DCs with or without addition of IL-2c. (C) Representative histogram plots and summary (mean ± SEM) graphs depicting pSTAT5-Y694 expression on plated DCs and T cells in both treatment groups. (D) 3×106 LPS-matured CD11c+ DCs from B16-flt3L-inoculated CD45.1+ B6 mice were adoptively transferred into naïve CD45.2+ B6 recipients and subsequently administered Rat Ig or IL-2c for 2 days. Representative histograms and summary bar graphs (mean ± SEM) depicting CD80 and CD86 expression in Rat Ig controls and IL-2c treated mice in adoptively transferred CD45.1+ DCs. (E) Representative histograms depicting CD25 and CD122 expression on transferred CD45.2+ DCs. Data are representative of three independent experiments with at least n=3 mice/group/experiment.
To investigate changes in B7-ligand expression on DCs after IL-2c1-3 in vivo, CD45.1+CD11c+ mature DCs were transferred into CD45.2+ recipients that were subsequently administered Rat Ig or IL-2c for 2 days. Due to the short in vivo lifespan of transferred DCs (38), spleens were harvested on D2 to compare surface expression of CD80 and CD86 on CD45.1+ DCs. Transferred DCs in IL-2c-treated hosts exhibited a marked down-regulation in CD80 and CD86 expression that was not observed in DC from Rat Ig treated mice (Fig. 3D) without any loss in DC numbers at the time of harvest (Fig. S3A, B). The decrease of surface B7-ligand expression was most likely not due to direct IL-2c signaling on DCs, based on low CD25 and CD122 expression on these cells in vivo (Fig. 3E). These data suggest that early IL-2c treatment down-regulates surface CD80 and CD86 expression on DC in an indirect manner.
Tregs down-modulate B7-ligands on DCs following IL-2c1-3
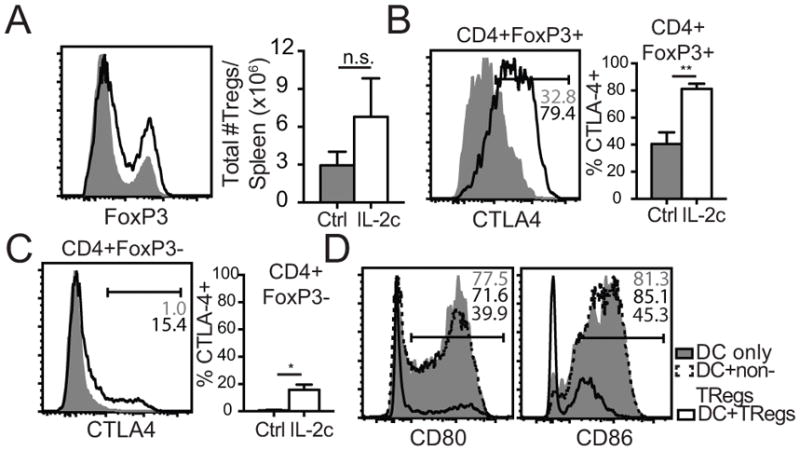
Recently, novel roles of Treg cells in promoting memory CD8 T cell progression have been described. Kalia et al. recently showed that transfer of Treg cells at the effector-to-memory transition suppressed effector programs through the CTLA-4-B7 axis to allow the generation of a memory population (54). CTLA-4, expressed constitutively on Treg cells (55), is known to terminate CD28-mediated co-stimulatory signals induced by interaction with B7 family members (56–59). To investigate whether IL-2c1-3 treatment led to an increase in Treg numbers, FoxP3 expression in CD4 T cells from mice receiving DC transfers was assessed at D2 in the spleen following early IL-2c treatment. IL-2c treatment led to a 2-fold increase in FoxP3-expressing Treg cells, as previously shown following DC+IL-2c4-6 treatment (27) (Fig. 4A). As IL-2, complexed to another mAb, JES6-1A12, has been demonstrated to stimulate Treg cells to express inhibitory molecules (60), CTLA-4 receptor expression was monitored on Treg (CD4+FoxP3+) and non-Treg (CD4+FoxP3−) cells from Rat Ig and early IL-2c-treated mice. As previously reported, CTLA-4 was detectable on some Treg cells in Rat Ig mice (Fig. 4B) with little CTLA-4 expression on non-Tregs (Fig. 4C). Two days of IL-2c treatment increased the percent of Treg cells expressing CTLA-4 receptor to 80% (Fig. 4B), with 15% of non-Tregs expressing lower levels of CTLA-4 receptor (Fig. 4C). These data suggest a potential role for CTLA-4 on either Treg or non-Treg populations to decrease B7-ligand expression on mature DCs during CD8 T cell priming.
Figure 4.
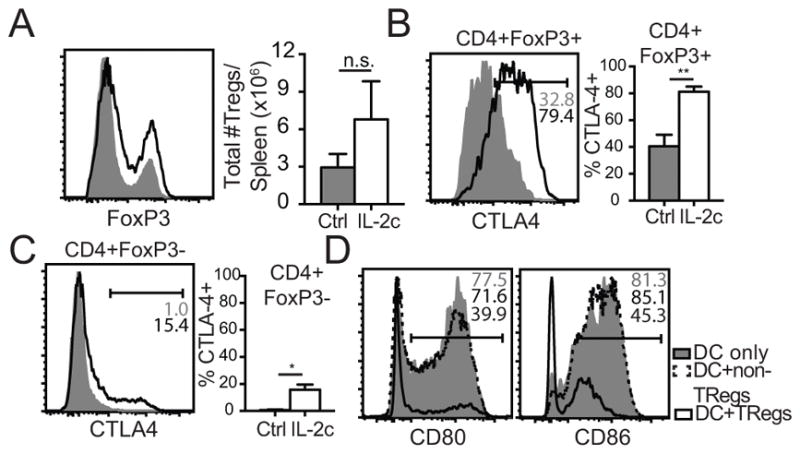
3×106 LPS-matured CD11c+ DCs from B16-flt3L inoculated CD45.1+ B6 mice were adoptively transferred into naïve CD45.2+ B6 recipients and subsequently administered Rat Ig or IL-2c for 2 days. (A) Representative histograms and summary bar graphs (mean ± SEM) of FoxP3+ cells previously gated on CD4+Thy1.2+ T cells in Rat Ig control and IL-2c treated mice. (B) Representative histograms and summary bar graph (mean ± SEM) of percent CTLA-4 positive Treg (CD4+ FoxP3+) cells at D2 post-DC transfer. (C) Same as (B) except percent CTLA-4+ non-Treg (CD4+FoxP3−) cells. (D) Treg or non-Treg cells were facs-sorted from FoxP3-GFP mice and incubated in a 1:2 ratio with LPS-matured DCs in the presence of 20nM bafilamycin A. Representative histograms of CD80 and CD86 expression on DC only, DC+non-Tregs, and DC+Tregs. Data are representative of at least 2 independent experiments with at least n=3 mice/group/experiment.
CTLA-4 was recently proposed to suppress co-stimulatory function through the CTLA-4-B7 axis in a predominantly cell-extrinsic manner (61). Indeed, Qureshi et al. demonstrated the capacity of CTLA-4 to trans-endocytose, internalize, and degrade CD80 and CD86 directly from the APC surface (62). To test whether Tregs or non-Tregs could directly remove B7-ligands from mature DCs, Treg and non-Treg cells were facs-sorted from FoxP3-GFP donor mice. Tregs or non-Tregs were then plated in a 1:2 ratio with LPS-matured DCs for 3 hours in the presence of Bafilomycin A (Baf A). Baf A was added to prevent phagolysosomal degradation of B7-ligands to allow their detection by flow cytometric analysis (62). Mature DCs plated alone expressed high CD80 and CD86 on their surface as seen previously (Fig. 3A). Incubation with non-Treg CD4 T cells had little to no effect on CD80 and CD86 expression on DCs (Fig. 4D). In contrast, DCs incubated in the presence of Treg cells lost 50–80% of surface B7-ligand expression (Fig. 4D, right). These in vitro studies, along with previously published data from Kalia et al. (54), suggest that Treg, but not non-Treg cells are capable of removing B7-ligands from the surface of DCs.
CTLA-4 blockade prevents enhanced memory formation following early IL-2c
To investigate whether down-regulation of B7-ligands on DCs occurred in a CTLA-4-dependent manner in vitro, we incubated DCs with Tregs and Tregs+αCTLA-4, measuring CLTA-4 binding to surface CD86. Compared to the Treg controls, αCTLA-4 treatment decreased CD86 binding (Fig. S3C) and led to higher retention of CD86 expression on DCs (Fig. S3D). To assess αCTLA-4 blockade in vivo, we repeated the DC transfer experiments with the addition of an IL-2c1-3+αCTLA-4 blockade group. As observed before, CD80 and CD86 expression decreased with IL-2c treatment alone (Fig. 5A, B); however, concurrent CTLA-4 blockade completely preserved CD80 expression and partially preserved CD86 expression on transferred DCs. These data suggest that up-regulated CTLA-4 on Treg cells following DC+IL-2c1-3 treatment permits trans-endocytosis of B7-ligands from the surface of DCs, potentially modulating co-stimulatory signals to T cells during priming that result in enhanced memory numbers.
Figure 5.
3×106 LPS-matured CD11c+ DCs from B16-flt3L inoculated CD45.1+ B6 mice were adoptively transferred into naïve CD45.2+ B6 recipients and subsequently administered Rat Ig, IL-2c, or IL-2c+αCTLA-4 mAb for 2 days. (A) Representative histogram plots of CD80 expression on transferred CD45.1+ DCs in Rat Ig control, IL-2c treated, and IL-2c+αCTLA-4 treated mice. (B) Same as (A) except CD86 expression. (C) 3×104 Thy1.1+ OT-I cells were adoptively transferred into naïve Thy1.2+ B6 mice and subsequently immunized with 5×105 DC-Ova257 followed by Rat Ig or IL-2c on D1–3 post-DC immunization ± αCTLA-4 blocking mAb. Total number of OT-I cells quantified per mL of blood longitudinally across 60 days in all treatment groups. (D) Representative flow plots of OT-I cell frequencies in the spleen at D60. (E) Summary bar graph (mean ± SEM) of total number of OT-I cells in the spleen at D60 post-DC immunization in all treatment groups. Data are representative of 2 independent experiment with at least n=10 mice/group.
The transient activation of Treg cells and subsequent down-modulation of B7-ligands on DCs upon IL-2c1-3 treatment led us to investigate whether CTLA-4 blockade would prevent the DC+IL-2c1-3-mediated increase in memory CD8 T cell numbers compared to DC only controls. First, we compared metabolic profiles of effector OT-I cells from DC+Early IL-2c and DC+Early IL-2c+αCTLA-4 to observe whether CTLA-4 blockade decreased memory potential early after DC immunization. Indeed, αCTLA-4 treatment led to lower maximum OCR levels (Fig. S4A) and thus, lower %SRC (Fig. S4B). Next, adoptively transferred OT-I cells were activated by DC, DC+IL-2c1-3, or DC+IL-2c1-3+αCTLA-4 and monitored over time. OT-I cells in both IL-2c1-3-treated groups exhibited similar enhanced expansion during the effector phase, but those that were treated with αCTLA-4 ultimately generated lower memory CD8 T cell numbers compared to DC+IL-2c1-3 alone that were not significantly increased from DC controls (Fig. 5C). Higher resolution analysis in the spleen corroborated findings in the blood, with a lower frequency (Fig. 5D) and number of OT-I cells (Fig. 5E) in the DC+IL-2c1-3+αCTLA-4-treated group compared to DC+IL-2c1-3 group. These data support the notion that early IL-2c treatment decreases co-stimulatory signals to CD8 T cells during priming by a CTLA-4 dependent mechanism to favor memory precursor formation during effector expansion, resulting in greater memory CD8 T cell numbers post-contraction.
Discussion
Using a controlled DC immunization coupled with relatively early (D1–3) or late (D4–6) alterations to IL-2 signaling, we define a mechanism by which Ag-specific memory CD8 T cells can be enhanced long-term. Although DC immunization is not currently used in the setting of prophylactic vaccinations, they have been approved for use in humans as a CD8 T cell-mediated cancer therapy (20). With the growing number of immunization strategies to combat chronic illness, DC immunization represents a viable platform to enhance Ag-specific memory CD8 T cell numbers in humans. Our studies initially show that inducing robust effector expansion with IL-2c4-6 signals does not predictably lead to increased memory CD8 T cell numbers due to greater induction of terminally differentiated effector cells. This is an important dissociation as vaccine efficacy is largely determined by the strength of the initial response, also referred to as vaccine immunogenicity. Our studies show that to successfully enhance the memory CD8 T cell population, vaccination strategies must deliver signals that maintain or increase the proportion of memory precursors rather than simply increase the numbers of terminally differentiated effector T cells observed at the peak of the response.
As previous studies suggest that memory CD8 T cell numbers are programmed during the priming phase (40), we administered DC with IL-2c1-3, which led to preservation of MPEC proportions in the context of enhanced IL-2-driven effector cell expansion. Although numerous studies have alluded to early programming of memory CD8 T cells (1, 40, 63, 64), it has been less clear what factors early on dictate changes in the ensuing memory population. Previous work from the Greenberg lab demonstrated that DCs exhibiting a mixture of mature and immature signals following TLR4/7/8 stimulation enhanced the generation of CD28-expressing memory cells from cultured human PBMCs (65). In addition to this, Laidlaw et al. demonstrated that Treg cell-derived IL-10 quelled inflammation and thus, the activation state of DCs, to allow the maturation of memory CD8 T cells following acute LCMV infection (66). In concordance with these previous findings, our data thus far support that, although complete absence of co-stimulation through CD28 results in T cell anergy, a decreased or curtailed B7-ligand expression in the context of DC+ early IL-2c immunization may enhance memory CD8 T cell generation. Our data correspond with the tunable threshold model of T cell activation, where different signal strengths determine effector and memory CD8 T cell programs (67–69). Despite this, our results do not necessarily disagree with the alternatively proposed autopilot model, stating that brief interactions with APCs in vitro will drive effector differentiation of naïve CD8 T cells (70). As demonstrated, DC immunization followed by both early and late IL-2c treatment results in robust effector expansion above DC controls (Fig. 1 and 2); however, decreasing co-stimulatory signal preserves the MPEC proportions in the effector phase to yield proportional memory CD8 T cell numbers post-contraction that is not seen with late IL-2c. It remains of great interest to determine the specific threshold level of B7-ligands required at the priming stage to induce memory versus terminal effector CD8 T cells. Our data provide novel insight into the immunological mechanisms that increase memory CD8 T cell numbers in a controlled immunization setting that can contribute to the development of predictable T cell vaccines.
Supplementary Material
Acknowledgments
We thank Brett Wagner and the Free Radical and Radiation Biology Core for technical support with metabolic assays and members of the Harty lab for helpful discussion.
Footnotes
Research reported in this publication was supported in part by the American Medical Association Foundation Seed Grant (MTK), the University of Iowa Holden Comprehensive Cancer Center (JTH) and the National Institutes of Health T32AI007485 (MTK, GSM) and AI4276 (JTH).
References
- 1.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nature reviews Immunology. 2008;8(2):107–119. doi: 10.1038/nri2251. Epub 2008/01/26. [DOI] [PubMed] [Google Scholar]
- 2.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nature reviews Immunology. 2002;2(4):251–262. doi: 10.1038/nri778. Epub 2002/05/11. [DOI] [PubMed] [Google Scholar]
- 3.Borowski AB, Boesteanu AC, Mueller YM, Carafides C, Topham DJ, Altman JD, Jennings SR, Katsikis PD. Memory CD8+ T cells require CD28 costimulation. J Immunol. 2007;179(10):6494–6503. doi: 10.4049/jimmunol.179.10.6494. [DOI] [PubMed] [Google Scholar]
- 4.Starbeck-Miller GR, Xue HH, Harty JT. IL-12 and type I interferon prolong the division of activated CD8 T cells by maintaining high-affinity IL-2 signaling in vivo. The Journal of experimental medicine. 2013 doi: 10.1084/jem.20130901. Epub 2013/12/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suresh M, Whitmire JK, Harrington LE, Larsen CP, Pearson TC, Altman JD, Ahmed R. Role of CD28-B7 interactions in generation and maintenance of CD8 T cell memory. J Immunol. 2001;167(10):5565–5573. doi: 10.4049/jimmunol.167.10.5565. [DOI] [PubMed] [Google Scholar]
- 6.Haring JS, Badovinac VP, Harty JT. Inflaming the CD8+ T cell response. Immunity. 2006;25(1):19–29. doi: 10.1016/j.immuni.2006.07.001. Epub 2006/07/25. [DOI] [PubMed] [Google Scholar]
- 7.Joffre O, Nolte MA, Sporri R, Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunological reviews. 2009;227(1):234–247. doi: 10.1111/j.1600-065X.2008.00718.x. Epub 2009/01/06. [DOI] [PubMed] [Google Scholar]
- 8.Keppler SJ, Rosenits K, Koegl T, Vucikuja S, Aichele P. Signal 3 cytokines as modulators of primary immune responses during infections: the interplay of type I IFN and IL-12 in CD8 T cell responses. PloS one. 2012;7(7):e40865. doi: 10.1371/journal.pone.0040865. Epub 2012/07/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim MT, Harty JT. Impact of Inflammatory Cytokines on Effector and Memory CD8+ T Cells. Frontiers in immunology. 2014;5:295. doi: 10.3389/fimmu.2014.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunological reviews. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. Epub 2006/07/11. [DOI] [PubMed] [Google Scholar]
- 11.Pham NL, Badovinac VP, Harty JT. Differential role of “Signal 3” inflammatory cytokines in regulating CD8 T cell expansion and differentiation in vivo. Frontiers in immunology. 2011;2:4. doi: 10.3389/fimmu.2011.00004. Epub 2011/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahlers JB, IM Memories that last forever: strategies for optimizing vaccine T-cell memory. Blood. 2010;115(9):12. doi: 10.1182/blood-2009-06-227546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nolz JC, Harty JT. Strategies and Implications for Prime-Boost Vaccination to Generate Memory CD8 T Cells. Adv Exp Med Biol. 2011;780:69–83. doi: 10.1007/978-1-4419-5632-3_7. [DOI] [PubMed] [Google Scholar]
- 14.Woodland DL. Jump-starting the immune system: prime-boosting comes of age. Trends in immunology. 2004;25(2):98–104. doi: 10.1016/j.it.2003.11.009. Epub 2004/04/23. [DOI] [PubMed] [Google Scholar]
- 15.Esser MT, Marchese RD, Kierstead LS, Tussey LG, Wang F, Chirmule N, Washabaugh MW. Memory T cells and vaccines. Vaccine. 2003;21(5–6):419–430. doi: 10.1016/s0264-410x(02)00407-3. Epub 2003/01/18. [DOI] [PubMed] [Google Scholar]
- 16.Koff WC, Johnson PR, Watkins DI, Burton DR, Lifson JD, Hasenkrug KJ, McDermott AB, Schultz A, Zamb TJ, Boyle R, Desrosiers RC. HIV vaccine design: insights from live attenuated SIV vaccines. Nature immunology. 2006;7(1):19–23. doi: 10.1038/ni1296. Epub 2005/12/17. [DOI] [PubMed] [Google Scholar]
- 17.Koup RA, Douek DC. Vaccine design for CD8 T lymphocyte responses. Cold Spring Harbor perspectives in medicine. 2011;1(1):a007252. doi: 10.1101/cshperspect.a007252. Epub 2012/01/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson HL, Amara RR. T cell vaccines for microbial infections. Nature medicine. 2005;11(4 Suppl):S25–32. doi: 10.1038/nm1212. Epub 2005/04/07. [DOI] [PubMed] [Google Scholar]
- 19.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nature reviews Immunology. 2008;8(4):247–258. doi: 10.1038/nri2274. Epub 2008/03/08. [DOI] [PubMed] [Google Scholar]
- 20.Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17(11):3520–3526. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 21.Sekaly RP. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? The Journal of experimental medicine. 2008;205(1):7–12. doi: 10.1084/jem.20072681. Epub 2008/01/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nature medicine. 2008;14(6):623–628. doi: 10.1038/nm.f.1774. Epub 2008/06/07. [DOI] [PubMed] [Google Scholar]
- 23.Harari A, Bart PA, Stohr W, Tapia G, Garcia M, Medjitna-Rais E, Burnet S, Cellerai C, Erlwein O, Barber T, Moog C, Liljestrom P, Wagner R, Wolf H, Kraehenbuhl JP, Esteban M, Heeney J, Frachette MJ, Tartaglia J, McCormack S, Babiker A, Weber J, Pantaleo G. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. The Journal of experimental medicine. 2008;205(1):63–77. doi: 10.1084/jem.20071331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plotkin SA, Plotkin SL. The development of vaccines: how the past led to the future. Nature reviews Microbiology. 2011;9(12):889–893. doi: 10.1038/nrmicro2668. [DOI] [PubMed] [Google Scholar]
- 25.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nature immunology. 2011;12(6):509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richer MJ, Nolz JC, Harty JT. Pathogen-specific inflammatory milieux tune the antigen sensitivity of CD8(+) T cells by enhancing T cell receptor signaling. Immunity. 2013;38(1):140–152. doi: 10.1016/j.immuni.2012.09.017. Epub 2012/12/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim MT, Richer MJ, Gross BP, Norian LA, Badovinac VP, Harty JT. Enhancing Dendritic Cell-based Immunotherapy with IL-2/Monoclonal Antibody Complexes for Control of Established Tumors. J Immunol. 2015 doi: 10.4049/jimmunol.1501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76(1):17–27. doi: 10.1016/0092-8674(94)90169-4. Epub 1994/01/14. [DOI] [PubMed] [Google Scholar]
- 29.Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342(6249):559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt NW, Podyminogin RL, Butler NS, Badovinac VP, Tucker BJ, Bahjat KS, Lauer P, Reyes-Sandoval A, Hutchings CL, Moore AC, Gilbert SC, Hill AV, Bartholomay LC, Harty JT. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(37):14017–14022. doi: 10.1073/pnas.0805452105. Epub 2008/09/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annual review of immunology. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. Epub 2006/11/30. [DOI] [PubMed] [Google Scholar]
- 32.Joshi NS, Kaech SM. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J Immunol. 2008;180(3):1309–1315. doi: 10.4049/jimmunol.180.3.1309. Epub 2008/01/23. [DOI] [PubMed] [Google Scholar]
- 33.Prlic M, Williams MA, Bevan MJ. Requirements for CD8 T-cell priming, memory generation and maintenance. Current opinion in immunology. 2007;19(3):315–319. doi: 10.1016/j.coi.2007.04.010. Epub 2007/04/17. [DOI] [PubMed] [Google Scholar]
- 34.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nature reviews Immunology. 2012;12(3):180–190. doi: 10.1038/nri3156. Epub 2012/02/22. [DOI] [PubMed] [Google Scholar]
- 35.Boyman O, Krieg C, Letourneau S, Webster K, Surh CD, Sprent J. Selectively expanding subsets of T cells in mice by injection of interleukin-2/antibody complexes: implications for transplantation tolerance. Transplantation proceedings. 2012;44(4):1032–1034. doi: 10.1016/j.transproceed.2012.01.093. Epub 2012/05/09. [DOI] [PubMed] [Google Scholar]
- 36.Letourneau S, van Leeuwen EM, Krieg C, Martin C, Pantaleo G, Sprent J, Surh CD, Boyman O. IL-2/anti-IL-2 antibody complexes show strong biological activity by avoiding interaction with IL-2 receptor alpha subunit CD25. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(5):2171–2176. doi: 10.1073/pnas.0909384107. Epub 2010/02/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J, Marrack P. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity. 2002;16(6):759–767. doi: 10.1016/s1074-7613(02)00322-9. Epub 2002/07/18. [DOI] [PubMed] [Google Scholar]
- 38.Kamath AT, Henri S, Battye F, Tough DF, Shortman K. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 2002;100(5):1734–1741. [PubMed] [Google Scholar]
- 39.Pham NL, Badovinac VP, Harty JT. A default pathway of memory CD8 T cell differentiation after dendritic cell immunization is deflected by encounter with inflammatory cytokines during antigen-driven proliferation. J Immunol. 2009;183(4):2337–2348. doi: 10.4049/jimmunol.0901203. Epub 2009/07/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Badovinac VP, Harty JT. Programming, demarcating, and manipulating CD8+ T-cell memory. Immunological reviews. 2006;211:67–80. doi: 10.1111/j.0105-2896.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 41.Castro I, Dee MJ, Malek TR. Transient enhanced IL-2R signaling early during priming rapidly amplifies development of functional CD8+ T effector-memory cells. J Immunol. 2012;189(9):4321–4330. doi: 10.4049/jimmunol.1202067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441(7095):890–893. doi: 10.1038/nature04790. Epub 2006/06/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nature immunology. 2005;6(12):1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 44.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27(2):281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keppler SJ, Theil K, Vucikuja S, Aichele P. Effector T-cell differentiation during viral and bacterial infections: Role of direct IL-12 signals for cell fate decision of CD8(+) T cells. European journal of immunology. 2009;39(7):1774–1783. doi: 10.1002/eji.200839093. Epub 2009/06/24. [DOI] [PubMed] [Google Scholar]
- 46.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nature immunology. 2003;4(12):1191–1198. doi: 10.1038/ni1009. Epub 2003/11/20. [DOI] [PubMed] [Google Scholar]
- 47.Xue HH, Kovanen PE, Pise-Masison CA, Berg M, Radovich MF, Brady JN, Leonard WJ. IL-2 negatively regulates IL-7 receptor alpha chain expression in activated T lymphocytes. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(21):13759–13764. doi: 10.1073/pnas.212214999. Epub 2002/10/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36(1):68–78. doi: 10.1016/j.immuni.2011.12.007. Epub 2011/12/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang CH, Curtis JD, Maggi LB, Jr, Faubert B, Villarino AV, O’Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, Weber JD, Pearce EJ, Jones RG, Pearce EL. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153(6):1239–1251. doi: 10.1016/j.cell.2013.05.016. Epub 2013/06/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gubser PM, Bantug GR, Razik L, Fischer M, Dimeloe S, Hoenger G, Durovic B, Jauch A, Hess C. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nature immunology. 2013;14(10):1064–1072. doi: 10.1038/ni.2687. Epub 2013/08/21. [DOI] [PubMed] [Google Scholar]
- 51.Driesen J, Popov A, Schultze JL. CD25 as an immune regulatory molecule expressed on myeloid dendritic cells. Immunobiology. 2008;213(9–10):849–858. doi: 10.1016/j.imbio.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 52.Liang D, Zuo A, Shao H, Born WK, O’Brien RL, Kaplan HJ, Sun D. Role of CD25+ dendritic cells in the generation of Th17 autoreactive T cells in autoimmune experimental uveitis. J Immunol. 2012;188(11):5785–5791. doi: 10.4049/jimmunol.1200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanarico N, Ciaramella A, Sacchi A, Bernasconi D, Bossu P, Mariani F, Colizzi V, Vendetti S. Human monocyte-derived dendritic cells differentiated in the presence of IL-2 produce proinflammatory cytokines and prime Th1 immune response. Journal of leukocyte biology. 2006;80(3):555–562. doi: 10.1189/jlb.1105690. [DOI] [PubMed] [Google Scholar]
- 54.Kalia V, Penny LA, Yuzefpolskiy Y, Baumann FM, Sarkar S. Quiescence of Memory CD8(+) T Cells Is Mediated by Regulatory T Cells through Inhibitory Receptor CTLA-4. Immunity. 2015;42(6):1116–1129. doi: 10.1016/j.immuni.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 55.Metzler B, Burkhart C, Wraith DC. Phenotypic analysis of CTLA-4 and CD28 expression during transient peptide-induced T cell activation in vivo. International immunology. 1999;11(5):667–675. doi: 10.1093/intimm/11.5.667. [DOI] [PubMed] [Google Scholar]
- 56.Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nature reviews Immunology. 2001;1(3):220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 57.Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annual review of immunology. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 58.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. The Journal of experimental medicine. 1995;182(2):459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1(5):405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 60.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. The Journal of experimental medicine. 2009;206(4):751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walker LS, Sansom DM. Confusing signals: recent progress in CTLA-4 biology. Trends in immunology. 2015;36(2):63–70. doi: 10.1016/j.it.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LS, Sansom DM. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332(6029):600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nature immunology. 2002;3(7):619–626. doi: 10.1038/ni804. Epub 2002/06/11. [DOI] [PubMed] [Google Scholar]
- 64.Xiao Z, Casey KA, Jameson SC, Curtsinger JM, Mescher MF. Programming for CD8 T cell memory development requires IL-12 or type I IFN. J Immunol. 2009;182(5):2786–2794. doi: 10.4049/jimmunol.0803484. Epub 2009/02/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pufnock JS, Cigal M, Rolczynski LS, Andersen-Nissen E, Wolfl M, McElrath MJ, Greenberg PD. Priming CD8+ T cells with dendritic cells matured using TLR4 and TLR7/8 ligands together enhances generation of CD8+ T cells retaining CD28. Blood. 2011;117(24):6542–6551. doi: 10.1182/blood-2010-11-317966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laidlaw BJ, Cui W, Amezquita RA, Gray SM, Guan T, Lu Y, Kobayashi Y, Flavell RA, Kleinstein SH, Craft J, Kaech SM. Production of IL-10 by CD4(+) regulatory T cells during the resolution of infection promotes the maturation of memory CD8(+) T cells. Nature immunology. 2015;16(8):871–879. doi: 10.1038/ni.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996;273(5271):104–106. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- 68.Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nature immunology. 2003;4(4):355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 69.Macagno A, Napolitani G, Lanzavecchia A, Sallusto F. Duration, combination and timing: the signal integration model of dendritic cell activation. Trends in immunology. 2007;28(5):227–233. doi: 10.1016/j.it.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 70.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nature immunology. 2001;2(5):423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.