Abstract
Epidemiological studies of blood pressure in men and women and in experimental animal models point to substantial sex differences in the occurrence of arterial hypertension as well as in the various manifestations of arterial hypertension, including myocardial infarction, stroke, retinopathy, chronic kidney failure, as well as hypertension-associated diseases (e.g. diabetes mellitus). Increasing evidence demonstrates that the endothelin (ET) system is a major player in the genesis of sex differences in cardiovascular and renal physiology and diseases. Sex differences in the ET system have been described in the vasculature, heart and kidney of humans and experimental animals. In the current review, we briefly describe the role of the ET system in the cardiovascular and renal systems. We also update information on sex differences at different levels of the ET system including synthesis, circulating and tissue levels, receptors, signaling pathways, ET actions, and responses to antagonists in different organs that contribute to blood pressure regulation. Knowledge of the mechanisms underlying sex differences in arterial hypertension can impact therapeutic strategies. Sex-targeted and/or sex-tailored approaches may improve treatment of cardiovascular and renal diseases.
Keywords: endothelin, sex, cardiovascular system, kidney, cardiovascular diseases
Graphical Abstract
In the current review, we will briefly describe the role of the ET system in the cardiovascular and renal systems, and update information on sex differences at different levels of the ET system including synthesis, circulating and tissue levels, receptors, signaling pathways, ET actions, and responses to antagonists in different organs that contribute to blood pressure regulation.
1. Introduction
According to the definition provided by the Department of Reproductive Health and Research, World Health Organization (source: WHO, Gender mainstreaming for health managers: a practical approach, 2011, available at: http://www.who.int/gender-equity-rights/knowledge/glossary/en/), “sex” refers to the different biological and physiological characteristics of males and females, such as reproductive organs, chromosomes, hormones, etc, whereas “gender” refers to the socially constructed characteristics of women and men – such as norms, roles and relationships of and between groups of women and men. Therefore, because the current review is focused on biological rather than social topics, the word “sex” will be used throughout this review.
Arterial hypertension is the single most important contributing factor to cardiovascular morbidity and mortality worldwide [1, 2]. Epidemiological studies of blood pressure in men and women and in experimental animal models point to substantial sex differences in the occurrence of arterial hypertension [3, 4]. In addition, sex differences are found in the various manifestations of arterial hypertension, with men having a higher risk of coronary heart disease than women, especially at younger ages [5], and women having greater susceptibility for developing stroke [6] and heart failure [7, 8].
The endothelin (ET) system is an important contributor to sex differences in arterial hypertension [9]. Sex differences in the ET system have been described in the vasculature and heart of human subjects and animals with arterial hypertension. Extensive evidence supports the central role of the kidney in the control of blood pressure and sodium (Na+) homeostasis. Elevated blood pressure is associated with a rightward-shift in the pressure-natriuresis curve. Sex differences in multiple intra renal signaling systems that contribute to blood pressure control, including ET, renin-angiotensin-aldosterone and nitric oxide signaling systems, have also been described.
In the current review, we focus on the ET system as a major player in the genesis of sex differences in cardiovascular and renal diseases. We will briefly review the role of ET in the cardiovascular and renal systems, and update information on sex differences in the ET system that may impact development and progression of arterial hypertension and renal diseases.
2. Role of ET-1 in the cardiovascular and renal systems
Since the discovery of endothelin-1 (ET-1) in 1988 [10], research has shown that the ET system includes a family of three endogenous 21-amino-acid peptides, ET-1, ET-2 (or vasoactive intestinal contractor, the mouse analogue) and ET-3, which interact with two G-protein-coupled receptors (GPCR) subtypes, ETA and ETB. ET-1 is generated from its precursor pre-pro–ET-1 in a two-step enzymatic pathway to produce the 38-amino-acid precursor big ET-1 or pro-ET-1, which is subsequently cleaved to yield ET-1 by an endothelin-converting enzyme (ECE). Alternatively, chymase, an enzyme found in mast cells, as well as matrix metalloproteinases (MMPs) can cleave big ET-1 to yield a 31-amino-acid ET-1(1-31) and a 32-amino-acid ET-1(1-32), respectively.
ET-1 is continuously released by constitutive pathways and most ET-1 actions are paracrine or autocrine in nature. Whereas most of ET-1 effects are produced by activation of ETA receptors, ETB receptors on vascular endothelial cells are particularly important in terminating ET-1 actions. ETB receptors remove ET-1 from the circulation, a process followed by ET-1 internalization and lysosomal degradation by the carboxypeptidase cathepsin A (reviewed by Barton and Yanagisawa [11]).
2.1. In the myocardium
ET-1 plays key roles in many aspects of cardiac physiology and pathology. ET-1 is vital for aortic arch formation during development, is required for cardiomyocyte survival and prevents myocyte loss during aging. ET-1 modulates coronary blood flow by regulation of vascular tone as well as cardiac muscle function (please refer to DrawneI et al. [12], for a very in-depth and elegant review on the cardiac ET system).
In the heart, ET-1 is synthesized and secreted by endothelial cells, myocytes and fibroblasts. ETA receptors are highly expressed in cardiac myocytes, and both ETA and ETB receptors are expressed in cardiac fibroblasts and endocardial endothelial cells. The human heart has a high density of ET receptors and endothelial cells generate the peptide within the lining of the coronary circulation and the endocardium [12]. Binding of ET-1 to ETA receptors on cardiomyocytes activates many signaling pathways, including protein kinase C (PKC), mitogen-activated protein kinases (MAPK) and G-protein receptor kinase (GRK), which produce intracellular calcium release, reactive oxygen species (ROS) generation and receptor internalization [13, 14]. ET-1 has positive inotropic and chronotropic effects, for example, ET-1 increases cardiac contractility and heart rate, and induces arrhythmias [15, 16]. ET-1 plays a role in cardiac remodeling, particularly maladaptive cardiac hypertrophy. Circulating plasma levels of ET-1 are positively correlated with severity of cardiac diseases and are considered a prognostic indicator of heart failure. It is well established that ET-1 induces left ventricular hypertrophy and congestive heart failure and that ET antagonists have positive effects in the treatment of pulmonary arterial hypertension, which is characterized by right ventricular heart failure (the most common cause of death in patients with this disease). However, ET antagonists have failed in the treatment of left ventricular heart failure [12, 17].
2.2. In the vasculature
In the vasculature, ETA receptors are mainly expressed in vascular smooth muscle cells (VSMCs), favoring short-term control of muscle tone and contributing to vasoconstriction, as well as long-term control of cell growth, adhesion, migration and intercellular matrix deposition [18]. ETB receptors are also expressed in VSMCs with similar actions. ETB receptors are expressed in the endothelial cells and, upon activation by ET-1, induce release of endothelial-derived vasodilator substances, including nitric oxide (NO), to favor vasodilation. The overall effect of ET-1 on vascular tone results from a balance between a direct vasoconstrictor effect via ETA and ETB receptors on smooth muscle cells and the vasodilation mediated by endothelial ETB receptors in endothelial cells (please refer to Rautureau & Schiffrin [19] for an in-depth and elegant review on the vascular ET system).
ET-1 also induces the production of growth factors and inflammatory mediators. ET-1 favors deposition of extracellular matrix components, including collagen and fibronectin, and stimulates ROS production by endothelial and smooth muscle cells. ET-1 activates transcriptional factors and stimulates the expression of many pro-inflammatory cytokines, including Interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), tumor necrosis fator-α (TNF-α)], and enzymes such as inducible nitric oxide synthase (iNOS), cyclo-oxygenase (COX), NADPH oxidases; and the production of adhesion molecules by endothelial cells leading to monocyte migration [19].
2.3. In the renal system
The renal ET-1 signaling system is involved in the control of renal hemodynamics as well as tubular function through activation of ETA and ETB receptors. ET-1 results in strong and sustained vasoconstriction, oxidative stress [20, 21], nephrin shedding, expression of pro-inflammatory factors in podocytes [22, 23], proteinuria [24], and reduces medullary blood flow through activation of ETA receptors [25]. On the contrary, activation of renal ETB receptors induces vasodilation and inhibits Na+ and water reabsorption resulting in subsequent natriuresis and diuresis. The ETB receptor-mediated natriuretic effects are NOS1-dependent [26]. Pharmacological blockade and genetic modification of the ETB receptor function induces salt-sensitive hypertension [27]. Collectively, ET-1 appears to have paradoxical effects on blood pressure regulation by acting either on the renal cortical ETA receptors promoting hypertension or renal medullary ETB receptors promoting hypotension.
2.4. In the immune system
A large body of evidence has accumulated over the last 10 years indicating that ET-1 is an important stimulus for inflammatory pathways. This is evident in a wide range of organ system diseases [28]. ET-1 induces proinflammatory mechanisms, superoxide anion production, cytokine secretion [29, 30], endoplasmic reticulum stress [31], and synthesis of cell adhesion molecules, such as soluble ICAM-1 [28]. Several studies have suggested a potential interaction between the ET and the immune systems in the pathogenesis of pulmonary hypertension [29], portal hypertension [32, 33], preeclampsia (elevated blood pressure in pregnancy) [34, 35], and myocardial infarction [36]. For example, TNF-α is an important stimulus for ET-1 in response to placental ischemia during preeclampsia [34]. In addition, chronic exposure to activated CD4(+) T cells in response to placental ischemia results in ET-1 activation as a mechanism to increase blood pressure during pregnancy [35]. In myocardial infarction patients, BQ-123 leads to reduction in plasma myeloperoxidase (MPO), a marker of neutrophil activation [36].
Additionally, research on several immune-mediated models of renal damage has demonstrated that the ET-1 system is a central contributor in disease progression [28, 37, 38]. Chronic infusion of a non-pressor dose of ET-1 increases glomerular and plasma soluble intercellular adhesion molecule 1 (ICAM-1) and monocyte chemoattractant protein 1 (MCP-1) and increases renal infiltration of macrophages and lymphocytes [38]. These ET-1 induced proinflammatory effects are attenuated by ETA receptor blockade. Similarly, ETA receptor blockade in diabetic rats provides anti-inflammatory actions by reducing hyperglycemia-dependent increases in early inflammatory markers such as MCP-1 and ICAM-1 [24]. In addition, ETA receptor activation mediates renal infiltration of T cells in angiotensin II-infused animals [39]. Furthermore, a recent clinical study has uncovered a new interaction between ET-1 and the immune system, with functional autoantibodies against ETA receptors involved in atherosclerotic pathophysiology [40]. These studies highlight an important role for ET-1/ETA receptor in inflammatory processes. However, the exact mechanism by which ET-1 induces inflammation and whether sex plays a role in this context remain undefined yet.
3. Sex differences in the ET-1 system
Sex-specific differences in age-related increases in blood pressure, in the prevalence of arterial hypertension [3, 4] and in the various manifestations of arterial hypertension have been consistently reported [1, 5–8]. Premenopausal women are largely protected against hypertension compared to age-matched men [4]. Experimental models of hypertension support the idea that males develop an earlier and more severe form of hypertension than females [41–44]. After menopause, blood pressure rises much faster in women, and, at this stage of life, the prevalence of hypertension is greater in women than men [45]. The prevalence of hypertension is higher in postmenopausal than premenopausal women, and the progression of renal diseases is less severe in women before menopause [46, 47], suggesting that gonadal hormones directly modulate sex differences in hypertension. Indeed, gonadal hormones modulate several pathways, including the ET system, that contribute to blood pressure control, by regulating the cardiovascular, renal and neuronal systems function [4, 46, 48–55].
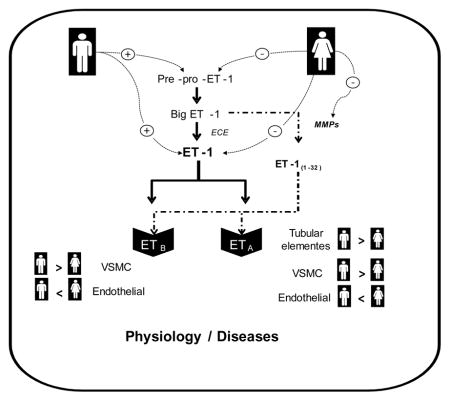
Sex differences in the ET system occur on different levels: expression of pre-pro-ET-1 and ECE activity/expression, plasma and tissue levels of ET-1, expression/activity of ETA and ETB receptors, effects of ET-1 on the cardiovascular and renal systems, responses to ETA and ETB receptors antagonists. Sex differences in the ET system also occur during the developmental programming of hypertension. This section will focus on the different aspects of sex differences in the ET system in the vasculature, heart and kidney. Whenever possible, the influence/contribution of sex hormones to these differences will be discussed.
3.1. Plasma and tissue levels of ET-1
Although sex differences are observed in blood pressure values in hypertensive adolescents, no significant changes in ET-1 levels have been found [56]. Sex differences in plasma ET-1 in hypertensive subjects are observed during the adult life, with men exhibiting higher plasma ET-1 levels than age-matched women [57, 58]. Healthy older women display higher ET-1 concentrations compared to healthy middle-aged women [59]. Indeed, aging is positively associated with increased ET-1 levels [60–63] and postmenopausal women exhibiting high testosterone levels also display high ET-1 levels, in comparison to those with lower testosterone levels, which may be relevant for increased blood pressure [64] and a proatherogenic profile [65].
Evidence points to a role for female gonadal hormones contributing to lower ET-1 plasma levels in females, compared to age-matched males. During the menstrual cycle, plasma ET-1 levels fluctuate. In the follicular and luteal phase, higher amounts of estrogen are secreted and lower plasma ET-1 levels are observed in comparison to the menstrual phase [63]. Pregnancy, another condition linked with increased levels of estrogen and progesterone, is also associated with decreased plasma ET-1 levels [57, 66–68]. Of importance, pregnant women with preeclampsia display increased levels of ET-1 [69, 70] and lower circulating progesterone, compared to normal pregnant women [71]. ETA receptor blockade attenuates hypertension associated with preeclampsia [72].
Whereas low doses of a progestagen-only (etonogestrel) contraceptive do not change plasma ET-1 concentrations [73], hormone replacement therapy with 17β-estradiol combined with progestins decreases plasma ET-1 in humans [74]. On the other hand, short-term administration of 17β-estradiol with different progestins does not significantly change circulating ET-1 levels in healthy menopausal women [75, 76]. Male-to-female transsexual patients treated with estradiol and cyproterone acetate also exhibit decreased levels of ET-1 [57].
In experimental animal models, long-term replacement therapy with estradiol (6 months) prevents increases in ET-1 levels induced by ovariectomy. This effect of estradiol is also observed in female rats that received nicotine and in women subjected to environmental smoke, suggesting a protective role of this hormone via down-regulation of the ET system [77]. Raloxifene, a selective estrogen receptor modulator, decreases ET-1 levels in aortas from aged ovariectomized rats [78] and decreases plasma ET-1 in healthy postmenopausal women [79, 80]. Raloxifene effects on plasma ET-1 are observed after 6-months of treatment [79, 80] but not after short periods of treatment (4 weeks) [81].
Androgens seem to positively modulate ET-1 expression since female-to-male transsexual patients treated with testosterone display increased ET-1 levels [57]. Hyperandrogenism is common in women with polycystic ovary syndrome, and these patients display higher levels of ET-1, compared to healthy and age-matched women [82–85]. Antagonism of ETA and ETB receptors augments vasodilation in polycystic ovary syndrome patients [86]. Conversely, patients with various forms of hypogonadism display significantly higher ET-1 levels in comparison with age-matched healthy males and testosterone therapy decreased ET-1 levels in these individuals [87].
3.2. Expression of pre-pro-ET-1 and ECE activity
Whereas 17β-estradiol inhibits ET-1 mRNA expression in various cell types, including endothelial cells, smooth muscle cells, cardiac myocytes, fibroblasts and mesangial cells [88–95], testosterone has been shown to increase ET-1 mRNA in human aortic endothelial cells [96]. Estrogen seems to modulate ET-1 gene expression and production also by indirect mechanisms. Estrogen increases nitric oxide synthase (NOS) activity and bioavailability of NO, an important factor that modulates ET-1 production [52], and inhibition of NOS partly attenuates the inhibitory effect of 17β-estradiol on ET-1 production [88, 97].
The effects of gonadal hormones on ET-1 production are also observed in experimental animal models. Deoxycorticosterone acetate (DOCA)-salt hypertensive male rats exhibit higher pre-pro-ET-1 mRNA compared to hypertensive females [98, 99], and ovariectomy augments pre-pro-ET-1 mRNA [98]. Ovariectomy also augments plasma ECE activity whereas 17β-estradiol replacement inhibits plasma ECE activity [100]. The negative modulatory effects of estrogen on ECE activity may be tissue-dependent, since acute 17β-estradiol-induced inhibition of ECE is greater in internal mammary arteries compared to saphenous veins [101]. Phytoestrogens, such as genistein and daidzein, inhibit ECE-1 mRNA expression in rat mesenteric arteries [102]. To date, there is no evidence showing a regulatory role of testosterone on ECE activity in the cardiovascular system. Of importance, ECE polymorphisms have been associated with sex-dependent cardiovascular risks. For example, a genetic polymorphism located in the ECE-1 gene promoter (ECE-1b C-338A) is associated with increased risk of coronary artery disease [103], carotid atherosclerosis [104] and ischemic stroke [105] in Chinese women.
3.3. ET receptors
Tissue distribution of ET receptors is different between males and females, generally favoring vasodilation in females, that is, relatively fewer ETA receptors compared to ETB. Both ETA and ETB receptors, as well as their ratio, are increased in saphenous veins from men, compared to women [106]. Vascular ETB receptors mRNA is increased in male DOCA-salt rats, compared to females [98, 99]. ETA receptor activation is modified by age in a sex-dependent manner. Middle-aged and older normotensive men are more responsive to vasoconstriction elicited by ETA receptors, compared to women, as demonstrated by forearm blood flow experiments [107].
Ovarian hormones also modulate ETB receptors. 17β-estradiol down-regulates ETB receptor mRNA in isolated rat cardiomyocytes of spontaneously hypertensive rats (SHR) [108]. Ovariectomy up-regulates ETB receptors in the ventricular myocardium of SHR [108] and in vessels from DOCA-salt rats [98].
Hormonal therapy with 17β-estradiol or conjugated equine estrogens reduces ETA receptors in rabbit thoracic aorta. However, the reduction of pre-pro-ET-1 (RNA expression) is only achieved when conjugated equine estrogens are administered in combination with norethisterone acetate [109]. In rabbit coronary arteries, long-term estrogen treatment selectively attenuates ET-1-induced vasoconstriction, possibly by increasing ETB gene expression. This effect is abolished by progestins, suggesting that progestins may oppose the beneficial effects of estrogen on the arterial wall [110]. In portal veins, expression of ETB receptors and ET-1 levels are enhanced in orchidectomized rats, and testosterone-replacement therapy completely reversed this effect [111].
Experiments conducted in the spotting-lethal (sl) rat, which carries a natural deletion in the ETB receptor gene, indicate that ETB receptor protects females during development of hypertension. Whereas elevation of systolic blood pressure by DOCA-salt treatment in wild-type rats is higher in males than in females, in the homozygous spotting-lethal (sl/sl) rats treatment with DOCA-salt similarly increases systolic blood pressure in male, female and ovariectomized rats [112]. Potential mechanisms involved in the protective effects of ETB receptors include modulation of vascular ROS generation [112] and renal NOS activity [113].
In rats, pregnancy increases the expression of endothelial ETB receptors in mesenteric arteries, which is followed by enhanced ETB receptor-mediated vasodilation. Increased endothelial ETB receptor function may be an important contributor to decreased vascular resistance during pregnancy [114]. The expression of ETA and ETB receptors during pregnancy may be modulated differently in pathological conditions, since pregnant rats submitted to chronic hypoxia develop preeclamptic symptoms, via enhancement of ET-1 and ETA receptor mediated signaling [115]. Male, but not female, rats under chronic infusion of Ang II display an attenuated natriuretic response to sarafotoxin 6c, an ETB receptor agonist, compared to normotensive rats, indicating impairment of ETB receptors function in male hypertensive rats. ET receptors function is maintained in female rats infused with Ang II [116]. Under high salt intake, administration of A-192621, an ETB receptor antagonist, increases blood pressure in both sexes, confirming that ETB receptors are important to limit blood pressure increases during high salt feeding. When high salt intake is associated with Ang II infusion, male rats have significantly higher mean arterial pressure compared to females and, in this case, ETB receptor antagonism abolishes sex differences in blood pressure control [117]. These studies reinforce the idea that in some forms of hypertension, female protection is conferred by differential ETB receptors function/expression. Female rats lacking functional ETB receptors everywhere except sympathetic nerves (ETB deficient rats) have a greater blood pressure response to acute stress than males when placed on high salt [118]. More work needs be performed to fully elucidate the role of ETA and ETB receptors in the sympathetic control of blood pressure in males and females.’
3.4. ET-1 effects in the cardiovascular system
During the reproductive age, vascular reactivity is functionally different between sexes. Competitive binding studies conducted in saphenous veins from patients who underwent coronary artery bypass graft surgery demonstrated a higher ratio of ET-1 binding both in ETA and ETB receptors in men, as compared to women [106]. Administration of the ETB receptor antagonist BQ-788 into the forearm skin of human subjects, by intradermal microdialysis, revealed that ETB receptors in men mediate tonic vasoconstriction, whereas ETB receptors in women mediate tonic vasodilation [119]. Human cerebral arteries from women, collected from patients undergoing neurological surgery of brain tumors or epilepsy, are significantly less responsive to ET-1, compared to arteries from men. Interestingly, expression of ETB receptor mRNA is higher in cerebral arteries from female subjects [120].
Increased sensitivity to ET-1 and to the selective ETB receptor agonist IRL-1620 has also been reported in conductance and resistance arteries from male, compared to female, DOCA-salt rats [121, 122]. In vivo, IRL-1620 induces vasodilation in mesenteric microvessels from control rats, marked vasoconstriction in male DOCA-salt rats and a very mild constriction in vessels from female DOCA-salt rats [122]. Changes in ETB-mediated vascular responses are associated with increased ET-1 and ETB receptor gene expression in male, but not in female, animals [99]. Furthermore, ovariectomy increases the vasoconstrictor responses to the ETB agonist, and estrogen-replacement therapy restores IRL-1620-induced responses [98], reinforcing the suggestion that the ovarian hormones modulate ET-1/ETB receptor vascular responses in salt-sensitive hypertension.
Intracellular pathways may also be sex-regulated during hypertension, resulting in differential vascular contractility. Augmented constriction in aorta and resistance mesenteric arteries from DOCA-salt male rats, compared to females, is associated with increased phosphorylation of ERK 1/2, a pathway activated by ET-1, and decreased mitogen-activated protein kinase phosphatase 1 (MKP-1) [41].
Sexual dimorphism in vascular reactivity to ET-1 is evident in other experimental models of hypertension. In SHR, aorta and mesenteric arterioles from males exhibit greater sensitivity to ET-1 compared to females. Vascular sensitivity to ET-1 is similar between female SHR and their normotensive controls [123]. Skeletal muscle arteries from female Yucatan pigs develop greater contractile force in response to ET-1 compared to arteries from males. Exercise training increases ET-1-induced contractions in arteries from males, but not in arteries from females [124]. Additionally, exercise training reduces vascular sensitivity to vasoconstrictor responses to ET-1, with the adaptation response being greater in male than in female [125].
Intracoronary infusion of estrogen mediates a greater increase in the coronary blood flow in angiographically normal middle-aged women, who simultaneously exhibit lower ET-1 levels [126]. In addition, 17β-estradiol attenuates ET-1-induced coronary artery constriction both in vitro and in vivo [127–129], whereas testosterone significantly potentiates responses elicited by ET-1 [129]. Testosterone effects are not inhibited by androgen receptor antagonists (flutamide or cyproterone acetate), indicating that testosterone effects are not mediated by classical genomic activities [129].
Sex-related differences in other vascular effects of ET-1 have been reported. ET-1 inhibits proliferation of smooth muscle cells derived from female, but not male, pig coronary arteries [130]. In a rodent model of brain ischemic injury, injection of ET-1 adjacent to the middle cerebral artery induces brain injury, with males having greater damage compared to females [131]. However, upon ET-1 stimulation, both testosterone in males, as well as 17β-estradiol in females prevent constriction-induced inward remodeling in isolated mesenteric arteries from rats, an effect dependent on NO and transglutaminase activity [132].
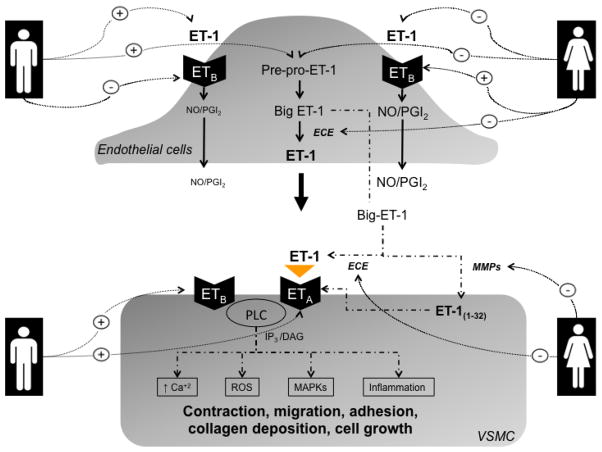
In the heart, cardiac adaptations to DOCA-salt hypertension display sex-specific dimorphism independent of blood pressure with male mice exhibiting greater left ventricular and renal hypertrophy compared with female mice [133]. On the other hand, DOCA-salt-treatment of estrogen receptor β deficient (ERβ−/−) mice produces greater cardiac hypertrophy in females than in males. Cardiac hypertrophy in female ERβ−/− mice treated with DOCA-salt is associated with greater collagen deposition and differential activation of ERK1/2 and p38, proteins of the MAPK signaling pathways [134]. Importantly, ET-1 is a key player in mediating cardiovascular abnormalities associated with DOCA-salt hypertension [135, 136]. Figure 1 summarizes the sex differences in ET system in the vasculature.
Figure 1.
Hypothetical scheme highlighting sexual dimorphism in ET system in the vasculature. Ca2+, calcium; DAG, diacylglycerol; ET-1, endothelin-1; ECE, endothelin converting enzyme; ETA/ETB, subtype A and subtype B endothelin receptors; IP3, inositol trisphosphate; MAPK, mitogen-activated protein kinases; MMPs, matrix metalloproteinases; NO, nitric oxide; PGI2, prostacyclin; PLC, phospholipase C; ROS, reactive oxygen species; VSMCs, vascular smooth muscle cells.
3. 5. ET-1 effects in the renal system
In the several last years, studies have provided evidence that the renal medullary ET-1 system contributes to sex differences in blood pressure. Nakano and Pollock [137] demonstrated that infusion of ET-1 into the renal medulla results in enhancement of Na+ excretion only in female rats. This sexual dimorphism can be related to the reduction in medullary blood flow in male rats that was not evident in female rats. Interestingly, ovariectomy attenuates this male-female difference [137], suggesting an important role for ovarian hormones in mediating sex differences in renal medullary ET-1 function. Additionally, Kittikulsuth et al. [116] found that intramedullary infusion of the ETB agonist, S6c, results in natriuresis in female Ang II hypertensive rats, but not in males. Moreover, renal medullary ET-1 infusion to female Ang II hypertensive rats stimulates natriuresis, which is partially blunted by ETA receptor antagonism [116]. These two studies highlights that medullary ETA receptors contribute to natriuresis in female rats lacking ETB receptors or infused chronically with Ang II. However, ETA receptor expression and Ca2+ signaling in inner medullary collecting duct (IMCD) are greater in male compared with female Sprague Dawley rats, whereas, IMCD ETB receptor binding is not sexually dimorphic [138]. Taken together, it is postulated that ETA dependent natriuresis demonstrated in female rats is related to ETA receptors located on other cell types [138].
Despite the evidence that the renal ET-1 system is sexually dimorphic and gonadectomy eliminates this dimorphism in animal model [139]), the roles of male and female sex steroids in mediating sexual dimorphism in renal ET-1 system have been poorly studied. Few studies have explored a direct interaction between male sex steroids and renal ET-1 system. Kalk et al. [140] demonstrated that the renal histopathological phenotype in male ET-1 transgenic mice with regard to glomerulosclerosis, proteinuria, perivascular fibrosis and immune cell immigration is ameliorated by castration, suggesting that the effects of ET-1 overexpression on renal tissue injury are modulated by androgens. Additionally, renal ET-1 system has been implicated in sex differences in renal ischemic susceptibility based on androgens particularly [141]. Thus, androgens may up-regulate ET-1 production promoting renal damage by causing renal vasoconstriction and mitogenesis that can contribute to kidney fibrosis [142].
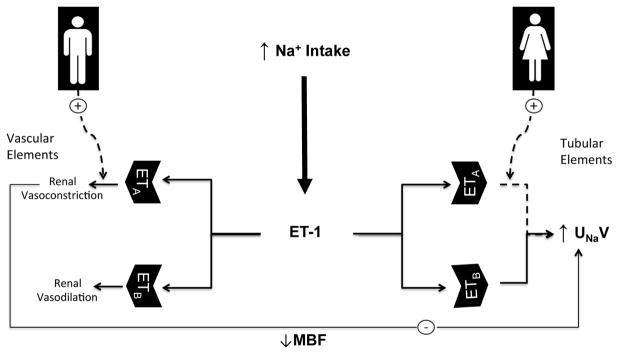
A role for the female sex steroids, estrogen and progesterone, in mediating sex differences in renal ET systems has been also suggested. Estradiol suppresses renal ET-1 overproduction and the consequent renal damage in acute renal failure [143]. This suppressive effect of estradiol on renal ET-1 overproduction is closely related to the NO pathway and is probably involved in the beneficial effect of estradiol on ischemia/reperfusion-induced renal injury [144]. Ba and Chaudry demonstrated that the administration of estradiol protects different organs, including the kidneys, against ischemia-reperfusion damage through a mechanism that involves, at least in part, estradiol-induced reduction in ET-1 renal vasoconstriction probably through activation of renal estrogen receptor-β [145]. Estradiol attenuated the inhibitory effect of ovariectomy on ETA and ETB receptor expression in the renal cortex [146]. Additionally, supplementation of OVX rats with estradiol and progesterone prevented the increase in renal inner medullary ETA and ETB receptor expression demonstrated in OVX rats [146]. The female sex appears to be protective against the progression of autosomal-dominant polycystic kidney disease, in association with changes in renal expression of ET-1 [139]. The difference in renal ET-1 levels between male and female rats with autosomal-dominant polycystic kidney disease (ADPKD) is attenuated by ovariectomy, but not orchiectomy [139], suggesting a central role for ovarian hormones in sexual dimorphism in renal ET-1 system in ADPKD. Moreover, an interaction between progesterone and the renal ET-1 system has been also suggested. Zhang et al. [147] showed that pregnancy and progesterone enhance renal ETA receptor expression. Recent studies support a role of renal ET-1 in the pathophysiology of preeclampsia [115, 148]. However, further studies are required to clarify the link between progesterone and the renal ET-1 particularly in the context of preeclampsia. Figure 2 summarizes the sex differences in ET system in the kidney.
Figure 2.
Hypothetical scheme highlighting sexual dimorphism in ET system in the kidney. ET-1, endothelin-1; ETA/ETB, subtype A and subtype B endothelin receptors; MBF, medullary blood flow; Na+, sodium; UNaV urinary sodium (Na2+) excretion.
3.6. Pressor effects and responses to ET receptor antagonists
Sex differences have been reported in the pressor effects of ET-1. In anesthetized rats, ET-1 produces rapid and transient falls in arterial blood pressure and hindquarter resistance, followed by long-lasting increases in blood pressure and hindquarter resistance. The initial ET-1-induced hypotension and vasodilation are similar in male and female rats. However, the pressor and hindquarter vasoconstrictor effects are significantly higher in male than female rats [149].
Bosentan, a mixed ETA and ETB receptor antagonist is more effective to reduce blood pressure in male, compared to female, DOCA-salt rats, supporting the idea that differential activation of ET-1 pathways contributes to higher blood pressure in male hypertensive subjects [122]. Ovariectomy augments the blood pressure-lowering effects of bosentan whereas estradiol replacement, combined or not with progesterone, restores ET-1 plasma levels and the blood pressure effects of bosentan [98]. Administration of ABT-627, an ETA receptor antagonist, to sl/sl rats for two weeks reduces blood pressure in a greater magnitude in females, compared to male rats [112].
A study looking at the pharmacokinetics and safety of macitentan, a dual ET-1 receptor antagonist, in healthy Caucasian and Japanese subjects, showed that Cmax values 1 for macitentan and its active metabolite, ACT-132577, are approximately 25% higher in Caucasian women, compared to Caucasian men. Additionally, Caucasians and Japanese women display a 15% higher exposure to ACT-132577, when compared to men. Although these pharmacokinetic changes are not considered clinically relevant, since clinical laboratory and electrocardiographic parameters are similar among the groups [150], it is possible that sex-related differences in ET antagonists’ pharmacokinetics may account for some of the differences in the effects of ET antagonists.
Obese female SHHF/Mcc-fa(cp) rats are resistant to the antihypertensive effects of irbesartan, an AT1 receptor antagonist, compared with males. The resistance to the depressor effects of irbesartan in obese females is associated with increased urinary ET-1 excretion [151]. In addition, a G5665T gene polymorphism of pre-pro-ET-1 in humans modifies responses to antihypertensive treatments in men, but not in women [152]. Changes in systolic blood pressure, after 12 weeks of treatment with either irbesartan or atenolol, are more than 2-fold greater in men carrying the T allele compared with those with the G/G genotype, whereas no significant differences in blood pressure changes between G/G and carriers of the T allele are seen among women [152].
Sex hormones may contribute to the differential response to ET antagonists in males and females. Ovariectomized rats treated with J-104132, a mixed ETA/ETB receptor antagonist, for two weeks exhibit decreased neointimal formation after balloon injury. Combination of J-104132 with an Ang II antagonist, but not with 17β-estradiol, further reduces neointimal formation, suggesting that the antagonism of ET receptors have estrogen-like vasoprotective actions in neointimal formation [153].
Several clinical studies showed that selective ETA receptor antagonists and combined ETA and ETB receptor antagonists lower blood presssure in hypertensive patients [154, 155] and reduce proteinuria and albuminuria in patients with chronic kidney diseases and [156, 157] and diabetic nephropathy [158, 159]. Despite the high therapeutic potential of ET receptor antagonists as antihypertensive and renoprotective agents, none of these antagonists has been approved for management of hypertension or nephropathies. This is mainly attributed to their side effects, particularly fluid retention [160]. However, fluid retension appears to be managed by proper dosing of the ET receptor antagonist along with concomitant adminstration of diuretics [161]. In general, the therapeutic potential of ET receptor antagonism for treatment of cardiovascular and renal diseases has been recently reviewed by several elegant reviews in 2015 [162–166]. Clinical trials focusing on sex-associated differences and confirmation in humans of data on sex differences in the ET-1 system obtained in experimental animals will help to establish sex-targeted or sex-tailored interventions for the treatment of cardiovascular and renal diseases.
3.7. ET and sex specific developmental programming of hypertension
Events occurring early in life, comprising the fetal period, contribute to long-term modulation in the cardiovascular functions, including blood pressure control [167–170]. Therefore, sex differences in adult blood pressure may result from sex-specific developmental programming, through several mechanisms including the ET system [171] (See Ojeda 2014 for an in-depth review on sex differences in the developmental programming of hypertension).
One factor that may modulate sex differences in the early life programming of ET system is stress. Stressors such as hypoxia are considered risk factors related to complications during pregnancy, and exposure to hypoxia during the fetal period augments cardiovascular disease in later-life [172]. Mesenteric resistance arteries from aged male rats, but not aged female rats, which were exposed to prenatal hypoxia during day 15 to 21 of pregnancy, display increased vasoconstriction to big ET-1, but not to ET-1, compared to their aged-matched controls. This response is almost completely abolished by an ECE antagonist (CGS35066). Interestingly, NOS inhibition potentiates big ET-1 contractility observed in male offspring that underwent intrauterine growth restriction (IUGR), demonstrating a modulatory role for NO. ET-1 antagonism results in more significant blood pressure-lowering effect in IUGR male offspring, compared to female IUGR offspring [173]. These findings indicate that hypoxia during the prenatal life favors vascular sex differences observed in the ET system, with long-term consequences [171, 173].
Male and female offspring from sheep administered the steroid betamethasone during pregnancy exhibit increased mean arterial pressure. Administration of ET-1 generates a more pronounced effect on vascular resistance and increases in blood pressure in female offspring compared to the male offspring [174]. These findings indicate that exposure to different drugs/agents during the antenatal phase can program vascular responses to ET-1 in adult, in a sex-specific manner.
Maternal separation, a model of early life stress, decreases expression of vascular ETA and ETB receptors in male rats. ET receptor blockade significantly blunts acute air jet stress-induced pressor response in control rats and in rats submitted to maternal separation. Acute air jet stress also increases plasma corticosterone levels in control, but not in rats submitted to maternal separation, an effect inhibited by ET receptor blockade [175]. Acute air jet stress-elicited pressor response involves ROS-dependent mechanisms, downstream of ETB receptor activation [176]. These data reinforce the idea that early life stress modulates the ET-1 system, and augments stress-induced pressor responses later in life [175].
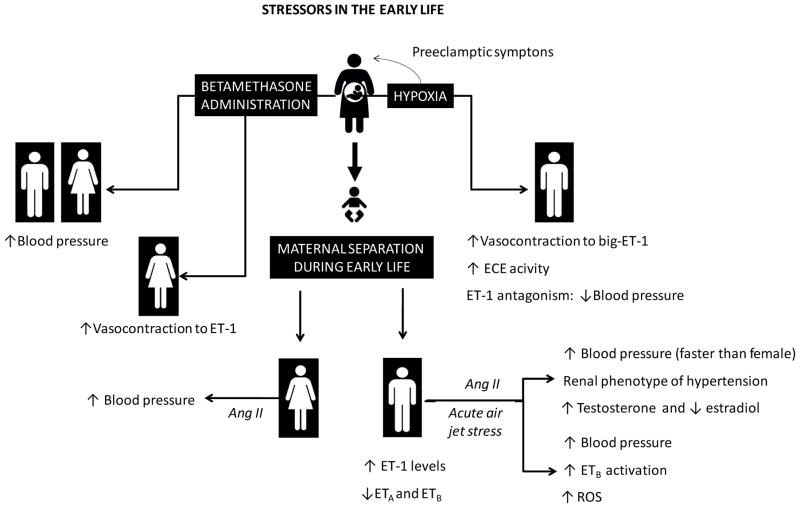
Human studies reinforce a positive relation between exposure to adverse childhood experiences and high ET-1 in young adults, but failed to show an interaction between sex-related adverse childhood experiences with ET-1 levels [177]. Therefore, it will be interesting in the future to describe which conditions may interfere in the sex-specific programming of blood pressure through the modulation of the ET-1 system components. Figure 3 summarizes the sex differences in ET system in development programming.
Figure 3.
Stressors in the early life and sex specific developmental programming of hypertension. Ang II, angiotensin II; ET-1, endothelin-1; ECE, endothelin converting enzyme; ETA/ETB, subtype A and subtype B endothelin receptors; ROS, reactive oxygen species.
3.8. ET and sex chromosomes
Importantly, sex differences in physiology and pathophysiology arise not only from direct effects of sex hormones, but also from sex chromosome genes. However, limited studies have addressed the potential role for the sex chromosomes in the development of cardiovascular and renal diseases. Using the four-core genotype model, Chen et al. have demonstrated that adiposity is linked to X chromosome [178]. Although blood pressure was not monitored in this particular study, it is well established that obesity has a hypertensive effect [179]. Recently, the use of the same mouse model has revealed that sex chromosome genes participate in the modulation of neural pathways underlying regulatory response to renin-angiotensin stimulation [180, 181]. Additionally, angiotensin-induced vasodilation and endothelium-derived hyperpolarizing factors require the XX chromosome sex complement [181]. It is very important to separate the contribution of the female sex steroids on the cardiovascular and renal ET system from the contribution made by sex chromosomal complement. However, this area has not been explored yet.
4. Conclusions and perspectives
Despite the fact that several studies have attracted the attention to sexual dimorphism in the molecular pathways involved in the pathophysiology of cardiovascular and renal diseases, the current national guidelines for treatments of hypertension and other cardiorenal diseases still recommend the same therapeutic approaches for both sexes. Thus, starting to think about the selection of sex-specific drugs for management of cardiovascular and renal diseases should be a central goal, which is even further supported by available data from clinical trials highlighting sex differences in the blood lowering capacity of some classes of antihypertensive medications [182–185].
Acknowledgments
This work was supported by a postdoctoral grant from the American Heart Association (15POST25090329 to EYG) and by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2004/13796-9, 2013/08216-2 to RCT), LÓREAL - For Women in Sciences (to FRG), Fundação de Amparo à Pesquisa do Estado de Mato Grosso (FAPEMAT 151371/2014 to FRG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 471675/2013-0 to FRG) as well as funding from the National Heart Lung and Blood Institute (HL69999 and HL95499 to DMP).
Footnotes
Cmax = maximum (or peak) serum concentration that a drug achieves in a specified compartment or test area of the body after the drug has been administrated and prior to the administration of a second dose.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
List of References
- 1.Kearney PM, et al. Worldwide prevalence of hypertension: a systematic review. J Hypertens. 2004;22(1):11–9. doi: 10.1097/00004872-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303(20):2043–50. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 3.Cutler JA, et al. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52(5):818–27. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 4.Sandberg K, Ji H. Sex differences in primary hypertension. Biol Sex Differ. 2012;3(1):7. doi: 10.1186/2042-6410-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM, et al. Lifetime risk of developing coronary heart disease. Lancet. 1999;353(9147):89–92. doi: 10.1016/S0140-6736(98)10279-9. [DOI] [PubMed] [Google Scholar]
- 6.Petrea RE, et al. Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke. 2009;40(4):1032–7. doi: 10.1161/STROKEAHA.108.542894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lloyd-Jones DM, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106(24):3068–72. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 8.Leening MJ, et al. Sex differences in lifetime risk and first manifestation of cardiovascular disease: prospective population based cohort study. BMJ. 2014;349:g5992. doi: 10.1136/bmj.g5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tostes RC, et al. Endothelin, sex and hypertension. Clin Sci (Lond) 2008;114(2):85–97. doi: 10.1042/CS20070169. [DOI] [PubMed] [Google Scholar]
- 10.Yanagisawa M, et al. A novel peptide vasoconstrictor, endothelin, is produced by vascular endothelium and modulates smooth muscle Ca2+ channels. J Hypertens Suppl. 1988;6(4):S188–91. doi: 10.1097/00004872-198812040-00056. [DOI] [PubMed] [Google Scholar]
- 11.Barton M, Yanagisawa M. Endothelin: 20 years from discovery to therapy. Can J Physiol Pharmacol. 2008;86(8):485–98. doi: 10.1139/Y08-059. [DOI] [PubMed] [Google Scholar]
- 12.Drawnel FM, Archer CR, Roderick HL. The role of the paracrine/autocrine mediator endothelin-1 in regulation of cardiac contractility and growth. Br J Pharmacol. 2013;168(2):296–317. doi: 10.1111/j.1476-5381.2012.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogoyevitch MA, Parker PJ, Sugden PH. Characterization of protein kinase C isotype expression in adult rat heart. Protein kinase C-epsilon is a major isotype present, and it is activated by phorbol esters, epinephrine, and endothelin. Circ Res. 1993;72(4):757–67. doi: 10.1161/01.res.72.4.757. [DOI] [PubMed] [Google Scholar]
- 14.Bogoyevitch MA, et al. Endothelin-1 and fibroblast growth factors stimulate the mitogen-activated protein kinase signaling cascade in cardiac myocytes. The potential role of the cascade in the integration of two signaling pathways leading to myocyte hypertrophy. J Biol Chem. 1994;269(2):1110–9. [PubMed] [Google Scholar]
- 15.Kelly RA, et al. Endothelin enhances the contractile responsiveness of adult rat ventricular myocytes to calcium by a pertussis toxin-sensitive pathway. J Clin Invest. 1990;86(4):1164–71. doi: 10.1172/JCI114822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baltogiannis GG, et al. Endothelin receptor--a blockade decreases ventricular arrhythmias after myocardial infarction in rats. Cardiovasc Res. 2005;67(4):647–54. doi: 10.1016/j.cardiores.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Cowburn PJ, et al. Comparison of selective ET(A) and ET(B) receptor antagonists in patients with chronic heart failure. Eur J Heart Fail. 2005;7(1):37–42. doi: 10.1016/j.ejheart.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Schiffrin EL, Touyz RM. Vascular biology of endothelin. J Cardiovasc Pharmacol. 1998;32(Suppl 3):S2–13. [PubMed] [Google Scholar]
- 19.Rautureau Y, Schiffrin EL. Endothelin in hypertension: an update. Curr Opin Nephrol Hypertens. 2012;21(2):128–36. doi: 10.1097/MNH.0b013e32834f0092. [DOI] [PubMed] [Google Scholar]
- 20.Elmarakby AA, et al. NADPH oxidase inhibition attenuates oxidative stress but not hypertension produced by chronic ET-1. Hypertension. 2005;45(2):283–7. doi: 10.1161/01.HYP.0000153051.56460.6a. [DOI] [PubMed] [Google Scholar]
- 21.Chen DD, et al. Endothelin 1 activation of endothelin A receptor/NADPH oxidase pathway and diminished antioxidants critically contribute to endothelial progenitor cell reduction and dysfunction in salt-sensitive hypertension. Hypertension. 2012;59(5):1037–43. doi: 10.1161/HYPERTENSIONAHA.111.183368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morigi M, et al. Shigatoxin-induced endothelin-1 expression in cultured podocytes autocrinally mediates actin remodeling. Am J Pathol. 2006;169(6):1965–75. doi: 10.2353/ajpath.2006.051331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fligny C, Barton M, Tharaux PL. Endothelin and podocyte injury in chronic kidney disease. Contrib Nephrol. 2011;172:120–38. doi: 10.1159/000328692. [DOI] [PubMed] [Google Scholar]
- 24.Saleh MA, et al. Endothelin receptor A-specific stimulation of glomerular inflammation and injury in a streptozotocin-induced rat model of diabetes. Diabetologia. 2011;54(4):979–88. doi: 10.1007/s00125-010-2021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohan DE. The renal medullary endothelin system in control of sodium and water excretion and systemic blood pressure. Curr Opin Nephrol Hypertens. 2006;15(1):34–40. doi: 10.1097/01.mnh.0000186852.15889.1a. [DOI] [PubMed] [Google Scholar]
- 26.Nakano D, Pollock JS, Pollock DM. Renal medullary ETB receptors produce diuresis and natriuresis via NOS1. Am J Physiol Renal Physiol. 2008;294(5):F1205–11. doi: 10.1152/ajprenal.00578.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kittikulsuth W, Sullivan JC, Pollock DM. ET-1 actions in the kidney: evidence for sex differences. Br J Pharmacol. 2013;168(2):318–26. doi: 10.1111/j.1476-5381.2012.01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saleh MA, Pollock DM. Endothelin in renal inflammation and hypertension. Contrib Nephrol. 2011;172:160–70. doi: 10.1159/000328696. [DOI] [PubMed] [Google Scholar]
- 29.Yeager ME, et al. Endothelin-1, the unfolded protein response, and persistent inflammation: role of pulmonary artery smooth muscle cells. Am J Respir Cell Mol Biol. 2012;46(1):14–22. doi: 10.1165/rcmb.2010-0506OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kowalczyk A, et al. The role of endothelin-1 and endothelin receptor antagonists in inflammatory response and sepsis. Arch Immunol Ther Exp (Warsz) 2015;63(1):41–52. doi: 10.1007/s00005-014-0310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Miguel C, Pollock JS. Does endoplasmic reticulum stress mediate endothelin-1-induced renal inflammation? Am J Physiol Regul Integr Comp Physiol. 2013;305(2):R107–9. doi: 10.1152/ajpregu.00184.2013. [DOI] [PubMed] [Google Scholar]
- 32.Nagasue N, et al. Production and release of endothelin-1 from the gut and spleen in portal hypertension due to cirrhosis. Hepatology. 2000;31(5):1107–14. doi: 10.1053/he.2000.6596. [DOI] [PubMed] [Google Scholar]
- 33.Kawanaka H, et al. Effect of laparoscopic splenectomy on portal haemodynamics in patients with liver cirrhosis and portal hypertension. Br J Surg. 2014;101(12):1585–93. doi: 10.1002/bjs.9622. [DOI] [PubMed] [Google Scholar]
- 34.LaMarca B, et al. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension. 2008;52(6):1161–7. doi: 10.1161/HYPERTENSIONAHA.108.120881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallace K, et al. Hypertension in response to CD4(+) T cells from reduced uterine perfusion pregnant rats is associated with activation of the endothelin-1 system. Am J Physiol Regul Integr Comp Physiol. 2012;303(2):R144–9. doi: 10.1152/ajpregu.00049.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adlbrecht C, et al. Peri-interventional endothelin--a receptor blockade improves long-term outcome in patients with ST-elevation acute myocardial infarction. Thromb Haemost. 2014;112(1):176–82. doi: 10.1160/TH13-10-0832. [DOI] [PubMed] [Google Scholar]
- 37.Orth SR, et al. Endothelin in renal diseases and cardiovascular remodeling in renal failure. Intern Med. 2001;40(4):285–91. doi: 10.2169/internalmedicine.40.285. [DOI] [PubMed] [Google Scholar]
- 38.Saleh MA, et al. Endothelin-1 increases glomerular permeability and inflammation independent of blood pressure in the rat. Hypertension. 2010;56(5):942–9. doi: 10.1161/HYPERTENSIONAHA.110.156570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boesen EI, et al. ETA activation mediates angiotensin II-induced infiltration of renal cortical T cells. J Am Soc Nephrol. 2011;22(12):2187–92. doi: 10.1681/ASN.2010020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albert C, et al. In arterial occlusive disease autoantibodies against ETAR and AT(1)R correlate with each other but are not associated with classical cardiovascular risk factors. Vasa. 2014;43(2):113–23. doi: 10.1024/0301-1526/a000337. [DOI] [PubMed] [Google Scholar]
- 41.Giachini FR, et al. Extracellular signal-regulated kinase 1/2 activation, via downregulation of mitogen-activated protein kinase phosphatase 1, mediates sex differences in desoxycorticosterone acetate-salt hypertension vascular reactivity. Hypertension. 2010;55(1):172–9. doi: 10.1161/HYPERTENSIONAHA.109.140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giachini FR, et al. Therapeutic targets in hypertension: is there a place for antagonists of the most potent vasoconstrictors? Expert Opin Ther Targets. 2008;12(3):327–39. doi: 10.1517/14728222.12.3.327. [DOI] [PubMed] [Google Scholar]
- 43.Cambotti LJ, et al. Neonatal gonadal hormones and blood pressure in the spontaneously hypertensive rat. Am J Physiol. 1984;247(2 Pt 1):E258–64. doi: 10.1152/ajpendo.1984.247.2.E258. [DOI] [PubMed] [Google Scholar]
- 44.Crofton JT, Share L. Gonadal hormones modulate deoxycorticosterone-salt hypertension in male and female rats. Hypertension. 1997;29(1 Pt 2):494–9. doi: 10.1161/01.hyp.29.1.494. [DOI] [PubMed] [Google Scholar]
- 45.Joyner MJ, Wallin BG, Charkoudian N. Sex differences and blood pressure regulation in humans. Exp Physiol. 2015 doi: 10.1113/EP085146. [DOI] [PubMed] [Google Scholar]
- 46.Pechere-Bertschi A, Burnier M. Gonadal steroids, salt-sensitivity and renal function. Curr Opin Nephrol Hypertens. 2007;16(1):16–21. doi: 10.1097/MNH.0b013e328011d7f6. [DOI] [PubMed] [Google Scholar]
- 47.Messerli FH, et al. Disparate cardiovascular findings in men and women with essential hypertension. Ann Intern Med. 1987;107(2):158–61. doi: 10.7326/0003-4819-107-2-158. [DOI] [PubMed] [Google Scholar]
- 48.Fortepiani LA, et al. Role of androgens in mediating renal injury in aging SHR. Hypertension. 2003;42(5):952–5. doi: 10.1161/01.HYP.0000099241.53121.7F. [DOI] [PubMed] [Google Scholar]
- 49.Reckelhoff JF, Fortepiani LA. Novel mechanisms responsible for postmenopausal hypertension. Hypertension. 2004;43(5):918–23. doi: 10.1161/01.HYP.0000124670.03674.15. [DOI] [PubMed] [Google Scholar]
- 50.Dubey RK, Jackson EK. Estrogen-induced cardiorenal protection: potential cellular, biochemical, and molecular mechanisms. Am J Physiol Renal Physiol. 2001;280(3):F365–88. doi: 10.1152/ajprenal.2001.280.3.F365. [DOI] [PubMed] [Google Scholar]
- 51.Khalil RA. Sex hormones as potential modulators of vascular function in hypertension. Hypertension. 2005;46(2):249–54. doi: 10.1161/01.HYP.0000172945.06681.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tostes RC, et al. Effects of estrogen on the vascular system. Braz J Med Biol Res. 2003;36(9):1143–58. doi: 10.1590/s0100-879x2003000900002. [DOI] [PubMed] [Google Scholar]
- 53.Mankhey RW, Bhatti F, Maric C. 17beta-Estradiol replacement improves renal function and pathology associated with diabetic nephropathy. Am J Physiol Renal Physiol. 2005;288(2):F399–405. doi: 10.1152/ajprenal.00195.2004. [DOI] [PubMed] [Google Scholar]
- 54.Hay M, Xue B, Johnson AK. Yes! Sex matters: sex, the brain and blood pressure. Curr Hypertens Rep. 2014;16(8):458. doi: 10.1007/s11906-014-0458-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maranon R, Reckelhoff JF. Sex and gender differences in control of blood pressure. Clin Sci (Lond) 2013;125(7):311–8. doi: 10.1042/CS20130140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Juhasz M, et al. Gender-related differences in adolescent hypertension and in target organ effects. J Womens Health (Larchmt) 2010;19(4):759–65. doi: 10.1089/jwh.2009.1407. [DOI] [PubMed] [Google Scholar]
- 57.Polderman KH, et al. Influence of sex hormones on plasma endothelin levels. Ann Intern Med. 1993;118(6):429–32. doi: 10.7326/0003-4819-118-6-199303150-00006. [DOI] [PubMed] [Google Scholar]
- 58.Miyauchi T, et al. Age- and sex-related variation of plasma endothelin-1 concentration in normal and hypertensive subjects. Am Heart J. 1992;123(4 Pt 1):1092–3. doi: 10.1016/0002-8703(92)90734-d. [DOI] [PubMed] [Google Scholar]
- 59.Maeda S, et al. Aerobic exercise training reduces plasma endothelin-1 concentration in older women. J Appl Physiol (1985) 2003;95(1):336–41. doi: 10.1152/japplphysiol.01016.2002. [DOI] [PubMed] [Google Scholar]
- 60.Barton M, et al. Anatomic heterogeneity of vascular aging: role of nitric oxide and endothelin. Hypertension. 1997;30(4):817–24. doi: 10.1161/01.hyp.30.4.817. [DOI] [PubMed] [Google Scholar]
- 61.Kumazaki T, et al. Expression of endothelin, fibronectin, and mortalin as aging and mortality markers. Exp Gerontol. 1997;32(1–2):95–103. doi: 10.1016/s0531-5565(96)00080-0. [DOI] [PubMed] [Google Scholar]
- 62.Goettsch W, et al. Increased expression of endothelin-1 and inducible nitric oxide synthase isoform II in aging arteries in vivo: implications for atherosclerosis. Biochem Biophys Res Commun. 2001;280(3):908–13. doi: 10.1006/bbrc.2000.4180. [DOI] [PubMed] [Google Scholar]
- 63.Lekontseva O, Chakrabarti S, Davidge ST. Endothelin in the female vasculature: a role in aging? Am J Physiol Regul Integr Comp Physiol. 2010;298(3):R509–16. doi: 10.1152/ajpregu.00656.2009. [DOI] [PubMed] [Google Scholar]
- 64.dos Santos RL, et al. Sex hormones in the cardiovascular system. Horm Mol Biol Clin Investig. 2014;18(2):89–103. doi: 10.1515/hmbci-2013-0048. [DOI] [PubMed] [Google Scholar]
- 65.Maturana MA, et al. Relationship between endogenous testosterone and cardiovascular risk in early postmenopausal women. Metabolism. 2008;57(7):961–5. doi: 10.1016/j.metabol.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 66.Carbonne B, et al. Changes in plasma and amniotic fluid endothelin levels during pregnancy: facts or artefacts? Eur J Obstet Gynecol Reprod Biol. 1998;76(1):15–9. doi: 10.1016/s0301-2115(97)00141-3. [DOI] [PubMed] [Google Scholar]
- 67.Lygnos MC, et al. Changes in maternal plasma levels of VEGF, bFGF, TGF-beta1, ET-1 and sKL during uncomplicated pregnancy, hypertensive pregnancy and gestational diabetes. In Vivo. 2006;20(1):157–63. [PubMed] [Google Scholar]
- 68.Pappa KI, et al. Maternal and fetal circulating sKL and ET-1 levels as function of normal labor at term. J Matern Fetal Neonatal Med. 2011;24(2):324–9. doi: 10.3109/14767058.2010.496502. [DOI] [PubMed] [Google Scholar]
- 69.Verdonk K, et al. Association studies suggest a key role for endothelin-1 in the pathogenesis of preeclampsia and the accompanying renin-angiotensin-aldosterone system suppression. Hypertension. 2015;65(6):1316–23. doi: 10.1161/HYPERTENSIONAHA.115.05267. [DOI] [PubMed] [Google Scholar]
- 70.Vinayagam V, et al. Plasma markers of endothelial dysfunction in patients with hypertensive disorders of pregnancy: a pilot study in a South Indian population. J Matern Fetal Neonatal Med. 2015:1–6. doi: 10.3109/14767058.2015.1075200. [DOI] [PubMed] [Google Scholar]
- 71.Kiprono LV, et al. Progesterone blunts vascular endothelial cell secretion of endothelin-1 in response to placental ischemia. Am J Obstet Gynecol. 2013;209(1):44 e1–6. doi: 10.1016/j.ajog.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tam Tam KB, et al. Endothelin type A receptor antagonist attenuates placental ischemia-induced hypertension and uterine vascular resistance. Am J Obstet Gynecol. 2011;204(4):330 e1–4. doi: 10.1016/j.ajog.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Merki-Feld GS, Imthurn B, Seifert B. Effects of the progestagen-only contraceptive implant Implanon on transforming growth factor beta1 and endothelin-1. Horm Metab Res. 2008;40(10):692–6. doi: 10.1055/s-2008-1073149. [DOI] [PubMed] [Google Scholar]
- 74.Best PJ, et al. The effect of estrogen replacement therapy on plasma nitric oxide and endothelin-1 levels in postmenopausal women. Ann Intern Med. 1998;128(4):285–8. doi: 10.7326/0003-4819-128-4-199802150-00006. [DOI] [PubMed] [Google Scholar]
- 75.Casanova G, et al. Effects of nonoral estradiol-micronized progesterone or low-dose oral estradiol-drospirenone therapy on metabolic variables and markers of endothelial function in early postmenopause. Fertil Steril. 2009;92(2):605–12. doi: 10.1016/j.fertnstert.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 76.de Kraker AT, et al. Short-term effects of two continuous combined oestrogen-progestogen therapies on several cardiovascular risk markers in healthy postmenopausal women: a randomised controlled trial. Eur J Obstet Gynecol Reprod Biol. 2009;142(2):139–44. doi: 10.1016/j.ejogrb.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 77.El-Seweidy MM, et al. Nicotine and vascular endothelial dysfunction in female ovariectomized rats: role of estrogen replacement therapy. J Pharm Pharmacol. 2012;64(1):108–19. doi: 10.1111/j.2042-7158.2011.01377.x. [DOI] [PubMed] [Google Scholar]
- 78.Cetinkaya Demir B, et al. Effect of raloxifene and atorvastatin in atherosclerotic process in ovariectomized rats. J Obstet Gynaecol Res. 2013;39(1):229–36. doi: 10.1111/j.1447-0756.2012.01969.x. [DOI] [PubMed] [Google Scholar]
- 79.Saitta A, et al. Randomized, double-blind, placebo-controlled study on effects of raloxifene and hormone replacement therapy on plasma no concentrations, endothelin-1 levels, and endothelium-dependent vasodilation in postmenopausal women. Arterioscler Thromb Vasc Biol. 2001;21(9):1512–9. doi: 10.1161/hq0901.095565. [DOI] [PubMed] [Google Scholar]
- 80.Saitta A, et al. Cardiovascular effects of raloxifene hydrochloride. Cardiovasc Drug Rev. 2001;19(1):57–74. doi: 10.1111/j.1527-3466.2001.tb00183.x. [DOI] [PubMed] [Google Scholar]
- 81.Cerquetani E, et al. Comparative vascular effects of hormone replacement therapy and raloxifene in women at increased cardiovascular risk. Gynecol Endocrinol. 2004;18(6):291–8. doi: 10.1080/09513590410001729888. [DOI] [PubMed] [Google Scholar]
- 82.Diamanti-Kandarakis E, et al. Increased endothelin-1 levels in women with polycystic ovary syndrome and the beneficial effect of metformin therapy. J Clin Endocrinol Metab. 2001;86(10):4666–73. doi: 10.1210/jcem.86.10.7904. [DOI] [PubMed] [Google Scholar]
- 83.Diamanti-Kandarakis E, et al. Inflammatory and endothelial markers in women with polycystic ovary syndrome. Eur J Clin Invest. 2006;36(10):691–7. doi: 10.1111/j.1365-2362.2006.01712.x. [DOI] [PubMed] [Google Scholar]
- 84.Toulis KA, et al. Meta-analysis of cardiovascular disease risk markers in women with polycystic ovary syndrome. Hum Reprod Update. 2011;17(6):741–60. doi: 10.1093/humupd/dmr025. [DOI] [PubMed] [Google Scholar]
- 85.Christakou C, et al. Strong and positive association of endothelin-1 with AGEs in PCOS: a causal relationship or a bystander? Hormones (Athens) 2011;10(4):292–7. doi: 10.14310/horm.2002.1320. [DOI] [PubMed] [Google Scholar]
- 86.Wenner MM, Taylor HS, Stachenfeld NS. Androgens influence microvascular dilation in PCOS through ET-A and ET-B receptors. Am J Physiol Endocrinol Metab. 2013;305(7):E818–25. doi: 10.1152/ajpendo.00343.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kumanov P, Tomova A, Kirilov G. Testosterone replacement therapy in male hypogonadism is not associated with increase of endothelin-1 levels. Int J Androl. 2007;30(1):41–7. doi: 10.1111/j.1365-2605.2006.00706.x. [DOI] [PubMed] [Google Scholar]
- 88.Akishita M, et al. Estrogen attenuates endothelin-1 production by bovine endothelial cells via estrogen receptor. Biochem Biophys Res Commun. 1998;251(1):17–21. doi: 10.1006/bbrc.1998.9409. [DOI] [PubMed] [Google Scholar]
- 89.Bilsel AS, et al. 17Beta-estradiol modulates endothelin-1 expression and release in human endothelial cells. Cardiovasc Res. 2000;46(3):579–84. doi: 10.1016/s0008-6363(00)00046-8. [DOI] [PubMed] [Google Scholar]
- 90.Wang X, et al. Gender and transcriptional regulation of NO synthase and ET-1 in porcine aortic endothelial cells. Am J Physiol. 1997;273(4 Pt 2):H1962–7. doi: 10.1152/ajpheart.1997.273.4.H1962. [DOI] [PubMed] [Google Scholar]
- 91.Juan SH, et al. 17beta-estradiol inhibits cyclic strain-induced endothelin-1 gene expression within vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287(3):H1254–61. doi: 10.1152/ajpheart.00723.2003. [DOI] [PubMed] [Google Scholar]
- 92.Dubey RK, et al. Estradiol metabolites inhibit endothelin synthesis by an estrogen receptor-independent mechanism. Hypertension. 2001;37(2 Pt 2):640–4. doi: 10.1161/01.hyp.37.2.640. [DOI] [PubMed] [Google Scholar]
- 93.Hong HJ, et al. 17beta-estradiol downregulates angiotensin-II-induced endothelin-1 gene expression in rat aortic smooth muscle cells. J Biomed Sci. 2004;11(1):27–36. doi: 10.1007/BF02256546. [DOI] [PubMed] [Google Scholar]
- 94.Chao HH, et al. Inhibition of angiotensin II induced endothelin-1 gene expression by 17-beta-oestradiol in rat cardiac fibroblasts. Heart. 2005;91(5):664–9. doi: 10.1136/hrt.2003.031898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Akishita M, et al. Estrogen inhibits endothelin-1 production and c-fos gene expression in rat aorta. Atherosclerosis. 1996;125(1):27–38. doi: 10.1016/0021-9150(96)05836-4. [DOI] [PubMed] [Google Scholar]
- 96.Pearson LJ, et al. Regulation of endothelin-1 release from human endothelial cells by sex steroids and angiotensin-II. Peptides. 2008;29(6):1057–61. doi: 10.1016/j.peptides.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 97.Tan Z, et al. Role of NO signal pathway in the inhibitory of 17beta-estradiol on the production of endothelin-1 in vascular smooth muscle cells. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2007;23(3):347–50. [PubMed] [Google Scholar]
- 98.David FL, et al. Ovarian hormones modulate endothelin-1 vascular reactivity and mRNA expression in DOCA-salt hypertensive rats. Hypertension. 2001;38(3 Pt 2):692–6. doi: 10.1161/01.hyp.38.3.692. [DOI] [PubMed] [Google Scholar]
- 99.David FL, et al. Gender differences in vascular expression of endothelin and ET(A)/ET(B) receptors, but not in calcium handling mechanisms, in deoxycorticosterone acetate-salt hypertension. Braz J Med Biol Res. 2002;35(9):1061–8. doi: 10.1590/s0100-879x2002000900006. [DOI] [PubMed] [Google Scholar]
- 100.Tan Z, et al. Mechanisms of 17beta-estradiol on the production of ET-1 in ovariectomized rats. Life Sci. 2003;73(21):2665–74. doi: 10.1016/s0024-3205(03)00605-2. [DOI] [PubMed] [Google Scholar]
- 101.Williams JG. Drugs and sport. Med Sci Law. 1975;15(1):9–15. doi: 10.1177/002580247501500103. [DOI] [PubMed] [Google Scholar]
- 102.Rodrigo MC, Martin DS, Eyster KM. Vascular ECE-1 mRNA expression decreases in response to estrogens. Life Sci. 2003;73(23):2973–83. doi: 10.1016/j.lfs.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 103.Wang LS, et al. Endothelin-converting enzyme-1b C-338A polymorphism is associated with the increased risk of coronary artery disease in Chinese population. Clin Chim Acta. 2007;384(1–2):75–9. doi: 10.1016/j.cca.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 104.Jin Z, et al. C-338A polymorphism of the endothelin-converting enzyme-1 gene and the susceptibility to carotid atherosclerosis. Microvasc Res. 2009;78(1):128–31. doi: 10.1016/j.mvr.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 105.Li R, et al. Association of endothelin-converting enzyme-1b C-338A polymorphism with increased risk of ischemic stroke in Chinese Han population. J Mol Neurosci. 2013;51(2):485–92. doi: 10.1007/s12031-013-0100-y. [DOI] [PubMed] [Google Scholar]
- 106.Ergul A, et al. Gender differences in the expression of endothelin receptors in human saphenous veins in vitro. J Pharmacol Exp Ther. 1998;285(2):511–7. [PubMed] [Google Scholar]
- 107.Stauffer BL, et al. Sex differences in endothelin-1-mediated vasoconstrictor tone in middle-aged and older adults. Am J Physiol Regul Integr Comp Physiol. 2010;298(2):R261–5. doi: 10.1152/ajpregu.00626.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nuedling S, et al. 17 Beta-estradiol regulates the expression of endothelin receptor type B in the heart. Br J Pharmacol. 2003;140(1):195–201. doi: 10.1038/sj.bjp.0705409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pedersen SH, et al. Hormone therapy modulates ET(A) mRNA expression in the aorta of ovariectomised New Zealand White rabbits. Gynecol Endocrinol. 2009;25(3):175–82. doi: 10.1080/09513590802549833. [DOI] [PubMed] [Google Scholar]
- 110.Pedersen SH, et al. Progestins oppose the effects of estradiol on the endothelin-1 receptor type B in coronary arteries from ovariectomized hyperlipidemic rabbits. Menopause. 2008;15(3):503–10. doi: 10.1097/gme.0b013e318156f803. [DOI] [PubMed] [Google Scholar]
- 111.de Rossignoli PS, et al. Orchidectomy enhances the expression of endothelin-1 and ETB receptors in rat portal vein. J Smooth Muscle Res. 2014;50:85–92. doi: 10.1540/jsmr.50.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kawanishi H, et al. Involvement of the endothelin ET(B) receptor in gender differences in deoxycorticosterone acetate-salt-induced hypertension. Clin Exp Pharmacol Physiol. 2007;34(4):280–5. doi: 10.1111/j.1440-1681.2007.04580.x. [DOI] [PubMed] [Google Scholar]
- 113.Taylor TA, et al. Gender differences in ET and NOS systems in ETB receptor-deficient rats: effect of a high salt diet. Hypertension. 2003;41(3 Pt 2):657–62. doi: 10.1161/01.HYP.0000048193.85814.78. [DOI] [PubMed] [Google Scholar]
- 114.Mazzuca MQ, Dang Y, Khalil RA. Enhanced endothelin receptor type B-mediated vasodilation and underlying [Ca(2)(+)]i in mesenteric microvessels of pregnant rats. Br J Pharmacol. 2013;169(6):1335–51. doi: 10.1111/bph.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhou J, et al. Gestational hypoxia induces preeclampsia-like symptoms via heightened endothelin-1 signaling in pregnant rats. Hypertension. 2013;62(3):599–607. doi: 10.1161/HYPERTENSIONAHA.113.01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kittikulsuth W, Pollock JS, Pollock DM. Sex differences in renal medullary endothelin receptor function in angiotensin II hypertensive rats. Hypertension. 2011;58(2):212–8. doi: 10.1161/HYPERTENSIONAHA.111.172734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kittikulsuth W, Looney SW, Pollock DM. Endothelin ET(B) receptors contribute to sex differences in blood pressure elevation in angiotensin II hypertensive rats on a high-salt diet. Clin Exp Pharmacol Physiol. 2013;40(6):362–70. doi: 10.1111/1440-1681.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Speed JS, et al. High salt diet increases the pressor response to stress in female, but not male ETB-receptor-deficient rats. Physiol Rep. 2015;3(3) doi: 10.14814/phy2.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kellogg DL, Jr, Liu Y, Pergola PE. Selected contribution: Gender differences in the endothelin-B receptor contribution to basal cutaneous vascular tone in humans. J Appl Physiol (1985) 2001;91(5):2407–11. doi: 10.1152/jappl.2001.91.5.2407. discussion 2389–90. [DOI] [PubMed] [Google Scholar]
- 120.Ahnstedt H, et al. Male-female differences in upregulation of vasoconstrictor responses in human cerebral arteries. PLoS One. 2013;8(4):e62698. doi: 10.1371/journal.pone.0062698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tostes RC, et al. Gender differences in vascular reactivity to endothelin-1 in deoxycorticosterone-salt hypertensive rats. J Cardiovasc Pharmacol. 2000;36(5 Suppl 1):S99–101. doi: 10.1097/00005344-200036051-00032. [DOI] [PubMed] [Google Scholar]
- 122.Tostes Passaglia RC, et al. Deoxycorticosterone acetate-salt hypertensive rats display gender-related differences in ET(B) receptor-mediated vascular responses. Br J Pharmacol. 2000;130(5):1092–8. doi: 10.1038/sj.bjp.0703389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fortes ZB, et al. Influence of sex on the reactivity to endothelin-1 and noradrenaline in spontaneously hypertensive rats. Clin Exp Hypertens A. 1991;13(5):807–16. doi: 10.3109/10641969109042084. [DOI] [PubMed] [Google Scholar]
- 124.Laughlin MH, et al. Interaction of gender and exercise training: vasomotor reactivity of porcine skeletal muscle arteries. J Appl Physiol (1985) 2001;90(1):216–27. doi: 10.1152/jappl.2001.90.1.216. [DOI] [PubMed] [Google Scholar]
- 125.Jones AW, Rubin LJ, Magliola L. Endothelin-1 sensitivity of porcine coronary arteries is reduced by exercise training and is gender dependent. J Appl Physiol (1985) 1999;87(3):1172–7. doi: 10.1152/jappl.1999.87.3.1172. [DOI] [PubMed] [Google Scholar]
- 126.Kallikazaros I, et al. Estrogen-induced improvement in coronary flow responses during atrial pacing in relation to endothelin-1 levels in postmenopausal women without coronary disease. Vasc Health Risk Manag. 2008;4(3):705–14. doi: 10.2147/vhrm.s2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lamping KG, Nuno DW. Effects of 17 beta-estradiol on coronary microvascular responses to endothelin-1. Am J Physiol. 1996;271(3 Pt 2):H1117–24. doi: 10.1152/ajpheart.1996.271.3.H1117. [DOI] [PubMed] [Google Scholar]
- 128.Sudhir K, et al. Physiological concentrations of estradiol attenuate endothelin 1-induced coronary vasoconstriction in vivo. Circulation. 1997;96(10):3626–32. doi: 10.1161/01.cir.96.10.3626. [DOI] [PubMed] [Google Scholar]
- 129.Teoh H, et al. Differential effects of 17beta-estradiol and testosterone on the contractile responses of porcine coronary arteries. Br J Pharmacol. 2000;129(7):1301–8. doi: 10.1038/sj.bjp.0703164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Antoniucci D, et al. Gender-related differences in proliferative responses of vascular smooth muscle cells to endothelin-1. Endothelium. 2001;8(2):137–45. doi: 10.3109/10623320109165322. [DOI] [PubMed] [Google Scholar]
- 131.Yager JY, et al. A new model for determining the influence of age and sex on functional recovery following hypoxic-ischemic brain damage. Dev Neurosci. 2005;27(2–4):112–20. doi: 10.1159/000085982. [DOI] [PubMed] [Google Scholar]
- 132.del Campo L, et al. Testosterone and beta-oestradiol prevent inward remodelling of rat small mesenteric arteries: role of NO and transglutaminase. Clin Sci (Lond) 2013;124(12):719–28. doi: 10.1042/CS20120700. [DOI] [PubMed] [Google Scholar]
- 133.Karatas A, et al. Deoxycorticosterone acetate-salt mice exhibit blood pressure-independent sexual dimorphism. Hypertension. 2008;51(4):1177–83. doi: 10.1161/HYPERTENSIONAHA.107.107938. [DOI] [PubMed] [Google Scholar]
- 134.Gurgen D, et al. Estrogen receptor-beta signals left ventricular hypertrophy sex differences in normotensive deoxycorticosterone acetate-salt mice. Hypertension. 2011;57(3):648–54. doi: 10.1161/HYPERTENSIONAHA.110.166157. [DOI] [PubMed] [Google Scholar]
- 135.Callera GE, et al. ETA receptor blockade decreases vascular superoxide generation in DOCA-salt hypertension. Hypertension. 2003;42(4):811–7. doi: 10.1161/01.HYP.0000088363.65943.6C. [DOI] [PubMed] [Google Scholar]
- 136.Callera GE, et al. ETA receptor mediates altered leukocyte-endothelial cell interaction and adhesion molecules expression in DOCA-salt rats. Hypertension. 2004;43(4):872–9. doi: 10.1161/01.HYP.0000117296.30296.14. [DOI] [PubMed] [Google Scholar]
- 137.Nakano D, Pollock DM. Contribution of endothelin A receptors in endothelin 1-dependent natriuresis in female rats. Hypertension. 2009;53(2):324–30. doi: 10.1161/HYPERTENSIONAHA.108.123687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jin C, et al. Sex differences in ET-1 receptor expression and Ca2+ signaling in the IMCD. Am J Physiol Renal Physiol. 2013;305(8):F1099–104. doi: 10.1152/ajprenal.00400.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stringer KD, et al. Gender hormones and the progression of experimental polycystic kidney disease. Kidney Int. 2005;68(4):1729–39. doi: 10.1111/j.1523-1755.2005.00589.x. [DOI] [PubMed] [Google Scholar]
- 140.Kalk P, et al. Renal phenotype of ET-1 transgenic mice is modulated by androgens. Eur J Med Res. 2009;14:55–8. doi: 10.1186/2047-783X-14-2-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Muller V, et al. Sexual dimorphism in renal ischemia-reperfusion injury in rats: possible role of endothelin. Kidney Int. 2002;62(4):1364–71. doi: 10.1111/j.1523-1755.2002.kid590.x. [DOI] [PubMed] [Google Scholar]
- 142.Dousdampanis P, et al. Role of testosterone in the pathogenesis, progression, prognosis and comorbidity of men with chronic kidney disease. Ther Apher Dial. 2014;18(3):220–30. doi: 10.1111/1744-9987.12101. [DOI] [PubMed] [Google Scholar]
- 143.Takaoka M, et al. Oestrogen protects against ischaemic acute renal failure in rats by suppressing renal endothelin-1 overproduction. Clin Sci (Lond) 2002;103(Suppl 48):434S–437S. doi: 10.1042/CS103S434S. [DOI] [PubMed] [Google Scholar]
- 144.Shibata Y, et al. Involvement of nitric oxide in the suppressive effect of 17beta-estradiol on endothelin-1 overproduction in ischemic acute renal failure. J Cardiovasc Pharmacol. 2004;44(Suppl 1):S459–61. doi: 10.1097/01.fjc.0000166315.38258.e1. [DOI] [PubMed] [Google Scholar]
- 145.Ba ZF, I, Chaudry H. Role of estrogen receptor subtypes in estrogen-induced organ-specific vasorelaxation after trauma-hemorrhage. Am J Physiol Heart Circ Physiol. 2008;295(5):H2061–7. doi: 10.1152/ajpheart.00707.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gohar EY, Yusuf C, Pollock DM. Ovarian hormones modulate endothelin A and B receptor expression. Life Sci. 2016 doi: 10.1016/j.lfs.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang Y, et al. Control of endothelin-a receptor expression by progesterone is enhanced by synergy with Gata2. Mol Endocrinol. 2013;27(6):892–908. doi: 10.1210/me.2012-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Craici IM, et al. Advances in the pathophysiology of pre-eclampsia and related podocyte injury. Kidney Int. 2014;86(2):275–85. doi: 10.1038/ki.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tatchum-Talom R, et al. Gender differences in hemodynamic responses to endothelin-1. J Cardiovasc Pharmacol. 2000;36(5 Suppl 1):S102–4. doi: 10.1097/00005344-200036051-00033. [DOI] [PubMed] [Google Scholar]
- 150.Bruderer S, et al. Pharmacokinetics of macitentan in caucasian and Japanese subjects: the influence of ethnicity and sex. Pharmacology. 2013;91(5–6):331–8. doi: 10.1159/000351704. [DOI] [PubMed] [Google Scholar]
- 151.Sharkey LC, et al. Obese female SHHF/Mcc-fa(cp) rats resist antihypertensive effects of renin-angiotensin system inhibition. Clin Exp Hypertens. 2001;23(3):227–39. doi: 10.1081/ceh-100102662. [DOI] [PubMed] [Google Scholar]
- 152.Hallberg P, et al. Gender-specific association between preproendothelin-1 genotype and reduction of systolic blood pressure during antihypertensive treatment--results from the Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA) Clin Cardiol. 2004;27(5):287–90. doi: 10.1002/clc.4960270510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kitada K, et al. Vasoprotective effects of an endothelin receptor antagonist in ovariectomized female rats. Life Sci. 2014;118(2):379–85. doi: 10.1016/j.lfs.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 154.Krum H, et al. The effect of an endothelin-receptor antagonist, bosentan, on blood pressure in patients with essential hypertension. Bosentan Hypertension Investigators. N Engl J Med. 1998;338(12):784–90. doi: 10.1056/NEJM199803193381202. [DOI] [PubMed] [Google Scholar]
- 155.Nakov R, et al. Darusentan: an effective endothelin A receptor antagonist for treatment of hypertension. Am J Hypertens. 2002;15(7 Pt 1):583–9. doi: 10.1016/s0895-7061(02)02933-3. [DOI] [PubMed] [Google Scholar]
- 156.Dhaun N, et al. Selective endothelin-A receptor antagonism reduces proteinuria, blood pressure, and arterial stiffness in chronic proteinuric kidney disease. Hypertension. 2011;57(4):772–9. doi: 10.1161/HYPERTENSIONAHA.110.167486. [DOI] [PubMed] [Google Scholar]
- 157.Dhaun N, et al. Blood pressure-independent reduction in proteinuria and arterial stiffness after acute endothelin-a receptor antagonism in chronic kidney disease. Hypertension. 2009;54(1):113–9. doi: 10.1161/HYPERTENSIONAHA.109.132670. [DOI] [PubMed] [Google Scholar]
- 158.Wenzel RR, et al. Avosentan reduces albumin excretion in diabetics with macroalbuminuria. J Am Soc Nephrol. 2009;20(3):655–64. doi: 10.1681/ASN.2008050482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kohan DE, et al. Addition of atrasentan to renin-angiotensin system blockade reduces albuminuria in diabetic nephropathy. J Am Soc Nephrol. 2011;22(4):763–72. doi: 10.1681/ASN.2010080869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Stuart D, et al. Myocardial, smooth muscle, nephron, and collecting duct gene targeting reveals the organ sites of endothelin A receptor antagonist fluid retention. J Pharmacol Exp Ther. 2013;346(2):182–9. doi: 10.1124/jpet.113.205286. [DOI] [PubMed] [Google Scholar]
- 161.Weber MA, et al. A selective endothelin-receptor antagonist to reduce blood pressure in patients with treatment-resistant hypertension: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;374(9699):1423–31. doi: 10.1016/S0140-6736(09)61500-2. [DOI] [PubMed] [Google Scholar]
- 162.Czopek A, et al. The therapeutic potential of endothelin receptor antagonism in kidney disease. Am J Physiol Regul Integr Comp Physiol. 2015 doi: 10.1152/ajpregu.00478.2015. p. ajpregu 00478 2015. [DOI] [PubMed] [Google Scholar]
- 163.Anguiano L, et al. Endothelin Blockade in Diabetic Kidney Disease. J Clin Med. 2015;4(6):1171–92. doi: 10.3390/jcm4061171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Laffin LJ, Bakris GL. Endothelin antagonism and hypertension: an evolving target. Semin Nephrol. 2015;35(2):168–75. doi: 10.1016/j.semnephrol.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 165.Maguire JJ, Davenport AP. Endothelin receptors and their antagonists. Semin Nephrol. 2015;35(2):125–36. doi: 10.1016/j.semnephrol.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Aversa M, Porter S, Granton J. Comparative safety and tolerability of endothelin receptor antagonists in pulmonary arterial hypertension. Drug Saf. 2015;38(5):419–35. doi: 10.1007/s40264-015-0275-y. [DOI] [PubMed] [Google Scholar]
- 167.Forsdahl A. Are poor living conditions in childhood and adolescence and important risk factor for arteriosclerotic heart disease? Int J Rehabil Res. 1979;2(2):238–9. doi: 10.1097/00004356-197905000-00008. [DOI] [PubMed] [Google Scholar]
- 168.Forsdahl A. Are poor living conditions in childhood and adolescence an important risk factor for arteriosclerotic heart disease? Br J Prev Soc Med. 1977;31(2):91–5. doi: 10.1136/jech.31.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Barker DJ, et al. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298(6673):564–7. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Barker DJ, Osmond C. Low birth weight and hypertension. BMJ. 1988;297(6641):134–5. doi: 10.1136/bmj.297.6641.134-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ojeda NB, Intapad S, Alexander BT. Sex differences in the developmental programming of hypertension. Acta Physiol (Oxf) 2014;210(2):307–16. doi: 10.1111/apha.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rueda-Clausen CF, Morton JS, Davidge ST. The early origins of cardiovascular health and disease: who, when, and how. Semin Reprod Med. 2011;29(3):197–210. doi: 10.1055/s-0031-1275520. [DOI] [PubMed] [Google Scholar]
- 173.Bourque SL, et al. Prenatal hypoxia causes long-term alterations in vascular endothelin-1 function in aged male, but not female, offspring. Hypertension. 2013;62(4):753–8. doi: 10.1161/HYPERTENSIONAHA.113.01516. [DOI] [PubMed] [Google Scholar]
- 174.Lee JH, et al. Antenatal betamethasone has a sex-dependent effect on the in vivo response to endothelin in adult sheep. Am J Physiol Regul Integr Comp Physiol. 2013;304(8):R581–7. doi: 10.1152/ajpregu.00579.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Loria AS, et al. Early life stress downregulates endothelin receptor expression and enhances acute stress-mediated blood pressure responses in adult rats. Am J Physiol Regul Integr Comp Physiol. 2010;299(1):R185–91. doi: 10.1152/ajpregu.00333.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.D’Angelo G, et al. Endothelin activation of reactive oxygen species mediates stress-induced pressor response in Dahl salt-sensitive prehypertensive rats. Hypertension. 2010;56(2):282–9. doi: 10.1161/HYPERTENSIONAHA.110.152629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Su S, et al. Adverse childhood experiences are associated with detrimental hemodynamics and elevated circulating endothelin-1 in adolescents and young adults. Hypertension. 2014;64(1):201–7. doi: 10.1161/HYPERTENSIONAHA.113.02755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chen X, et al. The number of x chromosomes causes sex differences in adiposity in mice. PLoS Genet. 2012;8(5):e1002709. doi: 10.1371/journal.pgen.1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hall JE, et al. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010;285(23):17271–6. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vivas L, Dadam FM, Caeiro XE. Sex differences in body fluid homeostasis: Sex chromosome complement influences on bradycardic baroreflex response and sodium depletion induced neural activity. Physiol Behav. 2015;152(Pt B):416–21. doi: 10.1016/j.physbeh.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 181.Pessoa BS, et al. Angiotensin II type 2 receptor- and acetylcholine-mediated relaxation: essential contribution of female sex hormones and chromosomes. Hypertension. 2015;66(2):396–402. doi: 10.1161/HYPERTENSIONAHA.115.05303. [DOI] [PubMed] [Google Scholar]
- 182.Falconnet C, et al. Gender difference in the response to an angiotensin-converting enzyme inhibitor and a diuretic in hypertensive patients of African descent. J Hypertens. 2004;22(6):1213–20. doi: 10.1097/00004872-200406000-00023. [DOI] [PubMed] [Google Scholar]
- 183.Saunders E, Cable G, Neutel J. Predictors of blood pressure response to angiotensin receptor blocker/diuretic combination therapy: a secondary analysis of the irbesartan/hydrochlorothiazide blood pressure reductions in diverse patient populations (INCLUSIVE) study. J Clin Hypertens (Greenwich) 2008;10(1):27–33. doi: 10.1111/j.1524-6175.2007.07195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zanchetti A, et al. Outcomes in subgroups of hypertensive patients treated with regimens based on valsartan and amlodipine: An analysis of findings from the VALUE trial. J Hypertens. 2006;24(11):2163–8. doi: 10.1097/01.hjh.0000249692.96488.46. [DOI] [PubMed] [Google Scholar]
- 185.Canzanello VJ, et al. Predictors of blood pressure response to the angiotensin receptor blocker candesartan in essential hypertension. Am J Hypertens. 2008;21(1):61–6. doi: 10.1038/ajh.2007.24. [DOI] [PubMed] [Google Scholar]