Abstract
Nutrient sensing receptors are key metabolic mediators of responses to dietary and endogenously derived nutrients. These receptors are largely G-protein coupled receptors (GPCRs) and many are gaining significant interest as drug targets with a potential therapeutic role in metabolic diseases. A distinct subclass of nutrient sensing GPCRs, two short chain fatty acid (SCFA) receptors (FFA2 and FFA3) are uniquely responsive to gut microbiota derived nutrients (such as acetate, propionate and butyrate). Pharmacologic, molecular and genetic studies have investigated their role in organismal glucose metabolism and recently in pancreatic beta-cell biology. Here we summarize the present knowledge on the role of these receptors as metabolic sensors in beta cell function and physiology, revealing new therapeutic opportunities for type 2 diabetes.
Keywords: beta cell mass, FFA2, FFA3, gut microbiota, insulin secretion, short chain fatty acids
“Let food be thy medicine and medicine be thy food”- Hippocrates, 460 BC-370 BC.
NUTRIENT SENSING RECEPTORS
Dietary nutrients beyond providing sustenance influence our health through immune and metabolic effects [1]. While their mode of action was previously thought to be through their intracellular metabolism [1], the discovery of membrane receptors that sense nutrients (nutrient sensors, see Glossary) has made it apparent that nutrients can act via these receptors to transmit cues to intracellular molecular pathways. These receptors generally belong to the G protein coupled receptor (GPCR) class [2], where the term ‘nutrient sensing GPCRs’ was originally suggested for the free fatty acid (FFA) receptors (see [2]). Along with FFA receptors (FFAR), nutrient sensing GPCRs also exist for amino acids [3] and carbohydrates [4].
The FFAR subclass binds to fatty acids or their derivatives, with each GPCR in this subclass exhibiting specificity for fatty acids (FAs) of a particular chain length. FFA1 (or GPR40), GPR84 and FFA4 (or GPR120) are activated by medium or long chain FAs (carbon length above 6); whereas, four other members of the FFAR family, FFA2 (previously GPR43), FFA3 (previously GPR41), GPR109a and OLFR78 bind short chain fatty acids (SCFAs, ranging from 1–6 carbons in length) [5, 6, 7]. While SCFAs are a source of energy and are primarily generated through gut microbiota fermentation, they also act systemically as signaling metabolites and gene transcription modulators (Box 1) [6]. The discovery of receptors specific to SCFAs has led to the attribution of the metabolic effects of the gut microbiota and SCFAs to these receptors. As the majority of published data in the SCFA receptor field has been on FFA2 and FFA3, we focus on these two GPCRs in this review (Table 1, see [7] for more details on GPR109a and OLFR78). Additionally, the potency of SCFAs for these other two receptors is much less than FFA2 and FFA3, and their role as SCFA receptors is just beginning to emerge [8, 9]. In the sections that follow, we highlight the basic pharmacology of FFA2 and FFA3, identify their biological roles with a focus on the pancreatic beta (β) cells, and explain how studies have begun to connect these GPCRs to the gut microbiota. The therapeutic potential of these receptors as novel metabolic targets for diabetes is also highlighted in the review.
BOX 1. The long and short on SCFAs.
Acetate, propionate and butyrate, the most abundant SCFAs in the body, are produced by the gut microbiota where a large percentage of SCFAs are immediately metabolized in the colon. SCFAs not metabolized by colonocytes are absorbed into the hepatic and portal venous systems and emerge from liver into the systemic circulation [6]. Broadly, SCFAs contribute to many biological pathways, including having metabolic, immune, and intestinal effects. Metabolically, SCFAs are rich energy sources for colonocytes, liver, muscle, kidney as well as brain and heart [60]. Moreover, dietary SCFA supplementation in rodents diminishes obesity and increases insulin sensitivity and energy expenditure [61, 62]. Acetate and propionate can also modulate energy intake by reducing appetite via central mechanisms [59, 63]. Beyond metabolism, SCFAs have been reported to regulate colonic regulatory T cells (Treg) and promote differentiation of naive CD4+ T cells to anti-inflammatory FOXP3+ Tregs [64], that then migrate to target tissues like brain and adipose suppressing inflammation and autoimmunity [65]. SCFAs also protect the integrity of the gut epithelium by competitively excluding the growth of pathogenic bacteria and preventing leakiness of the gut barrier [58]. Because of these findings, SCFAs have been considered as therapeutic modalities against intestinal disorders and against leaky gut derived metabolic endotoxemia associated with obesity, adipose inflammation and insulin resistance [66–68]. With these benefits, exploring the roles of SCFAs in coordinating various biological processes is an exciting area of research.
Table 1.
Summary of the short chain fatty acid receptors (FFA2 and -3), highlighting their tissue expression, endogenous and synthetic ligands, G-protein signaling/effector, and biological functions
| Receptor | Tissue expression | Endogenous ligands | Synthetic agonists | G protein & effector | Biological functionsa | Ref |
|---|---|---|---|---|---|---|
| FFA2 | Pancreatic islets, white adipocytes, enteroendocrine cells, immune cells | SCFAs (C2-C6)a acetate (pEC50 4.5/3.6)b propionate (pEC50 4.2/3.7)b |
CMTB (IC50 0.7/1.0)b PA (IC50 0.7/NA)b SCA14 (pEC50 3.2/NA)b SCA15 (pEC50 2.7/NA)b |
Gαi/o/cAMP , Gαq/11/Ca2+ | Insulin secretion ↑ | [11, 17, 19, 37–42, 45–49, 53] |
| GLP-1 secretion ↑ | ||||||
| GIP secretion ↑ | ||||||
| ghrelin secretion ↓ | ||||||
| leptin secretion ? | ||||||
| PYY secretion ↑ | ||||||
| β cell growth ↑ | ||||||
| insulin signaling in adipose ↓ | ||||||
| regulation of inflammatory responses | ||||||
| gut homeostasis ↑ | ||||||
| FFA3 | Pancreatic islets, enteroendocrine cells, sympathetic ganglia, enteric neurons, immune cells, white adipocytes | SCFAs (C3-C6)a valerate (pEC50 4.6/NA)b butyrate (pEC50 4.0/3.7)b propionate (pEC50 4.1/4.9)b |
MCPC (pEC50 3.9/4.3)b | Gαi/o/cAMP | Insulin secretion ↓ | [16, 18, 38, 43, 44, 46,50–55] |
| GLP-1 secretion ↑ | ||||||
| PYY secretion ↑ | ||||||
| intestinal gluconeogenesis ↑ | ||||||
| sympathetic outflow regulation | ||||||
| inflammation ↓ |
carbon chain length of SCFAs and particular SCFAs with a preference; ↑, promotes; ↓, decreases, ?, controversial
pEC50 or IC50 values for human receptor/mouse receptor as available from various functional assays [14, 15, 30, 31]
NA, not available
CMTB, 4-chloro-α-(1-methylethyl)-N-2-thiazolylbenzeneacetamide)
PA, ((S)-2-(4-chlorophenyl)-3,3-dimethyl-N-(5-phenylthiazol-2-yl)butanamide)
SCA, small carboxylic acid
MCPC, 1-methylcyclopropanecarboxylic acid
Pharmacology of Short Chain Fatty Acid Receptors, FFA2 and FFA3
Ligand selectivity
The SCFAs were identified as ligands for FFA2 and FFA3 over 10 years ago [10, 11]. Since then, a substantial effort has been placed into determining the potency of various SCFAs for both receptors. Prior to discussing these data, it is important to note what SCFA levels are in the blood, where the levels in humans and mice are similar and range from approximately 100–200 μM for acetate and 1–20 μM for propionate and butyrate [6, 12]. For FFA2, both acetate and propionate are the most potent agonists (Table 1) [10, 11, 13–15]. Longer SCFAs (4–7 carbons in length) and formate also show the ability to activate FFA2, albeit less potently than acetate or propionate [13–15]. With FFA3, slightly longer SCFAs have the greatest potency with valerate being the most potent, followed by butyrate [13], but this activity decreases as size increases to medium-chained acids. Another distinction is that SCFAs with branched alkyl groups such as isovalerate and isobutyrate tend to favor FFA3 over FFA2 [15]. An interesting observation is made when the alkyl chain is modified with unsaturated double or triple bonds [15]. When the double bond of the SCFAs is in the α,β-position, compounds have less potency for FFA3 while maintaining or increasing their effect on FFA2. The preference for FFA2 to bind acrylates and for FFA3 to be activated by branched alkanes suggests that the orthosteric binding pocket of FFA2 is smaller and possibly narrower than FFA3.
Role of FFA2 and FFA3 in Regulation of Metabolism
Owing to their expression in many metabolically relevant tissues, evaluation of the physiological roles of FFA2 and FFA3 is topical especially considering the emerging of role of the gut microbiota in metabolism. In the past one year, contributions of FFA2 and FFA3 to pancreatic β cell function including glucose stimulated insulin secretion (GSIS), β cell mass and to β cell responses to insulin resistance have been recognized [16–19], and this role of FFA2 and FFA3 in β cells is the primary focus of the following sections.
Islet expression of FFA2 and FFA3 and the influence of insulin resistance
Multiple initial observations suggested a role of both receptors in β cell function. First, Arena Pharmaceuticals reported in the patent literature that Ffar2 and Ffar3 levels were upregulated in the islets of db/db and ob/ob, and db/db mice, respectively [20, 21]. Subsequently, other groups observed increased expression of Ffar2 in islets during the insulin resistant phase of pregnancy [22, 23]. Furthermore, a mouse gene database profiling in islets, adipose, skeletal muscle, and hypothalamus relative to obesity, diabetes susceptibility, and age [24] revealed increased islet specific expression of Ffar2 in obese mice, independent of type 2 diabetes (T2D) susceptibility [17]. Collectively, these data suggest that β cell expressed FFA2 and possibly FFA3 may contribute to the β cell response to insulin resistance.
Role of FFA2 and FFA3 in insulin secretion
A primary function of β cells is to secrete insulin, where its principal secretagogue is glucose. Besides glucose, others nutrients including SCFAs have been reported to modulate GSIS and prior to the discovery of nutrient-sensing receptors, it was thought to be through their intracellular metabolism [25]. A range of studies over the past 50 years have observed SCFAs contribute to GSIS (see [26–29]); however, there was no clear consensus whether the effects are stimulatory or inhibitory. During 2015, three groups independently reported on the role of FFA2 and FFA3 in β cells [12, 16–19] providing evidence that these GPCRs can, in part, be the mode that SCFAs influence GSIS.
Continuing with the trend prior to the discovery of these receptors, reported outcomes in 2015 of how these nutrients and their receptors mediate GSIS were divergent. Tang et al [16], through studies using both mouse (MIN6) and human pancreatic β cell lines (EndoC-βH1) and a combination of genetic FFA2 and FFA3 knockout mouse models and human islets, reported that FFA2 and FFA3 are negative modulators of GSIS (using acetate as the activator of both receptors). Moreover, each of these receptors was observed to signal via a pertussis toxin (PTX)-sensitive Gαi/o pathway (see Box 2). Priyadarshini and Layden [18] confirmed that FFA3 mediates a PTX-sensitive inhibition of GSIS using mouse islets, SCFAs and a FFA3 agonist to probe the function of FFA3. However, Priyadarshini et al [17] and McNelis et al [19] reported that FFA2 signaling largely augments GSIS (and does not diminish GSIS) and does this through the Gα subunit class, Gαq/11. Next, we explore these opposing outcomes, which will help clarify these conflicting reports on how SCFAs and their GPCRs influence GSIS.
BOX 2. G protein binding profiles and signaling signatures.
Following the deorphanization of these receptors [10, 11], investigation using yeast containing different yeast/mammalian Gα chimeras indicated that human FFA2 (hFFA2) activates Gpa1p/Gα chimeras containing the C termini of mammalian Gα12, Gα13, Gα14, Gαi1, and Gαi3, suggesting that FFA2 activates the Gαi/o, Gαq/11, and Gα12/13 families of G proteins. Additionally, acetate provoked a dose-dependent increase in (35S)GTPγS binding in membranes prepared from HEK293T cells transfected with Gαo and hFFA2 [10, 11]. Le Poul et al [32] found that acetate or propionate promote FFA2 signaling to Gαq/11 and Gαi/o, through intracellular calcium and cAMP, respectively, in CHO-K1 and COS-7 cells expressing hFFA2. A (35S)GTPγS binding assay confirmed acetate and propionate coupling of FFA2 with Gαi/o in a cell-free assay, and pertussis toxin (PTX) inhibited the response [32]. In cells cotransfected with FFA2 and Gαqi5, there was an increase in the basal level of inositol phosphates over cells expressing only one of the plasmids, suggesting that FFA2 may have constitutive activity [32].
Despite amino acid sequence homology of FFA3 to FFA2, FFA3 couples exclusively through the PTX-sensitive Gαi/o family. Like FFA2, FFA3 was found to be constitutively active in yeast when co-expressed with Gpa1p/Gαi1 and Gpa1p/Gαi3 chimeras [10, 11]. FFA3 also responded to SCFAs when co-expressed in yeast with the Gpa1p/Gαo chimera, an effect not seen with FFA2 [10, 11]. Using transient co-transfection of hFFA3 and Gαi3 into HEK293T cells and transfection of a hFFA3-Gαi3 fusion protein, it was shown that propionate increased levels of Gαi3-bound (35S)GTPγS in a concentration-dependent manner [13]. Thus, FFA2 can couple to both Gαq/11 and Gαi/o protein classes whereas FFA3 can couple to Gαi/o protein class. With different G protein binding profiles, these receptors can uniquely affect diverse cellular processes with either antagonistic or synergistic outcomes.
Co-existence of these receptors in pancreatic β cells suggests that their activation could differently affect insulin secretion (Figure 1) and cell mass (Figure 2). For example, FFA2 signaling via two divergent G protein classes (Gαq/11 and Gαi/o) is anticipated to have dissonant effects on GSIS, FFA3 coupling solely to Gαi/o class would inhibit GSIS [69].
First, Priyadarshini et al [17] showed that acetate (at 1 mM) potentiated GSIS from wild type (WT) islets as compared to islets from FFA2 null (Ffar2−/−) mice in static insulin secretion studies. As these data conflicted with Tang et al [16], synthetic FFA2 agonists were used to probe the role of FFA2 in GSIS with mouse islets and it was observed that each had varying effects on GSIS; two agonists [15] potentiated GSIS in a FFA2-specific manner, while two other ligands CMTB (4-chloro-α-(1-methylethyl)-N-2-thiazolylbenzeneacetamide) [30] and PA ((S)-2-(4-chlorophenyl)-3,3-dimethyl-N-(5-phenylthiazol-2-yl)butanamide) [31]), both at 100 μM, inhibited GSIS from WT mouse islets. However, the inhibitory effects of CMTB and PA on GSIS were not exclusively through FFA2, indicating off-target effects. Further data through using pharmacological inhibitors of the respective G protein pathways showed that these FFA2 agonists mediate GSIS through coupling with Gαq/11 and likely Gαi/o in β cells (Figure 1 & Box 2) [32]. The other study, McNelis et al [19], observed that FFA2 mediates GSIS through a Gαq/11 dependent manner, where these results were obtained through studies with human islets, mouse islets, and MIN6 cells. However, they did not observe acetate (using 1 mM acetate) influenced GSIS but did find that PA (at 1 μM) stimulated insulin release in a Gαq/11 dependent manner. Additionally, coupling of FFA2 to Gαq/11 was further confirmed by PA-mediated accumulation of inositol-1,4,5-triphosphate (IP3), a second messenger downstream of Gαq/11 (Figure 1). Emerging from these two studies [17,19] is the important finding that FFA2 predominantly couples to Gαq/11, but both groups did observe evidence that FFA2 also couples Gαi/o.
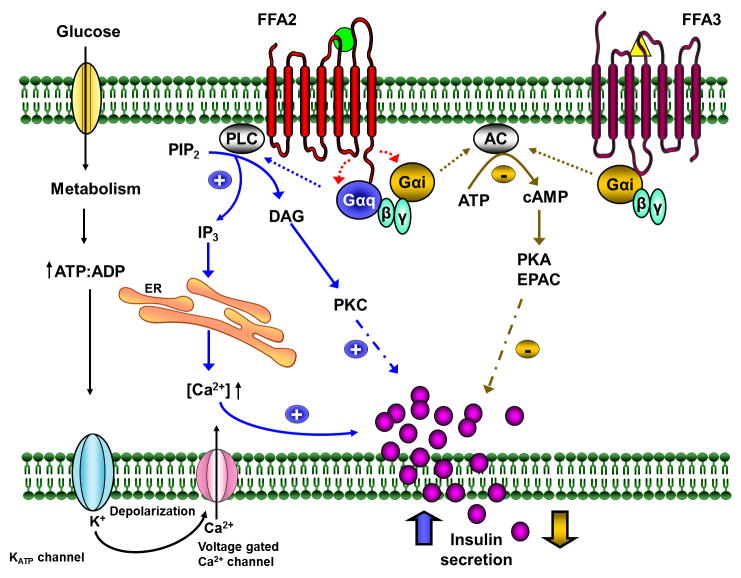
Figure 1.
Modulation of insulin secretion by FFA2 and FFA3.
Glucose once internalized in β cells is metabolized which results in elevation of ATP:ADP ratio, closure of KATP channels, cell membrane depolarization, opening of voltage dependent calcium channels (Ca2+ channels) causing calcium influx triggering insulin vesicle exocytosis. SCFAs (acetate and propionate) can bind to FFA2 and FFA3 either amplifying (in blue) or diminishing (in golden) glucose stimulated insulin secretion. Upon ligand activation of FFA2, Gαq/11 subunits activate PLC which hydrolyses PIP2 to DAG and IP3 which in turn activates PKC and releases calcium from ER stores, respectively, amplifying the insulin release. FFA2, like FFA3, can also couple with Gαi/o subunits and inhibit AC, which decreases the concentration of cAMP, inhibiting PKA and EPAC mediated insulin release.
Abbreviations: AC, adenylate cyclase; cAMP, cyclic AMP; DAG, diacylglycerol; EPAC, exchange protein directly activated by cAMP; ER, endoplasmic reticulum; FFA2, free fatty acid receptor 2; FFA3, free fatty acid receptor 3; IP3, inositol triphosphate; KATP channels, ATP sensitive potassium channels; PLC, phospholipase C; PIP2, phosphatidylinositol 4,5-bisphosphate; PKC, protein kinase C; PKA, protein kinase A; SCFAs, short chain fatty acids. Green circle for acetate and yellow triangle for propionate.
While these two studies concluded that FFA2 mostly couples with Gαq/11, differences existed between these studies which warrant exploration, in particular in the observed function of FFA2 in human islets. With human islets, Priyadarshini et al [17] showed data that acetate (at 1 mM) and SCA14, SCA15 and PA (each at 100 μM) did not affect GSIS, but the agonist, CMTB (at 100 μM), inhibited GSIS and that this inhibition was not PTX-sensitive. McNelis et al [19] showed PA (at 1 μM) augments GSIS with human islets in a PTX-insensitive manner. Additional data from Priyadarshini et al [17] confirmed that the FFA2 agonists used in their study can simultaneously activate both Gαq/11 and Gαi/o pathways in hFFA2 expressing CHO-K1 cells, suggesting the possibility that in human β cells, these agonists activate both pathways equally resulting in a net neutral effect on GSIS. Reasons for these different outcomes could be related to receptor pharmacology of FFA2 between humans and mice (see Box 3) or the lack of well characterized FFA2 agonists (see [33]). Additional investigations are needed to reconcile the differences in the human data between Priyadarshini et al [17] and McNelis et al [19] and to confirm the G-protein coupling preference in human islets. Another variable is that each of these human studies had only a small amount of donors, and differences very well may exist between islets from different donors in their response to different agonists (also, receptor expression may be influenced by unforeseen factors influencing receptor function). Further understanding of FFA2 signaling in human islets will ultimately be crucial to truly understand the therapeutic value of FFA2 in T2D.
BOX 3. Structural features define species specificity in receptor function.
It has been observed that differences exist in the selectivity and potency of SCFAs binding to FFA2 and FFA3 orthologs between species. For example, the activities of the human and mouse FFA2 (hFFA2 and mFFA2) orthologs show clear differences, with acetate being nearly 100x more potent against hFFA2 than hFFA3, but nearly equipotent against the two mouse orthologs [14]. Additionally, while propionate has similar potency for hFFA2 and hFFA3, it is approximately 10x more potent for mFFA3 than mFFA2.
While the crystal structures of FFA2 and FFA3 have not been solved, there is substantial mutagenesis data that provides information on the binding mode of SCFAs to these receptors (Figure I). One identified interaction between receptor and ligand is with the carboxylate group of SCFAs [13, 14, 70]. Specifically, interactions between the carboxylate groups (which are negatively charged at physiological pH) with the positively charged arginine residues in the orthosteric site of FFA2 have been identified. In particular, Arg180 and Arg255 are essential for acetate or propionate agonist activity towards FFA2. It was also found that His242 is required for FFA2 activation by SCFAs, presumably due to a favorable electrostatic-driven complex between His242 and these two arginine side chains in the orthosteric pocket. Interestingly, the recently reported FFA1 crystal structure [71] shows strong interactions between the carboxylate of the bound ligand (TAK-875) and Arg183 and Arg258. These two arginine residues are equivalent to Arg255/180 in FFA2, confirming this orthosteric carboxylate binding interaction. These structural data have been useful in identifying why SCFAs have unique interactions with hFFA2 and mFFA2. Notably, a third residue in mFFA2 was identified which has a significant effect on the potency of SCFAs against FFA2. In mFFA2, a Glu159 forms an “ionic lock” with Arg255/180 in a manner similar to that of FFA1 [14]. However, in hFFA2, this glutamate is replaced by glycine, abolishing the ionic interaction. Mutagenesis studies confirmed that this residue plays a critical role in determining the species selectivity towards SCFAs between mouse and human FFA2. These data are highly relevant because they may confound drug discovery efforts due to the reliance on non-human models [72].
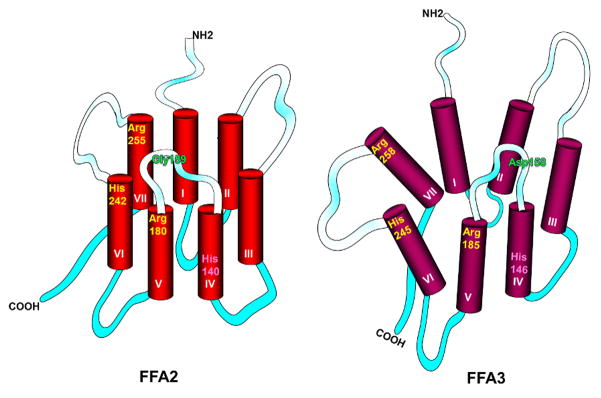
Figure I.
Key features of ligand binding sites of hFFA2 and hFFA3.
The binding pocket of FFA2 is smaller than FFA3, which conforms to the size selectivity of each receptor. Residues that play important role in SCFA recognition and binding are Arg 180 in FFA2 (Arg 185 in FFA3) at the top of transmembrane domain V (shown in yellow), His 242 in FFA2 (His 245 in FFA3) at the top of transmembrane domain VI and Arg 255 in FFA2 (Arg 258 in FFA3) at the top of transmembrane domain VII. These positively charged residues interact with the negatively charged carboxylate group of SCFAs facilitating their function. His 140 (His 146 in FFA3) (shown in pink) predicted to be lower in the binding pocket possibly plays a role in fatty acid chain length selectivity. Presence of an acidic non-conserved residue in extracellular loop 2 (connecting domains IV and V) is responsible for the variation in constitutive, ligand independent activity between human and rodent receptors. In orthologs lacking constitutive activity (mFFA2 and hFFA3), an extracellular ionic lock is formed between the arginine residues of the orthosteric binding site and an acidic residue in extracellular loop 2 (Glu159 in mFFA2 and Asp158 in hFFA3) whereas orthologs with high constitutive activity (hFFA2 and mFFA3 have Gly and Asn at corresponding positions, respectively) do not possess this interaction. For further explanation, see [13, 14, 15]. Green= residues that vary between the orthologs and play role in constitutive activity of the receptor. TM, transmembrane. Shaded segments= extracellular loops connecting the TM domains; solid aqua segments= intracellular loops.
As noted earlier in this review, insulin resistant states including that induced by high fat diet (HFD) influences receptor expression in islets in particular for FFA2 [17, 19]. An important question these data raises is whether this expression change alters receptor function, and insight into this possibility has been suggested. From Priyadarshini et al [17], islets from HFD fed Ffar2−/− mice showed a similar GSIS pattern to islets from normal chow fed mice in the presence of the FFA2 agonists, with the exception of one of the FFA2 agonists, PA, which increased GSIS in islets from WT mice fed a HFD, as opposed to its inhibitory effect on GSIS in islets from WT normal chow fed mouse islets. These data provide evidence of a possible change in G-protein coupling with FFA2 from HFD. Next, measuring IP3 and cAMP levels subsequent to PA stimulation, McNelis et al [19] observed that cAMP inhibition was similar in islets from HFD fed mice either on short term (8 week) or long term (14 week) regimen, but IP3 accumulation increased as function of time on HFD, where the authors postulated that for FFA2, the ratio of stimulatory to inhibitory G-protein signaling may depend upon the duration of HFD consumption. Considering these data [17, 19], we suggest a shift in the G-protein coupling preference of FFA2 (or changing in coupling) may be occurring, in the context of HFD. Moreover, our lack of understanding of the G-protein coupling preferences of FFA2 may explain differences between each of these studies. Taken together, it is apparent from these studies that both FFA2 and FFA3 regulate GSIS, where FFA2 does this through Gαq/11 and Gαi/o, and FFA3 through Gαi/o; however, a greater understanding is needed into what controls FFA2 coupling preferences.
Role of FFA2 and FFA3 in pancreatic β cell mass regulation
As with GSIS, Gαq/11 coupled GPCRs have been observed to augment β cell mass expansion in response to insulin resistance, whereas Gαi/o coupled GPCRs have been reported to restrict the β cell mass expansion [34, 35]. Not surprisingly, McNelis et al [19] observed reduced β cell mass in Ffar2−/− mice on a HFD compared to WT mice, lower β cell proliferation and reduced expression of insulin and the β cell transcription factors (Mafa, Pdx1, NeuroD) [19]. Consistent with these findings, PA or acetate stimulated the proliferation of MIN6 cells, insulin levels, and Pdx1 and NeuroD gene transcription, and was mediated via Gαq/11 (Figure 2, Key Figure). Likewise, one of the other groups reported that Ffar2−/− mice exhibited reduced β cell mass and β cell proliferation in response to pregnancy [12]; however, their mouse model had reduced β cell mass prior to HFD and also pregnancy. As opposed to these studies, Tang et al [16] observed no morphological difference in their FFA2 or FFA3 knockout islets, but directly measuring β cell mass was not done. While these data suggest a role for FFA2 in the regulation of β cell mass, the role, if any, of FFA3 remains unknown, as it has not been reported.
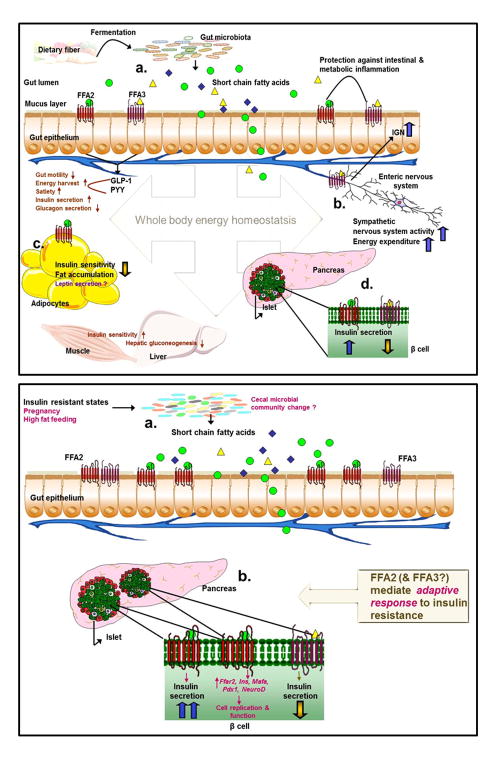
Figure 2.
Homeostatic role of FFA2 and FFA3 in energy metabolism, a global view (upper panel) and β cell-specific view (lower panel).
Gut microbial fermentation products, SCFAs, activate FFA2 and -3 on enteroendocrine L cells stimulating the release of GLP-1 and PYY, which affect energy absorption and metabolism via effects on gut function and outside the gut (a). Activation of FFA3 on the enteric neurons and sympathetic ganglia enhances intestinal gluconeogenesis and sympathetic outflow, reducing hepatic glucose production and increasing energy expenditure (b). Stimulation of FFA2 decreases insulin signaling in adipocytes with a consequent increase in energy expenditure in other tissues (c). In β cells, activation of FFA2 or FFA3 causes opposing affects on insulin secretion (d). Lower panel: Under states of insulin resistance (pregnancy and high fat feeding), gut microbiota composition changes may lead to altered cecal and plasma levels of SCFAs (a). In β cells, increased expression and activation of FFA2 causes compensatory increase in insulin secretion and transcription of factors responsible for increased β cell proliferation and mass, contributing to adaptive responses of the β cell to insulin resistance. The role of FFA3 in insulin resistance is less clear (b).
Abbreviations: IGN, intestinal gluconeogenesis; Ins, insulin; Mafa, v-maf musculoaponeurotic fibrosarcoma oncogene family, protein A; NeuroD, neuronal differentiation 1; Pdx1, pancreatic and duodenal homeobox 1; PYY, peptide YY; SCFAs, short chain fatty acids. Green circles= acetate; yellow triangles= propionate; blue diamonds= butyrate. Dark brown: effects secondary to receptor activation; purple: unclear roles of receptor signaling; magenta: insulin resistance mediated changes.
Role of FFA2 and FFA3 in insulin resistance
Considering the diverse outcomes published above, it is not surprising that diverse in vivo phenotypes have also been reported on the role of FFA2 and FFA3 in β cell function during insulin resistant states. For FFA2, Fuller et al, Priyadarshini et al and McNelis et al [12, 17, 19] in different models of insulin resistance, each observed an in vivo insulin secretion deficit. During the insulin resistant phase of pregnancy, at gestation day 15 (G15) [36], Ffar2−/− mice exhibited an in vivo insulin secretory defect, specifically, pregnant Ffar2−/− mice exhibited impaired glucose tolerance due to low glucose stimulated insulin secretion [12]. However, this group did not observe with male Ffar2−/− mice on a HFD impaired glucose tolerance; but did observe an in vivo deficit during hyperglycemic clamp studies while mice were on normal chow [17]. McNelis et al [19] showed an impaired glucose tolerance with diminished in vivo insulin secretion on HFD for Ffar2−/− mice. However, Tang et al [16], observed the opposite, Ffar2−/− and Ffar3−/− mice or Ffar2−/−;Ffar3−/− double knockout mice on HFD exhibited increased glucose tolerance and plasma insulin levels post glucose challenge, with the effects being more pronounced in the double knockouts. Why these opposing outcomes are observed is not clear, but we suspect this is due to our lack of understanding of the G-protein coupling with FFA2 and/or the confounding variable of FFA2 and FFA3 expression in tissues other than islets (Figure 2).
Non-pancreatic beta cell roles in metabolism
FFA2 and FFA3 can also influence metabolism through their expression in other tissues and cell types, for example immune cells (see [37]), adipose tissue, neural tissue and gut L cells. A role of these receptors in gut L cells emerged from studies with Ffar2−/− and Ffar3−/− mice, either on chow [12, 38] or on HFD [19] with these mice having decreased secretion of GLP-1 in response to an oral glucose challenge. McNelis et al [19] also reported lower circulating gastric inhibitory polypeptide (GIP) and PYY (peptide YY) in the Ffar2−/− mice, hormones involved in regulating β cell function and suppressing appetite, respectively. An incretin role for these receptors raises the concern that some of the β cell function can partly be attributed to this function. Thus it is necessary to distinguish the roles of FFA2 and possibly FFA3 in β cells versus L cells. It is important to mention, however, the roles of these receptors in incretin release in vivo has not been observed by all groups [16].
A role of FFA2 and FFA3 in adiposity has been reported, but is also debated. Early reports observed a reduction of body fat mass in Ffar2−/− mice on HFD, from altered energy expenditure [39]. These findings are directly contradicted by others [40], where it was observed that FFA2 inhibits insulin signaling and hence fat accumulation in adipose tissue. In this study [40], FFA2 ablation led to spontaneous obesity on normal chow, whereas overexpressing Ffar2 in adipose tissue led to a lean phenotype on a HFD, effects that were attributed to the gut microbiota. It is noteworthy that FFA2 has also been reported to increase leptin and decrease ghrelin secretion, two hormones that influence energy balance [41, 42]. FFA3 signaling has also been implicated in modulating body fat mass, although these effects are also far from clear with reports of Ffar3−/− mice having no change [38], loss [43] or gain of body fat mass [44]. In summary, contradictory results have also been obtained with both FFA2 and FFA3 knockout models on their role in the development of adiposity.
These receptors are also reported to influence the immune system, and for FFA3, in the nervous system. For example, Ffar2−/− mice exhibit exacerbated colitis, arthritis and asthma [45], while the stimulation of FFA2 affects immune cell recruitment, production of chemokines and cytokines [45–49]. These results indicate a role for FFA2 in inflammation, where the action of this receptor can seemingly be both pro- and anti-inflammatory, depending on the specific cell type [37]. Likewise, FFA3 has been reported to influence inflammatory responses in intestinal epithelial cells and lungs [50, 51]. And finally, FFA3 is expressed in sympathetic ganglion and the enteric nervous system [52, 53]. In the fed state, FFA3 agonism has been reported to increase energy expenditure via sympathetic outflow [52, 54]; while under fasting conditions, FFA3 signaling attenuates energy expenditure and in sympathetic outflow. FFA3 activation in peripheral nerves by propionate was also reported to have metabolic benefits such as improved glucose control, decreased adiposity, and decreased hepatic glucose production through increased intestinal gluconeogenesis [55]. Collectively, signaling of FFA2 and FFA3 in adipose, gut, neural, pancreatic β cells and inflammatory cells can affect overall metabolic homeostasis (Figure 2). As apparent, the conflicting data might arise from the multitude of effects at these different tissues, but, as discussed next, may also be affected by gut microbial activity through undefined mechanisms.
Considerations in understanding the gut microbiota to receptor relationship
Specific compositional and functional gut microbial signatures are associated with particular host metabolic phenotypes [56, 57]. One mechanism by which gut microbiota influences host metabolism is through production of metabolites, like SCFAs [58] which as highlighted above, can influence host physiology by their ligation with FFA2 and FFA3 (Box 1 & Figure 2). Unraveling this interface between gut microbiota and metabolic phenotypes through these receptors is emerging from rodent studies using receptor knockout models, models of gnotobiotic mice and from dietary intervention models [12, 19, 40, 55]. One example is that Ffar3−/− gnotobiotic or conventionally raised mice were leaner than WT mice due to reduced PYY levels, increased gut transit and decreased energy harvest from SCFAs [43], and this effect was influenced by the gut microbiota. Similar effects have been published by Kimura et al [40] where Ffar2−/− mice and HFD fed mice over expressing Ffar2 in adipose tissue had altered weight phenotypes that reverted to normal under germ free conditions or with antibiotic treatment. Metabolic phenotype and diet associated gut microbial shifts in Ffar2 mouse models have also been depicted in other studies [12, 19]. On a HFD, WT and Ffar2−/− mice showed distinct fecal bacterial phyla profiles with an observed increase ratio of Firmicutes/Bacteroidetes, a possible signature for human obesity [19]. Functional impacts of these changes in the gut microbiota in the WT and Ffar2−/− mice on HFD led to higher serum acetate levels [19].
The exact implication of this relationship between the gut microbiota and SCFAs through these receptors at the β cells remains unclear. In a pregnancy mouse model, where pregnancy is a state of insulin resistance, Fuller et al [12] provides an initial exploration of this relationship. While no significant overall gut microbial shifts were observed [12], changes in relative abundances of Actinobacteria and Bacteroidetes in the postpartum period occurred in WT mice. Also, functional changes were evident in plasma SCFA levels with acetate trending higher and propionate lower during pregnancy, which could be relevant to FFA2 activity, as acetate is a more potent ligand than propionate. Moreover, Ffar2−/− mice had gestational impaired glucose tolerance, possibly due to the absence of acetate-FFA2 signaling during pregnancy, which led to impaired in vivo insulin secretion and β cell proliferation [12]. As with other tissues that express FFA2 and FFA3, effects of gut microbial generated SCFAs may be influencing FFA2 and FFA3 function in pancreatic β cell function and growth [12, 16, 17, 19], thus representing a novel ‘gut/β cell axis’.
Concluding Remarks and Future Perspectives
FFA2 and FFA3 impact metabolism through their roles in β cells and other tissues. Complicating the understanding of the function of these receptors is the fact that FFA2 (and possibly FFA3) couple to multiple G proteins in vivo and exhibit species specific responses to ligands [19] (Box 2 and Box 3). Likewise, SCFAs exhibit functional redundancy for these receptors (Box 3) and also, SCFAs likely exert effects independent of these receptors [57, 59] (Box 1). Another challenging variable with the mouse models being used to decipher the function of these receptors is that FFA2 and FFA3 genes are located in close genetic proximity, thus, genetic knockout may modulate the expression of the other receptor [18, 42]. This calls for additional, more specific, tissue knockout models. To support these models, the development of selective specific synthetic agonists and antagonists that are characterized for species- and signaling-preferences are needed for detailed dissection of the physiological roles of these receptors. Furthermore, differences in the gut microbiota composition (between animal facilities) may lead to discordant phenotypes of these mouse models possibly through altering SCFA levels and consequently the function of these receptors; thus to eliminate this variable it may be necessary to control for the gut microbiota through gnotobiotic conditions when investigating these receptors.
Studies defining the pharmacology and metabolic function of FFA2 and FFA3 are yielding our initial understanding of the role of these receptors in the β cell and beyond. It is now demonstrated that FFA2 and FFA3 modulate GSIS, and FFA2 contributes to the regulation of β cell mass. Consistent evidence is still lacking and key questions remain (see Outstanding Questions Box). Moving forward, manipulation of FFA2 and/or FFA3 function in the β cells as targets for diabetes therapy still requires validation in animal models, with close translation to human tissue.
Outstanding Questions.
What are the main functions of FFA2 and/or FFA3 outside the β-cells, and how do these functions influence aspects of β-cell biology?
What factors regulate expression of FFA2 and FFA3 in β cells and outside of β cells?
Does increased mRNA expression of FFA2 and FFA3 lead to altered function?
What physiological and pathological conditions mediate Gα coupling preference for FFA2?
While FFA2 activation may be an initial adaptive mechanism under states of insulin resistance to compensate for increase in insulin requirements, what are the effects of its chronic activation?
Pharmacological studies suggest differences exist in the constitutive activity of each receptor. How does this constitutive activity influence their function in β-cells?
How do FFA2 and FFA3 mouse studies translate to humans?
Are biased FFA2-Gαq/11 agonists and/or FFA3 antagonists a reasonable approach to treat type 2 diabetes?
Trends Box.
FFA2 and FFA3, uniquely respond to ligands that are derived from the gut microbiota and thus, represent a biologic mechanism linking microbial composition (and its changes) to glycemic control and multi-tissue homeostasis.
FFA2 and FFA3 are novel effectors of glucose homeostasis in part due to their direct effects on insulin secretion and beta cell proliferation.
Associated challenges in establishment of the biology of these receptors are from the scarcity of selective agonists, insufficient mouse models and challenges in correlating changes in endogenous ligands of these receptors to specific gut microbial/dietary/metabolic profiles.
The still evolving novel gut-pancreas axis represents a potential therapeutic target for treatment of type 2 diabetes and related metabolic disorders.
Acknowledgments
BTL is supported by the National Institutes of Health under award number, R01DK104927-01A1, The University of Chicago DR&TC (P30DK020595) and Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Career Development (Grant no. 1IK2BX001587-01). MP is supported by an American Heart Association postdoctoral fellowship (#15POST22410016). A part of this work was performed by the Northwestern University Medicinal and Synthetic Chemistry Core (ChemCore) at the Center for Molecular Innovation and Drug Discovery (CMIDD), which is funded by the Chicago Biomedical Consortium with support from the Searle Funds at the Chicago Community Trust.
Glossary
- Gnotobiotic
is an organism with a defined microbial community such as germ free or colonized with a particular microbial species.
- Hyperglycemic clamp
involves clamping the plasma glucose concentration at a pre-specified elevated level through a specific glucose infusion rate, which is dependent on β cell’s ability to secrete insulin and the body’s ability to metabolize glucose.
- Insulin sensitivity
is the measure of responsiveness of organs to metabolic actions of insulin. Significantly reduced insulin sensitivity is used as prognostic parameter for type 2 diabetes and metabolic syndrome.
- Microbiota
represents the microbial community of a specific region.
- Nutrient sensors
are functionally and structurally diverse molecular machines either transmembrane (GPCRs) or cytosolic (AMPK, mTOR) that are specialized in detecting specific nutrients. Detection of the nutrient is followed by induction of a response, which may alter cellular physiology.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iyer A, Brown L, Whitehead JP, Prins JB, Fairlie DP. Nutrient and immune sensing are obligate pathways in metabolism, immunity, and disease. Faseb J. 2015;29(9):3612–3625. doi: 10.1096/fj.15-271155. [DOI] [PubMed] [Google Scholar]
- 2.Kotarsky K, Nilsson NE, Flodgren E, Owman C, Olde B. A human cell surface receptor activated by free fatty acids and thiazolidinedione drugs. Biochemical and biophysical research communications. 2003;301(2):406–410. doi: 10.1016/s0006-291x(02)03064-4. [DOI] [PubMed] [Google Scholar]
- 3.Wauson EM, Lorente-Rodriguez A, Cobb MH. Minireview: Nutrient sensing by G protein-coupled receptors. Molecular endocrinology. 2013;27(8):1188–1197. doi: 10.1210/me.2013-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Efeyan A, Comb WC, Sabatini DM. Nutrient-sensing mechanisms and pathways. Nature. 2015;517(7534):302–310. doi: 10.1038/nature14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hara T, Kashihara D, Ichimura A, Kimura I, Tsujimoto G, Hirasawa A. Role of free fatty acid receptors in the regulation of energy metabolism. Biochimica et biophysica acta. 2014;1841(9):1292–1300. doi: 10.1016/j.bbalip.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Layden BT, Angueira AR, Brodsky M, Durai V, Lowe WL., Jr Short chain fatty acids and their receptors: new metabolic targets. Translational research: the journal of laboratory and clinical medicine. 2013;161(3):131–140. doi: 10.1016/j.trsl.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Natarajan N, Pluznick JL. From microbe to man: the role of microbial short chain fatty acid metabolites in host cell biology. Am J Physiol Cell Physiol. 2014;307(11):C979–985. doi: 10.1152/ajpcell.00228.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(11):4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito M, Ren N, Kaplan R, Wu K, Wu TJ, et al. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. The Journal of biological chemistry. 2005;280(29):26649–26652. doi: 10.1074/jbc.C500213200. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson N, Kotarsky K, Owman C, Olde B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochemical and biophysical research communications. 2003;303(4):1047–1052. doi: 10.1016/s0006-291x(03)00488-1. [DOI] [PubMed] [Google Scholar]
- 11.Brown A, Goldsworthy S, Barnes A, Eilert M, Tcheang L, Daniels D, Muir A, Wigglesworth M, Kinghorn I, Fraser N, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. The Journal of biological chemistry. 2003;278(13):11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 12.Fuller M, Priyadarshini M, Gibbons SM, Angueira AR, Brodsky M, Hayes MG, Kovatcheva-Datchary P, Backhed F, Gilbert JA, Lowe WL, et al. The Short Chain Fatty Acid Receptor, FFA2, contributes to gestational glucose homeostasis. American journal of physiology. 2015 doi: 10.1152/ajpendo.00171.2015. ajpendo 00171 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoddart LA, Smith NJ, Jenkins L, Brown AJ, Milligan G. Conserved polar residues in transmembrane domains V, VI, and VII of free fatty acid receptor 2 and free fatty acid receptor 3 are required for the binding and function of short chain fatty acids. The Journal of biological chemistry. 2008;283(47):32913–32924. doi: 10.1074/jbc.M805601200. [DOI] [PubMed] [Google Scholar]
- 14.Hudson B, Tikhonova I, Pandey S, Ulven T, Milligan G. Extracellular ionic locks determine variation in constitutive activity and ligand potency between species orthologs of the free fatty acid receptors FFA2 and FFA3. The Journal of biological chemistry. 2012;287(49):41195–41209. doi: 10.1074/jbc.M112.396259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt J, Smith NJ, Christiansen E, Tikhonova IG, Grundmann M, Hudson BD, Ward RJ, Drewke C, Milligan G, Kostenis E, et al. Selective orthosteric free fatty acid receptor 2 (FFA2) agonists: identification of the structural and chemical requirements for selective activation of FFA2 versus FFA3. The Journal of biological chemistry. 2011;286(12):10628–10640. doi: 10.1074/jbc.M110.210872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang C, Ahmed K, Gille A, Lu S, Grone HJ, Tunaru S, Offermanns S. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nature medicine. 2015 doi: 10.1038/nm.3779. [DOI] [PubMed] [Google Scholar]
- 17.Priyadarshini M, Villa SR, Fuller M, Wicksteed B, Mackay CR, Alquier T, Poitout V, Mancebo H, Mirmira RG, Gilchrist A, et al. An acetate-specific GPCR, FFAR2, regulates insulin secretion. Molecular endocrinology. 2015;29:1055–1066. doi: 10.1210/me.2015-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Priyadarshini M, Layden BT. FFAR3 modulates insulin secretion and global gene expression in mouse islets. Islets. 2015 doi: 10.1080/19382014.2015.1045182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNelis JC, Lee YS, Mayoral R, van der Kant R, Johnson AM, Wollam J, Olefsky JM. GPR43 potentiates beta cell function in obesity. Diabetes. 2015;64:3203–3217. doi: 10.2337/db14-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leonard JN, Chu ZL, Bruce MA, Boatman PD. Gpr41 and modulators thereof for the treatment of insulin-related disorders. Patent. 2005 [Google Scholar]
- 21.Leonard JN, Hakak Y. Gpr43 and modulators thereof for the treatment of metabolic-related disorders. Patent. 2005 [Google Scholar]
- 22.Layden BT, Durai V, Newman MV, Marinelarena AM, Ahn CW, Feng G, Lin S, Zhang X, Kaufman DB, Jafari N, et al. Regulation of pancreatic islet gene expression in mouse islets by pregnancy. The Journal of endocrinology. 2010;207(3):265–279. doi: 10.1677/JOE-10-0298. [DOI] [PubMed] [Google Scholar]
- 23.Rieck S, White P, Schug J, Fox AJ, Smirnova O, Gao N, Gupta RK, Wang ZV, Scherer PE, Keller MP, et al. The transcriptional response of the islet to pregnancy in mice. Molecular endocrinology. 2009;23:1702–1710. doi: 10.1210/me.2009-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keller MP, Choi Y, Wang P, Davis DB, Rabaglia ME, Oler AT, Stapleton DS, Argmann C, Schueler KL, Edwards S, et al. A gene expression network model of type 2 diabetes links cell cycle regulation in islets with diabetes susceptibility. Genome research. 2008;18(5):706–716. doi: 10.1101/gr.074914.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prentki M, Matschinsky FM, Madiraju SR. Metabolic signaling in fuel-induced insulin secretion. Cell metabolism. 2013;18(2):162–185. doi: 10.1016/j.cmet.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Tiengo A, Valerio A, Molinari M, Meneghel A, Lapolla A. Effect of ethanol, acetaldehyde, and acetate on insulin and glucagon secretion in the perfused rat pancreas. Diabetes. 1981;30(9):705–709. doi: 10.2337/diab.30.9.705. [DOI] [PubMed] [Google Scholar]
- 27.Ximenes HM, Hirata AE, Rocha MS, Curi R, Carpinelli AR. Propionate inhibits glucose-induced insulin secretion in isolated rat pancreatic islets. Cell biochemistry and function. 2007;25(2):173–178. doi: 10.1002/cbf.1297. [DOI] [PubMed] [Google Scholar]
- 28.Shah JH, Wongsurawat N, Aran PP. Effect of ethanol on stimulus-induced insulin secretion and glucose tolerance. A study of mechanisms Diabetes. 1977;26(4):271–277. doi: 10.2337/diab.26.4.271. [DOI] [PubMed] [Google Scholar]
- 29.Patel DG, Singh SP. Effect of ethanol and its metabolites on glucose mediated insulin release from isolated islets of rats. Metabolism: clinical and experimental. 1979;28(1):85–89. doi: 10.1016/0026-0495(79)90173-2. [DOI] [PubMed] [Google Scholar]
- 30.Lee T, Schwandner R, Swaminath G, Weiszmann J, Cardozo M, Greenberg J, Jaeckel P, Ge H, Wang Y, Jiao X, et al. Identification and functional characterization of allosteric agonists for the G protein-coupled receptor FFA2. Molecular pharmacology. 2008;74(6):1599–1609. doi: 10.1124/mol.108.049536. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Jiao X, Kayser F, Liu J, Wang Z, Wanska M, Greenberg J, Weiszmann J, Ge H, Tian H, et al. The first synthetic agonists of FFA2: Discovery and SAR of phenylacetamides as allosteric modulators. Bioorganic & medicinal chemistry letters. 2010;20(2):493–498. doi: 10.1016/j.bmcl.2009.11.112. [DOI] [PubMed] [Google Scholar]
- 32.Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. The Journal of biological chemistry. 2003;278(28):25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 33.Bolognini D, Tobin AB, Milligan G, Moss CE. The Pharmacology and Function of Short Chain Fatty Acid Receptors. Molecular pharmacology. 2015 doi: 10.1124/mol.115.102301. [DOI] [PubMed] [Google Scholar]
- 34.Ahren B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nature reviews. 2009;8(5):369–385. doi: 10.1038/nrd2782. [DOI] [PubMed] [Google Scholar]
- 35.Berger M, Scheel DW, Macias H, Miyatsuka T, Kim H, Hoang P, Ku GM, Honig G, Liou A, Tang Y, et al. Galphai/o-coupled receptor signaling restricts pancreatic beta-cell expansion. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(9):2888–2893. doi: 10.1073/pnas.1319378112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karnik SK, Chen H, McLean GW, Heit JJ, Gu X, Zhang AY, Fontaine M, Yen MH, Kim SK. Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science (New York, NY. 2007;318(5851):806–809. doi: 10.1126/science.1146812. [DOI] [PubMed] [Google Scholar]
- 37.McKenzie CI, Mackay CR, Macia L. GPR43 - A Prototypic Metabolite Sensor Linking Metabolic and Inflammatory Diseases. Trends in endocrinology and metabolism: TEM. 2015;26(10):511–512. doi: 10.1016/j.tem.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 38.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61(2):364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bjursell M, Admyre T, Goransson M, Marley AE, Smith DM, Oscarsson J, Bohlooly YM. Improved glucose control and reduced body fat mass in free fatty acid receptor 2-deficient mice fed a high-fat diet. American journal of physiology. 2011;300(1):E211–220. doi: 10.1152/ajpendo.00229.2010. [DOI] [PubMed] [Google Scholar]
- 40.Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nature communications. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Engelstoft MS, Park WM, Sakata I, Kristensen LV, Husted AS, Osborne-Lawrence S, Piper PK, Walker AK, Pedersen MH, Nohr MK, et al. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Molecular metabolism. 2013;2(4):376–392. doi: 10.1016/j.molmet.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zaibi MS, Stocker CJ, O’Dowd J, Davies A, Bellahcene M, Cawthorne MA, Brown AJ, Smith DM, Arch JR. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS letters. 2010;584(11):2381–2386. doi: 10.1016/j.febslet.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 43.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(43):16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bellahcene M, O’Dowd JF, Wargent ET, Zaibi MS, Hislop DC, Ngala RA, Smith DM, Cawthorne MA, Stocker CJ, Arch JR. Male mice that lack the G-protein-coupled receptor GPR41 have low energy expenditure and increased body fat content. The British journal of nutrition. 2013;109(10):1755–1764. doi: 10.1017/S0007114512003923. [DOI] [PubMed] [Google Scholar]
- 45.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145(2):396–406. e391–310. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 47.Sina C, Gavrilova O, Forster M, Till A, Derer S, Hildebrand F, Raabe B, Chalaris A, Scheller J, Rehmann A, et al. G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J Immunol. 2009;183(11):7514–7522. doi: 10.4049/jimmunol.0900063. [DOI] [PubMed] [Google Scholar]
- 48.Vinolo MA, Ferguson GJ, Kulkarni S, Damoulakis G, Anderson K, Bohlooly YM, Stephens L, Hawkins PT, Curi R. SCFAs induce mouse neutrophil chemotaxis through the GPR43 receptor. PLoS ONE. 2011;6(6):e21205. doi: 10.1371/journal.pone.0021205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masui R, Sasaki M, Funaki Y, Ogasawara N, Mizuno M, Iida A, Izawa S, Kondo Y, Ito Y, Tamura Y, et al. G protein-coupled receptor 43 moderates gut inflammation through cytokine regulation from mononuclear cells. Inflammatory bowel diseases. 2013;19(13):2848–2856. doi: 10.1097/01.MIB.0000435444.14860.ea. [DOI] [PubMed] [Google Scholar]
- 50.Mirkovic B, Murray MA, Lavelle GM, Molloy K, Azim AA, Gunaratnam C, Healy F, Slattery D, McNally P, Hatch J, et al. Short-chain Fatty Acids Cause an IL-8 Response in Cystic Fibrosis Airways via Increased GPR41. American journal of respiratory and critical care medicine. 2015 doi: 10.1164/rccm.201505-0943OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nature medicine. 2014;20(2):159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 52.Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41) Proceedings of the National Academy of Sciences of the United States of America. 2011;108(19):8030–8035. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nohr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, Sichlau RM, Grunddal KV, Poulsen SS, Han S, et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013;154(10):3552–3564. doi: 10.1210/en.2013-1142. [DOI] [PubMed] [Google Scholar]
- 54.Inoue D, Kimura I, Wakabayashi M, Tsumoto H, Ozawa K, Hara T, Takei Y, Hirasawa A, Ishihama Y, Tsujimoto G. Short-chain fatty acid receptor GPR41-mediated activation of sympathetic neurons involves synapsin 2b phosphorylation. FEBS letters. 2012;586(10):1547–1554. doi: 10.1016/j.febslet.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 55.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Backhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156(1–2):84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 56.Cani PD, Everard A. Talking microbes: When gut bacteria interact with diet and host organs. Molecular nutrition & food research. 2016;60:58–66. doi: 10.1002/mnfr.201500406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nature reviews Endocrinology. 2015;11(10):577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 58.Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity. 2014;40(6):833–842. doi: 10.1016/j.immuni.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 59.Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, Anastasovska J, Ghourab S, Hankir M, Zhang S, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nature communications. 2014;5:3611. doi: 10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cummings JH, Rombeau JL, Sakata T, editors. Physiological and clinical aspects of short-chaain fatty acids. New York: Cambridge University Press; 1995. [Google Scholar]
- 61.Lin HV, Frassetto A, Kowalik EJ, Jr, Nawrocki AR, Lu MM, Kosinski JR, Hubert JA, Szeto D, Yao X, Forrest G, et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE. 2012;7(4):e35240. doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58(7):1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arora T, Sharma R, Frost G. Propionate. Anti-obesity and satiety enhancing factor? Appetite. 2011;56(2):511–515. doi: 10.1016/j.appet.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 64.Geuking MB, McCoy KD, Macpherson AJ. Metabolites from intestinal microbes shape Treg. Cell research. 2013;23(12):1339–1340. doi: 10.1038/cr.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bhutia YD, Ganapathy V. Short, but Smart: SCFAs Train T Cells in the Gut to Fight Autoimmunity in the Brain. Immunity. 2015;43(4):629–631. doi: 10.1016/j.immuni.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 67.Cani PD, Everard A. Harnessing Genes and Diet to Fine-Tune the Gut Microbial Fitness. Cell metabolism. 2015;22(5):754–756. doi: 10.1016/j.cmet.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 68.Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015;7(4):2839–2849. doi: 10.3390/nu7042839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Winzell MS, Ahren B. G-protein-coupled receptors and islet function-implications for treatment of type 2 diabetes. Pharmacology & therapeutics. 2007;116(3):437–448. doi: 10.1016/j.pharmthera.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 70.Swaminath G, Jaeckel P, Guo Q, Cardozo M, Weiszmann J, Lindberg R, Wang Y, Schwandner R, Li Y. Mutational analysis of G-protein coupled receptor - FFA2. Biochemical and biophysical research communications. 2011;405(1):122–127. doi: 10.1016/j.bbrc.2010.12.139. [DOI] [PubMed] [Google Scholar]
- 71.Srivastava A, Yano J, Hirozane Y, Kefala G, Gruswitz F, Snell G, Lane W, Ivetac A, Aertgeerts K, Nguyen J, et al. High-resolution structure of the human GPR40 receptor bound to allosteric agonist TAK-875. Nature. 2014;513(7516):124–127. doi: 10.1038/nature13494. [DOI] [PubMed] [Google Scholar]
- 72.Pizzonero M, Dupont S, Babel M, Beaumont S, Bienvenu N, Rol, Blanqué R, Blanqué, Cherel L, Christophe T, et al. Discovery and optimization of an azetidine chemical series as a free fatty acid receptor 2 (FFA2) antagonist: from hit to clinic. Journal of medicinal chemistry. 2014;57(23):10044–10057. doi: 10.1021/jm5012885. [DOI] [PubMed] [Google Scholar]