Abstract
Obesity is a growing public health problem and affects 35% US adults. Obesity increases the risk of many cancer types and is associated with poor outcomes. Clinical management of cancer patients has been essentially the same between normal weight and obese individuals. Understanding causal mechanisms by which obesity drives cancer initiation and progression is essential for the development of novel precision therapy for obese cancer patients. One caveat is that various mechanisms have been proposed for different cancer types for their progression under obesity. Since obesity is known to have global impact on inflammation, here we will summarize recent literature and discuss the potential of inflammation being the common causal mechanism to promote cancer promotion across cancer types.
Introduction: obesity, inflammation, and immune alterations
Obesity, as defined in adults by a body mass index (BMI) of greater than or equal to 30, is a growing public health problem worldwide, particularly in Western countries. The world health organization has reported that 13% of adults over the age of 18 are clinically obese, totaling more than 600 million people. In the US 34.9% of adults (age >20) are obese [1]. It has been estimated that nearly 20% of deaths in US adults between 1986 and 2006 were related to obesity [2]. The health risks from obesity arise from its association with the increased risk of several diseases including hypertension, type 2 diabetes, cardiovascular disease, osteoarthritis, kidney failure, liver disease and several types of cancer [3,4]. The link between obesity and increased cancer incidence and cancer-related deaths has been well established over the last two decades and it has been estimated that 14% of cancer deaths in men and 20% in woman are attributable to obesity [5]. As a common factor for many chronic diseases, different mechanisms have been used to explain how obesity drives their progression. Interestingly, chronic inflammation, a phenotype associated with obesity, has been known to be major factor that contributes to the disease progression of the above chronic conditions.
Obesity-associated inflammation is first triggered by excess nutrients, and is primarily localized in specialized metabolic tissues such as white adipose tissue [6], which acts as a major source of energy and is primarily composed of adipocytes. Adipocytes are endocrine cells that secrete a large range of cytokines, hormones and growth factors, referred to as adipokines, and specialize in the storage of energy as triglycerides in cytoplasmic lipid droplets [7]. Excess nutrients leads to activation of metabolic signaling pathways including c-Jun N-terminal kinase (JNK), nuclear factor κ B (NFκB), and protein kinase R [8,9]. Activation of these pathways leads to an induction of low-level of inflammatory cytokines resulting in a low-grade inflammatory response [6]. Excess nutrients and obesity also lead to the hyperplasia and hypertrophy of white adipose tissue adipocytes, as well as the extensive tissue remodeling and an increase in free fatty acids resulting in changes in adipokine production and a low-grade inflammatory response [10,11]. Obesity also leads to increased endoplasmic reticulum stress resulting in activation of the unfolded protein response, which leads to activation of NFκB, JNK and increased oxidative stress, and in turn the upregulation of inflammatory cytokines[12]. These pathways all contribute to the initiation of obesity associated inflammation. While obesity associated inflammation is primarily localized in white adipose tissue, other tissues have been shown to have increased inflammation under obesity, including the liver, pancreas, and brain[13].
This low-grade inflammatory response associated with obesity leads to changes in immune cell infiltration and polarization in white adipose tissue [14]. In particular, macrophages are the major innate immune cells that are recruited to white adipose tissue under obesity and are one of the major sources of inflammatory cytokines in obese white adipose tissue [15]. The recruitment of macrophages into obese white adipose tissue is mediated by a few different mechanisms [11], 1) adipose tissue macrophages and adipocytes secrete a milieu of elevated levels of chemokines, including CCL2, CCL3 and RANTES/CCL5, which promote the recruitment of macrophages into obese white adipose tissue; 2) obesity-induced adipocyte hypertrophy leads to increased adipocyte cell death, in turn recruiting macrophages to phagocytize the dead adipocytes; 3) adipocyte hypertrophy and cell death leads to increased levels of free fatty acids (FFAs), which act as TLR4 agonists and likely ligands for nod-like receptors to induce an inflammatory response and recruitment of macrophages in white adipose tissue. These mechanisms work in concert to induce a large increase in adipose tissue macrophages in obese white adipose tissue.
In addition to the increased adipose tissue macrophages in white adipose tissue from obese individuals, white adipose tissue can also shift the polarization of macrophages, from an anti-inflammatory M2-like phenotype in lean white adipose tissue, to a more pro-inflammatory M1-like phenotype in obese white adipose tissue [16]. This is partly due to the imbalance of obesity-related adipokines, i.e. the change in leptin and adiponectin ratio. Obese white adipose tissue tends to have an increase in the production of leptin, which is pro-inflammatory, proangiogenic, and pro-proliferative, and a decrease in adiponectin, which is anti-inflammatory, antiangiogenic and anti-proliferative [17,18]. The increased leptin level leads to monocyte differentiation into macrophages and repolarization of adipose tissue macrophages. Recent studies have suggested that adipose tissue macrophages in obese white adipose tissue may not be classically activated M1 macrophages. Using proteomic and other techniques, Kratz et al. has recently shown that adipose tissue macrophages from obese humans and animals have a distinct phenotype that express inflammatory cytokines associated with M1 macrophages but lacked other characteristics of M1 macrophages [19]. The pro-inflammatory adipose tissue macrophages in obese white adipose tissue recruit other immune cells, and along with adipocytes secrete more than 50 different cytokines, hormones and chemokines, all of which contributing to the chronic inflammation associated with obesity [10,11].
The impact of obesity on immunity is not limited to macrophages. Recent literature have identified a panel of immune alterations, including those from both adaptive and innate immunity, that are impacted by obesity, including the increased Th1 cell response [20], CD8 cytotoxic T cell response [21], natural killer (NK) cells [22] etc. as well as the decreased number of regulatory T cells [23]. The interaction between these cells in adipose tissues is very complex. For example, CD8 T cells have been shown to be the early event showing up in adipose tissue of diet-induced obesity, which plays an important role in further recruiting M1 macrophages and subsequent inflammation [21]. Th1 cytokines are also the known activator of M1 macrophages in general and this axis has been established in adipose tissue of obese individual and contributes to insulin resistance [24].
Among all the immune alterations, obesity-associated inflammation is of particular interest because the pathophysiology of many of the major human diseases associated with obesity, including type 2 diabetes, cardiovascular disease, and cancer, have been linked to inflammation [17,25,26]. Here we will discuss the effect of obesity on, and the role of obesity-associated inflammation in the carcinogenesis and disease progression of several major types of cancer.
Obesity and Cancer
Breast Cancer
Breast cancer is the second leading cause of cancer-related death among women in the U.S. (NCI SEER data) [27]. Based on the expression of estrogen receptor (ER), progesterone receptor (PR), and HER2, invasive breast cancer can be further classified as luminal type, HER2-positive, and triple negative breast cancer. There is a well-established increased risk of developing ER+ luminal breast cancer in postmenopausal obese women [28,29]. A recent meta-analysis of 25 studies found that obesity increased the risk of developing breast cancer in a non-linear dose-response in postmenopausal breast cancer [30]. Two recent meta-analysis have shown that obesity is associated with a worse clinical outcome in both pre- and post-menopausal women with a higher relative risk of total mortality in obese premenopausal patients than postmenopausal patients [31,32]. In addition, a retrospective review has shown that obesity is linked to the advanced TMN stage at time of diagnosis and a worse clinical outcome in both pre- and postmenopausal women [33]. Recent studies have also shown that obesity is associated with an increased prevalence of triple negative breast cancer, the most aggressive subtype of breast cancer; and it may serve, along with menopausal status, as a predictor for the sensitivity to neoadjuvant chemotherapy in triple negative breast cancer [34,35]. A recent prospective study has shown a link between obesity and the development of metastasis in breast cancer patients. This report also indicated that non-obese patients responded better to first-line metastatic chemotherapy treatment than obese patients [36]. Even though there are some other studies that have concluded differently on prognosis using smaller number of patients [28,37], it is a general recognition that obesity is associated with bad prognosis in breast cancer patients.
There have been several hypotheses to explain how obesity promotes breast cancer development and progression. However, there have been no studies showing a mechanistic link between obesity and breast cancer development or progression. One of the reasons for this is possibly the lack of good models for studying the effect of obesity on breast cancer. While FVB/N and Balb/C mice are permissive to breast cancer, they are relatively resistant to obesity. In contrast, C57BL/6 mice are susceptible to diet-induced obesity, but have very few breast cancer models available [38].
Adipose tissue derived estrogens
One possible link between obesity and the increased incidence of ER+ breast cancer in postmenopausal women is the elevated level of circulating estrogens from increased aromatization of androgens in adipose tissue [39]. This hypothesis, however, fails to fully explain the relationship between body mass and breast cancer incidence and poor clinical outcome, as the relationship is present in ER-negative breast cancers [34,36]. Also, some recent studies have indicated that circulating estrogens may protect against the development of breast cancer in obese women [40].
Metabolic syndrome, insulin and IGF-1
Another hypotheses is that obesity-associated metabolic syndrome results in elevated levels of insulin and insulin-like growth factor 1 (IGF-1) [41]. Studies have shown that obesity-associated metabolic syndrome and type 2 diabetes, is associated with an increased risk of breast cancer [42]. Epidemiological studies have shown a correlation between circulating levels of IGF-1 and the development of ER+ breast cancer [43]. IGF-1 is known to act as a mitogen in breast epithelial cells and is important in mammary gland development. Binding of IGF-1 to its receptor, IGF-1R, leads to activation of PI3K/AKT/mTOR and mitogen activated protein kinases (MAPKs) signaling [44]. IGF-1 signaling also induces activation of ERα, via activation S6 kinase down stream of mTOR, leading to enhanced estrogen signaling and the promotion of breast cancer [45]. However, the genetic evidence supporting insulin/IGF-1 signaling being the causal factors for obesity-driven cancer progression is lacking. It is known that the development of obesity-associated metabolic syndrome and insulin resistance is caused by obesity-associated inflammation [25], underscoring the potential importance of inflammation as the critical factor for obesity-driven cancer progression.
Balance between leptin and adiponectin
Other studies have looked at the role of elevated levels of leptin and decreased levels of adiponectin in obese patients. Leptin is known to be pro-tumorigenic and to act directly on breast epithelial cells to promote proliferation, by promoting estrogen signaling, activation of MAPKs and STAT3 signaling, and inhibition of apoptosis by activation of AKT signaling [46]. Leptin is also pro-angiogenic and has been shown to promote epithelial-mesenchymal transition (EMT) in breast cancer and to promote the self-renewal of mammary stem cells and breast cancer stem cells [47–49]. Adiponectin has been shown to be anti-tumorigenic in the breast, as systemically low level of adiponectin is associated with increased lymph node metastasis and more aggressive tumors [50]. Adiponectin can inhibit the proliferation of breast cancer cells by inhibiting MAPK and AKT signaling, promoting apoptosis, and downregulation of metastatic properties by inhibiting mTOR signaling via activation of AMPK [51]. Thus the shift in leptin and adiponectin balance is thought to play a role in obesity-associated breast cancer development and progression. Interestingly, leptin signaling is also involved in eliciting a pro-inflammatory circuit to promote cancer progression. Crosstalk between IL-1, leptin and notch has shown to promote proliferation, migration and upregulation of vascular endothelial growth factor and receptor 2 in breast cancer [52]. The genetic evidence of leptin signaling in obesity-driven breast cancer progression is established using animal models lacking either leptin or its receptor, but conclusions drawn from these results are debatable due to the impact of leptin signaling on normal mammary gland development and tumorigenesis in non-obese mice [53].
Inflammation
A growing amount of evidence supports a link between obesity-associated inflammation and breast cancer incidence and progression. Adipose tissue in the breast has been shown to be involved in obesity-associated inflammation, with increased adipocyte hyperplasia and cell death, cytokine production and macrophage infiltration [54]. Transcriptomic and Gene Set Enrichment analysis of data from luminal breast cancer patients has shown that obesity is associated with the enrichment in genes and pathways associated with inflammation and immune cell trafficking in obese patients compared to non-obese patients [55]. These pathways were also enriched in obese MMTV-TGFα/α mice, a model for luminal breast cancer in which obesity promotes tumor progression. A recent report has shown that interaction between adipocytes and breast cancer cells leads to the production of inflammatory cytokines and increase the number of cancer cells with tumor-forming capabilities [56]. The other hypotheses for the link between obesity and breast cancer as discussed above have also been linked with inflammation (Figure 1). IL-6, TNF-α, and prostaglandin E2, which are all elevated in obese patients, have been shown to increase the expression of aromatase and the production of estrogens in both adipose tissue and breast tissue [57,58]. As mentioned above, obesity-associated metabolic syndrome and elevated insulin levels are tightly linked with inflammation [25]. Leptin is also known to induce the expression of pro-inflammatory, pro-tumorigenic cytokines in macrophages, including IL-1, IL-6 and TNF-α [59]. Other studies have shown the concomitant elevation of IL-1β and leptin with increased body mass in rats that drives the growth of mammary epithelium [60] and that leptin expression in pre-adipocytes can be induced by IL-1 and TNF-α secreted from ATM [61].
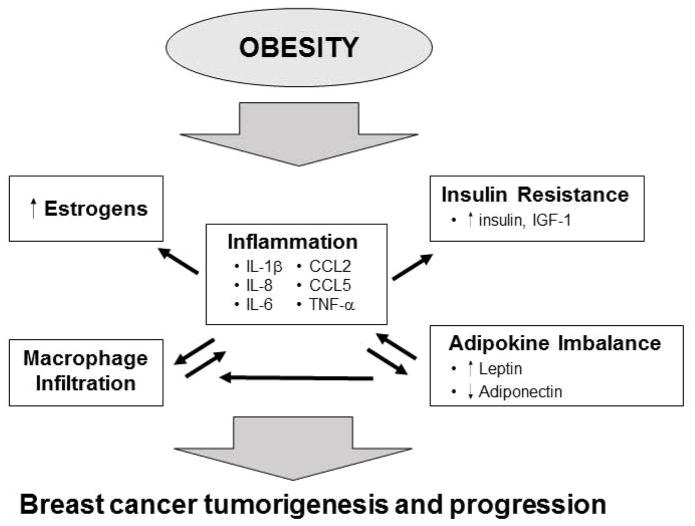
Figure 1. Obesity promotes breast cancer.
Obesity leads to macrophage infiltration and inflammation in white adipose tissue in the breast. This is associated with the upregulation of several pro-inflammatory and pro-tumorigenic cytokines. Inflammatory cytokines can promote the upregulation of aromatase and increased production of estrogens in stromal cells of the breast. Obesity-associated inflammation is also involved in the development of insulin resistance and increased IGF-1, which is a mitogen for breast epithelial cells. Obesity also leads to an increase in leptin and a decrease in adiponectin. Leptin expression is induced by inflammatory cytokines and can induce expression of inflammatory cytokines leading to increased inflammation. Leptin can promote breast cancer through increased proliferation, survival and angiogenesis. All of these factors likely contribute to a pro-tumorigenic microenvironment that promotes breast cancer.
Many studies have demonstrated that elevated levels of inflammatory cytokines including IL-6 [62], TNF-α{To, 2013 #218}, IL-8 [63] are associated with increased breast cancer tumor growth and poor patient outcomes. These same cytokines have all been shown to be upregulated in obese white adipose tissue [10,64]. While there is no direct evidence linking high levels of cytokines in obese patients to increased breast cancer incidence and poor outcome, there is plenty of correlative evidence suggesting a link. Gene ontology analysis of normal breast tissue has shown that pathways involving IL-6, IL-8 are enriched in obese patients, and that IL-6 from adipose stromal cells can promote breast cancer cell proliferation and migration [54,65]. Obese ovariectomized mice, in which there was increased breast cancer tumor growth in a syngeneic transplant model of Py230 breast cancer cells, had elevated levels of TNF-α in the mammary gland and TNF-α could induce Py230 growth in vitro [66]. However, while there is correlative evidence to suggest a possible role of elevated cytokines in obesity associated breast cancer incidence and progression, a recent study found no correlation between circulating levels of IL-6 and TNF-α and breast cancer risk in postmenopausal women [67]. While there is a potential connection between obesity-associated inflammation and breast cancer, it is yet to be determined the causal mechanism of how inflammation contributes to obesity-driven breast cancer progression. Future studies using genetically modified mice deficient in these cytokine pathways and immune cell types will need to be done to further define the requirement of inflammation in obesity-driven breast cancer progression.
Colorectal Cancer
The association between BMI and colorectal cancer incidence has been evaluated in several epidemiological studies, and it has been shown that the risk and incidence of colorectal cancer increase with BMI [4,68]. A meta-analysis of 30 prospective studies found an increased risk of colorectal cancer in obese men and women, though the association was higher in men [69]. While the relative risk of colorectal cancer with obesity is moderate, due to the high prevalence of obesity it is estimated that 35.4% of colorectal cancer cases in men and 20.8% in women in the U.S. are attributable to obesity [70]. The relationship between body mass and colorectal cancer development has also been studied in animal models, where both APCmin mice, a model for spontaneous colorectal cancer, and mice treated with azoxymethane, a carcinogen used to induce colorectal cancer, fed a high-fat diet had increased colon polyp formation[71,72].
The dysregulation of leptin and adiponectin during obesity may also play a role in obesity-associated colorectal cancer. A few epidemiological studies have examined the relationship between levels of circulating leptin and colorectal cancer risk, finding that high levels of leptin are associated with an increased risk of colon cancer, but not rectal cancer, in men and no association in women [73,74]. Leptin has also been shown to promote proliferation, survival and invasiveness of colon cancer cells through activation of MAPKs, PI3K, NF-κB and STAT3 signaling [75–77]. Inflammation and inflammatory cytokines are known to play a critical role in the development of both colitis-associated and sporadic colorectal cancer [78,79]. Colonic TNF-α expression is elevated in HFD-fed mice [80], and TNF-α is potent inducer of IL-6 which is known to play a role in promoting colorectal cancer [81]. A recent study found that leptin stimulated the production of IL-6 and TNF-α and promoted proliferation in colon epithelial cells in an mTOR-dependent manner [82]. Adiponectin can inhibit colorectal cancer cell growth through activation of AMPK [83], and loss of adiponectin enhances development of both colitis-associated colorectal cancer and in APCmin mice [84]. Furthermore, epidemiological studies have found that circulating levels of adiponectin is inversely correlated with risk of colorectal cancer [85]. However, other studies have found no significant correlation between adiponectin levels and colorectal cancer risk [86].
A growing amount of evidence has linked colorectal cancer to intestinal microbiota and dysfunction of the intestinal barrier [87,88]. Studies have also shown that altered gut microbiota is present in patients with adenomas of the colon, suggesting a role for dysbiosis in the early stages of colorectal cancer development [89,90]. The development of colorectal cancer in animal models has been shown to be linked to leakage of the intestinal barrier and activation of pro-inflammatory tumor promoting IL-23/IL-17 signaling [91]. A previous study also supported a role for intestinal barrier dysfunction and subsequent endotoxemia in the development and progression of colorectal cancer in APCmin mice [92]. Obesity is also associated with changes in the gut microbiome and dysfunction of intestinal barrier [93]. Pfalzer A.C. et al recently reported that genetically obese or diet-induced obese (mice fed a HFD) APC1638N colon tumor-bearing obese mice had altered gut microbiome compared to non-obese mice [94]. They identified a depletion of Parabaceroides distasonis, which has been shown to be anti-inflammatory in the gut and to reduce dextran sodium sulfate-induced inflammatory cytokines and colitis in mice [95]. HFD-fed mice have also been shown to have increased levels of circulating lipopolysaccharide (LPS), a marker of intestinal barrier dysfunction and endotoxemia, which has been shown to promote colorectal cancer development [96]. Another study has suggested that HFD-fed mice have increased colorectal cancer development primarily due to gut dysbiosis associated with obesity. They showed that fecal transfer from obese Kras (G12Dint) mice to normal-weight mice was sufficient to increase colorectal cancer development and progression [97]. These studies indicate that inflammation due to obesity-mediated gut dysbiosis and intestinal barrier dysfunction, along with changes in the balance between leptin and adiponectin, plays a role in the increased risk of colorectal cancer in obese individuals (Figure 2).
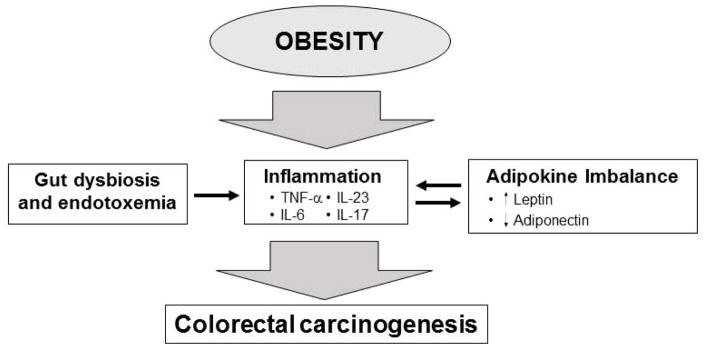
Figure 2. Obesity and colorectal cancer.
Obesity is associated with changes in the intestinal microbiome and dysfunction of the intestinal barrier. Gut dysbiosis and endotoxemia promotes inflammation through the upregulation inflammatory cytokines, particularly IL-6, TNF-α, IL-17 and IL-23, which promote CRC carcinogenesis. Obesity-associated changes in leptin and adiponectin also leads to increased inflammation and CRC carcinogenesis.
Liver Cancer
Liver cancer is the fifth most common type of cancer in men and the ninth most common in women worldwide, and is the second most common cause of cancer related death due to a yearly fatality ratio that is close to 1 and a 5 year-survival of less than 10% [98]. Hepatocellular carcinoma accounts for 75–90% of liver cancer, and usually arises in the context of chronic liver disease [98]. Globally, hepatitis B or C viral (HBV and HCV) infections are the most common cause of chronic liver disease and HCC [99]; however, in western countries non-alcoholic fatty liver disease (NAFLD) is the most common type of chronic liver disease and NAFLD-related cirrhosis is predicted to be the most common reason for liver transplantation within a decade [100]. NAFLD encompasses a range of progressive liver disease from hepatic steatosis to non-alcoholic steatohepatitis (NASH) which ultimately leads to cirrhosis [98,101]. NAFLD, NASH and NASH-related cirrhosis are associated with increased risk of HCC and studies have shown that about 2.6% of people with NASH-related cirrhosis develop HCC [102]. While cirrhosis is a major risk factor for HCC, a growing amount of evidence has shown that HCC can develop from individuals with NAFLD without the presence of cirrhosis [103]. The development of HCC in patients with non-cirrhotic NAFLD is problematic because non-cirrhotic NAFLD is often asymptomatic. As a result, these patients are not monitored for the development of HCC and HCC are commonly presented symptomatically with a larger tumor burden. Such patients had a median survival of just 7.2 months [104].
The development of NAFLD is closely correlated with obesity, obesity-associated metabolic syndrome and type 2 diabetes [105]. About one fourth of the population in the US have NAFLD, 8% of which progress into NASH which progresses into cirrhosis with 25% frequency [106]. The rate of NAFLD is much higher in obese individuals and type 2 diabetics. One study in Europe found that 94% of obese individuals had NAFLD, with 25% presenting some form of NASH [107]. As a major risk factor for the development of NAFLD and NASH, obesity is associated with an increased risk of developing HCC [4]. A meta-analysis of 11 cohort studies found that obesity was associated with increased HCC, with a relative risk of 1.89 [108]. One study found that the risk of death from liver cancer was 4.5 times higher in men with a BMI of greater than 35 compared to men with a normal BMI [4]. Given its prevalence, obesity, at least in the U.S. and other western countries, is likely to become the leading risk factor for HCC [109].
Excess caloric intake can result in increased triglyceride production in the liver that is greater than the ability of the liver to export, leading to accumulation of triglycerides in the form of lipid droplets in parenchymal hepatocytes and hepatic steatosis (the beginning stages of NAFLD) [105]. Obesity and lipid accumulation in liver leads to an increase in the levels of free fatty acids and other lipid metabolites, such as diacylglyceride and ceramide, which results in hepatocyte injury, endoplasmic reticulum (ER) stress and inflammation [105,110]. These factors all contribute to a cycle of hepatocyte cell death and compensatory proliferation and inflammation that result in the progression of hepatic steatosis to NASH and eventually NASH-related cirrhosis [109,110]. Increased levels of IL-6 and TNF-α have been shown to be major drivers of cell proliferation in NAFLD and NASH, and play important roles in the development of obesity-associated HCC [111,112]. These cycles of cell death and regeneration and the associated cell proliferation and inflammation create a pro-tumorigenic microenvironment that promotes the development of HCC (Figure 3) [98].
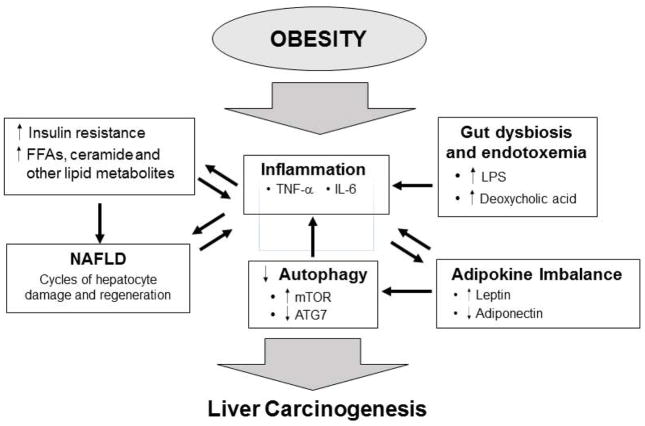
Figure 3. Obesity and liver cancer.
The development of liver cancer in obese patients is associated with chronic liver damage and NAFLD. Obesity leads to an accumulation of triglycerides in the liver (hepatic steatosis), which leads to increased free fatty acids and other lipid metabolites and in turn to hepatocyte cell death and regeneration. This process is associated with liver inflammation. Upregulation of leptin and down regulation of ATG7 result in decreased autophagy that leads to increased inflammation and oxidative stress. Gut dysbiosis and endotoxemia leads to increased inflammation. Cycles of liver damage and regeneration and the associated inflammation creates a microenvironment that promotes the development liver cancer.
Another mechanism involved in the progression of NAFLD to HCC in obese individuals is again the imbalance between leptin and adiponectin. Apart from its involvement in obesity-associated insulin resistance and inflammation, leptin can induce pro-tumorigenic signaling in the liver, including STAT3 and PI3K/Akt [113]. Of particular importance in HCC, is activation of PI3K/Akt and its downstream target mTOR, which has been shown to be active in about 40% of HCC patients [114]. The activation of mTOR by leptin and other factors in NAFLD leads not only to the proliferation and pro-survival signaling, but also to the inhibition of autophagy [115]. Autophagy is important for preventing oxidative stress by removing damaged organelles, including mitochondria, and the resolution of inflammation [116]. Thus the inhibition of autophagy leads to increased oxidative stress and inflammation resulting in liver damage [115,117]. Obesity also suppresses autophagy through the downregulation of autophagy related gene 7 (ATG7), which is associated with insulin resistance and liver damage [118]. Adiponectin has an antagonistic effect to leptin. It can inhibit the development of HCC by reducing activation of STAT3 and PI3K through activation of AMPK and upregulation of SOCS3 [119,120].
As mentioned previously obesity is associated with dysbiosis of the gut and increased intestinal permeability and endotoxemia. Bacterial products such as LPS can promote liver inflammation via the upregulation of inflammatory cytokines from Kupffer cells and liver-infiltrating macrophages which can promote the development of HCC [121]. A recent report by Kumar et al. indicated that obesity promoted hepatocarcinogenesis in the HCV oncogene NS5A-transgenic mice via the activation of TLR4 by LPS [122]. Activation of TLR4 by LPS led to activation of Nanog which cooperates with leptin-induced STAT3 to upregulate TWIST in CD133+/CD49f+/Nanog+ cells, thought to be tumor-initiating cells in alcohol-associated HCC [123]. Obesity-associated dysbiosis also contributes to the elevated level of deoxycholic acid, which can promote the secretion of inflammatory cytokines in hepatic stellate cells and promote the development of carcinogen-induced HCC [124].
Other cancers
Obesity is also associated with increased risk and progression of several other types of cancer. Obesity is associated with an increased risk of pancreatitis [125] and pancreatic cancer [126], as well as the increased mortality in pancreatic cancer patients [127]. The mechanisms by which obesity promotes pancreatic cancer is not completely known; however, they likely involve obesity-associated inflammation, insulin resistance, increased infiltration of macrophages and immunosuppressive cells, and dysregulation of autophagy [128–130]. Obesity is also associated with the incidence and mortality of endometrial and ovarian cancer [131,132]. Obesity likely promotes endothelial cancer through increased insulin/IGF signaling, increased estrogens, chronic inflammation and increased leptin mediated activation of PI3k/Akt/mTOR signaling [133,134]. Obesity is also associated with esophageal cancers, through due to a lack of good animal models, more studies will need to be done to verify a link between obesity and esophageal cancer and to identify a mechanism [135].
Obesity, inflammasome activation and cancer
One major source of inflammation in obese white adipose tissue is inflammasome activation in ATM. Inflammasomes are multiprotein complexes that activate IL-1β and IL-18 via Caspase 1 (Casp-1)-mediated cleavage in response to pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs). Inflammasomes consist of a NOD-like receptor, NLRP1, NLRP3 or NLRC4, or Pyrin family protein AIM2, the adapter protein ASC and Casp1 [136,137]. NLRP3 inflammasome activation and IL-1β have been shown to be involved in obesity associated adipose tissue inflammation, insulin resistance and type 2 diabetes [138,139]. Inflammasomes have also been shown to play a complex and tissue specific role in cancer [136,140]. IL-1β has been shown to promote proliferation and invasion of colon cancer cells [141]. Polymorphisms in the IL1B gene is associated with breast cancer progression and prognosis [142], and IL-1β is also upregulated in stromal cells in IDC of the breast compared to DCIS and is associated with a more aggressive phenotype [143,144]. Although there is no evidence for the role of inflammasome activation in obesity-associated cancer, there is evidence that IL-1β may play a role. Studies have shown that the inflammatory effect of leptin is dependent of IL-1β, as they do not occur in IL-1β−/− mice [145]. Furthermore, as mentioned earlier, IL-1β is elevated with increased body weight in obese rats and can promote expansion of mammary epithelium [60]. In a recent study, Arendt et al. found that interactions between adipocytes and macrophages via an IL-1β/CCL2/CXCL12 signaling nexus, promotes breast cancer angiogenesis and progression under obesity [146]. Inflammasome activation and IL-1β has been shown to be a driver of obesity-associated insulin resistance and type II diabetes, which is an independent risk factor for the development of obesity-associated cancer including breast, liver, and pancreatic [42,104,147]. Inflammasomes have also been shown to play a role in the development of pancreatitis, an important risk factor in the development of pancreatic cancer [148,149]. As such, an examination of the role of inflammasomes in obesity-associated cancer is warranted.
Perspective
Emerging evidence indicates that obesity-associated chronic inflammation is a cancer-promoting event, which is largely attributable to the innate immune cells especially macrophages. A potential paradox here is here that obesity-associated adipose tissue tends to accumulate M1-like polarized macrophages, the classic type of anti-tumor macrophages regularly described in the cancer literature. The pro-inflammatory microenvironment in the obesity-associated adipose tissue also accumulates the classic anti-tumor Th1 and CD8 T cells. Recent studies have shown that macrophage polarization is complex existing of a spectrum of polarization, with most tissue macrophages being somewhere between the extremes of an M1 and M2 polarization [150]. Data also suggests that individual macrophages within a tissue vary widely and that polarization changes based on signaling from the tumor microenvironment [150]. Therefore, one potential explanation could be that changes in the microenvironment during tumor development change adipose tissue macrophages from a more M1-like phenotype, to a more tumor-promoting M2-like like stage under obesity. It is likely that there are significant difference between the microenvironments from obese adipose tissues and obese tumor tissues. A side-by-side profiling of immune cell composition, cytokine production profile, and effector immune cell function within adipose tissues vs. tumor tissues should be performed in order to draw some meaning information.
Nevertheless, obesity increases the risk of developing several different types of cancer and is associated with a worse clinical outcome. The mechanisms by which obesity promotes caner have been proposed in a tissue-specific manner in most of the studies and involve the interplay between different signaling events. However, the underlying theme is the obesity-associated inflammation, which is known to promote the progression of several types of cancer described above and likely in some other cancer types as well. More studies need to be de done to identify causal mechanisms of obesity-associated cancers and to identify therapeutic targets to better treat obese cancer patients. Given the role of inflammation in obesity-associated cancers, a likely place to start would be anti-inflammatory therapies in obese patient populations.
Highlights.
Obesity is a growing public health problem associated with chronic inflammation, immune modulation and several chronic diseases
Obesity is associated with increased risk and progression of several cancers including breast, colon and liver
The mechanism by which obesity promotes cancer is complex and tissue-specific
An underlying theme in obesity-associated cancer is inflammation
Acknowledgments
W. Z. was supported by NIH grants K99/R00CA158055 and R01 CA200673, the V Scholar award from the V Foundation for Cancer Research, a Breast Cancer Research Grant and an Oberley Award from the Holden Comprehensive Cancer Center, University of Iowa. R. K. was supported by T32 AI007260 AI/NIAID.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masters RK, Reither EN, Powers DA, Yang YC, Burger AE, Link BG. The impact of obesity on US mortality levels: the importance of age and cohort factors in population estimates. Am J Public Health. 2013;103:1895–1901. doi: 10.2105/AJPH.2013.301379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin-Rodriguez E, Guillen-Grima F, Marti A, Brugos-Larumbe A. Comorbidity associated with obesity in a large population: The APNA study. Obes Res Clin Pract. 2015;9:435–447. doi: 10.1016/j.orcp.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 5.Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozoren N, Brady G, Meshinchi S, Jagirdar R, Gewirtz A, Akira S, et al. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006;281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- *6.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. A review of the mechanisms of obesity associated inflammation. The authors review how obesity induced an inflammtory response in greater detail then space will allow in this revew. [DOI] [PubMed] [Google Scholar]
- *7.Fasshauer M, Bluher M. Adipokines in health and disease. Trends Pharmacol Sci. 2015;36:461–470. doi: 10.1016/j.tips.2015.04.014. A comprehensive review of the role adipokines play in the pathophysiology of various diseases. [DOI] [PubMed] [Google Scholar]
- 8.Solinas G, Karin M. JNK1 and IKKbeta: molecular links between obesity and metabolic dysfunction. FASEB J. 2010;24:2596–2611. doi: 10.1096/fj.09-151340. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura T, Furuhashi M, Li P, Cao H, Tuncman G, Sonenberg N, Gorgun CZ, Hotamisligil GS. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell. 2010;140:338–348. doi: 10.1016/j.cell.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *10.Balistreri CR, Caruso C, Candore G. The role of adipose tissue and adipokines in obesity-related inflammatory diseases. Mediators Inflamm. 2010;2010:802078. doi: 10.1155/2010/802078. A review discussing the ole of adipokines in obesity-related diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cnop M, Foufelle F, Velloso LA. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med. 2012;18:59–68. doi: 10.1016/j.molmed.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Cildir G, Akincilar SC, Tergaonkar V. Chronic adipose tissue inflammation: all immune cells on the stage. Trends Mol Med. 2013;19:487–500. doi: 10.1016/j.molmed.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Han JM, Levings MK. Immune regulation in obesity-associated adipose inflammation. J Immunol. 2013;191:527–532. doi: 10.4049/jimmunol.1301035. [DOI] [PubMed] [Google Scholar]
- 15.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. Journal of Clinical Investigation. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *16.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. Journal of Clinical Investigation. 2007;117:175–184. doi: 10.1172/JCI29881. Authors characterized adipose tissue macrophages from lean and obese mice and foud that the surface macrophage polarization changes from a more M2-like pheonotype in lean mice to a more M1-like phenotype in obese mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 18.Ryan AS, Berman DM, Nicklas BJ, Sinha M, Gingerich RL, Meneilly GS, Egan JM, Elahi D. Plasma adiponectin and leptin levels, body composition, and glucose utilization in adult women with wide ranges of age and obesity. Diabetes Care. 2003;26:2383–2388. doi: 10.2337/diacare.26.8.2383. [DOI] [PubMed] [Google Scholar]
- **19.Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, Schoenfelt KQ, Kuzma JN, Larson I, Billing PS, et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–625. doi: 10.1016/j.cmet.2014.08.010. Authors used proteomic approaches to characterize ATMs from obese patients and mice. Authors found that classic M1 activation markers were absent from ATMs, insteand ATMs expressed markers of metabolically active macrophages, which displayed a balance between being proinflammatory cytokine prodction and lipid metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 22.Revelo XS, Tsai S, Lei H, Luck H, Ghazarian M, Tsui H, Shi SY, Schroer S, Luk CT, Lin GH, et al. Perforin is a novel immune regulator of obesity-related insulin resistance. Diabetes. 2015;64:90–103. doi: 10.2337/db13-1524. [DOI] [PubMed] [Google Scholar]
- 23.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *24.McLaughlin T, Liu LF, Lamendola C, Shen L, Morton J, Rivas H, Winer D, Tolentino L, Choi O, Zhang H, et al. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler Thromb Vasc Biol. 2014;34:2637–2643. doi: 10.1161/ATVBAHA.114.304636. Research paper finding increased NLRP3 inflammasome activation in monocyte-derived macrophages form patients with type 2 diabetes compated to healthy patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, et al. SEER Cancer Statistics Review. 1975–2010 [Google Scholar]
- 28.Healy LA, Ryan AM, Rowley S, Boyle T, Connolly E, Kennedy MJ, Reynolds JV. Obesity increases the risk of postmenopausal breast cancer and is associated with more advanced stage at presentation but no impact on survival. Breast J. 2010;16:95–97. doi: 10.1111/j.1524-4741.2009.00861.x. [DOI] [PubMed] [Google Scholar]
- 29.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **30.Xia X, Chen W, Li J, Chen X, Rui R, Liu C, Sun Y, Liu L, Gong J, Yuan P. Body mass index and risk of breast cancer: a nonlinear dose-response meta-analysis of prospective studies. Sci Rep. 2014;4:7480. doi: 10.1038/srep07480. A meta-analysis showing that obesity is associated with increased mortality rate in both pre-and postmenopausal breast cancer patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **31.Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, Navarro Rosenblatt D, Thune I, Vieira R, Norat T. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25:1901–1914. doi: 10.1093/annonc/mdu042. Meta-analysis of 82 studies identifying a negative correlation between body mass index and breast cancer survival. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **32.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627–635. doi: 10.1007/s10549-010-0990-0. Meta-analysis indicating that obese breast cancer patients have a shorter survival than non-obese patients. [DOI] [PubMed] [Google Scholar]
- 33.Kaviani A, Neishaboury M, Mohammadzadeh N, Ansari-Damavandi M, Jamei K. Effects of obesity on presentation of breast cancer, lymph node metastasis and patient survival: a retrospective review. Asian Pac J Cancer Prev. 2013;14:2225–2229. doi: 10.7314/apjcp.2013.14.4.2225. [DOI] [PubMed] [Google Scholar]
- 34.Rose DP, Vona-Davis L. Influence of obesity on breast cancer receptor status and prognosis. Expert Rev Anticancer Ther. 2009;9:1091–1101. doi: 10.1586/era.09.71. [DOI] [PubMed] [Google Scholar]
- **35.Bonsang-Kitzis H, Chaltier L, Belin L, Savignoni A, Rouzier R, Sablin MP, Lerebours F, Bidard FC, Cottu P, Sastre-Garau X, et al. Beyond Axillary Lymph Node Metastasis, BMI and Menopausal Status Are Prognostic Determinants for Triple-Negative Breast Cancer Treated by Neoadjuvant Chemotherapy. PLoS One. 2015;10:e0144359. doi: 10.1371/journal.pone.0144359. Analysis of 326 TNBC patients that received nepadjuvant chemotherapy. Authors identified BMI and postmenopausal status as an independent prognostic factor for metastasis free survival in these patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **36.Osman MA, Hennessy BT. Obesity Correlation With Metastases Development and Response to First-Line Metastatic Chemotherapy in Breast Cancer. Clin Med Insights Oncol. 2015;9:105–112. doi: 10.4137/CMO.S32812. Analysis of 118 patients with metastatic breast cancer. Authors found that obesity was associated with increased metastasis and that non-obese patients responded better to chemotherapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berclaz G, Li S, Price KN, Coates AS, Castiglione-Gertsch M, Rudenstam CM, Holmberg SB, Lindtner J, Erien D, Collins J, et al. Body mass index as a prognostic feature in operable breast cancer: the International Breast Cancer Study Group experience. Ann Oncol. 2004;15:875–884. doi: 10.1093/annonc/mdh222. [DOI] [PubMed] [Google Scholar]
- 38.Montgomery MK, Hallahan NL, Brown SH, Liu M, Mitchell TW, Cooney GJ, Turner N. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia. 2013;56:1129–1139. doi: 10.1007/s00125-013-2846-8. [DOI] [PubMed] [Google Scholar]
- 39.Lorincz AM, Sukumar S. Molecular links between obesity and breast cancer. Endocr Relat Cancer. 2006;13:279–292. doi: 10.1677/erc.1.00729. [DOI] [PubMed] [Google Scholar]
- 40.Suba Z. Circulatory estrogen level protects against breast cancer in obese women. Recent Pat Anticancer Drug Discov. 2013;8:154–167. doi: 10.2174/1574892811308020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *41.Farabaugh SM, Boone DN, Lee AV. Role of IGF1R in Breast Cancer Subtypes, Stemness, and Lineage Differentiation. Front Endocrinol (Lausanne) 2015;6:59. doi: 10.3389/fendo.2015.00059. Comprehensive review of the ole of insulin-like growth factor 1 and its receptor in breast cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esposito K, Chiodini P, Capuano A, Bellastella G, Maiorino MI, Rafaniello C, Giugliano D. Metabolic syndrome and postmenopausal breast cancer: systematic review and meta-analysis. Menopause. 2013;20:1301–1309. doi: 10.1097/GME.0b013e31828ce95d. [DOI] [PubMed] [Google Scholar]
- 43.Endogenous H, Key TJ, Appleby PN, Reeves GK, Roddam AW Breast Cancer Collaborative G. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010;11:530–542. doi: 10.1016/S1470-2045(10)70095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christopoulos PF, Msaouel P, Koutsilieris M. The role of the insulin-like growth factor-1 system in breast cancer. Mol Cancer. 2015;14:43. doi: 10.1186/s12943-015-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Becker MA, Ibrahim YH, Cui X, Lee AV, Yee D. The IGF pathway regulates ERalpha through a S6K1-dependent mechanism in breast cancer cells. Mol Endocrinol. 2011;25:516–528. doi: 10.1210/me.2010-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delort L, Rossary A, Farges MC, Vasson MP, Caldefie-Chezet F. Leptin, adipocytes and breast cancer: Focus on inflammation and anti-tumor immunity. Life Sci. 2015;140:37–48. doi: 10.1016/j.lfs.2015.04.012. [DOI] [PubMed] [Google Scholar]
- *47.Wang L, Tang C, Cao H, Li K, Pang X, Zhong L, Dang W, Tang H, Huang Y, Wei L, et al. Activation of IL-8 via PI3K/Akt-dependent pathway is involved in leptin-mediated epithelial-mesenchymal transition in human breast cancer cells. Cancer Biol Ther. 2015;16:1220–1230. doi: 10.1080/15384047.2015.1056409. Authors show that leptin promotes an epithelial to mesenchymal transition in breast cancer cells through the uprgulation of IL-8 via activation of PI3K/Akt signaling pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *48.Giordano C, Chemi F, Panza S, Barone I, Bonofiglio D, Lanzino M, Cordella A, Campana A, Hashim A, Rizza P, et al. Leptin as a mediator of tumor-stromal interactions promotes breast cancer stem cell activity. Oncotarget. 2015 doi: 10.18632/oncotarget.6014. Authros show that leptin produced by mammary adipocytes can promote increased breast cancer stem cell activity as evidenced by increased mammosphere formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *49.Esper RM, Dame M, McClintock S, Holt PR, Dannenberg AJ, Wicha MS, Brenner DE. Leptin and Adiponectin Modulate the Self-renewal of Normal Human Breast Epithelial Stem Cells. Cancer Prev Res (Phila) 2015;8:1174–1183. doi: 10.1158/1940-6207.CAPR-14-0334. Authors found that Leptin can promote the self renewal of mammary epithelial stem cells, while adiponectin suppresses it. This paper provides evidene that one possible reason for increased incidicene of breast cancer postmenopausal women is increased expansion of normal stem cells, which may act as tumor-initiatting cells upon further oncogenic hits, through increased leptin and decreased adiponectin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saxena NK, Sharma D. Metastasis suppression by adiponectin: LKB1 rises up to the challenge. Cell Adh Migr. 2010;4:358–362. doi: 10.4161/cam.4.3.11541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delort L, Jarde T, Dubois V, Vasson MP, Caldefie-Chezet F. New insights into anticarcinogenic properties of adiponectin: a potential therapeutic approach in breast cancer? Vitam Horm. 2012;90:397–417. doi: 10.1016/B978-0-12-398313-8.00015-4. [DOI] [PubMed] [Google Scholar]
- **52.Newman G, Gonzalez-Perez RR. Leptin-cytokine crosstalk in breast cancer. Mol Cell Endocrinol. 2014;382:570–582. doi: 10.1016/j.mce.2013.03.025. Review of the role of leptin in breast cancer and how its association with cytokine signaling. Reviews a previous paper (referenced below) where the authors shown cross talk between leptin, notch and IL-1 in promoting breast cancer agiogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin--a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst. 2002;94:1704–1711. doi: 10.1093/jnci/94.22.1704. [DOI] [PubMed] [Google Scholar]
- 54.Sun X, Casbas-Hernandez P, Bigelow C, Makowski L, Joseph Jerry D, Smith Schneider S, Troester MA. Normal breast tissue of obese women is enriched for macrophage markers and macrophage-associated gene expression. Breast Cancer Res Treat. 2012;131:1003–1012. doi: 10.1007/s10549-011-1789-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **55.Fuentes-Mattei E, Velazquez-Torres G, Phan L, Zhang F, Chou PC, Shin JH, Choi HH, Chen JS, Zhao R, Chen J, et al. Effects of obesity on transcriptomic changes and cancer hallmarks in estrogen receptor-positive breast cancer. J Natl Cancer Inst. 2014:106. doi: 10.1093/jnci/dju158. Transcripomic analysis of human ER+ breast cancer samples comparing expression in obese versus non-obese patients. Authors shown an enrichment for several oncogenic pathways includin immune cell traficking and inflammation in obese patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **56.Picon-Ruiz M, Pan C, Drews-Elger K, Jang K, Besser AH, Zhao D, Morata-Tarifa C, Kim M, Ince TA, Azzam DJ, et al. Interactions between Adipocytes and Breast Cancer Cells Stimulate Cytokine Production and Drive Src/Sox2/miR-302b-Mediated Malignant Progression. Cancer Res. 2016;76:491–504. doi: 10.1158/0008-5472.CAN-15-0927. Authors showed that interaction between adipocytes and breast cancer cells stimulated the production of inflammatory cytokines and malignant progression through a SOX2 dependent mechanism. [DOI] [PubMed] [Google Scholar]
- 57.Zhao Y, Nichols JE, Valdez R, Mendelson CR, Simpson ER. Tumor necrosis factor-alpha stimulates aromatase gene expression in human adipose stromal cells through use of an activating protein-1 binding site upstream of promoter 1.4. Mol Endocrinol. 1996;10:1350–1357. doi: 10.1210/mend.10.11.8923461. [DOI] [PubMed] [Google Scholar]
- 58.Subbaramaiah K, Morris PG, Zhou XK, Morrow M, Du B, Giri D, Kopelovich L, Hudis CA, Dannenberg AJ. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov. 2012;2:356–365. doi: 10.1158/2159-8290.CD-11-0241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Saxena NK, Sharma D. Multifaceted leptin network: the molecular connection between obesity and breast cancer. J Mammary Gland Biol Neoplasia. 2013;18:309–320. doi: 10.1007/s10911-013-9308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reichlin S, Chen G, Nicolson M. Blood to brain transfer of leptin in normal and interleukin-1beta-treated male rats. Endocrinology. 2000;141:1951–1954. doi: 10.1210/endo.141.6.7459. [DOI] [PubMed] [Google Scholar]
- 61.Simons PJ, van den Pangaart PS, van Roomen CP, Aerts JM, Boon L. Cytokine-mediated modulation of leptin and adiponectin secretion during in vitro adipogenesis: evidence that tumor necrosis factor-alpha- and interleukin-1beta-treated human preadipocytes are potent leptin producers. Cytokine. 2005;32:94–103. doi: 10.1016/j.cyto.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 62.Knupfer H, Preiss R. Significance of interleukin-6 (IL-6) in breast cancer (review) Breast Cancer Res Treat. 2007;102:129–135. doi: 10.1007/s10549-006-9328-3. [DOI] [PubMed] [Google Scholar]
- 63.De Larco JE, Wuertz BR, Rosner KA, Erickson SA, Gamache DE, Manivel JC, Furcht LT. A potential role for interleukin-8 in the metastatic phenotype of breast carcinoma cells. Am J Pathol. 2001;158:639–646. doi: 10.1016/S0002-9440(10)64005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gilbert CA, Slingerland JM. Cytokines, obesity, and cancer: new insights on mechanisms linking obesity to cancer risk and progression. Annu Rev Med. 2013;64:45–57. doi: 10.1146/annurev-med-121211-091527. [DOI] [PubMed] [Google Scholar]
- 65.Walter M, Liang S, Ghosh S, Hornsby PJ, Li R. Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene. 2009;28:2745–2755. doi: 10.1038/onc.2009.130. Authors show that IL-6 from adipose stromal cells can promote the migration and invasion of breast cancer cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chung H, Lee YS, Mayoral R, Oh DY, Siu JT, Webster NJ, Sears DD, Olefsky JM, Ellies LG. Omega-3 fatty acids reduce obesity-induced tumor progression independent of GPR120 in a mouse model of postmenopausal breast cancer. Oncogene. 2014 doi: 10.1038/onc.2014.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gunter MJ, Wang T, Cushman M, Xue X, Wassertheil-Smoller S, Strickler HD, Rohan TE, Manson JE, McTiernan A, Kaplan RC, et al. Circulating Adipokines and Inflammatory Markers and Postmenopausal Breast Cancer Risk. J Natl Cancer Inst. 2015:107. doi: 10.1093/jnci/djv169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **69.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007;86:556–565. doi: 10.1093/ajcn/86.3.556. Meta-analysis of studies btween 1966 and 2007 finding a link btween obesity and the risk of developing colorectal cancer in both men and women. [DOI] [PubMed] [Google Scholar]
- 70.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 71.Chen J, Huang XF. High fat diet-induced obesity increases the formation of colon polyps induced by azoxymethane in mice. Ann Transl Med. 2015;3:79. doi: 10.3978/j.issn.2305-5839.2015.03.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **72.Ngo HT, Hetland RB, Nygaard UC, Steffensen IL. Genetic and Diet-Induced Obesity Increased Intestinal Tumorigenesis in the Double Mutant Mouse Model Multiple Intestinal Neoplasia X Obese via Disturbed Glucose Regulation and Inflammation. J Obes. 2015;2015:343479. doi: 10.1155/2015/343479. Study showing that both genetically-induced and diet-induced obesity proomotes increased colorectal tumorigenesis in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stattin P, Palmqvist R, Soderberg S, Biessy C, Ardnor B, Hallmans G, Kaaks R, Olsson T. Plasma leptin and colorectal cancer risk: a prospective study in Northern Sweden. Oncol Rep. 2003;10:2015–2021. [PubMed] [Google Scholar]
- 74.Stocks T, Lukanova A, Johansson M, Rinaldi S, Palmqvist R, Hallmans G, Kaaks R, Stattin P. Components of the metabolic syndrome and colorectal cancer risk; a prospective study. Int J Obes (Lond) 2008;32:304–314. doi: 10.1038/sj.ijo.0803713. [DOI] [PubMed] [Google Scholar]
- 75.Rouet-Benzineb P, Aparicio T, Guilmeau S, Pouzet C, Descatoire V, Buyse M, Bado A. Leptin counteracts sodium butyrate-induced apoptosis in human colon cancer HT-29 cells via NF-kappaB signaling. J Biol Chem. 2004;279:16495–16502. doi: 10.1074/jbc.M312999200. [DOI] [PubMed] [Google Scholar]
- 76.Wang D, Chen J, Chen H, Duan Z, Xu Q, Wei M, Wang L, Zhong M. Leptin regulates proliferation and apoptosis of colorectal carcinoma through PI3K/Akt/mTOR signalling pathway. J Biosci. 2012;37:91–101. doi: 10.1007/s12038-011-9172-4. [DOI] [PubMed] [Google Scholar]
- 77.Uchiyama T, Takahashi H, Sugiyama M, Sakai E, Endo H, Hosono K, Yoneda K, Yoneda M, Inamori M, Nagashima Y, et al. Leptin receptor is involved in STAT3 activation in human colorectal adenoma. Cancer Sci. 2011;102:367–372. doi: 10.1111/j.1349-7006.2010.01803.x. [DOI] [PubMed] [Google Scholar]
- 78.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114. e2105. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 79.Moossavi S, Bishehsari F. Inflammation in sporadic colorectal cancer. Arch Iran Med. 2012;15:166–170. [PubMed] [Google Scholar]
- 80.Liu Z, Brooks RS, Ciappio ED, Kim SJ, Crott JW, Bennett G, Greenberg AS, Mason JB. Diet-induced obesity elevates colonic TNF-alpha in mice and is accompanied by an activation of Wnt signaling: a mechanism for obesity-associated colorectal cancer. J Nutr Biochem. 2012;23:1207–1213. doi: 10.1016/j.jnutbio.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26:54–74. doi: 10.1016/j.smim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- **82.Fazolini NP, Cruz AL, Werneck MB, Viola JP, Maya-Monteiro CM, Bozza PT. Leptin activation of mTOR pathway in intestinal epithelial cell triggers lipid droplet formation, cytokine production and increased cell proliferation. Cell Cycle. 2015;14:2667–2676. doi: 10.1080/15384101.2015.1041684. In this study, the authors whow that leptin signaling via mTOR acitvation oromotes increased inflammatory cytokine production and increased cell proliferation. This study provides evidence that increased leptin signaling in obese patients may play a role in the increased incidence of colorectal cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sugiyama M, Takahashi H, Hosono K, Endo H, Kato S, Yoneda K, Nozaki Y, Fujita K, Yoneda M, Wada K, et al. Adiponectin inhibits colorectal cancer cell growth through the AMPK/mTOR pathway. Int J Oncol. 2009;34:339–344. [PubMed] [Google Scholar]
- 84.Mutoh M, Teraoka N, Takasu S, Takahashi M, Onuma K, Yamamoto M, Kubota N, Iseki T, Kadowaki T, Sugimura T, et al. Loss of adiponectin promotes intestinal carcinogenesis in Min and wild-type mice. Gastroenterology. 2011;140:2000–2008. 2008 e2001–2002. doi: 10.1053/j.gastro.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 85.Wei EK, Giovannucci E, Fuchs CS, Willett WC, Mantzoros CS. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J Natl Cancer Inst. 2005;97:1688–1694. doi: 10.1093/jnci/dji376. [DOI] [PubMed] [Google Scholar]
- 86.Lukanova A, Soderberg S, Kaaks R, Jellum E, Stattin P. Serum adiponectin is not associated with risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:401–402. doi: 10.1158/1055-9965.EPI-05-0836. [DOI] [PubMed] [Google Scholar]
- 87.Vipperla K, O’Keefe SJ. Diet, microbiota, and dysbiosis: a ‘recipe’ for colorectal cancer. Food Funct. 2016 doi: 10.1039/c5fo01276g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *88.Yu YN, Fang JY. Gut Microbiota and Colorectal Cancer. Gastrointest Tumors. 2015;2:26–32. doi: 10.1159/000380892. A review on how the gut microbiome effects colorectal cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shen XJ, Rawls JF, Randall T, Burcal L, Mpande CN, Jenkins N, Jovov B, Abdo Z, Sandler RS, Keku TO. Molecular characterization of mucosal adherent bacteria and associations with colorectal adenomas. Gut Microbes. 2010;1:138–147. doi: 10.4161/gmic.1.3.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McCoy AN, Araujo-Perez F, Azcarate-Peril A, Yeh JJ, Sandler RS, Keku TO. Fusobacterium is associated with colorectal adenomas. PLoS One. 2013;8:e53653. doi: 10.1371/journal.pone.0053653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Puppa MJ, White JP, Sato S, Cairns M, Baynes JW, Carson JA. Gut barrier dysfunction in the Apc(Min/+) mouse model of colon cancer cachexia. Biochim Biophys Acta. 2011;1812:1601–1606. doi: 10.1016/j.bbadis.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Serino M, Luche E, Gres S, Baylac A, Berge M, Cenac C, Waget A, Klopp P, Iacovoni J, Klopp C, et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut. 2012;61:543–553. doi: 10.1136/gutjnl-2011-301012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **94.Pfalzer AC, Nesbeth PD, Parnell LD, Iyer LK, Liu Z, Kane AV, Chen CY, Tai AK, Bowman TA, Obin MS, et al. Diet- and Genetically-Induced Obesity Differentially Affect the Fecal Microbiome and Metabolome in Apc1638N Mice. PLoS One. 2015;10:e0135758. doi: 10.1371/journal.pone.0135758. Authors show that obesity led to changes in the gut microbiome in Apc1638N mice tumor bearing mice. They identified a depletion of Parabecteroides distasonis, which has previously been shown to have anti-inflammatory effects in the colon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kverka M, Zakostelska Z, Klimesova K, Sokol D, Hudcovic T, Hrncir T, Rossmann P, Mrazek J, Kopecny J, Verdu EF, et al. Oral administration of Parabacteroides distasonis antigens attenuates experimental murine colitis through modulation of immunity and microbiota composition. Clin Exp Immunol. 2011;163:250–259. doi: 10.1111/j.1365-2249.2010.04286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hersoug LG, Moller P, Loft S. Gut microbiota-derived lipopolysaccharide uptake and trafficking to adipose tissue: implications for inflammation and obesity. Obes Rev. 2015 doi: 10.1111/obr.12370. [DOI] [PubMed] [Google Scholar]
- **97.Schulz MD, Atay C, Heringer J, Romrig FK, Schwitalla S, Aydin B, Ziegler PK, Varga J, Reindl W, Pommerenke C, et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature. 2014;514:508–512. doi: 10.1038/nature13398. First paper showing that obesity-associated changes to the gut microbiome may promote colon tumorigenesis independently of obesity. Authors show that fecal transplant from obese tumor bearing mice was sufficient to increase tumorigenesis in non-obese tumor bearing mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marengo A, Rosso C, Bugianesi E. Liver Cancer: Connections with Obesity, Fatty Liver, and Cirrhosis. Annu Rev Med. 2016;67:103–117. doi: 10.1146/annurev-med-090514-013832. [DOI] [PubMed] [Google Scholar]
- 99.Bosch FX, Ribes J, Cleries R, Diaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9:191–211. v. doi: 10.1016/j.cld.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 100.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 101.Brown GT, Kleiner DE. Histopathology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Metabolism. 2015 doi: 10.1016/j.metabol.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 103.Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012;56:1384–1391. doi: 10.1016/j.jhep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- **104.Dyson J, Jaques B, Chattopadyhay D, Lochan R, Graham J, Das D, Aslam T, Patanwala I, Gaggar S, Cole M, et al. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol. 2014;60:110–117. doi: 10.1016/j.jhep.2013.08.011. Study looking at the recent increase in the incidence of hepatopcellular carcinomas and how this is assocaited with increased obesity and type 2 diabetes. [DOI] [PubMed] [Google Scholar]
- *105.Woo Baidal JA, Lavine JE. The intersection of nonalcoholic fatty liver disease and obesity. Sci Transl Med. 2016;8:323rv321. doi: 10.1126/scitranslmed.aad8390. A review of on the pathogenesis of non-alchoholic fatty liver disease and its relationship to obeisty. [DOI] [PubMed] [Google Scholar]
- 106.McCullough AJ. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:521–533. viii. doi: 10.1016/j.cld.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 107.Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13:511–531. doi: 10.1016/j.cld.2009.07.005. [DOI] [PubMed] [Google Scholar]
- **108.Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97:1005–1008. doi: 10.1038/sj.bjc.6603932. A meta-analysis of studies between 1966 and 2007 finding that obesity is assocaited with an increased risk of liver cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol. 2012;56:704–713. doi: 10.1016/j.jhep.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **110.Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–788. doi: 10.1002/hep.23719. A review of literature discussing how excess triglyceride accumulation in the liver leads to inflammation and development non-alchoholic steatohepatitis. [DOI] [PubMed] [Google Scholar]
- 111.Nakagawa H, Umemura A, Taniguchi K, Font-Burgada J, Dhar D, Ogata H, Zhong Z, Valasek MA, Seki E, Hidalgo J, et al. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell. 2014;26:331–343. doi: 10.1016/j.ccr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jiang N, Sun R, Sun Q. Leptin signaling molecular actions and drug target in hepatocellular carcinoma. Drug Des Devel Ther. 2014;8:2295–2302. doi: 10.2147/DDDT.S69004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C, Tovar V, Roayaie S, Minguez B, Sole M, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135:1972–1983. 1983 e1971–1911. doi: 10.1053/j.gastro.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim J, Guan KL. Regulation of the autophagy initiating kinase ULK1 by nutrients: roles of mTORC1 and AMPK. Cell Cycle. 2011;10:1337–1338. doi: 10.4161/cc.10.9.15291. [DOI] [PubMed] [Google Scholar]
- 116.White E, Karp C, Strohecker AM, Guo Y, Mathew R. Role of autophagy in suppression of inflammation and cancer. Curr Opin Cell Biol. 2010;22:212–217. doi: 10.1016/j.ceb.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Takamura A, Komatsu M, Hara T, Sakamoto A, Kishi C, Waguri S, Eishi Y, Hino O, Tanaka K, Mizushima N. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011;25:795–800. doi: 10.1101/gad.2016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11:467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sharma D, Wang J, Fu PP, Sharma S, Nagalingam A, Mells J, Handy J, Page AJ, Cohen C, Anania FA, et al. Adiponectin antagonizes the oncogenic actions of leptin in hepatocellular carcinogenesis. Hepatology. 2010;52:1713–1722. doi: 10.1002/hep.23892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Saxena NK, Fu PP, Nagalingam A, Wang J, Handy J, Cohen C, Tighiouart M, Sharma D, Anania FA. Adiponectin modulates C-jun N-terminal kinase and mammalian target of rapamycin and inhibits hepatocellular carcinoma. Gastroenterology. 2010;139:1762–1773. 1773 e1761–1765. doi: 10.1053/j.gastro.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13:800–812. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Uthaya Kumar DB, Chen CL, Liu JC, Feldman DE, Sher LS, French S, DiNorcia J, French SW, Naini BV, Junrungsee S, et al. TLR4 Signaling via NANOG Cooperates With STAT3 to Activate Twist1 and Promote Formation of Tumor-Initiating Stem-Like Cells in Livers of Mice. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Machida K, Tsukamoto H, Mkrtchyan H, Duan L, Dynnyk A, Liu HM, Asahina K, Govindarajan S, Ray R, Ou JH, et al. Toll-like receptor 4 mediates synergism between alcohol and HCV in hepatic oncogenesis involving stem cell marker Nanog. Proc Natl Acad Sci USA. 2009;106:1548–1553. doi: 10.1073/pnas.0807390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 125.Navina S, Acharya C, DeLany JP, Orlichenko LS, Baty CJ, Shiva SS, Durgampudi C, Karlsson JM, Lee K, Bae KT, et al. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med. 2011;3:107ra110. doi: 10.1126/scitranslmed.3002573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67:253–256. doi: 10.1017/S002966510800712X. Report by the World Cancer Research Fund and the American Institute for Cancer Research on the relationship between nutrition, physical activity and obesity and cancer. [DOI] [PubMed] [Google Scholar]
- 127.Majumder K, Gupta A, Arora N, Singh PP, Singh S. Premorbid Obesity and Mortality in Patients With Pancreatic Cancer: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2015 doi: 10.1016/j.cgh.2015.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Philip B, Roland CL, Daniluk J, Liu Y, Chatterjee D, Gomez SB, Ji B, Huang H, Wang H, Fleming JB, et al. A high-fat diet activates oncogenic Kras and COX2 to induce development of pancreatic ductal adenocarcinoma in mice. Gastroenterology. 2013;145:1449–1458. doi: 10.1053/j.gastro.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cox AD, Olive KP. Silencing the killers: paracrine immune suppression in pancreatic cancer. Cancer Cell. 2012;21:715–716. doi: 10.1016/j.ccr.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 130.Li D. Diabetes and pancreatic cancer. Mol Carcinog. 2012;51:64–74. doi: 10.1002/mc.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Secord AA, Hasselblad V, Von Gruenigen VE, Gehrig PA, Modesitt SC, Bae-Jump V, Havrilesky LJ. Body mass index and mortality in endometrial cancer: A systematic review and meta-analysis. Gynecol Oncol. 2016;140:184–190. doi: 10.1016/j.ygyno.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Olsen CM, Green AC, Whiteman DC, Sadeghi S, Kolahdooz F, Webb PM. Obesity and the risk of epithelial ovarian cancer: a systematic review and meta-analysis. Eur J Cancer. 2007;43:690–709. doi: 10.1016/j.ejca.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 133.Gu W, Chen C, Zhao KN. Obesity-associated endometrial and cervical cancers. Front Biosci (Elite Ed) 2013;5:109–118. doi: 10.2741/e600. [DOI] [PubMed] [Google Scholar]
- 134.Chen J, Zhao KN, Li R, Shao R, Chen C. Activation of PI3K/Akt/mTOR pathway and dual inhibitors of PI3K and mTOR in endometrial cancer. Curr Med Chem. 2014;21:3070–3080. doi: 10.2174/0929867321666140414095605. [DOI] [PubMed] [Google Scholar]
- 135.Long E, Beales IL. The role of obesity in oesophageal cancer development. Therap Adv Gastroenterol. 2014;7:247–268. doi: 10.1177/1756283X14538689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kolb R, Liu GH, Janowski AM, Sutterwala FS, Zhang W. Inflammasomes in cancer: a double-edged sword. Protein Cell. 2014;5:12–20. doi: 10.1007/s13238-013-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bauernfeind F, Ablasser A, Bartok E, Kim S, Schmid-Burgk J, Cavlar T, Hornung V. Inflammasomes: current understanding and open questions. Cell Mol Life Sci. 2011;68:765–783. doi: 10.1007/s00018-010-0567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stienstra R, Joosten LA, Koenen T, van Tits B, van Diepen JA, van den Berg SA, Rensen PC, Voshol PJ, Fantuzzi G, Hijmans A, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12:593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Janowski AM, Kolb R, Zhang W, Sutterwala FS. Beneficial and Detrimental Roles of NLRs in Carcinogenesis. Front Immunol. 2013;4:370. doi: 10.3389/fimmu.2013.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li Y, Wang L, Pappan L, Galliher-Beckley A, Shi J. IL-1beta promotes stemness and invasiveness of colon cancer cells through Zeb1 activation. Mol Cancer. 2012;11:87. doi: 10.1186/1476-4598-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu X, Wang Z, Yu J, Lei G, Wang S. Three polymorphisms in interleukin-1beta gene and risk for breast cancer: a meta-analysis. Breast Cancer Res Treat. 2010;124:821–825. doi: 10.1007/s10549-010-0910-3. [DOI] [PubMed] [Google Scholar]
- 143.Jin L, Yuan RQ, Fuchs A, Yao Y, Joseph A, Schwall R, Schnitt SJ, Guida A, Hastings HM, Andres J, et al. Expression of interleukin-1beta in human breast carcinoma. Cancer. 1997;80:421–434. doi: 10.1002/(sici)1097-0142(19970801)80:3<421::aid-cncr10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 144.Chavey C, Bibeau F, Gourgou-Bourgade S, Burlinchon S, Boissiere F, Laune D, Roques S, Lazennec G. Oestrogen receptor negative breast cancers exhibit high cytokine content. Breast Cancer Res. 2007;9:R15. doi: 10.1186/bcr1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Faggioni R, Fantuzzi G, Fuller J, Dinarello CA, Feingold KR, Grunfeld C. IL-1 beta mediates leptin induction during inflammation. Am J Physiol. 1998;274:R204–208. doi: 10.1152/ajpregu.1998.274.1.R204. [DOI] [PubMed] [Google Scholar]
- **146.Arendt LM, McCready J, Keller PJ, Baker DD, Naber SP, Seewaldt V, Kuperwasser C. Obesity Promotes Breast Cancer by CCL2-Mediated Macrophage Recruitment and Angiogenesis. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-13-0926. The authors used mice with humanized mammary fat pad and found that obesity promoted breast cancer tumor growth, macrophage recruitment and angiogenesis via a CCL2/IL-1β/CXCL12 signaling pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang F, Herrington M, Larsson J, Permert J. The relationship between diabetes and pancreatic cancer. Mol Cancer. 2003;2:4. doi: 10.1186/1476-4598-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Frossard JL, Lescuyer P, Pastor CM. Experimental evidence of obesity as a risk factor for severe acute pancreatitis. World J Gastroenterol. 2009;15:5260–5265. doi: 10.3748/wjg.15.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gu H, Werner J, Bergmann F, Whitcomb DC, Buchler MW, Fortunato F. Necro-inflammatory response of pancreatic acinar cells in the pathogenesis of acute alcoholic pancreatitis. Cell Death Dis. 2013;4:e816. doi: 10.1038/cddis.2013.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **150.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. A review about the complexity of macrophage polarization and a discussion on whetehr the M1 vs M2 polarization paradigm needs to be readdressed. [DOI] [PMC free article] [PubMed] [Google Scholar]