Abstract
During cognitive tasks requiring externally directed attention, activation in the default-network (DN) typically decreases below baseline levels (“deactivations”). Healthy aging is associated with reduced deactivations, which are usually attributed to a failure to suppress DN processes. Recent evidence instead suggests older adults may be more reliant on DN than young adults when performing these tasks.
Keywords: Default-network, deactivation, aging, mind-wandering, task-unrelated thought, memory
In one moment, a student may be directing her attention to a professor’s lecture, while in the next, she may be directing attention to making dinner plans for the evening. These examples illustrate the distinction between externally directed attention (attention to stimuli and events in the environment) and internally-directed attention (attention to thoughts and feelings). In recent years, functional magnetic resonance imaging (fMRI) studies have indicated that specific brain networks, the dorsal attention network (which includes superior parietal lobes and frontal eye fields), and the default-network (DN; which includes the medial prefrontal and medial temporal cortex, posterior cingulate cortex, inferior parietal lobes and lateral temporal cortices) are associated with externally-and internally-directed attention, respectively. Moreover, in young adults, there is now extensive evidence that these two networks often work in opposition [1]. Specifically, as requirements for externally directed attention increase, activation in dorsal attention network increases, whereas activation in DN decreases below baseline levels. Such deactivations in regions of DN are thought to reflect suppression of task-irrelevant internal processes (e.g. thinking of dinner plans) [1].
Healthy (non-demented) older adults aged 60 and above often exhibit reduced deactivations compared to young adults during tasks requiring externally directed attention, such as tasks assessing episodic memory encoding and working memory [2–7]. These effects have often been interpreted as failures of task-induced deactivations with age [2, 4, 6] although the precise mechanisms underlying this effect have remained elusive.
In one of the first studies [5] to assess age-related differences in deactivations, young adults, healthy older adults, and Alzheimer’s disease (AD) patients performed a semantic classification task intermixed with passive fixation (baseline). Results indicated that during semantic classification versus baseline, young adults exhibited the most deactivation in posterior cingulate cortex, older adults exhibited an intermediate level of deactivation and AD patients exhibited the least (for further discussion of the association between deactivations and AD-related pathology, see Box 1). Subsequent studies indicated that reduced deactivations occur not only when contrasting activation in a task versus baseline contrast – they also occur when comparing between-group differences in two tasks (e.g. a hard versus easy working memory task [7]). This observation is important because, whereas age-related differences in a task-baseline contrast could be attributable to baseline differences in cerebral blood flow or neurovascular coupling, age-related differences in a contrast between two experimental tasks (an age-group-by-task interaction) are more likely attributable to age-related differences in neural activity associated with cognitive processes tapped by different experimental tasks [8]. It is these age-group-by-task interactions in DN that are of interest in the current article.
Box 1. Association between deactivations and beta-amyloid deposition.
At around the same time that interest grew in age-related differences in deactivations, new technology allowed in-vivo measurement of beta-amyloid deposition, a biomarker for AD. Studies using this technology indicated that amyloid deposition is detectable prior to any behavioral symptoms in a significant percentage (20–33%) of non-demented individuals [4]. Additionally, amyloid is present in DN regions, and higher rates of amyloid correlate with decreased deactivations in DN regions [4]. These results raised the possibility that previous reports of reduced deactivations in samples of older relative to young adults are primarily or entirely attributable to individuals with high amyloid in the older sample. However, recent studies that can dissociate the effects of age and amyloid deposition on age-group-by-task interactions in DN have indicated that deactivations are present even in older adults with no evidence of amyloid deposition [13, 15] (although it remains possible that sub-threshold levels of amyloid, or other AD-related pathology contribute to these results). Moreover, whereas there is often an a-priori focus of amyloid-DN associations, it is important to note that amyloid sometimes has a larger impact on task-related activation in regions outside DN that are activated above-baseline [13].
When do older adults exhibit reduced deactivation?
There are two experimental paradigms in which age-group-by-task interactions in deactivation have consistently been observed. First, in studies that include a manipulation of task difficulty (e.g. a hard and an easy working memory task) [7], young and older adults typically exhibit similar levels of deactivation in the easy task, but older adults exhibit reduced deactivation in harder tasks. Thus, age-related reductions in deactivation occur primarily when cognitive resources are taxed [7]. However, these studies have rarely correlated brain activation to task performance, making it hard to determine whether decreased deactivation in aging contributes to, or impairs task performance.
The second paradigm in which age-group-by-task interactions in deactivation have consistently been observed is the subsequent memory paradigm. Here, activation is contrasted between encoding items (e.g. words, pictures) that are remembered versus forgotten in a later memory test. Subsequent memory studies have used two complementary types of brain-behavior associations. First, the subsequent memory contrast allows identification of regions that are more active when individuals successfully versus unsuccessfully encode information. Second, across-individual correlations between activation and task performance reveal brain regions that are activated in individuals performing the best overall. The brain regions identified as supporting performance in these two analyses need not correspond. For instance, consider an experimental task that can be performed using two distinct strategies, one of which is more efficient and will lead to higher performance than the other. In this scenario, brain regions involved in the less effective strategy may be more active during successful versus unsuccessful task performance (because this is one way to do the task) and negatively correlated with overall task performance (because this is not the optimal strategy) [11].
In the subsequent memory paradigm, young adults typically exhibit greater deactivation for subsequently remembered versus forgotten encoding items in DN regions, suggesting that deactivation in DN regions contributes to successful encoding in young adults [3, 6]. Compared to young adults, older adults exhibit decreased deactivation in DN regions while encoding items they will later remember. This results in a group-by-task interaction in which older adults exhibit either a reduced difference in activation between forgotten and remembered events compared to young adults in some studies, or even a reversal of the young adult effect in other studies, such that there is less deactivation for remembered versus forgotten items in older adults [3, 6, 11] (Figure 1). Furthermore, those older adults who exhibit the most deactivation during successful encoding tend to perform worst on the task overall [3, 6, 11]. Thus, DN regions contribute to task performance primarily in lower-performing older adults. Furthermore, subsequent memory and task-difficulty paradigms both suggest that age-related reductions in deactivations occur primarily when cognitive resources are taxed (in a harder versus an easy task, or in individuals performing more poorly on the task).
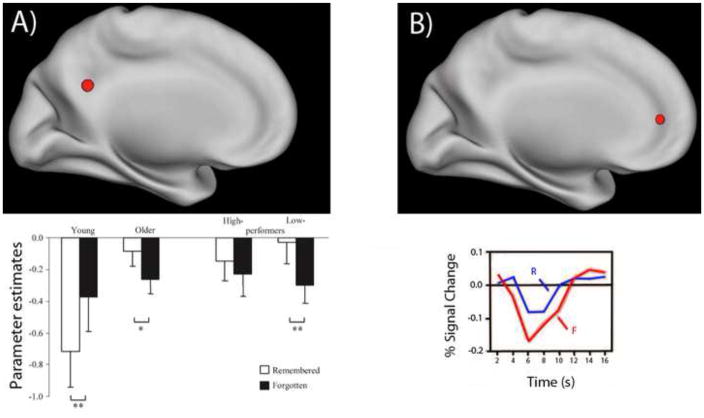
Figure 1. Reduced deactivations during events later remembered versus forgotten.
A) A representative example of an age-group-by-subsequent-memory interaction in a deactivated region (adapted from [3]). The bar graph displays parameter estimates in posterior cingulate cortex for encoding events subsequently remembered and forgotten in young adults, older adults, and also for sub-groups of high-performing and low-performing older adults. A reversal in subsequent memory effects with age (forgotten > remembered in young, remembered > forgotten in old) was observed, which is particularly evident in low-performing older adults. This interaction is attributable primarily to a difference in activation for remembered (rather than forgotten) events. See [11] for a meta-analysis indicating that such effects are consistently observed. B) Young adults, under certain conditions, can also exhibit reduced deactivation in a region that contributes to successful task performance (adapted from [14]). A region in medial prefrontal cortex was less deactivated for remembered (R) than forgotten (F) events. The encoding task was to judge the self-descriptiveness of adjectives, a task that differs from traditional semantic encoding tasks such as the animate/inanimate task used in [3].
Cognitive processes associated with reduced deactivations in aging
What are the cognitive processes associated with age-group-by-task interactions in deactivation in DN regions? One influential perspective is that healthy older adults exhibit reduced deactivations because they are less able than young adults to suppress task-irrelevant processes [e.g., 2]. Yet this proposal is inconsistent with the findings reviewed above indicating that age-related differences in deactivation occur when participants are successfully doing the task.
Further evidence against this proposal comes from behavioral studies that have consistently revealed that older adults exhibit a reduction in self-reported task-irrelevant thoughts during a variety of tasks [9]. Moreover, older adults sometimes outperform young adults in monotonous sustained attention tasks, providing objective evidence to corroborate self-reports. However, behavioral studies take place in quiet and comfortable environments, which differ from the noisy and less comfortable fMRI environment. It is possible that in this latter setting, older adults exhibit a higher frequency of task-irrelevant thoughts (e.g. because they are distracted by the scanner environment). Inconsistent with this idea, a recent study [10] found no significant age-related differences in online self-reports of task-irrelevant thoughts (personal thoughts, thoughts about the scanner environment and worries about task performance) during an fMRI episodic encoding task; task-unrelated thoughts were numerically lower in older versus young, consistent with behavioral studies. It is thus unlikely that age-related reductions in deactivations are attributable to increased task-unrelated thoughts.
Instead, we suggest that when cognitive resources are taxed, older adults increasingly rely on cognitive processes mediated by DN [see also, 7, 11]. The critical aspect of this proposal is that age-related reductions in DN represent task-relevant processes rather than task-irrelevant ones. Importantly, we are not suggesting that recruitment of DN in older adults is compensatory in the sense that those older adults that recruit DN the most will perform the best (as discussed earlier, the opposite seems to be true).
The precise corresponding cognitive processes engaged by older adults remain to be elucidated, and may differ based on the task. For example, whereas current evidence suggests that older adults do not mind-wander more than young adults during cognitive tasks (thoughts that have nothing to with, and were not triggered by any stimulus the current task, such as dinner plans), one possibility is that they exhibit more task-related thoughts and feelings (thoughts/feelings triggered by stimuli in the task itself, such as a picture of a sofa triggering a thought about how it looks similar to the one in my living room) [12]. Distinguishing which thoughts are task-related/unrelated is a thorny issue, and may vary based on the specific requirements of a task. Another possibility is that older adults rely more on prior knowledge, experience and schemas that they have accumulated over their longer lifespan (i.e. semantic memory) [7]. The suggestion that older adults sometimes use their greater knowledge in the face of declining cognitive efficiency is not new – however, these ideas have not yet been widely discussed by neuroimaging researchers in relation to age-related differences in DN activation. Our hope is that this article brings attention to these possibilities and stimulates research assessing their validity.
Acknowledgments
This word was supported by a National Institute on Aging grant AG08441 to D. Schacter and by a Fonds de Recherche Santé Québec postdoctoral training award to D. Maillet. We thank Preston Thakral, Karen Campbell and Paul Seli for helpful feedback.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anticevic A, et al. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16(12):584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grady CL, et al. Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci. 2006;18(2):227–241. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- 3.Duverne S, Motamedinia S, Rugg MD. The relationship between aging, performance, and the neural correlates of successful memory encoding. Cereb Cortex. 2009;19(3):733–744. doi: 10.1093/cercor/bhn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sperling RA, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63(2):178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lustig C, et al. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci USA. 2003;100(24):14504–9. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller SL, et al. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci U S A. 2008;105(6):2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner GR, Spreng RN. Prefrontal engagement and reduced default network suppression co-occur and are dynamically coupled in older adults: The default-executive coupling hypothesis of aging. J Cogn Neurosci. 2015;27(12):2462–2476. doi: 10.1162/jocn_a_00869. [DOI] [PubMed] [Google Scholar]
- 8.D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4(11):863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- 9.Maillet D, Schacter DL. From mind wandering to involuntary retrieval: Age-related differences in spontaneous cognitive processes. Neuropsychologia. 2016;80:142–156. doi: 10.1016/j.neuropsychologia.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maillet D, Rajah MN. Assessing the neural correlates of task-unrelated thoughts during episodic encoding and their association with subsequent memory in young and older adults. J Cogn Neurosci. 2016;8(6):826–841. doi: 10.1162/jocn_a_00935. [DOI] [PubMed] [Google Scholar]
- 11.Maillet D, Rajah MN. Age-related differences in brain activity in the subsequent memory paradigm: a meta-analysis. Neurosci Biobehav Rev. 2014;45:246–257. doi: 10.1016/j.neubiorev.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Maillet D, Schacter DL. When the mind wanders: Distinguishing stimulus-dependent from stimulus-independent thoughts during incidental encoding in young and older adults. Psychol Aging. doi: 10.1037/pag0000099. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mormino EC, et al. Abeta deposition in aging is associated with increases in brain activation during successful memory encoding. Cereb Cortex. 2012;22(8):1813–23. doi: 10.1093/cercor/bhr255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macrae CN, et al. Medial prefrontal activity predicts memory for self. Cereb Cortex. 2004;14(6):647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- 15.Elman JA, et al. Neural compensation in older people with brain amyloid-beta deposition. Nat Neurosci. 2014;17(10):1316–1318. doi: 10.1038/nn.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]