Abstract
Psoriasis vulgaris is an inflammatory skin disease caused by hyper-activated T-cells regulated by positive and negative mechanisms; while the former has been much studied, but the latter has not. We studied the regulatory mechanism mediated by myeloid-derived suppressor cells (MDSCs), especially having shown that MDSCs expanded in melanoma patients express DC-HIL, a critical mediator of T-cell suppressor function. We examined expansion of DC-HIL+ MDSCs in psoriasis and characterized their functional properties. Frequency of DC-HIL+ monocytic MDSCs (CD14+HLA-DRno/low) in blood and skin was markedly increased in psoriatic patients vs. healthy controls, but there was no statistically significant relationship with disease severity (PASI score). Blood DC-HIL+ MDSC levels in the untreated patients were significantly higher than the treated patients. Compared to melanoma-derived MDSCs, psoriatic MDSCs exhibited significantly reduced suppressor function, and were less dependent on DC-HIL, but capable of inhibiting proliferation and IFN-γ and IL-17 responses of autologous T-cells. Psoriatic MDSCs were functionally diverse among patients in their ability to suppress allogeneic T-cells and use of either IL-17/arginase I or IFN-γ/iNOS axis as suppressor mechanisms. Thus DC-HIL+ MDSCs are expanded in psoriasis patients, and their mechanistic heterogeneity and relative functional deficiency may contribute to the development of psoriasis.
Introduction
Psoriasis is a common immune-mediated, chronic inflammatory skin disease characterized by hyperproliferative keratinocytes (epidermal hyperplasia or acanthosis) and extensive infiltration of various leukocytes, including T-cells, dendritic cells (DC), macrophages, and neutrophils (Rivas Bejarano and Valdecantos, 2013). Among these leukocytes, T-cells play a central role in development of these characteristic clinical features. In particular, hyper-activated Th1 and Th17 responses are frequently observed in the blood and skin of psoriasis patients and have been considered to be responsible for psoriatic dermatitis (Di Cesare et al., 2009; Lowes et al., 2014; Ma et al., 2008). However, the pathogenesis of psoriasis still remains ambiguous, in particular, regarding mechanisms leading to persistence of T-cell hyperactivation.
T-cell activation is regulated by competing positive and negative co-regulatory signals delivered through interaction of co-regulatory receptors (expressed on T-cells) and their ligands (on antigen-presenting cells and non-lymphoid cells) (Wang and Chen, 2004). The positive regulators (or co-stimulators) include CD28:CD80/CD86, CD40:CD40L, and OX40:OX40L paring receptors (Briones et al., 2011; Chatzigeorgiou et al., 2009; Ishii et al., 2010); and the negative regulators (or co-inhibitors) include CTLA-4:CD80/CD86 and PD-1:PD-L1/PD-L2 (Egen et al., 2002; Francisco et al., 2010). In psoriatic patients, expression of co-stimulators is elevated significantly in hyper-activated T-cells and other leukocytes, compared to healthy controls (Ferenczi et al., 2000; Niu et al., 2015). Treatment of psoriatic patients or psoriatic skin grafts in SCID mice with co-stimulator-specific inhibitors (Ab or chemicals) reduces acanthosis and lymphocyte skin infiltrates (Abrams et al., 1999; Raychaudhuri et al., 2008), indicating that the co-stimulators are critically involved in the development of psoriatic skin. Although T-cell hyperactivation is considered to be also due to dysregulated expression of or deficiency in function of co-inhibitors, little is known about their contribution to pathogenesis.
Myeloid-derived suppressor cells (MDSCs) were originally identified by the CD11b+Gr1+ phenotype in tumor-bearing mice (Serafini et al., 2006), but in humans different surface markers are used; e.g., CD14+HLA-DRno/low and other phenotypes (Filipazzi et al., 2007) because of a lack of the Gr1 gene homologue. In healthy individuals, MDSCs consist of myeloid progenitors that differentiate into DC, granulocytes, and macrophages, so that MDSCs are a critical component in replenishing potent immune systems (Gabrilovich and Nagaraj, 2009). Note that in normal individuals, MDSCs are weakly immunosuppressive. By contrast, in cancer patients, MDSCs fail to differentiate, thereby proliferating and mobilizing from bone marrow to other organs, where they exert potent T-cell suppression (Frey, 2006; Youn and Gabrilovich, 2010). Recently, it was reported that expanded MDSC populations with suppressor function is also associated with inflammatory disorders, including alopecia areata (Marhaba et al., 2007; Singh et al., 2011), arthritis (Fujii et al., 2013), and infectious diseases such as Mycobacterial infections (du Plessis et al., 2013), but their exact role in these conditions has been debated (Cripps and Gorham, 2011; Cuenca et al., 2011).
We discovered a co-inhibitory pathway comprising of DC-HIL receptor on inflammatory Ag-presenting cells and its paired receptor syndecan-4 (SD-4) on effector/memory T-cells (Chung et al., 2007a; Chung et al., 2007b). Using mouse models, we showed the DC-HIL/SD-4 pathway to be a potent regulator of T cell-mediated immune responses in contact hypersensitivity, graft-versus-host disease, experimental autoimmune encephalomyelitis (EAE), and melanoma (Chung et al., 2014b; Chung et al., 2013). Recently, we found an expanded MDSC population in melanoma patients express DC-HIL, in which this expression positively correlates with melanoma stage progression; and DC-HIL is a critical mediator of MDSCs’ suppressor function (Turrentine et al., 2014). Thus, DC-HIL may serve as a marker of MDCSs’ immunosuppressive capacity.
To explore the possible contributions of MDSC-mediated suppression to psoriasis development, we examined expansion of DC-HIL+ MDSCs in psoriatic patients, its correlation with disease severity, and its functional properties. Data were interpreted in comparison with those of MDSCs from melanoma patients and normal controls. While exhibiting some similarities with their melanoma counterparts, psoriatic MDSCs had a reduced ability to suppress activation of autologous T-cells and exhibited heterogeneous suppressive mechanisms among patients. Their functional deficiency and mechanistic diversity indicate an array of impaired immunosuppression that may contribute to the autoreactivity in chronic psoriasis patients.
Results
Psoriasis induces expansion of DC-HIL+ monocytic MDSCs
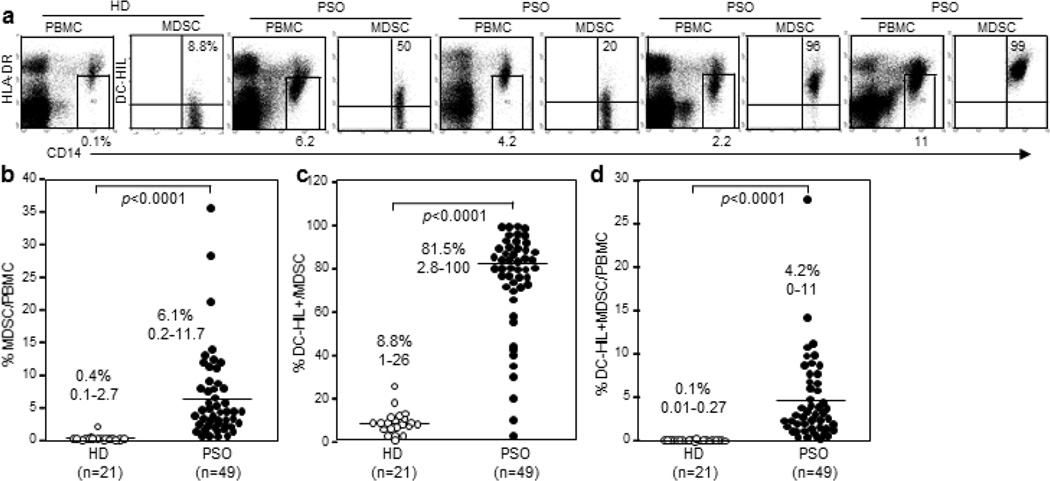
Since some inflammatory diseases can induce MDSC expansion as strongly as in cancer patients and because immunosuppressive MDSCs induced in melanoma patients express DC-HIL, we examined expansion of MDSCs and their DC-HIL expression in the blood of psoriatic patients vs. healthy controls. We recruited patients (n=49) and age/gender-matched healthy donors (n=21) (demographics are summarized in Table 1). At the time of blood draw, 19 patients were untreated, with 30 treated with various regimens (topical steroids, topical vitamin D analogues, topical calcineurin inhibitors, and/or narrow-band UVB). Blood samples were assayed by flow-cytometry for frequency (%) of CD14+HLA-DRno/low monocytic MDSCs among total PBMCs; and DC-HIL expression on the MDSCs (Figure 1a). These MDSCs were also CD15negCD33+CD11b+ (Supplementary Figure S1). MDSCs were significantly elevated in psoriatic patients, albeit with considerably high variation, compared to heathy controls (median and range 6.1%, 0.2–11.7% vs. 0.4%, 0.1–2.7%, p<0.0001) (Figure 1b). While MDSCs in healthy controls expressed DC-HIL at 8.8% (range of 1–26%, mean fluorescence intensity (MFI) of 5.7 ± 5.38), most psoriatic MDSCs expressed DC-HIL (81.5%, 2.8–100%, MFI of 81 ± 93, p<0.0001, Figure 1c). Using these data, we calculated % DC-HIL+ MDSCs in total PBMCs and found the population to be expanded significantly in psoriatic blood (4.2%, 0–11% vs. 0.1%, 0.01–0.27%, p<0.001, Figure 1d). This level was higher than in metastatic melanoma patients (2.6 ± 0.6% (Turrentine et al., 2014)). The T-cell ligand SD-4 of DC-HIL was also upregulated in CD4+ and CD8+ T cells of psoriatic patients (Supplementary Figure S2). Thus DC-HIL+ monocytic MDSCs were markedly proliferated in the blood of psoriatic patients.
Table 1.
Demographics of psoriasis patients and healthy controls used for study.
| Type of patients | Psoriasis patients | Healthy controls | ||
|---|---|---|---|---|
| Number of analyzed patients | 49 | 21 | ||
| Age (Mean +/− SD) | 52 +/− 12 | 53 +/− 13 | ||
| Gender | 22 Male, 27 Female | 11 Male, 10 Female | ||
| Race/Ethnicity | 21 Caucasian, 16 Hispanic, 7 Asian, 3 Middle Eastern, 2 African American |
11 Caucasian, 2 Hispanic, 8 Asian | ||
| Treatments | None | 19 (39%) | N/A | |
| Topical steroids | 29 (90%) | |||
| Topical vitamin D analogue | 6 (18%) | |||
| Topical calcineurin inhibitors | 3 (9%) | |||
| NBUVB | 12 (37% | |||
| Psoriasis Type | Plaque | 49 (100%) | N/A | |
| 1/49 pt had concurrent palmoplantar psoriasis | ||||
| PASI Median (Range) | 6.1 (0.4 – 45) | N/A | ||
Some patients were treated with multiple therapies.
NBUVB: Narrowband UVB light therapy.
Figure 1. Expansion of DC-HIL-expressing monocytic MDSCs in blood of psoriasis patients.
PBMCs freshly isolated from blood samples of healthy donors (HD) or psoriasis patients (PSO) were analyzed for expression of HLA-DR and CD14, and CD14+HLA-DRno/low cells were gated (shown by a small window) and examined for expression of DC-HIL vs. CD14. Flow-cytometric data shown are representative of each cohort (a), calculated for frequency (%) of MDSCs among total PBMCs (b), of DC-HIL+ cells among MDSCs (c), and of DC-HIL+ MDSCs among PBMCs (d) (median and range) and summarized in scatter graphs.
Relationship of DC-HIL+ MDSC blood levels with psoriatic severity and treatment
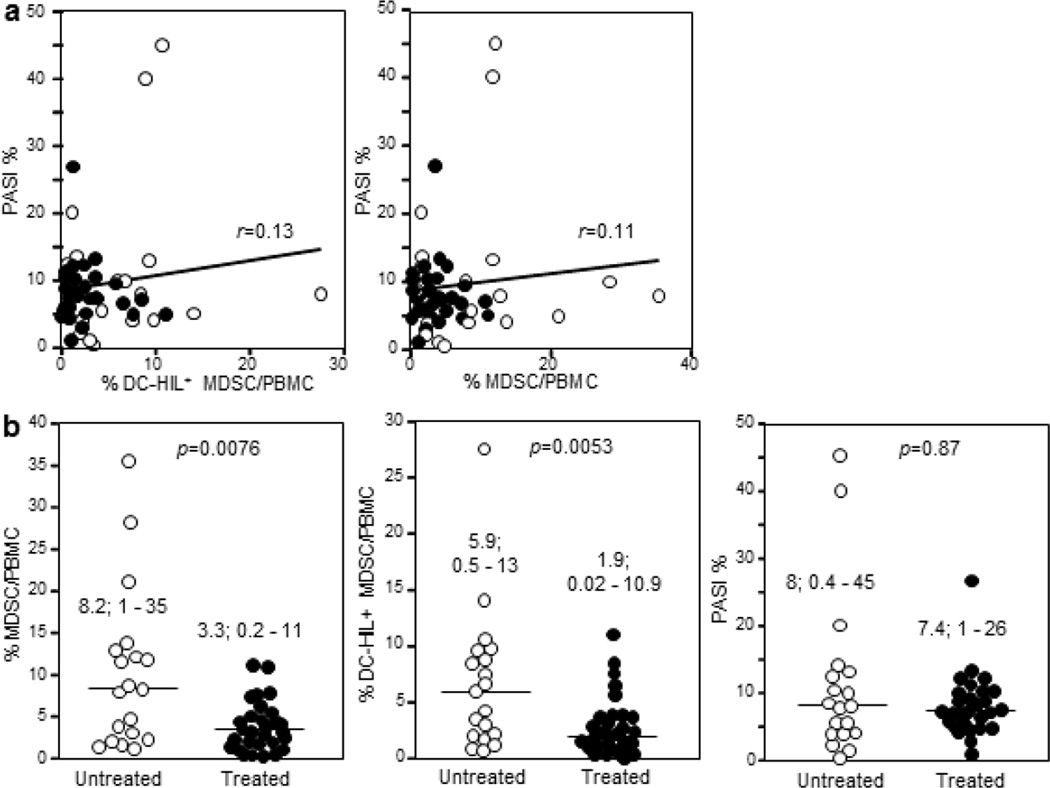
Given that % DC-HIL+ MDSCs/PBMCs positively correlated with melanoma cancer stages, we examined the relationship of % DC-HIL+ MDSCs/PBMCs with disease severity, assessed by Psoriasis Area Severity Index (PASI) scores (%) (Fredriksson and Pettersson, 1978). Statistical analysis using all patients revealed a very weak correlation between % DC-HIL+ MDSCs/PBMCs or % MDSCs/PBMCs with PASI scores (Spearman r = 0.13 or 0.11; and p=0.35 or 0.31, respectively, Figure 2a). We then sorted patients into untreated and treated groups, with variation in MDSC expansion analyzed (Figure 2b). Blood DC-HIL+ MDSC (or total MDSC) levels in the untreated group were higher than the treated group (median of 8.2% vs. 3.3% for % MDSCs/PBMCs, p=0.0076; and 5.9% vs. 1.9% for % DC-HIL+ MDSCs/PBMCs, p=0.0053), but fluctuate within a considerable range. There was no significant difference in PASI % between these two groups (p=0.87). Thus, there was no statistical correlation of DC-HIL+ MDSC expansion with disease activity, whereas treated psoriatic patients had decreased levels of DC-HIL+ MDSCs.
Figure 2. Expansion of DC-HIL+ MDSCs in untreated vs. treated patients.
(a) Correlations between % DC-HIL+ MDSCs/PBMCs (or % MDSC/PBMC) and % PASI in all patients are analyzed using Spearman’s coefficient r. (b) % MDSCs in PBMCs, % DC-HIL+ MDSCs in PBMCs or % PASI is compared between untreated (open circles) and treated patients (closed circles); median and range and statistical p values are shown.
Psoriatic DC-HIL+ MDSCs are T-cell suppressors, but less potent than melanoma-MDSCs
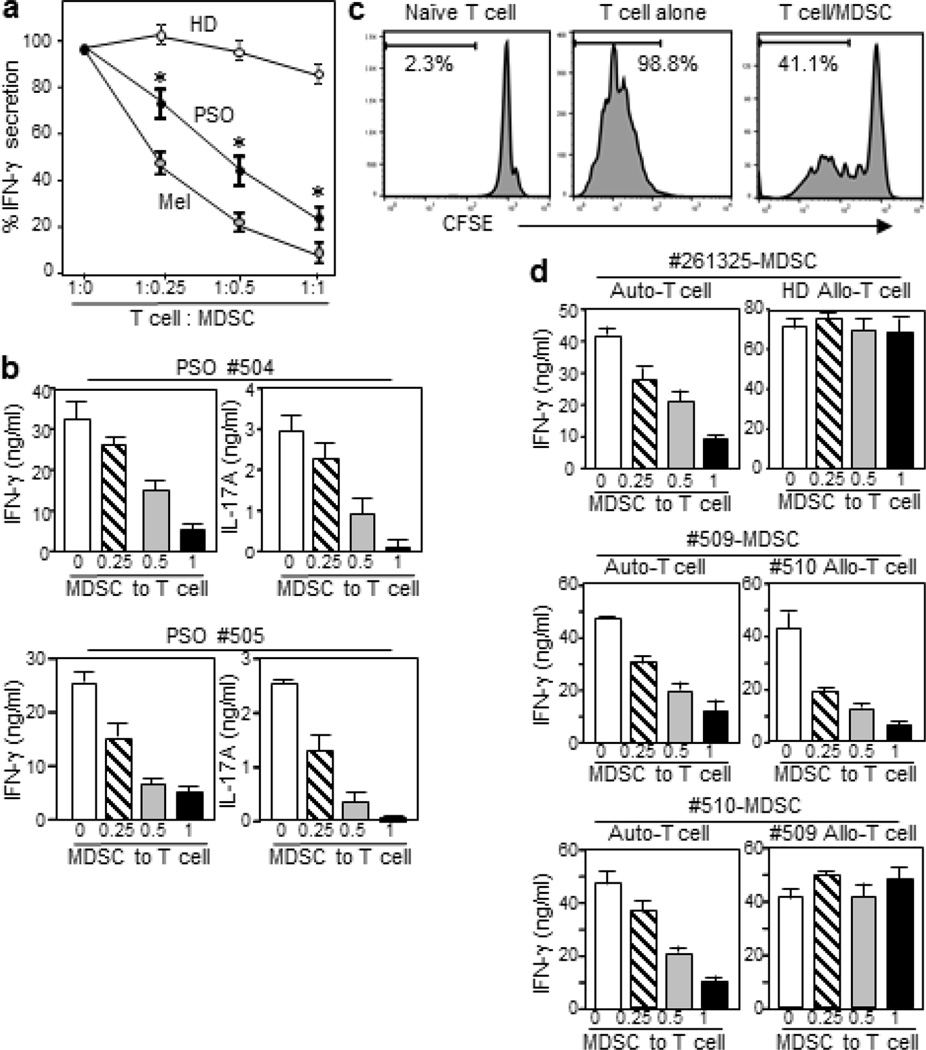
We examined the ability of psoriatic MDSCs to suppress activation of autologous T-cells (Figure 3a). MDSCs and T-cells were isolated from PBMCs of the same patient or healthy donor. As positive control, MDSCs from stage III melanoma patients were also examined. T-cells were co-cultured with increasing doses of purified MDSCs. Their activation was triggered by anti-CD2/CD3/CD28 co-stimulation, and IFN-γ response was measured. Whereas MDSCs from healthy donors exhibited a marginal effect on IFN-γ response, psoriatic MDSCs inhibited T-cell activation in a dose-dependent manner, with 70–80% decline at highest dose (1:1 cell ratio of MDSC: T-cell). However, this inhibition was weaker than that seen for MDSCs from melanoma patients, which suppressed T-cell activation stronger than psoriatic MDSCs at every dose point in cocultures. This outcome was also true for 2 other pairs of psoriasis and melanoma patients. Psoriatic MDSCs also suppressed proliferation and IL-17 response of autologous T-cells in a dose-dependent manner (Figures 3b and 3c).
Figure 3. Functional characterization of psoriatic MDSCs.
(a) CD14+HLA-DRneg MDSCs isolated from PSO, HD, or melanoma patient (Mel) were cocultured with autologous T-cells at varying cell ratios, with anti-CD2/CD3/CD28 Ab. IFN-γ secretion was measured (mean ± sd, n=3). Cultures of T-cell alone were 98 ± 7 ng/ml for HD, 45 ± 2.7 for PSO, and 58 ± 4.3 for Mel. Data shown are representative of 3 experiments using different patients. (b) In same assays but with 2 different patients, IFN-γ and IL-17A secretion in cocultures were measured. (c) Proliferation in cultures of T-cells alone and in 1:1 cocultures with MDSCs was analyzed by CFSE-dilution assay. (d) MDSCs from 3 different patients were cocultured with autologous or allogeneic T-cells isolated from HD or PSO, at varying cell ratios, and IFN-γ response was measured. *p<0.001.
Psoriatic MDSCs are heterogeneous in their suppressive mechanisms
We next characterized T cell-suppressor mechanisms of psoriatic MDSCs. To examine the influence of T-cells to MDSC effects, psoriatic MDSCs were allowed to suppress autologous or allogeneic T-cells isolated from a healthy donor. They suppressed IFN-γ response of autologous T-cells, but had no effect on allogeneic T-cells (n=3, Figure 3d). We then questioned whether psoriatic T-cells are susceptible to allogeneic MDSCs’ suppressor activity. In 2 patients, purified MDSCs and T-cells were switched and cocultured. MDSCs from both patients were potent suppressors of autologous T-cells, whereas these 2 patients’ MDSCs differed in their effects on allogeneic T-cells: MDSCs from patient #509 were equally potent for allogeneic/psoriatic T-cells, while those from patient #510 were not. We performed the same crisscross experiments using 4 additional pairs, and data showed clear-cut results that 10 patients (in total) were clearly sorted into the two types; MDSC from 30% of patients were insensitive to psoriatic allo-T cells, whereas 70% sensitive to allo-T cells (Supplementary Figure S3).
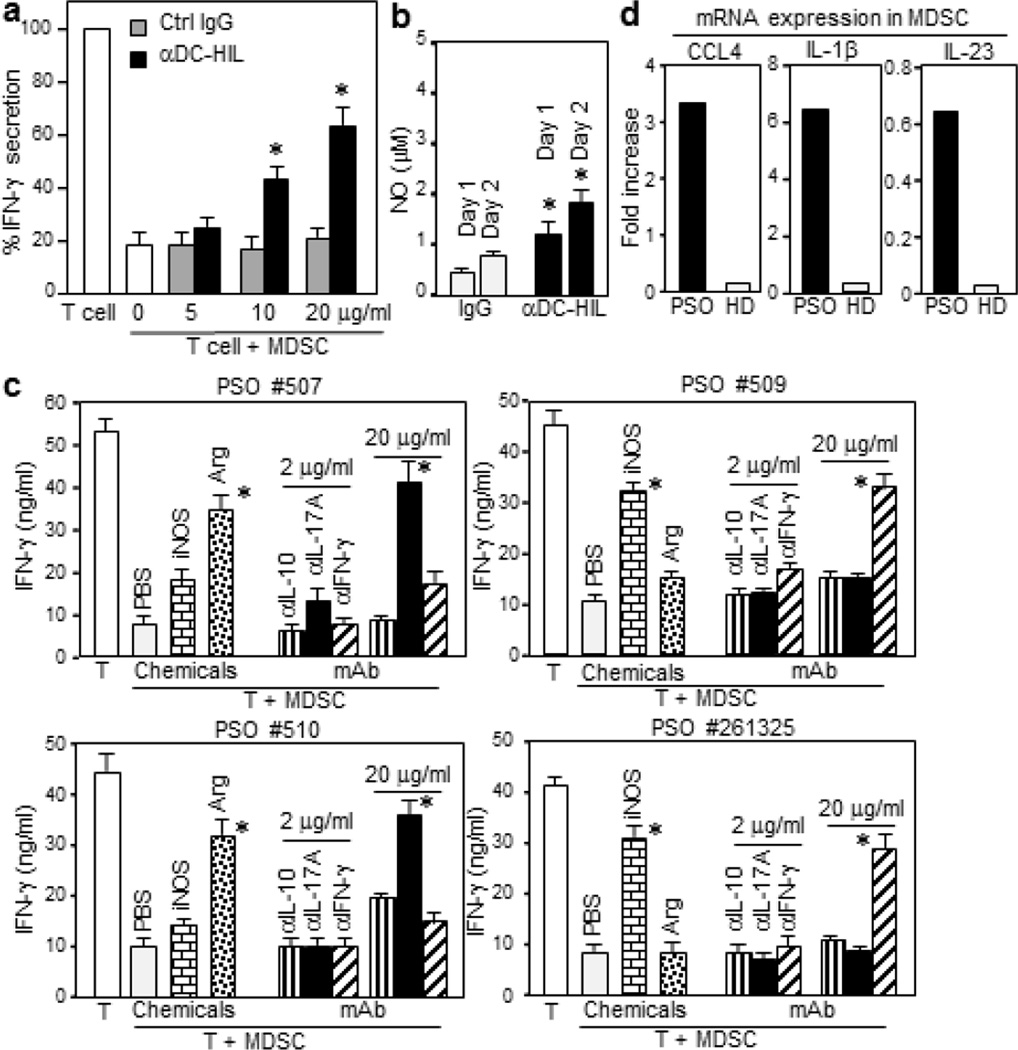
To examine whether DC-HIL mediates the suppressor function of psoriatic MDSCs, anti-DC-HIL mAb or control IgG was added to cocultures of MDSCs/T-cells (Figure 4a). In a dose-dependent manner, anti-DC-HIL mAb (but not control IgG) incompletely restored T-cell IFN-γ response (up to 63%) that was suppressed by MDSCs. This moderate effect by the mAb was not consistent with data of similar assays using melanoma-derived MDSCs, in which the same mAb reversed the suppressor activity almost completely (Turrentine et al., 2014). DC-HIL-triggered upregulation of nitric oxide (NO) in psoriatic MDSCs showed DC-HIL to be functional (Figure 4b): MDSC of healthy controls did not produce NO (≤0.003 µM) even after stimulation with anti-DC-HIL mAb. To probe mechanisms other than DC-HIL, we tested the effect of inhibitors to varying mediators on MDSC function (Figure 4c). The suppressor function of MDSCs from patients (#507 and #510) was reversed markedly by an arginase I-inhibitor and anti-IL-17A Ab at 20 µg/ml, but not by an iNOS-inhibitor, anti-IL-10 Ab, nor anti-IFN-γ Ab. By contrast, MDSCs from 2 other patients (#509 and #261325) had a completely opposite pattern: reversal by an iNOS-inhibitor and by anti-IFN-γ Ab at 20 µg/ml, but no effects by others. Our finding of CCL4, IL-1β and IL-23 gene expression in psoriatic MDSCs (Figure 4d) suggest involvement of Th17 in MDSC function. Thus psoriatic MDSCs employ heterogeneous mechanisms in exerting their suppressor function.
Figure 4. Mechanisms for the T-cell suppressor function of psoriatic MDSCs.
(a) Increasing doses of anti-DC-HIL mAb or control IgG were added to the coculture of MDSC/T-cell (1:1 cell ratio). IFN-γ amount is expressed as % relative to the T-cell alone culture (48 ng/ml). (b) Psoriatic MDSCs were cultured with immobilized anti-DC-HIL mAb or control IgG. At indicated days after culturing, cells were measured for the intracellular NO levels (mean ± sd, n=3). A second patient showed similar results. (c) The cocultures of 4 different patients were added with an inhibitor to iNOS or arginase I (Arg), or anti-cytokine Ab at 2 or 20 µg/ml. (d) MDSCs isolated from PSO or HD (a pool of 3 patients) were assayed for mRNA expression of CCL4, IL-1β, and IL-23. *p<0.001.
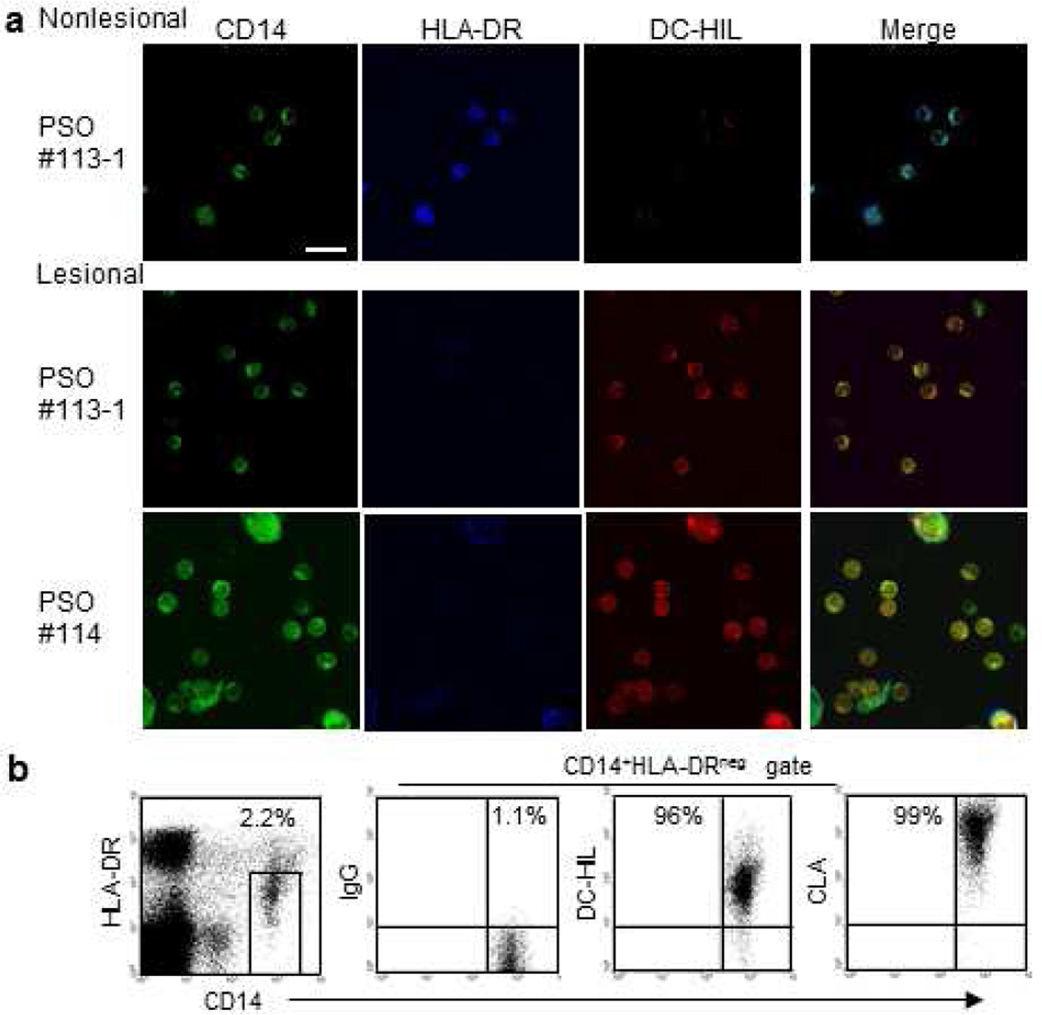
MDSCs are present among leukocytic infiltrates in lesional psoriatic skin
Because of technical difficulties to identify CD14+HLA-DRno/low MDSCs in immunofluorescent staining of skin specimens, we chose an explant culture, in which skin punch biopsies from nonlesional or lesional skin of patients (who were treated with topical corticosteroids) were cultured with GM-CSF and IL-4, which protect myeloid cells from apoptotic death in culture (Cheng et al., 2008) (Figure 5a). Two weeks after culture, emigrant cells from skin were harvested and examined for expression of CD14, HLA-DR, and DC-HIL. Most cells from nonlesional or lesional skin were CD14+ (~80%, detailed data are summarized in Supplementary Table S1). Cells from nonlesional skin co-expressed HLA-DRhi and DC-HILlow (80–90%, which are likely antigen-presenting cells), whereas cells from lesional skin were HLA-DRneg and DC-HILhi. These data suggest that monocytic MDSCs are recruited into lesional (but not nonlesional) skin. Skin-infiltration was supported by high expression of the skin-homing receptor cutaneous lymphocyte antigen (CLA) (Fuhlbrigge et al., 1997) on psoriatic MDSCs (Figure 5b).
Figure 5. DC-HIL-expressing MDSCs infiltrate in lesional skin of psoriatic patients.
(a) Punch biopsies from the nonlesional or lesional skin of same psoriasis patient (PSO #113-1 or #114) were explant cultured with GM-CSF/IL-4. Skin-emigrated cells were fluorescently stained for expression of CD14 (shown in green), HLA-DR (blue), and DC-HIL (red), and examined under confocal microscope: 100× magnification, scale bar = 1 µm. (b) CD14+HLA-DRno/low MDSCs in PBMCs of a psoriasis patient were gated in the dot-plot of HLA-DR vs. CD14 and examined by flow-cytometry for expression of CD14 vs. DC-HIL or CLA. Data shown are representative of four patients.
Discussion
Co-stimulatory signals are required for the full activation of T-cells and maintenance of effector T-cells, and also trigger expression of co-inhibitory receptors on T-cells and their ligands on antigen-presenting cells and keratinocytes. Thus the pro-inflammatory environment in psoriatic skin likely induces and activates co-inhibitory pathways. Persistent hyper-activation of T-cells in psoriasis could be due to repetitive and prolonged co-stimulation that overwhelms the inhibitory effects of co-inhibitors working correctly or due to a functional deficiency of co-inhibitors that permit T-cells to continue as effector cells. We addressed this issue through studying the highly potent suppressor function of MDSCs. We found that MDSCs proliferated in blood of psoriatic patients to a degree greater than in melanoma patients, and that these MDSCs infiltrated psoriatic lesional skin. Most of the expanded MDSCs expressed DC-HIL and were T-cell suppressors. However, psoriatic MDSCs differ from melanoma MDSCs in 4 aspects; first, psoriatic DC-HIL+ MDSCs do not further proliferate with increasing disease severity, unlike those in melanoma that increase in proportion to cancer stages (Turrentine et al., 2014); second, psoriatic DC-HIL+ MDCSs are less potent in suppressing T-cell activation compared to melanoma MDSCs; third, their suppressor function is less dependent on DC-HIL; and fourth, they are divergent in the use of inhibitory mediators among patients.
There are a few reports showing involvement of other co-inhibitors in the pathogenesis of psoriasis. Blood-circulating T-cells expressing CTLA-4 or PD-1 in psoriatic patients were increased significantly compared to controls, but the levels were not as high as in in vitro-activated T-cells from healthy controls (Ferenczi et al., 2000; Niu et al., 2015): Unfortunately, the functional properties were not studied. Th17 and Th1 cells in psoriasis did not efficiently induce the co-inhibitor Tim-3 upon in vitro activation, compared to those from healthy donors (Kanai et al., 2012). Lastly, significant associations of polymorphisms in CTLA-4 gene with susceptibility to psoriasis were found in Japanese and Polish Caucasian patients (Luszczek et al., 2008; Muto et al., 2011). Consistent with our findings, these reports support the possibility that deficient co-inhibitory function contributes to the etiology of psoriasis.
Like MDSCs, regulatory T-cells (Tregs) are also highly potent suppressors of T-cell function and critically involved in development of autoimmune diseases as well as cancers. The Foxp3+ Treg population was increased in circulation and lesional skin of psoriatic patients and positively correlates with disease severity (PASI) (Zhang et al., 2010). Surprisingly, psoriatic Tregs were incapable of suppressing IL-17 secretion by CD4+ T cells, although these cells inhibit proliferation and IFN-γ secretion by CD4+ T cells (Zhang et al., 2010). By contrast, other reports showed no statistical difference in circulating or skin-infiltrating Tregs between psoriasis patients and healthy controls (Bovenschen et al., 2006; Shehata and Elghandour, 2007). Unlike Tregs, DC-HIL+ MDSCs were markedly increased in psoriatic patients (very low levels in healthy controls). We also found that psoriatic MDSCs were potent suppressors of both IFN-γ and IL-17 responses. Since MDSCs can expand Tregs (Serafini et al., 2008), MDSCs may be more important than Tregs in regulating psoriatic inflammation.
It is well-established that cancer-induced MDSCs play a pathogenic role by blunting T-cell function. What about the role of expanded/activated MDSCs in psoriatic inflammation? In an EAE mouse model, we showed that DC-HIL gene-disrupted mice developed hyper-activated Th1 and Th17 responses and exacerbated autoimmune disease following immunization with MOG peptide (Chung et al., 2014a). Such worsened disease was alleviated by adoptive transfer of DC-HIL+ MDSCs isolated from EAE-affected WT mice. Similar results were found in a psoriatic mouse model (unpublished data), in which psoriasis-like acanthosis was induced by intradermal injection of IL-23 (Chan et al., 2006). These studies indicate that DC-HIL+ MDSCs protect hosts from these forms of inflammation. In contrast to the mouse model, there was no significant association of DC-HIL+ MDSC levels with disease severity (PASI), nor with serum IL-17A and IFN-γ levels (Supplementary Table S2), cytokines that positively correlate with severity (Michalak-Stoma et al., 2013). On the other hand, we found that psoriatic DC-HIL+ MDSCs exhibit significantly reduced suppressor capacity, compared to their melanoma counterparts. However, interpretation of these data may be limited because melanoma-MDSCs may be an inappropriate reference. Further studies are required to define the role of expanded MDSCs in psoriasis.
We found that psoriatic MDSCs are heterogeneous in their suppressor mechanisms in at least 2 ways: First, regarding suppression of allogeneic T-cell response, all psoriatic MDSCs tested (~30 patients) exhibited suppressor effects to autologous T-cells, whereas they are unable to suppress allogeneic T-cells from healthy donors (n=3). Moreover, MDSCs from some patients suppressed allogeneic/psoriatic T-cell response, but others did not. This difference may be ascribed to T-cells that may have been primed for susceptibility to MDSC-suppression. Secondly, in 4 patients, we found heterogeneity: MDSCs of some patients employed IL-17 and arginase I. In these cases, IL-17 may upregulate arginase I expression in MDSCs (He et al., 2010; Tran le et al., 2015). By contrast, MDSCs from other patients employed the IFN-γ/iNOS axis (Faure et al., 1999). It is unlikely that IL-17 is involved in selecting between the 2 mechanisms because all patients produced similar levels of serum IL-17A (11.6 to 25.3 pg/ml). Rather, the differences may be unique to the individual patient. It would be good to know which cytokines (or soluble factors) govern the selection of critical mediators and whether the precise mediators relate to psoriasis severity. Lack of commonly shared inhibitory mediators among psoriatic patients suggests diversity in the pathogenesis of this inflammatory disorder.
Despite elevated expression of DC-HIL and the DC-HIL ligand SD-4 on MDSC and T-cells, respectively, DC-HIL was not critical to the inhibitory mechanisms. This less critical role of MDSC in psoriasis (vs. in melanoma) may be explained by psoriatic T-cells expressing high-levels of IL-17 and/or IFN-γ, which are cytokines that can induce NO production (Miljkovic et al, 2004; Faure et al, 1999). Such induction may compromise the critical role of the DC-HIL pathway since we assume that its T cell-inhibitory effect is achieved primarily through NO and ROS production and more potent than SD-4-signaling (Chung et al, 2014b).
In sum, we found that blood DC-HIL+ monocytic MDSCs are expanded in psoriasis and that they infiltrated lesional skin. While DC-HIL expression did not correlate significantly with disease severity, MDSC-mediated immunosuppression contributed to regulating hyperactivated T-cells in psoriatic skin.
Materials and Methods
Subjects
We performed a cross-sectional study of 49 psoriasis patients and 21 healthy controls without inflammatory skin disease. Study protocols were approved by the institutional review boards of UT Southwestern Medical Center and Parkland Hospital, Dallas, TX. Inclusion criteria included: psoriasis patients or healthy controls >18 years of age; able to give written informed consent; able to give blood and/or skin samples. Exclusion criteria included patients on SC/IV systemic immunosuppressant medications. Patients were clinically evaluated for psoriasis subtype (plaque, majority of lesions plaque; guttate, majority of lesions raindrops; inverse, only involving intertriginous areas; pustular, majority of lesions erythematous patches with pustules; palmo-plantar, majority of lesions on palms and soles) and PASI; completed a clinical questionnaire about demographics, current and past psoriasis treatments (Table 1). From 2 patients, two 4 mm punch skin biopsies (from lesional and nonlesional skin) were procured. Melanoma patients (n=3) were diagnosed with cancer stage III.
Flow-cytometry
Blood was collected into tubes with sodium citrate (BD Vacutainer SST, BD Biosciences). PBMCs (5 × 105 cells) isolated from the blood samples were treated with human IgG (1 µg/ml for blocking) and incubated with 10 µg/ml 3D5 mouse anti-human DC-HIL mAb (Chung et al., 2009) or isotypic control mouse IgG and 1 µg/ml PE-anti-mouse IgG [F(ab´)2 fragment]. After washing, cells were further stained with APC-conjugated anti-HLA-DR and FITC-anti-CD14 Ab (each 2.5 µg/ml), and analyzed by flow-cytometry. Ab were purchased from eBiosciences (San Diego, CA) or Jackson ImmunoResearch (West Grove, PA).
T-cell suppression assays
CD14+HLA-DRneg and T-cells were freshly isolated from blood samples (20–30 ml) of the same donor. For crisscross experiments to switch MDSC and T-cells, 2 untreated patients were recruited on the same day. PBMCs were depleted of HLA-DR+ cells using biotinylated anti-HLA-DR Ab and anti-biotin microbeads (Miltenyi Biotec). The pass-through fraction was sorted into the CD14+ and CD14neg subfractions using anti-CD14 Ab-beads (Miltenyi); the former was used as MDSCs (containing >95% CD14+HLA-DRneg cells, Supplementary Figure S1) and the latter as T-cell preparations (~90% CD3+ cells). Autologous or allogeneic T-cells (5 × 104 cells/well) were cocultured with MDSCs at varying cell ratios with anti-CD2/CD3/CD28 beads (Miltenyi) (1.5 beads per T-cell) in microculture wells (triplicate) for 5 days. This assay was repeated twice with different individuals (all patients displayed >80% DC-HIL+ cells in total MDSCs; and all healthy donors with ~5%). T-cell proliferation was measured by CFSE-dilution assay. For inhibition studies, varying inhibitors were added to the same culture (1:1 cell ratio), including anti-DC-HIL mAb; anti-cytokine Ab; 0.5 mM N6-(1-iminoethyl)-L-lysine (to iNOS); 1 mM N-hydroxyl-nor-arginine (Arginase I). IFN-γ and/or IL-17A amount in the culture was determined by ELISA, and suppressive activity assessed by cytokine amount (%) relative to culture without suppressor cells. Most data shown were performed with blood of untreated patients.
Quantitative RT-PCR
PCR was performed (Chung et al., 2014b) using primers for CCL4, 5'-TCCTCGCAACTTTGTGGTAG-3' and 5'-TTCAGTTCCAGGTCATACACG-3'; for IL-1β, 5'-TCTACACCAATGCCCAACTC-3' and 5'-AAGTGAGTAGGAGAGGTGAGAG-3', and for IL-23, 5'-ATGTTCCCCATATCCAGTGTG-3' and 5'-GCTCCCCTGTGAAAATATCCG-3'. mRNA expression was shown as the expression level relative to GAPDH gene.
NO assay
Purified MDSCs (5 × 106) were cultured in microculture wells precoated with 3D5 mAb or control IgG (10 µg/ml). After 1 or 2 days of culture, cells were measured for NO production using Griess method (De Santo et al., 2005).
Skin explant cultures
For isolation of monocytes from skin, we modified a previously reported explant culture method (Clark et al., 2006) by replacing cytokines for T-cells with those for monocytes. Lesional and nonlesional skin punch biopsies from same patient were ex vivo cultured with GM-CSF and IL-4 (each 10 ng/ml) for 10–14 days with refeeding cytokines every 4 days, and skin-emigrated cells were harvested and cytospun onto slide glasses, followed by immunofluorescent staining with anti-DC-HIL mAb plus Alexa546-anti-mouse IgG, Alexa350-anti-HLA-DR, and Alexa488-anti-CD14 Ab (each 10 µg/ml). Staining was analyzed under a TCS-SP1 laser scanning confocal microscope (Leica Micro-systems, Bannockburn, IL).
Statistical analysis
Statistical analyses were performed using Mann Whitney U test or Spearman’s correlation; and student’s t test for evaluation of in vitro assays.
Supplementary Material
Acknowledgments
We thank Irene Dougherty and Lin-Chiang Tseng for technical and Therona Ramos for administrative assistance. This research was supported by National Institute of Health grant (AI064927-05).
Abbreviations
- MDSCs
myeloid-derived suppressor cells
- NO
nitric oxide
- SD-4
syndecan-4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors state no conflict of interest.
References
- Abrams JR, Lebwohl MG, Guzzo CA, Jegasothy BV, Goldfarb MT, Goffe BS, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. The Journal of clinical investigation. 1999;103:1243–1252. doi: 10.1172/JCI5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovenschen HJ, van Vlijmen-Willems IM, van de Kerkhof PC, van Erp PE. Identification of lesional CD4+ CD25+ Foxp3+ regulatory T cells in Psoriasis. Dermatology. 2006;213:111–117. doi: 10.1159/000093849. [DOI] [PubMed] [Google Scholar]
- Briones J, Novelli S, Sierra J. T-cell costimulatory molecules in acute-graft-versus host disease: therapeutic implications. Bone Marrow Research. 2011;2011:1–7. doi: 10.1155/2011/976793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, et al. L-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203:2577–2587. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzigeorgiou A, Lyberi M, Chatzilymperis G, Nezos A, Kamper E. CD40/CD40L signaling and its implication in health and disease. Biofactors. 2009;35:474–483. doi: 10.1002/biof.62. [DOI] [PubMed] [Google Scholar]
- Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JS, Bonkobara M, Tomihari M, Cruz PD, Jr, Ariizumi K. The DC-HIL/syndecan-4 pathway inhibits human allogeneic T-cell responses. Eur J Immunol. 2009;39:965–974. doi: 10.1002/eji.200838990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JS, Dougherty I, Cruz PD, Jr, Ariizumi K. Syndecan-4 mediates the coinhibitory function of DC-HIL on T cell activation. Journal of immunology (Baltimore, Md : 1950) 2007a;179:5778–5784. doi: 10.4049/jimmunol.179.9.5778. [DOI] [PubMed] [Google Scholar]
- Chung JS, Sato K, Dougherty II, Cruz PD, Jr, Ariizumi K. DC-HIL is a negative regulator of T lymphocyte activation. Blood. 2007b;109:4320–4327. doi: 10.1182/blood-2006-11-053769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JS, Tamura K, Akiyoshi H, Cruz PD, Jr, Ariizumi K. The DC-HIL/syndecan-4 pathway regulates autoimmune responses through myeloid-derived suppressor cells. Journal of immunology (Baltimore, Md : 1950) 2014a;192:2576–2584. doi: 10.4049/jimmunol.1301857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JS, Tamura K, Cruz PD, Jr, Ariizumi K. DC-HIL-expressing myelomonocytic cells are critical promoters of melanoma growth. J Invest Dermatol. 2014b;134:2784–2794. doi: 10.1038/jid.2014.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JS, Tomihari M, Tamura K, Kojima T, Cruz PD, Jr, Ariizumi K. The DC-HIL ligand syndecan-4 is a negative regulator of T-cell allo-reactivity responsible for graft-versus-host disease. Immunology. 2013;138:173–182. doi: 10.1111/imm.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, et al. The vast majority of CLA+ T cells are resident in normal skin. Journal of immunology (Baltimore, Md : 1950) 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- Cripps JG, Gorham JD. MDSC in autoimmunity. Int Immunopharmacol. 2011;11:789–793. doi: 10.1016/j.intimp.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moreno C, Scumpia PO, Laface DM, et al. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol Med. 2011;17:281–292. doi: 10.2119/molmed.2010.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santo C, Serafini P, Marigo I, Dolcetti L, Bolla M, Del Soldato P, et al. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4185–4190. doi: 10.1073/pnas.0409783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129:1339–1350. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- du Plessis N, Loebenberg L, Kriel M, von Groote-Bidlingmaier F, Ribechini E, Loxton AG, et al. Increased Frequency of Myeloid-derived Suppressor Cells during Active Tuberculosis and after Recent Mycobacterium tuberculosis Infection Suppresses T-Cell Function. Am J Respir Crit Care Med. 2013;188:724–732. doi: 10.1164/rccm.201302-0249OC. [DOI] [PubMed] [Google Scholar]
- Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- Faure V, Hecquet C, Courtois Y, Goureau O. Role of interferon regulatory factor-1 and mitogen-activated protein kinase pathways in the induction of nitric oxide synthase-2 in retinal pigmented epithelial cells. The Journal of biological chemistry. 1999;274:4794–4800. doi: 10.1074/jbc.274.8.4794. [DOI] [PubMed] [Google Scholar]
- Ferenczi K, Burack L, Pope M, Krueger JG, Austin LM. CD69, HLA-DR and the IL-2R identify persistently activated T cells in psoriasis vulgaris lesional skin: blood and skin comparisons by flow cytometry. J Autoimmun. 2000;14:63–78. doi: 10.1006/jaut.1999.0343. [DOI] [PubMed] [Google Scholar]
- Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson T, Pettersson U. Severe psoriasis--oral therapy with a new retinoid. Dermatologica. 1978;157:238–244. doi: 10.1159/000250839. [DOI] [PubMed] [Google Scholar]
- Frey AB. Myeloid suppressor cells regulate the adaptive immune response to cancer. The Journal of clinical investigation. 2006;116:2587–2590. doi: 10.1172/JCI29906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature. 1997;389:978–981. doi: 10.1038/40166. [DOI] [PubMed] [Google Scholar]
- Fujii W, Ashihara E, Hirai H, Nagahara H, Kajitani N, Fujioka K, et al. Myeloid-derived suppressor cells play crucial roles in the regulation of mouse collagen-induced arthritis. J Immunol. 2013;191:1073–1081. doi: 10.4049/jimmunol.1203535. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature reviews Immunology. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D, Li H, Yusuf N, Elmets CA, Li J, Mountz JD, et al. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. Journal of immunology (Baltimore, Md : 1950) 2010;184:2281–2288. doi: 10.4049/jimmunol.0902574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii N, Takahashi T, Soroosh P, Sugamura K. OX40-OX40 ligand interaction in T-cell-mediated immunity and immunopathology. Adv Immunol. 2010;105:63–98. doi: 10.1016/S0065-2776(10)05003-0. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Satoh T, Igawa K, Yokozeki H. Impaired expression of Tim-3 on Th17 and Th1 cells in psoriasis. Acta Derm Venereol. 2012;92:367–371. doi: 10.2340/00015555-1285. [DOI] [PubMed] [Google Scholar]
- Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of psoriasis. Annual review of immunology. 2014;32:227–255. doi: 10.1146/annurev-immunol-032713-120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luszczek W, Majorczyk E, Nockowski P, Plucinski P, Jasek M, Nowak I, et al. Distribution of the CTLA-4 single nucleotide polymorphisms CT60G>A and +49A>G in psoriasis vulgaris patients and control individuals from a Polish Caucasian population. Int J Immunogenet. 2008;35:51–55. doi: 10.1111/j.1744-313X.2007.00736.x. [DOI] [PubMed] [Google Scholar]
- Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. The Journal of clinical investigation. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhaba R, Vitacolonna M, Hildebrand D, Baniyash M, Freyschmidt-Paul P, Zoller M. The importance of myeloid-derived suppressor cells in the regulation of autoimmune effector cells by a chronic contact eczema. J Immunol. 2007;179:5071–5081. doi: 10.4049/jimmunol.179.8.5071. [DOI] [PubMed] [Google Scholar]
- Michalak-Stoma A, Bartosinska J, Kowal M, Juszkiewicz-Borowiec M, Gerkowicz A, Chodorowska G. Serum levels of selected Th17 and Th22 cytokines in psoriatic patients. Dis Markers. 2013;35:625–631. doi: 10.1155/2013/856056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto M, Deguchi H, Tanaka A, Inoue T, Ichimiya M. Association between T-lymphocyte regulatory gene CTLA4 single nucleotide polymorphism at position 49 in exon 1 and HLA-DRB1*08 in Japanese patients with psoriasis vulgaris. J Dermatol Sci. 2011;62:70–71. doi: 10.1016/j.jdermsci.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Niu J, Song Z, Yang X, Zhai Z, Zhong H, Hao F. Increased circulating follicular helper T cells and activated B cells correlate with disease severity in patients with psoriasis. J Eur Acad Dermatol Venereol. 2015 doi: 10.1111/jdv.13027. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri SP, Kundu-Raychaudhuri S, Tamura K, Masunaga T, Kubo K, Hanaoka K, et al. FR255734, a humanized, Fc-Silent, Anti-CD28 antibody, improves psoriasis in the SCID mouse-psoriasis xenograft model. J Invest Dermatol. 2008;128:1969–1976. doi: 10.1038/jid.2008.38. [DOI] [PubMed] [Google Scholar]
- Rivas Bejarano JJ, Valdecantos WC. Psoriasis as autoinflammatory disease. Dermatol Clin. 2013;31:445–460. doi: 10.1016/j.det.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer research. 2008;68:5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata IH, Elghandour TM. A possible pathogenic role of CD4+CD25+ T-regulatory cells in psoriasis. Egypt J Immunol. 2007;14:21–31. [PubMed] [Google Scholar]
- Singh V, Mueller U, Freyschmidt-Paul P, Zoller M. Delayed type hypersensitivity-induced myeloid-derived suppressor cells regulate autoreactive T cells. Eur J Immunol. 2011;41:2871–2882. doi: 10.1002/eji.201141696. [DOI] [PubMed] [Google Scholar]
- Tran le S, Mittal D, Mattarollo SR, Frazer IH. Interleukin-17A Promotes Arginase-1 Production and 2,4-Dinitrochlorobenzene-Induced Acute Hyperinflammation in Human Papillomavirus E7 Oncoprotein-Expressing Skin. J Innate Immun. 2015;7:392–404. doi: 10.1159/000374115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrentine J, Chung JS, Nezafati K, Tamura K, Harker-Murray A, Huth J, et al. DC-HIL+ CD14+ HLA-DRno/low cells are a potential blood marker and therapeutic target for melanoma. J Invest Dermatol. 2014;134:2839–2842. doi: 10.1038/jid.2014.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Chen L. Co-signaling molecules of the B7-CD28 family in positive and negative regulation of T lymphocyte responses. Microbes Infect. 2004;6:759–766. doi: 10.1016/j.micinf.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40:2969–2975. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yang XQ, Cheng J, Hui RS, Gao TW. Increased Th17 cells are accompanied by FoxP3(+) Treg cell accumulation and correlated with psoriasis disease severity. Clin Immunol. 2010;135:108–117. doi: 10.1016/j.clim.2009.11.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.