Abstract
Huntington's disease (HD) is a genetic neurological disorder that causes severe and progressive motor, cognitive, psychiatric, and metabolic symptoms. There is a robust, significant elevation in circulating levels of the stress hormone, cortisol, in HD patients; however the causes and consequences of this elevation are largely uncharacterized. Here, we evaluated whether elevated levels of corticosterone, the rodent homolog of cortisol, contributed to the development of symptomology in transgenic HD mice. Wild-type (WT) and transgenic R6/2 mice were given either: 1) adrenalectomy with WT level corticosterone replacement (10ng/ml), 2) adrenalectomy with high HD-level corticosterone replacement (60ng/ml), or 3) sham surgery without replacement. R6/2 mice on HD-level replacement showed severe and rapid weight loss (p<.05) and a shorter latency to death (p<.01) relative to the HD mice on WT-level replacement. We further evaluated basal and stress-induced levels of circulating corticosterone in R6/2 mice throughout the course of their life. We found that R6/2 transgenic HD mice display a spontaneous elevation in circulating corticosterone levels that became significant at 10 weeks of age. Furthermore, we identified significant dysregulation of circadian rhythmicity of corticosterone release measured over a 24 hour period compared to wildtype controls. Unexpectedly, we found that R6/2 transgenic mice show a blunted corticosterone response to restraint stress, compared to wild-type mice. Together, this data provides further evidence that HPA-axis activity is abnormal in R6/2 mice, and highlights the important role that cortisol plays in HD symptom development. Our findings suggest that cortisol-reducing therapeutics may be of value in improving HD patient quality of life.
Keywords: Huntington's disease, cortisol, corticosterone, R6/2 transgenic mice, HPA axis, circadian dysfunction
Introduction
Huntington's disease (HD) is an autosomal dominantly inherited neurological disorder that is caused by an expansion in the CAG region of the human HTT gene (Huntington Disease Collaborative Research Group, 1993). This expansion confers a toxic gain of function on the encoded protein, mutant huntingtin (mHTT). mHTT is expressed ubiquitously, but in particularly high levels in the brain, leading to widespread neurodegeneration. The striatum, cortex, hippocampus and hypothalamus are particularly affected and show inclusion body formation, cell loss, and gliosis (Gourfinkel-An et al., 1998; Heinsen et al., 1994; Vonsattel et al., 1985). The hallmark symptom of HD is chorea, with patients showing uncontrollable hyperkinetic movements. However, patients also show particularly severe psychiatric, cognitive, and metabolic symptoms (Anderson and Marder, 2001 ; Lawrence et al., 1996; Tabrizi et al., 2009). Psychiatric symptoms are particularly prevalent, including high rates of depression and anxiety (Anderson et al., 2001; Levy et al., 1998; Pflanz et al., 1991). Cognitive symptoms include deficits in memory, executive function, and impulse control (Lawrence et al., 1996; Tabrizi et al., 2009). Patients also show metabolic symptoms, including severe weight loss and diabetes (Aziz et al., 2008; Petersen and Bjorkqvist, 2006; Trejo et al., 2004; van der Burg et al., 2009). HD typically onsets in midlife, and symptoms become increasingly severe over 10-15 years, ultimately leading to death.
HD patients show a two-fold increase in circulating basal levels of the glucocorticoid, cortisol (Heuser et al., 1991; Saleh et al., 2009). Likewise, R6/2 transgenic HD mice show a three-fold elevation in corticosterone, the rodent homolog of cortisol (both now abbreviated CORT), and these levels increase in parallel with HD symptom progression (Bjorkqvist et al., 2006). R6/2 mice also show hypertrophy of the adrenal gland, consistent with HPA-axis hyperactivity (Bjorkqvist et al., 2006). However, the consequences of elevated CORT on HD symptoms, as well as the mechanisms that underlie it, are largely unexplored. Because chronic high levels of circulating CORT in the non-HD population leads to Cushing's disease, a disorder characterized by neuropathological changes and cognitive, psychiatric and metabolic disturbance (Orth, 1995; Valassi et al., 2012), we postulated that elevated CORT in HD may be exacerbating HD symptom development and/or accelerating disease progression. There is recent empirical support that elevated CORT can exacerbate HD symptoms; Mo and colleagues (2014) showed that increasing CORT levels in R6/1 transgenic mice, a model wherein CORT is not naturally elevated, exacerbates cognitive deficits and reduces hippocampal neurogenesis (Mo et al., 2014a). Additionally, female R6/1 mice, show an aberrant, persistent elevation in CORT following restraint stress (Du et al., 2012). Thus, in the current study, we investigated the role of chronic, elevated CORT in metabolic symptom progression (weight loss), as well as broad HD symptom progression (motor dysfunction and lifespan) in the R6/2 mouse model, which more accurately mirrors the spontaneous elevation in CORT shown by HD patients. Additionally, we characterized the circadian pattern of plasma CORT release, as well as the stress induced CORT response, in the R6/2 transgenic mouse model of HD.
Materials and Methods
Animals
All animals were group housed with littermates (3-5 mice per cage) under controlled conditions of temperature and light (12 hour light/dark cycle). Food and water were provided ad libitum. R6/2 mice (stock # 002810, carrying 160±5 cag repeats) were obtained from Jackson Laboratories (Bar Harbor, ME) and bred in the vivarium at the ONPRC. Wild-type males were mated with ovary transplanted wild-type females. Transgenic mice were genotyped using primers specific for the mutant human HTT transgene (forward primer 5’ TCATCAGCTTTTCCAGGGTCGCCAT and reverse primer 5’ CGCAGGCTAGGGCTGTCAATCATGCT), and age-matched wild-type littermates were used for the indicated experiments. A subset of mice from the colony that participated in the current studies were used to assess CAG repeat length (n=12), showing a mean length of 165 ± 5 (SD) repeats (Laragen Sequencing and Genotyping, Los Angeles, CA). Body weights of all animals were recorded weekly. All experimental procedures were performed according to ONPRC and OHSU Institutional Animal Care and Use Committee.
Adrenalectomy Surgery
Mice were anesthetized with 3% isoflurane. A 2cm × 2cm square was shaved on their lower dorsal surface and an incision was made in the skin at the animal's dorsal midline (this opening was used for the removal of both adrenal glands). Next, a small (3-5mm) incision in the muscular wall was made, directly above the kidney. Small tweezers were used to grasp and gently remove the adrenal gland. This procedure was repeated for the animal's contralateral side (bilateral adrenalectomy). The muscle incisions were closed using suture and the dorsal skin was closed with wound clips.
Corticosterone Replacement
Corticosterone replacement was provided in the animals’ drinking water, which was available ad-libitum. Corticosterone (Sigma, cat. #27840) was dissolved in a small volume of ethanol (0.6%), and then added to dH20. Since the aldosterone producing cells of the adrenal gland are lost to adrenalectomy, NaCl (0.9%) was also added to replace salt loss. Sucrose (2.0%) was also added to increase palatability of the solution. A dose of 10ug/ml corticosterone was used for the physiological/WT level replacement group and 60ug/ml of corticosterone was used for the high/HD level replacement group. Vehicle alone (2% sucrose, 0.9% NaCl, and 0.6% ethanol in dH20) was given to the sham surgery animals. All treatments were balanced for sex and genotype, with 2 WT and 2 HD age-matched mice randomized and caged together from the colony, into each male and female cage. A total of 48 mice were used in this study, with n=16 (4 HD males, 4 HD females, 4 WT males, and 4 WT females) for each of the three treatments. The experimenter was blind to the three different experimental conditions and genotypes. Replacement solution intake (volume) was monitored daily for each cage, and averaged over the week (and mathematically adjusted for all 4 mice in a weekly intake/mouse/week score – this was further adjusted with the loss of mice due to early death).
Restraint Stress
Mice were restrained for 30 minutes in modified 50mL conical tubes (Bale et al., 2002). Several air-holes were drilled into the bottom of each tube, tubes were shortened to restrict forward and backward movement and were closed at the end. Tube lengths were 7.3cm (for mice ≤20g), 8.1cm (mice between 20g – 25g), and 9.0cm (for mice >25g). Mice underwent restraint stress at 4, 7, and 10 weeks of age.
Behavioral Measures – Open Field
Mice were placed singly in a dimly lit, automated infra-red activity arenas (40.64 x 40.64 cm) for 15 minutes (Med Associates, St. Albans, VT). Each open field arena was enclosed in a sound attenuating chamber. Mice were habituated to the room for 1 hour before testing, and all testing occurred in the afternoon between 4-6pm. Distance traveled during the 15 minute test period was measured. Mice were assayed in the open field at weeks 6, 8, and 10.
Blood Collection and processing
Blood was collected by saphenous venipuncture in the dose response and adrenalectomy studies. Mice were gently restrained, their left hind-limb extended and wiped with antibiotic ointment (to visualize the vein), the vein was punctured using a 30g needle, and whole blood was collected into heparinized collection tubes and processed for plasma. The tail clip method was utilized for serial blood measurements in the circadian corticosterone and stress-induced corticosterone studies. The tip of the tail was clipped (0.5mm), and approximately 20ul of whole blood was collected from the tip and collected into heparinized capillary tubes, and processed for plasma. The incision site was disrupted for subsequent sampling within the same week. Handling of mice during tail-clip method blood collection was minimized, and typically lasted approximately 30 seconds. All plasma was assayed for corticosterone by the ONPRC Endocrine Technology CORE using an in-house radio-immuno assay. For the circadian CORT experiment, blood was collected every 20 hours over 5 days for a total of 6 collections (6pm, 2pm, 10am, 6am, 2am, and 10pm). Blood was collected every 20 hours, versus every 4, to avoid artificially elevating plasma corticosterone levels due to frequent repeated handling stress. This procedure was repeated for each mouse included in the study, at each age (4, 7, and 10 weeks); thus, each mouse had a total of 18 blood collections. A total of 28 mice were used for the circadian CORT experiment, including 6 WT females, 5 WT males, 6 HD females, and 11 HD males. More HD than WT mice were included to account for possible premature deaths before the end of the experiment. For the stress induced CORT experiment, blood was collected every 30 minutes, starting immediately before the mice were placed into restrainer tubes. Blood was collected 5 times (0, 30, 60, 90, 120min) for each mouse at 3 ages (4, 7, and 10 weeks of age), which was carried out between 11am and 2pm. A total of 30 mice were used in this experiment, including 4 WT females, 8 WT males, 12 HD females, and 6 HD males. For both the circadian and the stress-induced CORT studies, the experimenter was blind to genotype at the time of blood collection and assay.
Statistical analysis
All statistical analyses were performed by using JMP Version 11 (SAS Institute Inc.). A repeated measures mixed-model was used for all repeated measures (dependent variables: bodyweight, open field, circadian CORT, stress induced CORT; independent variables: genotype, sex, treatment, time-point and/or age). A proportional hazards model was used to analyze the survivorship data, which allows for post-hoc multiple pairwise comparisons between groups. A three-way ANOVA was used to assess the effects of genotype, sex, and treatment group on plasma CORT levels. Post-hoc contrasts were used to assess differences between WT and HD mice in the circadian corticosterone experiment at each possible age/time combination (i.e. HD vs WT mice at 10pm at 10 weeks of age), and alpha level was Bonferroni adjusted to 0.002 (.05/18 comparisons – p<.002 was considered statistically significant). For all other analyses, Tukey's post-hoc comparisons were performed when statistically significant fixed effects were detected (p < 0.05 was considered statistically significant).
Results
Corticosterone Replacement Dose Response
Prior to assessing the effect of differing levels of CORT on HD symptom progression, we performed an initial dose response study to establish CORT replacement doses that would achieve WT and HD-levels of plasma CORT following adrenalectomy surgery in R6/2 mice. Animals received CORT replacement in their drinking water. Target levels were based on previously published data demonstrating that basal CORT levels of 50-100ng/ml for WT R6/2 mice and 600ng/ml for transgenic R6/2 mice (Bjorkqvist et al., 2006). Additionally, we added low levels of sucrose to the CORT replacement solution to increase palatability and thus increase our likelihood of achieving target HD-levels of circulating CORT. Two concentrations of sucrose supplementation were tested (2% and 5%) in addition to four concentrations of CORT replacement (10, 20, 60, and 80ug/ml). CORT replacement solutions also contained sodium chloride (at 0.9%) to prevent salt-loss due to the removal aldosterone producing cells following the adrenalectomy.
Transgenic and wild-type R6/2 Mice were adrenalectomized at 8-9 weeks of age, and allocated to one of four CORT-replacement groups: 10, 20, 60, or 80ug/ml dose replacement. These solutions were first supplemented with 2% sucrose for the first week, then 5% for the second week. Blood was collected at midnight on the last night of supplementation and analyzed for plasma CORT. Replacement with 2% sucrose supplementation achieved the following plasma CORT levels: 65.1±14.7ng/ml for 10ug/ml dose, 145.1±47.1ng/ml for the 20ug/ml dose, 458.1±100.0ng/ml for the 60ug/ml dose, and 404.2±198.7ng/ml dose. Replacement with 5% sucrose achieved the following plasma CORT levels: 63.4±19.5ng/ml for 10ug/ml dose, 147.5±83.5ng/ml, 674.6±193.0ng/ml, 1240.3±441.5ng/ml (Supplemental Fig. 1). The 10ug/ml and 60ug/ml doses supplemented with 2% sucrose were selected for subsequent studies as they achieved plasma CORT within or near the desired range. 2% instead of 5% sucrose supplementation was selected with the goal of avoiding elevating plasma CORT above the levels described previously (Bjorkqvist et al., 2006).
Effect of adrenalectomy and corticosterone replacement on HD symptoms
R6/2 and wild-type mice underwent adrenalectomy surgery (ADX) at 6 weeks of age, followed by CORT replacement in water at physiological/WT levels (10ug/ml) or high/HD levels (60ug/ml). An additional group of R6/2 and WT mice underwent sham surgery at 6 weeks of age, and vehicle only (water, 2% sucrose and 0.9% NaCl) was provided instead of CORT replacement. Given that chronically elevated levels of glucocorticoids lead to metabolic abnormalities and muscle wasting in healthy individuals, we hypothesized that high level CORT replacement would exacerbate weight loss in HD mice. Indeed, R6/2 mice on the 60ug/ml CORT replacement showed a significant reduction in bodyweight (Fig. 1b) relative to sham mice starting at week 8, and continuing through the end of the study (p<.05 for each time-point); By week 11, HD mice on 60ug/ml CORT replacement showed a 15.4% reduction in bodyweight, while sham HD mice had yet to lose weight (4.1% increase over baseline level). Mice on HD-level (60ug/ml) replacement showed significantly reduced bodyweight relative to those on WT-level (10ug/ml) replacement at weeks 10 and 11 (p<.05), at which point they showed a 2.5% reduction from baseline. There was no difference in bodyweight between sham R6/2 mice and those on WT-level (10ug/ml) replacement (p>.05). In contrast to the R6/2 mice, all WT controls gained weight throughout the study, regardless of treatment. At the 11 week timepoint, there was a small but significant reduction in weight-gain in those WT mice on low-dose CORT, relative to those WT mice treated with HD-level CORT replacement or sham surgery (Supplemental Fig. 2, p<.05 for both pairwise comparisons).
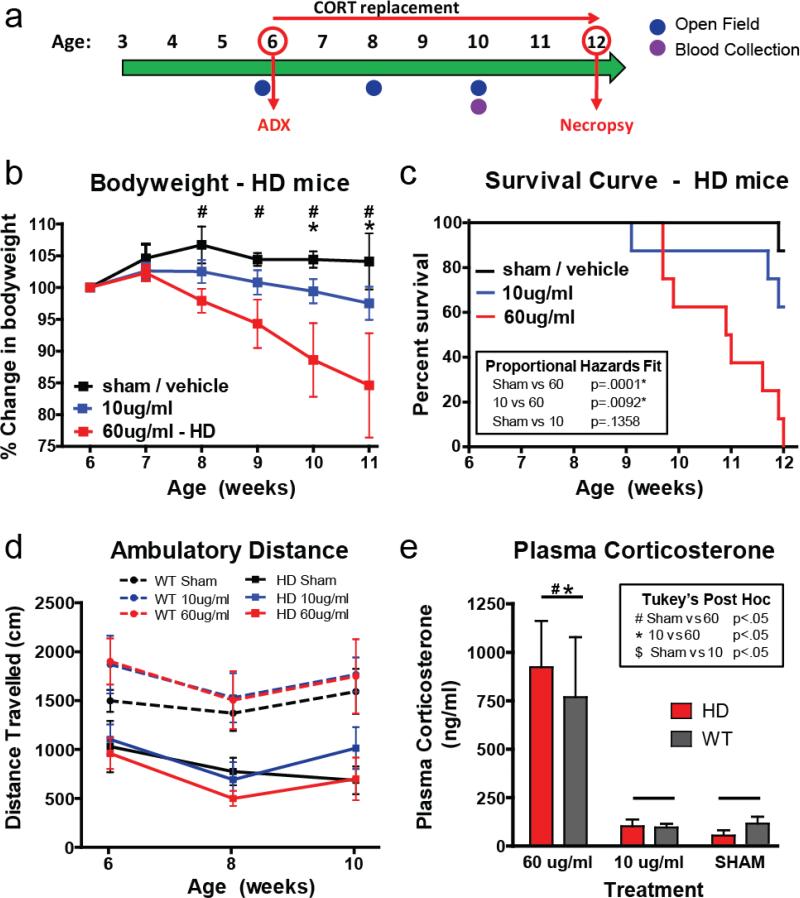
Figure 1. Adrenalectomy and corticosterone replacement.
(a) Timeline for experiment (b) HD mice on high/HD level replacement showed a progressive reduction in bodyweight starting at 8 weeks of age relative to sham HD mice (Tukey's post-hoc p<.05, indicated by #) and starting at week 10 relative to physiological/WT level replacement in adrenalectomized HD mice (Tukey's post-hoc, p<.05, indicated by *). (c) HD mice on high/HD level CORT replacement showed a significant reduction in survival relative to sham treated HD mice (p<.001) and HD mice on physiological/WT-level corticosterone replacement (p<.01). (d) Although R6/2 showed a reduction in distance traveled relative to WT mice, there was no effect of treatment on either R6/2 or WT mice (p>.05). (e) As expected, plasma CORT was higher in mice on the high/HD-level corticosterone replacement than the sham treated mice and physiological/WT-level corticosterone replacement groups. There was no difference in plasma corticosterone levels between R6/2 and WT mice in any of the treatment groups (p>.05).
Changes in motor behavior were assessed in the open field, both before (baseline at 6 weeks of age) and following surgery/treatment at 8 and 10 weeks of age. R6/2 mice show a clear motor phenotype, as indicated by reductions in ambulatory activity in the open field. While the characteristic reduction in distance travelled was detected in the R6/2 mice here (894.17 ± 181.57 cm for R6/2 mice and 1609.05 ± 140.64 cm for WT, p<.05), there was no effect of CORT treatment on motor performance (i.e. no genotype*treatment interaction, p>.05, Fig. 1d). Thus, the progressive motor symptoms shown in R6/2 mice are not affected by changes in circulating glucocorticoid levels.
In addition to weight loss, we hypothesized that increased CORT levels would hasten the latency to death. R6/2 transgenic mice have a shortened lifespan compared to WT mice, typically dying between the ages of 12-14 weeks. We found a dramatic increase in mortality in the HD-level replacement group, with 100% of mice (8/8) dying before the pre-determined endpoint of the study at 12 weeks of age (Fig. 1c). In contrast, only 37% (3/8) of the HD mice on WT-level replacement and 13% (1/8) of the sham treated HD mice died before the study's endpoint. Using a proportional hazards survivorship analysis, which allows between group comparisons, there was a significant reduction in survivorship in the HD-level replacement group relative to the sham operated (p<.001) and the WT-level replacement (p<.01) groups. There was no difference in survivorship between the WT-level replacement and the sham groups (p>.05). Together these data demonstrate that high levels of CORT can exacerbate the weight-loss and early death phenotype of transgenic R6/2 mice, but does not affect their motor phenotype.
To confirm that corticosterone replacement doses achieved the desired target plasma levels, blood was collected from all mice at 10 weeks of age (Fig. 1e). Since mice drink water in a diurnal pattern, with most drinking occurring during the night/dark, blood was collected at midnight - the expected time of peak plasma CORT from drinking. We also measured CORT replacement solution intake in this experiment (See Supplemental Fig. 3), which showed that there was an increase in intake in the high-CORT replacement mice at the 10 week timepoint (Tukey's p<.05 for both comparisons). Although this likely elevated the plasma CORT levels in the high-dose group, there was still a very clear difference between low and high-dose treatment plasma levels, similar to the values expected. Mean plasma CORT values for mice on high/HD-level (60ug/ml) replacement was 768.9 ± 310.1 ng/ml for WT and 922.9 ± 239.5 ng/ml for R6/2, which were not statistically different between genotypes (p>.05). Mean plasma CORT values for mice on physiological/WT (10ug/ml) replacement was 96.2 ± 18.6 ng/ml for WT and 119.8 ± 30.5 ng/ml for R6/2, which was also not statistically different between genotypes (p>.05). We found that mice on high/HD-level replacement showed significantly higher plasma CORT levels relative to those on physiological/WT replacement (p>.05) and sham treated mice (p<.05). Interestingly, we found no genotype differences in the sham treatment group: WT mice had a mean of 116.3 ± 34.7 ng/ml, while R6/2 mice had a mean of 53.6 ± 28.0 ng/ml. We hypothesized that the lack of elevated CORT seen in the HD sham treated mouse, which was predicted to be elevated based on previously published data, resulted from the time of day that we chose for blood collection. Therefore, we performed a follow-up study to characterize the circadian pattern of CORT release in R6/2 mice.
Circadian corticosterone release
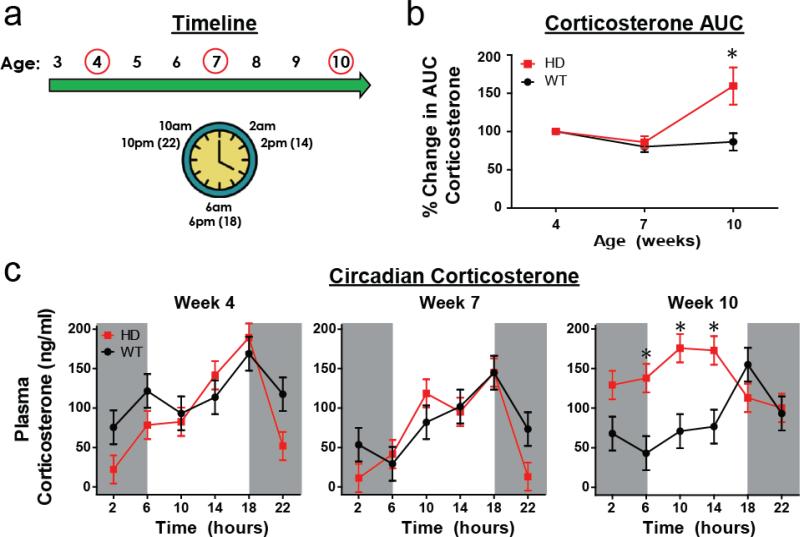
CORT is released from the adrenal gland in a circadian pattern, with peak levels occurring at the time an organism wakes (morning for humans, evening/lights-out for rodents). This pattern of release is regulated by the suprachiasmatic nucleus (SCN), which regulates the HPA-axis at the level of the paraventricular nucleus of the hypothalamus. While R6/2 mice have been previously shown to have a progressive increase in plasma CORT levels at a single clock-time, it is unclear whether or not there is a change in the circadian pattern of CORT release. Given that R6/2 mice also have a progressive disruption in the SCN, and in circadian behavior (Morton et al., 2005), we hypothesized that the circadian pattern of CORT release would likewise show a progressive disruption. Therefore, we characterized the circadian pattern of CORT release in R6/2 mice at 4, 7, and 10 weeks of age. Blood was collected at 6am, 10am, 2pm, 6pm, 10pm, and 2am at each age (Fig. 2a) from transgenic R6/2 mice and WT controls. Area under the curve (AUC) values were generated from the circadian profile for each mouse at each age, detailing the total amount of CORT that each mouse produces over a 24 hour period. We found that male WT mice had mean CORT AUC values of 1421 ±154, 1277 ± 130, and 1398 ± 245 at 4, 7, and 10 weeks of age, respectively, while male R6/2 mice had mean CORT AUC values of 1584 ± 170, 1156 ± 67 and 2575 ± 365 at 4, 7, and 10 weeks of age. Female WT mice had CORT AUC values of 3424 ± 642, 2182 ± 317 and 2117 ± 152 at 4, 7, and 10 weeks of age and female R6/2 mice had CORT AUC values of 2727 ± 440, 2327 ± 278 and 3230 ± 1057 at 4, 7, and 10 weeks of age, respectively. Percent change in CORT AUC values from week 4 were calculated and analyzed statistically. Relative to week 4, HD mice showed a significant 159.5 ± 24.3% increase in CORT AUC levels at week 10 (Fig. 2b, Tukey's p<.05), although there was no change at week 7 (p>.05). As expected, WT mice showed consistent AUC CORT levels across all ages (no significant difference in % change AUC CORT at 7 or 10 weeks of age, p>.05). Male and female mice did not show any differences in AUC CORT % change, regardless of age or genotype (Sex: p=.78; sex*genotype: p=.51; sex*age*genotype interaction: p=.15).
Figure 2. Circadian corticosterone.
(a) Timeline for data collection - mice underwent repeated blood collection at 4, 7, and 10 weeks of age. At each age, blood was collected from each mouse at 2am, 6am, 10am, 2pm (14hrs), 6pm (18hrs), and 10pm (22hrs). (b) R6/2 mice show an increase in area under the curve (AUC) corticosterone at 10 weeks of age relative to WT mice (p<.05). (c) R6/2 mice showed abnormalities in their circadian profile of plasma corticosterone at week 10 only. R6/2 mice showed elevated CORT levels at 6am, 10am, and 2pm (14hrs) relative to WT mice of the same age (Bonferroni adjusted alpha = 0.0028, significant contrasts all p<.001). At all other timepoints, within each age, there was no difference in plasma corticosterone between R6/2 and WT mice. There was no effect of sex (p>.05), and thus the data is collapsed across sex. Mice were on a 12:12 light cycle, with gray shading reflecting clock times with lights out (from 6pm to 6am).
The light-cycle for all mice was 12:12 (lights on at 6am and lights out at 6pm) and circadian corticosterone levels are expected to peak at 6pm when lights go out. We found no difference in the circadian CORT profile of WT and HD mice at 4 or 7 weeks of age, with both genotypes showing the expected peak level occurring at 6pm (18hrs) (Fig. 2c). However, at 10 weeks there is a clear and significant disruption in the circadian CORT profile in R6/2 mice – the peak mean level no longer occurs at 18hrs, but instead occurs at 10hrs (10am). Also at 10 weeks of age, R6/2 mice also showed significantly elevated CORT at 6, 10, and 14hrs (p<.001 for each pairwise contrast) relative to WT mice (Genotype*Age*Timepoint interaction: p<.05) (Fig. 2C). There was no significant effect of sex, or any of its interactions (all p>.05), and thus the data presented is collapsed across sex (values for males and females are presented separately in Supplemental Fig. 4). Although there is not a fragmentation of the circadian pattern of CORT release, the peak is clearly shifted to an earlier clock time and widened, with R6/2 mice showing a sustained elevation. Together, the circadian data demonstrate that there is indeed an alteration in the circadian pattern of CORT release in late-stage R6/2 mice, and that R6/2 mice do show an elevation in overall plasma CORT levels across a 24 hour period even when accounting for the disruption in circadian release.
Stress induced corticosterone release
In addition to the basal circadian pattern of CORT release, all types of stressors (psychological, physiological, etc.) also activate the HPA-axis, leading to a short-term release of CORT. Although R6/2 mice show an elevation in basal CORT release, it is unclear whether or not they show an exaggerated stress-induced activation of the HPA-axis leading to increased secretion of CORT. Thus, in a third experiment, we assessed whether restraint stress would lead to an acute increase in CORT release in R6/2 mice relative to WT mice and whether there is a disease-associated delay returning back to basal levels.
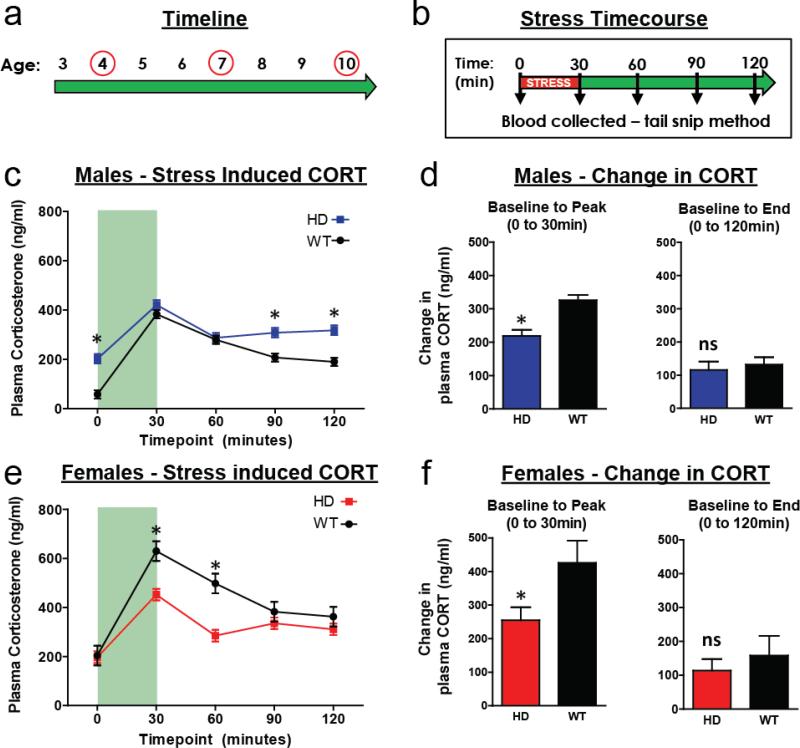
To address this, R6/2 and WT mice were subjected to 30 minutes of restraint stress at 4, 7, and 10 weeks of age (Fig. 3a). Blood was collected at baseline (prior to the stressor – 0min) and four subsequent times following the stressor (30, 60, 90, and 120min. – see Fig. 3b). There was no significant effect of age, or any of its interactions, on plasma CORT levels (all p>.05), and thus the data presented in Fig. 3 is collapsed across age (data for both sexes at all ages and timepoints are provided in Supplemental Fig. 5). Relative to male WT mice, male R6/2 mice showed elevated plasma CORT levels at baseline (0min.), and at the 90min. and 120min. time-points (Fig. 3c, Tukey's p<.05 for all). Conversely, female R6/2 mice showed lower plasma CORT levels relative to WT females at the 30min. and 60min. time points (Fig. 3f, Tukey's p<.05 for both). To test our hypothesis that R6/2 mice show an augmented stress-induced CORT response, we accounted for the baseline difference shown between R6/2 and WT mice by calculating the absolute difference between baseline (0min) and peak (30min) plasma CORT levels. Contrary to our hypothesis, both male and female R6/2 mice have a blunted stress-induced CORT response relative to WT mice (Fig. 3d and 3f, p<.05). We also found that CORT values similarly returned to baseline for both WT and R6/2 mice, regardless of sex (difference between 0min. and 120min., Fig. 3d and 3f, p>.05). As with the males, female R6/2 and WT mice had a similar reduction towards baseline at the 120min. time point (Fig. 3f, p>.05). These data demonstrate that elevated CORT in R6/2 mice isn't simply due to general hyperactivity of the HPA-axis. If it were, we would expect there to be both an increase in resting CORT levels (which there is at 10 weeks), as well as stress-induced CORT release. In fact, these data demonstrate that R6/2 mice have an attenuated CORT response to restraint stress, and suggests that they have intact negative feedback of the HPA-axis as their levels normalized similarly to WT mice.
Figure 3. Stress induced corticosterone release.
(a) Mice underwent restraint stress and blood collection protocol at 4, 7, and 10 weeks of age. (b) Mice were placed in restrainer tubes for 30 minutes, with blood collected at baseline (time = 0min), immediately following restraint stress (30min), and for the next 90 minutes at 30 minute intervals (time 60, 90, 120min). There was not a significant effect of age, and thus data is collapsed across the three different ages. (c) Male R6/2 mice showed elevated CORT levels at time 0, 90, and 120 (Tukey's post-hoc, p<.05), relative to WT males. (d) Absolute difference from baseline to peak (30min) plasma CORT values shows that male R6/2 mice have a blunted CORT response to stress, when baseline differences are accounted for (p<.05). Absolute difference from baseline to end (0 to 120min) shows a small persistent elevation in CORT at the end of the time-course, that is not different between WT and R6/2 males (p>.05). (e) Female R6/2 mice showed lower plasma CORT values at time 30 and 60min, relative to WT females (Tukey's post-hoc, p<.05). (f) Absolute difference from baseline to peak (0 to 30min) plasma CORT values shows that female R6/2 mice have a blunted CORT response to stress, when baseline differences are accounted for (p<.05). Absolute difference from baseline to end (0 to 120min) shows a small persistent elevation in CORT at the end of the time-course, that is not different between WT and R6/2 females (p>.05). For both (c) and (e), there was no effect of age or any age-interactions (all p>.05) on plasma CORT levels, and thus the data presented is collapsed across age. Light green shading in (c) and (d) reflects the time that the mice were undergoing restraint stress.
Adrenal gland weights
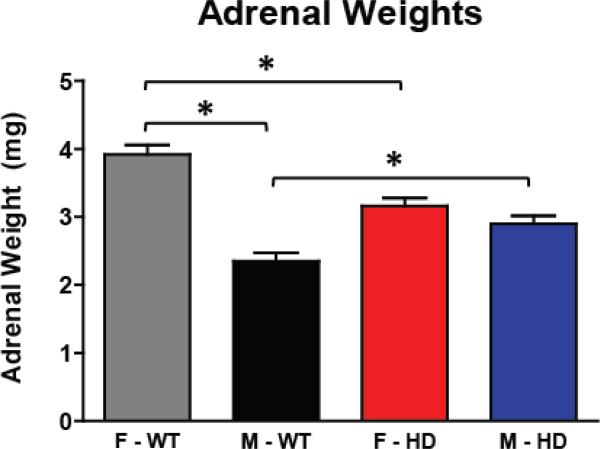
We collected adrenal glands from mice in both the circadian and stress induced CORT experiments to assess whether there is a change in adrenal gland weight due to genotype (circadian experiment) and whether this weight could be affected by exposure to repeated restraint stress (stress-induced CORT experiment). Previous studies have shown that R6/2 mice demonstrate adrenal gland hypertrophy, as measured by an increase in adrenal gland weight. It is also well established that WT female mice have larger adrenal glands than males (Bielohuby et al., 2007). Here, mice were sacrificed at 12 weeks of age and adrenal gland weights were measured at necropsy. The effect of experiment (circadian and stress), sex (male and female), and genotype (WT and HD), and their interactions on adrenal gland weight were assessed. There were no differences in adrenal gland weight between the two studies (p>.05 for experiment and all of its interactions), suggesting that repeated restraint stress did not have an effect on adrenal gland weight in either WT or HD mice. Since there was no difference between experiments, data was collapsed across the two studies (Fig. 4). We did, however, find that both sex and genotype influenced adrenal gland weight (p<.05 sex*genotype interaction). R6/2 male mice showed significantly larger adrenal gland weight relative to WT males (2.90±0.1mg for R6/2 and 2.35±0.12mg for WT; Tukey's post-hoc p<.05). Conversely, R6/2 females had significantly smaller adrenal weights than WT females (3.16±0.12mg for R6/2 and 3.92±0.14mg for WT; Tukey's post-hoc p<.05). As has been shown previously, female WT mice had significantly larger adrenal glands compared to male WT mice (Tukey's post-hoc p<.05). There was no statistical difference in adrenal gland weight between male and female R6/2 mice (Tukey's post-hoc p>.05). Together, this data shows that there is a sex-specific difference in adrenal gland weight, with female HD mice showing adrenal hypertrophy and male HD mice showing adrenal atrophy.
Figure 4. Adrenal Gland Weights.
Mice were sacrificed at 12 weeks of age in the Circadian and Stress-induced CORT experiments, and wet adrenal gland weights were measured. Female R6/2 mice had significantly smaller adrenal glands than did WT females, while male R6/2 mice had significantly larger adrenal glands compared to WT males (p<.05). There was no difference in adrenal gland weight between R6/2 males and females (p>.05), while WT females had larger adrenal glands than WT males (p<.05). There was no difference in adrenal gland weight between the two experiments, and thus data is collapsed across experiments.
Discussion
Although numerous studies have shown that HD patients have elevated plasma cortisol (Aziz et al., 2009; Bjorkqvist et al., 2006; Heuser et al., 1991; Saleh et al., 2009), the causes and consequences of this elevation remain largely unknown. Given that persistent hypercortisolinemia in otherwise healthy individuals can cause metabolic, cognitive, and psychiatric symptoms, it is possible that hypercortisolinemia is contributing to some of the same symptoms in HD patients. Abnormalities in corticosterone homeostasis, the rodent homolog of cortisol, have been demonstrated in the R6/2 (Bjorkqvist et al., 2006) and the R6/1 (Du et al., 2012) transgenic HD mouse models of HD. Experimental elevation of CORT in the male R6/1 HD mouse exacerbates cognitive symptoms, reduces hippocampal neurogenesis, and slows weight gain (Mo et al., 2014a). Similarly, short-term stress in the R6/1 model exacerbates memory impairment in female HD mice (Mo et al., 2013), while long term stress reduces weight gain in male and female HD mice, reduces saccharine preference in female HD mice (a depressive-like measure of anhedonia), and reduces olfactory sensitivity (Mo et al., 2014b). In the series of experiments presented here, we extend these findings and demonstrate that elevated corticosterone in the R6/2 model dramatically exacerbates the weight loss phenotype in these mice and also shortens lifespan. Additionally, we extended previous findings that corticosterone homeostasis is altered in R6/2 HD mice, confirming that CORT levels are elevated at end stage of disease. Further, we demonstrate that there is a clear disruption in the circadian pattern of CORT release and an attenuated CORT response to restraint stress in R6/2 HD mice.
One of the most significant findings from these experiments is that experimentally elevated CORT dramatically reduced bodyweight in transgenic R6/2 mice. Elevated CORT in human HD patients is significantly correlated with reduced BMI (Aziz et al., 2008). Although this human study was correlational, our finding in R6/2 mice demonstrates causality – that artificially elevated CORT has the ability to exacerbate the weight loss phenotype associated with the HD gene. HD patients, like R6/2 mice, are in a state of negative energy balance, showing increased hunger and food intake and simultaneous weight loss (Farrer and Yu, 1985; Trejo et al., 2004; van der Burg et al., 2008). Weight loss is progressive in patients, typically starting before the onset of motor symptoms, and by end stage leads to severe cachexia (Djousse et al., 2002; Farrer et al., 1985; Kosinski et al., 2007; van der Burg et al., 2009). Although the weight loss phenotype is well documented in HD, the mechanisms by which mutant huntingtin toxicity leads to weight loss in patients is still poorly understood (van der Burg et al., 2009). It is not caused by energy expenditure due to chorea, nor is it due to insufficient nutritional intake (Mochel et al., 2007; Sanberg et al., 1981). In fact, HD patients have significantly higher caloric intake than controls (Farrer et al., 1985; Trejo et al., 2004). It has been speculated that mutant huntingtin toxicity in peripheral tissues, such as muscle, pancreas and adipose tissue may be the source of weight loss in HD (Bjorkqvist et al., 2005; Phan et al., 2009; van der Burg et al., 2008; van der Burg et al., 2009; van der Burg et al., 2011). It has also been postulated that hypothalamic pathology may be causal to metabolic changes in patients (Hult et al., 2011; Petersen et al., 2005). As a steroid hormone, CORT reaches virtually all tissues in the brain and body, and has potent metabolic effects on an organism. In individuals with chronically elevated CORT there is a dramatic shift in whole body metabolism and composition, with a decrease in skeletal muscle mass (wasting), a simultaneous increase in body fat, and robust insulin resistance (Orth, 1995; Valassi et al., 2012). Although it is likely that mutant huntingtin toxicity in the periphery and/or the hypothalamus is sufficient to cause a metabolic phenotype in HD, this phenotype is probably multifactorial and elevated CORT may play an important role.
Another key finding is that high dose CORT treatment significantly shortened lifespan in R6/2 mice. This transgenic mouse model of HD is one of the most severe - mice typically die between 13-16 weeks of age, after developing a progressive motor, cognitive, and metabolic phenotype. Although lifespan is severely truncated, the immediate cause of death is unclear for these mice, as it has never been systematically evaluated (Li et al., 2005). Considering that increased BMI is correlated with a slower progression of disease in human patients (Myers et al., 1991), it may be possible that the converse is true – that elevated CORT hastens the progression to death in R6/2 mice by exacerbating the weight-loss phenotype.
One surprising finding from the adrenalectomy experiments was that we failed to detect an elevation in CORT in 10 week old sham treated (intact) R6/2 mice (Fig. 1e). It has been previously shown that R6/2 mice show a progressive elevation in circulating CORT, starting at approximately 6 weeks of age and resulting in approximately a three-fold increase by end stage (Bjorkqvist et al., 2006). In the same study, the authors found that late-stage human HD patients also show a progressive increase in urinary CORT, starting at clinical stage III and further worsening at clinical stage IV (Bjorkqvist et al., 2006). We hypothesized that our failure to detect an increase in our adrenalectomy experiment was due to the time that we collected blood (midnight). However, it remained unclear whether or not R6/2 mice simply show an increase in blood CORT only at the time it peaks (lights off), whether there is a persistent elevation in CORT at all clock times, or whether there is a dysregulation in the time of CORT peak in this transgenic line that has a progressive disturbance in circadian rhythms (Morton, 2013; Morton et al., 2005). Thus, we decided to further characterize the circadian pattern of CORT release in R6/2 mice at three different ages: 4, 7, and 10 weeks of age.
We found a clear alteration in the circadian pattern of release of CORT in R6/2 mice at 10 weeks of age. This is a novel finding in HD mice, and may represent a generalized disruption in circadian rhythms which becomes pronounced in late stage R6/2 mice. In early stage human HD patients, the 24 hour profile of CORT release also shows an increase in AUC levels (Aziz et al., 2009). While there was no difference in peak levels between human patients and controls, CORT levels became elevated earlier than expected and persisted at a level until later in the day (Aziz et al., 2009). Our finding is similar, with R6/2 mice showing a persistent, significant elevation in CORT which starts at a much earlier clock time (6am) and lasting throughout most the day (until 2pm). At 6pm, the expected time of peak CORT levels, plasma levels in R6/2 mice lowered to the peak WT levels. As with the human studies, this early elevation and persistence in high CORT levels accumulates to an overall AUC increase in 24 hour CORT. From a practical point of view, these data demonstrate that measuring blood levels of CORT in individuals with HD may be more complicated than taking a single measure at a single clock time. Although there is an overall increase in CORT, as indicated by the AUC analysis, no differences would have been detected from samples taken at 6pm (lights-out) or 10pm alone. This likely explains our failure to detect a difference in the midnight CORT measure from sham treated mice in the adrenalectomy. Since the circadian rhythm of CORT release is altered, it might be more ideal to take a more long-term measure of the hormone, such as CORT from hair samples (Thomson et al., 2010), which is not sensitive to diurnal variation. Although there are numerous human studies showing that HD patients show an elevation in CORT, there are also some that fail to detect an increase. It is possible that abnormalities in the circadian pattern of CORT release may have obscured what the true differences are between human HD subjects and controls in these studies. More studies investigating the circadian pattern of CORT release in human subjects across disease stages could provide great insight into the discrepancies in these studies, as well as offer a better understanding of how CORT may influence symptom progression in HD.
In contrast with the circadian CORT findings, we found that R6/2 mice show a reduction in stress-induced CORT release, and that they similarly return to baseline levels following the stressor (Fig. 3d and 3f). Unlike the circadian experiment, the pattern of stress-induced CORT release was stable and did not change with age. Both of these findings were unexpected. Although stress-induced CORT release had never been previously investigated in R6/2 mice, we hypothesized that previous reports of elevated CORT in R6/2 mice reflected a general hyperactivity of the HPA-axis. We therefore expected to see an increase in both basal and stress-induced CORT release in these mice, as well as longer time for CORT levels to normalize in mice undergoing stress. The restraint-stress paradigm has been utilized in R6/1 studies investigating CORT homeostasis at an early (pre-motor dysfunction) stage (Du et al., 2012). These studies demonstrated that there was not a different response in stress-induced peak CORT levels between WT and HD R6/1 mice, although female HD mice failed to normalize to pre-stress CORT levels following the stressor (Du et al., 2012). While there is no clear explanation for the divergent findings between the two strains, it is possible that the R6/2 is simply a better model of CORT disruption in the human disease, as they show a spontaneous increase in CORT levels, similar to human HD patients. It is also possible that alterations in CORT homeostasis may simply occur at a later stage in R6/1 mice than have been previously investigated. One unexpected finding was that female mice showed high basal CORT (approximately 200ng/ml), which was not different between genotypes. Although it is possible that this elevation is associated with stress at handling, we purposefully used a blood collection method that is rapid (blood collection from tail tip, which takes less than 30 seconds) and doesn't require anesthesia. This method is similar, if not quicker, to the tail-incision method described by Sadler and Bailey (2013), which is designed to reduce the possibility of elevating CORT levels through handling. Interestingly, we did not find a sex difference in adrenal gland weight between male and female HD mice, although there was a clear and expected difference between in WT mice, with females showing significantly larger adrenal glands. Although the reason for this sex effect is unclear, it confirms that sex can be an important factor and should be accounted for in HD neuroendocrine studies, as has been previously shown in stress-induced CORT release in R6/1 mice (Du et al., 2012).
While CORT release and HPA-axis function have been assessed in multiple rodent models of HD, all have failed to recapitulate the basal elevation in CORT shown in human patients (Du et al., 2012; Hult et al., 2013). As this phenomena may be occurring during late stages of disease, and may show abnormal circadian patterns of release, further assessment of CORT in other mouse models at later time-points are warranted. Considering that the mechanism that underlies these changes in HPA-axis activity is poorly understood, particularly in human patients, the R6/2 mouse may provide insights. We found that female R6/2 mice show hypertrophy of the adrenal gland, consistent with a previous study showing adrenal hypertrophy and increased ACTH immune-reactivity in the pituitary gland (Bjorkqvist et al., 2006). While these changes may be directly causal to an elevation in CORT, it is also possible that these changes are simply a consequence of hyper-activation of the HPA-axis by upstream brain regions that regulate it. One candidate is the SCN, the circadian pacemaker in the brain. The SCN shows clear pathology in HD (Fahrenkrug et al., 2007; Morton et al., 2005; van Wamelen et al., 2013), and regulates the circadian rhythm of HPA-axis activation which leads to a diurnal pattern of CORT release. Although speculative, it is possible that changes in the SCN could lead to overstimulation of the HPA-axis, leading to the increased blood levels of ACTH and adrenal hypertrophy shown in R6/2 mice (Bjorkqvist et al., 2006). This is consistent with our data showing that elevated CORT co-occurs with disruption in the circadian pattern of release, and that it occurs at a later stage of disease when SCN and circadian abnormalities are more pronounced. This is also consistent with our stress-induced CORT data, which showed a hypoactive HPA-axis response to restraint in HD mice, further suggesting that the HPA-axis is not just simply overactive. However, further complicating the matter is that stress-induced activation of the HPA-axis is mediated by a variety of brain regions, including the amygdala, limbic cortex, and hippocampus (Ulrich-Lai and Herman, 2009) – all of which show pathology in R6/2 mice. It is possible that pathology in these upstream regions may impair stress-induced HPA-axis activation, leading to a blunted CORT response.
Although numerous studies have shown alterations in cortisol in clinical HD, there is not a clear consensus in the field over the magnitude and timing of that change. Heuser (1991) was the first to show abnormal levels of CORT in HD, with symptomatic patients showing a two-fold increase in basal plasma levels relative to healthy controls. Subsequent studies also showed an elevation in plasma and urinary CORT (Aziz et al., 2009; Bjorkqvist et al., 2006; Leblhuber et al., 1995; Saleh et al., 2009), ranging from a small 10% increase (Leblhuber et al., 1995) to a 3-fold increase in late stage patients (Bjorkqvist et al., 2006). Bjorkqvist (2006) showed a clear progressive phenotype of elevated urinary cortisol in a large clinical sample (n=68 for controls, n=82 for HD), with pre-symptomatic patients showing a mild decrease in CORT levels, clinical stage I/II patients showing no difference from controls, stage III patients showing a 2-fold increase, and stage IV patients with a 3-fold increase. Saleh et al. (2009) conducted the largest neuroendocrine study in HD to date and showed a 50% increase in serum cortisol levels in HD patients (n=217) relative to age and sex matched WT controls. This study included subjects from all clinical stages, but failed to detect any association between disease progression and the magnitude of the elevation of CORT. Aziz (2009) has run the most detailed analysis of basal HPA-axis activity in a clinical HD sample, collecting blood samples from patients over 24-hours in 10 minute intervals. As expected, healthy controls showed an elevation in plasma CORT in the early morning (starting at approximately 5am), which return to nadir levels by noon. HD patients showed an early rise in plasma CORT (approximately 1am) and a delayed return to basal levels (approximately 5pm). Accordingly, they found a significant 50% elevation in 24 hour area-under-the-curve cortisol in patients. The limitations to this study is that it was a very small sample of early stage HD patients (n=8) and healthy controls (n=8). Although these early studies were largely consistent, more recent studies have failed to replicate these findings. Two recent studies have shown the same finding - a small increase in post-waking salivary cortisol in pre-symptomatic HD-mutation carriers, but not in symptomatic HD patients (Hubers et al., 2015; van et al., 2010). Another two studies have shown either no difference (Shirbin et al., 2013a) or a small decrease in cortisol in early symptomatic patients (Shirbin et al., 2013b). One possible difference in methodology that may account for these inconsistent in findings is that the earlier studies measured blood levels of CORT, which seem to be more consistently elevated, while the studies showing null or small increases in cortisol rely on salivary measures of the hormone. However, it would be surprising that this would explain the differences, as salivary and blood levels of cortisol are typically in agreement (Vining et al., 1983). Although purely speculative, perhaps salivary levels of cortisol aren't as indicative of plasma levels of the hormone in the clinical HD population due to pathophysiological mechanisms germane to the disease. Additionally, another recent study failed to show an elevation in plasma or urinary cortisol in a clinical HD population (Kalliolia et al., 2014). One limitation in these studies is that they largely represent earlier stages in the progression of the disease – and perhaps aren't detecting hypercortisolinemia that may develop at later stages. Another limitation is that many of the patients are medicated in many of these studies, which also may influence cortisol (Saleh et al., 2009). Hopefully future clinical studies will clarify these inconsistencies, and better take into account disease stage, 24-hour pattern of release, as well as methodology for cortisol measurement.
HD patients have a devastating course of disease and the dopamine antagonist Tetrabenazine, which reduces motor symptoms, is currently the only FDA-approved treatment. There are currently no treatments which slow the disease progression. Although helpful, treatments for weight-loss are largely limited to increasing caloric intake through nutritional supplementation (Trejo et al., 2005). The key findings here, that elevated CORT exacerbates the metabolic phenotype and hastens time to death in R6/2 mice, highlight that the HPA-axis may be a novel therapeutic target for HD. More specifically, these data suggest that normalizing CORT levels may be of therapeutic benefit in HD patients, potentially slowing weight loss, increasing BMI, and potentially slowing the course of disease. Ongoing studies in our laboratory are assessing whether lowering CORT to WT levels in HD mice can prevent or slow the development of the myriad motor, cognitive and metabolic phenotypes that are germane to HD.
Supplementary Material
Highlights.
High level corticosterone treatment in adrenalectomized R6/2 mice exacerbates weight loss and shortens lifespan
R6/2 mice show a progressive increase in spontaneous corticosterone release and a disruption in the circadian pattern of corticosterone release
R6/2 mice show a blunted corticosterone response to restraint stress
Acknowledgements
We would like to thank the ONPRC Endocrinology Core for their evaluation of the HD mouse blood samples. Additionally, we thank Drs. Andrey Ryabinin, Deb Finn, Charlie Meshul and Penelope Hogarth for their thoughtful comments and critiques on the study design. We also thank Jordan Lueras and the Small Animal Laboratory Unit for mouse colony management and animal care. This research was supported by a generous donation from Quentin and Bee Neufeld (JLM), ONPRC Core Grant P51OD011092 (JLM) and funding from a Ruth L. Kirschstein National Research Service Award (F31NS092281, BDD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Anderson KE, Marder KS. An overview of psychiatric symptoms in Huntington's disease. Curr. Psychiatry Rep. 2001;3:379–388. doi: 10.1007/s11920-996-0030-2. [DOI] [PubMed] [Google Scholar]
- 2.Aziz NA, Pijl H, Frolich M, van der Graaf AW, Roelfsema F, Roos RA. Increased hypothalamic-pituitary-adrenal axis activity in Huntington's disease. J. Clin. Endocrinol. Metab. 2009;94:1223–1228. doi: 10.1210/jc.2008-2543. [DOI] [PubMed] [Google Scholar]
- 3.Aziz NA, van der Burg JM, Landwehrmeyer GB, Brundin P, Stijnen T, Roos RA. Weight loss in Huntington disease increases with higher CAG repeat number. Neurology. 2008;71:1506–1513. doi: 10.1212/01.wnl.0000334276.09729.0e. [DOI] [PubMed] [Google Scholar]
- 4.Bale TL, Picetti R, Contarino A, Koob GF, Vale WW, Lee KF. Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. J. Neurosci. 2002;22:193–199. doi: 10.1523/JNEUROSCI.22-01-00193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bielohuby M, Herbach N, Wanke R, Maser-Gluth C, Beuschlein F, Wolf E, Hoeflich A. Growth analysis of the mouse adrenal gland from weaning to adulthood: time- and gender-dependent alterations of cell size and number in the cortical compartment. Am. J. Physiol Endocrinol. Metab. 2007;293:E139–E146. doi: 10.1152/ajpendo.00705.2006. [DOI] [PubMed] [Google Scholar]
- 6.Bjorkqvist M, Fex M, Renstrom E, Wierup N, Petersen A, Gil J, Bacos K, Popovic N, Li JY, Sundler F, Brundin P, Mulder H. The R6/2 transgenic mouse model of Huntington's disease develops diabetes due to deficient beta-cell mass and exocytosis. Hum. Mol. Genet. 2005;14:565–574. doi: 10.1093/hmg/ddi053. [DOI] [PubMed] [Google Scholar]
- 7.Bjorkqvist M, Petersen A, Bacos K, Isaacs J, Norlen P, Gil J, Popovic N, Sundler F, Bates GP, Tabrizi SJ, Brundin P, Mulder H. Progressive alterations in the hypothalamic-pituitary-adrenal axis in the R6/2 transgenic mouse model of Huntington's disease. Hum. Mol. Genet. 2006;15:1713–1721. doi: 10.1093/hmg/ddl094. [DOI] [PubMed] [Google Scholar]
- 8.Djousse L, Knowlton B, Cupples LA, Marder K, Shoulson I, Myers RH. Weight loss in early stage of Huntington's disease. Neurology. 2002;59:1325–1330. doi: 10.1212/01.wnl.0000031791.10922.cf. [DOI] [PubMed] [Google Scholar]
- 9.Du X, Leang L, Mustafa T, Renoir T, Pang TY, Hannan AJ. Environmental enrichment rescues female-specific hyperactivity of the hypothalamic-pituitary-adrenal axis in a model of Huntington's disease. Transl. Psychiatry. 2012;2:e133. doi: 10.1038/tp.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fahrenkrug J, Popovic N, Georg B, Brundin P, Hannibal J. Decreased VIP and VPAC2 receptor expression in the biological clock of the R6/2 Huntington's disease mouse. J. Mol. Neurosci. 2007;31:139–148. doi: 10.1385/jmn/31:02:139. [DOI] [PubMed] [Google Scholar]
- 11.Farrer LA, Yu PL. Anthropometric discrimination among affected, at-risk, and not-at-risk individuals in families with Huntington disease. Am. J. Med. Genet. 1985;21:307–316. doi: 10.1002/ajmg.1320210213. [DOI] [PubMed] [Google Scholar]
- 12.Gourfinkel-An I, Cancel G, Duyckaerts C, Faucheux B, Hauw JJ, Trottier Y, Brice A, Agid Y, Hirsch EC. Neuronal distribution of intranuclear inclusions in Huntington's disease with adult onset. Neuroreport. 1998;9:1823–1826. doi: 10.1097/00001756-199806010-00028. [DOI] [PubMed] [Google Scholar]
- 13.Heinsen H, Strik M, Bauer M, Luther K, Ulmar G, Gangnus D, Jungkunz G, Eisenmenger W, Gotz M. Cortical and striatal neurone number in Huntington's disease. Acta Neuropathol. 1994;88:320–333. doi: 10.1007/BF00310376. [DOI] [PubMed] [Google Scholar]
- 14.Heuser IJ, Chase TN, Mouradian MM. The limbic-hypothalamic-pituitary-adrenal axis in Huntington's disease. Biol. Psychiatry. 1991;30:943–952. doi: 10.1016/0006-3223(91)90007-9. [DOI] [PubMed] [Google Scholar]
- 15.Hubers AA, van der Mast RC, Pereira AM, Roos RA, Veen LJ, Cobbaert CM, van DE, Giltay EJ. Hypothalamic-pituitary-adrenal axis functioning in Huntington's disease and its association with depressive symptoms and suicidality. J. Neuroendocrinol. 2015;27:234–244. doi: 10.1111/jne.12255. [DOI] [PubMed] [Google Scholar]
- 16.Hult LS, Nilsson N, Soylu R, Kirik D, Petersen A. Hypothalamic expression of mutant huntingtin contributes to the development of depressive-like behavior in the BAC transgenic mouse model of Huntington's disease. Hum. Mol. Genet. 2013;22:3485–3497. doi: 10.1093/hmg/ddt203. [DOI] [PubMed] [Google Scholar]
- 17.Hult S, Soylu R, Bjorklund T, Belgardt BF, Mauer J, Bruning JC, Kirik D, Petersen A. Mutant huntingtin causes metabolic imbalance by disruption of hypothalamic neurocircuits. Cell Metab. 2011;13:428–439. doi: 10.1016/j.cmet.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Huntington Disease Collaborative Research Group A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 19.Kalliolia E, Silajdzic E, Nambron R, Hill NR, Doshi A, Frost C, Watt H, Hindmarsh P, Bjorkqvist M, Warner TT. Plasma melatonin is reduced in Huntington's disease. Mov Disord. 2014;29:1511–1515. doi: 10.1002/mds.26003. [DOI] [PubMed] [Google Scholar]
- 20.Kosinski CM, Schlangen C, Gellerich FN, Gizatullina Z, Deschauer M, Schiefer J, Young AB, Landwehrmeyer GB, Toyka KV, Sellhaus B, Lindenberg KS. Myopathy as a first symptom of Huntington's disease in a Marathon runner. Mov Disord. 2007;22:1637–1640. doi: 10.1002/mds.21550. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence AD, Sahakian BJ, Hodges JR, Rosser AE, Lange KW, Robbins TW. Executive and mnemonic functions in early Huntington's disease. Brain. 1996;119(Pt 5):1633–1645. doi: 10.1093/brain/119.5.1633. [DOI] [PubMed] [Google Scholar]
- 22.Leblhuber F, Peichl M, Neubauer C, Reisecker F, Steinparz FX, Windhager E, Maschek W. Serum dehydroepiandrosterone and cortisol measurements in Huntington's chorea. J. Neurol. Sci. 1995;132:76–79. doi: 10.1016/0022-510x(95)00114-h. [DOI] [PubMed] [Google Scholar]
- 23.Levy ML, Cummings JL, Fairbanks LA, Masterman D, Miller BL, Craig AH, Paulsen JS, Litvan I. Apathy is not depression. J. Neuropsychiatry Clin. Neurosci. 1998;10:314–319. doi: 10.1176/jnp.10.3.314. [DOI] [PubMed] [Google Scholar]
- 24.Li JY, Popovic N, Brundin P. The use of the R6 transgenic mouse models of Huntington's disease in attempts to develop novel therapeutic strategies. NeuroRx. 2005;2:447–464. doi: 10.1602/neurorx.2.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mo C, Pang TY, Ransome MI, Hill RA, Renoir T, Hannan AJ. High stress hormone levels accelerate the onset of memory deficits in male Huntington's disease mice. Neurobiol. Dis. 2014a;69:248–262. doi: 10.1016/j.nbd.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Mo C, Renoir T, Hannan AJ. Effects of chronic stress on the onset and progression of Huntington's disease in transgenic mice. Neurobiol. Dis. 2014b;71:81–94. doi: 10.1016/j.nbd.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Mo C, Renoir T, Pang TY, Hannan AJ. Short-term memory acquisition in female Huntington's disease mice is vulnerable to acute stress. Behav. Brain Res. 2013;253:318–322. doi: 10.1016/j.bbr.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 28.Mochel F, Charles P, Seguin F, Barritault J, Coussieu C, Perin L, Le BY, Gervais C, Carcelain G, Vassault A, Feingold J, Rabier D, Durr A. Early energy deficit in Huntington disease: identification of a plasma biomarker traceable during disease progression. PLoS. One. 2007;2:e647. doi: 10.1371/journal.pone.0000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morton AJ. Circadian and sleep disorder in Huntington's disease. Exp. Neurol. 2013;243:34–44. doi: 10.1016/j.expneurol.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Morton AJ, Wood NI, Hastings MH, Hurelbrink C, Barker RA, Maywood ES. Disintegration of the sleep-wake cycle and circadian timing in Huntington's disease. J. Neurosci. 2005;25:157–163. doi: 10.1523/JNEUROSCI.3842-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myers RH, Sax DS, Koroshetz WJ, Mastromauro C, Cupples LA, Kiely DK, Pettengill FK, Bird ED. Factors associated with slow progression in Huntington's disease. Arch. Neurol. 1991;48:800–804. doi: 10.1001/archneur.1991.00530200036015. [DOI] [PubMed] [Google Scholar]
- 32.Orth DN. Cushing's syndrome. N. Engl. J. Med. 1995;332:791–803. doi: 10.1056/NEJM199503233321207. [DOI] [PubMed] [Google Scholar]
- 33.Petersen A, Bjorkqvist M. Hypothalamic-endocrine aspects in Huntington's disease. Eur. J. Neurosci. 2006;24:961–967. doi: 10.1111/j.1460-9568.2006.04985.x. [DOI] [PubMed] [Google Scholar]
- 34.Petersen A, Gil J, Maat-Schieman ML, Bjorkqvist M, Tanila H, Araujo IM, Smith R, Popovic N, Wierup N, Norlen P, Li JY, Roos RA, Sundler F, Mulder H, Brundin P. Orexin loss in Huntington's disease. Hum. Mol. Genet. 2005;14:39–47. doi: 10.1093/hmg/ddi004. [DOI] [PubMed] [Google Scholar]
- 35.Pflanz S, Besson JA, Ebmeier KP, Simpson S. The clinical manifestation of mental disorder in Huntington's disease: a retrospective case record study of disease progression. Acta Psychiatr. Scand. 1991;83:53–60. doi: 10.1111/j.1600-0447.1991.tb05511.x. [DOI] [PubMed] [Google Scholar]
- 36.Phan J, Hickey MA, Zhang P, Chesselet MF, Reue K. Adipose tissue dysfunction tracks disease progression in two Huntington's disease mouse models. Hum. Mol. Genet. 2009;18:1006–1016. doi: 10.1093/hmg/ddn428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadler AM, Bailey SJ. Validation of a refined technique for taking repeated blood samples from juvenile and adult mice. Lab Anim. 2013;47:316–319. doi: 10.1177/0023677213494366. [DOI] [PubMed] [Google Scholar]
- 38.Saleh N, Moutereau S, Durr A, Krystkowiak P, Azulay JP, Tranchant C, Broussolle E, Morin F, Bachoud-Levi AC, Maison P. Neuroendocrine disturbances in Huntington's disease. PLoS. One. 2009;4:e4962. doi: 10.1371/journal.pone.0004962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanberg PR, Fibiger HC, Mark RF. Body weight and dietary factors in Huntington's disease patients compared with matched controls. Med. J. Aust. 1981;1:407–409. doi: 10.5694/j.1326-5377.1981.tb135681.x. [DOI] [PubMed] [Google Scholar]
- 40.Shirbin CA, Chua P, Churchyard A, Hannan AJ, Lowndes G, Stout JC. The relationship between cortisol and verbal memory in the early stages of Huntington's disease. J. Neurol. 2013a;260:891–902. doi: 10.1007/s00415-012-6732-y. [DOI] [PubMed] [Google Scholar]
- 41.Shirbin CA, Chua P, Churchyard A, Lowndes G, Hannan AJ, Pang TY, Chiu E, Stout JC. Cortisol and depression in pre-diagnosed and early stage Huntington's disease. Psychoneuroendocrinology. 2013b;38:2439–2447. doi: 10.1016/j.psyneuen.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 42.Tabrizi SJ, Langbehn DR, Leavitt BR, Roos RA, Durr A, Craufurd D, Kennard C, Hicks SL, Fox NC, Scahill RI, Borowsky B, Tobin AJ, Rosas HD, Johnson H, Reilmann R, Landwehrmeyer B, Stout JC. Biological and clinical manifestations of Huntington's disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol. 2009;8:791–801. doi: 10.1016/S1474-4422(09)70170-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomson S, Koren G, Fraser LA, Rieder M, Friedman TC, Van Uum SH. Hair analysis provides a historical record of cortisol levels in Cushing's syndrome. Exp. Clin. Endocrinol. Diabetes. 2010;118:133–138. doi: 10.1055/s-0029-1220771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trejo A, Boll MC, Alonso ME, Ochoa A, Velasquez L. Use of oral nutritional supplements in patients with Huntington's disease. Nutrition. 2005;21:889–894. doi: 10.1016/j.nut.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 45.Trejo A, Tarrats RM, Alonso ME, Boll MC, Ochoa A, Velasquez L. Assessment of the nutrition status of patients with Huntington's disease. Nutrition. 2004;20:192–196. doi: 10.1016/j.nut.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 46.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valassi E, Crespo I, Santos A, Webb SM. Clinical consequences of Cushing's syndrome. Pituitary. 2012;15:319–329. doi: 10.1007/s11102-012-0394-8. [DOI] [PubMed] [Google Scholar]
- 48.van der Burg JM, Bacos K, Wood NI, Lindqvist A, Wierup N, Woodman B, Wamsteeker JI, Smith R, Deierborg T, Kuhar MJ, Bates GP, Mulder H, Erlanson-Albertsson C, Morton AJ, Brundin P, Petersen A, Bjorkqvist M. Increased metabolism in the R6/2 mouse model of Huntington's disease. Neurobiol. Dis. 2008;29:41–51. doi: 10.1016/j.nbd.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 49.van der Burg JM, Bjorkqvist M, Brundin P. Beyond the brain: widespread pathology in Huntington's disease. Lancet Neurol. 2009;8:765–774. doi: 10.1016/S1474-4422(09)70178-4. [DOI] [PubMed] [Google Scholar]
- 50.van der Burg JM, Winqvist A, Aziz NA, Maat-Schieman ML, Roos RA, Bates GP, Brundin P, Bjorkqvist M, Wierup N. Gastrointestinal dysfunction contributes to weight loss in Huntington's disease mice. Neurobiol. Dis. 2011 doi: 10.1016/j.nbd.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 51.van Wamelen DJ, Aziz NA, Anink JJ, van SR, Angeloni D, Fraschini F, Jockers R, Roos RA, Swaab DF. Suprachiasmatic nucleus neuropeptide expression in patients with Huntington's Disease. Sleep. 2013;36:117–125. doi: 10.5665/sleep.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van DE, Selis MA, Giltay EJ, Zitman FG, Roos RA, van PH, van der Mast RC. Hypothalamic-pituitary-adrenal axis functioning in Huntington's disease mutation carriers compared with mutation-negative first-degree controls. Brain Res. Bull. 2010;83:232–237. doi: 10.1016/j.brainresbull.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 53.Vining RF, McGinley RA, Maksvytis JJ, Ho KY. Salivary cortisol: a better measure of adrenal cortical function than serum cortisol. Ann. Clin. Biochem. 1983;20(Pt 6):329–335. doi: 10.1177/000456328302000601. [DOI] [PubMed] [Google Scholar]
- 54.Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP., Jr. Neuropathological classification of Huntington's disease. J Neuropathol. Exp. Neurol. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.