Abstract
Goblet cells within the conjunctival epithelium are specialized cells that secrete mucins onto the surface of the eye. Recent research has demonstrated new characteristics of the cells, including factors influencing their differentiation, their gene products and their functions at the ocular surface. The following review summarizes the newly discovered aspects of the role of Spdef, a member of the Ets transcription factor family in conjunctival goblet cell differentiation, the newly discovered goblet cell products including claudin2, the Wnt inhibitor Frzb, and the transmembrane mucin Muc16. The current concepts of conjunctival goblet cell function, including debris removal and immune surveillance are reviewed, as are changes in the goblet cell population in ocular surface diseases. Major remaining questions regarding conjunctival cell biology are discussed.
Keywords: Goblet Cell, Goblet Cell Differentiation, Conjunctival Epithelium, Ocular Surface, SPDEF, Dry Eye
1. Introduction
A continuous layer of cells termed the epithelium covers the surface of the human body. The epithelial surface is primarily of the dry, keratinized and stratified type on the outer epidermal surface of the body, but as the epithelium involutes internally to cover the ocular surface, and the respiratory, gastrointestinal, and urogenital surfaces, it becomes a wet surfaced and non-keratinized, mucosal epithelium. At the transition zones from outer to internal epithelium, the wet surfaced epithelia remain stratified but become a simple single cell layer upon reaching more protected internal surfaces. If one were to travel over the entire surface of the epithelium covering the body surfaces, including the involuted epithelial glands, one would encounter an astounding variety of specialized epithelial cells. One such specialized cell present within several regions of the internal wet surfaced mucosal epithelia of the body is the mucin producing goblet cell. In drying, cicatrizing diseases of the ocular surface, which in their most severe stages have the potential of resulting in corneal blindness, goblet cell numbers are reduced; thus understanding their differentiation in the conjunctival epithelium is imperative.
Goblet cells are found intercalated within the epithelia of the conjunctiva, respiratory epithelium, and gastrointestinal epithelium. In the respiratory epithelia they are found as single cells interspersed in the columnar epithelium of the conducting tubes of the trachea, bronchi and large bronchioles and in the gastrointestinal epithelium they are present within the columnar epithelium of the stomach, and small and large intestine. Uniquely, in the conjunctiva, goblet cells are interspersed within a stratified epithelium.
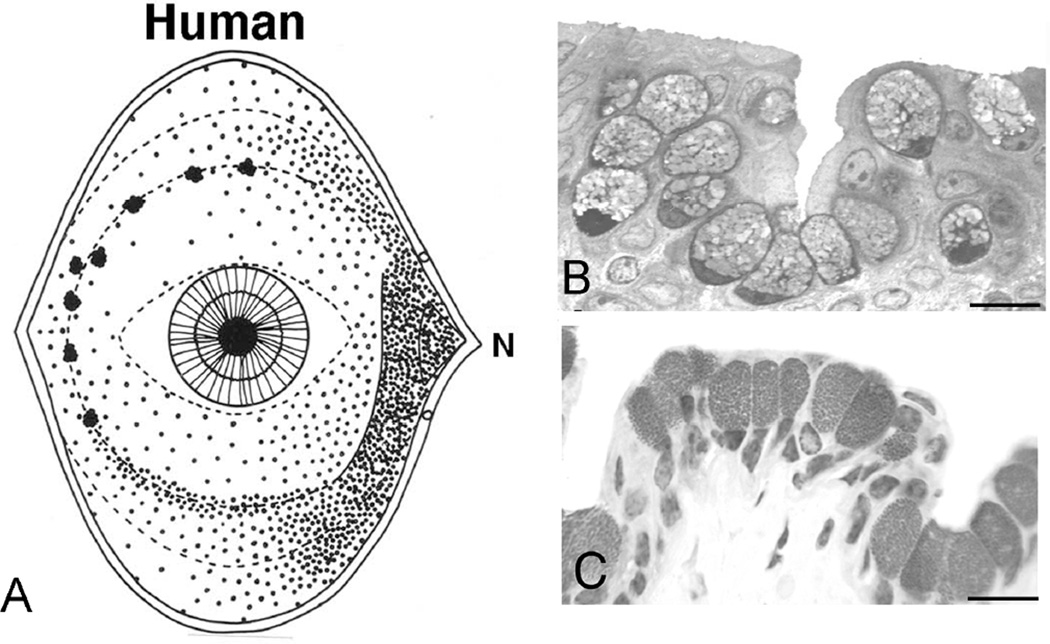
The distribution patterns of goblet cells within the conjunctival epithelium are species specific. In humans (Fig. 1A) and in dogs, the greatest density of goblet cells per area of epithelium is in the nasal region, with fewer of the cells in superior and inferior bulbar regions(Kessing, 1968; Moore et al., 1987). In humans, goblet cells can occur individually or within clusters, albeit in regions with sparser numbers, individual cells are predominant (Fig. 1B, 2A) (Gipson and Tisdale, 1997; Greiner et al., 1981). In the so called lid wiper region at the tarsal lid border adjacent to the mucocutaneous junction, goblet cells were recently found to occur individually or in clusters, and they were also present within cryptal epithelial infoldings (Fig. 1 B) (Knop et al., 2012). In rodents, goblet cells occur in basketlike clusters (Fig. 1C, Fig. 2 and Gipson and Tisdale, 1997).
Figure 1.
Goblet cell distribution on the human ocular surface (A, after (Kessing, 1968) and histologic appearance of goblet cells in (B) human and (C) mouse conjunctival epithelium. Note that the goblet cells in humans can appear singly in regions of sparse density and that they can also occur in clusters in the forniceal region as shown in A. Such a cluster is present in the section of human conjunctiva in B. In mice (C), clusters are present throughout the conjunctiva. Bars, B=10 µm, C=20 µm.
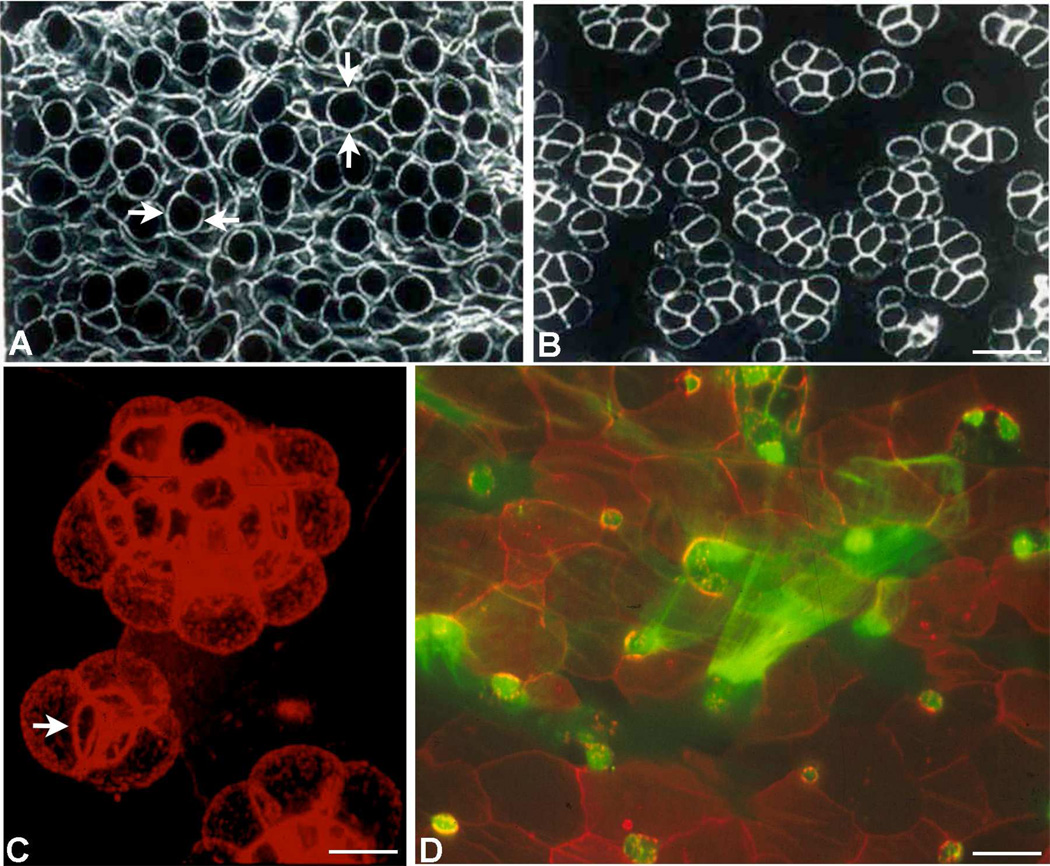
Figure 2.
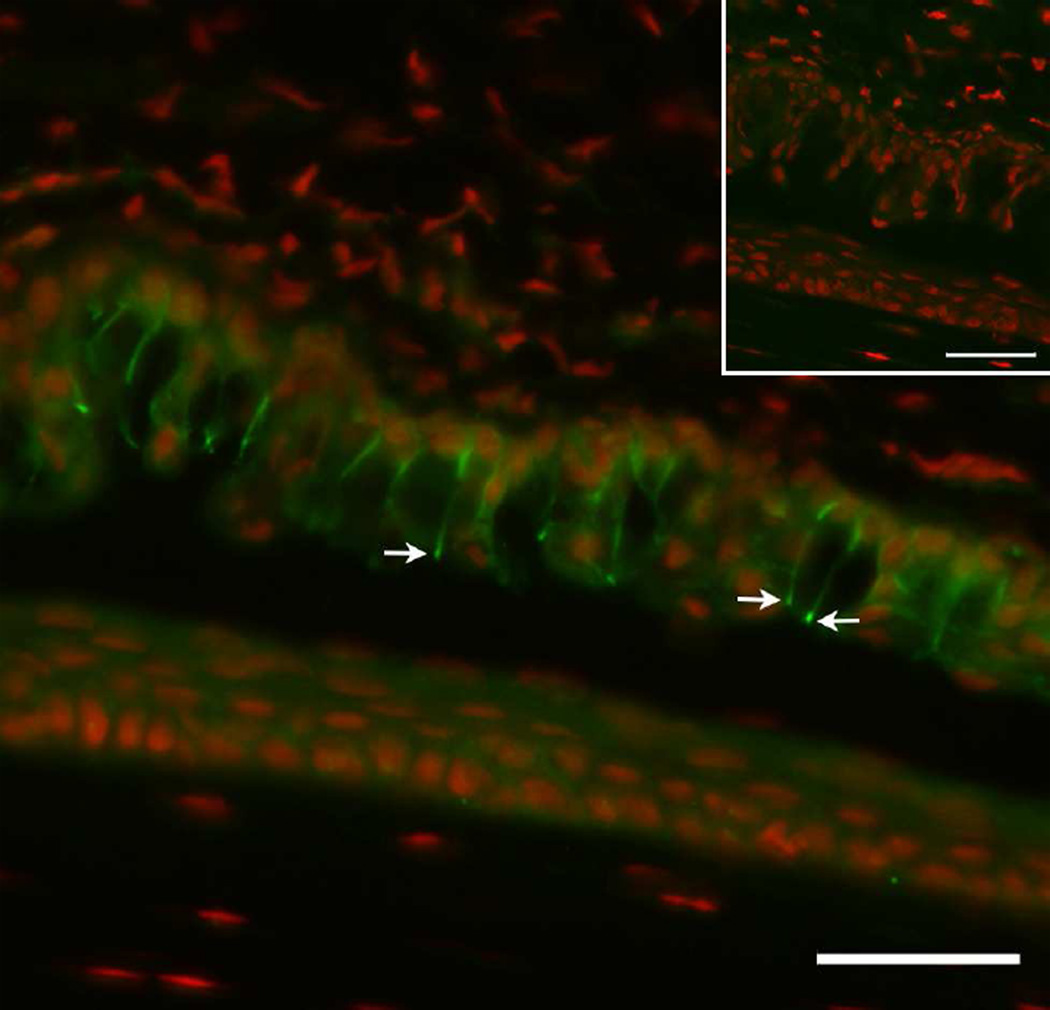
Confocal microscopy images of whole mounts of conjunctival epithelium labeled with phalloidin to demonstrate the actin cytoskeleton in goblet cells (after Gipson and Tisdale, 1997) in human (A), mouse (B), and rat (C, D). Note that in humans (A), goblet cells appear singly within the tissue (arrows) whereas in mice and rats, the goblet cells appear in clusters (B, C). The stacked image in C demonstrates the three dimensional structure of the cluster and demonstrates an apical band of actin around the orifice of the goblet cell cluster (arrow) suggesting an acinus like arrangement of cells. In D the apical surface of rat conjunctiva was double labeled with phalloidin in red and with fluorescein labeled UEA lectin in green to demonstrate mucin being secreted from goblet cells. Details of tissue preparation can be found in Gipson and Tisdale, 1997. Bars, A, B=10 µm, C=5µm, D=20µm.
2. Conjunctival goblet cell structure
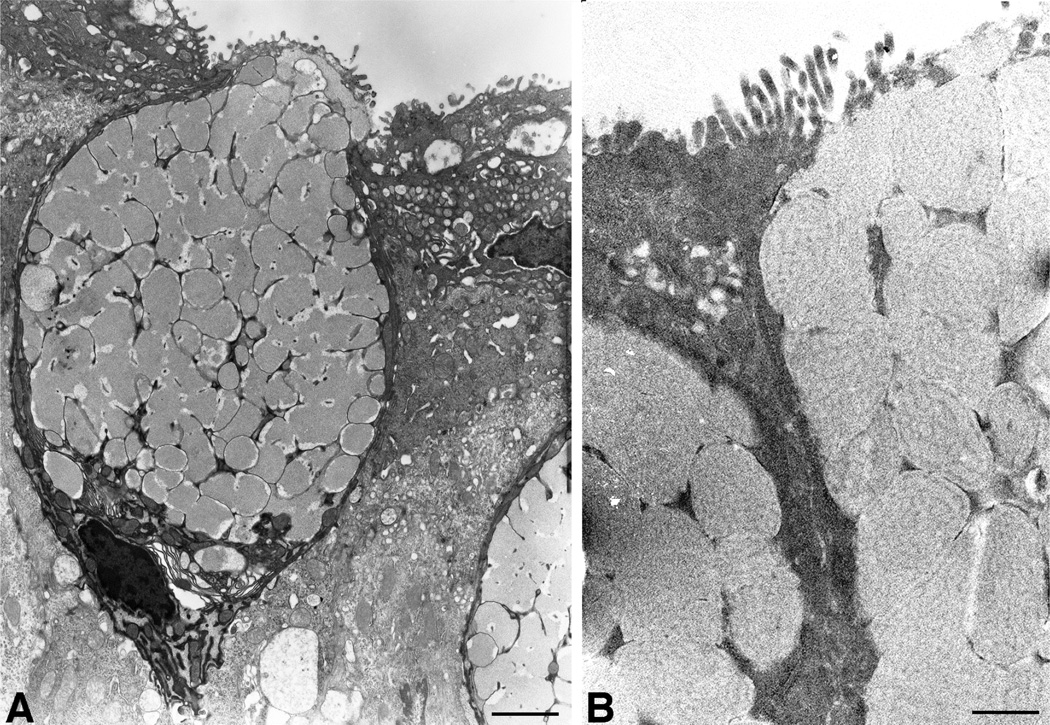
Goblet cells of the conjunctiva are plump, rounded cells, the basal membrane of which is in contact with the epithelial basement membrane, thus the goblet cell extends the entire thickness of the stratified epithelium to the apical surface (Fig. 3A). Mucin granules fill the cell leaving a thin rim of cytoplasm, rich in golgi apparati, and a nucleus crowded toward the basal cell membrane of the cell (Fig. 3A). High resolution transmission electron microscopy demonstrates a granule membrane and a “filamentous” substructure to the mucin granule content (Fig. 3B), which may reflect the packaging of the large secretory mucin glycoprotein, MUC5AC in humans (Inatomi et al., 1996), or Muc5ac or 5b in mice (Marko et al., 2014), within the granule. Packaging of the very large, heavily O-glycosylated mucins within the mucin granule is dependent on having a high concentration of multivalent cations within the granule (Perez-Vilar, 2007) and disruption of calcium levels in epithelia due to vitamin D receptor knockdown, alters goblet cell mucin levels and mucin granules in the conjunctiva of the mice (Paz et al., 2003). Membranes surrounding mucin granules in the human conjunctival goblet cells were recently demonstrated to bind antibodies to the membrane anchored mucin MUC16 (Fig. 4) (Gipson et al., 2015). The function of this membrane-associated mucin at the granule membrane is unknown. In humans but not mice, MUC16 is present on the apical cell membrane of apical cells of the cornea and conjunctival epithelium where it provides barrier function(Gipson et al., 2014) but also a dysadhesive function, preventing adherence of cells and pathogens and secreted mucin (MUC5AC) to the ocular surface (Govindarajan and Gipson, 2010). Perhaps the function of MUC16 in the goblet cell mucin granule membrane may be to facilitate expulsion of the secretory mucins from the granule membrane upon secretion.
Figure 3.
Electron micrographs of human conjunctival goblet cells at low (A) and higher magnification (B). Note the plump appearance of the polarized goblet cell in A, with the cell content taken up by mucin granules. A thin layer of cytoplasm is present along the cell periphery with the nucleus displaced toward the base of the cell. The cytoplasm at the cell base around the nucleus is enriched in golgi and endoplasmic reticulum. A high resolution image of mucin filled mucin graules in B shows a chain-like filamentous network of the mucin. Bars, A=1.5µm, B=0.5µm.
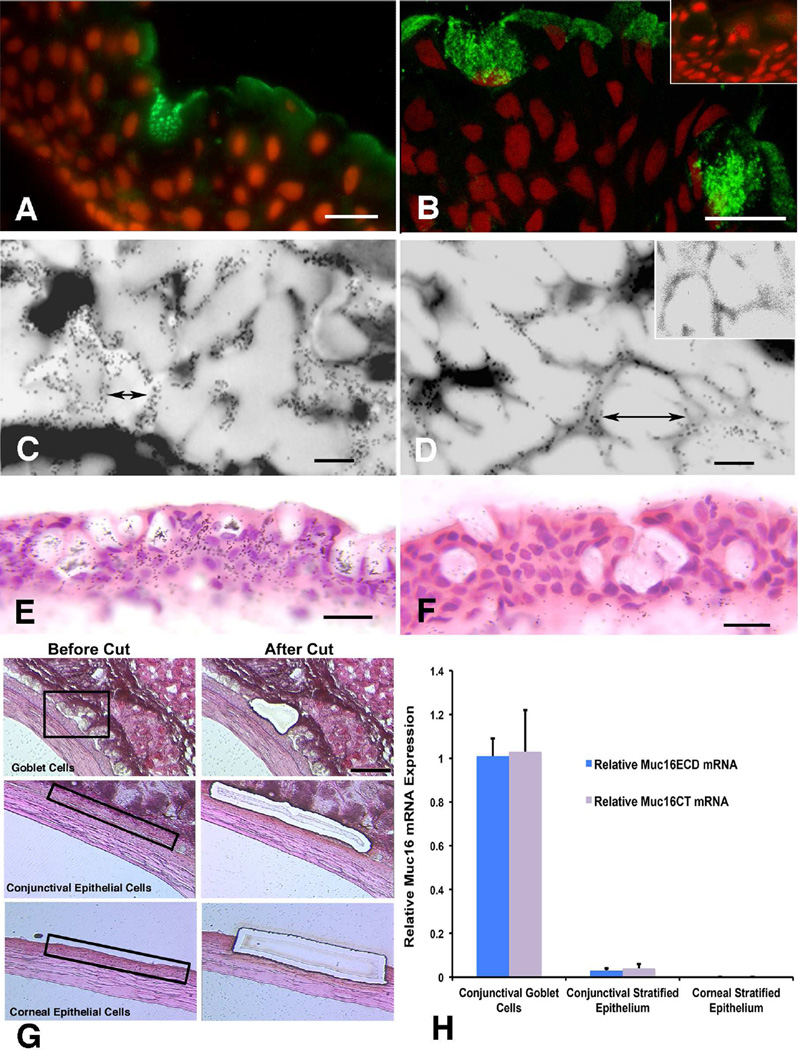
Figure 4.
Demonstration of the membrane –anchored mucin MUC16 protein and message in human goblet cells (A, B, C, D, E and F) and message in laser captured mouse conjunctival goblet cells (G, H). (Reprinted from Gipson et al., 2016, with permission.) Immunohistochemical localization of MUC16 in human goblet cells using two different antibodies (A) antibody OC125 and (B) antibody M11 shows the mucin to be localized to mucin granules. Bars=18um. By immunoelectron microscopy, using antibody H185, which recognizes a carbohydrate epitope on MUC16 (Argueso et al., 2003a), the mucin appears to be localized to the mucin granule membrane (C, D, inset in D shows 10 nm gold secondary antibody control). Bars=0.2um. In situ hybriization of MUC16 message is shown in human conjunctival goblet cells in E, using an S35 labeled antisense oligoprobe with F showing the control Sense probe. Bars=10um. G shows the method of dissection of goblet cell clusters from mouse conjunctiva as well as control conjunctival and corneal epithelium using laser microdissection. RNA from the goblet cells and the conjunctival and corneal keratinocytes was isolated from the dissected tissue and analyzed by qRT-PCR for levels of Muc16 ectodomain (Muc16ECD) and Muc16 cytoplasmic tail (Muc16CT). As noted in the graph, goblet cells have full length Muc16 but unlike the human, in the mouse neither the conjunctival nor corneal keratinocytes express the mucin.
Phalloidin staining of whole mounts of conjunctival epithelium show that an actin filament rich zonula adherens surrounds the apical-basal border of the cell (Fig. 2C) (Gipson and Tisdale, 1997) and in those species where goblet cell clusters predominate, the zonula adherens of adjacent cells form an outer circular annulus around the goblet cell cluster. This outer ring of actin appears to form an orifice to the acinar-like basket of goblet cells (Fig. 2C). These actin structures may be useful in propelling mucin from the cell as demonstrated in Figure 2D or alternatively keep the orifice closed to prevent mucin granule expulsion.
Goblet cells form tight junctions with neighboring stratified epithelial cells (Gipson et al., 2005). Recent data indicates that in the human and mouse, the conjunctival goblet cell expresses specific claudins, transmembrane components of tight junctions that associate with ZO-1 and form the junction “strands” visible in freeze fracture preparations. In humans, claudin 10 has been demonstrated to surround some of the conjunctival goblet cells (Yoshida et al., 2009) and in mice both immunohistochemical data (Fig. 5) and data derived by subtractive microarray of mRNA of laser captured conjunctival epithelium with or without goblet cells (Table 1) demonstrate that claudin 2 is goblet cell specific (Supplemental data, (Marko et al., 2013). Interestingly both Claudins 2 and 10 belong to the so-called “poreforming” members of the claudin family that regulate paracellular transport. They are found primarily in “leaky” epithelia (for review see(Gunzel and Yu, 2013; Krause et al., 2008). Perhaps these “pore-forming” claudins on conjunctival goblet cells regulate fluid flow to or from the tear film into the epithelium. It has been hypothesized that there is a conjunctival source of fluid for the tear film, but the source and mechanism by which the fluid moves into the tear film from the conjunctiva has been unclear (Mircheff, 1994). The fluid moving from the conjunctival tissue by way of the perimeter of the goblet cell may facilitate mucin hydration and expulsion from the goblet cell upon its secretion from the cell. It is well known that mucins dramatically expand upon hydration at time of secretion from the goblet cell.
Figure 5.
Localization of claudin 2, a member of the “leaky” class of tight junction proteins to the conjunctival goblet cell in the mouse (arrows). Note that the protein appears at the apical surface but also down the lateral membrane of the cell. Inset is secondary antibody control. The primary antibody was a rabbit anti-claudin 2 polyclonal (Invitrogen, Catalog #51-6100) used at 1:100, with a secondary fluoresceinated donkey anti rabbit IgG used at 1:100. Bar=20µm.
Table 1.
Genes Downregulated in Conjunctival Epithelium of Spdef −/− Mice that Lack Goblet Cells Compared to Spdef +/+ Mice (>20 Fold Change; p <0.01)
| Gene Name | NCBI Accession Number |
Gene Symbol | Fold Difference |
|---|---|---|---|
| mucin 5 subtypes A and C | NM_010844 | Muc5AC | −12.40.99 |
| SAM pointed domain containing containing ets transcription factor |
NM_013891 | Spdef | −324.46 |
| endoplasmic reticulum (ER) nucleus signaling 2 |
NM_012016 | Ern2 | −129.31 |
| frizzled-related protein | NM_011356 | Frzb | −115.67 |
| lectin, mannose-binding 1 like | NM_199222.3 | Lman1l | −102.77 |
| forkhead box A3 | NM_008260 | FoxA3 | −59 |
| chondroadherin | NM_007689 | Chad | −49.16 |
| protein tyrosine phosphatase, receptor type, N polypeptide 2 |
NM_011215 | Ptprn2 | −46.62 |
| claudin 2 | NM_016675 | Cldn2 | −44.81 |
| mucin 16 | XM_011242635.1 | Muc16 | −42.5 |
| trefoil factor 1 | NM_009362 | Tff1 | −35.55 |
| sulfotransferase family cytosolic, 1C, member 1 |
NM_018751 | Sult1c1 | −30.16 |
| glucosaminyl (N-acetyl) transferase 3, mucin type |
NM__028087 | Gcnt3 | −29.49 |
| four and a half LIM domains 1 | NM_001077361 | Fhl1 | −29.23 |
| family with sequence similarity 196, member B |
NM_001025382 | Fam196b | −27.81 |
| carbonic anhydrase 8 (CARP) | NM_007592 | Car8 | −22.52 |
| transmembrane protein 213 | NM_029921 | Tmem213 | −20.65 |
3. Location of conjunctival stem cells and goblet cell lineage
There has been intense interest in and research on the limbal basal cells as adult stem cells for the corneal epithelium, (for review see(Di Girolamo, 2015). There has, however, been less interest in the conjunctival epithelial adult stem cell population and differentiation of its specialized goblet cell. Pioneering experimental studies done by Wei et al. compared corneal and conjunctival epithelial lineages and location of conjunctival epithelial stem cells. Using cultures of rabbit cornea and conjunctival epithelium, they demonstrated that cells from corneal or conjunctival regions express only corneal and conjunctival differentiation markers respectively, suggesting the two areas have differing lineages (Wei et al., 1996; Wei et al., 1993). Using Sencar mice, the same group demonstrated that slow cycling, tritiated thymidine label retaining cells were present in all regions of the conjunctival epithelium but were most numerous in the fornicial epithelium (Wei et al., 1995) suggesting the fornix as a site enriched in adult stem cells in the conjunctiva. In addition the paper demonstrated that goblet cells in the Sencar mice also retained the tritiated thymidine label indicating that they are mitotic. Importantly, as regards goblet cell differentiation within the conjunctival epithelium, later studies by Wei et al. (Wei et al., 1997) demonstrated that single cell derived clonal cultures of rabbit conjunctival epithelial cells, when injected into BALB C athymic mice, differentiated into both keratinocytes and goblet cells, demonstrating that the conjunctival keratinocyte is bipotent and gives rise to goblet cells.
In other pioneering work, Pellegrini et al. using human conjunctival epithelial cell cultures found so called “conjunctival stem cells” with “holoclone culture characteristics” to be uniformly distributed in bulbar and fornicial conjunctiva, and they corroborated the work of Wei et al. (Wei et al., 1997) showing that conjunctival keratinocytes and goblet cells derive from a common bipotent progenitor. Interestingly though, they suggest that “conjunctival keratinocytes with high proliferative capacity give rise to goblet cells at least twice in their life and, at rather precise times of their life history, namely at 45–50 cell doublings and at approximately 15 cell doublings before senescence….conjunctival keratinocyte differentiation into goblet cells appears to be dependent upon an intrinsic “cell doubling clock”(Pellegrini et al., 1999).
More recently, a study of the location of long-term BrdU label-retaining cells of the rabbit conjunctiva, reported that the BrdU-labeled cells appeared to be concentrated at the mucocutaneous junction with the authors suggesting that epithelial stem/progenitor cells at the mucocutaneous junction serve as the source of transient amplifying cells that migrate towards the fornix (Su et al., 2011). An even more recent study of human conjunctival epithelium by Stewart et al. studied location of stem cell-like cells using colony-forming efficiency, expression of stem cell markers ABCG2, ΔNp63, and Hsp70 in cultures from multiple regions of the conjunctiva, as well as ABCG2 localization in fixed tissue. All assays consistently demonstrated stem cell character throughout the tissue but with highest levels of colony forming efficiency and label in the medial canthal and inferior forniceal areas (P < 0.01 for each) (Stewart et al., 2015). Interestingly, this site is the site of the greatest goblet cell density in humans as demonstrated by Kessing (Kessing, 1968) and shown in Figure 1A. Taken together these studies demonstrated that conjunctival and corneal lineages are distinct, that the stem cell population of the conjunctiva is distributed through the conjunctiva with an enrichment in the medial canthus, lower fornix in humans, and that conjunctival keratinocytes are bipotent giving rise to both keratinocytes and goblet cells. Despite these efforts, a biologic marker of the conjunctival epithelial stem cell and thus its precise location within the conjunctiva has not been identified to date.
But what regulates goblet cell differentiation from precursor conjunctival keratinocytes? Is there, as Pelligrini proposed, an intrinsic developmental clock within keratinocytes that regulates goblet cell differentiation, or can goblet cell differentiation be influenced extrinsically? Recent data from other mucosal epithelia in which goblet cells differentiate as well as from studies of conjunctival epithelium suggest that goblet cell differentiation can be manipulated extrinsically. Probably the best examples come from the tracheal bronchial epithelium in which goblet cell hyperplasia occurs in response to chronic pulmonary diseases including asthma, cystic fibrosis and chronic obstructive pulmonary disease (Park et al., 2007) and from the gut in response to parasitic infection(Ponce-Macotela et al., 2008). During chronic injury or exposure to allergens, epithelial cells lining the lung undergo metaplasia/hyperplasia associated with goblet cell hyperplasia and mucus hypersecretion, which are mediated by various cytokines and growth factors including IL-4, IL-13, and inducers of EGF signaling (for review see Park et al., 2007). Based on these data De Paiva et al. studied the effect of IL13 on conjunctival goblet cells in the mouse and found that the cytokine, derived primarily from natural killer T cells can increase goblet cell numbers (De Paiva et al., 2011). As demonstrated by Chen et al., IL13 in the lung enhances expression of the transcription factor SPDEF, the transcription factor that induces goblet cell differentiation (Chen et al., 2009).
4. Goblet cell differentiation
Differentiation of specialized epithelial cells from an adult stem cell reservoir has been the subject of much recent research, especially in the skin (Hsu et al., 2014), respiratory tree (Wansleeben et al., 2013) and gut (Barker et al., 2008). Similarly, differentiation of goblet cells within the wet surfaced mucosae has been extensively studied in the respiratory (Chen et al., 2014; Chen et al., 2009) and gastrointestinal epithelia (Gregorieff et al., 2009; Katz et al., 2002; Noah et al., 2010). Each mucosal region now appears to have adult stem cell reservoirs and recent data, including that from the conjunctiva (Marko et al., 2013), indicate that a common factor, a member of the ets family of transcription factors known as “sterile alpha motif pointed domain Ets factor” or “SAM pointed domain Ets factor” (SPDEF) is involved in goblet cell differentiation in wet surfaced mucosae.
4.1 SPDEF, a transcription factor that induces goblet cell differentiation
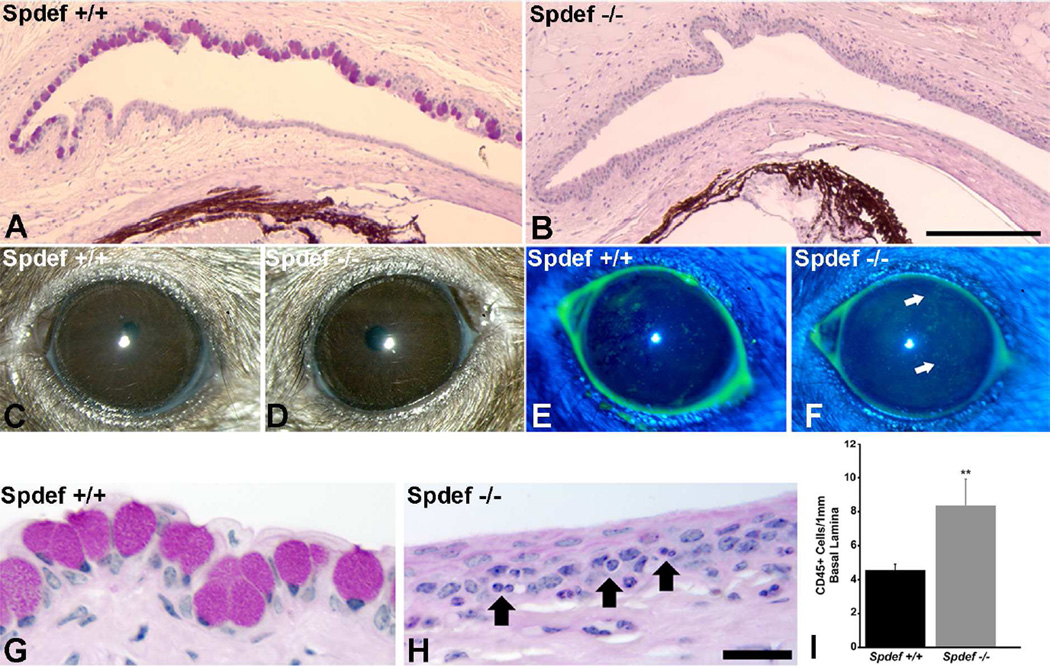
SPDEF, the SAM pointed domain ETS factor, a member of the large family of transcription factors that share the feature of a highly conserved DNA binding domain, has been demonstrated to be a common factor required for goblet cell differentiation in the gut (Gregorieff et al., 2009);(Noah et al., 2010) respiratory tree(Park et al., 2007); Chen et al., 2009) and in the conjunctiva (Fig. 6 and (Marko et al., 2013). Mice null for SPDEF lack goblet cells in all wet surfaced mucosae, as shown in the conjunctiva (Fig. 6), but curiously the mice reproduce, and appear grossly to lack a phenotype. There is however a mild ocular surface phenotype with conjunctival inflammation, expression of inflammatory and stress proteins, and fluorescein staining of the ocular surface (Fig. 6 and described in detail under “Functions of goblet cells” see below). These mice show several characteristics of dry eye making it a model for the disease, and are helpful in discerning functions of goblet cells at the ocular surface as well as goblet cell specific proteins.
Figure 6.
Demonstation of lack of goblet cells and phenotype of Spdef null mice. Comparison of the histology of conjunctiva of wild type (A) and Spdef−/− mice (B) show that goblet cells are lacking in the Spdef null mice. C and D demonstrate lack of overt phenotype in the null mice, but fluorescein staining revealed a significant increase in punctate staining (E, F (Marko et al., 2013). Histology demonstrated an increase in inflammatory cells within the conjunctival epithelium of the Spdef null mice (compare G and H), and enumeration of the number of CD45+ cells within the tissue by immunohistochemistry demonstrated a significant increase in their numbers within the Spdef−/− conjunctival epithelium compared to wild type control. Data and images after (Marko et al., 2013). Bar A, B=200µm, G, H =20µm.
4.2 Factors influencing SPDEF expression
The combined action of Notch and Wnt cascades of tethered and secreted signaling molecule families have been shown to be involved in epithelial differentiation and goblet cell differentiation (van Es et al., 2005). Several notch regulated, Kruppel like factor transcription factor family members, KLF4 and KLF5, have been correlated with gut and respiratory system epithelial goblet cell differentiation. Knockdowns or interference with each of these signaling cascades and transcription factors have resulted in loss of goblet cells in these mucosae. Data from the studies in the gut and respiratory tree laid the foundation for study of conjunctival epithelial and goblet cell differentiation. A very helpful review of similarities and differences between goblet cell differentiation in the mucosal systems was recently published (McCauley and Guasch, 2015).
In regard to conjunctival goblet cell differentiation, involvement of the Notch pathway has been demonstrated in several studies. Zhang et al. showed that conditional inhibition of canonical Notch signaling accomplished by over expressing a dominant negative mastermind-like1, a transcriptional coactivator in the Notch signaling pathway, effects conjunctival identity and goblet cell differentiation (Zhang et al., 2013). In addition to the loss of goblet cells, Notch inhibition induced conjunctival hyperplasia and abberant desquamation. In other Notch related work disruption in the mouse of either KLF4 or KLF5, members of the Notch regulated Kruppel like-family of transcription factors that are zinc finger DNA-binding proteins, each led to loss of goblet cells not only in the gut (Bell et al., 2013; Katz et al., 2002) but in the conjunctival epithelium as well (Swamynathan et al., 2007; Kenchegowda et al., 2011). Swamynathan et al. (Swamynathan et al., 2007) also demonstrated that KLF4 deficient mice have decreased Spdef message, which may explain the lack of goblet cells in these mice. In the KLF4 deficient mice, corneal fragility and stromal edema were also noted (Swamynathan et al., 2007) and in the KLF5 deficient mice, eyelids were also defective, meibomian glands were malformed, and the cornea was abnormal (Kenchegowda et al., 2011). That multiple developmental aberrations derive from the loss of Notch activity and the Klf4 and Klf5 transcription factors suggests that their involvement is upstream in the cascade of activation of factors accomplishing epithelial differentiation, not just goblet cell differentiation.
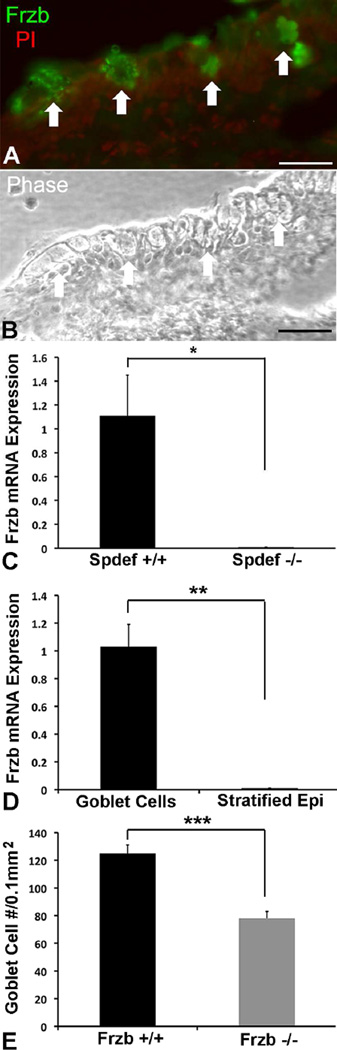
Study of Wnt pathway responsive genes has led to the current understanding of goblet cell differentiation. Spdef, the transcription factor associated with goblet cell differentiation, is such a Wnt responsive gene (Gregorieff et al., 2009). Demonstration of a potential role of the Wnt pathway in conjunctival goblet cell differentiation comes from recent work in our laboratory. Searching for goblet cell specific genes, a subtrative microarray analysis was done comparing conjunctival epithelial gene expression in wild type and spdef −/− mice lacking goblet cells. Several Wnt pathway genes , including Frzb, Dixdc1, Wnt5b and Wnt11, were significantly downregulated in the Spdef null conjunctival epithelium (Marko et al., 2013) suggesting they may be involved in either conjunctival epithelial homeostasis and/or goblet cell differentiation by way of Spdef expression. Frzb, an extracellular antagonist of the canonical Wnt signaling pathway, was the most highly downregulated of the four Wnt associated genes at 116 fold lower than in wild type. The high level of reduction of Frzb expression suggested that it is a goblet cell specific gene and investigations using immunohistochemistry and mRNA derived from goblet cells by laser capture microdisection (Fig. 7) verified that Frzb is a conjunctival goblet cell gene (Marko et al., 2013). Subsequently we obtained Frzb null mice, a kind gift of Professor Frank P Luyten, Katholieke Universiteit Leuven, Leuven Belgium (Lories et al., 2007), to determine if goblet cell numbers are altered in the conjunctivas of these mice. As demonstrated in Figure 7 E, goblet cell numbers in these mice are significantly reduced, suggesting that the Wnt antagonist is regulating a Wnt pathway cascade involved in goblet cell differentiation in the conjunctiva.
Figure 7.
Goblet cells express the WNT antagonist Frzb and mice null for Frzb have fewer conjunctival goblet cells. Antibodies to Frzb bind to goblet cells in the mouse conjunctiva (A). Arrows demonstrate position of goblet cells. B shows a phase contrast image of the same section as A. Frzb mRNA could not be detected in RNA isolated from conjunctival epithelium obtained by laser capture of the fornix of the conjunctiva of Spdef −/− mice contrary to wild type control (C). In wild type mice, Frzb mRNA was detected in RNA obtained from goblet cell clusters obtained by laser capture microdissection, whereas none was detected in the stratified epithelia outside the goblet cell areas (D). Goblet cell numbers were significantly decreased in conjunctivas of Frzb null mice. Counts of numbers of goblet cells were as per (Marko et al, 2014) and mice were a kind gift of Professor Frank P Luyten, Katholieke Universiteit Leuven, Leuven Belgium (Lories et al., 2007). Immunohistochemistry data and laser capture methods were as per (Marko et al., 2013). Bar=20µm. *p<0.05, **P<0.01, ***p<0.001
Recently an additional pathway, functional in conjunctival epithelial homeostasis and goblet cell differentiation, has been identified - the TGF beta signaling pathway. Conditional deletion of TGF beta signaling in K14 expressing cells in mice induces conjunctival epithelial hyperplasia and conjunctival goblet cell expansion(McCauley et al., 2014). The data from this study also demonstate that Spdef levels are increased in the mice suggesting that TGF beta suppresses Spdef transcription. Indeed, McCauley et al. further demonstrated that Smad3 (which forms a complex with Smad2 and Smad4 after TGF-beta binds its beta receptor 1 and phosphorylates Smad2) binds to the Spdef promotor to prevent Spdef transcription.
Another potential player in conjunctival goblet cell differentiation relative to Spdef, is the DNA binding, transcription activator FOXA3, also known as forkhead box protein A3. In the airway epithelia (Park et al., 2007); (Chen et al., 2009), and in the conjunctiva (McCauley et al., 2014) it has been demonstrated that Spdef and FoxA3 reciprocally regulate one another. More recently, again in airway epithelia, FoxA3 was shown to induce goblet cell metaplasia independently of Spdef (Chen et al., 2014). As in the lung (Park et al., 2007), in the conjunctiva, Fox A3 is highly downregulated in Spdef null mice (Table 1 and (Marko et al., 2013) indicating that Spdef is involved in regulation of FoxA3 but it is not known if FoxA3 can independently induce goblet cell differentiation in the conjunctiva.
As mentioned above, IL13 has been demonstated to induce goblet cell differentiation. In the airway epithelia, the induction of goblet cell differentiation by IL13 is through upregulation of Spdef expression mediated by the JAK-STAT pathway (Park et al., 2007). It is not known if the IL13 induction of conjunctival goblet cell differentiation (De Paiva et al., 2011)is through a similar mechanism.
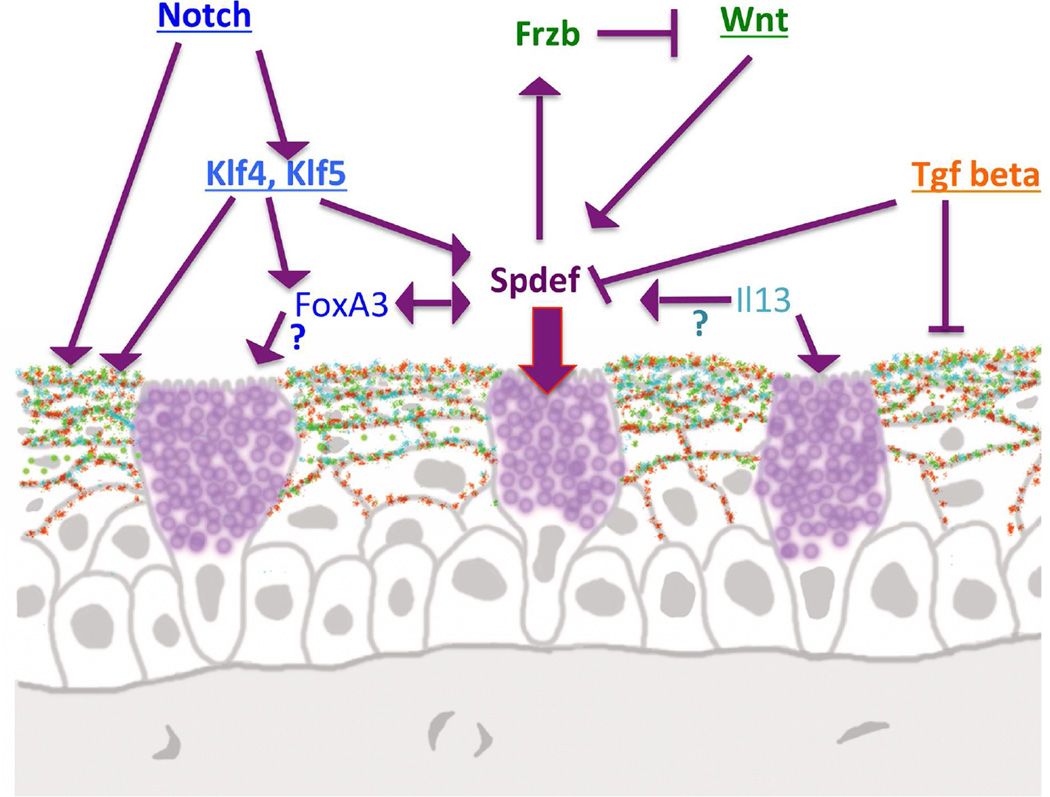
In summary, of the factors that have been demonsrated to date to decrease or cause a total loss of conjunctival goblet cell differentiation when abrogated, abrogation of SPDEF appears to cause the least disturbance of the conjunctival epithelium or cause other morphologic aberrations, suggesting that the SPDEF transcription factor is, within the signaling/transcription factor heirarchy that brings about goblet cell differentiation, down stream, close to the end point of the pathway that turns on goblet cell genes. Figure 8 summarizes recent data obtained regarding factors effecting goblet cell differentiation. McCauley and Gausch (McCauley and Guasch, 2015) have summarized the similarities and differences in factors effecting goblet cell differentiation in the gut, respiratory tree and conjunctiva, pointing out the similarity of the role of Spdef in goblet cell differentiation in all three muscosal surfaces. Spdef is perhaps to date a unique example of a common transcription factor controlling development of a specific epithelial cell type across several different regions of the mucosal epithelial surface.
Figure 8.
Diagram demonstrating factors known to date to be involved in goblet cell differentiation in the conjunctiva. Spdef appears to be a central transcription factor involved in goblet cell differentiation, being influenced by both the Notch, Wnt, and TGF beta pathways. It is not known if the IL13 induced increase in conjunctival goblet cell numbers (De Paiva et al., 2011) is through Spdef as it is in the airway epithelia (Chen et al., 2009). Similarly, a role for FoxA3 in goblet cell differentiation in airway epithelia has been demonstrated but a role for FoxA3 in conjunctival goblet cell differentiation has not been demonstrated. The FoxA3 gene is, however, highly down regulated in Spdef null mice that lack goblet cells indicating that it is Spdef regulated (Marko et al., 2013).
5. Goblet cell function at the ocular surface
At the human ocular surface, reduction of goblet cells within the conjunctiva has been demonstrated in the most severe drying cicatrizing diseases that often end in corneal keratinization and opacity (Nelson and Wright, 1986). Perhaps because of this correlation, the function of goblet cells, particularly as it relates to mucin secretion at the ocular surface, has been assumed to be vital for corneal/ocular surface function. Specific functions attributed to goblet cells, as a result of their secretion of mucins are, lubrication, maintenance of surface wetting, maintenance of tear film across the epithelium, and prevention of infection (For review see (Fahy and Dickey, 2010; Gipson and Argueso, 2003; Roy et al., 2014). Direct testing, however, of specific goblet cell function at the ocular surface has not been possible until the recent development of the Spdef null mice (Gregorieff et al., 2009) which lack goblet cells in the conjunctiva (Fig. 6 and (Marko et al., 2013). Study of the ocular surface phenotype in these mice has yielded clues to goblet cell function at the ocular surface and has yielded information on heretofore unknown goblet cell products.
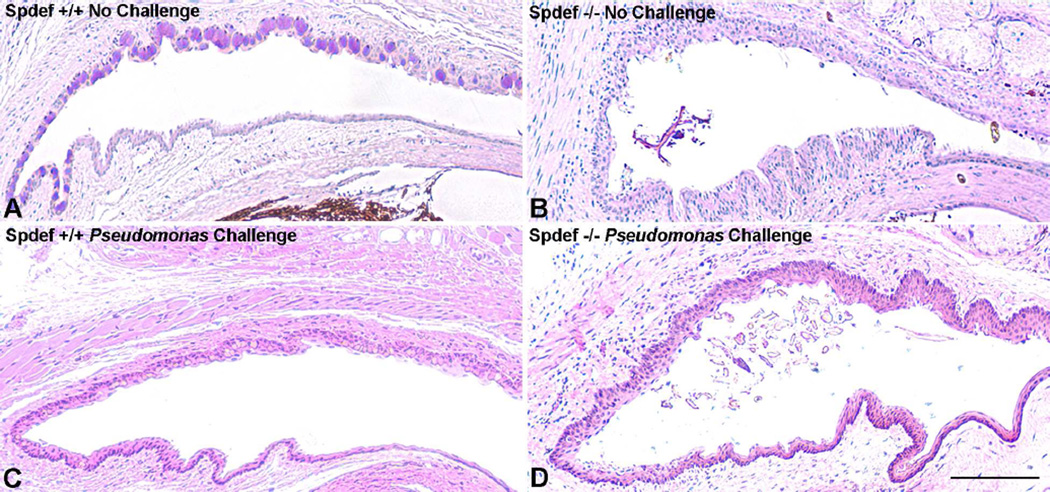
Firstly, and surprisingly, the Spdef−/− mice show no overt phenotype. Upon closer examination of the ocular surface, however, the mice show increased fluorescein staining of the cornea, increased tear volume and presence of increased inflammatory cells in the conjunctival epithelium (Fig. 6 and (Marko et al., 2013). In addition, as shown in this manuscript, debris accumulates in the conjunctival cul-de sac of the Spdef null mice compared to wild type mice (Table 2 and Fig. 9). The data demonstrate the importance of the goblet cell in clearance of debris from the ocular surface.
Table 2.
Incidence of Debris Accumulation in Conjunctival Cul-de-sac of Wild Type and Spdef Null Mice
| Genotype | No Challenge | Pseudomonas Challenge |
|---|---|---|
| Spdef +/+ | 0/19 | 0/10 |
| Spdef −/− | 10/15 | 4/10 |
Figure 9.
Accumulation of debris in conjunctival cul-de-sac of Spdef null mice compared to wild type. The conjunctival cul-de-sac of wildtype mice (A) show no debris compared to that of the Spdef null mouse (B). Debris was not present in the conjunctival cul-de-sac of wild type mice (C) even after challenge by application of Pseudomonas aeruginosa strain 6294 twice daily for three days (at 1×107 colony forming units /ml in normal saline). Debris did accumulate in the Spdef mice after Pseudomonas challenge (D). See Table 2 for compilation of data. Bar=20µm.
To test the function of goblet cells in preventing infection, we challenged the ocular surface of Spdef null (which show increased fluorescein staining-thus barrier defects) and wild type mice by application of Pseudomonas aeruginosa, strain 6294, a bacterium known to infect mice after surface wounding (Preston et al., 1997). Surprisingly, twice daily application for 3 days, of Pseudomonas aeruginosa, strain 6294 (at 1×107 colony forming units /ml in normal saline, N=16 in 3 separate experiments) did not cause ocular infection despite accumulation of debris in the cul-de-sac, of the conjunctiva (Table 2 and Fig.9). These data suggest that goblet cell secretions are not absolutely required to prevent infection as was previously thought (Fahy and Dickey, 2010; Gipson and Argueso, 2003; Roy et al., 2014).
These data correlate with data on Muc5AC and 5B null mice in which no infection was noted at the ocular surface (Marko et al., 2014) and on data from Spdef null mice in which no infections were reported in the gut (Gregorieff et al., 2009);(Noah et al., 2010) or respiratory tree(Park et al., 2007); Chen et al., 2009) . Lack of infection in these experimental mice suggests that other components of the mucosal surface such as membrane-anchored mucins, and surface antimicrobials may be sufficient barriers to prevent infection.
That goblet cells are important in maintaining conjunctival epithelial homeostasis comes from data from subtractive microarray analysis of conjunctival epithelial RNA comparing Spdef to wild type mice. In response to lack of goblet cells, the conjunctival epithelium showed increased expression of genes related to epithelial stress, keratinization, and inflammation, several of which are upregulated in human dry eye making it a model for study of dry eye (Marko et al., 2013).
Recently a new function has been ascribed to small intestine as well as colonic goblet cells - that of luminal antigen presentation to dendritic cells (Knoop et al., 2015; McDole et al., 2012). So called GAPs, “goblet cell-associated antigen passages”, form, post mucin secretion in intestinal goblet cells, to deliver antigen to underlying dendritic cells. This activity has been hypothesized to be responsible for development of immune tolerance and invokes goblet cells as players in intestinal immune homeostasis. Whether conjunctival goblet cells have such properties remains to be determined. In undifferentiated cultures of mouse conjunctival epithelium which the authors designate as goblet cells, but which on hematoxylin and eosin staining of sections of the cultures appear to be stratified, flat cells lacking goblet cell mucin packets (see Fig 6, (Contreras-Ruiz and Masli, 2015), Masli et al. report that the conjunctival cells express TGF beta2. They suggest that TGF beta 2 containing culture media from these cells induces dendritic cells in co-culture to alter their phenotype to a tolerogenic one. Definitive proof of such interactions between conjunctival goblet cells and dendritic cells requires in vivo verification. The presence of GAPS in conjunctival goblet cells and their role in providing immune tolerance at the ocular surface remains to be determined.
In summary, data from testing of goblet cell function in Spdef null mice, suggest that goblet cells, through secretions onto the ocular surface, are required to prevent corneal surface damage as evidenced by fluorescein staining and clearance of surface debris. Potentially they have a role in development of ocular surface immune tolerance by microbial or tear component sensing through “GAPS” but that role remains to be conclusively proven. Surprisingly, lack of goblet cells did not cause the anticipated corneal keratinization seen in humans with diseases in which goblet cells are severely diminished or lacking, nor did we obtain evidence of a role of goblet cells in preventing infection when the mice were challenged with Pseudomonas aeruginosa.
6. Goblet cell products and specific cell markers
6.1 Mucins
It has long been known that goblet cells secrete mucins onto wet surfaced epithelia. Mucins are a component of mucus, which, on the respiratory, gastrointestinal and reproductive tract epithelial surfaces is an opaque viscous gel. At the ocular surface, mucin is secreted by conjunctival goblet cells but a thick opaque viscous gel does not form due perhaps to the fact that upon secretion MUC5AC is of lower molecular weight (Spurr-Michaud et al., 2007). In fact mucus does not form an adherent layer on most mucosal epithelial surfaces, save the stomach and the colon where mucins form a tightly adherent thick protective layer driven to the epithelial surface, in the instance of the colon, by the water absorption from the colon content (Ermund et al., 2013). Other wet surface mucosae regions have a non-adherent mucus which is moved over the surface of the epithelium by mucosae specific methods; by eyelid blink, ciliary action on the trachea and bronchi, and peristaltic movement in the gut.
Mucins are defined as heavily O-glycosylated glycoproteins that have tandem repeats of amino acids rich in serine, threonine and proline in their protein backbones. There are at least 20 mucin genes encoding different mucin gene products. They are termed MUCs in humans and Mucs in mice, and they fall into two categories: secreted and membrane-anchored(For review of mucin nomenclature and the structure/ function of the two classes of mucins, secreted and membrane-anchored, see (Gipson et al., 2004; Govindarajan and Gipson, 2010; Rachagani et al., 2009; Thornton et al., 2008).
In humans, the mucin gene expressed by human conjunctival goblet cells is the secreted polymeric mucin MUC5AC (Inatomi et al., 1996). Another secreted mucin MUC2 has been described as present in the conjunctival goblet cells, but the levels of this mucin are some 5900 fold less than that of MUC5AC (McKenzie et al., 2000). In mice by comparison, two populations of goblet cells are present in the conjunctiva, those that express Muc5AC, and a smaller population that express the secretory mucin Muc5B (Marko et al, 2014). Recent studies testing the function of these two mucins on mouse ocular surface were done using mice null for either Muc5AC or Muc5B. No discernible phenotype was noted in either mouse, however there was a significant upregulation of Muc5b in the Muc5AC null mice, so in neither mouse was there a complete lack of secreted mucin making assessment of specific mucin difficult (Marko et al., 2014). These data also suggest that Muc5AC and Muc5b serve the same function at the mouse ocular surface.
As stated above in the section on Goblet Cell Structure, we recently demonstrated that the membrane-anchored mucin MUC16 is expressed by both human and mouse goblet cells (Fig. 4 and(Gipson et al., 2015). This mucin, the largest of the membrane-anchored class and also the largest glycoprotein in humans at 22,152 amino acids, is present in the glycocalyx of the apical surface of human cornea and conjunctival epithelium(Govindarajan and Gipson, 2010). The mouse homologue is much smaller at 8,873 amino acids and it is not expressed by corneal and conjunctival keratinocytes (Gipson et al., 2015). Thus in the mouse conjunctiva, Muc16 is a goblet cell specific protein.
6.2 Non-mucin proteins
Other proteins expressed in and specific to the goblet cells in conjunctival epithelia include trefoil factor’s 1and 3, (TFF1, TFF3) (Langer et al., 1999). Goblet cell derived trefoil factors at the ocular surface have been shown, to enhance corneal epithelial wound healing in vitro and in vivo (Paulsen et al., 2008). In the gut they have also been shown to bind secreted mucins and purportedly stabilize mucin networks (for review see (Kim and Ho, 2010).
An enzyme involved in glycosylation of mucins has been demonstrated to be a conjunctival goblet cell product in humans (Argueso et al., 2003b). This enzyme is the GalNAc-transferase-T6. Enzymes in the GalNAc-Transferases family are responsible for the initial step in O-glycosylation and are thus involved in glycosylation of the mucins within the goblet cell. In conjunctival epithelium from patients with ocular cicatricial pemphigoid, (now called mucous membrane pemphigoid), GalNAc-transferase-T6 appears to be lost coincident with keratinization of the ocular surface (Argueso et al., 2003b).
Based on immunochemical assay, Keratin 7 has been reported to be a goblet cell specific marker (Krenzer and Freddo, 1997). Immunohistochemical assay of the keratin has been subsequently used to identify goblet cells in cultures of conjunctival epithelial cells (Shatos et al., 2001; Tukler Henriksson et al., 2015). In recent comparative microarray studies of conjunctival RNA from wild type mice and Spdef null mice lacking goblet cells, no change in keratin 7-gene expression could be found with the loss of the goblet cells suggesting that the keratin may not be goblet cell specific (Marko et al., 2013). Microarray data from the study is available at the National Center for Biotechnology Information Gene Expression Omnibus (http://www.ncbi.nim.nih.gov/projects/geo;accession number GSE44101). In the original images published by Krenzer and Freddo, one can see binding of the K7 antibody to cells outside the goblet cell (see Fig. 1C Krenzer and Freddo, 1997). Validation of keratin 7 as a marker restricted to goblet cells can be validated by comparing RNA isolated from goblet cells to that of keratinocytes isolated by laser microdissection, or by use of in situ hybridization.
Validation of immunohistochemical binding studies of Keratin 7 and use of keratin 7 as a marker of goblet cells, can be done using molecular methodologies in which either native tissue goblet cell RNA is isolated by laser capture methodologies and screened for keratin 7 message, or by in situ hybridization studies localizing the Keratin 7 message to goblet cells.
As mentioned above, Claudin 10 has been demonstrated by immunohistochemistry to be localized to human conjunctival goblet cell tight junctions (Yoshida et al., 2009). We demonstrate here (Fig. 5 and(Marko et al., 2013) that claudin 2, is a goblet cell specific gene but in the mouse only. As both these claudins belong to the “leaky, pore-forming” class of tight junction proteins that are permeable to water and have cation selectivity, a goblet cell specific junction appears to be characteristic of the cell. The function of this type of tight junction at the conjunctival surface is unclear, but recent data using vitamin D receptor (VDR) null mice, indicate that Claudin 2 is directly regulated by VDR (Zhang et al., 2015). Previously we demonstrated alteration of goblet cell mucin production and mucin granule morphology in the VDR null mice (Paz et al., 2003).
6.3 Recently identified and candidate proteins
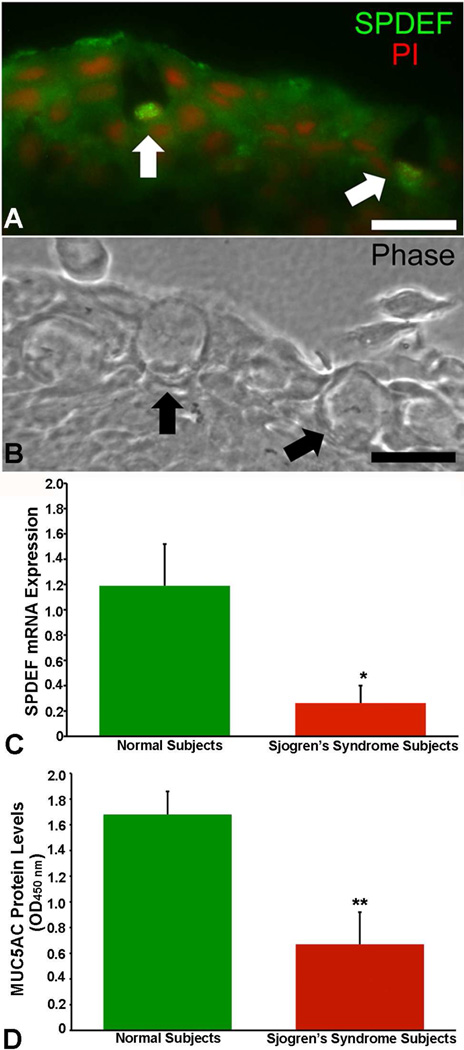
Several candidates for goblet cell specific products arose from the comparative microarray analysis of conjunctival epithelial mRNA from wild type mice and Spdef−/− mice lacking goblet cells(Marko et al., 2013). A number of genes showed dramatic downregulation in the epithelium lacking goblet cells, making them candidates for goblet cell specific expression (Marko et al., 2013). A list of those genes with greater that 20 fold downregulation are listed in Table 1. These down regulated genes included not only the mucins Muc5AC, and Spdef, the later shown by immunohistochemistry to be present in the conjunctival goblet cell nucleus (Fig. 10), but another gene known to be involved in mucin production/goblet cell differentiation in the lung, FoxA3 (Chen et al., 2014). It is not clear in the conjunctiva how FoxA3 effects goblet cell differentiation (Fig. 8).
Figure 10.
SPDEF is localized to conjunctival goblet cells while conjunctival epithelia from patients with Sjogrens Dry Eye have decreased levels of message for both Spdef and the goblet cell mucin MUC5AC. A and B are photomicrographs of human conjunctival epithelium, with A showing fluoresceinated antibody immunolocalization of Spdef, with goblet cells indicated by arrows, and nuclei localized with Propidium iodide (PI). B is a phase contrast image of the same section as in A. Bar=20µm. C and D show significantly lower levels of SPDEF message and MUC5AC protein levels in impression cytology samples of conjunctival epithelium from normal subjects and Sjogrens dry eye subjects, respectively. (*P<0.05, **P<0.01) SPDEF immunolocalization data and SPDEF message data from Marko et al, 2013. MUC5AC data from (Argueso et al., 2002).
In addition the array identified four WNT family genes, one of which Frzb, also known as SFRP3, was dramatically downregulated by 115 fold, making it a candidate goblet cell specific gene. As reported (Marko et al., 2013), and demonstrated above in the section on goblet cell differentiation this Wnt antagonist is goblet cell specific and mice lacking the gene have significantly fewer goblet cells (Fig. 7). The inhibitor has been shown to be a goblet cell specific marker in the mouse since both Frzb message and protein has been demonstrated to be present in the goblet cell. Whether this antagonist is also specific to the human conjunctival goblet cell remains to be determined and may be a fruitful avenue for future research.
There are several other candidate genes in the comparative array data that show dramatic down regulation that are worth future study as either potential goblet cell specific markers or for their function in goblet cells (Table 1). Several of these include, in order of the degree of down regulation, endoplasmic reticulum (ER) to nucleus signaling 2, lectin mannose-binding 1 like, and chondroadherin, known to be a component of cartilage.
Use of valid goblet cell markers for future research on goblet cell differentiation is important in moving the field forward. Table 3 lists data on validated conjunctival goblet proteins along with proteins for which partial validation is available. Validation of goblet cell markers should include demonstration of both protein and message data, not just immunohistochemical data. This is particularly important for goblet cells since the mucins produced and stored by the cells are by nature “sticky” (Cone, 2009) and bind antibodies indiscriminately (Moniaux et al., 2004). Additionally mucin antibodies are notoriously difficult to characterize and often lack clear demonstration of specificity. It is also “good practice” to routinely assess RNA and protein levels of the putative goblet cell markers marker as one does experimental work to discern goblet cell functions both in vivo and in vitro.
Table 3.
List of Conjunctival Goblet Cell Proteins
| Gene/Protein | Species | Methods of Assay | Reference |
|---|---|---|---|
| A. Validated Markers = Demonstration of mRNA and Protein in Goblet Cells | |||
| MUC5AC mucin | Human, Rat, Mouse |
ISH, IH | (Inatomi et al., 1996) (Tei et al., 1999) Marko et al., 2014) |
| Frzb | Mouse | LCM – PCR, IH | (Marko et al., 2013) |
| Spdef | Human, Mouse | LCM – PCR, IH | (Marko et al., 2013) |
| Muc16 | Human*, Mouse | IH, IEM, ISH, LCM – PCR | (Gipson et al., 2015) |
| B. Proteins with Protein Data Only | |||
| Keratin 7 | Human | IH | (Krenzer and Freddo, 1997) |
| TFF1, TFF3 | Human | IH | (Langer et al., 1999) |
| Claudin 10 Claudin 2 |
Human Mouse |
IH IH |
(Yoshida et al., 2009) This manuscript |
| GalNAc-T6 | Human | IH | (Argueso et al., 2003b) |
MUC16 is also present in human corneal and conjunctival stratified epithelium, so it is not goblet cell specific in humans
ISH = In situ Hybridization
IH = Immunohistochemistry
LCM – PCR = Laser Capture Microdissection of Goblet Cells and RNA assayed by RT-PCR
IEM = Immunoelectron Microscopy
7. Models for study of goblet cell function/biology
Since the ocular surface and conjunctiva is exposed to the outside environment, it is particularly amenable to sampling compared to other mucosal sites. This has facilitated studies of the human conjunctival epithelium by biopsy and surface epithelial sampling by impression cytology. These methods have facilitated study of goblet cell numbers per unit area in a number of clinical studies (Nelson and Wright, 1986) as well as mucin gene expression in normal (Inatomi et al., 1995; Inatomi et al., 1996) and diseased conjunctiva (Danjo et al., 1998; Gipson et al., 2011). While assay of human conjunctiva is critical for discovery of human relevant goblet cell characteristics, the biopsy and impression cytology sampling does not allow for in depth experimental manipulation. For these reasons experimental animal models and tissue culture methods have been developed to study goblet cell biology.
Mouse models have been particularly useful in bringing new information regarding conjunctival goblet cell differentiation and function. As cited above, the Spdef −/−, Muc5ac −/−, Muc5b−/−, conditional Tgb2 −/−, conditional Notch −/−, and conditional Kruppel Like Factor −/− mice have all been used to study conjunctival goblet cell biology. The Spdef −/− mouse with its ocular surface phenotype that mimics in several aspects, human mild to moderate dry eye represents a convenient model for the disease since it does not require injection of scopolamine and exposure to a dry eye chamber to induce ocular surface inflammation characteristic of dry eye (Marko et al., 2013). Future research dissecting out pathways that regulate Spdef and the search for markers of progenitors of goblet cells will undoubtedly require additional mouse models.
Culture methods for airway epithelium (tracheal, bronchial) in which the epithelial cells fully differentiate to polarize, and produce both ciliated cells and interspersed goblet cells have been developed (Fulcher et al., 2005). The cultures were used to immortalize bronchial epithelial cells at first passage by inserting both hTERT and the protooncogene Bmi-1; these cells exhibited extended cell life span, and also differentiated to form a pseudostratified epithelium, with apical basal polarization, numerous mucous secretory cells (goblet cells) and some ciliated cells (Fulcher et al., 2009). These culture models in which goblet cells differentiate have been used extensively to study airway epithelia epithelial cell differentiation and function, especially as it relates to cystic fibrosis. In the gut, goblet cells have been cultured in a polarized monolayer from an adenoma cell line (Phillips et al., 1988), but more recently a culture method was developed that allows maintenance of high-purity Lgr5+ intestinal epithelial stem cells in vitro. In the intestine, adult stems cell markers have been identified (Lgr5+) and the cells are maintained in vivo by their direct association with Paneth cells. Yin et al., discovered that two small molecules, CHIR99021 and valproic acid, that target Wnt, Notch and BMP pathway are sufficient to maintain the stem cells in vitro allowing study of differentiation of the cells into mature enterocytes, goblet cells and Paneth cells (Yin et al., 2014). By comparison to methods for airway and gut epithelia, culture of conjunctival epithelium to fully differentiate into a differentiated, polarized epithelium with mature goblet cells interspersed, has to date not been accomplished and is a promising area of future research.
Several culture methods for conjunctival epithelium have been reported but they do not produce fully differentiated polarized goblet cells. A human hTERT, p53 and p16 abrogated, immortalized conjunctival epithelial cell line has been developed, which, when cultured with serum, stratifies to produce 3–5 layers of conjunctival epithelial keratinocytes that are polarized apically, and that express membrane-anchored mucins apically comparable to the native tissue (Gipson et al., 2003). Despite some expression of MUC5AC, no fully differentiated goblet cells have been grown from the cell line (Gipson et al., 2003). Since the conjunctival cells that were hTERT transfected were at fourth passage (Rheinwald et al., 2002), the bipotent stem cells that give rise to both keratinocytes and goblet cells may have already been lost. Development of an hTERT immortalized conjunctival epithelial cell line from initial primary culture is needed.
Primary cultures of epithelia from minced rat, mouse, and human forniceal conjunctiva have been used to study factors that induce goblet cell secretion and factors effecting that secretion (for example see(Hodges et al., 2012). The cell populations in the cultures show Muc5AC and keratin 7 immunohistochemically and are termed goblet cells. Histology of these cells show stratified layers of cells that do not appear to be polarized nor does one see in published figures a mucin granule filled cytoplasm. Thus the cells of the culture system lack classic goblet cell morphology (Contreras-Ruiz and Masli, 2015; Shatos et al., 2001; Tukler Henriksson et al., 2015).
In order to gain information on conjunctival goblet cell differentiation, improved cell culture systems are needed in which stem cells can give rise to both differentiated keratinocytes and fully differentiated goblet cells within the same culture. Such a mixed culture that mimics native epithelia is essential for understanding conjunctival stem cell differentiation. As cited above, in the small intestine epithelial stem cell maintenance requires that the stem cell be in contact with Paneth cells to maintain its stem cell characteristics (Yin et al., 2014). Since goblet cell populations appear to be most dense in conjunctival epithelial regions that show Brdu label retention (in mice, (Wei et al., 1995) as well as areas that show binding of stem cell markers (in humans,(Stewart et al., 2015), is it possible that adult stem cells in the conjunctiva interact with goblet cells? In human drying cicatrizing diseases, loss of goblet cells coincides with keratinization of the ocular surface epithelium.
8. Alterations of goblet cells in ocular surface disease
Unlike the lung where goblet cell hyperplasia accompanies major lung diseases such as COPD, cystic fibrosis, chronic bronchitis and asthma (Kim and Criner, 2013; Rubin, 2014) diseases affecting goblet cell numbers at the ocular surface, are primarily ones in which the goblet cells numbers are decreased compared to normal controls. Impression cytology sampling of dry eye diseases of several etiologies including, Keratoconjunctivitis sicca (KCS), radiation KCS, Blepharitis with KCS demonstrated lower numbers than controls and in the cicatrizing diseases, including ocular cicatricial pemphigoid and Stevens Johnson syndrome goblet cell numbers have been reported to be reduced by 95% (Nelson and Wright, 1986). In Sjogrens Syndrome dry eye, goblet cell numbers (Kunert et al., 2002) as well as expression of MUC5AC (Argueso et al., 2002), and SPDEF (Figure 10(Marko et al., 2013) have been demonstrated to be reduced, thus goblet cell numbers may be dependent on the level of SPDEF in this human dry eye disease. Factors influencing SPDEF production in the conjunctiva may be useful therapies for treating drying and cicatrizing dry eye disease.
Goblet cell numbers were also shown to be decreased in patients with Graft Versus Host Disease (GVHD) dry eye compared to patients with allogeneic hematopoietic stem cell transplantation without dry eye as well as normal subjects (Wang et al., 2010). Patients with GVHD dry eye and fewer goblet cells also showed significantly higher conjunctival inflammatory cells. A significant decrease in MUC5AC expression in patients with atopic keratoconjunctivitis has also been noted (Dogru et al., 2008). This reduction in goblet cell product is also associated with inflammation. Inflammation and the immune system’s influence in regulation of goblet cell numbers has been studied extensively in mouse models of dry eye (for review see (Pflugfelder et al., 2013) where it has been demonstrated that IL13 derived from NK or NKT cells induces goblet cell differentiation (De Paiva et al., 2011), but curiously interferon gamma production, a cytokine that plays a role in inducing damage in Sjogrens dry eye, is also produced by NK or NKT cells. Seemingly a complex balance in cytokine production may be responsible for regulating the inflammatory response to dry eye, which in turn regulates goblet cell differentiation (Pflugfelder et al., 2013).
Vitamin A has long been known to effect mucosal epithelial differentiation and its deficiency impacts ocular surface epithelial health and causes loss of goblet cells in the conjunctiva (Sommer, 1983). Indeed conjunctival impression cytology analysis has been used to assess vitamin A status as indicated by number of goblet cells and presence of large squamous cells in the conjunctival specimens (Natadisastra et al., 1988). Retinoic acid has been demonstrated both in rats (Tei et al., 2000) and in human conjunctival epithelium in vitro to effect mucin gene expression (Hori et al., 2005).
In summary many forms of dry eye disease show diminution of conjunctival goblet cell numbers. Understanding goblet cell differentiation in the conjunctiva in the context of the stratified conjunctival epithelium is, by comparison to knowledge regarding the airway and intestinal epithelium, in its infancy. Development of methods to understand conjunctival differentiation will undoubtedly yield clues for treatments for human ocular surfaces diseases, particularly the drying and cicatrizing forms. The field would profit greatly from direct studies of conjunctival goblet cell differentiation from adult stem cells in human relevant models.
9. Future directions/Remaining questions
Many questions remain regarding the conjunctival goblet cells. First of all, what is its life history? How long does a conjunctival goblet cell live? Does the cell fill and secrete repeatedly? Does the conjunctival goblet cell exhibit baseline continuous secretion as appears to be in the case in the human bronchial epithelium (Zhu et al., 2015) or does it secrete bolus fashion only when stimulated? What is the relationship between conjunctival goblet cells and the conjunctival keratinocyte? These questions about the fundamental biology of the conjunctival goblet cell have not been addressed.
In order to address such fundamental questions better culture methods of conjunctival epithelium are needed. Such culture models would, as do models for airway epithelium and gut epithelium, incorporate conjunctival epithelial stem cells that produce a differentiated conjunctival epithelium with both stratified epithelial keratinocytes and goblet cells. Since the mucins are excellent differentiation markers, both membrane mucins and the goblet cell specific mucin MUC5AC can be used to determine differentiation of cultures, but both message level and protein content need to be assayed. Identification of a conjunctival adult stem cell marker would tremendously facilitate development of such culture systems and development of an immortalized cell line that differentiates into both conjunctival keratinocytes as well as goblet cells is needed.
Acknowledgments
The work reported in this manuscript was supported by a grant, NIH NEI EY003306. The author wishes to acknowledge the assistance of Sandra Spurr-Michaud and Ann Tisdale, long term staff in the Gipson Lab, as well as Christina Marko, whose post-doctoral research is reviewed in this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Argueso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjögren’s syndrome. Invest Ophthalmol Vis Sci. 2002;43:1004–1011. [PubMed] [Google Scholar]
- Argueso P, Spurr-Michaud S, Russo CL, Tisdale A, Gipson IK. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Investigative ophthalmology & visual science. 2003a;44:2487–2495. doi: 10.1167/iovs.02-0862. http://dx.doi.org/10.1167/iovs.02-0862. [DOI] [PubMed] [Google Scholar]
- Argueso P, Tisdale A, Mandel U, Letko E, Foster CS, Gipson IK. The cell-layer- and cell-type-specific distribution of GalNAc-transferases in the ocular surface epithelia is altered during keratinization. Investigative ophthalmology & visual science. 2003b;44:86–92. doi: 10.1167/iovs.02-0181. http://dx.doi.org/10.1167/iovs.02-0181. [DOI] [PubMed] [Google Scholar]
- Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes & development. 2008;22:1856–1864. doi: 10.1101/gad.1674008. http://dx.doi.org/10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SM, Zhang L, Xu Y, Besnard V, Wert SE, Shroyer N, Whitsett JA. Kruppel-like factor 5 controls villus formation and initiation of cytodifferentiation in the embryonic intestinal epithelium. Developmental biology. 2013;375:128–139. doi: 10.1016/j.ydbio.2012.12.010. http://dx.doi.org/10.1016/j.ydbio.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Korfhagen TR, Karp CL, Impey S, Xu Y, Randell SH, Kitzmiller J, Maeda Y, Haitchi HM, Sridharan A, Senft AP, Whitsett JA. Foxa3 induces goblet cell metaplasia and inhibits innate antiviral immunity. American journal of respiratory and critical care medicine. 2014;189:301–313. doi: 10.1164/rccm.201306-1181OC. http://dx.doi.org/10.1164/rccm.201306-1181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, Gregorieff A, Clevers H, Whitsett JA. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. The Journal of clinical investigation. 2009;119:2914–2924. doi: 10.1172/JCI39731. http://dx.doi.org/10.1172/JCI39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RA. Barrier properties of mucus. Advanced drug delivery reviews. 2009;61:75–85. doi: 10.1016/j.addr.2008.09.008. http://dx.doi.org/10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Contreras-Ruiz L, Masli S. Immunomodulatory cross-talk between conjunctival goblet cells and dendritic cells. PloS one. 2015;10:e0120284. doi: 10.1371/journal.pone.0120284. http://dx.doi.org/10.1371/journal.pone.0120284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danjo Y, Watanabe H, Tisdale AS, George M, Tsumura T, Abelson MB, Gipson IK. Alteration of mucin in human conjunctival epithelia in dry eye. Invest Ophthalmol Vis Sci. 1998;39:2602–2609. [PubMed] [Google Scholar]
- De Paiva CS, Raince JK, McClellan AJ, Shanmugam KP, Pangelinan SB, Volpe EA, Corrales RM, Farley WJ, Corry DB, Li DQ, Pflugfelder SC. Homeostatic control of conjunctival mucosal goblet cells by NKT-derived IL-13. Mucosal immunology. 2011;4:397–408. doi: 10.1038/mi.2010.82. http://dx.doi.org/10.1038/mi.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Girolamo N. Moving epithelia: Tracking the fate of mammalian limbal epithelial stem cells. Progress in retinal and eye research. 2015;48:203–225. doi: 10.1016/j.preteyeres.2015.04.002. http://dx.doi.org/10.1016/j.preteyeres.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Dogru M, Matsumoto Y, Okada N, Igarashi A, Fukagawa K, Shimazaki J, Tsubota K, Fujishima H. Alterations of the ocular surface epithelial MUC16 and goblet cell MUC5AC in patients with atopic keratoconjunctivitis. Allergy. 2008;63:1324–1334. doi: 10.1111/j.1398-9995.2008.01781.x. http://dx.doi.org/10.1111/j.1398-9995.2008.01781.x. [DOI] [PubMed] [Google Scholar]
- Ermund A, Schutte A, Johansson ME, Gustafsson JK, Hansson GC. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. American journal of physiology. Gastrointestinal and liver physiology. 2013;305:G341–G347. doi: 10.1152/ajpgi.00046.2013. http://dx.doi.org/10.1152/ajpgi.00046.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy JV, Dickey BF. Airway mucus function and dysfunction. The New England journal of medicine. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. http://dx.doi.org/10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods in molecular medicine. 2005;107:183–206. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- Fulcher ML, Gabriel SE, Olsen JC, Tatreau JR, Gentzsch M, Livanos E, Saavedra MT, Salmon P, Randell SH. Novel human bronchial epithelial cell lines for cystic fibrosis research. American journal of physiology. Lung cellular and molecular physiology. 2009;296:L82–L91. doi: 10.1152/ajplung.90314.2008. http://dx.doi.org/10.1152/ajplung.90314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson I, Argueso P. The role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol. 2003;231:1–49. doi: 10.1016/s0074-7696(03)31001-0. http://dx.doi.org/10.1016/S0074-7696(03)31001-0. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Hori Y, Argueso P. Character of ocular surface mucins and their alteration in dry eye disease. Ocul Surf. 2004;2:131–148. doi: 10.1016/s1542-0124(12)70149-0. http://dx.doi.org/10.1016/S1542-0124(12)70149-0. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Joyce NC, Zieske JD. The Anatomy and Cell Biology of the Human Cornea, Limbus, Conjunctiva, and Adnexa. In: Foster CS, et al., editors. Smolin and Thoft’s The Cornea:scientific foundations and clinical practice. Lippincott Williams and wilkins; Phildelphia, PA: 2005. pp. 1–35. [Google Scholar]
- Gipson IK, Spurr-Michaud S, Argueso P, Tisdale A, Ng TF, Russo CL. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2496–2506. doi: 10.1167/iovs.02-0851. http://dx.doi.org/10.1167/iovs.02-0851. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Spurr-Michaud S, Tisdale A. Human conjunctival goblet cells express the membrane associated mucin MUC16: Localization to mucin granules. Experimental eye research. 2015;145:230–234. doi: 10.1016/j.exer.2015.12.009. http://dx.doi.org/10.1016/j.exer.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson IK, Spurr-Michaud S, Tisdale A, Menon BB. Comparison of the transmembrane mucins MUC1 and MUC16 in epithelial barrier function. PloS one. 2014;9:e100393. doi: 10.1371/journal.pone.0100393. http://dx.doi.org/10.1371/journal.pone.0100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson IK, Spurr-Michaud SJ, Senchyna M, Ritter R, 3rd, Schaumberg D. Comparison of mucin levels at the ocular surface of postmenopausal women with and without a history of dry eye. Cornea. 2011;30:1346–1352. doi: 10.1097/ICO.0b013e31820d852a. http://dx.doi.org/10.1097/ICO.0b013e31820d852a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson IK, Tisdale AS. Visualization of conjunctival goblet cell actin cytoskeleton and mucin content in tissue whole mounts. Exp Eye Res. 1997;65:407–415. doi: 10.1006/exer.1997.0351. http://dx.doi.org/10.1006/exer.1997.0351. [DOI] [PubMed] [Google Scholar]
- Govindarajan B, Gipson IK. Membrane-tethered mucins have multiple functions on the ocular surface. Exp Eye Res. 2010;90:655–663. doi: 10.1016/j.exer.2010.02.014. http://dx.doi.org/10.1016/j.exer.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorieff A, Stange DE, Kujala P, Begthel H, van den Born M, Korving J, Peters PJ, Clevers H. The ets-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology. 2009;137:1333–1345. doi: 10.1053/j.gastro.2009.06.044. e1331-1333. http://dx.doi.org/10.1053/j.gastro.2009.06.044. [DOI] [PubMed] [Google Scholar]
- Greiner JV, Henriquez AS, Covington HI, Weidman TA, Allansmith MR. Goblet cells of the human conjunctiva. Arch Ophthalmol. 1981;99:2190–2197. doi: 10.1001/archopht.1981.03930021066016. http://dx.doi.org/10.1001/archopht.1981.03930021066016. [DOI] [PubMed] [Google Scholar]
- Gunzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiological reviews. 2013;93:525–569. doi: 10.1152/physrev.00019.2012. http://dx.doi.org/10.1152/physrev.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges RR, Bair JA, Carozza RB, Li D, Shatos MA, Dartt DA. Signaling pathways used by EGF to stimulate conjunctival goblet cell secretion. Experimental eye research. 2012;103:99–113. doi: 10.1016/j.exer.2012.08.010. http://dx.doi.org/10.1016/j.exer.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori Y, Spurr-Michaud SJ, Russo CL, Argueso P, Gipson IK. Effect of retinoic acid on gene expression in human conjunctival epithelium: secretory phospholipase A2 mediates retinoic acid induction of MUC16. Invest Ophthalmol Vis Sci. 2005;46:4050–4061. doi: 10.1167/iovs.05-0627. http://dx.doi.org/10.1167/iovs.05-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Li L, Fuchs E. Emerging interactions between skin stem cells and their niches. Nature medicine. 2014;20:847–856. doi: 10.1038/nm.3643. http://dx.doi.org/10.1038/nm.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inatomi T, Spurr-Michaud S, Tisdale AS, Gipson IK. Human corneal and conjunctival epithelia express MUC1 mucin. Invest Ophthalmol Vis Sci. 1995;36:1818–1827. [PubMed] [Google Scholar]
- Inatomi T, Spurr-Michaud S, Tisdale AS, Zhan Q, Feldman ST, Gipson IK. Expression of secretory mucin genes by human conjunctival epithelia. Invest Ophthalmol Vis Sci. 1996;37:1684–1692. [PubMed] [Google Scholar]
- Katz JP, Perreault N, Goldstein BG, Lee CS, Labosky PA, Yang VW, Kaestner KH. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129:2619–2628. doi: 10.1242/dev.129.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenchegowda D, Swamynathan S, Gupta D, Wan H, Whitsett J, Swamynathan SK. Conditional disruption of mouse Klf5 results in defective eyelids with malformed meibomian glands, abnormal cornea and loss of conjunctival goblet cells. Developmental biology. 2011;356:5–18. doi: 10.1016/j.ydbio.2011.05.005. http://dx.doi.org/10.1016/j.ydbio.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessing S. Mucous gland system of the conjunctiva: A quantitative normal anatomical study. Acta Ophthalmol suppl. 1968;95:1–133. [PubMed] [Google Scholar]
- Kim V, Criner GJ. Chronic bronchitis and chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2013;187:228–237. doi: 10.1164/rccm.201210-1843CI. http://dx.doi.org/10.1164/rccm.201210-1843CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Current gastroenterology reports. 2010;12:319–330. doi: 10.1007/s11894-010-0131-2. http://dx.doi.org/10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoop KA, McDonald KG, McCrate S, McDole JR, Newberry RD. Microbial sensing by goblet cells controls immune surveillance of luminal antigens in the colon. Mucosal immunology. 2015;8:198–210. doi: 10.1038/mi.2014.58. http://dx.doi.org/10.1038/mi.2014.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop N, Korb DR, Blackie CA, Knop E. The lid wiper contains goblet cells and goblet cell crypts for ocular surface lubrication during the blink. Cornea. 2012;31:668–679. doi: 10.1097/ICO.0b013e31823f8d8c. http://dx.doi.org/10.1097/ICO.0b013e31823f8d8c. [DOI] [PubMed] [Google Scholar]
- Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochimica et biophysica acta. 2008;1778:631–645. doi: 10.1016/j.bbamem.2007.10.018. http://dx.doi.org/10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Krenzer KL, Freddo TF. Cytokeratin expression in normal human bulbar conjunctiva obtained by impression cytology. Invest Ophthalmol Vis Sci. 1997;38:142–152. [PubMed] [Google Scholar]
- Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002;120:330–337. doi: 10.1001/archopht.120.3.330. http://dx.doi.org/10.1001/archopht.120.3.330. [DOI] [PubMed] [Google Scholar]
- Langer G, Jagla W, Behrens-Baumann W, Walter S, Hoffmann W. Secretory peptides TFF1 and TFF3 synthesized in human conjunctival goblet cells. Investigative ophthalmology & visual science. 1999;40:2220–2224. [PubMed] [Google Scholar]
- Lories RJ, Peeters J, Bakker A, Tylzanowski P, Derese I, Schrooten J, Thomas JT, Luyten FP. Articular cartilage and biomechanical properties of the long bones in Frzb-knockout mice. Arthritis and rheumatism. 2007;56:4095–4103. doi: 10.1002/art.23137. http://dx.doi.org/10.1002/art.23137. [DOI] [PubMed] [Google Scholar]
- Marko CK, Menon BB, Chen G, Whitsett JA, Clevers H, Gipson IK. Spdef null mice lack conjunctival goblet cells and provide a model of dry eye. The American journal of pathology. 2013;183:35–48. doi: 10.1016/j.ajpath.2013.03.017. http://dx.doi.org/10.1016/j.ajpath.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marko CK, Tisdale AS, Spurr-Michaud S, Evans C, Gipson IK. The ocular surface phenotype of Muc5ac and Muc5b null mice. Investigative ophthalmology & visual science. 2014;55:291–300. doi: 10.1167/iovs.13-13194. http://dx.doi.org/10.1167/iovs.13-13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley HA, Guasch G. Three cheers for the goblet cell: maintaining homeostasis in mucosal epithelia. Trends in molecular medicine. 2015;21:492–503. doi: 10.1016/j.molmed.2015.06.003. http://dx.doi.org/10.1242/dev.117804. [DOI] [PubMed] [Google Scholar]
- McCauley HA, Liu CY, Attia AC, Wikenheiser-Brokamp KA, Zhang Y, Whitsett JA, Guasch G. TGFbeta signaling inhibits goblet cell differentiation via SPDEF in conjunctival epithelium. Development. 2014;141:4628–4639. doi: 10.1242/dev.117804. http://dx.doi.org/10.1242/dev.117804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, Newberry RD, Miller MJ. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345–349. doi: 10.1038/nature10863. http://dx.doi.org/10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie RW, Jumblatt JE, Jumblatt MM. Quantification of MUC2 and MUC5AC transcripts in human conjunctiva. Invest Ophthalmol Vis Sci. 2000;41:703–708. [PubMed] [Google Scholar]
- Mircheff AK. Water and Electrolyte Secretion and Fluid Modification. In: Albert DM, editor. Principles and Practice of Ophthalmology. Philadelphia, PA: W. B. Saunders; 1994. pp. 466–472. [Google Scholar]
- Moniaux N, Varshney GC, Chauhan SC, Copin MC, Jiain M, Wittel UA, Andiranifahanana M, Aubert JP, Batra SK. Generation and characterization of anti-MUC4 monoclonal antibodies reactive with normal and cancer cells in humans. J Histochem Cytochem. 2004;52:253–261. doi: 10.1177/002215540405200213. http://dx.doi.org/10.1177/002215540405200213. [DOI] [PubMed] [Google Scholar]
- Moore CP, Wilsman NJ, Nordheim EV, Majors LJ, Collier LL. Density and distribution of canine conjunctival goblet cells. Investigative ophthalmology & visual science. 1987;28:1925–1932. [PubMed] [Google Scholar]
- Natadisastra G, Wittpenn JR, Muhilal, West KP, Jr, Mele L, Sommer A. Impression cytology: a practical index of vitamin A status. The American journal of clinical nutrition. 1988;48:695–701. doi: 10.1093/ajcn/48.3.695. [DOI] [PubMed] [Google Scholar]
- Nelson JD, Wright JC. Impression cytology of the ocular surface in keratoconjunctivitis sicca. In: Holly FJ, editor. The Preocular Tear Film in Health, Disease, and Contact Lens Wear. Texas Dry Eye Institute; 1986. pp. 140–156. [Google Scholar]
- Noah TK, Kazanjian A, Whitsett J, Shroyer NF. SAM pointed domain ETS factor (SPDEF) regulates terminal differentiation and maturation of intestinal goblet cells. Experimental cell research. 2010;316:452–465. doi: 10.1016/j.yexcr.2009.09.020. http://dx.doi.org/10.1016/j.yexcr.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KS, Korfhagen TR, Bruno MD, Kitzmiller JA, Wan H, Wert SE, Khurana Hershey GK, Chen G, Whitsett JA. SPDEF regulates goblet cell hyperplasia in the airway epithelium. The Journal of clinical investigation. 2007;117:978–988. doi: 10.1172/JCI29176. http://dx.doi.org/10.1172/JCI29176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen FP, Woon CW, Varoga D, Jansen A, Garreis F, Jager K, Amm M, Podolsky DK, Steven P, Barker NP, Sel S. Intestinal trefoil factor/TFF3 promotes re-epithelialization of corneal wounds. The Journal of biological chemistry. 2008;283:13418–13427. doi: 10.1074/jbc.M800177200. http://dx.doi.org/10.1074/jbc.M800177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz HB, Tisdale AS, Danjo Y, Spurr-Michaud SJ, Argueso P, Gipson IK. The role of calcium in mucin packaging within goblet cells. Exp Eye Res. 2003;77:69–75. doi: 10.1016/s0014-4835(03)00084-8. http://dx.doi.org/10.1016/S0014-4835(03)00084-8. [DOI] [PubMed] [Google Scholar]
- Pellegrini G, Golisano O, Paterna P, Lambiase A, Bonini S, Rama P, De Luca M. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol. 1999;145:769–782. doi: 10.1083/jcb.145.4.769. http://dx.doi.org/10.1083/jcb.145.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Vilar J. Mucin granule intraluminal organization. American journal of respiratory cell and molecular biology. 2007;36:183–190. doi: 10.1165/rcmb.2006-0291TR. http://dx.doi.org/10.1165/rcmb.2006-0291TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugfelder SC, Corrales RM, de Paiva CS. T helper cytokines in dry eye disease. Experimental eye research. 2013;117:118–125. doi: 10.1016/j.exer.2013.08.013. http://dx.doi.org/10.1016/j.exer.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TE, Huet C, Bilbo PR, Podolsky DK, Louvard D, Neutra MR. Human intestinal goblet cells in monolayer culture: characterization of a mucus-secreting subclone derived from the HT29 colon adenocarcinoma cell line. Gastroenterology. 1988;94:1390–1403. doi: 10.1016/0016-5085(88)90678-6. [DOI] [PubMed] [Google Scholar]
- Ponce-Macotela M, Gonzalez-Maciel A, Reynoso-Robles R, Martinez-Gordillo MN. Goblet cells: are they an unspecific barrier against Giardia intestinalis or a gate? Parasitology research. 2008;102:509–513. doi: 10.1007/s00436-007-0790-6. http://dx.doi.org/10.1007/s00436-007-0790-6. [DOI] [PubMed] [Google Scholar]
- Preston MJ, Gerceker AA, Koles NL, Pollack M, Pier GB. Prophylactic and therapeutic efficacy of immunoglobulin G antibodies to Pseudomonas aeruginosa lipopolysaccharide against murine experimental corneal infection. Investigative ophthalmology & visual science. 1997;38:1418–1425. [PubMed] [Google Scholar]
- Rachagani S, Torres MP, Moniaux N, Batra SK. Current status of mucins in the diagnosis and therapy of cancer. BioFactors. 2009;35:509–527. doi: 10.1002/biof.64. http://dx.doi.org/10.1002/biof.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald JG, Hahn WC, Ramsey MR, Wu JY, Guo Z, Tsao H, De Luca M, Catricala C, O’Toole KM. A two-stage, p16INK4A- and p53-dependent keratinocyte senescence mechanism that limits replicative potential indepedent of telomere status. Molec Cell Biol. 2002;22:5157–5172. doi: 10.1128/MCB.22.14.5157-5172.2002. http://dx.doi.org/10.1128/MCB.22.14.5157-5172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, Alexander SN, Bellinghausen LK, Song AS, Petrova YM, Tuvim MJ, Adachi R, Romo I, Bordt AS, Bowden MG, Sisson JH, Woodruff PG, Thornton DJ, Rousseau K, De la Garza MM, Moghaddam SJ, Karmouty-Quintana H, Blackburn MR, Drouin SM, Davis CW, Terrell KA, Grubb BR, O’Neal WK, Flores SC, Cota-Gomez A, Lozupone CA, Donnelly JM, Watson AM, Hennessy CE, Keith RC, Yang IV, Barthel L, Henson PM, Janssen WJ, Schwartz DA, Boucher RC, Dickey BF, Evans CM. Muc5b is required for airway defence. Nature. 2014;505:412–416. doi: 10.1038/nature12807. http://dx.doi.org/10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BK. Secretion properties, clearance, and therapy in airway disease. Translational respiratory medicine. 2014;2:6. doi: 10.1186/2213-0802-2-6. http://dx.doi.org/10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatos MA, Rios JD, Tepavcevic V, Kano H, Hodges R, Dartt DA. Isolation, characterization, and propagation of rat conjunctival goblet cells in vitro. Investigative ophthalmology & visual science. 2001;42:1455–1464. [PubMed] [Google Scholar]
- Sommer A. Effects of vitamin A deficiency on the ocular surface. Ophthalmology. 1983;90:592–600. doi: 10.1016/s0161-6420(83)34512-7. http://dx.doi.org/10.1016/S0161-6420(83)34512-7. [DOI] [PubMed] [Google Scholar]
- Spurr-Michaud S, Argueso P, Gipson I. Assay of mucins in human tear fluid. Experimental eye research. 2007;84:939–950. doi: 10.1016/j.exer.2007.01.018. http://dx.doi.org/10.1016/j.exer.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RM, Sheridan CM, Hiscott PS, Czanner G, Kaye SB. Human Conjunctival Stem Cells are Predominantly Located in the Medial Canthal and Inferior Forniceal Areas. Investigative ophthalmology & visual science. 2015;56:2021–2030. doi: 10.1167/iovs.14-16266. http://dx.doi.org/10.1167/iovs.14-16266. [DOI] [PubMed] [Google Scholar]
- Su L, Cui H, Xu C, Xie X, Chen Q, Gao X. Putative rabbit conjunctival epithelial stem/progenitor cells preferentially reside in palpebral conjunctiva. Current eye research. 2011;36:797–803. doi: 10.3109/02713683.2011.593727. http://dx.doi.org/10.3109/02713683.2011.593727. [DOI] [PubMed] [Google Scholar]
- Swamynathan SK, Katz JP, Kaestner KH, Ashery-Padan R, Crawford MA, Piatigorsky J. Conditional deletion of the mouse Klf4 gene results in corneal epithelial fragility, stromal edema, and loss of conjunctival goblet cells. Molecular and cellular biology. 2007;27:182–194. doi: 10.1128/MCB.00846-06. http://dx.doi.org/10.1128/MCB.00846-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tei M, Moccia R, Gipson IK. Developmental expression of mucin genes ASGP (rMuc4) and rMuc5AC by the rat ocular surface epithelium. Invest Ophthalmol Vis Sci. 1999;40:1944–1951. [PubMed] [Google Scholar]
- Tei M, Spurr-Michaud SJ, Tisdale AS, Gipson IK. Vitamin A deficiency alters the expression of mucin genes by the rat ocular surface epithelium. Invest Ophthalmol Vis Sci. 2000;41:82–88. [PubMed] [Google Scholar]
- Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annual review of physiology. 2008;70:459–486. doi: 10.1146/annurev.physiol.70.113006.100702. http://dx.doi.org/10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- Tukler Henriksson J, Coursey TG, Corry DB, De Paiva CS, Pflugfelder SC. IL-13 Stimulates Proliferation and Expression of Mucin and Immunomodulatory Genes in Cultured Conjunctival Goblet Cells. Investigative ophthalmology & visual science. 2015;56:4186–4197. doi: 10.1167/iovs.14-15496. http://dx.doi.org/10.1167/iovs.14-15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. http://dx.doi.org/10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ogawa Y, Dogru M, Tatematsu Y, Uchino M, Kamoi M, Okada N, Okamoto S, Tsubota K. Baseline profiles of ocular surface and tear dynamics after allogeneic hematopoietic stem cell transplantation in patients with or without chronic GVHD-related dry eye. Bone marrow transplantation. 2010;45:1077–1083. doi: 10.1038/bmt.2009.312. http://dx.doi.org/10.1038/bmt.2009.312. [DOI] [PubMed] [Google Scholar]
- Wansleeben C, Barkauskas CE, Rock JR, Hogan BL. Stem cells of the adult lung: their development and role in homeostasis, regeneration, and disease. Wiley interdisciplinary reviews. Developmental biology. 2013;2:131–148. doi: 10.1002/wdev.58. http://dx.doi.org/10.1002/wdev.58. [DOI] [PubMed] [Google Scholar]
- Wei Z-B, Cotsarelis G, Sun T-T, Lavker RM. Label-retaining cells are preferentially located in fornical epithelium: Implications for conjunctival epithelial homeostasis. Invest. Ophthalmol. Vis. Sci. 1995;36:236–246. [PubMed] [Google Scholar]
- Wei Z-G, Lin T, Sun T-T, Lavker RM. Clonal analysis of the in vivo differentiation potential of keratinocytes. Invest Ophthalmol Vis Sci. 1997;38:753–761. [PubMed] [Google Scholar]
- Wei Z-G, Sun T-T, Lavker RM. Rabbit conjunctival and corneal epithelial cells belong to two separate lineages. Invest. Ophthalmol. Vis. Sci. 1996;37:523–533. [PubMed] [Google Scholar]
- Wei Z-G, Wu R-L, Lavker RM, Sun T-T. In vitro growth and differentiation of rabbit bulbar, fornix, and palpebral conjunctival epithelia. Implications on conjunctival epithelial transdifferentiation and stem cells. Invest. Ophthalmol. Vis. Sci. 1993;34:1814–1828. [PubMed] [Google Scholar]
- Yin X, Farin HF, van Es JH, Clevers H, Langer R, Karp JM. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nature methods. 2014;11:106–112. doi: 10.1038/nmeth.2737. http://dx.doi.org/10.1038/nmeth.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Ban Y, Kinoshita S. Tight junction transmembrane protein claudin subtype expression and distribution in human corneal and conjunctival epithelium. Investigative ophthalmology & visual science. 2009;50:2103–2108. doi: 10.1167/iovs.08-3046. http://dx.doi.org/10.1167/iovs.08-3046. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lam O, Nguyen MT, Ng G, Pear WS, Ai W, Wang IJ, Kao WW, Liu CY. Mastermind-like transcriptional co-activator-mediated Notch signaling is indispensable for maintaining conjunctival epithelial identity. Development. 2013;140:594–605. doi: 10.1242/dev.082842. http://dx.doi.org/10.1242/dev.082842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YG, Wu S, Lu R, Zhou D, Zhou J, Carmeliet G, Petrof E, Claud EC, Sun J. Tight junction CLDN2 gene is a direct target of the vitamin D receptor. Scientific reports. 2015;5:10642. doi: 10.1038/srep10642. http://dx.doi.org/10.1038/srep10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Abdullah LH, Doyle SP, Nguyen K, Ribeiro CM, Vasquez PA, Forest MG, Lethem MI, Dickey BF, Davis CW. Baseline Goblet Cell Mucin Secretion in the Airways Exceeds Stimulated Secretion over Extended Time Periods, and Is Sensitive to Shear Stress and Intracellular Mucin Stores. PloS one. 2015;10:e0127267. doi: 10.1371/journal.pone.0127267. http://dx.doi.org/10.1371/journal.pone.0127267. [DOI] [PMC free article] [PubMed] [Google Scholar]