Abstract
Sickle cell disease (SCD) is a genetic hematologic disorder that is characterized by a variety of potentially life threatening acute and chronic complications. Currently, hydroxyurea is the only clinically approved pharmacological therapy for the treatment of SCD, and the continued prevalence of severe disease complications underscores the desperate need for the development of new therapeutic agents. Central features of the sickle cell disease milieu, including hypoxia, oxidative stress, and thrombosis, are established enhancers of endothelin-1 (ET-1) synthesis. This conceptual connection between ET-1 and SCD was confirmed by multiple studies that demonstrated markedly elevated plasma and urinary levels of ET-1 in SCD patients. Direct evidence for the involvement of ET-1 signaling in the development of SCD pathologies has come from studies using endothelin receptor antagonists in SCD mice. This review summarizes recent studies that have implicated ET-1 signaling as a mechanistic contributor to renal, vascular, pulmonary, and nociceptive complications of sickle cell disease and discusses the potential for the use of ET receptor antagonists in the treatment of SCD.
Keywords: Endothelin-1, sickle cell disease, nephropathy, pain, pulmonary hypertension
Introduction
Sickle cell disease (SCD) is a common genetic hematologic disorder that affects millions of people worldwide1. The medical management of SCD has led to a steady improvement in the lifespan of patients throughout the past fifty years, and as a result, a variety of chronic complications have emerged as major medical issues in SCD2,3. The only clinically approved disease-specific drug for the treatment of SCD is hydroxyurea, which is thought to primarily function to induce expression of fetal hemoglobin, ultimately leading to a reduction in red blood cell sickling3,4. This treatment strategy has been demonstrated to produce a significant reduction in disease severity5,6. Despite this, patients with SCD continue to suffer from chronic end-organ damage as well as painful, and potentially lethal, acute exacerbations of the disease, known as vaso-occlusive crises (VOC). These acute and chronic disease manifestations continue to affect SCD patients due to the lack of pharmacological therapies targeted to mechanisms underlying their etiology. Thus, there is clearly a large unmet therapeutic need in SCD.
Endothelin-1 (ET-1) is a 21 amino acid peptide produced by a variety of cell types throughout the body and acts by binding to two distinct receptor subtypes, ETA and ETB7. ET-1 was originally identified as a sub-nanomolar potent vasoconstrictor8, and it is now additionally known to be a pro-inflammatory9, mitogenic10, natriuretic11, and nociceptive mediator12. These often detrimental actions of ET-1 are mediated primarily by activation of the ETA receptor, and in some tissues can be counter-balanced by activation of the ETB receptor13. The cellular synthesis of ET-1 has been demonstrated to be increased in the setting of hypoxia14, oxidative stress15, decreased nitric oxide bioavailability16, and thrombosis17, all of which are well-established features of the SCD milieu18-21. The first evidence supporting a connection between ET-1 and SCD came from a study demonstrating that exposure of endothelial cells to sickle erythrocytes increases their synthesis of ET-122. This discovery led to multiple published reports that documented markedly increased plasma and urinary levels of ET-1 in SCD patients23-28. Notably, plasma ET-1 levels were shown to be elevated to a significantly greater extent in SCD patients experiencing VOC. Together, these data clearly indicate that elevated ET-1 levels are a biomarker of the SCD process but do not directly implicate ET-1 signaling as a contributing factor to the pathophysiology of SCD.
Further evidence to support a connection between ET-1 and SCD comes from two gene polymorphism studies in SCD patients. The first study demonstrated that an ET-1 gene polymorphism is associated with risk of VOC in SCD patients29, and the second demonstrated an association of another ET-1 gene polymorphism with SCD when compared to controls in an African cohort30. Together, these studies implicate ET-1 as an important disease-modifying gene in SCD, and suggest that differential ET-1 signaling can modulate SCD severity.
The first direct evidence to suggest that ET-1 plays a mechanistic role in SCD pathology came from a published report in which SCD mice were treated with the dual ETA/ETB receptor antagonist, bosentan31. This study demonstrated that bosentan had protective effects in both kidneys and lungs by preventing both vascular congestion and inflammation following VOC, and additionally, demonstrated a complete protection from VOC-mediated mortality in SCD mice. Of note, the magnitude of the effects observed by Sabaa et al. suggested that ET-1 signaling is a major contributory mechanism to central features of SCD pathophysiology.
Collectively, these studies established the basis for investigation of mechanisms by which ET-1 contributes to SCD pathophysiology, and a growing number of labs are working in this area32-34. Importantly, ET-1 is an established mechanistic mediator of chronic kidney disease, vascular dysfunction, pulmonary hypertension, and chronic pain, all of which occur in SCD. Based on these associations, the National Institutes of Health's National Heart Lung and Blood Institute has established a center to investigate the therapeutic potential for endothelin receptor antagonists in SCD by funding an Excellence in Hemaglobinopathies Research Award (NIH Grant U01HL117684). The current review focuses on evidence implicating ET-1 as a major player in the pathogenesis, treatment, and prevention of sickle nephropathy, as well as in the pain hypersensitivity and pulmonary complication of SCD (Figure 1).
Figure 1.
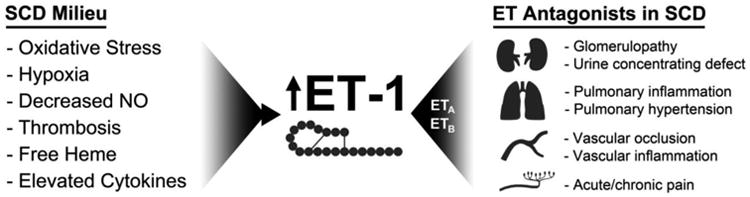
Factors known to cause elevated ET-1 expression are salient features of the SCD milieu, and ET-1 is an established mediator of multiple organ-specific pathologies that occur in SCD.
Therapeutic potential of endothelin antagonists in sickle cell nephropathy
Sickle cell nephropathy (SN) represents one of the most serious complications of SCD, accounting for up to 18% of mortality in patients35,36. SN is characterized by structural and functional abnormalities in the kidney, and these abnormalities vary in incidence and onset. An early-onset urine concentration defect occurs in nearly all SCD patients37. This disease manifestation is reversible with blood transfusion therapy in the first decade of life but irreversible later, due to rarefication of the capillary network and loss of tubule segments that establish the hyperosmotic environment within the renal medulla38-40. Hematuria and progressive glomerulopathy occurs in a large subset of patients41. Glomerulopathy in SN leads to subsequent proteinuria and reduced GFR, which are risk factors for increased mortality among patients with SCD42. Although the pathophysiology of SN remains to be fully elucidated, chronic hemolysis-related endothelial dysfunction is currently considered as one of the key mechanistic factors contributing to the disease process43,44.
In the renal medulla, ET-1 is an established mediator of natriuresis and diuresis by signaling to ETB receptors in the collecting duct, with subsequent action on epithelial sodium channels and aquaporins45-47. Additionally, ET-1 can regulate medullary vascular tone by acting on microvascular ETA receptors48. The microenvironment of the renal medulla is characterized by an oxygen tension that is among the lowest of any tissue in the body, and this oxygen tension is known to be decreased further in a variety of disease states49. Hypoxia increases ET-1 expression within the renal medulla, and in SCD, hypoxia contributes to red blood cell sickling and vascular congestion in the vasa recta50. Thus, in SCD, hypoxia-induced ET-1 production in the renal medulla may exacerbate vascular congestion, leading to a further reduction in interstitial osmolality and a resultant impairment in urinary concentrating ability. Results from our laboratory support this hypothesis. We generated chimeric endothelial-specific ET-1 knockout mice that express human hemoglobin S via bone marrow transplantation with marrow derived from humanized SCD mice. Humanized SCD mice have mouse globin genes knocked out and targeted insertion of human globin genes at the same loci, resulting in mice expressing human hemoglobin S51. Using our chimeric endothelial-specific ET-1 KO SCD mice, we demonstrated preserved urine-concentrating ability, indicating that endothelial-derived ET-1 is a major mediator of renal medullary dysfunction SCD mice52. The mechanism of the effect in this genetic system may be through preservation of medullary blood flow, which ultimately could prevent damage in the renal medulla. ET receptor antagonists are known to exert pro-angiogenic effects, which could limit capillary rarefication in the renal medulla, and prevent the urine concentrating defect via another mechanism53. Thus, future studies are needed to determine if ET-1 receptor blockade may display a similar therapeutic effect in SCD.
Urinary ET-1 excretion is an index of renal ET-1 production, and in SCD patients, elevated urinary ET-1 excretion correlates with microalbuminuria28. This observation suggests a pathophysiological link between increased renal ET-1 production and impairment of glomerular function in SCD. In addition, abundant evidence for endothelial dysfunction in SCD43,44 suggests that dysfunction in the glomerular endothelium may promote ET-1 release from the glomerular endothelial cells, thereby causing glomerular damage and albuminuria. Previous studies in our laboratory showed that hypoxia specifically increases glomerular ET-1 expression in C57BL6J mice, but not in endothelial-specific ET-1 knockout mice54, suggesting a potential mechanism for ET-1-induced nephropathy in SCD. However, despite many studies implicating ET-1 in numerous glomerulopathies, the mechanism by which elevated ET-1 production contributes to glomerular dysfunction in SN remains unknown55-57. Sabaa et al.31 first demonstrated the potential importance of ET receptor antagonism as a preventive or therapeutic approach in SN. Using doppler ultrasound to study the renal hemodynamics of bosentan treated SCD mice, investigators demonstrated a substantial decrease in vascular resistance during hypoxia/reoxygenation-induced VOC, renal microvascular congestion, and systemic inflammation31. More recently, chronic bosentan therapy was shown to attenuate both glomerulomegaly and glomerulosclerosis, as well as prevent glomerular lesions in SCD mice58. Results from our laboratory support these observations. We have recently demonstrated a beneficial effect of the ETA receptor antagonism, using ambrisentan, on glomerular injury in humanized SCD mice. Interestingly, our results showed that long-term treatment with ambrisentan fully prevented both elevated glomerular permeability to albumin as well as albuminuria and proteinuria in humanized SCD mice59. Moreover, as reactive oxygen species are implicated in a long-term pathology of SCD60, we have shown that chronic administration of ET-1 increases glomerular reactive oxygen species production through the ETA receptor54. This effect was prevented by ETA receptor blockade in SCD mice61, providing a confirmation of ETA-dependent reactive oxygen species mediated glomerular injury in SCD. These results suggest that ET-1, via ETA receptor activation, leads to glomerular injury, and suggest that chronic ETA antagonism should be considered as a prospective therapy to prevent the onset of SN in SCD patients.
Together, these studies suggest a critical role of ET-1 in initiating and/or mediating renal injury in SCD and highlight the potential for chronic ET receptor antagonism as a pharmacological intervention to prevent the development and delay progression of renal involvement in SCD.
Therapeutic potential of endothelin antagonists in chronic pain management in SCD
Pain is a chronic complication of SCD that is acutely exacerbated during VOC, and associated with inflammation and tissue injury62,63. Analogously, ET-1 is chronically elevated in the plasma of SCD patients at baseline, further elevated during the course of painful VOC, and significantly reduced at asymptomatic follow-up25. In children with SCD, both plasma ET-1 level and the ratio of plasma ET-164 to the peptide vasodilator apelin65 have been demonstrated to be positively correlated with baseline pain rating. In a mouse model of SCD, dorsal root ganglia, where cell bodies of sensory neurons are located, were found to have elevated ET-1 expression at the mRNA level66. The involvement of ET-1 in the elicitation of pain in peripheral locations has been well documented by administration of exogenous ET-1 in both humans and animal models of nociception67-71. Moreover, several studies have demonstrated the ability of ET receptor antagonists to alleviate clinical pain in patients with systemic sclerosis and Raynaud's phenomenon, two conditions in which disease manifestations share some similarity with SCD72-74. Nevertheless, two other studies have demonstrated negative results in these conditions75,76. Painful skin ulcers, which are seen in systemic sclerosis and have been shown to respond to ET antagonism, also occur in SCD73. A case report documented completed remission of ulcers in a SCD patient treated with bosentan, an effect that may be related to the pro-angiogenic effect of ET receptor antagonism77. Taken together the current body of evidence establish a strong foundation for the hypothesis that ET-1 signaling contributes to hyperalgesia in SCD.
The mechanisms underlying a potential role of ET-1 signaling in the pathogenesis of pain in SCD remain unknown. This understanding will be particularly important when considering translating preclinical findings to humans, as both ETA and dual ETA/ETB receptor antagonists are available for clinical use. In animal models of nociception, peripheral ETA receptors can be generally considered to mediate hyperalgesia, suggesting that ETA antagonism may be the appropriate approach in SCD78. However, the role of peripheral ETB receptors is less clear, as ETB receptors have been demonstrated to have pro-algesic79, neutral80, and analgesic effects81 depending on experimental conditions. Therefore, the use of dual ETA/ETB receptor antagonists in the treatment of pain in SCD could produce additional benefit over ETA antagonism alone or, alternatively, may decrease or eliminate the analgesic effect of ETA antagonism. A recent comprehensive review has discussed mechanisms by which ET-1 signaling induces nociception78. Thus, this review will focus on new findings that may be mechanistically related to a role of ET-1 signaling in hyperalgesia in SCD patients.
Recently it has been shown that transient receptor potential vanilloid 1 (TRPV1) channels mediate mechanical sensitization of nociceptor terminals, resulting in mechanical allodynia in SCD mice82 Inflammatory mediators, including ET-1, released following VOC events can maintain the persistent sensitization of TRPV1 channels in SCD83,84 Moreover, other studies showed that subcutaneous administration of ET-1, via direct sensitization of TRPV1, results in prolonged mechanical allodynia85 Interestingly, ETA receptors are co-localized with TRPV1 channels in HEK293 cells, and ET-1 leads to potentiation of TRPV1 activity via ETA receptors84 Furthermore, studies performed by Yamamoto et al. also highlight the importance of ET-1 in peripheral nociceptive signaling associated with pain. Investigators demonstrated that in sensory neurons, ET-1 induces Ca2+-dependent activation of protein kinase Cε, which is well known to phosphorylate TRPV1 channels86,87. These studies establish the basis for the hypothesis that in SCD, elevated ET-1 production results in excessive ETA receptor activation on sensory neurons, resulting in potentiation of TRPV1 activity, and ultimately pain hypersensitivity. Future studies of this hypothesis may provide evidence for the use of ET receptor antagonists as an analgesic therapy in SCD. Importantly, ET receptor antagonists may provide a non-habit-forming alternative to opioid analgesics that are currently widely used in the treatment of pain in SCD.
Therapeutic potential of endothelin antagonists in pulmonary complications in SCD
Pulmonary hypertension is a serious SCD complication that occurs in a subset of SCD patients and is a risk factor for mortality in SCD88. ET-1 is a well-established mediator of pulmonary hypertension, and ET receptor antagonists represent an approved therapy for this disease33,89. Based on the success of ET receptor antagonism in treating pulmonary hypertension arising from other etiologies, a clinical trial was initiated to investigate the therapeutic efficacy of bosentan to treat pulmonary hypertension in SCD90. Unfortunately, this clinical trial was prematurely discontinued because of the small sample size. However, bosentan was well tolerated in this small SCD cohort. Moreover, a study in SCD mice demonstrated that bosentan decreased lung injury scores following hypoxia/normoxia31. These studies suggest potential therapeutic benefit of ET receptor antagonists in pulmonary complications in SCD.
The current knowledge about the ET receptor subtypes and their functions raises the question: Should patients with SCD be treated with a selective or combined ET receptor antagonist? There have been many studies investigating the efficacy of ET receptor antagonists in pulmonary hypertension, but there have been only limited studies to investigate the advantage of targeting specific ET receptors to treat chronic lung disease in patients with SCD33. Pulmonary ETB receptors clear approximately 40% of circulating ET-1 in patients with pulmonary hypertension91, as well as inhibit ET converting enzyme-192. Therefore, ETB receptor antagonism could have detrimental effects. Knowledge about general ETA and ETB receptor functions provides a basis for the idea that selective ETA receptor antagonism could represent a superior strategy the treatment of pulmonary complications in SCD by allowing ETB mediated anti-inflammatory and nitric oxide-producing effects. Furthermore, a recent report supports this argument by demonstrating that ETA receptor blockade protects against pneumolysin-induced barrier dysfunction in SCD93, thus linking ET-1 with lung injury pathways in SCD through the activation of ETA receptors. However, it also has been reported that in the setting of prolonged elevated ET-1 expression in conditions of reduced nitric oxide bioavailability, ETB receptor signaling may contribute to additional reactive oxygen and nitrogen species, potentially adding to the effect of nitric oxide dysregulation in SCD94,95. Moreover, changes in the expression and signaling role of ET receptors in condition such as pulmonary hypertension in SCD may deviate significantly from physiological conditions. ETB receptor expression is known to be upregulated in pulmonary hypertension96. Further, the recent concept of ET receptors heterodimerisation97 suggests that the ETB receptors may adopt the function of the ETA receptors98 and may provide justification for use of dual ET receptor antagonist in treating pulmonary hypertension in SCD. Additionally, ETB receptor mediated activation of Gardos channels on red blood cells stimulates dehydration, which can contribute to sickling of hemoglobin S99,100 and potentially lead to VOC events and progression of the disease. Further investigations are warranted to resolve the question of the optimal ET receptor antagonism strategy to prevent pulmonary injury in SCD.
Conclusion
The studies herein reviewed have provided direct evidence that ET-1 is a mechanistic mediator of diverse complications that occur in distinct organ systems in SCD. Together, they suggest that elevated ET-1 signaling may be a central mechanism linking many salient features of SCD pathophysiology. This concept provides great potential for conducting more detailed studies on the clinical benefit of ET receptor antagonism in both the management of acute exacerbations and chronic complication in SCD patients. This is particularly critical. Additionally, as elevated ET-1 expression has been demonstrated across organ systems in SCD, endothelin converting enzyme inhibition could also be explored in the treatment of SCD. New therapies are desperately needed for the treatment of SCD, where an ideal therapeutic agent would target an upstream mechanism of organ dysfunction in order to provide maximal benefit. Currently, a pilot trial of the ETA receptor antagonist, ambrisentan, is underway in a cohort of SCD patients with evidence of renal complications (NIH Grant U01HL117684). This study will determine the safety and tolerability of ETA antagonism is SCD patients, and may provide evidence on the efficacy of this intervention to reduce SCD-mediated renal dysfunction. Future studies are needed to investigate the mechanism(s) underlying the involvement of ET-1 signaling in the herein described SCD complications and to determine if ET-1 plays a role in other SCD complications, such as stroke and skin ulceration.
Acknowledgments
The authors would like to thank Dr. Jennifer S. Pollock and Dr. David M. Pollock for contributing to the development of this review. This work was supported by U01 HL117684 to Dr. Jennifer S. Pollock and Dr. David M. Pollock and F30 DK107194 to Brandon M. Fox.
Footnotes
Conflict of interest: The authors declare no conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weatherall DJ, Clegg JB. Inherited haemoglobin disorders: an increasing global health problem. Bulletin of the World Health Organization. 2001;79:704–712. [PMC free article] [PubMed] [Google Scholar]
- 2.Lanzkron S, Carroll CP, Haywood C., Jr Mortality rates and age at death from sickle cell disease: U.S., 1979-2005. Public health reports. 2013;128:110–116. doi: 10.1177/003335491312800206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yawn BP, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. Jama. 2014;312:1033–1048. doi: 10.1001/jama.2014.10517. [DOI] [PubMed] [Google Scholar]
- 4.McGann PT, Ware RE. Hydroxyurea therapy for sickle cell anemia. Expert opinion on drug safety. 2015:1–10. doi: 10.1517/14740338.2015.1088827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanzkron S, et al. Systematic review: Hydroxyurea for the treatment of adults with sickle cell disease. Annals of internal medicine. 2008;148:939–955. doi: 10.7326/0003-4819-148-12-200806170-00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strouse JJ, Heeney MM. Hydroxyurea for the treatment of sickle cell disease: efficacy, barriers, toxicity, and management in children. Pediatric blood & cancer. 2012;59:365–371. doi: 10.1002/pbc.24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yanagisawa M, Masaki T. Molecular biology and biochemistry of the endothelins. Trends in pharmacological sciences. 1989;10:374–378. doi: 10.1016/0165-6147(89)90011-4. [DOI] [PubMed] [Google Scholar]
- 8.Yanagisawa M, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 9.Kowalczyk A, Kleniewska P, Kolodziejczyk M, Skibska B, Goraca A. The role of endothelin-1 and endothelin receptor antagonists in inflammatory response and sepsis. Archivum immunologiae et therapiae experimentalis. 2015;63:41–52. doi: 10.1007/s00005-014-0310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosano L, Spinella F, Bagnato A. Endothelin 1 in cancer: biological implications and therapeutic opportunities. Nature reviews Cancer. 2013;13:637–651. doi: 10.1038/nrc3546. [DOI] [PubMed] [Google Scholar]
- 11.Speed JS, Fox BM, Johnston JG, Pollock DM. Endothelin and renal ion and water transport. Seminars in nephrology. 2015;35:137–144. doi: 10.1016/j.semnephrol.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khodorova A, Montmayeur JP, Strichartz G. Endothelin receptors and pain. The journal of pain : official journal of the American Pain Society. 2009;10:4–28. doi: 10.1016/j.jpain.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maguire JJ, Davenport AP. Endothelin receptors and their antagonists. Seminars in nephrology. 2015;35:125–136. doi: 10.1016/j.semnephrol.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kourembanas S, Marsden PA, McQuillan LP, Faller DV. Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. The Journal of clinical investigation. 1991;88:1054–1057. doi: 10.1172/JCI115367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahler J, et al. Oxidative stress increases endothelin-1 synthesis in human coronary artery smooth muscle cells. Journal of cardiovascular pharmacology. 2001;38:49–57. doi: 10.1097/00005344-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Ahlborg G, Lundberg JM. Nitric oxide-endothelin-1 interaction in humans. Journal of applied physiology. 1997;82:1593–1600. doi: 10.1152/jappl.1997.82.5.1593. [DOI] [PubMed] [Google Scholar]
- 17.Schini VB, Hendrickson H, Heublein DM, Burnett JC, Jr, Vanhoutte PM. Thrombin enhances the release of endothelin from cultured porcine aortic endothelial cells. European journal of pharmacology. 1989;165:333–334. doi: 10.1016/0014-2999(89)90733-4. [DOI] [PubMed] [Google Scholar]
- 18.Sun K, Xia Y. New insights into sickle cell disease: a disease of hypoxia. Current opinion in hematology. 2013;20:215–221. doi: 10.1097/MOH.0b013e32835f55f9. [DOI] [PubMed] [Google Scholar]
- 19.Nur E, et al. Oxidative stress in sickle cell disease; pathophysiology and potential implications for disease management. American journal of hematology. 2011;86:484–489. doi: 10.1002/ajh.22012. [DOI] [PubMed] [Google Scholar]
- 20.Eberhardt RT, et al. Sickle cell anemia is associated with reduced nitric oxide bioactivity in peripheral conduit and resistance vessels. American journal of hematology. 2003;74:104–111. doi: 10.1002/ajh.10387. [DOI] [PubMed] [Google Scholar]
- 21.Chantrathammachart P, Pawlinski R. Tissue factor and thrombin in sickle cell anemia. Thrombosis research. 2012;129 Suppl 2:S70–72. doi: 10.1016/j.thromres.2012.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phelan M, Perrine SP, Brauer M, Faller DV. Sickle erythrocytes, after sickling, regulate the expression of the endothelin-1 gene and protein in human endothelial cells in culture. The Journal of clinical investigation. 1995;96:1145–1151. doi: 10.1172/JCI118102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammerman SI, et al. Endothelin-1 production during the acute chest syndrome in sickle cell disease. American journal of respiratory and critical care medicine. 1997;156:280–285. doi: 10.1164/ajrccm.156.1.9611085. [DOI] [PubMed] [Google Scholar]
- 24.Werdehoff SG, Moore RB, Hoff CJ, Fillingim E, Hackman AM. Elevated plasma endothelin-1 levels in sickle cell anemia: relationships to oxygen saturation and left ventricular hypertrophy. American journal of hematology. 1998;58:195–199. doi: 10.1002/(sici)1096-8652(199807)58:3<195::aid-ajh6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 25.Graido-Gonzalez E, et al. Plasma endothelin-1, cytokine, and prostaglandin E2 levels in sickle cell disease and acute vaso-occlusive sickle crisis. Blood. 1998;92:2551–2555. [PubMed] [Google Scholar]
- 26.Rybicki AC, Benjamin LJ. Increased levels of endothelin-1 in plasma of sickle cell anemia patients. Blood. 1998;92:2594–2596. [PubMed] [Google Scholar]
- 27.Lapoumeroulie C, et al. Decreased plasma endothelin-1 levels in children with sickle cell disease treated with hydroxyurea. Haematologica. 2005;90:401–403. [PubMed] [Google Scholar]
- 28.Tharaux PL, et al. Urinary endothelin-1 as a marker of renal damage in sickle cell disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2005;20:2408–2413. doi: 10.1093/ndt/gfi111. [DOI] [PubMed] [Google Scholar]
- 29.Thakur TJ, et al. Endothelin-1 but not Endothelial Nitric Oxide Synthase Gene Polymorphism is Associated with Sickle Cell Disease in Africa. Gene regulation and systems biology. 2014;8:119–126. doi: 10.4137/GRSB.S14836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaar V, et al. ET-1 and ecNOS gene polymorphisms andsusceptibility to acute chest syndrome and painful vaso-occlusive crises in children with sickle cell anemia. Haematologica. 2006;91:1277–1278. [PubMed] [Google Scholar]
- 31.Sabaa N, et al. Endothelin receptor antagonism prevents hypoxia-induced mortality and morbidity in a mouse model of sickle-cell disease. The Journal of clinical investigation. 2008;118:1924–1933. doi: 10.1172/JCI33308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lutz B, Meiler SE, Bekker A, Tao YX. Updated Mechanisms of Sickle Cell Disease-Associated Chronic pain. Translational perioperative and pain medicine. 2015;2:8–17. [PMC free article] [PubMed] [Google Scholar]
- 33.Minniti CP, et al. Endothelin receptor antagonists for pulmonary hypertension in adult patients with sickle cell disease. British journal of haematology. 2009;147:737–743. doi: 10.1111/j.1365-2141.2009.07906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tharaux PL. Endothelin in renal injury due to sickle cell disease. Contributions to nephrology. 2011;172:185–199. doi: 10.1159/000328699. [DOI] [PubMed] [Google Scholar]
- 35.Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999-2009) Pediatric blood & cancer. 2013;60:1482–1486. doi: 10.1002/pbc.24557. [DOI] [PubMed] [Google Scholar]
- 36.Platt OS, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 37.Miller ST, et al. Urine concentrating ability in infants with sickle cell disease: baseline data from the phase III trial of hydroxyurea (BABY HUG) Pediatric blood & cancer. 2010;54:265–268. doi: 10.1002/pbc.22189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Itano HA, Keitel HG, Thompson D. Hyposthenuria in sickle cell anemia: a reversible renal defect. The Journal of clinical investigation. 1956;35:998–1007. doi: 10.1172/JCI103360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hatch FE, Culbertson JW, Diggs LW. Nature of the renal concentrating defect in sickle cell disease. The Journal of clinical investigation. 1967;46:336–345. doi: 10.1172/JCI105535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Statius van Eps LW, Pinedo-Veels C, de Vries GH, de Koning J. Nature of concentrating defect in sickle-cell nephropathy. Microradioangiographic studies. Lancet. 1970;1:450–452. doi: 10.1016/s0140-6736(70)90836-6. [DOI] [PubMed] [Google Scholar]
- 41.Nath KA, Hebbel RP. Sickle cell disease: renal manifestations and mechanisms. Nat Rev Nephrol. 2015;11:161–171. doi: 10.1038/nrneph.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elmariah H, et al. Factors associated with survival in a contemporary adult sickle cell disease cohort. American journal of hematology. 2014;89:530–535. doi: 10.1002/ajh.23683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nath KA, Katusic ZS, Gladwin MT. The perfusion paradox and vascular instability in sickle cell disease. Microcirculation. 2004;11:179–193. doi: 10.1080/10739680490278592. [DOI] [PubMed] [Google Scholar]
- 44.Nath KA, Katusic ZS. Vasculature and kidney complications in sickle cell disease. Journal of the American Society of Nephrology : JASN. 2012;23:781–784. doi: 10.1681/ASN.2011101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ge Y, et al. Collecting duct-specific knockout of endothelin-1 alters vasopressin regulation of urine osmolality. Am J Physiol Renal Physiol. 2005;288:F912–920. doi: 10.1152/ajprenal.00432.2004. [DOI] [PubMed] [Google Scholar]
- 46.Ge Y, et al. Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. Am J Physiol Renal Physiol. 2006;291:F1274–1280. doi: 10.1152/ajprenal.00190.2006. [DOI] [PubMed] [Google Scholar]
- 47.Nadler SP, Zimpelmann JA, Hebert RL. Endothelin inhibits vasopressin-stimulated water permeability in rat terminal inner medullary collecting duct. The Journal of clinical investigation. 1992;90:1458–1466. doi: 10.1172/JCI116013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakano D, Pollock DM. Contribution of endothelin A receptors in endothelin 1-dependent natriuresis in female rats. Hypertension. 2009;53:324–330. doi: 10.1161/HYPERTENSIONAHA.108.123687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brezis M, Rosen S. Hypoxia of the renal medulla--its implications for disease. The New England journal of medicine. 1995;332:647–655. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 50.Miller RL, Kohan DE. Hypoxia regulates endothelin-1 production by the inner medullary collecting duct. J Lab Clin Med. 1998;131:45–48. doi: 10.1016/s0022-2143(98)90076-2. [DOI] [PubMed] [Google Scholar]
- 51.Wu LC, et al. Correction of sickle cell disease by homologous recombination in embryonic stem cells. Blood. 2006;108:1183–1188. doi: 10.1182/blood-2006-02-004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fox BM, H BJ, Sun Ch W, Townes T, Yanagisawa M, Pollock DM, Pollock JS. 14th International Conference on Endothelin: Physiology, Pathophysiology and Therapeutics. Physiologist. [PubMed] [Google Scholar]
- 53.Iglarz M, Silvestre JS, Duriez M, Henrion D, Levy BI. Chronic blockade of endothelin receptors improves ischemia-induced angiogenesis in rat hindlimbs through activation of vascular endothelial growth factor-no pathway. Arterioscler Thromb Vasc Biol. 2001;21:1598–1603. doi: 10.1161/hq1001.097065. [DOI] [PubMed] [Google Scholar]
- 54.Heimlich JB, et al. ET-1 increases reactive oxygen species following hypoxia and high-salt diet in the mouse glomerulus. Acta physiologica. 2015;213:722–730. doi: 10.1111/apha.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saleh MA, Pollock JS, Pollock DM. Distinct actions of endothelin A-selective versus combined endothelin A/B receptor antagonists in early diabetic kidney disease. The Journal of pharmacology and experimental therapeutics. 2011;338:263–270. doi: 10.1124/jpet.111.178988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barton M. Therapeutic potential of endothelin receptor antagonists for chronic proteinuric renal disease in humans. Biochimica et biophysica acta. 2010;1802:1203–1213. doi: 10.1016/j.bbadis.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 57.Fligny C, Barton M, Tharaux PL. Endothelin and podocyte injury in chronic kidney disease. Contributions to nephrology. 2011;172:120–138. doi: 10.1159/000328692. [DOI] [PubMed] [Google Scholar]
- 58.Lenoir O, S N, Henrique C, Guyonnet L, Fligny C, Audard V, Tharaux PL. 14th International Conference on Endothelin: Physiology, Pathophysiology and Therapeutics. Physiologist. [Google Scholar]
- 59.Kasztan M, S ChW, Townes TM, Pollock DM. 14th International Conference on Endothelin: Physiology, Pathophysiology and Therapeutics. Physiologist. [PubMed] [Google Scholar]
- 60.Aslan M, et al. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:15215–15220. doi: 10.1073/pnas.221292098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heimlich JB, S JS, O'Connor PM, Pollock JS, Townes TM, Meiler SE, Kutlar A, Pollock DM. Endothelin-1 contributes to the oxidative stress and renal dysfunction in sickle cell disease. British Journal of Pharmacology. 2015 doi: 10.1111/bph.13380. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barr TP, Kam S, Khodorova A, Montmayeur JP, Strichartz GR. New perspectives on the endothelin axis in pain. Pharmacological research. 2011;63:532–540. doi: 10.1016/j.phrs.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang ZJ, Wilkie DJ, Molokie R. Neurobiological mechanisms of pain in sickle cell disease. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2010;2010:403–408. doi: 10.1182/asheducation-2010.1.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schlenz AM, et al. Sensitization to acute procedural pain in pediatric sickle cell disease: modulation by painful vaso-occlusive episodes, age, and endothelin-1. The journal of pain : official journal of the American Pain Society. 2012;13:656–665. doi: 10.1016/j.jpain.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith TP, Schlenz AM, Schatz JC, Maitra R, Sweitzer SM. Modulation of pain in pediatric sickle cell disease: understanding the balance between endothelin mediated vasoconstriction and apelin mediated vasodilation. Blood Cells Mol Dis. 2015;54:155–159. doi: 10.1016/j.bcmd.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zappia KJ, Garrison SR, Hillery CA, Stucky CL. Cold hypersensitivity increases with age in mice with sickle cell disease. Pain. 2014;155:2476–2485. doi: 10.1016/j.pain.2014.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gokin AP, et al. Local injection of endothelin-1 produces pain-like behavior and excitation of nociceptors in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:5358–5366. doi: 10.1523/JNEUROSCI.21-14-05358.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shrestha S, et al. Local antinociception induced by endothelin-1 in the hairy skin of the rat's back. The journal of pain : official journal of the American Pain Society. 2009;10:702–714. doi: 10.1016/j.jpain.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dahlof B, Gustafsson D, Hedner T, Jern S, Hansson L. Regional haemodynamic effects of endothelin-1 in rat and man: unexpected adverse reaction. J Hypertens. 1990;8:811–817. doi: 10.1097/00004872-199009000-00004. [DOI] [PubMed] [Google Scholar]
- 70.Ferreira SH, Romitelli M, de Nucci G. Endothelin-1 participation in overt and inflammatory pain. Journal of cardiovascular pharmacology. 1989;13 Suppl 5:S220–222. doi: 10.1097/00005344-198900135-00065. [DOI] [PubMed] [Google Scholar]
- 71.Hans G, Deseure K, Robert D, De Hert S. Neurosensory changes in a human model of endothelin-1 induced pain: a behavioral study. Neurosci Lett. 2007;418:117–121. doi: 10.1016/j.neulet.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 72.Selenko-Gebauer N, Duschek N, Minimair G, Stingl G, Karlhofer F. Successful treatment of patients with severe secondary Raynaud's phenomenon with the endothelin receptor antagonist bosentan. Rheumatology (Oxford) 2006;45 Suppl 3:iii45–48. doi: 10.1093/rheumatology/kel290. [DOI] [PubMed] [Google Scholar]
- 73.Gholam P, Sehr T, Enk A, Hartmann M. Successful treatment of systemic-sclerosis-related digital ulcers with a selective endothelin type A receptor antagonist (sitaxentan) Dermatology. 2009;219:171–173. doi: 10.1159/000228318. [DOI] [PubMed] [Google Scholar]
- 74.Parisi S, et al. Efficacy of bosentan in the treatment of Raynaud's phenomenon in patients with systemic sclerosis never treated with prostanoids. Reumatismo. 2013;65:286–291. doi: 10.4081/reumatismo.2013.691. [DOI] [PubMed] [Google Scholar]
- 75.Bose N, Bena J, Chatterjee S. Evaluation of the effect of ambrisentan on digital microvascular flow in patients with systemic sclerosis using laser Doppler perfusion imaging: a 12-week randomized double-blind placebo controlled trial. Arthritis Res Ther. 2015;17:44. doi: 10.1186/s13075-015-0558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nguyen VA, et al. Effect of the dual endothelin receptor antagonist bosentan on Raynaud's phenomenon secondary to systemic sclerosis: a double-blind prospective, randomized, placebo-controlled pilot study. Rheumatology (Oxford) 2010;49:583–587. doi: 10.1093/rheumatology/kep413. [DOI] [PubMed] [Google Scholar]
- 77.Lionnet F, et al. Efficacy of the endothelin receptor blocker bosentan for refractory sickle cell leg ulcers. Br J Haematol. 2008;142:991–992. doi: 10.1111/j.1365-2141.2008.07206.x. [DOI] [PubMed] [Google Scholar]
- 78.Smith TP, Haymond T, Smith SN, Sweitzer SM. Evidence for the endothelin system as an emerging therapeutic target for the treatment of chronic pain. J Pain Res. 2014;7:531–545. doi: 10.2147/JPR.S65923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baamonde A, et al. Involvement of endogenous endothelins in thermal and mechanical inflammatory hyperalgesia in mice. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:245–251. doi: 10.1007/s00210-003-0841-1. [DOI] [PubMed] [Google Scholar]
- 80.Menendez L, Lastra A, Hidalgo A, Baamonde A. Nociceptive reaction and thermal hyperalgesia induced by local ET-1 in mice: a behavioral and Fos study. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:28–34. doi: 10.1007/s00210-002-0655-6. [DOI] [PubMed] [Google Scholar]
- 81.Khodorova A, et al. Endothelin-B receptor activation triggers an endogenous analgesic cascade at sites of peripheral injury. Nat Med. 2003;9:1055–1061. doi: 10.1038/nm885. [DOI] [PubMed] [Google Scholar]
- 82.Hillery CA, et al. Transient receptor potential vanilloid 1 mediates pain in mice with severe sickle cell disease. Blood. 2011;118:3376–3383. doi: 10.1182/blood-2010-12-327429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nature reviews Drug discovery. 2007;6:357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- 84.Plant TD, Zollner C, Mousa SA, Oksche A. Endothelin-1 potentiates capsaicin-induced TRPV1 currents via the endothelin A receptor. Experimental biology and medicine. 2006;231:1161–1164. [PubMed] [Google Scholar]
- 85.Balonov K, Khodorova A, Strichartz GR. Tactile allodynia initiated by local subcutaneous endothelin-1 is prolonged by activation of TRPV-1 receptors. Experimental biology and medicine. 2006;231:1165–1170. [PubMed] [Google Scholar]
- 86.Yamamoto H, Kawamata T, Ninomiya T, Omote K, Namiki A. Endothelin-1 enhances capsaicin-evoked intracellular Ca2+ response via activation of endothelin a receptor in a protein kinase Cepsilon-dependent manner in dorsal root ganglion neurons. Neuroscience. 2006;137:949–960. doi: 10.1016/j.neuroscience.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 87.Yamamoto T, et al. Endothelin receptor-mediated responses in trigeminal ganglion neurons. Journal of dental research. 2013;92:335–339. doi: 10.1177/0022034513478428. [DOI] [PubMed] [Google Scholar]
- 88.Gladwin MT, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. The New England journal of medicine. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 89.Galie N, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117:3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 90.Barst RJ, et al. Exercise capacity and haemodynamics in patients with sickle cell disease with pulmonary hypertension treated with bosentan: results of the ASSET studies. British journal of haematology. 2010;149:426–435. doi: 10.1111/j.1365-2141.2010.08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dupuis J, et al. Reduced pulmonary clearance of endothelin-1 in pulmonary hypertension. American heart journal. 1998;135:614–620. doi: 10.1016/s0002-8703(98)70276-5. [DOI] [PubMed] [Google Scholar]
- 92.Naomi S, et al. Endothelin-1 inhibits endothelin-converting enzyme-1 expression in cultured rat pulmonary endothelial cells. Circulation. 1998;97:234–236. doi: 10.1161/01.cir.97.3.234. [DOI] [PubMed] [Google Scholar]
- 93.Xiao H, G D, Gorshkov B, Dickerson C, Lucas R, Meiler S. 14th International Conference on Endothelin: Physiology, Pathophysiology and Therapeutics. Physiologist. [PubMed] [Google Scholar]
- 94.Dong F, et al. Endothelin-1 enhances oxidative stress, cell proliferation and reduces apoptosis in human umbilical vein endothelial cells: role of ETB receptor, NADPH oxidase and caveolin-1. Br J Pharmacol. 2005;145:323–333. doi: 10.1038/sj.bjp.0706193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stankova J, D'Orleans-Juste P, Rola-Pleszczynski M. ET-1 induces IL-6 gene expression in human umbilical vein endothelial cells: synergistic effect of IL-1. The American journal of physiology. 1996;271:C1073–1078. doi: 10.1152/ajpcell.1996.271.4.C1073. [DOI] [PubMed] [Google Scholar]
- 96.Davie N, et al. ET(A) and ET(B) receptors modulate the proliferation of human pulmonary artery smooth muscle cells. American journal of respiratory and critical care medicine. 2002;165:398–405. doi: 10.1164/ajrccm.165.3.2104059. [DOI] [PubMed] [Google Scholar]
- 97.Thorin E, Clozel M. The cardiovascular physiology and pharmacology of endothelin-1. Advances in pharmacology. 2010;60:1–26. doi: 10.1016/B978-0-12-385061-4.00001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gregan B, Schaefer M, Rosenthal W, Oksche A. Fluorescence resonance energy transfer analysis reveals the existence of endothelin-A and endothelin-B receptor homodimers. Journal of cardiovascular pharmacology. 2004;44 Suppl 1:S30–33. doi: 10.1097/01.fjc.0000166218.35168.79. [DOI] [PubMed] [Google Scholar]
- 99.Rivera A, Jarolim P, Brugnara C. Modulation of Gardos channel activity by cytokines in sickle erythrocytes. Blood. 2002;99:357–603. doi: 10.1182/blood.v99.1.357. [DOI] [PubMed] [Google Scholar]
- 100.Rivera A. Reduced sickle erythrocyte dehydration in vivo by endothelin-1 receptor antagonists. American journal of physiology Cell physiology. 2007;293:C960–966. doi: 10.1152/ajpcell.00530.2006. [DOI] [PubMed] [Google Scholar]


