Abstract
G protein-coupled receptors (GPCRs) regulate virtually all metabolic processes including glucose and energy homeostasis. Recently, the use of designer GPCRs referred to as DREADDs (designer receptors exclusively activated by designer drug) has made it possible to dissect metabolically relevant GPCR signaling pathways in a temporally and spatially controlled fashion in vivo.
Keywords: G protein-coupled receptor, designer GPCR, DREADD, metabolism, diabetes, obesity
The DREADD Concept
G protein-coupled receptors (GPCRs) are cell surface receptors that are targeted by an extraordinarily large number of clinically important drugs. Many studies suggest that distinct members of the GPCR superfamily represent potential targets for the treatment of various metabolic disorders including obesity and type 2 diabetes (T2D) [1]. However, research in this area has been hampered by the fact that a particular GPCR is usually expressed in multiple tissues and cell types, making it difficult to predict the in vivo metabolic consequences of targeting a specific GPCR with selective ligands.
During the past few years, designer GPCRs referred to as DREADDs (designer receptors exclusively activated by designer drugs) have emerged as powerful novel tools to study the physiological relevance of distinct GPCR signaling pathways in specific tissues in vivo. Structurally, DREADDs are mutant muscarinic acetylcholine receptors that can be activated with high potency by clozapine-N-oxide (CNO), an otherwise pharmacologically inert agent [2,3]. However, DREADDs cannot be activated by acetylcholine, the endogenous muscarinic receptor agonist [2,3].
Following agonist binding, GPCRs can activate different classes of heterotrimeric G proteins, which can be subdivided into four major functional groups: Gq, Gi, Gs, and G12. Moreover, accumulating evidence suggests that GPCRs can also initiate β-arrestin-dependent (G protein-independent) signaling. Thus, the physiological outcome of activating a specific GPCR (or DREADD) in a particular tissue, may also be modulated by β-arrestin-dependent signaling pathways.
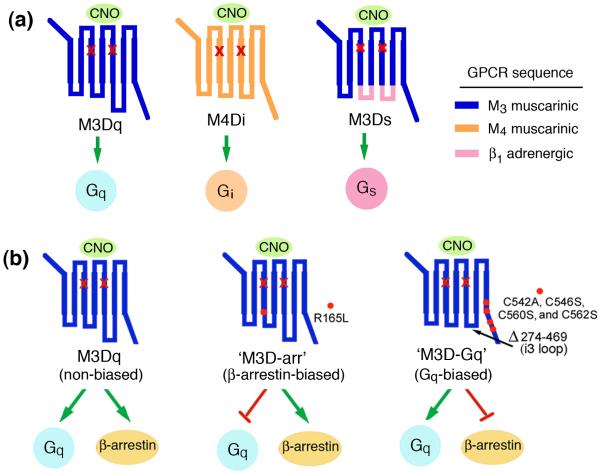
At present, the most commonly used DREADDs are mutant muscarinic receptors that stimulate Gq-, Gi-, or Gs-type heterotrimeric G proteins (Fig. 1a). More recently, two novel M3 muscarinic receptor-based DREADDs have been developed (Fig. 1b). One of these new designer receptors selectively stimulates β-arrestin-dependent signaling without activating G proteins [4], whereas the other construct selectively stimulates G proteins of the Gq family but lacks the ability to interact with β-arrestins [5]. In the following, I will briefly review several studies that have employed DREADD technology to identify metabolically important GPCR signaling pathways in vivo. Because of space constraints, I will focus on only three cell types that are of central importance for glucose and energy homeostasis: pancreatic β-cells, hepatocytes, and AgRP neurons of the hypothalamus (Fig. 2).
Figure 1.
Structure and coupling properties of muscarinic receptor-based DREADDs. All depicted DREADDs contain the same two point mutations (Y->C and A->G) in transmembrane helices 3 and 5, respectively (red `x marks') [2–5]. The designer receptors shown are unable to bind acetylcholine, the endogenous muscarinic receptor agonist, but can be activated by CNO with high potency and efficacy. (a) Structure and G protein coupling properties of the M3Dq [2], M4Di [2], and M3Ds [3] DREADDs. (b) Structure of Gq- and β-arrestin-biased DREADDs. M3Dq couples to Gq-type G proteins but can also initiate β-arrestin-dependent signaling [4]. The M3D-arr DREADD (center panel) represents a β-arrestin-biased version of M3Dq [4]. M3D-Gq (right panel) functions as a Gq-biased designer receptor [5]. Amino acid numbers refer to the rat M3 muscarinic receptor sequence.
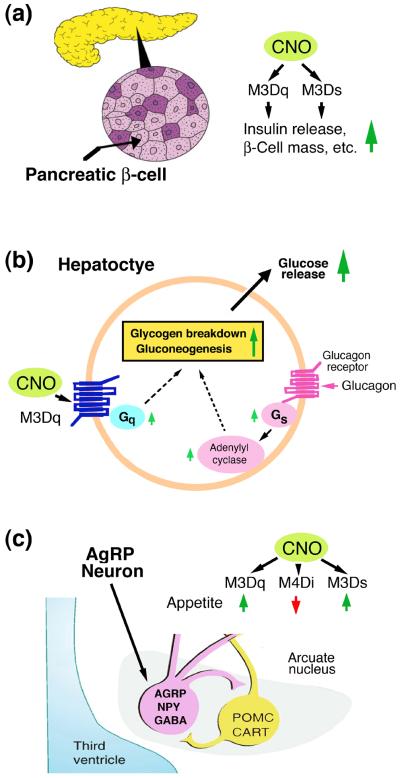
Figure 2.
Expression of DREADDs in various metabolically relevant cell types in the mouse.
The indicated DREADDs (M3Dq, M4Di, and M3Ds) were selectively expressed in mouse β-cells (a), hepatocytes (b), and AgRP neurons (c) of the arcuate nucleus of the hypothalamus. Some of the key metabolic phenotypes observed after CNO treatment are highlighted (see text for more details).
Modulation of β-Cell Function by DREADDs
The function of pancreatic β-cells is regulated by a large number of GPCRs [6]. Detailed metabolic studies with transgenic mice expressing M3Dq (Gq DREADD) selectively in pancreatic β-cells have led to the identification of novel pathways crucial for the regulation of β-cell function [3,7] (Fig. 2a). For example, chronic CNO treatment of these mutant mice resulted in pronounced improvements in β-cell function, including the upregulation of many genes critical for β-cell function [7]. Moreover, chronic stimulation of β-cell M3Dq led to a significant increase in pancreatic insulin content due to enhanced β-cell proliferation and changes in gene expression promoting insulin synthesis [7]. Chronic activation of β-cell M3Dq also greatly ameliorated streptozotocin-induced diabetes and metabolic deficits observed with mice maintained on a high-fat diet [7]. Additional in vivo and in vitro studies strongly suggested the existence of a novel signaling pathway through which activation of β-cell Gq triggers enhanced expression and function of insulin receptor substrate 2 (IRS2) and that IRS2-dependent downstream signaling plays a key role in mediating the improved β-cell function observed after chronic activation of M3Dq [7]. In acute CNO administration studies, transgenic mice that expressed the M3Ds designer construct (Gs DREADD) in a β-cell-selective fashion showed similar beneficial metabolic phenotypes as the β-cell M3Dq mutant mice [3]. However, the long-term effects of stimulating M3Ds in mouse β-cells have not been investigated so far. These findings strongly suggest that therapeutic strategies aimed at enhancing signaling through β-cell Gq and Gs should prove useful for the treatment of diabetes.
Expression of a Gq DREADD in Hepatocytes
The liver plays a central role in regulating whole body glucose homeostasis. It is well known that glucagon-mediated activation of hepatocyte glucagon receptors, which selectively activate the stimulatory G protein, Gs, strongly promotes hepatic glucose production (HGP; Fig. 2b). Interestingly, CNO treatment of transgenic mice expressing the M3Dq DREADD selectively in hepatocytes led to pronounced increases in blood glucose levels, due to enhanced hepatic gluconeogenesis and glycogen breakdown [8] (Fig. 2b). A recent study employing Gq- and β-arrestin-biased DREADDs (Fig. 1b) demonstrated that the M3Dq-mediated increases in HGP are caused by the activation of Gq-type G proteins and do not seem to be modulated by β-arrestins [5]. These observations suggest that inhibitors of Gq-mediated signaling in hepatocytes may prove clinically useful for suppressing HGP and hyperglycemia in T2D, a disease which is characterized by unphysiologically high hepatic glucose output.
Expression of DREADDs in AgRP Neurons
Obese individuals are at a high risk of developing T2D. In order to guide the design of novel appetite-suppressing drugs, it is critical to dissect the neuronal circuits that regulate food intake under physiological and pathophysiological conditions. Recently, several studies have used DREADD technology to study a subpopulation of hypothalamic neurons located in the arcuate nucleus of the hypothalamus which synthesize and release agouti-related peptide (AgRP), a neuropeptide endowed with potent orexigenic activity (Fig. 2c). AgRP neurons also store and release two additional appetite-stimulating agents, neuropeptide Y (NPY) and GABA [9] (Fig. 2c).
The activity of AgRP neurons, like that of essentially all other cell types is predicted to be regulated by GPCRs with different G protein coupling properties. CNO treatment of mice that selectively expressed the M3Dq DREADD in AgRP neurons triggered neuronal depolarization, associated with a pronounced increase in food intake [10] (Fig. 2c). In contrast, administration of CNO to mice that selectively expressed the M4Di DREADD in AgRP neurons inhibited the activity of AgRP neurons and caused a reduction in food intake [10] (Fig. 2c). In a recent study, Nakajima et al. [11] expressed the M3Ds construct (Gs DREADD; Fig. 1a), selectively in mouse AgRP neurons. CNO treatment of these animals led to a robust and sustained increase in food intake (Fig. 2c). Additional studies suggested that the mechanisms through which the Gq- and Gs-linked DREADDs stimulate appetite are clearly distinct [10,11]. In contrast to M3Dq-induced feeding, the orexigenic effect triggered by M3Ds activation lasted for several days following a single CNO injection and was almost entirely dependent on the release of AgRP [11]. In any case, these findings suggest that drugs capable of blocking signaling through endogenous Gq- and Gs-linked GPCRs expressed by AgRP neurons may prove beneficial as novel appetite-suppressing drugs.
During the past few years, DREADD technology has also been instrumental to map several other central pathways that regulate whole body glucose and energy homeostasis. Because of space constrains, I am unable to discuss these studies in this short article [for a recent review, see [12]).
Comparison of DREADD Technology With Optogenetic Approaches
Like DREADD technology, optogenetic techniques have enabled neuroscientists to inhibit or activate specific sets of neurons to map neural circuitries that regulate feeding behavior and energy expenditure [13,14]. These studies have shown that the activation or inhibition of specific neuronal subpopulations by either DREADD technology or optogenetic approaches leads to similar physiological outcomes [14]. In several instances, these two technologies have been used in parallel to provide converging evidence for the existence of specific neuronal pathways regulating food intake, energy expenditure, and other physiological functions [14].
Typically, CNO treatment of experimental animals expressing DREADDs in a distinct cell type or brain region results in long-lasting physiological effects (several hours) [12–14]. Since DREADD technology involves the drug-dependent activation of specific (designer) GPCRs, it is of particular relevance for potential translational applications (GPCRs are the most common targets of clinically used drugs). In contrast, optogenetic techniques allow the dissection of neural circuitries on a millisecond time scale. Although technically more challenging, optogenetic approaches offer the additional advantage that changes in neuronal activity can be triggered by the simple, light-mediated opening of distinct ion channels or pumps, thus facilitating the interpretation of experimental data. On the other hand, ligand-activated DREADDs are predicted to modulate the activity of many downstream signaling pathways and ion channels and may also trigger G protein-independent effects (e.g. β-arrestin-dependent signaling) that may affect neuronal function in a complex fashion. Moreover, in contrast to the use of light as activating `agent' in optogenetics, the chemical entities used as DREADD ligands may undergo conversion to biologically active metabolites in some species (including human) or under certain experimental conditions.
Concluding Remarks
DREADD technology has proven extremely useful to map metabolically relevant signaling pathways in different tissues in vivo. Importantly, this new chemogenetic approach makes it possible to assess the in vivo metabolic consequences of activating distinct GPCR cascades at the cellular level. These DREADD studies provide a rational basis for the development of novel classes of drugs that can activate or inhibit specific endogenous GPCRs for the treatment of diabetes, obesity, and related pathophysiological conditions.
Acknowledgements
I would like to thank all present and past members of my laboratory who were involved in parts of the DREADD work covered in this short review. Moreover, I apologize to all authors whose work I was unable to cite due to space limitations. The author's own research reviewed in this article was supported by the Intramural Research Program of the NIH, NIDDK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oh da Y, Olefsky JM. G protein-coupled receptors as targets for anti-diabetic therapeutics. Nat. Rev. Drug Discov. 2016;15:161–172. doi: 10.1038/nrd.2015.4. [DOI] [PubMed] [Google Scholar]
- 2.Armbruster BN, et al. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. U.S.A. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guettier JM, et al. A chemical-genetic approach to study G protein regulation of β cell function in vivo. Proc. Natl. Acad. Sci. U.S.A. 2009;106:19197–19202. doi: 10.1073/pnas.0906593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakajima K, Wess J. Design and functional characterization of a novel, arrestin-biased designer G protein-coupled receptor. Mol. Pharmacol. 2012;82:575–582. doi: 10.1124/mol.112.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu J, et al. A G protein-biased designer G protein-coupled receptor useful for studying the physiological relevance of Gq/11-dependent signaling pathways. J. Biol. Chem. 2016 Feb 5; doi: 10.1074/jbc.M115.702282. 2016 pii: jbc.M115.702282 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahrén B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat. Rev. Drug. Discov. 2009;8:369–385. doi: 10.1038/nrd2782. [DOI] [PubMed] [Google Scholar]
- 7.Jain S, et al. Chronic activation of a designer Gq-coupled receptor improves β-cell function. J. Clin. Invest. 2013;123:1750–1762. doi: 10.1172/JCI66432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li JH, et al. A novel experimental strategy to assess the metabolic effects of selective activation of a Gq-coupled receptor in hepatocytes in vivo. Endocrinology. 2013;154:3539–3551. doi: 10.1210/en.2012-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krashes MJ, et al. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metab. 2013;18:588–595. doi: 10.1016/j.cmet.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krashes MJ, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakajima K, et al. Gs-coupled GPCR signalling in AgRP neurons triggers sustained increase in food intake. Nat. Commun. 2016;7:10268. doi: 10.1038/ncomms10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urban DJ, Roth BL. DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annu. Rev. Pharmacol. Toxicol. 2015;55:399–417. doi: 10.1146/annurev-pharmtox-010814-124803. [DOI] [PubMed] [Google Scholar]
- 13.Roth BL. DREADDs for neuroscientists. Neuron. 2016;89:683–694. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacol. Rev. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]