Abstract
Recent optogenetic studies demonstrated that phasic dopamine release in the nucleus accumbens may play a causal role in multiple aspects of natural and drug reward-related behaviors. The role of tonic dopamine release in reward consummatory behavior remains unclear. The current study used a combinatorial viral-mediated gene delivery approach to express ChR2 on mesolimbic dopamine neurons in rats. We used optical activation of this dopamine circuit to mimic tonic dopamine release in the nucleus accumbens and to explore the causal relationship between this form of dopamine signaling within the ventral tegmental area (VTA)-nucleus accumbens projection and consumption of a natural reward. Using a two bottle choice paradigm (sucrose vs. water), the experiments revealed that tonic optogenetic stimulation of mesolimbic dopamine transmission significantly decreased reward consummatory behaviors. Specifically, there was a significant decrease in the number of bouts, licks and amount of sucrose obtained during the drinking session. Notably, activation of VTA dopamine cell bodies or dopamine terminals in the nucleus accumbens resulted in identical behavioral consequences. No changes in the water intake were evident under the same experimental conditions. Collectively, these data demonstrate that tonic optogenetic stimulation of VTA-nucleus accumbens dopamine release is sufficient to inhibit reward consummatory behavior, possibly by preventing this circuit from engaging in phasic activity that is thought to be essential for reward-based behaviors.
Dopamine release in the nucleus accumbens is involved in all types of reward-related behaviors, including reward learning, seeking and intake (Schultz et al., 1997; Hyland et al., 2002; Phillips et al., 2003b; Roitman et al., 2004; Wise, 2004; Stuber et al., 2005). This neurotransmitter can be released naturally with two main patterns: phasic and tonic (Grace, 1991; Schultz, 1998; Grace, 2000; Wightman and Robinson, 2002). Tonic dopamine firing occurs at a low frequency of ~5 Hz and results in steady-state dopamine concentrations that are lower than 50 nM (Parsons and Justice, 1992; Justice, 1993). In contrast, burst firing of dopaminergic neurons, at frequencies of more than 30 Hz, leads to large, transient increases in dopamine concentrations, which may significantly exceed 50 nM (Grace and Bunney, 1983, 1984; Freeman et al., 1985; Wightman and Zimmerman, 1990; Hyland et al., 2002; Wightman and Robinson, 2002; Aragona et al., 2008). These distinct characteristics of phasic and tonic dopamine release suggest divergent roles for each pattern in the control of dopamine-related behaviors.
It is well documented that accumbal dopamine is released in a phasic fashion in response to reward presentation (Day et al., 2007; Brown et al., 2011; Flagel et al., 2011) or cues that signal reinforcement availability (Roitman et al., 2004; Owesson-White et al., 2008; Beyene et al., 2010; Jones et al., 2010). Phasic dopamine release was also found to be temporally related to lever-pressing for reward delivery (Phillips et al., 2003b; Wassum et al., 2013). Remarkably, the propensity of a reward-paired cue to increase lever pressing can be predicted by the amplitude of phasic dopamine release, suggesting a possible mechanism through which cues initiate reward-seeking behavior (Wassum et al., 2013). Several other studies support this notion. For example, it has been shown that evoked dopamine transients can trigger a lever-press for cocaine (Phillips et al., 2003b). Additionally, alterations in conditioning-associated tonic dopamine release were negatively correlated with changes in the effort needed to obtain a reward (Ostlund et al., 2011). Other findings suggest that phasic dopamine release encodes the full range of reward prediction error necessary for reinforcement learning (Bayer and Glimcher, 2005; Hart et al., 2014). Altogether, multiple studies have revealed strong associations between changes in subsecond dopamine release in the nucleus accumbens and different types of reward-related behaviors.
Recently, the emergence of optogenetics has allowed us to better explore the causal relationship between accumbal dopamine and behavior. Indeed, optogenetic activation of VTA dopamine neurons can selectively induce dopamine release in accumbal terminal fields with very high temporal and spatial precision. Moreover, optical stimulation of the VTA was shown to mimic phasic and tonic dopamine release (Tsai et al., 2009; Bass et al., 2013). Using this approach, it was shown that phasic, but not tonic, dopamine release is solely responsible for the development of conditioned place preference (Tsai et al., 2009). Furthermore, phasic activation of dopaminergic neurons causally enhances positive reinforcing actions in a food-seeking task (Adamantidis et al., 2011). Moreover, phasic activation was sufficient to reactivate previously extinguished food-seeking behavior in the absence of external cues (Adamantidis et al., 2011) and to enhance the initiation of approach behavior without long-term motivational regulation (Ilango et al., 2014). Optogenetic activation of dopamine neurons, mimicking a prediction error, was sufficient to cause long-lasting increases in cue-elicited, reward-seeking behavior (Steinberg et al., 2013). These finding establish a causal role for fast dopamine signaling in reward learning. While phasic dopamine release in the nucleus accumbens plays a causal role in multiple aspects of reward learning, seeking and intake behavior, the role of tonic dopamine release in reward-related activities is still unclear. In our previous optogenetic study, we revealed that tonic, but not phasic, activation of the VTA attenuated alcohol intake in an intermittent drinking procedure (Bass et al., 2013). The aim of the present study was to explore whether driving mesolimbic dopamine transmission into the tonic mode affects natural reward consumption in a similar fashion.
EXPERIMENTAL PROCEDURES
Animals
Adult (90-150 days old) male Long-Evans rats, weighing 300-380g, were housed in a temperature-controlled vivarium in acrylic cages on a 12/12 h light/dark cycle (lights out at 6:00 PM). Food and water were available ad libitum, though food was withheld during experimental sessions (30 min), conducted during the light cycle. Prior to surgery, rats were group-housed and then individually housed following viral infusion and optical cannula implantation. All animal procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Viral constructs and packaging
Virus packaging and titering was previously described (Bass et al., 2010; Bass et al., 2013). A standard triple transfection packaging protocol was used to package viruses to generate pseudotyped AAV2/10 (Xiao et al., 1998). The three plasmids were an AAV2 plasmid, which contained the transgene to be packaged, pHelper (Stratagene, La Jolla, CA) provided adenoviral helper functions, and an AAV2/10 rep/cap plasmid containing the AAV2 replicase and AAV10 capsid genes (Gao et al., 2002; De et al., 2006). The EF1α-DIO-ChR2-EYFP-pAAV and TH-iCRE-pACP plasmids were previously described (Gompf et al., 2015). Briefly, Cre recombinase expression is driven by a rat tyrosine hydroxylase (TH) promoter, which restricts expression to TH+ neurons (Oh et al., 2008; Gompf et al., 2015). In the second construct ChR2-EYFP is driven by a strong, generalized promoter (EF1α), but this transgene is oriented in a DIO configuration which requires Cre recombinase to reorient it to an active, drivable position in relation to the EF1α promoter. When co-infused into the VTA, the Cre is expressed only in TH+ (dopaminergic) neurons which are then the only cells that express ChR2-EYFP.
Stereotaxic virus injection
Naïve subjects were anesthetized using ketamine hydrochloride (100 mg/kg, i.p.) and xylazine hydrochloride (20 mg/kg, i.p.). Once placed in a stereotaxic frame, the scalp was shaved and wiped with iodine. The skull was uncovered by making an incision centrally along the scalp. Two small drill holes were fashioned for 2 skull screws to stabilize a cement cap. A final hole was drilled on the right side above either the VTA (from bregma: anterior 5.8 mm; lateral, 0.7 mm) or nucleus accumbens (from bregma: anterior 1.3 mm; lateral, 1.3 mm) into which an optic-fluid cannula (OFC) (Doric Lenses, Canada) was implanted (DV, 7.3 mm) (Fig. 1). Next, a combination of DIO-ChR2-EYFP-AAV2/10 and TH-iCRE-AAV2/10 were coinjected (X:X, 1:3 μl total) gradually into the VTA (DV, 7.3mm) over 13 min through the OFC via a Hamilton syringe. Previously we have shown that this combinatorial system restricts ChR2 expression to dopaminergic neurons in the VTA (Gompf et al., 2015). Dental cement stabilized by skull screws was used to cover the exposed skull. Subjects were returned to their home cages for recovery once the cement was dry.
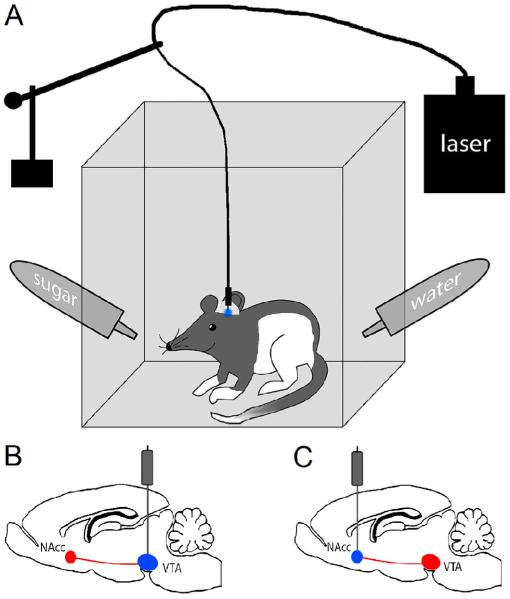
Figure 1. Schematic of the behavioral set-up designed to evaluate the effect of optogenetic stimulation of the VTA-nucleus accumbens dopamine circuit on consummatory behaviors.
Panel A represents a schematic picture of a rodent cage that was optimized to allow stimulation of targeted brain areas through implantable optical cannulas, which were connected to a laser via the patch cord, during 30 min drinking sessions. A counter-balanced lever arm with attached optical commutator allowed rats to move freely in the drinking chamber. Panel B and C represent schematic pictures of rat brains with the optical activation of the ventral tegmental area (VTA) and nucleus accumbens (NAcc) through implanted cannulas, respectively.
Sucrose drinking behavior
Sucrose (3%) and water preference was measured using a two-bottle drinking procedure (Bass et al., 2013; Chaudhury et al., 2013; Tye et al., 2013). Two groups of rats (6-7 rats per group) with ChR2 expression in the mesolimbic pathway and optic-fluid cannulas (Doric Lenses, Canada) implanted into the VTA or nucleus accumbens were prepared for these experiments. Subjects were placed in a cage (MED Associates, St. Albans, VT, USA) and given access to sucrose and water during 30 min testing periods every Monday, Wednesday, and Friday, having access only to water on other days (Figure 1A). Sucrose and water were given in graduated drinking tubes (MED Associates, St. Albans, VT, USA) and the position of the bottles was alternated on each drinking day to control for potential side preference. Using custom-made lickometers, sucrose and water consumption was measured as the number of licks, bouts and total intake (g/kg) after each drinking session. In addition, the latency to the first lick was also measured for both sucrose and water.
The control (no stimulation) and experimental (with optical stimulation) set for both experimental groups (targeted the VTA or nucleus accumbens) consisted of the same animals tested on different days in a balanced manner in order to provide a within-subject control design for sucrose and water consumption. This design allowed us to avoid the influence of multiple factors, including individual differences, and therefore, more clearly dissect effects of tonic stimulation on drinking behavior. Rats were habituated to the optical cable two weeks before stimulations. The stimulation was applied for the first 10 minutes of the drinking session only. Therefore, sucrose still remained accessible for the last 20 minutes of each session, providing rats with the opportunity to compensate for the amount of sucrose that was not obtained during the stimulation. No aversive effects of optical activation of the VTA or nucleus accumbens were observed.
Fast-scan cyclic voltammetry recordings
Extracellular dopamine concentrations, before and following optical (7 rats per group) and electrical (6 rats per group) stimulation of the VTA, were measured using fast-scan cyclic voltammetry (FSCV). The experiments with optical stimulation were performed at different time points following viral infusion, specifically at 4 weeks (4 rats) and 8 weeks (3 rats). No doubt, a greater spread of ChR2 expression can be observed with a longer incubation period. However, light penetration is always restricted to the same distance (≈1 mm) below the end of the optical fiber, and therefore, the signal is relatively the same at these two time periods. Since there was no difference in dopamine response between these two time points, data were analyzed together. Experiments with electrical stimulation were performed on rats matched to the same strain, age and weight as the ones on which optical stimulation was performed.
All subjects were secured in a stereotaxic frame following urethane (1.5 g/kg, i.p.) anesthesia. After the scalp was shaved and cleaned, the skull was uncovered by making a central incision along the scalp. One hole was fashioned above the nucleus accumbens (from bregma: anterior, 1.3 mm; lateral, 1.3 mm) and another hole above the ipsilateral VTA (from bregma: posterior, 5.8 mm; lateral, 0.7 mm). A final hole was placed on the contralateral hemisphere for the implantation of an Ag/AgCl reference electrode connected to a voltammetric amplifier (UNC Electronics Design Facility, Chapel Hill, NC). A carbon fiber microelectrode (exposed fiber length: 100 μ m; diameter: 6 μ m) connected to the voltage amplifier and secured to the stereotaxic frame arm was lowered into the hole drilled above the nucleus accumbens (ventral, 7.4 mm).
Electrical stimulation was obtained using a bipolar stimulating electrode that was inserted into the hole above the VTA (ventral, 7.5-8.5 mm) and connected to a voltage output box. Optical stimulation was achieved via an optical fiber (diameter: 200 μ m) inserted in the hole above the VTA (ventral, 7.5-8.5 mm) connected to a laser (Viasho, China). Optical (average power = 3.5 mW) and electrical (current = 350 μA) stimulations consisted of 250 rectangular 4 ms pulses at 5 Hz, and began 5 seconds into each recording. Voltammetric recordings occurred at the carbon fiber electrode every 100 ms for 75 seconds by applying a triangular waveform (-0.4 to +1.3V, 400 V/s). Oxidation and reduction peaks were observed at +0.6 V and -0.2 V respectively (vs. Ag/AgCl reference) identifying dopamine as the released chemical. Data were digitized (National Instruments, Austin, TX) and stored on a computer. Calibration of the carbon fiber electrodes was performed with known concentrations of dopamine (5, 10 μM) in vitro (Budygin et al., 2001b; Phillips et al., 2003a).
Optical stimulation during sucrose drinking
The sessions with optical stimulation of sucrose drinking rats were performed 4 weeks following viral transfection. This interval was necessary to obtain the level of ChR2 expression that is sufficient for effective stimulation of dopamine release (Bass et al., 2010; 2013). The optical setup had a laser at wavelength 473 nm (Beijing Viasho Technology Co., Ltd, Beijing, China) with a 100 mW maximum power output. A programmable function generator (Hewlett-Packard model 8116A) provided control signals to modulate the laser via the TTL input control port on the laser power supply. Parameters of the light presentation for tonic stimulation were 3000 light pulses at 5 Hz (total light exposure of 10 minutes). The optical pulse procedure began manually by firing a pulse generator (Systron-Donner Model 100C) which activated a digital delay generator (SRS Model DG535). The digital delay generator was used to ensure the function generator was appropriately gated to select a finite number of pulses from the continuous waveforms typically produced by the function generator. A series of 5 Hz square pulses were produced by the function generator. The total number of pulses in one data stream (250) was gated by the digital delay generator because the temporal length of a gate pulse received by the function generator dictated the number of square pulses produced by the function generator for each trigger. Individual pulses had a temporal width of 4 ms within each series of pulses. A commercial power meter (Thorlabs, Newton, NJ) was used to measure the laser power output.
Immunohistochemistry
The rats were sacrificed for histology at different time points (30 to 60 days) following viral transfection. They were anesthetized with a combination of ketamine (100 mg/kg) and xylazine (10 mg/kg) and then transcardially perfused with 10% normal buffered formalin. After removal, brains were soaked overnight in fixative at 4º C and then incubated in a 25% sucrose solution overnight until the brains sank. Fifty μ m thick sections were obtained on an American Optical 860 sliding microtome. Free-floating coronal sections which contained the midbrain were processed for immunohistochemistry. Briefly, sections were washed in PBS for 5 min followed by 3× 10 min rinses in PBS + 0.5% triton X-100. Primary antibody diluted in PBS + 0.3% triton X-100 was applied overnight at 4ºC while shaking. Primary antibodies used were mouse anti-tyrosine hydroxylase (ImmunoStar #22941) at a 1:4000 dilution and a rabbit anti-GFP (Invitrogen #A6455, also cross reacts with EYFP) at a 1:2000 dilution. The following day, sections underwent 3 × 10 min PBS rinses and then were incubated with secondary antibodies of Alexa 555 donkey anti-mouse (Invitrogen, #A31570, 1:4000) and Alexa 488 goat anti-rabbit (Invitrogen #A11034, 1:2000) at room temperature for 2 hours while shaking. A last set of 3× 10min PBS rinses were applied to the sections which were then mounted onto slides and coverslipped with Prolong Gold media. Slides were visualized via a Zeiss LSM 710 confocal microscope.
Statistical analysis
Data were analyzed in GraphPad Prism (GraphPad Software, San Diego, CA). 2-way ANOVAs, two-tailed unpaired t test with Welch’s correction and nonparametric Wilcoxon matched pairs signed rank test were used to determine statistical significance. The data were presented as mean ± SEM, and the criterion of significance was set at P<0.05.
RESULTS
Confirmation of ChR2 expression on dopaminergic neurons
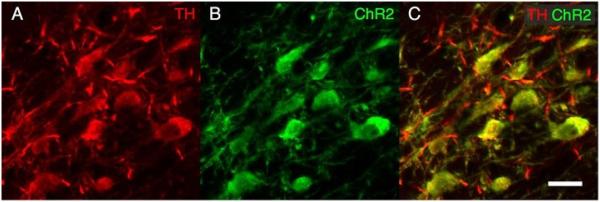
Immunohistochemistry for GFP and TH revealed robust ChR2-EYFP expression throughout the VTA with EYFP+ terminals apparent in the nucleus accumbens. Co-localization of TH and EGFP was restricted to TH+ neurons in the VTA (Fig. 2).
Figure 2. Characterization of ChR2 expression in the VTA.
Immunohistochemistry for TH (A) and EYFP (B) in the VTA revealed that ChR2-EYFP was largely restricted to dopaminergic neurons. Co-localization is apparent in panel (C). Scale bar represents 25 μm.
Mimicking and detection of tonic dopamine release in rat nucleus accumbens
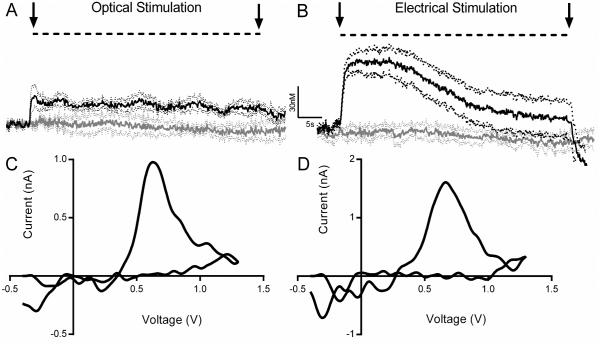
FSCV in anesthetized rats was used to detect changes in extracellular dopamine concentrations in the nucleus accumbens in response to tonic stimulation patterns. We compared the effect of optogenetic stimulation of dopamine cell bodies with consequences observed following their electrical stimulation that has been traditionally used for many years (Budygin et al., 1999; Budygin et al., 2000; Budygin et al., 2001a; Jones et al., 2006; Budygin, 2007; Oleson et al., 2008; Bass et al., 2010; Pattison et al., 2011; Pattison et al., 2012; Bass et al., 2013). As illustrated in Figure 3, tonic optogenetic (A) and electrical (B) VTA stimulation evoked significant increases in accumbal dopamine levels. An evaluation of the areas under each curve of the dopamine transients found no difference between optogenetic and electrical activation (P>0.05, n=6; Mann Whitney test). However, the area under each curve during the first 20 seconds of stimulation was significantly greater for electrically-evoked dopamine traces compared with optically-induced signals (15.9±3.0 vs. 6.1±1.0, P<0.05, n=6; Mann Whitney test). Notably, the dopamine concentration evoked by optical stimulation remained quite stable during the entire stimulus train (50s), while dopamine following electrical stimulation was less steady. Thus, similar to the dopamine response during the first second of optical stimulation, electrically-evoked dopamine increased transiently but then plateaued for 15 s and finally, gradually decreased. A 2-way RM ANOVA revealed a significant interaction (F(354,1888)=4.957, P<0.0001) and a main effect of time (F(188,1888)=8.715, P<0.0001, n=6-7). This main effect of time was driven by electrical stimulation (F(2.593,15.56)=22.88, P<0.0001, n=6) but not optical stimulation (F(2.382,11.91)=2.782, P=0.0959, n=7), according to a 1-way RM ANOVA. The difference in dopamine response induced by optical and electrical stimulation may be based on the specificity of these approaches. While optogenetic stimulation only activates dopamine neurons, electrical stimulation will also activate glutamatergic, and GABAergic neurons within the VTA and these cells may influence dopamine release kinetics. Moreover, electrical stimulation applied with these specific parameters may trigger a greater dopamine efflux in comparison with the optical stimulation used in this study.
Figure 3. Low frequency optogenetic and electrical activation of VTA dopaminergic neurons mimics tonic dopamine release patterns in the nucleus accumbens.
Dopamine effluxes were evoked by 5 Hz, 250 pulses of optical (A) or electrical (B) stimulation of the VTA. These data are presented as a mean ± SEM. Averaged dopamine concentrations are denoted by solid lines (upper bold curves are recordings with stimulations) and dots (lower bold curves are recordings with no stimulations), while SEMs are represented by broken lines. Optically- and electrically-evoked dopamine was identified by their background-subtracted cyclic voltammograms (C and D, respectively).
An electrical activation of dopamine axons is capable of inducing a depletion of releasable dopamine in the terminal field under some stimulating parameters (Michael et al., 1987; Nicolaysen et al., 1988). Therefore, it was important to figure out whether optogenetic stimulation, selected for behavioral experiments, caused dopamine depletion. The experiments revealed no depletion under our stimulation protocol. Dopamine was effectively released during the entire period of stimulation (10 minutes). There was some significant reduction (from 45.7±3.1 nM for the first minute to 29±3.5 nM during the last minute of VTA stimulation) in the maximal amplitude of optically-induced dopamine efflux at the end of the 10 min stimulation (F(1.099,3.296)=14.62, P<0.05, n=4; RM one-way ANOVA). However, dopamine recovered to 100% (46.0±3.9 nM) within 1 minute after stimulation was terminated.
Effects of tonic activation of VTA-accumbal dopamine release on sucrose drinking behaviors
Subjects were presented with two bottles containing water and a 3% sucrose solution for 30 min drinking sessions. Figure 4 represents average sucrose (3%) and water licks during 18 drinking sessions measured before optical stimulation. There was a significant difference between sucrose and water consumption (F (1, 101) = 559.5; P < 0.0001). Importantly, no significant changes in the number of licks were found after virus injection surgery (between 11th and 12th drinking session – see Fig. 4).
Figure 4. Average number of licks for sucrose and water during an intermittent 2-bottle choice drinking assay.
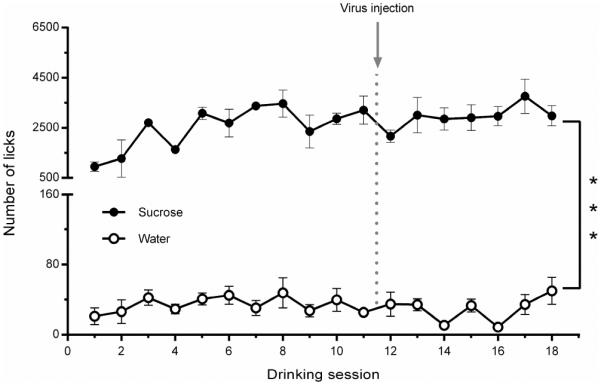
Average daily sucrose (3%) and water licks during 18 drinking sessions were measured before experiments with optical stimulation. No significant changes in the number of licks were found after the virus injection surgery (between the 11th and 12th drinking sessions). There was a dramatic difference between sucrose and water preference, which was also unchanged following the surgery.
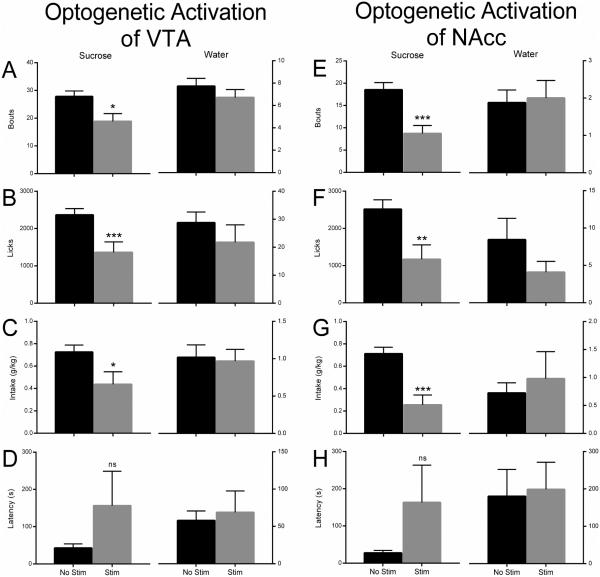
Multiple behavioral parameters were measured for an entire 30 minute session. For the first 10 minutes of the session, a 5 Hz optical stimulation was applied to VTA dopamine cell bodies or accumbal dopamine terminals. These data were compared to non-stimulated sessions (Figs. 5, 6). Since drinking measurements satisfied the criterion of a normal distribution (one-sample Kolmogorov-Smirnov test), a two-tailed unpaired t-test with Welch’s correction was used to analyze the results presented in Figure 6. Each visit to one of the two bottles was recorded as a single bout which could consist of one or more licks. The number of sucrose drinking bouts was significantly lower following VTA stimulation compared to results from non-stimulated control sessions (t(22.88)=2.614, P<0.05). In contrast, no difference was observed in the number of water drinking bouts between stimulated and non-stimulated sessions (Fig. 6A; t(36.35)=1.024, P=0.313). Similarly, accumbal stimulation resulted in significantly fewer sucrose bouts when compared to non-stimulated controls (t(25.37)=4.080, P<0.001) and, again no difference in the number of water bouts was observed (Fig. 6E; t(18.93)=0.2146, P=0.832). When the overall number of licks was analyzed, a pattern similar to that observed with bout behavior was noted (Fig. 6B, F). Significantly fewer licks of the sucrose solution were recorded following optical stimulation of the VTA (Fig.6B; t(19.71)=3.054, P<0.01) or nucleus accumbens (Fig. 6F; t(18.70)=2.912, P<0.01) compared to non-stimulated controls. Additionally, no change in water licks was observed following stimulation of either VTA (Fig 6B; t(23.35)=0.9578, P>0.05) or nucleus accumbens (Fig. 6F; t(26.34)=1.357, P>0.05) compared to non-stimulated sessions. Next, the solution intake of each bottle was calculated (Fig. 6C, G). Intake of the 3% sucrose solution was significantly lower following optical stimulation of the VTA (Fig. 6C; t(18.19)=2.244, P<0.05) or nucleus accumbens (Fig. 6G; t(15.43)=4.304, P<0.001). There was no difference in water intake following VTA (Fig. 6C; t(40.33)=0.215, P>0.05) or accumbal (Fig. 6G; t(11.66)=0.497, P>0.05) stimulation compared to non-stimulated control sessions. Finally, latency to the first bout was evaluated (Fig. 6D, H). There was no difference found between VTA stimulated sessions compared to non-stimulated periods for either the sucrose solution (t(14.41)=1.224, P>0.05) or water (Fig. 6D; t(18.53)=0.350, P>0.05). Additionally, accumbal stimulation did not alter latency compared to non-stimulated controls for either sucrose (t(10.09)=1.345, P>0.05) or water (Fig. 6H; t(23.82)=0.181, P>0.05).
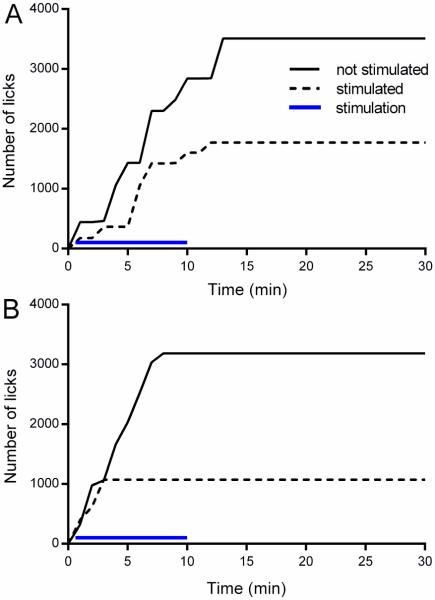
Figure 5. Representative cumulative records of sucrose licking during 30 minute drinking sessions.
The upper panel shows drinking patterns of a single rat during 2 separate sessions, which were performed 2 days apart, with or without optical stimulation of the VTA (A). The lower panel demonstrates drinking patterns of a different rat from analogous sessions, where the nucleus accumbens was optically stimulated during one session (B). The solid black line indicates the response of the subject during the session in which there was no stimulation; the dashed black line shows the response of the subject during the session in which there was stimulation; the blue line indicates time of stimulation.
Figure 6. Tonic optogenetic stimulation of VTA dopamine cells or nucleus accumbens dopamine terminals significantly attenuates sucrose, but not water, drinking measures.
Drinking was assessed using a two-bottle choice procedure and optical stimulation was delivered either to VTA dopamine cells or nucleus accumbens (NAcc) dopamine terminals. The number of drinking episodes, termed bouts (A, E), the total number of licks (B, F), intake (C, G), and the latency to the first bout (D, H) for sucrose and water were compared between stimulated and non-stimulated sessions. Following optical stimulation in either the VTA or NAcc, subjects showed significantly attenuated sucrose intake as evidenced by a decreased number of bouts, licks, and sucrose intake when compared to non-stimulated sessions. However, the latency to the first lick was not significantly altered following stimulation of either brain area. Optical stimulation of either the VTA or NAcc did not affect any water drinking measures. ***P<0.001, **P<0.01,*P<0.05; n=6-7 rats per group.
It is important to notice that multiple measures were obtained from single rats for the t-test analysis of consummatory behavior. This approach, termed “nested data analysis” is quite commonly used in the field of neuroscience research (Aarts et al., 2014). However, depending on the number of observations per object and the degree of dependence, this analysis can overestimate statistical significance (Aarts et al., 2014). Therefore, we performed an alternative statistical analysis of the data to confirm a significant difference in consummatory measures. The data were taken from separate animals in the experiments, where a single measure from a single rat (n=6-7 per group) was taken first during a control session and then during a subsequent session with optical stimulation. This allowed us to re-analyze the difference between unstimulated and stimulated consummatory parameters by the use of a nonparametric Wilcoxon matched pairs signed rank test. According to this analysis, activation of the VTA resulted in a significant decrease in the number of licks (from 2732±392 to 1185±309, P=0.031, n=6) and consequently the intake was reduced (from 0.81±0.14 to 0.43±0.14 g/kg, P=0.033, n=6). Similarly, the decreases in the number of licks (from 2059±542 to 629±296, P=0.016, n=7) and in the intake (from 0.60±0.15 to 0.18±0.12 g/kg, P=0.016, n=7) were observed following optical stimulation of the nucleus accumbens. Therefore, both analyses revealed statistically significant inhibition of consummatory measures.
DISCUSSION
We used a previously characterized combinatorial viral approach (Gompf et al., 2015) to selectively express ChR2 in VTA dopamine neurons of Long-Evans rats to evaluate the role of tonic dopamine release on the consumption of sucrose, a natural reward. The experiments revealed that optogenetically driving mesolimbic dopamine transmission into the tonic mode results in a significant decline in multiple reward consummatory behaviors, including the number of sucrose drinking bouts, the number of licks and the amount of sucrose obtained during the entire drinking session. Importantly, these inhibitory effects were observed following tonic stimulation of either VTA dopamine cell bodies or accumbal dopamine terminals. Moreover, optogenetic stimulation of the VTA-accumbal circuitry had no effect on water consumption under the same experimental conditions. These data demonstrate that delivery of tonic stimulation within the VTA-nucleus accumbens pathway is sufficient to deter reward consummatory behavior.
Sugar is a powerful natural reinforcer with some addictive potential (Avena et al., 2008). In animal models, such as a two bottle choice procedure, rodents quickly establish a preference for sucrose solutions over other fluids (Chaudhury et al., 2013; Tye et al., 2013). Following a month of intermittent drinking, animals show behavioral patterns quite similar to changes observed during addictive drug exposure (Avena et al., 2008). Moreover, these behaviors are associated with tonic increases in accumbal dopamine release (Rada et al., 2005; Avena et al., 2006; Ghitza et al., 2006). However, the causal relationship between tonic dopamine fluctuations in the nucleus accumbens and sucrose intake has not previously been examined. In order to assess this relationship, we optogenetically mimicked a tonic dopamine rise during the first 10 minutes of the drinking session. The activation of the VTA at 5 Hz, which induced a relatively low but sustained elevation in accumbal dopamine concentration selectively reduced sucrose, but not water, consumption. The lack of significant effect on water intake likely reflected the fact that animals were not water restricted during these studies. Since subjects consumed very little water during the limited access preference test, a floor effect would have likely impeded our ability to detect an inhibitory effect of the stimulation on water intake. Importantly, as we did not observe an increase in water intake, the experimental design allowed us to confirm that rats did not simply shift their drinking from sucrose to water. Prior studies have established that if the motivational valence of water is increased by water restriction, modulation of accumbal dopamine receptors does indeed significantly influence water intake (Ljungberg, 1989).
These results are similar to our prior findings with ethanol drinking rats, where the same stimulation protocol caused a significant decrease in the total amount of 20% ethanol consumed throughout the entire session (Bass et al., 2013). Notably, in both studies, ethanol or sucrose solutions were accessible for the last 2/3 of each drinking session, when the optogenetic stimulation was absent. Nevertheless, the rats did not compensate for their diminished intake during the first 10 minutes while the stimulation was applied. Therefore, attentional disruption of rodent behavior, which could be due to optogenetic activation of VTA dopamine cells, is unlikely to be responsible for the observed effects on sucrose drinking. Importantly, high-frequency optogenetic stimulation of dopamine cell bodies in the VTA that induced a marked dopamine response (Tsai et al., 2009; Bass et al., 2013), did not affect any ethanol drinking measures in the two bottle choice test.
The one striking difference between the effects of tonic VTA dopamine cell activation on sucrose and ethanol drinking behavior was on the latency to the first lick during the drinking sessions. Whereas tonic VTA stimulation resulted in a two-fold delay for the first lick during ethanol sessions, this parameter was highly variable but not significantly different from the control when sucrose was available. This dissimilarity may be based on the different values of natural (sucrose) and drug (ethanol) reinforcers in this particular behavioral paradigm.
The VTA and the nucleus accumbens, two highly connected brain areas, are essential for the manifestation of natural as well as drug reward-related behaviors (Stuber et al., 2012). However, the VTA sends dopamine projections not only to the nucleus accumbens but also to the amygdala, prefrontal cortex and hippocampus (Beckstead et al., 1979; Swanson, 1982). Changes in dopamine transmission induced by VTA activation in some of these other brain areas could influence the observed behavioral changes in this study, and the results may not be exclusively attributed to the VTA-nucleus accumbens projections. We explored this possibility by directly stimulating dopamine terminals in the nucleus accumbens of sucrose drinking rats using the same behavioral paradigm described above. These experiments showed an identical reduction in all sucrose drinking measures, including the number of bouts, licks and intake, indicating indistinguishable effects between dopamine cell body and nucleus accumbens dopamine terminal activation on sucrose drinking behavior. Therefore, tonic dopamine release in the nucleus accumbens can be causally linked with the attenuation of sucrose consummatory behaviors. These data provide a mechanistic link to previous findings, including how alterations in tonic dopamine efflux, measured by microdialysis in the nucleus accumbens core, were negatively correlated with changes in the effort required to obtain reward (Ostlund et al., 2011). In fact, earlier microdialysis studies demonstrated increases in accumbal dopamine concentration during and after the consummatory behaviors (Church et al., 1987; Radhakishun et al., 1988; Wilson et al., 1995). In contrast to measures of intake, anticipatory and seeking aspects of feeding behaviors were not robustly associated with increases in tonic dopamine release in the nucleus accumbens (Wilson et al., 1995).
Phasic dopamine release in the nucleus accumbens promotes reward-seeking and -taking actions (Phillips et al., 2003b; Roitman et al., 2004; Wassum et al., 2012; Wassum et al., 2013; du Hoffmann and Nicola, 2014; Ko and Wanat, 2016). Importantly, these transitory dopamine effluxes, which are reliably detected with voltammetry during reinforced behaviors, do not convert to substantial changes in tonic dopamine (Floresco et al., 2003). However, significant changes in tonic dopamine can influence phasic dopamine release through a D2 dopamine autoreceptor-mediated feedback mechanism (Grace, 1991; Phillips et al., 2003b; Oleson et al., 2008). Terminal autoreceptors in the nucleus accumbens are activated when tonic dopamine release is increased, resulting in inhibition of phasic dopamine. Consequently, we can speculate that the shift of accumbal dopamine neurotransmission to tonic patterns suppresses phasic dopamine release triggered by the contextual stimuli, which are tightly linked with the drinking environment. The outcome of these specific alterations in dopamine signaling may then lead to the observed decrease in reward consummatory behavior. Collectively, these findings provide evidence that dopamine transmission in the VTA-nucleus accumbens circuit plays a causal role in reward intake behavior through interplay between tonic and phasic modes of dopamine release.
Tonic dopamine increases in rat nucleus accumbens can be optogenetically mimicked
Increase in tonic accumbal dopamine release results in declined sucrose intake
Increase in tonic accumbal dopamine release does not alter water intake
Optogenetically-induced increase in tonic dopamine inhibits reward consumption
Acknowledgements
This study was funded by NIH grants AA022449 (EAB), AA17531 (JLW), DA024763 (CEB) and by the Russian Science Foundation (grant# 4-50-00069) (RRG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarts E, Verhage M, Veenvliet JV, Dolan CV, van der Sluis S. A solution to dependency: using multilevel analysis to accommodate nested data. Nat Neurosci. 2014;17:491–496. doi: 10.1038/nn.3648. [DOI] [PubMed] [Google Scholar]
- Adamantidis AR, Tsai H-C, Boutrel B, Zhang F, Stuber GD, Budygin EA, Touriño C, Bonci A, Deisseroth K, de Lecea L. Optogenetic Interrogation of Dopaminergic Modulation of the Multiple Phases of Reward-Seeking Behavior. J Neurosci. 2011;31:10829–10835. doi: 10.1523/JNEUROSCI.2246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential Enhancement of Dopamine Transmission within the Nucleus Accumbens Shell by Cocaine Is Attributable to a Direct Increase in Phasic Dopamine Release Events. J Neurosci. 2008;28:8821–8831. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Rada P, Moise N, Hoebel BG. Sucrose sham feeding on a binge schedule releases accumbens dopamine repeatedly and eliminates the acetylcholine satiety response. Neuroscience. 2006;139:813–820. doi: 10.1016/j.neuroscience.2005.12.037. [DOI] [PubMed] [Google Scholar]
- Bass CE, Grinevich VP, Gioia D, Day-Brown JD, Bonin KD, Stuber GD, Weiner JL, Budygin EA. Optogenetic stimulation of VTA dopamine neurons reveals that tonic but not phasic patterns of dopamine transmission reduce ethanol self-administration. Front Behav Neurosci. 2013;7:173. doi: 10.3389/fnbeh.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass CE, Grinevich VP, Vance ZB, Sullivan RP, Bonin KD, Budygin EA. Optogenetic control of striatal dopamine release in rats. J Neurochem. 2010;114:1344–1352. doi: 10.1111/j.1471-4159.2010.06850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer HM, Glimcher PW. Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron. 2005;47:129–141. doi: 10.1016/j.neuron.2005.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead RM, Domesick VB, Nauta WJ. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res. 1979;175:191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- Beyene M, Carelli RM, Wightman RM. Cue-evoked dopamine release in the nucleus accumbens shell tracks reinforcer magnitude during intracranial self-stimulation. Neuroscience. 2010;169:1682–1688. doi: 10.1016/j.neuroscience.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HD, McCutcheon JE, Cone JJ, Ragozzino ME, Roitman MF. Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. Eur J Neurosci. 2011;34:1997–2006. doi: 10.1111/j.1460-9568.2011.07914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budygin EA. Dopamine uptake inhibition is positively correlated with cocaine-induced stereotyped behavior. Neurosci Lett. 2007;429:55–58. doi: 10.1016/j.neulet.2007.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budygin EA, Gainetdinov RR, Kilpatrick MR, Rayevsky KS, Mannisto PT, Wightman RM. Effect of tolcapone, a catechol-O-methyltransferase inhibitor, on striatal dopaminergic transmission during blockade of dopamine uptake. Eur J Pharmacol. 1999;370:125–131. doi: 10.1016/s0014-2999(99)00084-9. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Kilpatrick MR, Gainetdinov RR, Wightman RM. Correlation between behavior and extracellular dopamine levels in rat striatum: comparison of microdialysis and fast-scan cyclic voltammetry. Neurosci Lett. 2000;281:9–12. doi: 10.1016/s0304-3940(00)00813-2. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Phillips PE, Robinson DL, Kennedy AP, Gainetdinov RR, Wightman RM. Effect of acute ethanol on striatal dopamine neurotransmission in ambulatory rats. J Pharmacol Exp Ther. 2001a;297:27–34. [PubMed] [Google Scholar]
- Budygin EA, Phillips PE, Wightman RM, Jones SR. Terminal effects of ethanol on dopamine dynamics in rat nucleus accumbens: an in vitro voltammetric study. Synapse. 2001b;42:77–79. doi: 10.1002/syn.1101. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo MK, Neve RL, Friedman JM, Russo SJ, Deisseroth K, Nestler EJ, Han MH. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church WH, Justice JB, Jr., Neill DB. Detecting behaviorally relevant changes in extracellular dopamine with microdialysis. Brain Res. 1987;412:397–399. doi: 10.1016/0006-8993(87)91150-4. [DOI] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- De BP, Heguy A, Hackett NR, Ferris B, Leopold PL, Lee J, Pierre L, Gao G, Wilson JM, Crystal RG. High levels of persistent expression of alpha1-antitrypsin mediated by the nonhuman primate serotype rh.10 adeno-associated virus despite preexisting immunity to common human adeno-associated viruses. Mol Ther. 2006;13:67–76. doi: 10.1016/j.ymthe.2005.09.003. [DOI] [PubMed] [Google Scholar]
- du Hoffmann J, Nicola SM. Dopamine invigorates reward seeking by promoting cue-evoked excitation in the nucleus accumbens. J Neurosci. 2014;34:14349–14364. doi: 10.1523/JNEUROSCI.3492-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Freeman AS, Meltzer LT, Bunney BS. Firing properties of substantia nigra dopaminergic neurons in freely moving rats. Life Sci. 1985;36:1983–1994. doi: 10.1016/0024-3205(85)90448-5. [DOI] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Prokopenko VF, West MO, Fabbricatore AT. Higher magnitude accumbal phasic firing changes among core neurons exhibiting tonic firing increases during cocaine self-administration. Neuroscience. 2006;137:1075–1085. doi: 10.1016/j.neuroscience.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Gompf HS, Budygin EA, Fuller PM, Bass CE. Targeted genetic manipulations of neuronal subtypes using promoter-specific combinatorial AAVs in wild-type animals. Front Behav Neurosci. 2015;9:152. doi: 10.3389/fnbeh.2015.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Grace AA. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction. 2000;95(Suppl 2):S119–128. doi: 10.1080/09652140050111690. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience. 1983;10:301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AS, Rutledge RB, Glimcher PW, Phillips PE. Phasic dopamine release in the rat nucleus accumbens symmetrically encodes a reward prediction error term. J Neurosci. 2014;34:698–704. doi: 10.1523/JNEUROSCI.2489-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Ilango A, Kesner AJ, Broker CJ, Wang DV, Ikemoto S. Phasic excitation of ventral tegmental dopamine neurons potentiates the initiation of conditioned approach behavior: parametric and reinforcement-schedule analyses. Front Behav Neurosci. 2014;8:155. doi: 10.3389/fnbeh.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JL, Day JJ, Aragona BJ, Wheeler RA, Wightman RM, Carelli RM. Basolateral Amygdala Modulates Terminal Dopamine Release in the Nucleus Accumbens and Conditioned Responding. Biol Psychiat. 2010;67:737–744. doi: 10.1016/j.biopsych.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Mathews TA, Budygin EA. Effect of moderate ethanol dose on dopamine uptake in rat nucleus accumbens in vivo. Synapse. 2006;60:251–255. doi: 10.1002/syn.20294. [DOI] [PubMed] [Google Scholar]
- Justice JB., Jr. Quantitative microdialysis of neurotransmitters. J Neurosci Meth. 1993;48:263–276. doi: 10.1016/0165-0270(93)90097-b. [DOI] [PubMed] [Google Scholar]
- Ko D, Wanat MJ. Phasic Dopamine Transmission Reflects Initiation Vigor and Exerted Effort in an Action- and Region-Specific Manner. J Neurosci. 2016;36:2202–2211. doi: 10.1523/JNEUROSCI.1279-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg T. Attenuation of Water Intake and Operant Responding by Dopamine D2 Antagonists: Raclopride Provides Important Cues for Understanding the Functional Mechanism of Action. Pharmacol Toxicol. 1989;65:9–12. doi: 10.1111/j.1600-0773.1989.tb01117.x. [DOI] [PubMed] [Google Scholar]
- Michael AC, Ikeda M, Justice JB., Jr Mechanisms contributing to the recovery of striatal releasable dopamine following MFB stimulation. Brain Res. 1987;421:325–335. doi: 10.1016/0006-8993(87)91302-3. [DOI] [PubMed] [Google Scholar]
- Nicolaysen LC, Ikeda M, Justice JB, Jr, Neill DB. Dopamine release at behaviorally relevant parameters of nigrostriatal stimulation: effects of current and frequency. Brain Res. 1988;460:50–59. doi: 10.1016/0006-8993(88)90428-3. [DOI] [PubMed] [Google Scholar]
- Oh MS, Hong SJ, Huh Y, Kim KS. Expression of transgenes in midbrain dopamine neurons using the tyrosine hydroxylase promoter. Gene Ther. 2008;16:437–440. doi: 10.1038/gt.2008.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Talluri S, Childers SR, Smith JE, Roberts DCS, Bonin KD, Budygin EA. Dopamine Uptake Changes Associated with Cocaine Self-Administration. Neuropsychopharmacol. 2008;34:1174–1184. doi: 10.1038/npp.2008.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Wassum KM, Murphy NP, Balleine BW, Maidment NT. Extracellular Dopamine Levels in Striatal Subregions Track Shifts in Motivation and Response Cost during Instrumental Conditioning. J Neurosci. 2011;31:200–207. doi: 10.1523/JNEUROSCI.4759-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owesson-White CA, Cheer JF, Beyene M, Carelli RM, Wightman RM. Dynamic changes in accumbens dopamine correlate with learning during intracranial self-stimulation. P Natl Acad Sci USA. 2008;105:11957–11962. doi: 10.1073/pnas.0803896105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, Justice JB. Extracellular Concentration and In Vivo Recovery of Dopamine in the Nucleus Accumbens Using Microdialysis. J Neurochem. 1992;58:212–218. doi: 10.1111/j.1471-4159.1992.tb09298.x. [DOI] [PubMed] [Google Scholar]
- Pattison LP, Bonin KD, Hemby SE, Budygin EA. Speedball induced changes in electrically stimulated dopamine overflow in rat nucleus accumbens. Neuropharmacology. 2011;60:312–317. doi: 10.1016/j.neuropharm.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattison LP, McIntosh S, Budygin EA, Hemby SE. Differential regulation of accumbal dopamine transmission in rats following cocaine, heroin and speedball self-administration. J Neurochem. 2012;122:138–146. doi: 10.1111/j.1471-4159.2012.07738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Johns JM, Lubin DA, Budygin EA, Gainetdinov RR, Lieberman JA, Wightman RM. Presynaptic dopaminergic function is largely unaltered in mesolimbic and mesostriatal terminals of adult rats that were prenatally exposed to cocaine. Brain Res. 2003a;961:63–72. doi: 10.1016/s0006-8993(02)03840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PEM, Stuber GD, Heien MLAV, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003b;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Radhakishun FS, van Ree JM, Westerink BH. Scheduled eating increases dopamine release in the nucleus accumbens of food-deprived rats as assessed with on-line brain dialysis. Neurosci Lett. 1988;85:351–356. doi: 10.1016/0304-3940(88)90591-5. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PEM, Wightman RM, Carelli RM. Dopamine Operates as a Subsecond Modulator of Food Seeking. J Neurosci. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive Reward Signal of Dopamine Neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A Neural Substrate of Prediction and Reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH. A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci. 2013;16:966–973. doi: 10.1038/nn.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Britt JP, Bonci A. Optogenetic Modulation of Neural Circuits that Underlie Reward Seeking. Biol Psychiat. 2012;71:1061–1067. doi: 10.1016/j.biopsych.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Wightman RM, Carelli RM. Extinction of Cocaine Self-Administration Reveals Functionally and Temporally Distinct Dopaminergic Signals in the Nucleus Accumbens. Neuron. 2005;46:661–669. doi: 10.1016/j.neuron.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, Kim SY, Adhikari A, Thompson KR, Andalman AS, Gunaydin LA, Witten IB, Deisseroth K. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Loewinger GC, Maidment NT. Phasic mesolimbic dopamine release tracks reward seeking during expression of Pavlovian-to-instrumental transfer. Biol Psychiat. 2013;73:747–755. doi: 10.1016/j.biopsych.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Maidment NT. Phasic mesolimbic dopamine signaling precedes and predicts performance of a self-initiated action sequence task. Biol Psychiat. 2012;71:846–854. doi: 10.1016/j.biopsych.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, Robinson DL. Transient changes in mesolimbic dopamine and their association with 'reward'. J Neurochem. 2002;82:721–735. doi: 10.1046/j.1471-4159.2002.01005.x. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Zimmerman JB. Control of dopamine extracellular concentration in rat striatum by impulse flow and uptake. Brain Res Rev. 1990;15:135–144. doi: 10.1016/0165-0173(90)90015-g. [DOI] [PubMed] [Google Scholar]
- Wilson C, Nomikos GG, Collu M, Fibiger HC. Dopaminergic correlates of motivated behavior: importance of drive. J Neurosci. 1995;15:5169–5178. doi: 10.1523/JNEUROSCI.15-07-05169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]