Abstract
Hematopoietic stem and progenitor cells (HSPCs) function to replenish the immune cell repertoire under steady-state conditions, and in response to inflammation due to infection or stress. While the bone marrow serves as the primary niche for hematopoiesis, extramedullary mobilization and differentiation of HSPCs occurs in the spleen during acute Plasmodium infection –n a critical step in the host immune response. Here, we identified an atypical HSPC population in the spleen of C57BL/6 mice, with a Lineage−Sca-1+c-kit− (LSK−) phenotype that proliferates in response to infection with non-lethal Plasmodium yoelii 17X. Infection-derived LSK− cells upon transfer into naïve congenic mice were found to differentiate predominantly into mature follicular B cells. However, when transferred into infection-matched hosts, infection-derived LSK− cells gave rise to B cells capable of entering into a germinal center reaction, and developed into memory B cells and antibody-secreting cells that were capable of producing parasite-specific antibodies. Differentiation of LSK− cells into B cells in vitro was enhanced in the presence of parasitized RBC lysate, suggesting that LSK− cells expand and differentiate in direct response to the parasite. However, the ability of LSK− cells to differentiate into B cells was not dependent on MyD88 as myd88−/− LSK− cell expansion and differentiation remained unaffected after Plasmodium infection. Collectively, these data identify a population of atypical lymphoid progenitors that differentiate into B-lymphocytes in the spleen, and are capable of contributing to the ongoing humoral immune response against Plasmodium infection.
Introduction
The importance of B cells and the antibodies they produce in controlling blood-stage Plasmodium infection, and providing long-term protection against clinical disease is well established in murine and human studies (1-7). Yet, during the acute stage of Plasmodium infection in mice B-lymphopoiesis in the bone marrow is down-regulated rapidly, resulting in a 95% depletion of B-cell progenitor populations in the bone marrow during peak parasitemia (8). Furthermore, production of common lymphoid progenitors (CLPs) in the bone marrow declines (8, 9), and only towards the end of acute stage infection do they start to repopulate the bone marrow (10). The observed decline in lymphopoiesis and erythropoiesis (11-14) in the bone marrow occurs in conjunction with increased production of granulocytes and monocytes, with interferon-γ playing an instrumental role in skewing hematopoiesis toward neutrophil and monocyte production (10, 15).
In steady state conditions, hematopoietic stem and progenitor cell (HSPC) populations lacking lineage specific markers (Lin−) can be found in the spleen of naïve mice (16, 17). Similarly, low numbers of HSPCs have also been identified in the spleen of adult pigs, baboons and humans (18). Thus, there is sufficient evidence suggesting that this organ can actively participate in extramedullary hematopoiesis. In support of this idea, dys-erythropoiesis observed in the bone marrow during blood-stage Plasmodium infection is compensated to some extent by extramedullary erythropoiesis in the spleen (19) and liver (20). The spleen also supports differentiation of dendritic cell populations from progenitor cells in mice (21-24). Furthermore, HSPCs located within the splenic red pulp can clonally expand and differentiate into Ly6Chi monocytes, as shown in a model of experimental atherosclerosis and an endotoxin challenge model (25). With regard to lymphocyte development, distinct progenitors for B-1 and B-2 cells have been identified in the spleen of adult mice, and expansion and differentiation of B-1 progenitors into mature B-1 cells occurred in direct response to LPS stimulation (26). Additionally, under conditions of inflammation reduction of B cell progenitors in the bone marrow coincides with their mobilization to the blood and spleen (8, 9, 27, 28). Whether these displaced bone marrow progenitors are able to continue their differentiation upon arrival in the spleen is unclear. Regardless, these findings highlight the capacity of the splenic microenvironment to support erythroid, myeloid and lymphoid development, particularly under conditions of stress and inflammation.
The classical model of lymphopoiesis is a simplified linear model of differentiation. Hence, all lymphoid committed progenitors were initially thought to be derived directly from CLPs, but several studies over the last decade have provided evidence that challenge this paradigm (10, 29-33). Progenitor cells other than CLPs have been found to generate lymphoid cells. For instance, a bi-potent progenitor cell type has been described to possess B cell and myeloid cell potential (32). Also, a subset of common myeloid progenitors expressing Flt3 on their surface displays T cell, but not B cell potential (30, 31, 33). Moreover, in vitro and in vivo studies utilizing a bone marrow-derived LSK− cell population from naive mice have indicated that these cells exhibit B and T cell lineage potential (30). Thus, there are potentially redundant pathways for generating lymphoid cells, and various hematopoietic progenitor cells possess a plastic phenotype that allows them to generate cells of either myeloid or lymphoid lineage.
In order to address how the mouse is able to generate new mature B cells during infection with Plasmodium despite an interruption in lymphopoiesis in the bone marrow, the ability of the spleen to serve as a site for extramedullary lymphopoiesis was investigated. In this report, we demonstrate that acute malaria infection results in expansion of an atypical lymphoid progenitor population in the spleen defined by its expression of stem cell antigen-1 (Sca-1) and its lack of expression of lineage markers or c-kit (Lin−Sca-1+c-kit−). Similar to a LSK− cell population previously described in the bone marrow (29-31, 34), this splenic LSK− progenitor population preferentially gave rise to mature B cells upon transfer into naïve mice; while transfer into infected recipients resulted in production of multiple B cell populations, including germinal center (GC) B cells, memory B cells and antibody-secreting cells, that were capable of producing parasite-specific antibodies. The emergence and expansion of this atypical lymphoid progenitor population in the spleen illustrates the ability of this tissue to adapt to changes induced by inflammation, and demonstrates its capacity to support B lymphocyte development and differentiation during an active infection.
Material and Methods
Mice and infections
Female C57BL/6J, C57BL/6-Tg (UBC-GFP)30Scha/J (Ubc-GFP Tg), B6.SJL-PtprcaPep3b/BoyJ (CD45.1+), and B6.129P2(SJL)-Myd88tm1Defr (Myd88 deficient) mice were purchased from The Jackson Laboratory, while male BALB/c mice were purchased from Harlan Laboratories. All mice were housed and bred in specific-pathogen free facilities at the University of Arkansas for Medical Sciences in accordance with institutional guidelines. For infection with P. yoelii 17X, male BALB/c mice were infected with parasitized red blood cells (RBCs) derived from frozen stocks. Subsequently, 105 parasitized erythrocytes derived from the passage were intraperitoneally injected into experimental female mice to establish infection. Parasitemia was evaluated by counting Giemsa (Harleco, Millipore) stained thin-blood smears.
Flow cytometry and antibodies
Single cell suspensions were achieved by passing the spleen through a 40 μm cell strainer, or flushing the bone marrow with RPMI media. The cell-suspension was then treated with a 0.86% NH4Cl solution for 10 min at room-temperature to lyse erythrocytes, followed by re-suspension in complete RPMI (RPMI 1640 supplemented with 10% FBS, 1% non-essential amino acids, 1% sodium pyruvate, 1% L-glutamate, 1% penicillin-streptomycin, and 0.1% β-mercaptoethanol). Phenotypic analysis of cell populations was performed by staining single-cell suspensions with fluorochrome-conjugated or biotinylated monoclonal antibodies, followed by acquisition of cells on a LSRFortessa flow cytometer (Becton Dickinson) and analysis using FlowJo software (version X, Treestar Inc.). Fluorescent-minus-one (FMO) controls were used to set the gates for negative populations, as well as for histograms representing background staining. Briefly, 3×106 cells were incubated with Fc block (10% 2.4G2 Fc block, 0.5% normal rat IgG, and 0.5% normal mouse IgG) in FACS buffer (0.2% BSA and 0.2% 0.5M EDTA in 1X PBS) for 10 min. The surface staining was performed using appropriate dilutions of antibodies in FACS buffer for 30 min at 4°C. For intracellular staining of Ki-67, the Foxp3 fixation/permeabilization kit (eBioscience) was used; whereas intracellular GFP staining was performed using 0.1% saponin in FACS buffer.
The antibodies CD93-biotin (AA4.1), IgD-PE (11-26c), CD73-PE (cBioTY/11.8), CD43-PE (eBioR2/60), CD45.2-biotin (104), CD3e-PCPCy5.5 (145-2c11), CD11c-PCPCy5.5 (B418), Ter-119-PCPCy5.5 (TER-119), CD11b-PCPCy5.5 (M1/70), CD5-PCPCy5.5 (53-7.3), NK1.1-PCPCy5.5 (PK136), CD8α-PCPCy5.5 (53-6.7), B220-AF700 (RA3-6B2), CD4-AF700 (RMA-5), CD38-PE-Cy7 (90), Ki-67-APC (SolA15), c-kit-PE-Cy7 (2B8), CD23-APC (B3B4), GL-7-eF660 (GL-7), CD90.2-eF450 (53-2.1), CD21/35-eF450 (eBio4E3), IL-7Rα-eF450 (A7R34), CD24-biotin (M1/69) and Streptavidin (SA)-APC were purchased from eBioscience (San Diego, CA). Antibodies - Sca-1-FITC (D7), CD19-BV650 (6D5), IgM-BV421 (RMM-1), CD90.2 (30-H12)-eF450, CD38-Pacific Blue (90), CD11c-PE-Cy7 (N418), and SA-BV650 were purchased from Biolegend (San Diego, CA), while CD138-BV711 (281-2) and Flt3-PE-CF594 (A2F10.1), were purchased from BD Biosciences (San Jose, CA). Unconjugated anti-GFP rabbit monoclonal antibody and secondary Alexa Fluor 488 conjugated goat anti-rabbit IgG were purchased from Thermo Fischer Scientific Inc. (Rockford, IL).
Cell sorting
For sorting, single cell suspensions from the spleen or bone marrow were enriched for Lin− cells, using biotinylated monoclonal antibodies against lineage markers (CD3e, B220, Ter-119, CD11b, and CD11c), followed by incubation with Streptavidin microbeads (Miltenyi) and negative selection on a magnetic column using an autoMACS pro separator (Miltenyi). The lineage negative fractions were subsequently stained with fluorochrome-conjugated antibodies to Lineage markers (Ter-119, B220, CD3e, CD4, CD5, NK1.1, Gr-1, CD11b, and CD11c), Sca-1, c-kit, Thy1.2, and IL-7Rα. Dead cells (excluded by counterstaining with live/dead fixable dye; eBioscience) and doublets were gated out prior to sorting on a FACSAria II (Becton Dickinson). LSK− cells and CLPs were collected by sorting for Lin−Sca-1+c-kit−Thy1.2− (LSK− cells) and Lin−Sca-1loc-kitloIL-7Rα+ (CLPs) populations. Additionally during adoptive transfer experiments using donor Ubc-GFP Tg mice, sorted cells were ensured to be GFP+ based on their fluorescence.
In vitro stromal cell co-culture and lymphoid differentiation assay
OP9 stromal cells were maintained in α-MEM (Invitrogen) supplemented with 10% FBS, 5% Horse serum, 1% L-glutamate, and 1% penicillin/streptomycin (Complete OP9 medium). The cells were seeded into 96-well flat-bottomed, sterile tissue-culture plates (BD Biosciences) and allowed to grow till 80% confluence whereupon they were irradiated with 30 Gy. After an hour of rest at 37°C, LSK− cells were sorted (100 cells per well) directly onto OP9 stromal cell monolayers. Complete OP9 medium with or without recombinant growth factors - IL-7 (10 ng/ml) (eBioscience), Flt3 ligand (10 ng/ml) (eBioscience), P. yoelii infected RBC lysate (1.25×107 cells/ml), or uninfected RBC (nRBC) lysate (1.25×107 cells/ml) were then added. The cells were incubated for 4, 7 and 10 days at 37°C in 5% CO2, followed by analysis by flow cytometry for the presence of CD19+B220+ B cells.
In vivo adoptive transfer
For adoptive transfer experiments sorted LSK− cells were resuspended in an appropriate volume of endotoxin-free, sterile PBS (Life Technologies). The cells were transferred into the venous sinus retro-orbitally for all recipient mice, following anesthetization with isoflurane, at a concentration of 1×104 cells per mouse (unless otherwise specified).
ELISpot assays
The frequency of antigen-specific antibody-secreting cells (ASCs) was assessed using a previously described method (35). Briefly, 96-well multi-screen filter plates (MAHAS4510, Millipore) were coated with recombinant P. yoelii MSP-119 protein (2.5 μg/ml), or P. yoelii infected RBC lysate (2×105 cells/ml), overnight at 4°C. Plates were then washed with sterile PBS and incubated with complete RPMI for 30 min at 37°C. After washing, plates were incubated with serially diluted cells from bone marrow or spleen (transferred population based on CD45.2 marker, as well as endogenous population based on CD45.1 marker) for 16-20 hour at 37°C in 5% CO2. After incubation, plates were washed again and incubated with 5% FBS in sterile PBS for 30 min at 37°C. Next, wells were washed and antigen bound antibodies were detected with alkaline phosphate conjugated anti-mouse IgG or anti-mouse IgM antibodies (Southern Biotech) for 2 hour at 37°C and developed with BCIP-NBT (Sigma-Aldrich). After washing off developing substrate, spots in each well were counted using an AID ELISpot Reader (AID GmbH). The number of spots in control wells was subtracted from the number of spots in experimental wells and the frequency of antibody-secreting cells (ASCs) was calculated by normalizing to 2×105 cells plated.
Quantitative RT-PCR
Splenic derived LSK− cells were sorted into RNA lysis buffer (QIAGEN) and RNA was isolated using an RNeasy mini kit following the manufacturer’s protocol. The resulting RNA from each sample was DNase treated using RNase-free DNase (Promega), followed by cDNA preparation using SuperScript II Reverse Transcriptase (Life Technologies). For real time qRT-PCR, cDNA from an equivalent number of cells was mixed with SYBR Green master-mix (Bio-Rad) and appropriate primer sets; analysis was performed using a StepOne™ Real-Time PCR System and StepOne™ Software.
Statistical analysis
One-way analysis of variance (ANOVA) was used for comparing data obtained from a group of Plasmodium infected mice, followed by Kruskal-Wallis test for multiple comparisons between the groups; whereas two-way ANOVA was used for comparison between two or more groups, followed by Bonferroni’s test for multiple comparisons between the groups, unless otherwise mentioned. All the statistical analysis was performed using GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA).
Results
Plasmodium yoelii infection induces expansion of LSK− progenitors in the spleen
Plasmodium yoelii 17X blood-stage infection induces an acute non-lethal systemic infection in C57BL/6 mice, characterized by high parasitemia and subsequent anemia. In addition, acute changes in hematopoiesis occur, including an acute disruption of lymphopoiesis in the bone marrow defined by a sharp decline in the number of CLPs and other B cell precursor populations (pre-pro-B, pro-B, pre-B, and immature B cells) ((9), Supplemental Fig. 1, 2), corroborating with similar observations seen in a P. chabaudi chabaudi AS model of infection (8, 10). It was only after three weeks post-infection that B cell precursor populations started to return in the bone marrow (Supplemental Fig. 2). Within the spleen there was a similar decline in the total number of mature B cell precursors during acute infection, with the exception of T1 B cells, which increased in number above that of naïve mice at day 7 post-infection (p.i.) before rapidly decreasing by day 11 p.i. (Supplemental Fig. 1, 2). Additionally, the decline in transitional B cell populations in the spleen correlated with an early burst of plasmablast and plasma cell expansion that peaked at day 7 post-infection (p.i.) (Supplemental Fig. 2; (36, 37)). These events lead to a decline in mature B cell numbers in the spleen (Supplemental Fig. 2). In spite of these events GC development and parasite-specific Ab production initiate before recovery of B cell lymphopoiesis in the bone marrow (Supplemental Fig. 2). Therefore, it was of interest to determine if the host possesses a mechanism to compensate for the loss of normal B cell production after Plasmodium infection.
While there was a general decline in mature B cell precursor numbers in the spleen during acute infection, there was an expansion in Lin− cells in the spleen (Supplemental Fig. 2). To determine the composition of these Lin− cells, they were analyzed for the presence or absence of surface markers used to define various hematopoietic progenitor populations. A Lin− cell population, which can be defined by high Sca-1 expression and a lack of c-kit and Thy1.2 expression (LSK−), was found to expand in the spleen during acute P. yoelii infection with their total cell numbers peaking on day 7 p.i. (Fig. 1a-c). Importantly, expansion of LSK− cells in the spleen was also observed following i.p. infection of C57BL/6 mice with P. chabaudi AS and another protozoan parasite Toxoplasma gondii (Supplemental Fig. 3). Thus, expansion of LSK− cells within the spleen was not associated specifically with P. yoelii infection, and may be part of a general inflammatory response associated with systemic inflammation.
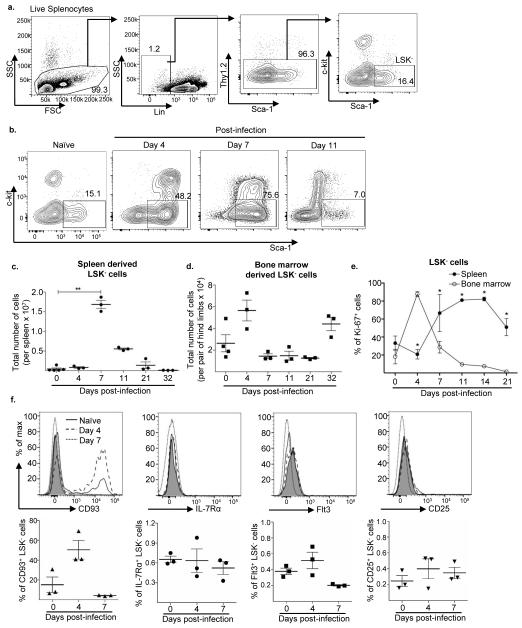
Figure 1. A LSK− progenitor population emerges in the spleen after P. yoelii infection.
a. Splenocytes from naïve C57BL/6 mice were stained with the indicated antibodies and the gating strategy used to select for Lin−Sca-1+c-kit− (LSK−) cells is shown. Lineage (Lin) cocktail included antibodies to CD3, CD4, CD5, B220, CD11b, CD11c, Ter-119, NK1.1, and Gr-1. b. Representative flow plots of LSK− cells from the spleen of C57BL/6 mice infected with P. yoelii 17X at days 0, 4, 7 and 11 p.i. Total number of LSK− progenitor cells in the c. spleen and d. bone marrow after P. yoelii 17X infection of C57BL/6 mice at days 0, 4, 7, 11, 21 and 32 p.i. **, p = 0.0062 (Kruskal-wallis non-parametric ANOVA). e. Percentage of LSK− cells expressing nuclear antigen Ki-67 from the spleen (filled circle) and bone marrow (empty circle) of C57BL/6 mice following P. yoelii 17X infection at days 0, 4, 7, 11, 14, and 21 p.i. *, p<0.001 (two-way ANOVA). f. Representative histogram plots and percentage of splenic LSK− cells from C57BL/6 mice showing expression of CD93 (triangle), IL-7Rα (circle), Flt3 (square), and CD25 (inverted triangle) at days 0 (solid line), 4 (dashed line), and 7 (dotted line) after P. yoelii 17X infection. The FMO (fluorescent minus one) controls are shown as grey filled histograms. Data are representative of three independent experiments with n = 3 mice per group/time point; Error bars (c-f) S.E.M.
As a progenitor population with a similar phenotype and a preference for differentiating into lymphoid cells has previously been described within the bone marrow of naïve mice (29-31, 34), cells with a LSK− phenotype were examined at this site during the course of infection. While there was a decline in LSK− cell numbers in the bone marrow at day 7 p.i., this was preceded by an expansion at day 4 p.i. (Fig. 1d). The rapid expansion of this cell population at day 4 p.i. suggests that these cells may initially proliferate in the bone marrow prior to migration to the spleen. To determine if LSK− cells were expanding within the bone marrow and spleen, LSK− cells were stained for the nuclear antigen Ki-67, which is expressed during all active phases of the cell cycle, but not in resting cells (38). As expected a large percentage of LSK− cells in the bone marrow were positive for Ki-67 staining at day 4 p.i., followed by a sharp decline by day 7 p.i. (Fig. 1e), correlating with their decrease in cell number (Fig. 1d). In the spleen, a 2-fold increase in the percentage of LSK− cells expressing Ki-67 was noted compared to naïve mice by day 7 p.i. (Fig. 1e), suggesting that expansion in LSK− cell numbers in the spleen could be due to a combination of resident LSK− cell proliferation and recruitment of additional LSK− cells from the bone marrow that continue to proliferate upon arrival in the spleen.
Additionally, a proportion of bone marrow-derived LSK− cells have been previously described to express IL-7Rα and Flt3, two receptors involved in lymphoid progenitor differentiation, as well as CD93 (AA4.1) and CD25 (30, 31, 39); therefore, to determine if LSK− cells within the spleen are phenotypically similar to those found in the bone marrow splenic LSK− cells were examined for expression of these markers. Consistent with previous findings (31), a proportion of bone marrow LSK− cells from naïve mice showed expression of CD93 (Supplemental Fig. 3), a c-type lectin expressed by a number of hematopoietic progenitor cells and B cell precursors in the bone marrow, and immature B cells in the spleen (40, 41). Also, small sub-populations of bone marrow derived LSK− cells from naïve mice showed expression of the receptors IL-7Rα, Flt3 and CD25 (Supplemental Fig. 3). Subsequent analysis of splenic LSK− cells from naïve mice revealed expression of CD93 within a small sub-population; however, expression of IL-7Rα, Flt3 and CD25 was substantially lacking (Fig. 1f). Infection with P. yoelii 17X resulted in an increase in CD93 expression on bone marrow LSK− cells at day 4 p.i.; however expression of this marker declined by day 7. The percentage of bone marrow LSK− cells expressing IL-7Rα, Flt3 and CD25 declined at day 4 p.i., but by day 7 the percentage of cells expressing these markers was similar to or higher than that seen on naive LSK− cells (Supplemental Fig. 3). With the exception of CD93 expression at day 4 p.i., there was no change in expression of IL-7Rα, Flt3 and CD25 by LSK− cells in the spleen (Fig. 1f). This data indicates that the LSK− cell population in the spleen is not as phenotypically diverse as their bone marrow counterparts. Also, the lack of IL-7Rα and Flt3 expression by splenic LSK− cells suggests that alternative factors dictate the fate of these cells.
Splenic LSK− cells preferentially differentiate into B cells
Bone marrow LSK− cells have previously been shown to have a lymphoid biased potential in vitro and in vivo (30, 31). Specifically, culturing of bone marrow LSK− cells in the presence of OP9 stromal cells results in their differentiation primarily into CD19+B220+ B cells, while culturing them with OP9 stromal cells that ectopically express Notch ligand, Delta-like 1 (DL-1) promotes T cell development (30, 31). In vivo, bone marrow LSK− cells were found to favor B cell production, however T cells and NK cells were also generated upon transfer into an irradiated host (30, 31). To determine whether splenic LSK− cells are capable of differentiating into B cells, LSK− cells sorted from the spleens of naïve mice and mice infected with P. yoelii 17X were co-cultured with OP9 stromal cells. The co-cultures were supplemented with or without IL-7 and Flt3 ligand (Flt3-L), growth factors that support B cell differentiation (42). Bone marrow LSK− cells and CLPs from naïve mice were used as controls for these studies. Splenic LSK− cells from uninfected and P. yoelii infected mice differentiated into CD19+B220+ B cells successfully in the presence of OP9 stromal cells, even in the absence of IL-7 and Flt3-L (Fig. 2a, b), as did CLPs (Fig. 2c) and LSK− cells from the bone marrow (Fig. 2d). However, CLPs yielded more B cells than either splenic or bone marrow derived LSK− cells. Addition of IL-7, Flt3-L or their combination enhanced differentiation of CLPs (Fig. 2c) and bone marrow derived LSK− cells by day 7 (Fig. 2d), whereas IL-7, Flt3-L or their combination only had a marginal or no effect on B cell differentiation of splenic LSK− cells (Fig. 2a, b), which was anticipated based on lack of expression of the receptors for these growth factors by LSK− cells (Fig. 1e). Together these results indicate that splenic LSK− cells are capable of differentiating into B cells in vitro, though less effectively than bone marrow CLPs and LSK− cells, and are less responsive to Flt3 and IL-7Rα signaling.
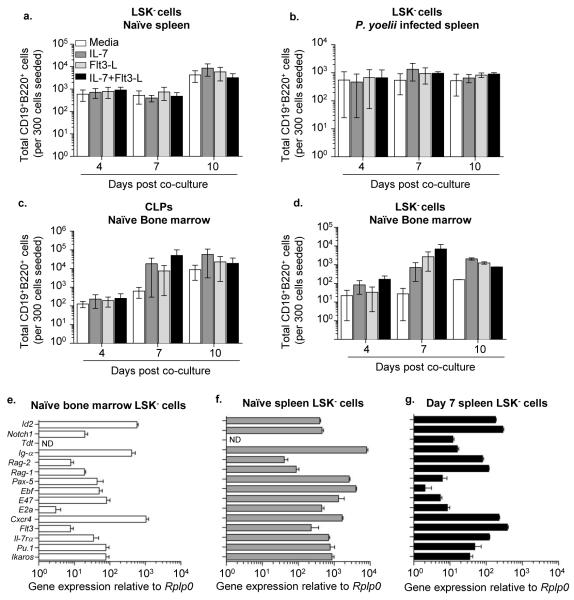
Figure 2. LSK− cells have the potential to differentiate into B cells in vitro.
Total number of live CD19+B220+ B cells produced by LSK− cells from the spleen of a. naïve mice and b. day 7 P. yoelii 17X infected C57BL/6 mice, c. CLPs and d. LSK− cells from bone marrow of naïve mice, in the presence of OP9 stromal cells. The analysis was done after co-culture for 4, 7, and 10 days in media supplemented with IL-7, Flt3 Ligand (Flt3-L) or their combination. Data are pooled from four independent experiments. Splenic LSK− cells were sorted from the spleens of n = 3 naïve mice that were subsequently pooled together, or n = 1 mouse infected with P. yoelii 17X at day 7 p.i. Bone marrow from n = 3 naïve mice were pooled for sorting bone marrow derived LSK− cells or CLPs. RNA was isolated from sorted cells, and expression of lymphoid specific genes in LSK− cells from e. bone marrow, and f. spleen of naïve mice and g. spleen from day 7 P. yoelii 17X infected mice was analyzed by quantitative RT-PCR and was displayed relative to the expression of Rplp0 (Ribosomal protein P0). Data are representative of two independent experiments; Error bars (e-g) S.E.M.
As the in vitro data suggested splenic-derived LSK− cells have lymphoid potential, they were further examined for expression of genes associated with early lymphoid development. Similar to bone marrow LSK− cells, the splenic progenitor cells obtained from naïve mice expressed the Rag genes (Rag1 and Rag2) and a number of genes associated with B cell development – E2a coded E47, EBF, Pax5 and Ig-α ((31), Fig. 2e,f). Expression of these B cell associated genes was also maintained in splenic LSK− cells isolated from P. yoelii infected mice (day 7) (Fig. 2g), lending additional support to their lymphoid potential. In addition to genes associated with B cell development, transcripts for genes associated with other hematopoietic lineages were expressed by bone marrow and splenic LSK− cells. Furthermore, although the majority of splenic LSK− cells did not express detectable amounts of Flt3 or IL-7Rα, transcripts of these two genes were found in LSK− cells from naïve and infected mice. Lastly, Tdt RNA was not detected in naïve splenic LSK− cells, but its expression was induced in these progenitor cells during active infection (Fig. 2g), indicating that the transcripts for major proteins involved in B-cell receptor gene rearrangement are expressed by LSK− cells during Plasmodium infection.
Since the OP9 stromal cell co-culture system is biased towards B cell development, the fate of these splenic progenitor cells in vivo was evaluated. LSK− cells were sorted from the spleen of Ubc-GFP Tg mice, infected for one week with P. yoelii 17X, and were transferred intravenously (i.v.) into naïve congenic (CD45.1+) mice (10,000 cells per recipient) (Fig. 3a). All cells derived from these transgenic mice constitutively express GFP (43). Recipient mice were sacrificed three weeks post-transfer to determine the fate of donor-derived (GFP+) LSK− cells. Analysis of the spleen of recipient mice revealed that the majority of recovered GFP+ cells were B cells (CD19+B220+), whereas the remaining donor-derived cells that were recovered expressed a CD19+B220lo/− phenotype that was associated with low CD11b and CD11c expression (Fig. 3b, c). No GFP+ cells with a T cell phenotype were detected. Examination of IgM and IgD expression on these GFP+ B cells indicated that while a majority of B cells displayed a mature phenotype (IgM+IgD+), a proportion down-regulated IgM and IgD expression, suggesting that these cells are an activated population of committed B cells undergoing functional differentiation (Fig. 3b, d). The majority of GFP+ B cells also showed down-regulation of CD93, a marker associated with immature B cells (44), further confirming their mature phenotype (Fig. 3b). However, a small proportion of donor-derived cells showed an immature B cell phenotype (CD93+IgM+IgD−), suggesting a linear differentiation of donor LSK− cells into B cells. Furthermore, donor-derived B cells displayed varied surface-expression of CD23 and CD21/35, with approximately 59% of the population displaying a CD23+CD21/35lo phenotype characteristic of follicular B cells and 14% displaying a CD23−CD21/35hi phenotype of marginal zone B cells (Fig. 3b, e). These results confirm that splenic derived LSK− cells isolated from P. yoelii infected mice are a lymphoid progenitor population that preferentially differentiates into B cells upon transfer into a naïve host and that these progenitor cells can give rise to follicular and marginal zone B cells.
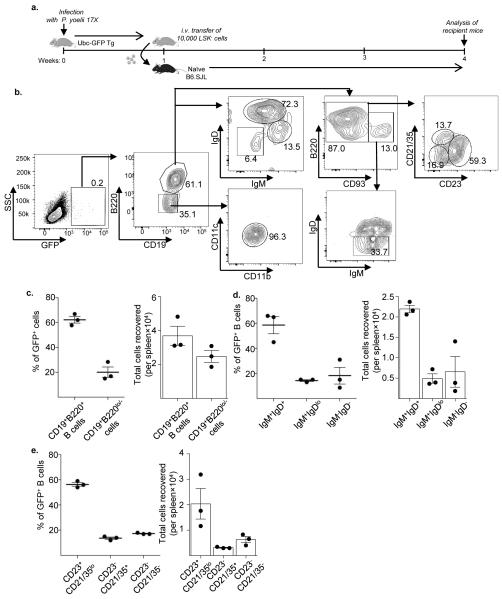
Figure 3. Infection-induced LSK− cells differentiate into B cells upon transfer into naïve mice.
a. LSK− cells were sorted from the spleen of an Ubc-GFP Tg mouse, one-week post P. yoelii 17X infection, and 10,000 cells were adoptively transferred into naïve congenic B6.SJL (CD45.1) mice. Three weeks post-transfer, splenocytes were harvested from recipients and stained with antibodies for GFP, CD19, B220, CD93, IgM, IgD, CD23, CD21/35, CD11b and CD11c. b. The gating strategy shown here was used to sub-analyze the donor (GFP+) cell population. c. The percentage and total number of donor-derived (GFP+) cells displaying a B cell (CD19+B220+) or CD19+B220lo/− phenotype in recipient mice. The percentage and total number of donor-derived GFP+ B cells displaying a d. mature B cell (IgM+IgD+), immature B cell (IgMhiIgDlo) or activated B cell (IgM−IgD−) phenotype; and a e. Follicular B cell (CD23+CD21/35−), marginal zone B cell (CD21/35+) or immature B cell (CD23−CD21/35−) cell phenotype in the spleen of recipient naïve mice three weeks after transfer. Data are representative of two independent experiments with n = 3 mice per group/time point; Error bars (c-e) S.E.M.
Splenic LSK− derived B cells produce parasite-specific antibodies
The finding that splenic LSK− cells not only differentiate into mature B cells, but also show signs of activation suggests that in addition to replenishing the B cell pool during this infection these LSK− derived B cells might be activated in direct response to infection, leading to production of parasite-specific antibodies. To test this idea 10,000 splenic LSK− cells were sorted from CD45.2+ mice infected for one week with P. yoelii 17X and transferred into congenic CD45.1+ mice at the same stage of infection or naïve congenic mice (Fig. 4a). Mice were infected with a GFP expressing P. yoelii 17X to insure that no parasite infected LSK− cells were transferred into naïve mice. At three weeks post-transfer (day 28 p.i.), after resolution of infection, recipient mice were analyzed to determine the fate of donor-derived (CD45.2+) LSK− cells. Similar to what was seen upon transfer of this population into naïve mice, LSK− cells primarily differentiated into CD19+B220+ B cells in infected recipients, and a similar number of CD45.2+ B cells were recovered from the spleen of naïve and infected recipients (Fig. 4b). The ability of splenic LSK− cells to differentiate into B cells was rapid as immature and mature B cells could be detected in the spleen of recipient mice as early as 24 hours post-transfer (Supplemental Fig. 4). If LSK− derived B cells are being activated in a T-cell dependent fashion, they should have the capacity to differentiate into GC B cells. Indeed, a small proportion of recovered CD45.2+ B cells from infected recipient mice displayed markers associated with a GC B cell phenotype (GL-7+CD38lo), whereas very few CD45.2+ GC B cells were apparent in the spleens of naïve recipient mice (Fig. 4c). Donor-derived CD45.2+CD138+B220lo/− cells that represent plasmablast and plasma cells were found in the spleens of naïve and infected recipient mice; however, there were a higher proportion and number of these class-switched (swIg+) cells in infected recipient mice compared to naïve mice (Fig. 4d). The expression of the cell surface marker CD73, an ecto-5′-nucleotidase, on B cells is associated with IgM+ and swIg+ memory B cells that have a high rate of somatic hypermutation (SHM; (45, 46)) and express activation-induced cytidine deaminase (47), an enzyme required for SHM and class-switch recombination. Hence, CD73 serves as a useful marker for distinguishing memory B cells that have come from the GC reaction, as this is the primary site of SHM. CD45.2+ B cells expressing CD73 were found in a much higher proportion in infected recipient mice (Fig. 4e). While naïve and infected recipient mice generated comparable numbers of CD45.2+IgM+ memory B cells there was a 30-fold higher number of CD45.2+swIg+ memory B cells recovered from infected recipient mice (Fig. 4e).
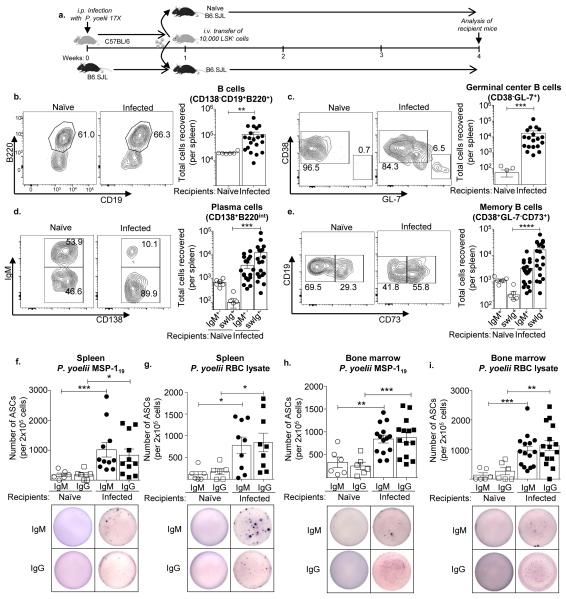
Figure 4. LSK− cell derived B cells produce parasite-specific antibodies.
a. LSK− cells were sorted from the spleen of CD45.2+ mice, one-week post P. yoelii 17X infection, and 10,000 cells were transferred into congenic B6.SJL (CD45.1) mice at same stage of infection (day 7). Three weeks later the spleens of recipient mice were harvested and stained with the indicated fluorochrome conjugated antibodies to sub-analyze the donor (CD45.2+) cell population. Representative flow plot analysis and total cell number of donor derived (CD45.2+) b. Total B cells (CD19+B220+), **, p=0.0033 c. Germinal center B cells (CD19+B220+GL7+CD38−), ***, p=0.0001 d. Plasma cells (CD138+B220lo/−) ***, p=0.0003 and e. Memory B cells (CD19+B220+CD38+GL-7−CD73+) ****, p<0.0001 (Mann-Whitney U test) in the spleen of recipient naïve (hollow circle) and Plasmodium yoelii 17X infected B6.SJL mice (filled circle), three weeks post-transfer. B cells were examined for CD138 and B220 expression and CD138+B220lo/− cells representing plasmablasts and plasma cells were gated on prior to analyzing IgM expression on these CD138+ cells. LSK− cells were sorted from the spleen of Ubc-GFP Tg mice, one-week post P. yoelii 17X infection, and 10,000 cells were transferred into naïve congenic B6.SJL (CD45.1) or B6.SJL mice at the same stage of infection (day 7). At week three post-transfer (day 28 p.i.), donor-derived GFP+ cells sorted from the spleen (f, g) and bone marrow (h, i) of recipient mice were used to determine the number of parasite-specific IgM (circle) or IgG (square) antibody secreting cells (ASCs) by ELISpot (P. yoelii MSP-119 (f, h) and P. yoelii infected RBC lysate (g, i)). *p<0.05, **p<0.001, ***p≤0.001 (Mann-Whitney U test). Representative ELISpot images from the assay are shown. Data are pooled from five (b-e) or four (f-i) independent experiments, n = 6-20 mice/group; Error bars (b-i) S.E.M.
The formation of GC B cells along with class switched plasmablasts, plasma cells and memory B cells indicate that B cells derived from splenic LSK− cells are activated in a T-cell dependent manner after Plasmodium infection, but whether these cells can produce parasite-specific antibodies is another question. To determine specificity of LSK− derived B cells, ELISpot assays were performed using the 19kDa P. yoelii merozoite surface protein-1 (MSP-119) and P. yoelii-infected red blood cell lysate (pRBC lysate). In these experiments, LSK− cells were sorted from the spleen of Ubc-GFP Tg mice one week after infection and transferred into naïve or infection-matched congenic (CD45.1+) mice. At three weeks post-transfer (day 28 p.i.) donor-derived cells were sorted based on GFP and CD45.2 expression from the spleen and bone marrow of recipient mice. ELISpot analysis showed that donor derived LSK− cells gave rise to parasite-specific IgM+ and class-switched IgG+ antibody-secreting cells (ASCs) that were present in both tissues of recipient mice (Fig. 4f-i). While it remains possible that some contaminating host cells contribute to the spots observed in the ELISpot assays, the use of GFP-labeled congenically marked donor LSK− cells should have effectively minimized the recovery of any recipient cells during the sort, indicating that the majority of the parasite-specific IgM+ and class-switched IgG+ ASCs detected are of donor origin. Taken together, these results confirm the capacity of infection-induced LSK− cells to differentiate into B cells that may well participate in the ongoing humoral response against Plasmodium through production of parasite-specific ASCs.
Bone marrow LSK− cells can home to the spleen and differentiate into B cells after Plasmodium infection
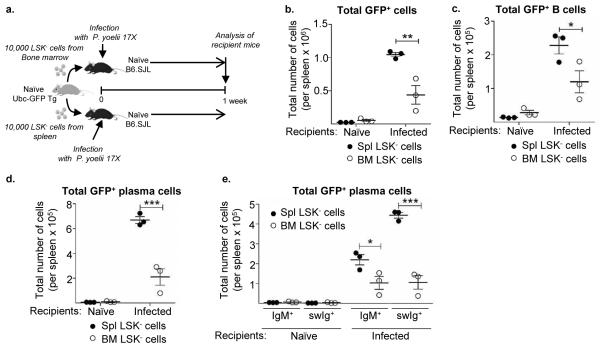
As LSK− cells are found in the spleen and bone marrow of naïve resting mice, it is unclear if LSK− cell expansion in the spleen after infection is the direct result of proliferation of the endogenous population, or whether LSK− cells migrate from the bone marrow to the spleen and undergo extensive proliferation upon their arrival. It is also possible that endogenous and recruited LSK− cells proliferate and differentiate in the spleen. To compare the ability of endogenous splenic LSK− cells and bone marrow LSK− cells to home to the spleen and differentiate into B cells after infection, these populations were sorted from naïve Ubc-GFP Tg mice and transferred into CD45.1+ mice prior to infection with P. yoelii 17X (Fig. 5a). One week after infection the spleen was examined for GFP+ cells. While a similar number of donor-derived GFP+ cells were recovered from the spleen of naïve congenic mice, a significantly higher number of GFP+ cells were found in the spleen of infected mice that received splenic LSK− cells compared to bone marrow-derived LSK− cells (Fig. 5b). While a proportion of transferred splenic and bone marrow derived LSK− cells maintained their progenitor phenotype the majority of LSK− cells differentiated into B cells and plasma cells (Fig. 5c-e). In naïve recipients, bone marrow LSK− cells showed a slightly greater propensity for differentiating into B cells compared to splenic LSK− cells. However, in the spleen of infected recipients, splenic LSK− cells gave rise to a significantly higher number of B cells than bone marrow derived progenitors (Fig. 5c), suggesting the positive impact of infection on splenic LSK− cell differentiation. Furthermore, in the spleen of infected recipients splenic LSK− cells differentiated into a significantly higher number of plasma cells compared to their bone marrow counterpart (Fig. 5d). In addition, while bone marrow derived LSK− cells produced a similar frequency of IgM+ and swIg+ plasma cells, splenic LSK− cells produced a greater frequency of swIg+ plasma cells than IgM+ plasma cells (Fig. 5e). Collectively, this data indicates that splenic and bone marrow LSK− cells can home to the spleen after transfer and are capable of differentiating into B cells; however, in response to infection with P. yoelii 17X transferred splenic LSK− cells expanded more readily than bone marrow LSK− cells, resulting in an increased number of progeny B cells and swIg+ plasma cells. Thus, proliferation of endogenous splenic LSK− cells, as well as recruitment and expansion of bone marrow derived LSK− cells together can contribute to the overall increase in LSK− progenitor cells in the spleen after infection.
Figure 5. Bone marrow and splenic LSK− cells give rise to B cells after infection.
a. Ten thousand LSK− cells sorted from the spleen or bone marrow of naïve Ubc-GFP Tg mice were transferred separately into congenic B6.SJL (CD45.1) recipient mice. A subset of recipient mice was then infected with P. yoelii 17X following adoptive transfer. One-week later the spleens of recipient mice were harvested to analyze the donor-derived (GFP+) cell populations. b. Total number of donor derived (GFP+) cells and c. GFP+ B cells (CD19+B220+). d. Total number of GFP+ plasmablasts/plasma cells (CD138+B220lo/−) and e. GFP+ IgM producing (CD138+B220lo/−IgM+) and class-switched antibody producing (CD138+B220lo/−IgM−) plasmablasts/plasma cells from spleens of mice receiving splenic LSK− cells (filled circle) or bone marrow derived LSK− cells (hollow circle). *, p<0.05, **, p<0.005, ***, p<0.0001 (two-way ANOVA). Data are representative of two independent experiments with n = 3 mice per group/time point; Error bars (b-e) S.E.M.
Parasite-derived material directly contributes to LSK− cell differentiation
HSCs have been shown to express a number of Toll-like receptors (TLRs; (48-52)) and thus they possess the capacity to sense and respond to pathogenic infections. As TLRs have been shown to be involved in innate sensing of Plasmodium (53, 54), we sought to determine if the parasite itself has any effect on expansion and differentiation of splenic LSK− cells. Utilizing the OP9 stromal cell co-culture assay described previously, LSK− cells were sorted from the spleen of naïve and P. yoelii 17X infected mice, with CLPs and LSK− cells sorted from the bone marrow of naïve mice, serving as controls once again. This time lysate material derived from P. yoelii infected RBCs or normal RBCs (nRBC) were added to the co-culture media and the ability of pRBC lysate to influence B cell differentiation was monitored. While addition of nRBC lysate had no effect on the output of B cells derived from splenic LSK− cells beyond that seen with media alone, addition of pRBC lysate enhanced B cell production over time by naïve and infection derived splenic LSK− cells (Fig. 6a, b). The observed effect of pRBC lysate on LSK− cells could be direct; alternatively, as LSK− cells differentiate into B cells the lysate may induce further expansion of differentiated B cells in the cultures. However, neither nRBC lysate nor pRBC lysate enhanced B cell output from bone marrow CLPs or LSK− cells in this assay (Fig. 6c, d). Thus, an unknown component of the parasite itself, acting directly on splenic LSK− cells, promotes their expansion and differentiation into B cells in vitro.
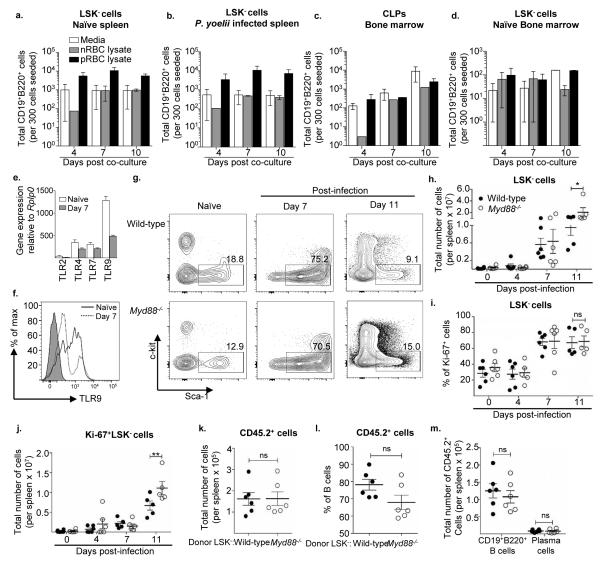
Figure 6. Splenic LSK− cell differentiation into B cells occurs in a MyD88-independent manner.
Total number of live CD19+B220+ B cells produced by LSK− cells from the spleen of a. naïve mice and b. day 7 P. yoelii 17X infected C57BL/6 mice, and c. CLPs and d. LSK− cells from bone marrow of naïve mice in the presence of OP9 stromal cells. The analysis was done after co-culture for 4, 7, and 10 days in media supplemented with. P. yoelii 17X infected RBC lysate (pRBC lysate) or normal RBC lysate (nRBC lysate). Data are representative of three experiments with similar results. Splenic LSK− cells were sorted from the spleen of n = 3 naïve mice that were subsequently pooled or n = 1 mouse infected with P. yoelii 17X for 7 days. RNA was isolated from sorted LSK− cells to generate cDNA. e. Quantitative analysis of TLR2, TLR4, TLR7, and TLR9 mRNA expression relative to the expression of Rplp0 in splenic LSK− cells from naïve mice and day 7 P. yoelii infected mice by RT-PCR. f. Representative histogram plot showing expression of TLR9 by splenic LSK− cells from naïve mice (solid line) and day 7 P. yoelii infected mice (dotted line), FMO control was shown as grey filled histogram. g. Representative flow plots of LSK− cells from spleens of naïve, day 7, and day 11 P. yoelii 17X infected wild-type (WT) and Myd88−/− mice. h. Total number of LSK− cells, and i. percentage of and j. total number of Ki-67 expressing LSK− cells from the spleen of naïve or P. yoelii 17X infected WT (filled circle) and Myd88−/− (hollow circle) mice after day 4, 7 and 11 p.i. *, p<0.05, **p<0.01 (two-way ANOVA). LSK− cells were sorted from the spleens of naïve WT and myd88−/− mice (pooled from n=3 mice) and 10,000 cells were transferred separately into naïve congenic B6.SJL (CD45.1) recipient mice followed by infection with P. yoelii. At two weeks p.i., the spleens of recipient mice were harvested to analyze the donor (CD45.2+) cell population. k. Total number of donor-derived (CD45.2+) cells. l. Percentage of donor-derived CD45.2+ cells with a B cell phenotype. ns, p>0.05 (non-parametric unpaired two-tailed t test). m. Total number of CD45.2+ B cells (CD138−CD19+B220+) and plasma cells (CD138+B220lo/−). ns, p>0.05 (two-way ANOVA). Data are representative of three independent experiments (a-d). Data are pooled from two independent experiments (h-m) with n = 5-6 mice per group/ time point; Error bars (h-m) S.E.M.
As splenic LSK− cells expanded and differentiated more vigorously into B cells in the presence of pRBC lysate, this suggested that LSK− cells are activated and induced by sensing the presence of parasite specific molecules. Real time quantitative PCR was used to determine if LSK− cells express Tlr genes, including TLR2, TLR4, TLR7 and TLR9, TLRs that have been implicated in the immune response against Plasmodium (53, 54). Transcripts for TLR4, TLR7 and TLR9, but not TLR2, were found to be present in splenic LSK− cells from naïve and P. yoelii 17X infected mice (Fig. 6e). Expression of TLR9 by splenic LSK− cells was confirmed by flow cytometry, which showed that infection resulted in down-regulation of this TLR (Fig. 6e, f), indicating the potential role of this or other TLRs in activating LSK− cells. As the adaptor protein MyD88 is downstream of the majority of TLR signaling pathways, mice deficient in MyD88 were infected with P. yoelii 17X and expansion of splenic LSK− cells was examined. A similar percentage and number of splenic LSK− cells were found in naïve wild-type (WT) and Myd88−/− mice (Fig. 6g, h). In response to infection a similar percentage of LSK− cells were found in the spleen of WT and Myd88−/− mice at day 7 and 11 p.i. (Fig. 6g). However, at day 11 p.i. a significantly higher amount of LSK− cells were found in Myd88−/− mice compared to WT mice (Fig. 6h). The expansion in LSK− cell numbers in Myd88−/− mice at day 11 p.i. was not due to a difference in the proportion of cells in cell cycle, as no difference in the percentage of Ki-67+ LSK− cells was noted at the time points examined (Fig. 6i). Based on cell counts a higher number of Ki-67+ LSK− cells were found in Myd88−/− mice at day 11 p.i., compared to WT mice (Fig. 6j). These differences in splenic LSK− cell numbers in Myd88−/− mice could not be explained by differences in bone marrow LSK− cell numbers, as there was no difference in the percentage or number of LSK− cells at this site during infection (data not shown). These results indicate that MyD88 signaling is not absolutely required for LSK− cell expansion; however in its absence higher numbers of LSK− cells accumulate in the spleen of Myd88−/− mice than WT mice during Plasmodium infection.
The increased accumulation in LSK− cells at day 11 p.i. in Myd88−/− mice could be an indication that MyD88-dependent signaling is required for LSK− cell differentiation. In order to test this idea LSK− cells were sorted from the spleen of naïve WT or Myd88−/− mice, transferred into naïve congenic (CD45.1+) mice, and development of progenitor cells was assessed two weeks following P. yoelii 17X infection. A comparable number of donor-derived (CD45.2+) cells were found in mice receiving WT or Myd88−/− LSK− cells (Fig. 6k). Additionally, analysis of recovered CD45.2+ cells showed that a similar frequency of B cells was generated in the presence or absence of MyD88 signaling (Fig. 6l). Furthermore, there was no difference in the number of B cells or plasma cells produced by donor WT and Myd88−/− splenic LSK− cells (Fig. 6m). These results indicate that MyD88 signaling is not required for activation of LSK− cells and their subsequent differentiation into B cells. While these results do not rule out a role for MyD88 in LSK− cell activation and differentiation they do suggest that additional pattern recognition receptors (PRRs) expressed by LSK− cells are involved in these processes.
Discussion
Lymphoid progenitors are hematopoietic progenitor cells of the bone marrow capable of differentiating into B-, T-, and innate lymphoid cells. Although different populations (CLPs, lympho-myeloid multipotent progenitors, and lymphoid biased progenitors) of hematopoietic progenitor cells have been found to have lymphoid potential (29-31, 55-57), CLPs are the predominant lymphoid progenitor that develops into mature B and T cells. In the case of Plasmodium infection, lymphoid progenitor numbers wane in the bone marrow within one-week post-infection (Supplemental Fig. 2, (8-10)), including their subsequent B cell precursor populations (8). These changes in lymphopoiesis eventually lead to a reduction in mature B cell numbers in the spleen during infection (Supplemental Fig. 2), suggesting that de novo B cell production is abandoned during times of intense inflammation. However, there is also evidence that naïve B cell numbers in the blood do not decline over the course of Plasmodium infection (58), indicating that some degree of B cell development is preserved. Although HSPCs are rarely found in the adult mouse spleen, their contribution to reconstitution of irradiated mice has been reported previously (16, 23). Splenic HSPCs, in spite of having a weaker contribution to hematopoiesis compared to their bone marrow counterparts (59), are suggested to play an important role during emergency conditions (19, 60, 61). Here, decline in lymphopoiesis with malaria infection coincided with expansion of an atypical population of HSPCs in the bone marrow and spleen that gave rise to mature B cells that participated in the humoral immune response through production of parasite-specific antibodies.
Transfer of splenic derived LSK− cells from Plasmodium infected mice into naïve or infection-matched mice resulted in differentiation of these progenitor cells into various types of mature B cells, including follicular and marginal zone. However, neither splenic or bone marrow LSK− cells ever gave rise to B cells with a B-1 phenotype (data not shown), indicating that these splenic B cells are a B-2 progenitor. Ghosn et al. (26) has previously identified a B-2 progenitor in the adult spleen of Balb/c mice with a CD5−CD11b−Gr-1−Ig−CD19−B220lo/− phenotype that were able to reconstitute the B-2 pool when transferred into Rag−/− mice; however, the authors did not phenotype these cells any further, so it is unclear if the splenic LSK− cells described here are the same B-2 progenitor population. Nevertheless, this data supports our finding that the adult spleen serves as a reservoir for HSPCs with a preference for developing into B-2 cells under conditions of inflammation.
One striking feature of the LSK− population is up-regulation of Sca-1 after infection. This protein has served as a useful marker for identification and sorting of HSCs from the bone marrow, yet the function of Sca-1 is currently unclear. Sca-1 has previously been shown to be induced by inflammatory cytokines, such as Type I and II interferons (10, 62), and its upregulation may be associated with cell activation. Whether expression of this receptor is necessary for commitment of these progenitor cells to B cell differentiation is unknown, although overexpression of Sca-1 had been shown to inhibit myeloid differentiation (62). Therefore, upregulation of this protein may favor development of this progenitor population into lymphoid cells rather than myeloid cells.
The identified splenic HSPC population was found to have a phenotype of Lin−Sca-1+c-kit− (LSK−) that initially resembled a similar population of LSK− cells found in the bone marrow with a lymphoid biased potential (29-31). However, there were notable phenotypic differences between splenic and bone marrow LSK− cells. While LSK− cells from the bone marrow and spleen of naïve mice express RNA for the Rag1/2 genes, as well as a number of genes involved in B cell differentiation, they did not express TdT. The DNA polymerase TdT is required for addition of N-nucleotides to V, D, and J exons during B-cell receptor gene recombination to provide junctional diversity (63). However, infection of mice with P. yoelii resulted in expression of TdT RNA by splenic LSK− cells, indicating that these cells are capable of expressing the necessary proteins required for B-cell receptor rearrangement and differentiation. Bone marrow derived LSK− cells have been described to have a heterogeneous expression pattern for IL-7Rα, Flt3, CD25 and CD93 (30, 31); however, splenic LSK− cells from naïve and infected mice lacked expression of IL-7Rα and Flt3, although RNA transcripts were detected, two important receptors associated with development of lymphoid progenitors (64), and CD25 while maintaining a heterogeneous expression pattern for CD93. It has previously been shown that hematopoietic progenitor cells can respond to cytokine stimulation despite an inability to detect receptor expression (65). This could offer one explanation as to why bone marrow LSK− cells, and to a far lesser degree splenic LSK− cells, show increased production of B cells in the OP9 stromal cell assays upon addition of IL-7 and/or Flt3L even though only a small proportion of LSK− cells express the receptors for these cytokines. In addition, the marginal ability of splenic LSK− cells to respond to these cytokines compared to their bone marrow counterparts indicates that an inherent difference exists between these two populations. Despite absence of three significant receptors (IL-7Rα, Flt3 and c-kit) required for lymphoid development, splenic LSK− cells were capable of differentiating into B cells in vitro and in vivo, indicating that splenic LSK− cell differentiation into mature B cells follows a separate pathway of development or alternative growth factors are required for stimulating differentiation.
This raises the question as to what controls B cell differentiation at this site? One likely candidate is B-cell activating factor (BAFF or BlyS), which is known to support positive selection and maturation of B cells in secondary lymphoid organs (66-69); however addition of exogenous BAFF to in vitro OP9 stromal cell assays did not enhance B cell differentiation above the numbers produced with media alone (data not shown). Thus, it remains unclear what factor or factors, as well as the cell type that produces them, supports LSK− differentiation into B cells. It is likely that a stromal cell population within the spleen regulates these events, as stromal cells play a critical role in B cell development in the bone marrow (70). There are numerous stromal cell populations within the spleen, including follicular dendritic cells, fibroblast reticular cells and marginal reticular cells that have been shown to support marginal zone B cell, dendritic cell and follicular helper T cell development in the spleen (71, 72). Our initial transfer studies focused on evaluating differentiation of LSK− cells three weeks post-transfer; however, transfer of splenic LSK− cells from the spleen of infected mice resulted in differentiation of these progenitor cells into mature B cells as quickly as 24-48 hours post-transfer (Supplemental Fig. 4). Normal B cell development in the bone marrow is a tightly regulated process involving negative selection to remove B cells with auto-reactive B-cell receptors. The finding that LSK− cells develop into mature B cells in such an inflammatory environment after infection and in a short timeframe raises the question as to whether regulatory constraints exist in the spleen to limit the chances of B cells that recognize self-antigens from developing into mature B cells. If these events are not tightly regulated in the spleen then extramedullary B cell development could contribute to production of autoreactive B cells. Future work will be needed to address these fundamental questions of B cell development in the spleen.
While addition of IL-7 and Flt3L did not enhance expansion and differentiation of splenic LSK− cells into mature B cells, the finding that pRBC lysate enhances B cell output in OP9 co-culture assays indicates the potential involvement of a PRR in sensing parasite derived material. Indeed, previous studies have shown expression of TLRs on the surface of hematopoietic progenitor cells (48, 50-52, 73), and stimulation by TLR ligands leads to their expansion and differentiation into myeloid cells in humans and mice (48, 52, 74, 75). However, the ability of LSK− cells to expand and differentiate into B cells in the absence of MyD88 after P. yoelii infection indicates that the parasite could be sensed by alternative PRRs that do not utilize MyD88 signaling. Support for this comes from findings that the absence of MyD88 does not impact the outcome of Plasmodium infection in mice (76) and the involvement of inflammasomes activated by pathogen or danger associated molecular patterns that do not utilize MyD88 for signal transduction events (77). Also, stimulation of Nod2 in human CD34+ cells by muramyl dipeptide triggers differentiation into CD11c+ myeloid cells (74), indicating that stimulation of other PRRs by their ligands can induce differentiation of progenitor cells. While stimulation of TLRs on HSPCs primarily induces myeloid differentiation there is precedence that signaling through TLR4 and TLR2 can promote B-cell maturation in vitro (78). The findings in this study indicate that the parasite itself plays an important role in promoting B cell development, and lends support to the idea that PRR stimulation on HSPCs plays a vital role in directing development of particular innate and adaptive immune cell populations required to boost the immune response against microbial pathogens.
While the original description of bone marrow LSK− cells provided no physiological role for this cell type, our findings now indicate that expansion of this population in the bone marrow and spleen is driven by inflammation and serves as a form of emergency lymphopoiesis. While there are numerous examples of emergency myelopoiesis during infection (79-82), including malaria infection (10, 15), this is the first example in which hematopoietic progenitor cells in the spleen have been shown to play an active role in replenishing lymphoid cells during an active infection. Normally, the adaptive immune system meets the need for increased B and T cell numbers through clonal expansion of antigen-specific cells. However, activation of large numbers of B cells, which is a characteristic of the early immune response to malaria, leads to rapid turnover in B cell numbers. In order to compensate for this loss the hematopoietic compartment may have developed alternative mechanisms to continue production of mature B cells within the spleen that are capable of feeding into the germinal center response, which develops after the initial burst of plasmablast and plasma cell production. Support for this comes from the finding that splenic LSK− cell numbers peak at the same time as the early burst in plasmablast and plasma cell production, and just prior to the emergence of germinal center B cells (Fig. 1 and Supplemental Fig. 2). The effector response of LSK− derived B cells described here in malaria-infected hosts suggests that this progenitor population may play a role in the resolution of infection through production of B cells that produce antigen-specific antibodies. The surge of this lymphoid progenitor population at the site of infection, especially during the dearth of B cell progenitors in the bone marrow lends additional support to the argument that the spleen provides a niche for extramedullary hematopoiesis during inflammatory conditions. As these progenitor cells also expand in the spleen in response to T. gondii infection future work will be needed to determine if this is a generalized inflammatory response associated with all or select pathogens that induce a systemic inflammatory response, which these two parasites cause, and to determine LSK− cell fate after infection by T. gondii or other pathogens. Lastly, further understanding of B cell developmental and differentiation pathways taken by splenic LSK− cells, as well as the cell types that support this process will provide new insights into the differential control of the hematopoietic compartment in the spleen during infection.
Supplementary Material
Acknowledgement
We thank William Weidanz for providing us with Plasmodium yoelii 17X. We thank the MR4, as part of the BEI Resource Repository, National Institute of Allergy and Infectious Diseases, National Institutes of Health, for providing Plasmodium yoelii 17XNL PYGFP (MRA-817), contributed by A. Rodriguez. We thank Daohong Zhou (University of Arkansas for Medical Sciences) for providing us with OP9 stromal cells. We also thank Dr. James Burns Jr. (Drexel University College of Medicine) for providing recombinant MSP-119 protein. A special thanks to Andrea Harris for her technical assistance as part of the flow cytometry core.
This work was supported by the Arkansas Biosciences Institute and National Institutes of Health (NIH) Grant AI090179 (J.S.S.), P20-GM103625-Project 3 (to J.S.S.) and NS072298 (E.H.W.). The flow cytometry core is supported by the Translational Research Institute (Grant UL1-TR000039; NIH National Center for Research Resources and National Center for Advancing Translation Sciences) and the UAMS Center for Microbial Pathogenesis and Host Inflammatory Responses (Grant P20-GM103625; NIH National Institute of General Medical Sciences Centers of Biomedical Research Excellence).
References
- 1.Langhorne J, Cross C, Seixas E, Li C, von der Weid T. A role for B cells in the development of T cell helper function in a malaria infection in mice. Proc Natl Acad Sci U S A. 1998;95:1730–1734. doi: 10.1073/pnas.95.4.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen S, Mc GI, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 3.Sabchareon A, Burnouf T, Ouattara D, Attanath P, Bouharoun-Tayoun H, Chantavanich P, Foucault C, Chongsuphajaisiddhi T, Druilhe P. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg. 1991;45:297–308. doi: 10.4269/ajtmh.1991.45.297. [DOI] [PubMed] [Google Scholar]
- 4.Weinbaum FI, Evans CB, Tigelaar RE. Immunity to Plasmodium berghei yoelii in mice. I. The course of infection in T cell and B cell deficient mice. J Immunol. 1976;117:1999–2005. [PubMed] [Google Scholar]
- 5.Grun JL, Weidanz WP. Immunity to Plasmodium chabaudi adami in the B-cell-deficient mouse. Nature. 1981;290:143–145. doi: 10.1038/290143a0. [DOI] [PubMed] [Google Scholar]
- 6.von der Weid T, Honarvar N, Langhorne J. Gene-targeted mice lacking B cells are unable to eliminate a blood stage malaria infection. J Immunol. 1996;156:2510–2516. [PubMed] [Google Scholar]
- 7.Roberts DW, Rank RG, Weidanz WP, Finerty JF. Prevention of recrudescent malaria in nude mice by thymic grafting or by treatment with hyperimmune serum. Infect Immun. 1977;16:821–826. doi: 10.1128/iai.16.3.821-826.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bockstal V, Geurts N, Magez S. Acute Disruption of Bone Marrow B Lymphopoiesis and Apoptosis of Transitional and Marginal Zone B Cells in the Spleen following a Blood-Stage Plasmodium chabaudi Infection in Mice. J Parasitol Res. 2011;2011:534697. doi: 10.1155/2011/534697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagaoka H, Gonzalez-Aseguinolaza G, Tsuji M, Nussenzweig MC. Immunization and infection change the number of recombination activating gene (RAG)-expressing B cells in the periphery by altering immature lymphocyte production. J Exp Med. 2000;191:2113–2120. doi: 10.1084/jem.191.12.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belyaev NN, Brown DE, Diaz AI, Rae A, Jarra W, Thompson J, Langhorne J, Potocnik AJ. Induction of an IL7-R(+)c-Kit(hi) myelolymphoid progenitor critically dependent on IFN-gamma signaling during acute malaria. Nat Immunol. 2010;11:477–485. doi: 10.1038/ni.1869. [DOI] [PubMed] [Google Scholar]
- 11.Weatherall DJ, Abdalla S, Pippard MJ. The anaemia of Plasmodium falciparum malaria. Ciba Found Symp. 1983;94:74–97. doi: 10.1002/9780470715444.ch6. [DOI] [PubMed] [Google Scholar]
- 12.Kurtzhals JA, Rodrigues O, Addae M, Commey JO, Nkrumah FK, Hviid L. Reversible suppression of bone marrow response to erythropoietin in Plasmodium falciparum malaria. Br J Haematol. 1997;97:169–174. doi: 10.1046/j.1365-2141.1997.82654.x. [DOI] [PubMed] [Google Scholar]
- 13.Chang KH, Tam M, Stevenson MM. Inappropriately low reticulocytosis in severe malarial anemia correlates with suppression in the development of late erythroid precursors. Blood. 2004;103:3727–3735. doi: 10.1182/blood-2003-08-2887. [DOI] [PubMed] [Google Scholar]
- 14.Casals-Pascual C, Kai O, Cheung JO, Williams S, Lowe B, Nyanoti M, Williams TN, Maitland K, Molyneux M, Newton CR, Peshu N, Watt SM, Roberts DJ. Suppression of erythropoiesis in malarial anemia is associated with hemozoin in vitro and in vivo. Blood. 2006;108:2569–2577. doi: 10.1182/blood-2006-05-018697. [DOI] [PubMed] [Google Scholar]
- 15.Belyaev NN, Biro J, Langhorne J, Potocnik AJ. Extramedullary myelopoiesis in malaria depends on mobilization of myeloid-restricted progenitors by IFN-gamma induced chemokines. PLoS Pathog. 2013;9:e1003406. doi: 10.1371/journal.ppat.1003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolber FM, Leonard E, Michael S, Orschell-Traycoff CM, Yoder MC, Srour EF. Roles of spleen and liver in development of the murine hematopoietic system. Exp Hematol. 2002;30:1010–1019. doi: 10.1016/s0301-472x(02)00881-0. [DOI] [PubMed] [Google Scholar]
- 17.Tan JK, O'Neill HC. Investigation of murine spleen as a niche for hematopoiesis. Transplantation. 2010;89:140–145. doi: 10.1097/TP.0b013e3181c42f70. [DOI] [PubMed] [Google Scholar]
- 18.Dor FJ, Ramirez ML, Parmar K, Altman EL, Huang CA, Down JD, Cooper DK. Primitive hematopoietic cell populations reside in the spleen: Studies in the pig, baboon, and human. Exp Hematol. 2006;34:1573–1582. doi: 10.1016/j.exphem.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 19.Seixas E, Ostler D. Plasmodium chabaudi chabaudi (AS): differential cellular responses to infection in resistant and susceptible mice. Exp Parasitol. 2005;110:394–405. doi: 10.1016/j.exppara.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 20.Halder RC, Abe T, Mannoor MK, Morshed SR, Ariyasinghe A, Watanabe H, Kawamura H, Sekikawa H, Hamada H, Nishiyama Y, Ishikawa H, Toba K, Abo T. Onset of hepatic erythropoiesis after malarial infection in mice. Parasitol Int. 2003;52:259–268. doi: 10.1016/s1383-5769(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 21.O'Neill HC, Griffiths KL, Periasamy P, Hinton RA, Hey YY, Petvises S, Tan JK. Spleen as a site for hematopoiesis of a distinct antigen presenting cell type. Stem Cells Int. 2011;2011:954275. doi: 10.4061/2011/954275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petvises S, O'Neill HC. Characterisation of dendritic cells arising from progenitors endogenous to murine spleen. PLoS One. 2014;9:e88311. doi: 10.1371/journal.pone.0088311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan JK, O'Neill HC. Haematopoietic stem cells in spleen have distinct differentiative potential for antigen presenting cells. J Cell Mol Med. 2010;14:2144–2150. doi: 10.1111/j.1582-4934.2009.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig M. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, Mulligan-Kehoe MJ, Libby P, Nahrendorf M, Pittet MJ, Weissleder R, Swirski FK. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125:364–374. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosn EE, Sadate-Ngatchou P, Yang Y, Herzenberg LA. Distinct progenitors for B-1 and B-2 cells are present in adult mouse spleen. Proc Natl Acad Sci U S A. 2011;108:2879–2884. doi: 10.1073/pnas.1019764108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueda Y, Yang K, Foster SJ, Kondo M, Kelsoe G. Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J Exp Med. 2004;199:47–58. doi: 10.1084/jem.20031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201:1771–1780. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fossati V, Kumar R, Snoeck HW. Progenitor cell origin plays a role in fate choices of mature B cells. J Immunol. 2010;184:1251–1260. doi: 10.4049/jimmunol.0901922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar R, Fossati V, Israel M, Snoeck HW. Lin-Sca1+kit- bone marrow cells contain early lymphoid-committed precursors that are distinct from common lymphoid progenitors. J Immunol. 2008;181:7507–7513. doi: 10.4049/jimmunol.181.11.7507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harman BC, Northrup DL, Allman D. Resolution of unique Sca-1highc-Kit- lymphoid-biased progenitors in adult bone marrow. J Immunol. 2008;181:7514–7524. doi: 10.4049/jimmunol.181.11.7514. [DOI] [PubMed] [Google Scholar]
- 32.Montecino-Rodriguez E, Leathers H, Dorshkind K. Bipotential B-macrophage progenitors are present in adult bone marrow. Nat Immunol. 2001;2:83–88. doi: 10.1038/83210. [DOI] [PubMed] [Google Scholar]
- 33.Chi AW, Chavez A, Xu L, Weber BN, Shestova O, Schaffer A, Wertheim G, Pear WS, Izon D, Bhandoola A. Identification of Flt3(+)CD150(−) myeloid progenitors in adult mouse bone marrow that harbor T lymphoid developmental potential. Blood. 2011;118:2723–2732. doi: 10.1182/blood-2010-09-309989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Randall TD, Weissman IL. Characterization of a population of cells in the bone marrow that phenotypically mimics hematopoietic stem cells: resting stem cells or mystery population? Stem Cells. 1998;16:38–48. doi: 10.1002/stem.160038. [DOI] [PubMed] [Google Scholar]
- 35.Czerkinsky CC, Nilsson LA, Nygren H, Ouchterlony O, Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;65:109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- 36.Langhorne J, Kim KJ, Asofsky R. Distribution of immunoglobulin isotypes in the nonspecific B-cell response induced by infection with Plasmodium chabaudi adami and Plasmodium yoelii. Cell Immunol. 1985;90:251–257. doi: 10.1016/0008-8749(85)90187-x. [DOI] [PubMed] [Google Scholar]
- 37.Castillo-Mendez SI, Zago CA, Sardinha LR, Freitas do Rosario AP, Alvarez JM, D'Imperio Lima MR. Characterization of the spleen B-cell compartment at the early and late blood-stage Plasmodium chabaudi malaria. Scand J Immunol. 2007;66:309–319. doi: 10.1111/j.1365-3083.2007.01972.x. [DOI] [PubMed] [Google Scholar]
- 38.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 39.Brickshawana A, Shapiro VS, Kita H, Pease LR. Lineage(−)Sca1+c-Kit(−)CD25+ cells are IL-33-responsive type 2 innate cells in the mouse bone marrow. J Immunol. 2011;187:5795–5804. doi: 10.4049/jimmunol.1102242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li YS, Wasserman R, Hayakawa K, Hardy RR. Identification of the earliest B lineage stage in mouse bone marrow. Immunity. 1996;5:527–535. doi: 10.1016/s1074-7613(00)80268-x. [DOI] [PubMed] [Google Scholar]
- 41.Jordan CT, McKearn JP, Lemischka IR. Cellular and developmental properties of fetal hematopoietic stem cells. Cell. 1990;61:953–963. doi: 10.1016/0092-8674(90)90061-i. [DOI] [PubMed] [Google Scholar]
- 42.Vieira P, Cumano A. Differentiation of B lymphocytes from hematopoietic stem cells. Methods Mol Biol. 2004;271:67–76. doi: 10.1385/1-59259-796-3:067. [DOI] [PubMed] [Google Scholar]
- 43.Hayakawa J, Migita M, Ueda T, Shimada T, Fukunaga Y. Generation of a chimeric mouse reconstituted with green fluorescent protein-positive bone marrow cells: a useful model for studying the behavior of bone marrow cells in regeneration in vivo. Int J Hematol. 2003;77:456–462. doi: 10.1007/BF02986613. [DOI] [PubMed] [Google Scholar]
- 44.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 45.Anderson SM, Tomayko MM, Ahuja A, Haberman AM, Shlomchik MJ. New markers for murine memory B cells that define mutated and unmutated subsets. J Exp Med. 2007;204:2103–2114. doi: 10.1084/jem.20062571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomayko MM, Steinel NC, Anderson SM, Shlomchik MJ. Cutting edge: Hierarchy of maturity of murine memory B cell subsets. J Immunol. 2010;185:7146–7150. doi: 10.4049/jimmunol.1002163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dogan I, Bertocci B, Vilmont V, Delbos F, Megret J, Storck S, Reynaud CA, Weill JC. Multiple layers of B cell memory with different effector functions. Nat Immunol. 2009;10:1292–1299. doi: 10.1038/ni.1814. [DOI] [PubMed] [Google Scholar]
- 48.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yanez A, Murciano C, O'Connor JE, Gozalbo D, Gil ML. Candida albicans triggers proliferation and differentiation of hematopoietic stem and progenitor cells by a MyD88-dependent signaling. Microbes Infect. 2009;11:531–535. doi: 10.1016/j.micinf.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Yanez A, Flores A, Murciano C, O'Connor JE, Gozalbo D, Gil ML. Signalling through TLR2/MyD88 induces differentiation of murine bone marrow stem and progenitor cells to functional phagocytes in response to Candida albicans. Cell Microbiol. 2010;12:114–128. doi: 10.1111/j.1462-5822.2009.01382.x. [DOI] [PubMed] [Google Scholar]
- 51.Choi HH, Kim KK, Kim KD, Kim HJ, Jo EK, Song CH. Effects of mycobacterial infection on proliferation of hematopoietic precursor cells. Microbes Infect. 2011;13:1252–1260. doi: 10.1016/j.micinf.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Megias J, Yanez A, Moriano S, O'Connor JE, Gozalbo D, Gil ML. Direct Toll-like receptor-mediated stimulation of hematopoietic stem and progenitor cells occurs in vivo and promotes differentiation toward macrophages. Stem Cells. 2012;30:1486–1495. doi: 10.1002/stem.1110. [DOI] [PubMed] [Google Scholar]
- 53.Ghosh D, Stumhofer JS. Do you see what I see: Recognition of protozoan parasites by Toll-like receptors. Curr Immunol Rev. 2013;9:129–140. doi: 10.2174/1573395509666131203225929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ropert C, Franklin BS, Gazzinelli RT. Role of TLRs/MyD88 in host resistance and pathogenesis during protozoan infection: lessons from malaria. Semin Immunopathol. 2008;30:41–51. doi: 10.1007/s00281-007-0103-2. [DOI] [PubMed] [Google Scholar]
- 55.Katsura Y. Redefinition of lymphoid progenitors. Nat Rev Immunol. 2002;2:127–132. doi: 10.1038/nri721. [DOI] [PubMed] [Google Scholar]
- 56.Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, Anderson K, Sitnicka E, Sasaki Y, Sigvardsson M, Jacobsen SE. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 57.Yang GX, Lian ZX, Chuang YH, Shu SA, Moritoki Y, Lan R, Wakabayashi K, Ansari AA, Dorshkind K, Ikehara S, Gershwin ME. Generation of functionally distinct B lymphocytes from common myeloid progenitors. Clin Exp Immunol. 2007;150:349–357. doi: 10.1111/j.1365-2249.2007.03493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Achtman AH, Khan M, MacLennan IC, Langhorne J. Plasmodium chabaudi chabaudi infection in mice induces strong B cell responses and striking but temporary changes in splenic cell distribution. J Immunol. 2003;171:317–324. doi: 10.4049/jimmunol.171.1.317. [DOI] [PubMed] [Google Scholar]
- 59.Morita Y, Iseki A, Okamura S, Suzuki S, Nakauchi H, Ema H. Functional characterization of hematopoietic stem cells in the spleen. Exp Hematol. 2011;39:351–359. e353. doi: 10.1016/j.exphem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 60.Bozzini CE, Barrio Rendo ME, Devoto FC, Epper CE. Studies on medullary and extramedullary erythropoiesis in the adult mouse. Am J Physiol. 1970;219:724–728. doi: 10.1152/ajplegacy.1970.219.3.724. [DOI] [PubMed] [Google Scholar]
- 61.Paulson RF, Shi L, Wu DC. Stress erythropoiesis: new signals and new stress progenitor cells. Curr Opin Hematol. 2011;18:139–145. doi: 10.1097/MOH.0b013e32834521c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bradfute SB, Graubert TA, Goodell MA. Roles of Sca-1 in hematopoietic stem/progenitor cell function. Exp Hematol. 2005;33:836–843. doi: 10.1016/j.exphem.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 63.Li YS, Hayakawa K, Hardy RR. The regulated expression of B lineage associated genes during B cell differentiation in bone marrow and fetal liver. J Exp Med. 1993;178:951–960. doi: 10.1084/jem.178.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ray RJ, Paige CJ, Furlonger C, Lyman SD, Rottapel R. Flt3 ligand supports the differentiation of early B cell progenitors in the presence of interleukin-11 and interleukin-7. Eur J Immunol. 1996;26:1504–1510. doi: 10.1002/eji.1830260715. [DOI] [PubMed] [Google Scholar]
- 65.Walker F, Nicola NA, Metcalf D, Burgess AW. Hierarchical down-modulation of hemopoietic growth factor receptors. Cell. 1985;43:269–276. doi: 10.1016/0092-8674(85)90032-7. [DOI] [PubMed] [Google Scholar]
- 66.Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea H, Valmori D, Romero P, Werner-Favre C, Zubler RH, Browning JL, Tschopp J. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 68.Gross JA, Dillon SR, Mudri S, Johnston J, Littau A, Roque R, Rixon M, Schou O, Foley KP, Haugen H, McMillen S, Waggie K, Schreckhise RW, Shoemaker K, Vu T, Moore M, Grossman A, Clegg CH. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15:289–302. doi: 10.1016/s1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 69.Meyer-Bahlburg A, Andrews SF, Yu KO, Porcelli SA, Rawlings DJ. Characterization of a late transitional B cell population highly sensitive to BAFF-mediated homeostatic proliferation. J Exp Med. 2008;205:155–168. doi: 10.1084/jem.20071088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dorshkind K, Landreth KS. Regulation of B cell differentiation by bone marrow stromal cells. Int J Cell Cloning. 1992;10:12–17. doi: 10.1002/stem.5530100104. [DOI] [PubMed] [Google Scholar]
- 71.den Haan JM, Mebius RE, Kraal G. Stromal cells of the mouse spleen. Front Immunol. 2012;3:201. doi: 10.3389/fimmu.2012.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fasnacht N, Huang HY, Koch U, Favre S, Auderset F, Chai Q, Onder L, Kallert S, Pinschewer DD, MacDonald HR, Tacchini-Cottier F, Ludewig B, Luther SA, Radtke F. Specific fibroblastic niches in secondary lymphoid organs orchestrate distinct Notch-regulated immune responses. J Exp Med. 2014;211:2265–2279. doi: 10.1084/jem.20132528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Welner RS, Pelayo R, Nagai Y, Garrett KP, Wuest TR, Carr DJ, Borghesi LA, Farrar MA, Kincade PW. Lymphoid precursors are directed to produce dendritic cells as a result of TLR9 ligation during herpes infection. Blood. 2008;112:3753–3761. doi: 10.1182/blood-2008-04-151506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sioud M, Floisand Y. TLR agonists induce the differentiation of human bone marrow CD34+ progenitors into CD11c+ CD80/86+ DC capable of inducing a Th1-type response. Eur J Immunol. 2007;37:2834–2846. doi: 10.1002/eji.200737112. [DOI] [PubMed] [Google Scholar]
- 75.Sioud M, Floisand Y, Forfang L, Lund-Johansen F. Signaling through toll-like receptor 7/8 induces the differentiation of human bone marrow CD34+ progenitor cells along the myeloid lineage. J Mol Biol. 2006;364:945–954. doi: 10.1016/j.jmb.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 76.Franklin BS, Rodrigues SO, Antonelli LR, Oliveira RV, Goncalves AM, Sales-Junior PA, Valente EP, Alvarez-Leite JI, Ropert C, Golenbock DT, Gazzinelli RT. MyD88-dependent activation of dendritic cells and CD4(+) T lymphocytes mediates symptoms, but is not required for the immunological control of parasites during rodent malaria. Microbes Infect. 2007;9:881–890. doi: 10.1016/j.micinf.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 77.Griffith JW, Sun T, McIntosh MT, Bucala R. Pure Hemozoin is inflammatory in vivo and activates the NALP3 inflammasome via release of uric acid. J Immunol. 2009;183:5208–5220. doi: 10.4049/jimmunol.0713552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hayashi EA, Akira S, Nobrega A. Role of TLR in B cell development: signaling through TLR4 promotes B cell maturation and is inhibited by TLR2. J Immunol. 2005;174:6639–6647. doi: 10.4049/jimmunol.174.11.6639. [DOI] [PubMed] [Google Scholar]
- 79.MacNamara KC, Oduro K, Martin O, Jones DD, McLaughlin M, Choi K, Borjesson DL, Winslow GM. Infection-induced myelopoiesis during intracellular bacterial infection is critically dependent upon IFN-gamma signaling. J Immunol. 2011;186:1032–1043. doi: 10.4049/jimmunol.1001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buechler MB, Teal TH, Elkon KB, Hamerman JA. Cutting edge: Type I IFN drives emergency myelopoiesis and peripheral myeloid expansion during chronic TLR7 signaling. J Immunol. 2013;190:886–891. doi: 10.4049/jimmunol.1202739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yanez A, Goodridge HS, Gozalbo D, Gil ML. TLRs control hematopoiesis during infection. Eur J Immunol. 2013;43:2526–2533. doi: 10.1002/eji.201343833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cuenca AG, Cuenca AL, Gentile LF, Efron PA, Islam S, Moldawer LL, Kays DW, Larson SD. Delayed emergency myelopoiesis following polymicrobial sepsis in neonates. Innate Immun. 2015;21:386–391. doi: 10.1177/1753425914542445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao X, et al. Brief report: interferon-gamma induces expansion of Lin(−)Sca-1(+)C-Kit(+) Cells. Stem Cells. 2010;28(1):122–6. doi: 10.1002/stem.252. [DOI] [PubMed] [Google Scholar]
- 84.Essers MA, et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458(7240):904–8. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.