Abstract
A successful pregnancy depends upon the maintenance of tolerance at the fetal-maternal interface; strong inflammation in the placental bed is generally associated with adverse fetal outcomes. Among the mechanisms that foster tolerance and limit inflammation, the fetal immune system favors Th2 or regulatory responses over Th1 responses. The unintended consequence of this functional program is high susceptibility to infections. Human Vδ2 T cells mount innate-like responses to a broad range of microorganisms and are poised for Th1 responses before birth. In infants they likely play a key role in protection against pathogens by exerting early Th1 effector functions, improving function of other innate cells, and promoting Th1 polarization of adaptive responses. However, their propensity to release Th1 mediators may require careful regulation during fetal life, to avoid exaggerated pro-inflammatory responses. We investigated molecules with the potential to act as a rheostat for fetal Vδ2 cells. PD1 is a negative regulator of T cell responses and a determinant of tolerance, particularly at the fetal-maternal interface. Neonatal Vδ2 cells up-regulate PD1 shortly after activation and, unlike their adult counterparts, express this molecule for at least 28 days. Engagement of PD1 by one of its ligands, PDL1, effectively dampens TCR-mediated responses (TNF-α production and degranulation) by neonatal Vδ2 cells and may thus help maintain their activity within safe limits. PD1 expression by neonatal Vδ2 cells is inversely associated with promoter DNA methylation. Prolonged PD1 expression may be part of a functional program to control Vδ2 cell inflammatory responses during fetal life.
Introduction
The fetus develops in a semi-allogeneic environment and must have mechanisms for maintaining immune tolerance to avoid rejecting maternal tissues (1). This is achieved through a functional program that skews adaptive immunity toward Th2 responses (2), prevents strong Th1 responses in order to limit inflammation [reviewed in (3, 4)] and promotes tolerance to several foreign antigens encountered in utero (1). Inflammatory responses during gestation are strongly associated with negative fetal outcomes including preterm birth or pregnancy loss (5). The unintended consequences of mechanisms that promote tolerance and suppress inflammation include high susceptibility to intracellular pathogens in infancy (6) with high morbidity and mortality during the first two years of life, and poor responses to some vaccines administered shortly after birth [reviewed in (7, 8)]. Our understanding of molecular mechanisms used by the fetal immune system to promote tolerance or suppression is limited. In this study, we focus on a potential mechanism for controlling fetal gammadelta (γδ) T cells that may provide broader insight into the regulatory mechanisms at the maternal fetal interface.
Human, adult peripheral blood Vδ2 T cells, a subset of γδ lymphocytes, mount rapid innate-like responses to a broad array of microorganisms including mycobacteria and plasmodia species. Activated Vδ2 T cells produce abundant Th1 cytokines (9–11), enhance NK cytotoxicity (12, 13), and favor DC maturation (14–16) to bridge innate and adaptive immunity. Previous reports showed that Vδ2 T cells in neonates mount responses qualitatively similar to their adult counterparts, though these responses are lower in magnitude (at least in in some experimental settings) (17–19) for reasons that are still unknown. A recent study showed that Vδ2 lymphocytes are already poised for rapid Th1 responses before birth (20). Moreover, Vδ2 T cells use cytokines of myeloid origin, such as IL-23 (18) or IL-15 (21, 22), to sustain their own CD4-independent proliferation; this may be extremely valuable during immune responses in early life, when adaptive responses are still skewed. We also know that Vδ2 T cells are a significant component of immune responses to the tuberculosis vaccine Bacille Calmette-Guérin (BCG) (17, 23, 24), which is administered routinely to neonates in sub-Saharan Africa at birth. Thanks to these functional properties, the Vδ2 lymphocytes may play key roles in the first line of defense during early life and we need to study fetal regulation of these cells to understand their impact on neonatal immunity.
Poorly regulated Vδ2 T cell activation could be a threat during fetal life. Tight control mechanisms are likely needed to prevent excessive Vδ2 T cell pro-inflammatory responses in utero and shortly after birth (when colonization of the gut by commensal microbiota may release large amounts of Vδ2-stimulating compounds). In healthy newborns that were not prenatally exposed to microbial agents, a relatively small fraction of Vδ2 cells expresses surface NKG2A (21), an inhibitory receptor for adult Vδ2 lymphocytes. We are now investigating molecular mechanisms controlling Vδ2 T cell responses before birth. We focused on PD1 because this negative regulator and its ligand, PDL1, play important roles in maintaining tolerance at the feto-maternal interface (25–30) and are known to modulate adult γδ cell responses to tumor cells (31, 32). We observed that PD1 is upregulated by a large fraction of activated neonatal Vδ2 T cells and expression is maintained for at least 4 weeks after in vitro stimulation. Concomitant engagement of the γδ TCR and PD1 dampens TCR-mediated signals, inhibiting TNF-α production and degranulation. Multiple molecular mechanisms including DNA methylation contribute to PD1 regulation.
Prolonged PD1 expression may ensure that fetal/neonatal Vδ2 T cells can be controlled by checkpoint regulation. This may be part of a functional program most important during perinatal development that enables Vδ2 cell responses to microbial threats while preventing excessive pro-inflammatory reactions.
Materials and Methods
Specimen collection and mononuclear cell isolation
Cord blood (CB) specimens were obtained from three sources. The Department of Obstetrics, Gynecology and Reproductive Services, University of Maryland Medical Center provided specimens as de-identified discarded material in the context of a protocol that was considered exempt by our university Institutional Review Board. Only healthy full term (≥37 weeks gestation by ultrasound) pregnancies were included in this study. Women were excluded if they were HIV-positive, HCV-positive, HBV-positive, immunosuppressed, had autoimmune disease, had proteinuric pre-eclampsia, used illegal drugs during pregnancy, had traveled to a malaria endemic region during pregnancy, had multiple gestation or if the newborn was below the 10th percentile weight for gestational age. AllCells (Emeryville, CA) provided cryo-preserved specimens collected from HIV-negative, HBV-negative, HCV-negative women. Finally, CB samples were collected at the Ndirande Government Health Center, in Blantyre, Malawi, from HIV-negative women who had been enrolled in a clinical trial (ClinicalTrials.gov Identifier: NCT01443130) during the first or second trimester of gestation. The specimens included in the current study were collected from term deliveries of women who tested negative for placental malaria infection (by placental histology and qPCR). The clinical trial study procedures were approved by the IRB of the University of Maryland and by the College of Medicine Research Ethic Committee, University of Malawi in Blantyre, Malawi.
Adult peripheral blood specimens were purchased from the New York blood bank or collected from healthy volunteers at the University of Maryland after obtaining a signed informed consent form from each participant in the context of a protocol approved by the University of Maryland Institutional Review Board.
Cord blood was collected from the umbilical vein of delivered placentas soon after uncomplicated births. Umbilical cord blood (15–60 ml) was collected using a sterile cord blood collection unit (Pall medical, Scottsdale, AZ) after wiping the cord to remove maternal blood and sterilizing the collection site with ethanol. Cord blood or peripheral blood was diluted with phosphate buffered saline, PBS (Lonza, Walkersville, MD) and layered over Lymphocyte Separation Medium (LSM, Corning, Tewksbury, MA) density gradient to isolate cord blood or peripheral blood mononuclear cells (CBMC and PBMC respectively). CBMC were counted using Tuerk solution (Sigma-Aldrich, St Louis, MO). Viability was assessed by trypan blue exclusion. CBMC or PBMC were frozen at 1×107 cells/ml, in 90% fetal bovine serum (Gibco, Life Technologies, Carlsbad, CA), 10% DMSO (Sigma-Aldrich) freezing medium. All specimens were stored at −130°C before use.
Cell culture
CBMC or PBMC were resuspended at 1.5×106 cells/ml in complete medium, RPMI 1640 supplemented with 10% FBS (Gibco, Life technologies), 2 mM L-glutamine, 100 IU/ml penicillin-streptomycin (Lonza). In order to expand Vγ9Vδ2 T lymphocytes, cultures were treated with zoledronic acid monohydrate, ZOL (Sigma-Aldrich) at 0.5 µM, in the presence of 100 IU/ml of human recombinant interleukin 2 (IL-2) (Tecin, NIH reagent program, NIH, Bethesda, MD). Cells were incubated for 14 days at 37°C with 5% CO2 and fresh cytokine was added every 3 days; fresh medium was added on days 7 and 10. After 14 days, cells were harvested and viability was assessed by trypan blue exclusion. For time course experiments, CBMC were harvested on days 1, 2, 3, 5, 7, 10, and 14 for phenotype analysis. On day 14, a fraction of the CBMC was used to determine Vδ2 T cell phenotype, the remaining cells were sorted on a FACSAria (BD Biosciences, San Jose, CA) and used for RNA and/or genomic DNA.
For some specimens, cell culture was prolonged up to 28 days in resting conditions. From day 14, cytokines were added every three days (10U/ml of IL-2), and medium was replaced when necessary. On day 28, cell viability was assessed by trypan blue exclusion, then Vδ2 T cells were sorted and used for genomic RNA and/or genomic DNA.
Flow cytometry
Ex vivo CBMC or expanded Vδ2 lymphocytes were resuspended in PBS-10% FBS and stained at 4°C with directly conjugated monoclonal antibodies. After 15 minutes, cells were washed with PBS-10% FBS and resuspended in PBS-10% FBS with 1% paraformaldehyde. (1–3)×105 lymphocytes (gated on the basis of forward and side scatter profiles) were collected for each sample on a FACSCalibur (BD Biosciences) and results were analyzed with FlowJo software (Tristar, San Jose, CA).
The following monoclonal antibodies were used for four-color staining: anti-Vδ2 PE or FITC (clone B6), anti-PD1 PE-Cy7 (clone EH12.2H7), anti-CD56 PE (clone HCD56), anti-PDL1 APC (clone 29E2A3) purchased from Biolegend (San Diego, CA); anti-CD16 FITC (clone 3G8), anti-CD70 PE (clone Ki-24), anti-CD80 PE (clone L307.4), anti-CD57 APC (clone NK1) purchased from BD biosciences.
For cell sorting, CBMC were stained either with anti-Vδ2 alone or with a combination of anti-Vδ2 PE and anti-PD1 PECy7, then resuspended at (1–2)×107 cells/ml in PBS-1% FBS. An AriaII flow cytometer (BD Biosciences) was used for sorting purposes. Sorted Vδ2 T cells were routinely >95% pure.
Granule mobilization assay
After 16 days in culture, CBMC were resuspended at 2×106 cells/ml in fresh complete medium and re-stimulated in 96-well plates pre-coated with anti-γδ TCR (clone B1.1, eBioscience, San Diego, CA). Plates were coated overnight at 4°C with anti-γδ TCR (diluted 1:250 in PBS, 50 µl/well) and with different concentrations (0.4, 2 and 10 µg/ml) of human recombinant PDL1-Fc (Sino biological, China) or, for a subset of specimens, with human recombinant CD19-Fc, 2 µg/ml (ACROBiosystems, Newark, DE). CMBC were plated in triplicate (100 µl/well) with anti-CD107a FITC (clone H4A3, BD Biosciences, 5 µl/well) and GolgiPlug (brefeldin A, 1 µg/ml, BD Biosciences). After a 6-hour incubation, cells were collected, washed once with cold PBS, and stained for membrane markers. After staining of surface markers, cells were permeabilized by incubating for 20 minutes at 4°C with fixation/permeabilization solution (BD Biosciences). Cells were then washed twice with 1× Perm/wash buffer (BD Biosciences). Anti-human TNF-α APC (clone MAb11, BD Biosciences) was added for 40 minutes at room temperature. Finally, cells were washed once with Perm/wash buffer. At least 105 lymphocytes were collected for each sample.
RNA extraction, RT-PCR, qPCR
After a 14- or 28-day expansion, (1–2)×106 Vδ2 cells were sorted directly in Trizol (Life Technologies, Carlsbad, CA) and total RNA was extracted using the Direct-zol RNA miniprep (Zymo Research, Irvine, CA) as described by the manufacturer. Total RNA (500 ng) was converted into cDNA using the iScript cDNA Synthesis kit (BioRad Laboratories, Hercules, CA), according to manufacturer’s instruction. cDNA was diluted with one volume of water for target gene amplifications, while 1/1000 dilutions were prepared for reference gene amplifications. We set up real-time PCR reactions according to the iTag Universal SYBR Green Supermix protocol (BioRad Laboratories). Reactions were performed using the StepOnePlus default thermal cycler program (Applied Biosystems, Foster City, CA). Results were analyzed with the StepOne software v2.3 (Applied Biosystems). We selected 18S ribosomal RNA as a reference housekeeping gene. The variation between runs was normalized using as reference a cDNA specimen from tonsil mononuclear cells for PD1, or a cDNA specimen from adult PBMC for T-box protein expressed in T cells (T-bet) reactions. The relative expression of target genes was analyzed using the comparative method [2^(−ΔΔCt)] (33). We used the following primer pairs for target genes: PD1 forward 5'-ACGAGGGACAATAGGAGCCA-3', reverse 5'-GGCATACTCCGTCTGCTCAG-3'; T-bet forward 5'-TTGAGGTGAACGACGGAGAG-3', reverse 5'-CCAAGGAATTGACAGTTGGGT-3'. For 18S ribosomal RNA we amplified two non-overlapping segments with the following primer pairs: 18S1 forward 5'-AGAAACGGCTACCACA-3', reverse 5'-CACCAGACTTGCCCTCCA-3'; 18S2: forward 5'-CAGCCACCCGAGATTGAGCA-3', reverse 5'-TAGTAGCGACGGGCGGTGTG-3'. The primer concentration was optimized in preliminary experiments (200nM for the two target genes, 350 and 300 nM for 18S1 and 18S2 respectively). The efficiency of each primer pair is within the recommended range.
Genomic DNA extraction and bisulfite conversion
Genomic DNA (gDNA) was extracted with Quick-gDNA MiniPrep (Zymo Research) from (0.5–3)×106 sorted Vδ2 T cells. We used EpiTect Bisulfite kit (Qiagen, Valencia, CA) to perform bisulfite conversion, with 500 ng of gDNA per reaction according to the manufacturer’s recommendation. A reference sample was used to ensure that comparable bisulfite conversion efficiency was achieved by different lots of the EpiTect Bisulfite kit. The Conserved Region C (CR-C) of the PD1 promoter was amplified from bisulfite-converted DNA using the following primers: forward 5’-AAGTTATTTATATAGTTTTATATTTTTGAG-3’; reverse 5’-CACACCATAACCACAATTCCAAATCTTTCC-3’. This primer pair yields a 555 bp amplicon that spans from –1192 to –637 of the PD1 promoter. For some experiments, we only amplified the CR-C portion proximal to the transcription initiation site using an alternate forward primer (alt-forward 5’TTAGTAGTTTTATTTTTTTTGTTTTTTATT-3’) and the same reverse primer, which generate a 297 bp amplicon. The methylation status of all CG pairs present in the CR-C amplicons was determined by Sanger sequencing.
Cloning and sequencing of PD1 promoter amplicons
PD1 amplicons were purified by gel extraction, using the QIAquick Gel Extraction Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Purified products were denatured (1 minute at 94°C), then incubated for 30 minutes at 72°C with 2mM MgCl2, 0.2mM dATPs, and 2.5 units of AmpliTaq Gold (Promega, Madison, WI), then ligated into a pCR2.1 vector (Invitrogen, Carlsbad, CA). Ligated vectors were transfected into TOP10F’ competent cells (TA Cloning Kit, Invitrogen, Carlsbad, CA), and bacterial colonies representing a library of PD1 amplicons were grown overnight on agar plates containing 50µg/ml ampicillin, 500 µM IPTG and 80 µg/ml X-Gal (Promega, Madison, WI). Colonies containing recombinant plasmids were cultured overnight in LB media and bacterial suspensions were used as a template to amplify the fragment of the PD1 promoter inserted in the plasmid. M13 polymerase chain reactions were performed using: 2µl of bacterial suspension as template, 100nM of M13 forward (5’ GTAAAACGACGGCCAG 3’) and M13 reverse primer (5’ CAGGAAACAGCTATGAC 3’), 0.2mM dNTPs, 2mM MgCl2, 10mM Tris pH 8.8, 50mM KCl, 0.1%Triton X-100 and 1 unit of AmpliTaq Gold (Promega). PCR were run with the following profile: denaturation 1 min at 94°C; 25 cycles (30 s at 94°C, 1 min at 60°C, 1 min at 72°C); extension: 10 min at 72°C. PCR products were purified by size exclusion, after running through a bed of Sephacryl S400 (GE Healthcare, Uppsala, Sweden) packed (200 µl/well) into 96-well MultiScreen HTS filtration plates (Millipore, Billerica, MA).
Sequencing reactions were performed with a Big Dye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems), and M13F or M13R oligonucleotide primers for each sample. Sequencing reactions were run with the following profile: denaturation: 1 min at 94°C; 25 cycles (30s at 96°C, 20s at 50°C, 4 min at 60°C). Sequences were loaded on an automated sequencer ABI3700 and analyzed using Sequencher (GeneCodes Corporation, Ann Harbor, MI) and Mesquite v3.02 (W.&D. Maddison) softwares.
Statistical analysis
Statistical analyses were performed using the software GraphPad Prism V5.0f (GraphPad Software, La Jolla, CA). For each measured variable, D'Agostino & Pearson omnibus normality test was performed to assess whether values were normally distributed. Differences between means (for normally distributed variables) or medians (for variables displaying a non-Gaussian distribution) were evaluated using a student’s t-test or Mann-Whitney test. For paired groups, paired t-test or Wilcoxon matched pairs signed-rank test were used respectively. For comparisons of multiple groups we used Kruskal-Wallis test with Dunn’s multiple comparison post-test.
Results
Cord blood Vδ2 T cells express the negative regulator PD1 for 28 days after the initial stimulation
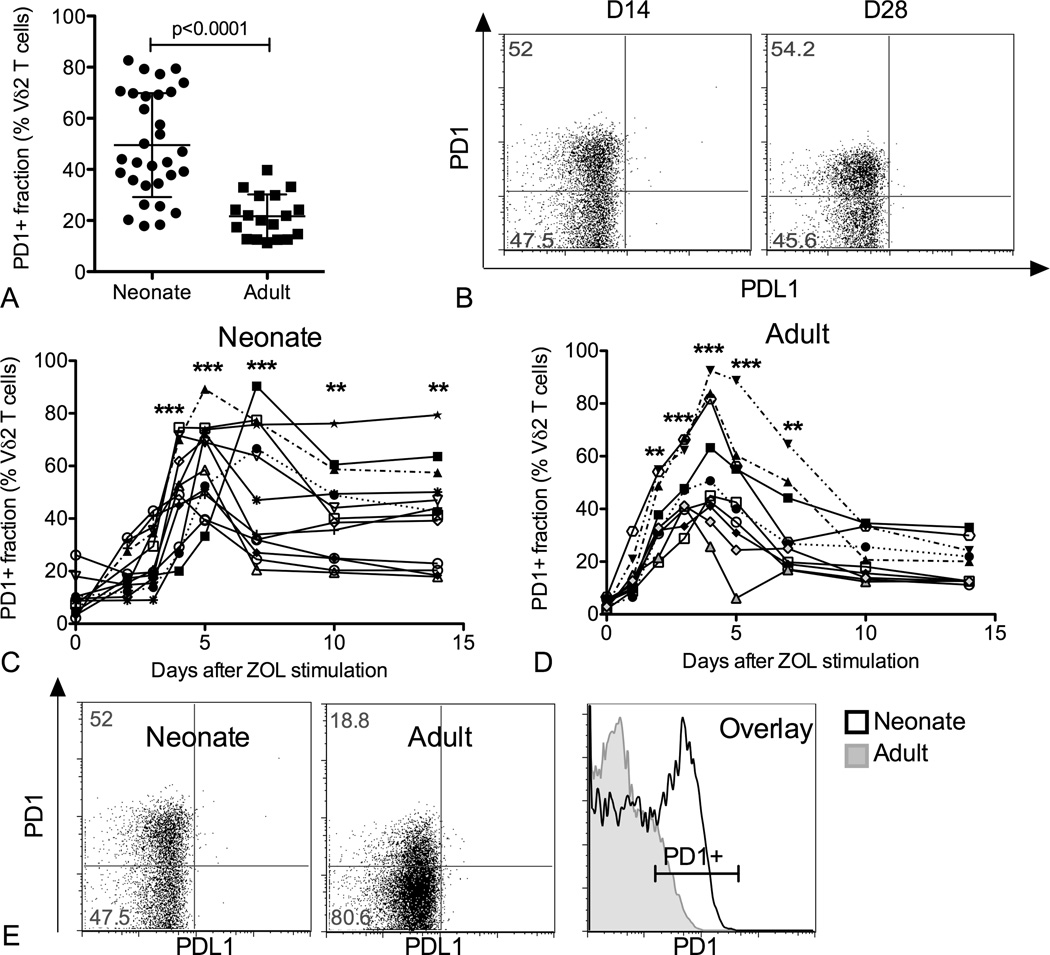
In order to identify inhibitory molecules responsible for modulating neonatal Vδ2 cell responses, we treated CBMC and PBMC with zoledronic acid (ZOL) plus IL-2 (100 U/ml) and compared Vδ2 T cell phenotype for neonatal and adult cultures 14 days after stimulation. While NKG2A was expressed on a relatively small fraction of Vδ2 T cells (<20% on average, data not shown), the immue checkpoint molecule PD1 was present on a large fraction of neonatal Vδ2 lymphocytes (49.5%±20.4) and a smaller fraction of adult Vδ2 lymphocytes (21.8%±8.5%) (Figure 1A). Importantly, PD1 remained on neonatal Vδ2 T cells up to 28 days after the initial triggering event and the proportions of PD1+ cells at 14 or 28 days post stimulation were remarkably similar (Figure 1B). There was no correlation between the proportion of PD1+ Vδ2 cells and the extent of neonatal Vδ2 T cell expansion (data not shown).
Figure 1.
Neonatal Vδ2 T cells are characterized by prolonged PD1 expression after activation. Cord blood mononuclear cells (CBMC) or peripheral blood mononuclear cells (PBMC) were treated with ZOL (0.5µM) plus IL-2 (100 U/ml) to stimulate and expand Vδ2 T cells. Expression of several co-stimulatory and inhibitory markers was analyzed over time. PD1 expression was monitored by flow cytometry at multiple time points (days 1, 2, 3, 4, 5, 7, 10, 14, 21, 28) after PBMC or CBMC stimulation. (A) The scatter plot shows individual values, mean and SD for the proportion of PD1+ Vδ2 T cells in each group 14 days after stimulation (N=31-19). Differences between medians were analyzed by Mann-Whitney U test. (B) The dot plots show the results for a representative CB specimen 14 and 28 days after activation (N=12). The line plots depict the time course for PD1 expression in (C) cord blood versus (D) adult Vδ2 T cells (N = 12 and 9, respectively). Each line represents an individual. Differences between time points were analyzed by Kruskal-Wallis test. ** p<0.01, *** p<0.001. (E) The dot plots show PD1 fluorescence intensity for a neonatal and an adult specimen of Vδ2 T cells 14 days after activation. The histogram overlays the PD1 fluorescence intensity profiles for the two specimens shown in the dot plots.
We then monitored PD1 in time course experiments to follow the expression kinetics. For both adult and neonatal specimens we observed small fractions of PD1+ Vδ2 T cells ex vivo The values for adults were lower than previously reported (34), probably due to our use of frozen specimens (35), different combination of fluorochromes and differences in instrumentation. For both groups PD1 expression peaked around 4–5 days after ZOL stimulation, reaching comparable proportions of positive cells, and declined between days 5 and 10 after activation (Figure 1C,D). After day 10 PD1 expression remained stable but the proportion of PD1+ cells was lower for adult than for neonatal Vδ2 cells. Also the MFI of PD1+ cells was lower among adult Vδ2 lymphocytes (Figure 1E) and decreased further between days 14 and 28 (data not shown). This suggested a delay in the down-modulation of PD1 in neonatal Vδ2 T cells that could be due to an active process (if PD1 gene expression is maintained) or a passive process (if PD1 protein lingers on the plasma membrane due to a slow turnover).
Consistent with a previous report (32), Vδ2 T cells also expressed PDL1 with peak levels occurring 4 days after activation followed by rapid down-modulation between days 5 and 7 (Supplemental Figure 1). However, because the antibody clone we used (29E2A3) does not bind to PDL1 when CD80 is concurrently expressed on the cell surface (36) and CD80 is expressed by Vδ2 T cells starting around day 3, we could not conclusively determine PDL1 expression kinetics in our system.
PD1 engagement concurrent with γδ TCR triggering inhibits neonatal Vδ2 T cell responses
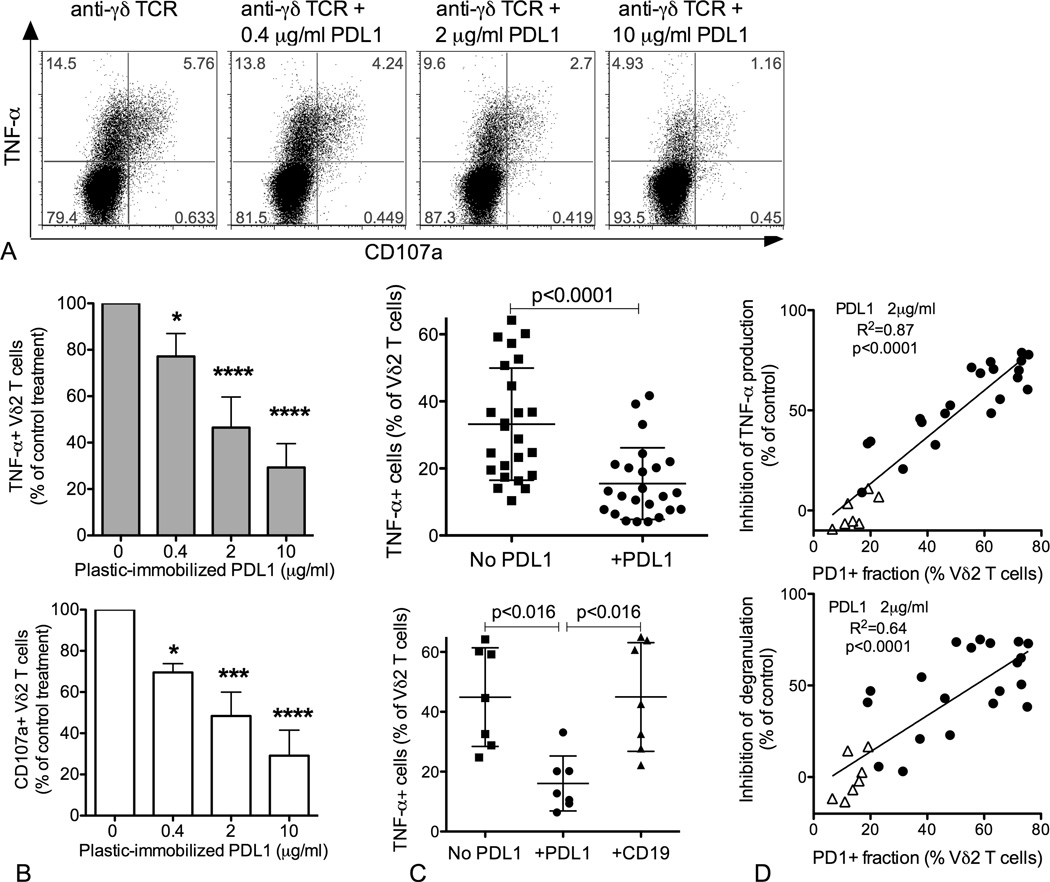
To test whether PD1 acts as a negative regulator of neonatal Vδ2 T cell responses, we assessed whether engaging PD1 during anti-γδ TCR restimulation would inhibit TNF-α production and cytotoxic granule mobilization. Plates were coated with anti-γδ TCR alone or in combination with three doses of human recombinant PDL1-Fc (0.4, 2, and 10µg/ml). The fraction of Vδ2 T cells that responded to anti-TCR stimulation by producing TNF-α or releasing granules (CD107a+) was typically less than 50% (Fig. 2A). In the presence of plastic-immobilized PDL1-Fc, both TNF-α production and granule mobilization were inhibited in a dose-dependent manner (Figure 2 A–B). After treating several samples with intermediate doses of PDL1-Fc, we observed a significant decrease in the fraction of TNF-α+ and CD107a+ Vδ2 T cells (Figure 2C, top panel, and data not shown). A subset of specimens were also stimulated with anti-γδ TCR in the presence of plastic-immobilized human recombinant CD19-Fc (2µg/ml), which served as an irrelevant Fc-tagged control protein. Consistent with a specific effect of PDL1 on TCR-mediated stimulation, CD19-Fc did not affect production of TNF-α (Figure 2C, bottom panel) or mobilization of cytotoxic granules (data not shown). Notably, the inhibition of TNF-α production and degranulation was directly proportional to the fraction of PD1+ Vδ2 T cells, with a strong positive correlation between this variable and the percent inhibition of TNF-α production or degranulation (Figure 2D, top and bottom panel respectively). In addition to 21 cord blood samples, we also tested PD1− mediated inhibition on 7 adult donors with a small fraction of PD1+ Vδ2 cells, and the results (shown by the triangles in Figure 2D) were consistent with the correlation observed for cord blood specimens.
Figure 2.
PD1 engagement concomitant with TCR stimulation inhibits TNF-α production and granule mobilization. CBMC were treated with ZOL (0.5µM) + IL-2 (100U/ml), then rested for 2 days with low IL-2 (10 U/ml). After 16 days of culture, Vδ2 T cells were re-stimulated with plastic-immobilized anti-γδ TCR antibody (2 µg/ml) in the absence or presence of plastic-immobilized human PDL1-Fc. Expression of TNF-α and CD107a was evaluated after a 6 hour stimulation in the presence of brefeldin A. (A) Dot plots show the effect of increasing doses of PDL1-Fc (0.4, 2, and 10 µg/ml) for a representative sample. (B) The bar graphs display the effect of increasing doses of PDL1-Fc (0.4, 2, and 10 µg/ml) on TNF-α production and CD107a mobilization, in the top and bottom panel respectively (N=5). Differences between doses were analyzed by ANOVA. * p<0.05, *** p<0.001, **** p<0.0001. (C) The top scatter plot shows individual values, mean and SD for the proportion of TNF-α+ Vδ2 T cells after anti-γδ TCR stimulation with or without a single dose of PDL1-Fc (2 µg/ml). For a subset of specimens (N=7, bottom panel), plastic immobilized human CD19-Fc was used as a negative control. D) The simple linear regression analysis shows a significant direct correlation between the proportion of PD1+ Vδ2 T cells and the extent of inhibition of TNF-α production (top panel) and granule mobilization (bottom panel) upon PD1 engagement. N= 21 for neonatal (circles) and 7 (triangles) for adult Vδ2 T cell cultures.
The PDL1 molecule used for these experiments was fused to a human Fc portion that could bind and signal through CD16 (FcγRIII). A sizable fraction of adult Vδ2 T cells express CD16 after expansion but the proportion of CD16+ cells among cord blood Vδ2 lymphocytes was relatively low (16.9% on average versus 42% for adults, Supplemental Figure 2A). This is consistent, with the result that CD19-Fc control protein did not cause an increase in the proportion of TNF-α+ or CD107+ neontal Vδ2 cells (Figure 2C, bottom panel). To test whether the binding of a Fc tagged protein to CD16 has a costimulatory effect for adult Vδ2 cells, a subset of adult specimens included in the inhibition assay (Figure 2C) were also restimulated in the presence of plastic immobilized CD19-Fc. We observed only a slight enhancement of TNF-α production and degranulation in the presence of CD19-Fc (10%–15% increase compared to the TCR treatment) for 2 adult Vδ2 cell specimens (Supplemental Figure 2B) and no effect for a third. This may indicate that six-hours are not enough to see a significant effect of CD16-mediated costimulation on cytokine production or degranulation
PD1 mRNA levels are elevated in neonatal Vδ2 T cells up to 28 days after activation
Remarkable stability of the PD1+ subset between 14 and 28 days after stimulation may have been due to slow turnover of PD1 protein or to active PD1 gene transcription. We monitored PD1 mRNA levels 14 and 28 days after activation in Vδ2 cells sorted from CBMC or PBMC cultures. We used the same tonsil mononuclear cell specimen as a reference in all RT-PCR assays in order to normalize for variation between runs, and used the comparative method (ΔΔCt) for analyses (33). The levels of PD1 mRNA were higher in neonatal than in adult Vδ2 cells at both time points. Although neonatal mRNA levels declined between 14 and 28 days, differences between time points were not statistically significant (Figure 3).
Figure 3.
PD1 mRNA levels are higher in neonatal than in adult Vδ2 T cells both 14 and 28 days after stimulation. 14 and 28 days after ZOL stimulation, Vδ2 lymphocytes from CBMC or PBMC cultures were sorted directly into Trizol for mRNA extraction; PD1 mRNA levels were analyzed using RT-PCR (N=8 for both adult and neonatal Vδ2 samples). The box plot shows 25th, 50th, 75th percentiles and means. Whiskers are drawn according to the Tukey method. The difference between means was analyzed using an unpaired Student's t-test, differences between medians were analyzed using Mann-Whitney U test or Wilcoxon matched pairs signed-rank test for unpaired and paired groups respectively.
PD1 expression in neonatal Vδ2 cells is associated with DNA hypo-methylation in the conserved region C of the PD1 promoter
PD1 expression in CD8 T cells is regulated by DNA methylation during responses to viral infections (37). Moreover, high mRNA levels are associated with an open chromatin structure and DNA hypo-methylation. In order to test whether DNA methylation is associated with differences in PD1 expression in our system, we added an optimized dose of hydralazine (an inhibitor of the DNA methyl-transferases) to CBMC cultures from days 7 to 10, and on day 10 we monitored PD1 levels. Hydralazine induced a small but significant increase in the fraction of neonatal PD1+ Vδ2 T cells compared to control treatment (ZOL only) (Figure 4A).
Figure 4.
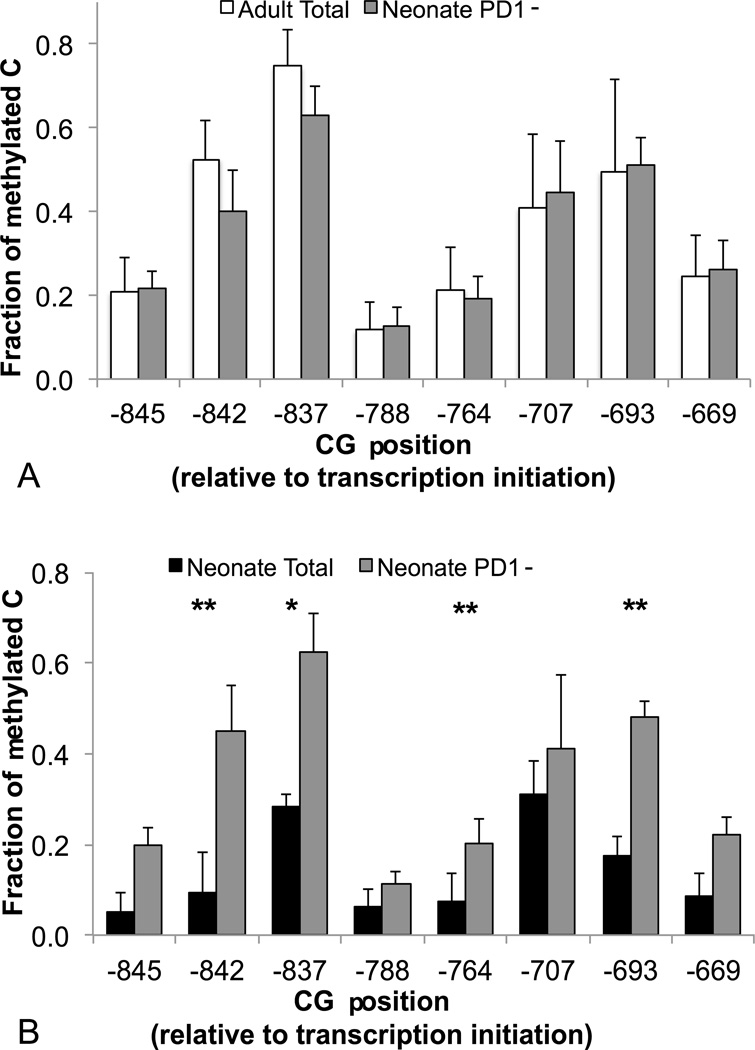
Low DNA methylation at the PD1 promoter is associated with PD1 expression in Vδ2 T cells. (A) Seven days after stimulation, hydralazine was added to one well of CBMC culture (15µM), while a second well was left untreated as a negative control. PD1 expression by Vδ2 T cells was monitored 3 days after hydralazine treatment (N=9). The scatter plot shows paired values for individual specimens. Two weeks after stimulation, Vδ2 cells were sorted from PBMC or CBMC (> 97% purity) and their genomic DNA was used for methylation analysis. After bisulfite conversion, an amplicon that spans the whole regulatory region CR-C (Conserved Region C, 550 bp) or a shorter 297 bp amplicon at the proximal end of CR-C (boxed in panel B) in the PD1 promoter were generated by PCR. 36–44 amplicons were analyzed for each sample and each time point by Sanger sequencing. (B) The bar graph shows, for each CG pair in the CR-C region, the fraction of methylated cytosine averaged for 9 adults and 12 neonates. Error bars represent SD. Only p<0.001 (***) and p<0.0001 (****) are shown in the chart. (C) The plot shows the inverse correlation between methylation levels at positions −837 and −842 and the proportion of neonatal PD1+ Vδ2 T cells. (D) For 5 CB samples, promoter methylation was monitored 14 and 28 days after stimulation. The methylation levels for the region between −845 and −669 are shown in the bar graph as means + SD. Differences between means were analyzed using unpaired or paired Student’s t-test.
In order to confirm that DNA methylation is associated with PD1 expression levels, we performed PD1 promoter methylation analysis using bisulfite conversion followed by Sanger sequencing. We selected primers for the conserved region C (CR-C), which had been shown to be involved in PD1 regulation in CD8 T cells (37), and we sequenced 36–46 clones per donor, for 12 neonates and 9 adults. We sorted the population of interest based on the expression of the Vδ2 chain, since after expansion with ZOL virtually all (97% on average) Vδ2+ cells also express the Vγ9 chain (data not shown). Using sorted Vδ2 T cells (>95% pure), we found that the PD1 CR-C was hypo-methylated in neonatal cells compared to adult cells (Figure 4B). We observed marked differences in methylation levels between adult and neonatal cells for the portion of the CR-C proximal to the transcription initiation site. Remarkably, there were significant inverse correlations between methylation levels at two adjacent CG pairs (positions −842 and −837) and the proportion of PD1+ Vδ2 T cells in CBMC cultures (R2=0.72, p=0.0005; R2=0.62, p=0.0023, respectively, Figure 4C). The average methylation levels for positions −842 through −669 were significantly associated with the proportion of PD1+ Vδ2 T cells (Supplemental Figure 3), indicating that the whole proximal portion of CR-C may be involved in the regulation of PD1 expression.
The conserved region C of the PD1 promoter remains hypo-methylated in neonatal Vδ2 cells up to 28 days after activation
We studied DNA methylation on days 14 and 28 in five cord blood specimens. Our analyses concentrated on the proximal CR-C (Figure 4D) in an effort to explain why PD1 levels are stable up to 28 days after stimulation. We observed no changes in methylation levels between days 14 and 28 for any CG pairs in the CR-C, suggesting that delayed re-methylation is involved in the prolonged expression of PD1.
CR-C methylation levels are higher in the PD1− fraction of neonatal Vδ2 cells
To prove that higher methylation levels in adult Vδ2 T cells are due to the predominance of PD1− cells in these specimens, we compared adult total Vδ2 cells to neonatal Vδ2 PD1− cells (typical results for the sorting are shown in Supplemental Figure 4). Analysis of the PD1− fraction for five CB specimens showed comparable promoter methylation levels in neonatal Vδ2 PD1− cells and adult total Vδ2 cells, with the exception of position −837 that was less methylated in neonatal cells (Figure 5A).
Figure 5.
The PD1− fraction of neonatal Vδ2 T cells displays higher methylation levels at the PD1 promoter than total neonatal Vδ2 T cells. PD1 promoter methylation levels were compared for PD1− neonatal Vδ2 T cells and (A) total adult Vδ2 cells (N=6 for neonatal and N=9 for adult Vδ2 cells) or (B) paired total neonatal Vδ2 T cells (N=3). Differences between means were analyzed using unpaired or paired Student’s t-test. * p<0.05, ** p<0.01.
For some of the cord blood specimens included in Figure 4, we attempted to perform methylation analysis for both PD1+ and PD1− Vδ2 T cells, but this experiment posed serious technical issues. Vδ2 T cells sorted on day 14 undergo apoptosis quickly. Sorting based on both Vδ2 and PD1 expression increased processing time and rendered PD1+ cells more susceptible to apoptosis, resulting in large proportions of dead cells in the PD1+ fractions. For this reason we compared the PD1− fraction to total Vδ2 T cells containing various proportions of PD1+ cells. As expected, sequences with higher CR-C methylation were enriched in the PD1− fraction compared to the total population (Figure 5B).
T-bet is present at similar levels in neonatal and adult Vδ2 T cells
T-bet is known to inhibit PD1 expression in the context of chronic TCR stimulation in CD8 T cells (38). However, T-bet is already expressed in fetal Vδ2 T cells at high levels (18), so it is unlikely that varying levels of this transcription factor would determine differential PD1 expression. In order to test our hypothesis, we monitored T-bet protein and mRNA levels in Vδ2 T cells. Flow cytometry showed that all Vδ2 T lymphocytes were T-bet+ after expansion, with similar amounts of protein in both adult and neonatal cells (as suggested by comparable MFI, Figure 6A). Sorted neonatal Vδ2 cells tended to have lower T-bet mRNA levels compared to their adult counterparts, but the difference was not statistically significant (Figure 6B). While PD1 promoter occupancy studies would be required to completely rule out T-bet involvement in PD1 regulation in this system, these results support our hypothesis and confirm previous reports that fetal Vδ2 T cells are poised for Th1 responses (18, 20).
Figure 6.
Neonatal and adult Vδ2 T cells express comparable levels of T-bet protein and mRNA. (A) The expression levels of T-bet for adult (N=4) and neonatal specimens (N=3) were compared by flow cytometry 14 days after ZOL stimulation. The dot plots show the expression of T-bet versus PD1 for Vδ2 T cells. The histogram overlays T-bet fluorescence intensity profiles for adult (dotted line) and neonatal (solid line) Vδ2 T cells. (B) mRNA levels were analyzed using the comparative method for sorted neonatal and adult Vδ2 T cells (N=8 and 10 respectively). The box plot shows 25th, 50th, 75th percentiles and means. Whiskers are drawn according to the Tukey method. The difference between means was analyzed using an unpaired Student’s t-test.
Discussion
In the current study we showed that PD1 is expressed by neonatal Vδ2 T cells and acts as a negative regulator of responses triggered by γδ TCR engagement. Lower methylation levels in the PD1 promoter region were associated with larger fractions of neonatal PD1+ Vδ2 lymphocytes and we observed an inverse correlation between PD1 promoter methylation levels and proportions of neonatal PD1+ Vδ2 T cells. In neonatal Vδ2 lymphocytes, the PD1 promoter remained hypo-methylated up to 28 days after stimulation. Compared to the entire Vδ2 population, the PD1− subset of neonatal Vδ2 lymphocytes displayed higher methylation levels that were similar to methylation levels observed for adult Vδ2 cells.
Our results support a model where DNA methylation is key to regulating PD1 expression in Vδ2 T cells. However, based on results obtained for CD8 T cells, we anticipate that other mechanisms also impact PD1 regulation. Both intrinsic and extrinsic factors likely contribute to differences between adult and neonatal Vδ2 T cells. Intrinsic factors include the pool of positive and negative regulators of PD-1 transcription (38–42) that may differ in adult versus neonatal cells. Extrinsic factors include higher expression of PDL1 in PBMC compared to CBMC cultures, which would drive negative selection of PD1+ cells (by cell cycle arrest, apoptosis or a combination of both) and bias the proliferating Vδ2 lymphocytes towards a PD1− population. Results published in a previous report (32), and consistent with our current findings, argue against this possibility, but do not disprove it conclusively. PD1+ Vδ2 cells, sorted three days after activation and cultured as a purified population, progressively down-modulated PD1 expression (32). PD1 down-regulation did not require the presence of any other cell type (32). Nevertheless, Vδ2 T cells express PDL1, which may contribute to the emergence of a PD1− population by providing selective advantage to the subset of cells that down-regulated PD1 first. We monitored PDL1 over time in PBMC cultures, observing kinetics consistent with Iwasaky’s study (32). However, for our analyses we used the anti-PDL1 monoclonal antibody clone 29E.2A3, which does not bind PDL1 when CD80 is also present on the cell surface (36). Since Vδ2 lymphocytes in our cultures express both CD80 and PDL1, possibly at the same time, we may have underestimated the expression of PDL1, thus in future studies we will confirm our results with anti-PDL1 clone 5H1 (36), a non-commercial antibody established by Dr. Chen’s laboratory (43).
Our results are consistent with the hypothesis that prolonged PD1 expression allows fine-tuning of Vδ2 T cell responses in the perinatal period. PD1 expression in our neonatal cultures is maintained by Vδ2 cells for at least 28 days after treating with a low dose of zoledronic acid, in the absence of chronic or repeated stimulation. It is unlikely that PD1 in this context represents an exhaustion marker as it does in the context of hematologic malignancies (31). If PD1 in our system is expressed by highly activated cells similar to what was observed by Iwasaki and colleagues (32), our results point to a model where neonatal Vδ2 lymphocytes maintain high activation levels for longer intervals compared to their adult counterparts. Thus, PD1 may function as a rheostat that maintains fetal/neonatal Vδ2 T cell responses within safe limits. Vδ2 T cell are poised to mount rapid Th1 responses even before birth (20), they are sensitive to sub-nanomolar concentrations of microbial phosphoantigens (44) and can sustain their proliferation using myeloid-derived cytokines instead of IL-2 (18, 21, 22). These characteristics make them an ideal first line of defense against pathogens during the first few months of life. Without an effective control mechanism, fetal Vδ2 cells might become engaged in a positive pro-inflammatory feedback loop (particularly in the context of a chronic/prolonged infection) that would be deleterious for the fetus. Prolonged PD1 expression provides a way to modulate Vδ2 T cell function that depends on the microenvironment; in particular, the levels of PDL1 in the system could fine-tune the extent of PD1 engagement. In the context of prenatal Vδ2 lymphocyte stimulation, the effects of PD1 engagement may range from modest inhibition of cytokine production with persistence of the PD1+ subset (in a mildly inflammatory environment with low PDL1 levels) to induction of apoptosis, deletion of the most activated clones and reduction of Vδ2 T cell frequency (in highly inflammatory environments that induce robust PDL1 up-regulation (45–48)). Either persistence of PD1 expression after birth or deletion of the most responsive clones could attenuate Vδ2 lymphocyte responses to pathogens shortly after birth, and hinder their role in the response to BCG vaccination. Activation of Vδ2 lymphocytes in a mildly pro-inflammatory environment might help explain our previous observations that in utero exposure to P. falciparum antigens during placental malaria negatively affects neonatal Vδ2 T cell responses to antigens without causing a significant change in the proportion of these cells in cord blood (49). On the other hand, activation of Vδ2 T cells in a highly pro-inflammatory environment with consequent PD1− mediated apoptosis may be responsible for recent results showing that recurrent clinical malaria in Ugandan children is associated with a progressive loss of Vδ2 T cells (50).
To understand the role of PD1 in Vδ2 lymphocyte regulation during perinatal life we need to study neonates exposed in utero to pathogens that elicit Vδ2 T cells. In this context, a number of factors could introduce bias in the analyses by affecting PD1/PDL1 expression with possible functional consequences. Gestational age, chorioamnionitis or even modality of delivery (delivery with labor versus elective C-section) are potential confounders. The women we enrolled in this study all delivered at term, did not show signs of chorioamnionitis and did not undergo elective C-section, but we do not have this type of information for the commercial specimens obtained from Allcells. Though we cannot exclude that we introduced a bias in our study, this seems unlikely considering that the Allcells specimens were not significantly different for any of the parameters analyzed from the samples we collected. The importance of factors related to the genetic background versus the microbial environment experienced by the mother should also be addressed. Our current investigation, including neonates born to healthy women from two different geographic regions (sub-Saharan Africa and North America), did not reveal significant differences between the groups in terms of PD1 expression ex vivo or after expansion. However, the power of our analysis was not adequate to provide a conclusive answer, since this was not the primary objective of the study. The factors listed above will require specific evaluation in future studies. Finally it would be interesting to investigate the impact of microbial colonization in the first few weeks after birth on PDL1 expression as previously described in a murine model (51).
We hypothesize that PD1 regulation is part of a more complex transcriptional and epigenetic program that translates into Vδ2 T cell properties best adapted for perinatal life. We seek to unravel this program and understand the mechanisms regulating Vδ2 T cell function in the perinatal period to better exploit their potential for improving responses to pathogens, and possibly vaccines, in early life.
Supplementary Material
Acknowledgments
We thank the Department of Obstetrics, Gynecology and Reproductive Services, University of Maryland Medical Center, and the Ndirande Health Center, Blantyre, Malawi for assistance with cord blood collection. We thank Dr. Karl B. Seydel and the Molecular Core at the University of Malawi, College of Medicine, in Blantyre, Malawi for the outstanding technical support. We are very grateful to Drs. Yutaka Tagaya and Juan Carlos Zapata at the IHV Flow Cytometry Core for their support with Vδ2 T cell sorting.
This work was supported by the U.S. Public Health Service grant AI104702 (CC).
Glossary
- PD1
Programmed Death 1
- BCG
Bacille Calmette-Guérin
- CBMC
Cord blood mononuclear cells
- ZOL
Zoledronic acid
References
- 1.Burt TD. Fetal regulatory T cells and peripheral immune tolerance in utero: implications for development and disease. American journal of reproductive immunology. 2013;69:346–358. doi: 10.1111/aji.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30:585–591. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adkins B. Neonatal immunology: responses to pathogenic microorganisms and epigenetics reveal an "immunodiverse" developmental state. Immunologic research. 2013;57:246–257. doi: 10.1007/s12026-013-8439-2. [DOI] [PubMed] [Google Scholar]
- 4.Dowling DJ, Levy O. Ontogeny of early life immunity. Trends Immunol. 2014;35:299–310. doi: 10.1016/j.it.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kemp MW. Preterm birth, intrauterine infection, and fetal inflammation. Frontiers in immunology. 2014;5:574. doi: 10.3389/fimmu.2014.00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elahi S, Ertelt JM, Kinder JM, Jiang TT, Zhang X, Xin L, Chaturvedi V, Strong BS, Qualls JE, Steinbrecher KA, Kalfa TA, Shaaban AF, Way SS. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature. 2013;504:158–162. doi: 10.1038/nature12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.PrabhuDas M, Adkins B, Gans H, King C, Levy O, Ramilo O, Siegrist CA. Challenges in infant immunity: implications for responses to infection and vaccines. Nat Immunol. 2011;12:189–194. doi: 10.1038/ni0311-189. [DOI] [PubMed] [Google Scholar]
- 8.Wood N, Siegrist CA. Neonatal immunization: where do we stand? Current opinion in infectious diseases. 2011;24:190–195. doi: 10.1097/QCO.0b013e328345d563. [DOI] [PubMed] [Google Scholar]
- 9.Lang F, Peyrat MA, Constant P, Davodeau F, David-Ameline J, Poquet Y, Vie H, Fournie JJ, Bonneville M. Early activation of human V gamma 9V delta 2 T cell broad cytotoxicity and TNF production by nonpeptidic mycobacterial ligands. J Immunol. 1995;154:5986–5994. [PubMed] [Google Scholar]
- 10.Barnes PF, Abrams JS, Lu S, Sieling PA, Rea TH, Modlin RL. Patterns of cytokine production by mycobacterium-reactive human T-cell clones. Infect Immun. 1993;61:197–203. doi: 10.1128/iai.61.1.197-203.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Follows GA, Munk ME, Gatrill AJ, Conradt P, Kaufmann SH. Gamma interferon and interleukin 2, but not interleukin 4, are detectable in gamma/delta T-cell cultures after activation with bacteria. Infect Immun. 1992;60:1229–1231. doi: 10.1128/iai.60.3.1229-1231.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cairo C, Surendran N, Harris KM, Mazan-Mamczarz K, Sakoda Y, Diaz-Mendez F, Tamada K, Gartenhaus RB, Mann DL, Pauza CD. Vgamma2Vdelta2 T cell Costimulation Increases NK cell Killing of Monocyte-derived Dendritic Cells. Immunology. 2014 doi: 10.1111/imm.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maniar A, Zhang X, Lin W, Gastman BR, Pauza CD, Strome SE, Chapoval AI. Human gammadelta T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood. 2010;116:1726–1733. doi: 10.1182/blood-2009-07-234211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conti L, Casetti R, Cardone M, Varano B, Martino A, Belardelli F, Poccia F, Gessani S. Reciprocal activating interaction between dendritic cells and pamidronate-stimulated gammadelta T cells: role of CD86 and inflammatory cytokines. J Immunol. 2005;174:252–260. doi: 10.4049/jimmunol.174.1.252. [DOI] [PubMed] [Google Scholar]
- 15.Martino A, Casetti R, D'Alessandri A, Sacchi A, Poccia F. Complementary function of gamma delta T-lymphocytes and dendritic cells in the response to isopentenyl-pyrophosphate and lipopolysaccharide antigens. J Clin Immunol. 2005;25:230–237. doi: 10.1007/s10875-005-4080-8. [DOI] [PubMed] [Google Scholar]
- 16.Dunne MR, Madrigal-Estebas L, Tobin LM, Doherty DG. (E)-4-hydroxy-3-methyl-but-2 enyl pyrophosphate-stimulated Vgamma9Vdelta2 T cells possess T helper type 1-promoting adjuvant activity for human monocyte-derived dendritic cells. Cancer Immunol Immunother. 2010;59:1109–1120. doi: 10.1007/s00262-010-0839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cairo C, Mancino G, Cappelli G, Pauza CD, Galli E, Brunetti E, Colizzi V. Vdelta2 T-lymphocyte responses in cord blood samples from Italy and Cote d'Ivoire. Immunology. 2008;124:380–387. doi: 10.1111/j.1365-2567.2007.02784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moens E, Brouwer M, Dimova T, Goldman M, Willems F, Vermijlen D. IL-23R and TCR signaling drives the generation of neonatal V{gamma}9V{delta}2 T cells expressing high levels of cytotoxic mediators and producing IFN-{gamma} and IL-17. J Leukoc Biol. 2011;89:743–752. doi: 10.1189/jlb.0910501. [DOI] [PubMed] [Google Scholar]
- 19.Morita CT, Parker CM, Brenner MB, Band H. TCR usage and functional capabilities of human gamma delta T cells at birth. J Immunol. 1994;153:3979–3988. [PubMed] [Google Scholar]
- 20.Dimova T, Brouwer M, Gosselin F, Tassignon J, Leo O, Donner C, Marchant A, Vermijlen D. Effector Vgamma9Vdelta2 T cells dominate the human fetal gammadelta T-cell repertoire. Proc Natl Acad Sci U S A. 2015;112:E556–E565. doi: 10.1073/pnas.1412058112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cairo C, Sagnia B, Cappelli G, Colizzi V, Leke RG, Leke RJ, Pauza CD. Human cord blood gammadelta T cells expressing public Vgamma2 chains dominate the response to bisphosphonate plus interleukin-15. Immunology. 2013;138:346–360. doi: 10.1111/imm.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia VE, Jullien D, Song M, Uyemura K, Shuai K, Morita CT, Modlin RL. IL-15 enhances the response of human gamma delta T cells to nonpeptide [correction of nonpetide] microbial antigens. Journal of immunology. 1998;160:4322–4329. [PubMed] [Google Scholar]
- 23.Mazzola TN, Da Silva MT, Moreno YM, Lima SC, Carniel EF, Morcillo AM, Antonio MA, Zanolli ML, Netto AA, Blotta MH, Raw I, Vilela MM. Robust gammadelta+ T cell expansion in infants immunized at birth with BCG vaccine. Vaccine. 2007;25:6313–6320. doi: 10.1016/j.vaccine.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 24.Tastan Y, Arvas A, Demir G, Alikasifoglu M, Gur E, Kiray E. Influence of Bacillus Calmette-Guerin vaccination at birth and 2 months old age on the peripheral blood T-cell subpopulations [gamma/delta and alpha-beta T cell] Pediatr Allergy Immunol. 2005;16:624–629. doi: 10.1111/j.1399-3038.2005.00329.x. [DOI] [PubMed] [Google Scholar]
- 25.D'Addio F, Riella LV, Mfarrej BG, Chabtini L, Adams LT, Yeung M, Yagita H, Azuma M, Sayegh MH, Guleria I. The link between the PDL1 costimulatory pathway and Th17 in fetomaternal tolerance. J Immunol. 2011;187:4530–4541. doi: 10.4049/jimmunol.1002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guleria I, Khosroshahi A, Ansari MJ, Habicht A, Azuma M, Yagita H, Noelle RJ, Coyle A, Mellor AL, Khoury SJ, Sayegh MH. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J Exp Med. 2005;202:231–237. doi: 10.1084/jem.20050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Habicht A, Dada S, Jurewicz M, Fife BT, Yagita H, Azuma M, Sayegh MH, Guleria I. A link between PDL1 and T regulatory cells in fetomaternal tolerance. J Immunol. 2007;179:5211–5219. doi: 10.4049/jimmunol.179.8.5211. [DOI] [PubMed] [Google Scholar]
- 28.Riella LV, Dada S, Chabtini L, Smith B, Huang L, Dakle P, Mfarrej B, D'Addio F, Adams LT, Kochupurakkal N, Vergani A, Fiorina P, Mellor AL, Sharpe AH, Yagita H, Guleria I. B7h (ICOS-L) maintains tolerance at the fetomaternal interface. Am J Pathol. 2013;182:2204–2213. doi: 10.1016/j.ajpath.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tripathi S, Guleria I. Role of PD1/PDL1 pathway, and TH17 and treg cells in maternal tolerance to the fetus. Biomedical journal. 2015;38:25–31. doi: 10.4103/2319-4170.143511. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105:9331–9336. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castella B, Foglietta M, Sciancalepore P, Rigoni M, Coscia M, Griggio V, Vitale C, Ferracini R, Saraci E, Omede P, Riganti C, Palumbo A, Boccadoro M, Massaia M. Anergic bone marrow Vgamma9Vdelta2 T cells as early and long-lasting markers of PD-1-targetable microenvironment-induced immune suppression in human myeloma. Oncoimmunology. 2015;4:e1047580. doi: 10.1080/2162402X.2015.1047580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwasaki M, Tanaka Y, Kobayashi H, Murata-Hirai K, Miyabe H, Sugie T, Toi M, Minato N. Expression and function of PD-1 in human gammadelta T cells that recognize phosphoantigens. Eur J Immunol. 2011;41:345–355. doi: 10.1002/eji.201040959. [DOI] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Gertner-Dardenne J, Fauriat C, Orlanducci F, Thibult ML, Pastor S, Fitzgibbon J, Bouabdallah R, Xerri L, Olive D. The co-receptor BTLA negatively regulates human Vgamma9Vdelta2 T-cell proliferation: a potential way of immune escape for lymphoma cells. Blood. 2013;122:922–931. doi: 10.1182/blood-2012-11-464685. [DOI] [PubMed] [Google Scholar]
- 35.Campbell DE, Tustin NB, Riedel E, Tustin R, 3rd, Taylor J, Murray J, Douglas SD. Cryopreservation decreases receptor PD-1 and ligand PD-L1 coinhibitory expression on peripheral blood mononuclear cell-derived T cells and monocytes. Clin Vaccine Immunol. 2009;16:1648–1653. doi: 10.1128/CVI.00259-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haile ST, Dalal SP, Clements V, Tamada K, Ostrand-Rosenberg S. Soluble CD80 restores T cell activation and overcomes tumor cell programmed death ligand 1-mediated immune suppression. J Immunol. 2013;191:2829–2836. doi: 10.4049/jimmunol.1202777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Youngblood B, Oestreich KJ, Ha SJ, Duraiswamy J, Akondy RS, West EE, Wei Z, Lu P, Austin JW, Riley JL, Boss JM, Ahmed R. Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8(+) T cells. Immunity. 2011;35:400–412. doi: 10.1016/j.immuni.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kao C, Oestreich KJ, Paley MA, Crawford A, Angelosanto JM, Ali MA, Intlekofer AM, Boss JM, Reiner SL, Weinmann AS, Wherry EJ. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol. 2011;12:663–671. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu P, Youngblood BA, Austin JW, Mohammed AU, Butler R, Ahmed R, Boss JM. Blimp-1 represses CD8 T cell expression of PD-1 using a feed-forward transcriptional circuit during acute viral infection. J Exp Med. 2014;211:515–527. doi: 10.1084/jem.20130208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathieu M, Cotta-Grand N, Daudelin JF, Thebault P, Labrecque N. Notch signaling regulates PD-1 expression during CD8(+) T-cell activation. Immunol Cell Biol. 2013;91:82–88. doi: 10.1038/icb.2012.53. [DOI] [PubMed] [Google Scholar]
- 41.Oestreich KJ, Yoon H, Ahmed R, Boss JM. NFATc1 regulates PD-1 expression upon T cell activation. J Immunol. 2008;181:4832–4839. doi: 10.4049/jimmunol.181.7.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park B, Chattergoon M, Pan F, Pardoll D, Cox A. TGF-b1 enhances T cell PD-1 expression through a Smad3-dependant increase in transcription. (IRM4P.499) The Journal of Immunology. 2014;192:S61.66. [Google Scholar]
- 43.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nature medicine. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 44.Eberl M, Hintz M, Reichenberg A, Kollas AK, Wiesner J, Jomaa H. Microbial isoprenoid biosynthesis and human gammadelta T cell activation. FEBS Lett. 2003;544:4–10. doi: 10.1016/s0014-5793(03)00483-6. [DOI] [PubMed] [Google Scholar]
- 45.de Kleijn S, Langereis JD, Leentjens J, Kox M, Netea MG, Koenderman L, Ferwerda G, Pickkers P, Hermans PW. IFN-gamma-stimulated neutrophils suppress lymphocyte proliferation through expression of PD-L1. PLoS One. 2013;8:e72249. doi: 10.1371/journal.pone.0072249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin P, Zhao Y, Liu H, Chen J, Ren J, Jin J, Bedognetti D, Liu S, Wang E, Marincola F, Stroncek D. Interferon-gamma and Tumor Necrosis Factor-alpha Polarize Bone Marrow Stromal Cells Uniformly to a Th1 Phenotype. Sci Rep. 2016;6:26345. doi: 10.1038/srep26345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ou JN, Wiedeman AE, Stevens AM. TNF-alpha and TGF-beta counter-regulate PD-L1 expression on monocytes in systemic lupus erythematosus. Sci Rep. 2012;2:295. doi: 10.1038/srep00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wan B, Nie H, Liu A, Feng G, He D, Xu R, Zhang Q, Dong C, Zhang JZ. Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J Immunol. 2006;177:8844–8850. doi: 10.4049/jimmunol.177.12.8844. [DOI] [PubMed] [Google Scholar]
- 49.Cairo C, Longinaker N, Cappelli G, Leke RG, Ondo MM, Djokam R, Fogako J, Leke RJ, Sagnia B, Sosso S, Colizzi V, Pauza CD. Cord blood Vgamma2Vdelta2 T cells provide a molecular marker for the influence of pregnancy-associated malaria on neonatal immunity. J Infect Dis. 2014;209:1653–1662. doi: 10.1093/infdis/jit802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farrington LA, Jagannathan P, McIntyre TI, Vance HM, Bowen K, Boyle MJ, Nankya F, Wamala S, Auma A, Nalubega M, Sikyomu E, Naluwu K, Bigira V, Kapisi J, Dorsey G, Kamya MR, Feeney ME. Frequent malaria drives progressive Vdelta2 T cell loss, dysfunction, and CD16 upregulation during early childhood. J Infect Dis. 2015 doi: 10.1093/infdis/jiv600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gollwitzer ES, Saglani S, Trompette A, Yadava K, Sherburn R, McCoy KD, Nicod LP, Lloyd CM, Marsland BJ. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nature medicine. 2014;20:642–647. doi: 10.1038/nm.3568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.