Abstract
Hypoxic and adenosine rich tumor microenvironments represent an important barrier that must be overcome to enable T and NK cells to reject tumors. The A2A adenosine receptor (A2AR) on activated immune cells was identified as a critical and non-redundant mediator of physiological immunosuppression. Observations showing that tumor-protecting A2AR also suppress and redirect the anti-tumor immune response pointed to the importance of inhibiting this pathway to improve cancer immunotherapy. We advocated i) blocking immunosuppressive Adenosine-A2AR-cAMP–mediated intracellular signaling by A2AR antagonists and ii) weakening Hypoxia-HIF-1α-mediated accumulation of extracellular adenosine by oxygenation agents that also inhibit CD39/CD73 adenosine-generating enzymes. In view of commencing clinical trials of synthetic A2AR antagonists in combination with cancer immunotherapies, we discuss their promise and exclusion criteria.
Introduction
At the center of the current excitement surrounding cancer immunotherapy are spectacular examples of tumor rejection in some patients by T cell-based immunotherapies and immune-checkpoint inhibitors [1]. The introduction of CTLA-4 and PD-1 blocking antibodies as FDA-approved drugs that target not the tumor, but cells of the immune system, represents a new approach in the development of cancer therapies.
However, there is room to further improve clinical outcomes. The hypoxic and adenosine rich “Hypoxia-A2-Adenosinergic” tumor microenvironment (TME) (Fig. 1) is now considered an important barrier that must be overcome in order to enable tumor-reactive T cells and Natural Killer (NK) cells to infiltrate and kill tumors. This is because anti-tumor T cells are still inhibited by other immunosuppressive mechanisms even after blockade of CTLA-4 and PD1. Currently, several pharmaceutical companies, including Novartis, AstraZeneca and others are preparing for clinical trials where immunotherapeutic drugs such as anti-PD1 monoclonal antibody (mAb), would be combined with synthetic A2A adenosine receptor (A2AR) antagonists to weaken the Hypoxia-A2-Adenosinergic immunosuppression.
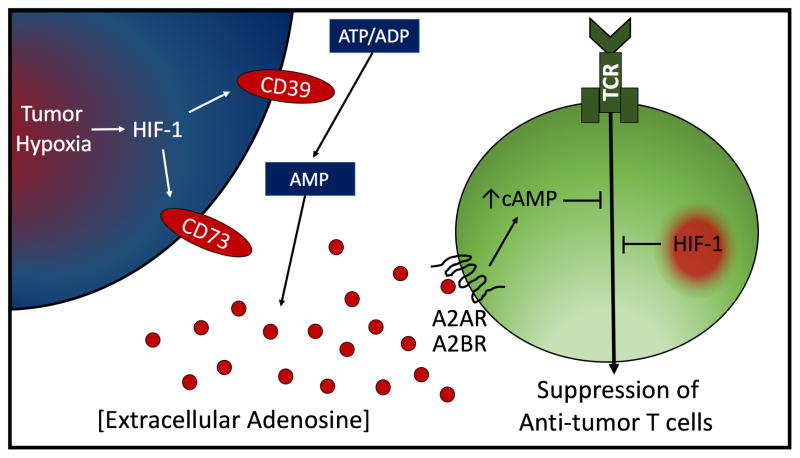
Figure 1. Intratumoral Hypoxia→HIF-1α driven and A2A/A2B Adenosine Receptor-mediated suppression of anti-tumor T cells.
Shown are the HIF-1α regulated ecto-enzymes CD39/CD73 which act in tandem to generate extracellular adenosine. Adenosine triggers the accumulation of immunosuppressive intracellular cAMP by signaling through high affinity A2AR and low affinity A2BR. HIF-1α is also shown to suppress cells of the adaptive immune system [20].
This clinical focus on A2AR puts a premium on a better understanding of how A2AR functions in the regulation of the immune response, including the anti-tumor immune response. We will summarize the studies of the anti-hypoxia A2-adenosinergic co-adjuvants (Fig. 2), which target both anti-tumor immune cells and the TME. Blockade of this pathway can prevent the inhibition of anti-tumor T and NK cells by the weakening of the Adenosine-A2AR signaling (Fig. 1 and Fig. 2).
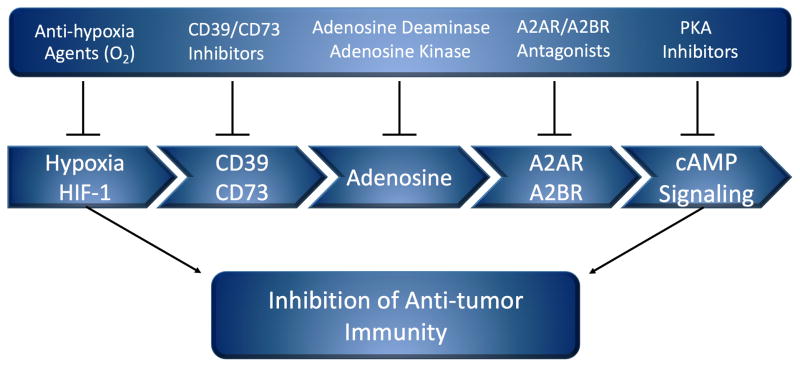
Figure 2. Anti-hypoxia A2-adenosinergic coadjuvants to enable the effector functions of anti-tumor T cells.
Shown are the individual classes of drugs that inhibit the upstream and downstream stages of Hypoxia-HIF-1α driven and A2A/A2B Adenosine Receptor-mediated suppression of anti-tumor T cells. Under consideration for clinical trials are i) anti-hypoxia treatments such as oxygenation agents ii) inhibitors of CD39 and/or CD73 to prevent the generation of extracellular adenosine iii) enzymes that degrade extracellular adenosine and iv) A2AR antagonists.
Investigations of blocking A2AR to improve immunotherapy complemented the long-term studies and important advances of Bruce Cronstein and co-authors. These studies were motivated by the opposite aim; to decrease the inflammatory damage to normal tissues by pharmacologically recruiting A2AR and A2BR on overactive myeloid cells (reviewed in [2]). The field of anti-Hypoxia-A2-Adenosinergic treatments (“Co-adjuvants”) to improve cancer immunotherapy began following the genetic in vivo evidence that A2AR on T cells and myeloid cells are negative physiological regulators of virtually all types of tested effector functions [6–8]. Similarly, HIF-1α was found to be inhibitory in cells of the adaptive immune system [6–8].
It is now well established that hypoxic and adenosine-rich TMEs strongly inhibit anti-tumor T and NK cells. Our initial studies provided the proof-of principle to combine the immunotherapies of cancer with synthetic or natural antagonists of A2AR [9]. We also demonstrated the feasibility of inhibiting the accumulation of extracellular adenosine in inflamed tissues by oxygenation agents that reprogram the TME away from immunosuppression and toward an immunopermissive phenotype [8].
These original studies were reviewed and interpreted in [10–12] and they provided the necessary justification for other scientists to invest in the further development of the anti-hypoxia-A2-adenosinergic drugs by focusing on CD39/CD73–Adenosine-A2AR axis[13–19].
Discovery of A2A-adenosinergic protection of normal and cancerous tissues from immune cells
The long-term interest in understanding the biochemical mechanisms of cancerous tissue protection was triggered by the “Hellstom paradox”, describing the coexistence of tumors and anti-tumor lymphocytes in the same cancer patients [20].
We started with the consideration to target intracellular cAMP, based on established evidence that cAMP was inhibitory to lymphocytes (reviewed in [21]). We hypothesized and then demonstrated [12][22–26] that increases in intracellular cAMP may explain the Hellstrom Paradox. The most important remaining question was regarding the identification of a cAMP elevating G-protein coupled receptor (GPCR) and its ligand [21].
The initial studies of the pharmacological effects of the cAMP-elevating G-protein coupled high affinity A2AR on T cells supported the view that A2AR could be among many other GPCR candidates that could serve as physiological negative regulators of the immune response (reviewed in [2, 12, 27]). However, only in vivo genetic studies in animals with A2AR gene-deletion could establish whether A2AR was indeed inhibiting activated immune cells at physiological and pathophysiological levels of extracellular adenosine. These studies conclusively demonstrated the critical and non-redundant role of A2AR in the protection of normal tissues from collateral damage during the anti-pathogen immune response [5].
Importantly, genetic targeting of A2AR was also recapitulated by pharmacological treatments with synthetic (ZM 241385) and natural (caffeine) A2AR antagonists. These studies were the first to suggest and provide the proof-of-principle for targeting the Hypoxia → Adenosine → A2AR pathway as a strategy to prevent the inhibition of anti-tumor T cells in the TME [5].
The available data regarding the inhibitory role of A2AR in activated immune cells in vivo suggested that all activated immune cells–including anti-tumor immune cells–might be under inhibitory influence of adenosine-A2AR axis in vivo. However, additional studies were required to establish the crucial role of A2AR in the protection of cancerous tissues during the anti-tumor immune response [9,12].
In support of our original hypothesis, it was found that genetic deletion of A2AR resulted in rejection of established tumors in approximately 60% of A2AR-deficient mice by unleashing the otherwise inhibited anti-tumor immune cells. These data positioned the A2AR as promising therapeutic targets to improve the immunotherapy of cancer, even though the immunosuppressive role of A2AR and A2BR in anti-tumor killer cells had been explicitly excluded from considerations by pharmacological studies of others [29].
Using antagonists of A2AR to prevent the inhibition of anti-tumor T and NK cells
Anti-A2-adenosinergic pre-clinical studies provided proof of principle for the use of A2AR antagonists, which recapitulated–although not as strongly–genetic deletion of A2AR [9]. It was found that targeting of A2AR by antagonists de-inhibits CD8+ T cells, facilitating their antitumor effector functions. This was due to the prevention of the adenosine-triggered cAMP elevation and reversal of the adenosine-mediated inhibition of activated T cells and other immune cells in vivo.
Enhanced tumor rejection by T cells with A2AR-targeted siRNA suggested that the effects of A2AR are T cell-autonomous [9]. In these studies, ZM241385 (synthetic antagonist of A2AR/A2BR) and the natural antagonist 1,3,7 trimethylxanthine (caffeine) were effective in i) promoting T cell-mediated tumor regression ii) increasing tumor cell apoptosis and iii) preventing tumor neo-angiogenesis by enhancing IFN-γ production. This suggested that A2AR antagonists not only unleash perforin and Fas ligand-mediated cytotoxicity, but that increased IFN-γ production may also starve tumors by denying nutritional supply [9].
The limitation of these early studies of A2AR in preclinical models of cancer immunotherapy was the lack of availability of a selective synthetic A2AR antagonist with a long in vivo half-life. Thus, survival was not previously reported with short-lived A2AR antagonists, in contrast to the improved survival shown in tumor-bearing mice with genetic deletion of A2AR [9].
The recent commercial availability of the long-lived A2AR antagonists Preladenant, or SCH58261, allowed others to confirm and extend our studies demonstrating the original proof-of principle. Several groups of scientists supported the targeting of A2AR by demonstrating that blocking A2AR genetically or by synthetic A2AR antagonists, resulted in powerful inhibition of tumor metastasis. This was due to the unleashing of the anti-tumor immune cells from tolerization by A2AR. In their studies, Powell and co-authors [13] showed that A2AR promotes peripheral tolerance and that deletion of A2AR improves immunotherapy-enabled tumor rejection in mice [14]. The case for translating A2AR antagonists into clinical trials of cancer immunotherapy was also greatly strengthened by cancer immunologists in Australia, Canada, and the USA [16, 19, 30–34]
More advanced synthetic A2AR antagonists for cancer immunotherapy now exist since their development was motivated by observations of A2AR involvement in the neurobiology and pathogenesis of Parkinson’s disease [35]. These A2AR antagonists were demonstrated to be safe and well tolerated in extensive clinical trials, including phase III. While not approved by the FDA in the US, the A2AR antagonist KW6002 was approved in Japan.
A2AR antagonists improve anti-tumor effects if combined with blockade of immunological negative regulators CTLA-4/PD1
Current interest of Pharma was strengthened greatly by the studies of mechanisms of immunosuppression of human cancers that are resistant to chemotherapy [30]. Authors demonstrated that tumors with high expression of adenosine-generating CD73 are resistant to chemotherapy and immunotherapy. However, these tumors can be rejected by T and NK cells if chemotherapy or immunotherapy is combined with antagonists of A2AR [30].
In these studies, authors demonstrate that A2AR/A2BR antagonists were effective in reducing metastasis of tumors expressing CD73. Additionally, A2AR inhibitors unleashed anti-tumor effector functions and promoted NK cell function by increasing Perforin-mediated cytotoxicity and increasing the expression of Granzyme B in NK cells in vivo [30]. This suggests the therapeutic potential of an A2AR/A2BR blockade strategy for the treatment of CD73(+) metastatic tumors [30].
Directly feeding into current clinical trials, A2AR antagonists were also shown to improve the effects of blockade of CTLA-4/PD-1 [33, 36]. These anti-tumor effects were strongly dependent on NK cells and IFNγ, although CD8+ T cells and perforin also played a role [31, 36]. Overall, these results provide strong rationale for the use A2AR with immunological checkpoint inhibitors for the treatment of residual and metastatic disease. Importantly, these studies also offered a novel approach and biomarkers to stratify patients for this immunotherapy by selecting only patients with tumors expressing high levels of adenosine-generating CD73 [32, 36].
Considerations of important properties of A2AR antagonists
The studies presented here strongly support the feasibility and promise of the weakening of hypoxia-adenosinergic signaling as a way to prevent tumor resistance to tumor-reactive T cells. The advantage of existing A2AR antagonists [35, 37, 38] is demonstrated in their safety profile in healthy volunteers and patients with Parkinson’s disease. The efficacy in cancer immunotherapy can be predicted from sophisticated imaging studies of the occupation of A2AR binding sites by synthetic A2AR antagonists in human tissues in vivo.
Among the most important properties of an antagonist is the requirement for the high level of occupation of the A2AR at safe and well-tolerated doses. This requirement should be met during the evaluation of new synthetic A2AR antagonists prior to human clinical trials. This is important to emphasize due to the associated risk with rushing A2AR antagonists into clinical development prematurely with under-developed and poorly characterized drugs.
Future directions in the development of A2AR antagonists for cancer therapies may address the need in blood brain barrier-impermeable drugs since existing A2AR antagonists have been developed specifically to act in the brain of Parkinson’s disease patients [37]. This may prevent the potential neurological side effects in cancer patients with tumors in anatomical locations other than the brain, and increase the number of patients eligible for this therapy.
Also to be addressed is the poor aqueous solubility and photoisomerization, a known limitation of existing A2AR antagonists of this class. One way to solve this problem was offered by an one-pot route to 8-substituted xanthines from 5,6-diaminouracils and carboxaldehydes with good yields [39]. As an example, these limitations were addressed in the modification of the drug approved in Japan, KW-6002, which was converted to a PEG derivative. Importantly, it was shown to be a functional derivative in in vitro bioassays used to confirm efficacy. It was also observed that the PEGylated version had much better aqueous solubility and was inert to photoisomerization [39].
Facilitating competitive A2AR antagonism by lowering adenosine in TME
The promise of A2AR antagonists as anti-tumor treatments attracted attention to the upstream events of the hypoxia-adenosinergic pathway, the generation of extracellular adenosine by the tandem of ecto-enzymes CD39-CD73 (Figure 1).
Thus, the targeting of the upstream hypoxia-HIF-1 [8] and CD39/CD73-adenosine stages of this pathway are the subject of many ongoing preclinical and clinical investigations. The adenosine-generating ecto-enzymes CD39/CD73 as drug targets have been extensively reviewed by leading scientists in this field [15–19]. Increased levels of CD39 [15, 18, 47] and CD73-generated tumor-protecting extracellular adenosine [15, 16, 30, 32, 48–51] may signal through A2AR/A2BR to induce suppression of anti-tumor immune cells [5, 8, 9, 12, 19, 31].
A very important advance from fundamental biochemical and pre-clinical studies toward human cancer was in the uncovering of the connection between the adenosine-rich TME and immunosuppression in human cancer patients. This was done by extensive bioinformatics analysis of more than 6000 data sets from individual triple negative breast cancers (TNBC), which are also resistant to chemotherapies [19, 32, 53]. These studies have demonstrated the predictive power of CD73 expression as being predominantly associated with poor prognosis, emphasizing the correlation between tumor protection from chemotherapy and the overproduction of extracellular adenosine by the high levels of expression of these ecto-enzymes.
Several major studies have demonstrated that the inhibition of adenosine generation by CD73 mAbs or by small molecule drugs (APCP) significantly enhances the anti-tumor activity of T cells and NK cells [15,16]. Indeed, these data have stimulated AstraZeneca to prepare clinical trials with MEDI9447, a mAb against CD73, alone and in combination with MEDI4736 (durvalumab), a monoclonal antibody directed against PD-L1, in advanced solid tumors (ClinicalTrials.gov Identifier: NCT02503774).
Taken together, these data support and extend the implications of the reported anti-tumor effects of A2AR antagonists [9], strongly suggesting that the combination of CD39/CD73 inhibitors with A2AR or A2BR antagonists should be tested during the immunotherapy of cancer.
Considerations of the A2B Adenosine Receptor
A2BR was known to be immunosuppressive after pharmacological activation and after stimulation by pathophysiological levels of adenosine in inflamed tissues [41]. A2BR has also been implicated in the regulation of myeloid cells, including myeloid derived suppressor cells (MDSC) [42, 43, 54]. A2BR has been shown to promote the development of MDSC, which can be a source of the CD73-generated immunosuppressive adenosine [44, 45]. It is expected that A2BR may also contribute to the inhibition of human T cells, which do express both A2AR and A2BR. Studies by Morello’s group indicated that A2BR had a significant role in tumor progression [44, 45]. Similar studies by Ryzhov and colleagues [46] explored the important role of A2BR in tumor development in studies of Lewis lung carcinoma in A2BR-deficient mice.
Concluding remarks
There is an important advantage of synthetic small molecule inhibitors of anti-Hypoxia-A2-Adenosinergic drugs over mAbs that block immunological negative regulators. In the case of side effects, it is impossible to remove antibodies from patients due to their long persistence. In contrast, synthetic A2AR antagonists can be used as a once-a-day small molecule, in which treatment can be immediately ceased to avoid adverse side effects.
It is important to note, there could be potential side effects of A2AR antagonists as was described in “Caveats and cautions for the therapeutic targeting of the anti-inflammatory A2 adenosine receptors” [58]. These drugs must be used carefully since unleashing anti-tumor immune cells may be accompanied by increased auto-immunity in cancer patients if this treatment coincides with simultaneous acute inflammation or sub-threshold auto-immunity. The observations of autoimmunity during melanoma rejection in A2AR-deficient mice [15] suggest that A2AR in T cells is also important in preventing autoimmunity. Since unleashing the anti-tumor immune cells may be accompanied by an increased auto-immunity, we propose that episodes of even sub-threshold levels of ongoing inflammation should be considered among the exclusion criteria [58].
It would be interesting to test whether drugs that act as inhibitors of adenine nucleotide release and reuptake might also affect anti-tumor activity. Specifically, adenosine reuptake inhibitors (AdoRI) that block the action of one or more of the equilibrative nucleoside transporters (ENTs), may lead to increased extracellular concentrations of adenosine and immunosuppressive effects. These drugs should therefore be excluded in patients who are treated with A2AR antagonists. Future studies are needed to determine the effects of such drugs on the anti-tumor immune response.
There is a paucity of well-characterized synthetic A2AR antagonists and need in being careful in the pharmaceutical development, preclinical studies, and clinical trials of novel drugs [55–56]. Additionally, there is a need in the development and characterization of A2AR antagonists with a long in vivo half-life. The development of new A2AR antagonists is facilitated by important advances in structure-based A2AR antagonist design that are taking advantage of the recently revealed molecular basis of GPCR-ligand binding and activation [54].
Highlights.
A2AR and Hypoxia in TME are important barriers that must be overcome in cancer immunotherapy
A2AR on immune cells are critical and non-redundant mediators of physiological immunosuppression
Blocking immunosuppressive A2AR-mediated signaling may improve current cancer immunotherapy
Footnotes
Disclosure of conflict of interest Michail Sitkovsky is author of Intellectual Property to use of inhibitors of adenosine receptor signaling.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yang JC, Rosenberg SA. Adoptive T-Cell Therapy for Cancer. Adv Immunol. 2016;130:279–294. doi: 10.1016/bs.ai.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2*.Hasko G, Cronstein B. Regulation of inflammation by adenosine. Front Immunol. 2013;4:85. doi: 10.3389/fimmu.2013.00085. This is important overview of the major findings in the field of inflammatory tissue damage in studies of adenosine receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koshiba M, Kojima H, Huang S, Apasov S, Sitkovsky MV. Memory of extracellular adenosine A2A purinergic receptor-mediated signaling in murine T cells. J Biol Chem. 1997;272:25881–25889. doi: 10.1074/jbc.272.41.25881. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong JM, Chen JF, Schwarzschild MA, Apasov S, Smith PT, Caldwell C, Chen P, Figler H, Sullivan G, Fink S, et al. Gene dose effect reveals no Gs-coupled A2A adenosine receptor reserve in murine T-lymphocytes: studies of cells from A2A-receptor-gene- deficient mice. Biochem J. 2001;354:123–130. doi: 10.1042/0264-6021:3540123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 6.Lukashev D, Ohta A, Apasov S, Chen JF, Sitkovsky M. Cutting edge: Physiologic attenuation of proinflammatory transcription by the Gs protein-coupled A2A adenosine receptor in vivo. J Immunol. 2004;173:21–24. doi: 10.4049/jimmunol.173.1.21. [DOI] [PubMed] [Google Scholar]
- 7.Kojima H, Gu H, Nomura S, Caldwell CC, Kobata T, Carmeliet P, Semenza GL, Sitkovsky MV. Abnormal B lymphocyte development and autoimmunity in hypoxia-inducible factor 1alpha -deficient chimeric mice. Proc Natl Acad Sci U S A. 2002;99:2170–2174. doi: 10.1073/pnas.052706699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8*.Hatfield SM, Kjaergaard J, Lukashev D, Schreiber TH, Belikoff B, Abbott R, Sethumadhavan, Shalini, Philbrook P, Ko K, Cannici R, et al. Immunological mechanisms of the anti-tumor effects of supplemental oxygenation. Science Translational Medicine. 2015;7:277. doi: 10.1126/scitranslmed.aaa1260. This is a paradigm shifting set of observations that offer promising future direction in improving the immunotherapies of cancer by supplemental oxygenation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 11.Sitkovsky MV. T regulatory cells: hypoxia-adenosinergic suppression and redirection of the immune response. Trends Immunol. 2009;30:102–108. doi: 10.1016/j.it.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Sitkovsky MV, Hatfield S, Abbott R, Belikoff B, Lukashev D, Ohta A. Hostile, hypoxia-A2-adenosinergic tumor biology as the next barrier to overcome for tumor immunologists. Cancer Immunol Res. 2014;2:598–605. doi: 10.1158/2326-6066.CIR-14-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, Drake CG, Powell JD. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waickman AT, Alme A, Senaldi L, Zarek PE, Horton M, Powell JD. Enhancement of tumor immunotherapy by deletion of the A2A adenosine receptor. Cancer Immunol Immunother. 2012;61:917–926. doi: 10.1007/s00262-011-1155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, Dwyer KM, Smyth MJ. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A. 2010;107:1547–1552. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin D, Fan J, Wang L, Thompson LF, Liu A, Daniel BJ, Shin T, Curiel TJ, Zhang B. CD73 on Tumor Cells Impairs Antitumor T-Cell Responses: A Novel Mechanism of Tumor-Induced Immune Suppression. Cancer Res. 2010 doi: 10.1158/0008-5472.CAN-09-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunzli BM, Bernlochner MI, Rath S, Kaser S, Csizmadia E, Enjyoji K, Cowan P, d’Apice A, Dwyer K, Rosenberg R, et al. Impact of CD39 and purinergic signalling on the growth and metastasis of colorectal cancer. Purinergic Signal. 2011;7:231–241. doi: 10.1007/s11302-011-9228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng L, Sun X, Csizmadia E, Han L, Bian S, Murakami T, Wang X, Robson SC, Wu Y. Vascular CD39/ENTPD1 directly promotes tumor cell growth by scavenging extracellular adenosine triphosphate. Neoplasia. 2011;13:206–216. doi: 10.1593/neo.101332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young A, Mittal D, Stagg J, Smyth MJ. Targeting cancer-derived adenosine: new therapeutic approaches. Cancer Discov. 2014;4:879–888. doi: 10.1158/2159-8290.CD-14-0341. [DOI] [PubMed] [Google Scholar]
- 20.Hellstrom I, Hellstrom KE, Pierce GE, Yang JP. Cellular and humoral immunity to different types of human neoplasms. Nature. 1968;220:1352–1354. doi: 10.1038/2201352a0. [DOI] [PubMed] [Google Scholar]
- 21.Sitkovsky MV. Use of the A(2A) adenosine receptor as a physiological immunosuppressor and to engineer inflammation in vivo. Biochem Pharmacol. 2003;65:493–501. doi: 10.1016/s0006-2952(02)01548-4. [DOI] [PubMed] [Google Scholar]
- 22.Takayama H, Trenn G, Sitkovsky MV. Locus of inhibitory action of cAMP-dependent protein kinase in the antigen-receptor triggered cytotoxic T-lymphocyte activation pathway. J Biol Chem. 1988;263:2330–2336. [PubMed] [Google Scholar]
- 23.Sitkovsky MV, Trenn G, Takayama H. Cyclic AMP-dependent protein kinase as a part of the possible down-regulating pathway in the antigen receptor-regulated cytotoxic T lymphocyte conjugate formation and granule exocytosis. Ann NY Acad Sci. 1988;532:350–358. doi: 10.1111/j.1749-6632.1988.tb36352.x. [DOI] [PubMed] [Google Scholar]
- 24.Takayama H, Sitkovsky MV. Potential use of an antagonist of cAMP-dependent protein kinase to block inhibition and modulate T-cell receptor-triggered activation of cytotoxic T-lymphocytes. J Pharm Sci. 1989;78:8–10. doi: 10.1002/jps.2600780104. [DOI] [PubMed] [Google Scholar]
- 25.Neary CL, Nesterova M, Cho YS, Cheadle C, Becker KG, Cho-Chung YS. Protein kinase A isozyme switching: eliciting differential cAMP signaling and tumor reversion. Oncogene. 2004;23:8847–8856. doi: 10.1038/sj.onc.1208165. [DOI] [PubMed] [Google Scholar]
- 26.Klotz KN, Kachler S. Inhibitors of membranous adenylyl cyclases with affinity for adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 2016;389:349–352. doi: 10.1007/s00210-015-1197-z. [DOI] [PubMed] [Google Scholar]
- 27.Koshiba M, Kojima H, Huang S, Apasov S, Sitkovsky MV. Memory of extracellular adenosine/A2a purinergic receptor-mediated signalling in murine T cells. J Biol Chem. 1997;272:25881–25889. doi: 10.1074/jbc.272.41.25881. [DOI] [PubMed] [Google Scholar]
- 28.Williams BA, Manzer A, Blay J, Hoskin DW. Adenosine acts through a novel extracellular receptor to inhibit granule exocytosis by natural killer cells. Biochem Biophys Res Commun. 1997;231:264–269. doi: 10.1006/bbrc.1997.6077. [DOI] [PubMed] [Google Scholar]
- 29.Hoskin DW, Butler JJ, Drapeau D, Haeryfar SM, Blay J. Adenosine acts through an A3 receptor to prevent the induction of murine anti-CD3-activated killer T cells. Int J Cancer. 2002;99:386–395. doi: 10.1002/ijc.10325. [DOI] [PubMed] [Google Scholar]
- 30*.Beavis PA, Divisekera U, Paget C, Chow MT, John LB, Devaud C, Dwyer K, Stagg J, Smyth MJ, Darcy PK. Blockade of A2A receptors potently suppresses the metastasis of CD73+ tumors. Proc Natl Acad Sci U S A. 2013;110:14711–14716. doi: 10.1073/pnas.1308209110. This paper was critical in extending the evidence for the promise of A2AR antagonists in therapies of cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mittal D, Young A, Stannard K, Yong M, Teng MW, Allard B, Stagg J, Smyth MJ. Antimetastatic effects of blocking PD-1 and the adenosine A2A receptor. Cancer Res. 2014;74:3652–3658. doi: 10.1158/0008-5472.CAN-14-0957. [DOI] [PubMed] [Google Scholar]
- 32**.Loi S, Pommey S, Haibe-Kains B, Beavis PA, Darcy PK, Smyth MJ, Stagg J. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc Natl Acad Sci U S A. 2013;110:11091–11096. doi: 10.1073/pnas.1222251110. This paradigm shifting set of observations introduces the expression of adenosine generating ecto-enzymes as a poor prognosis factor and further justifies combination of anti-hypoxia-adenosinergic drugs with chemotherapies and immunotherapies of cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whiteside TL, Jackson EK. Adenosine and prostaglandin e2 production by human inducible regulatory T cells in health and disease. Front Immunol. 2013;4:212. doi: 10.3389/fimmu.2013.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuler PJ, Saze Z, Hong CS, Muller L, Gillespie DG, Cheng D, Harasymczuk M, Mandapathil M, Lang S, Jackson EK, et al. Human CD4+ CD39+ regulatory T cells produce adenosine upon co-expression of surface CD73 or contact with CD73+ exosomes or CD73+ cells. Clin Exp Immunol. 2014;177:531–543. doi: 10.1111/cei.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fredholm BB, APIJ, Jacobson KA, Linden J, Muller CE. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors--an update. Pharmacol Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young A, Mittal D, Stannard K, Yong M, Teng MW, Allard B, Stagg J, Smyth MJ. Co-blockade of immune checkpoints and adenosine A receptor suppresses metastasis. Oncoimmunology. 2014;3:e958952. doi: 10.4161/21624011.2014.958952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller CE, Jacobson KA. Xanthines as adenosine receptor antagonists. Handb Exp Pharmacol. 2011:151–199. doi: 10.1007/978-3-642-13443-2_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets--what are the challenges? Nat Rev Drug Discov. 2013;12:265–286. doi: 10.1038/nrd3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas R, Lee J, Chevalier V, Sadler S, Selesniemi K, Hatfield S, Sitkovsky M, Ondrechen MJ, Jones GB. Design and evaluation of xanthine based adenosine receptor antagonists: potential hypoxia targeted immunotherapies. Bioorg Med Chem. 2013;21:7453–7464. doi: 10.1016/j.bmc.2013.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohta A, Sitkovsky M. Caveats in promising therapeutic targeting of the anti-inflammatory A2 adenosine receptors: The notes of caution. Commentary/Opinion. Nature Reviews Drug Discovery. 2006 doi: 10.1038/nrd1983-C1. [DOI] [Google Scholar]
- 41.Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St Hilaire C, Seldin DC, Toselli P, et al. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest. 2006;116:1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, Pisarev V, Sherman S, Sporn MB, Gabrilovich D. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest. 2011;121:4015–4029. doi: 10.1172/JCI45862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43*.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125:3356–3364. doi: 10.1172/JCI80005. This authoritative opinion by creators of this field point to the yet to be fully uncovered important influences of these cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sorrentino R, Pinto A, Morello S. The adenosinergic system in cancer: Key therapeutic target. Oncoimmunology. 2013;2:e22448. doi: 10.4161/onci.22448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iannone R, Miele L, Maiolino P, Pinto A, Morello S. Blockade of A2b adenosine receptor reduces tumor growth and immune suppression mediated by myeloid-derived suppressor cells in a mouse model of melanoma. Neoplasia. 2013;15:1400–1409. doi: 10.1593/neo.131748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryzhov S, Novitskiy SV, Zaynagetdinov R, Goldstein AE, Carbone DP, Biaggioni I, Dikov MM, Feoktistov I. Host A(2B) adenosine receptors promote carcinoma growth. Neoplasia. 2008;10:987–995. doi: 10.1593/neo.08478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med. 2013;368:1260. doi: 10.1056/NEJMc1300259. [DOI] [PubMed] [Google Scholar]
- 48*.Allard B, Pommey S, Smyth MJ, Stagg J. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin Cancer Res. 2013;19:5626–5635. doi: 10.1158/1078-0432.CCR-13-0545. Authors provide the rationale for current clinical trials to combine these two antibody treatments with drugs that inhibit adenosine-A2AR axis. [DOI] [PubMed] [Google Scholar]
- 49.Stagg J, Beavis PA, Divisekera U, Liu MC, Moller A, Darcy PK, Smyth MJ. CD73-deficient mice are resistant to carcinogenesis. Cancer Res. 2012;72:2190–2196. doi: 10.1158/0008-5472.CAN-12-0420. [DOI] [PubMed] [Google Scholar]
- 50.Stagg J, Divisekera U, Duret H, Sparwasser T, Teng MW, Darcy PK, Smyth MJ. CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res. 2011;71:2892–2900. doi: 10.1158/0008-5472.CAN-10-4246. [DOI] [PubMed] [Google Scholar]
- 51.Qin L, Thompson LF, Kuzel TM, Zhang B. Requirement of NK cells for selective A2A receptor blockade to suppress CD73+ tumor metastasis. Immunotherapy. 2014;6:19–21. doi: 10.2217/imt.13.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freundlieb M, Zimmermann H, Muller CE. A new, sensitive ecto-5′-nucleotidase assay for compound screening. Anal Biochem. 2014;446:53–58. doi: 10.1016/j.ab.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 53.Zhang B, Song B, Wang X, Chang XS, Pang T, Zhang X, Yin K, Fang GE. The expression and clinical significance of CD73 molecule in human rectal adenocarcinoma. Tumour Biol. 2015;36:5459–5466. doi: 10.1007/s13277-015-3212-x. [DOI] [PubMed] [Google Scholar]
- 54.Cekic C, Day YJ, Sag D, Linden J. Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Res. 2014 Dec 15;74(24):7250–9. doi: 10.1158/0008-5472.CAN-13-3583. [DOI] [PMC free article] [PubMed] [Google Scholar]