Abstract
Recent synthetic approaches to understanding the origin of life have yielded insights into plausible pathways for the emergence of the first cells. Here we review current experiments with implications for the origin of life, emphasizing the ability of unexpected physical processes to facilitate the self-assembly and self-replication of the first biological systems. These laboratory efforts have uncovered novel physical mechanisms for the emergence of homochirality, the concentration and purification of prebiotic building blocks, and the ability of the first cells to assemble, grow, divide, and acquire greater complexity. In the absence of evolved biochemical capabilities, such physical processes must have played an essential role in early biology.
Keywords: prebiotic, homochirality, RNA world, thermophoresis, protocell
Introduction
One of the many challenges to developing an understanding of how life began lies in the complexity of extant biology. Even the simplest cells feature hundreds of essential genes (22) coding for proteins and nucleic acids that perform tasks ranging from building block synthesis to cell division. The spontaneous emergence of ordered biological systems had, by definition, to occur without such complex machinery and thus depended on simple physical and chemical processes, both intrinsic and environmental, for self-assembly and reproduction. Recent experimental work has uncovered plausible physical pathways and mechanisms for at least some steps in both the origin and early evolution of life. Given the diverse geochemical microenvironments of the early earth, and the varied geophysical means of chemical transport between them, there is considerable potential for unexpected physicochemical phenomena to assist in the origin of life. Such processes allowed for the synthesis of constitutionally and chirally enriched biomolecules, their self-assembly into primitive biological systems, and the ability of those systems to self-replicate and evolve into the complex cells that followed; all tasks that have long since been taken over by evolved biological machinery.
Physical mechanisms for the emergence of homochirality
Biology is universally chiral: proteins are composed almost entirely of L-amino acids while nucleic acids use D-ribose as their sugar component. Even prebiotic models of non-enzymatic RNA polymerization require a source of chirally pure nucleotides, because L-nucleotides act as chain terminators for template-directed primer-extension of D-RNA (33). Modern enzymes easily synthesize chiral metabolites, but if homochirality had to predate the first living systems, how could it have been generated abiotically?
An abiotic origin of homochirality probably involved two sequential steps: an initial breaking of chiral symmetry and generation of a small enantiomeric excess (ee) in certain key compounds, followed by the action of physical or chemical processes that amplified the initial ee to a state approaching homochirality. Meteorite samples that contain amino acids with significant chiral asymmetry (up to 15–18% ee) provide direct evidence that physical processes can break chiral symmetry (15, 23). These striking findings have yet to be full explained. The most commonly cited hypothesis is that circularly polarized UV light (CPL) leads to enantioselective photolysis. Intense sources of highly polarized extreme UV are thought to be relatively common in interstellar molecular clouds, where meteoritic amino acids or their chiral precursors may have formed. Experimentally, CPL from synchrotron radiation has been used to generate a small ee (< 3%) in racemic samples of the amino acid leucine (51). Unfortunately, the fraction of the input leucine that was degraded during the generation of the 3% ee could not be measured, so the efficiency of this process remains unclear.
Although we know from the analysis of meteoritic organics that chiral symmetry can be broken extraterrestrially, it is possible that this symmetry was also broken in specific terrestrial environments. A local symmetry breaking process would circumvent the need for the deposition of chiral compounds onto the early earth on a scale sufficient to influence the course of planetary organic chemistry. Rosenberg et. al. recently proposed that polarized secondary electrons, which are generated when a magnetic substrate (such as a bed of iron ore particles) is irradiated, can result in a strongly enantioselective photolysis of adsorbed molecules (67). In a model reaction, they demonstrated that the rate of photocleavage of the C-O bond in R vs. S 2-butanol adsorbed on a magnetized alloy can differ by up to 10% depending on the spin polarization of the secondary electrons.
Recent years have seen a virtual explosion of experimental research focused on robust mechanisms for the amplification of a small initial ee to subsequent enantiopurity. Chemical processes in which a chiral product both catalyzes its own formation and inhibits the formation of its enantiomer were proposed over 50 years ago as a means of achieving enantiopurity given a small ee excess (18). The Soai reaction, an alkylation of pyrimidyl aldehydes, was the first demonstration of such a chemical process and has thus been extensively studied as a model system (5, 73). This work has provided experimental validation and a specific chemical mechanism for the theoretically proposed requirements for chiral amplification (18). However, a purely chemical route for chiral amplification is currently constrained to a limited set of asymmetric autocatalytic reactions. In a surprising series of developments, simple phase transitions seem to offer much more general mechanisms of chiral amplification. Viedma first convincingly demonstrated such a physical amplification process by showing that abrasive grinding of a racemic slurry of chiral crystals (composed of achiral sodium chlorate) in equilibrium with an achiral solution phase leads inexorably to a state of complete chiral purity of the crystal phase (82). This remarkable process is extremely robust: chiral purity in the solid phase emerges even without an initial ee, indicating that stochastic fluctuations in crystal composition are sufficient to break the chiral symmetry.
Viedma’s experiments were performed on chiral crystals of achiral molecules. For such a process to apply for chiral molecules, such as biological building blocks, there has to be an accompanying chemical means of solution phase racemization. Blackmond and colleagues have recently demonstrated that, by introducing a base-catalyzed solution phase racemization, the same crystal grinding process can lead to chiral amplification of amino acid crystals with an initially small (< 10%) ee (57, 83). Since racemization occurs only in solution, chiral conversion acts preferentially on the faster dissolving crystal species. Asymmetry in dissolution leads to asymmetry in chiral conversion via racemization, which in turn amplifies the chirality of the dominant chiral species.
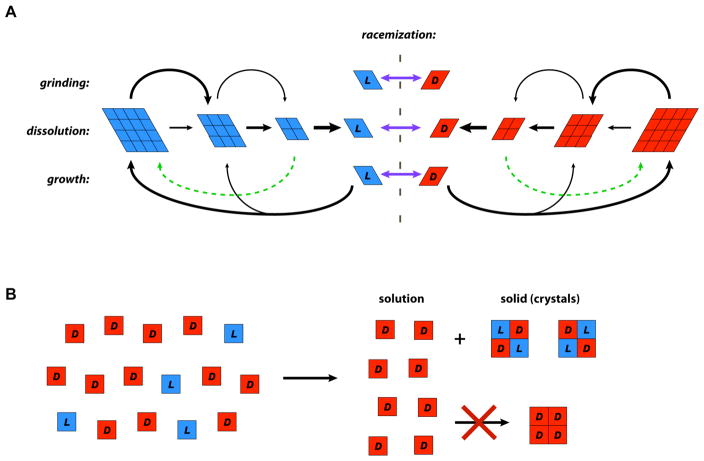
The mechanism by which asymmetry is amplified during crystal grinding experiments is still being debated (Schematic in Figure 1A). It is generally agreed that abrasive grinding converts large crystals to small crystals, thereby driving cycles of dissolution and recrystallization during which small crystals tend to dissolve while larger crystals tend to grow. This is the classical Ostwald ripening effect, in which differences in surface energy drive the growth of larger crystals at the expense of smaller crystals. One hypothesis is that the dissolution of small crystals to monomers coupled with growth of larger crystals generates a cycle that is sufficient to lead to chiral amplification (58). However, it is unclear how purely first order processes could lead to amplification, as both dissolution and recrystallization would be proportional to the crystal surface area, thus maintaining any initial ee indefinitely. An alternative proposal is that the processes of abrasive grinding and dissolution generate chiral microcrystals, which can then either dissolve into monomers or be incorporated directly into larger crystals of the same chirality (49). If even a small fraction of crystal growth is mediated by such a process, which is second order because it is dependent on the concentration of both large crystals and micro-crystals of a given chirality, then crystals of the major chirality would increase in abundance until homo-chirality of the crystal phase was reached. Future mechanistic studies examining the kinetics of these phase transitions and the exact conditions under which chiral enrichment does (and does not) proceed should help to resolve these questions.
Figure 1.
Physical mechanisms of chiral amplification based on phase transitions. (A) Schematic of processes involved in crystal grinding experiments. Abrasive grinding of a racemic slurry of L and D crystals with glass beads leads to the amplification of an initially small ee to a nearly-homochiral state. Identical processes occur for crystals of both L (left) and D (right) enantiomers, with rapid solution phase racemization (purple arrows) maintaining a racemic pool of monomers in solution. Abrasive grinding and dissolution lead to large crystals breaking into smaller crystals which eventually dissolve into chiral monomers. Growth by monomer addition occurs preferentially on larger crystals due to their lower surface energy compared to smaller crystals. A second-order pathway for growth by the addition of small crystal clusters (green, dashed arrow) has been proposed to explain the inexorable drive to homochirality. (B) Enantioenrichment of compounds that crystallize as a racemate. If a solution with an initial ee (left) is concentrated, crystallization of the racemate (right) leaves monomers of the chirality that is in excess in solution, because conglomerate (chiral) crystals are energetically disfavored relative to crystals of the racemate. Therefore, the solution phase becomes highly enriched in the chirality of the initial ee.
Chiral amplification of an amino acid by crystal grinding requires that the racemic amino acid crystallizes into two separate enantiopure crystal phases (referred to as a conglomerate), which is the case for only two of the proteinogenic amino acids (aspartic acid and threonine). In separate experiments Blackmond (39) and Breslow (8) demonstrated an alternative physical mechanism for asymmetric chiral enrichment in the other 18 amino acids, which form crystals of a 1:1 D:L racemate. Given an amino acid solution with a small ee, precipitation of the racemate leads to a solution phase eutectic containing the excess molecules of the major enantiomer. In this case, chiral enrichment is controlled thermodynamically: formation of enantiopure crystals is energetically disfavored, causing the excess enantiomer to stay in solution while the racemate precipitates (Figure 1B). Amplification thus depends on the energy, and thus solubility, difference between the precipitation of racemate and conglomerate. Small molecule additives, such as dicarboxylic acids, have been found in some cases been found to enhance this effect by co-crystallizing with amino acids and further stabilizing the racemic crystal lattice (40). Eutectic chiral enrichment has also been demonstrated for three of the four standard nucleosides, A, C and U, which crystallize as the racemate, but not for G which crystallizes as a conglomerate (7).
Homochirality did not necessarily need to arise independently in multiple biological building blocks; if a chiral molecule can act as an asymmetric catalyst, then homochirality could be transferred to other chiral building blocks. For example, the amino acid proline is well known to act as an asymmetric catalyst for a variety of aldol condensations (45). Pizzarello and Weber found that L-isovaline (found in meteorites with 15% ee) and, to a lesser extent, L-alanine can serve as asymmetric catalysts for sugar synthesis, such as the condensation of glycoaldehyde with formaldehyde to generate glyceraldehyde, or the condensation of two molecules of gylcoaldehyde to form tetroses (60, 85). However, in these cases the ee of the sugar products was always much less than the ee of the amino acid. For the proposed prebiotic pathway for nucleotide synthesis discussed below, the chirality of the input glyceraldehyde determines the chirality of the nucleotide that is ultimately generated, so that a source of chirally pure glyceraldehyde would lead to the synthesis of chirally pure nucleotides. We note that glyceraldehyde forms remarkably stable DL dimers in aqueous solutions (16, 71). This could potentially lead to enantioenriched reaction products either by favoring reaction with the excess enantiomer, which is not sequestered in DL dimers, or by phase separation of the dimer racemate, leaving the excess enantiomer in solution.
Physical mechanisms for the self-assembly of biological materials
Purification of building blocks and their polymers
The now-famous Miller-Urey experiment (52) launched the field of prebiotic chemistry. In the 50 years since, synthetic approaches to prebiotic chemistry have sought to define reasonable conditions for the formation of the first biomolecules by finding robust chemical pathways for the abiotic synthesis of biological building blocks and plausible geophysical processes for the purification of these products and their key intermediates. The field has focused on nucleic acids in recent years, primarily because of the RNA world hypothesis (88), which has gained wide acceptance with the discovery of extant RNA enzymes (ribozymes) (24, 41, 75), including the finding that the ribosome is a ribozyme (75), and the isolation of a wide variety of functional RNAs in vitro (87).
It remains unclear whether RNA could have provided the genetic basis for the very first living systems, or must have been preceded by an earlier genetic polymer that was easier to synthesize, replicate, or more stable to hydrolysis. To address this, there has been a long-standing effort to demonstrate a prebiotically plausible synthesis of nucleotides and oligonucleotides (oligomers). Prebiotic chemists have traditionally approached nucleotide synthesis by finding independent pathways for sugar and nucleobase synthesis. This approach seems logical in that nucleotides are complex molecules and a ‘synthesis by parts’ approach seems most likely to achieve robust yields. The synthesis of ribose from formaldehyde via the formose reaction (10, 20) and pathways for purine (84) and pyrimidine (66) nucleobase synthesis have long been established. However, the formose reaction features notoriously complex mixtures of products, and ribose is quite unstable under common reaction conditions (42). This problem is ameliorated by the finding that simpler mixtures are generated in the presence of borate, which stabilizes cis-diol containing sugars, such as ribose (65). However, the most serious problem is that the joining of the sugar and nucleobase is inefficient for purines (19) and has not been demonstrated for pyrimidines. The lack of a prebiotically plausible total synthesis for any of the nucleotides has been a primary argument against the RNA-first hypothesis (72) and has led to searches for simpler genetic polymers (32, 70).
A major advance now tells us that the longstanding assumption that nitrogenous (nucleobase) and oxygenous (sugar) chemistry must occur separately was misguided. In a recent report, Sutherland and colleagues have described a prebiotically plausible synthesis for the pyrimidine ribonucleotides (61), which begins with the reaction of glycoaldehyde with cyanamide to form the key intermediate 2-aminooxazole. This intermediate is in effect part sugar and part nucleobase, and subsequent reactions elaborate the full nucleotide structure from this chimeric product. The yields for each step in the pathway are generally good, but there are also intriguing physical methods for purification at three stages in the pathway. 2-aminooxazole has a very high vapor pressure, and thus could have been purified directly by sublimation and condensation, perhaps during diurnal temperature cycles or in an appropriate geological setting in which hot and cold surfaces are in close proximity. The next step in the pathway is the formation of arabinose-aminooxazoline by condensation of 2-aminooxazole with glyceraldehyde. This reaction generates two new chiral centers, and thus four diastereomeric products are formed, with the ribo- and arabino-aminooxazolines being predominant. The desired arabino compound could potentially be prebiotically purified by spontaneous crystallization of the much less soluble ribose-aminooxazoline side-product (74), leaving the arabinose product in solution. In the last step of nucleotide synthesis in the Sutherland pathway, another physical process, UV irradiation, purifies the cytidine nucleotide by selective hydrolysis of the primary side-products, including the α anomer. Remarkably, this UV irradiation also leads to the partial conversion of cytidine to uridine, thereby providing a route to a second nucleotide. It would obviously be very satisfying if similar pathways could be found for the purine ribonucleotides, thus establishing a plausible synthesis for all four RNA monomers. The polymerization of the ribonucleotide monomers into oligomers is the final step in the spontaneous assembly of RNA. The products of the Sutherland pathway are 2′-3′ cyclic nucleotides. These are known to condense into RNA chains, but only under strongly dehydrating conditions, and with poor regiospecificity so that a mixture of 2′-5′ and 3′-5′ linkages is generated (81). Plausible mechanisms for chemically activating the available nucleotides could lead to more efficient polymerization. 5′ monophosphate nucleotides activated with good leaving groups (e.g. imidazole, adenine, 1-methyladenine) are efficiently polymerized by inorganic catalysts, most dramatically montmorillonite clay (17). In these experiments, both the catalyst and leaving group are critical for polymerization efficiency, while the leaving group largely determines regioselectivity. Using specific leaving groups, mostly notably 1-methyladenine, yields for 3′-5′ linkages of greater than 80% have been reported (62).
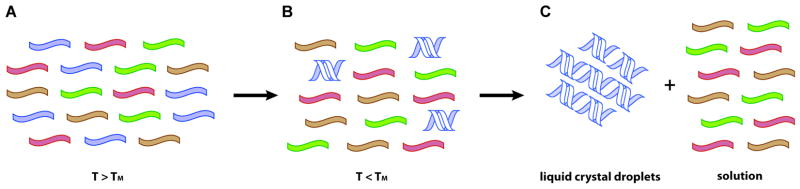
Oligomer purity and (functional) length would have almost certainly been limited by the specificity of the condensation chemistry and the purity of the monomer pool. Work on the origin of homochirality has demonstrated the power of phase transitions to purify molecular building blocks. Could similar phenomena apply to the purification of polymers? In a series of elegant experiments, Clark and colleagues demonstrated that short double-stranded DNA (dsDNA) oligomers of 5–20 bases can phase separate from a solution of single-stranded DNA (ssDNA) oligonucleotides by forming liquid crystal (LC) aggregates (54, 90). This aggregation is based on the co-axial stacking of short dsDNA segments, whose ends are hydrophobic due to the exposed terminal nucleobases, into longer DNA duplexes. However, this end-to-end interaction is insufficient by itself for LC formation; purified dsDNA spontaneously aggregates only at higher concentrations. The key to LC formation is the depletion force resulting from the flexible ssDNA in solution, whose entropy is maximized by minimizing the volume occupancy of the rigid dsDNA through LC formation. This is analogous to the well-studied phenomenon of spontaneous colloid aggregation in solutions of concentrated polymers (79). Hence, the impurity of the initial solution, with non-helical ssDNA contaminating dsDNA, promotes aggregation by entropically favoring self-assembly. As a result, oligomers that can form double-stranded helices could in principle be purified away from oligomers with defective or racemic nucleotides, or incorrect linkages, which would not be able to hybridize as well and would therefore remain in the isotropic (solution) phase (Figure 2). Identical processes should apply to other, similarly rigid polymers (such as double-stranded RNA) and could be enhanced by other flexible crowding agents (such as polypeptides). An important direction for future research is to experimentally explore the ability of LC phase separation to purify uniform dsRNA from complex prebiotically plausible mixtures of oligomers. Mechanisms for subsequently separating the LC aggregates from bulk solution, perhaps based on high-affinity adsorption onto charged mineral surfaces, would also be helpful, as would an examination of ligation chemistry within the end to end LC aggregates. The authors propose that such a process could generate longer homogeneous oligomers by favoring ligation of the duplex-forming oligomers. In such a processes, we note that the nucleobases of the hybridized RNA would also be protected by base pairing from highly reactive activating agents such as cyanoimidazole, which could then lead to selective inactivation of the single strands in the solution.
Figure 2.
Schematic for nucleic acid oligomer purification by liquid crystal formation. (A) A heterogeneous population of single-stranded oligomers contains some strands (blue) that can assemble to form duplex segments, and an excess of non-functional strands (red, green, and brown) that cannot form duplexes due to incorrect linkages, modified bases, or the incorporation of L nucleotides. (B) Below the melting temperature of the oligomers, duplex forming strands hybridize to complementary sequences and form short double-stranded helices. (C) At sufficient concentrations, the short double-stranded nucleic acids phase separate into liquid crystal droplets due to preferential co-axial stacking and depletion forces from the single strands in solutions.
The concentration problem
The increased local order resulting from self-assembly processes would have required an environmental source of energy to allow self-assembly to occur spontaneously. An easy way to lower the entropic cost of self-assembly is to maintain locally high concentrations of building blocks. Prebiotic self-assembly processes such as membrane formation and template-directed polymerization thus necessitate high (~mM) monomer concentrations due to the weak interactions involved. Similarly, any process that involves precipitation requires saturating monomer concentrations. Concentration is also a crucial parameter for prebiotic chemistry; since water is ever-present as a competing nucleophile, high reactant concentrations are often needed to favor product formation over hydrolysis.
If prebiotic building blocks were washed into and accumulated in dilute aqueous reservoirs, how could they be concentrated to the degree necessary for self-assembly into polymers or cell-like structures? The most intuitive solution to this “concentration problem” is simply solvent evaporation (56) in environments such as ponds or tidal pools, creating a thickened ‘primordial soup’ within which prebiotic chemistry and assembly could occur. A common phase transition, freezing, also provides a simple and surprisingly effective concentration mechanism as a result of the formation of pure water-ice crystals and the concentration of solutes in the thin layer of eutectic solution between the crystal grains. Polymerization is greatly enhanced in the eutectic phase when dilute solutions of activated nucleotides are frozen (53, 76, 78). Other concentration methods are more specific, such as mineral surfaces that increase effective local concentrations via adsorption. The aldolization of glycoaldehyde phosphate into tetrose diphosphates and hexose triphosphates was shown to occur at 1000-fold lower concentrations when the glycoaldehyde phosphate was first adsorbed on common metal hydroxide minerals (59).
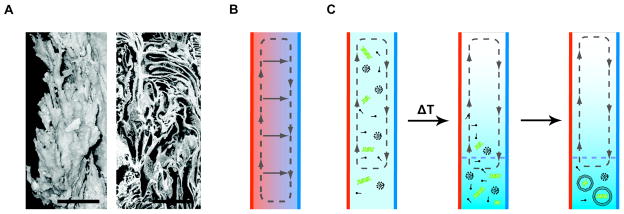
A very different concentration mechanism proposed by Braun and colleagues involves the natural formation of thermal diffusion columns in a hydrothermal setting (1). Explorations of off-axis, alkaline vents have revealed unusually porous rock structures, containing a network of narrow channels (38) (Figure 3A). Due to their proximity to both hot vent water and cold ocean water, significant temperature gradients can develop across such channels. A lateral thermal gradient across a vertical channel results in the coupling of two physical processes: 1) a vertical convective flow as cold fluid sinks and hot fluid rises and 2) the thermal diffusion (or thermophoresis) of molecules along the temperature gradient (Figure 3B). This coupling can lead to large accumulations of solutes in both gases and liquids, and was used as an industrial process (known as Clusius-Dickel separations or thermal diffusion columns) in the middle of the 20th century (31). Braun and colleagues performed numerical simulations that predicted extreme accumulations (> 1010 fold enrichment) of nucleotides and polynucleotides based on experimental measurements of thermal diffusivities. We have recently validated this concentration mechanism experimentally by observing the accumulation of small molecules (> 1000 fold enrichment) in response to the imposition of a transverse temperature gradient across glass microchannels (9).
Figure 3.
Molecular concentration by thermal diffusion columns in a hydrothermal vent setting. (A) Image (left, scale bar represents 3.5 cm) of a vent chimney (composed of calcium carbonate) taken from the Lost City alkaline hydrothermal field. Cross-sectional micrographs (right, scale bar represents 500 μm) show that the rock contains numerous pores and channels, which are proximal to both hot vent water and cold ocean water. Such structures have been proposed to act as sites for natural thermal diffusion columns. Figure modified, with permission, from Reference 39. (B) Schematic for a thermal diffusion column. A lateral temperature gradient across a vertical channel induces convective flow (dashed line) due to buoyancy effects. Thermophoresis (solid lines) also occurs along the direction of the temperature gradient. The coupling of these two processes can lead to significant concentration of selected molecular species towards the bottom of the channel. (C) Schematic for the assembly of cell-like structures from a dilute prebiotic reservoir in a thermal diffusion column. Prebiotic chemical processes generate a dilute solution containing simple lipids, which exist as monomers or micelles at low concentrations, and nucleic acids (represented as green helices) (left). A temperature gradient causes both components to become concentrated. Once their concentration exceeds a characteristic threshold (below the dashed line), the lipids self-assemble into bilayer vesicles, which encapsulate the concentrated nucleic acids in the solution (right).
The concentration of solutes in response to a temperature gradient is intriguing for a number of reasons. First, the accumulation is specific in that for a given temperature gradient, the concentration effect depends on the coupling between the channel diameter and the solute thermal diffusivity. Thus, unlike evaporation or freezing, which concentrate all solutes and thus invariably generate brine-like solutions, a given thermal diffusion column only concentrates a relatively narrow range of molecules based on their molecular (size) and thermal diffusivities. Furthermore, it is a steady state system: the constant input of thermal energy via heating of the channel wall keeps the local concentration continuously high. Hydrothermal systems have long been proposed as sites for prebiotic chemistry, suggesting that the channels in vent precipitates could have coupled prebiotic synthesis with self-assembly. We demonstrated that tehrmophoresis could facilitate the concentration-dependent process of fatty acid vesicle self-assembly, a model system for the formation of early cell-like structures (see below) (9). Model microchannels were able to concentrate dilute solutions of fatty acids leading to the formation of fatty acid vesicles. In a second experiment, nucleic acid oligomers were also included in the initial solution and co-concentrated with the fatty acids, leading to the self-assembly of vesicles encapsulating the oligomers. These experiments demonstrate that simple and surprising physical phenomena could have provided the necessary energy for the assembly of cell-like structures through the concentration of dilute solutions of building blocks (Figure 3C). The localized nature of the process allows it to guide the self-assembly of these structures through the co-concentration of multiple biological components, e.g. lipids and genetic polymers. However, marine environments feature very high salt concentrations, which generally inhibit thermal diffusion. Seawater also contains high concentrations of divalent salts, which prevent membrane assembly from fatty acids. These constraints suggest that analogous fresh-water hydrothermal systems would have provided a more suitable environment.
Cellular replication
Primitive membranes and their physical properties
Biological systems require some means of preventing their genetic information from diffusing away and any evolved biochemical (e.g. catalytic) complexity from being wasted on neighboring (non-self) molecules in the environment. Therefore, compartmentalization has long been recognized as a physical pre-requisite for Darwinian evolution. A variety of physical barriers to diffusion (e.g. surface adsorption, emulsion droplets, hydrogels) could have initially served as compartments. All modern cells, however, use bilayer membranes composed primarily of di-acyl or di-alkyl glycerolphospholipids for encapsulation. This is not a coincidence; the dynamic structure of fluid membranes seems particularly well suited for growth, division, and propagation. However, phospholipids form membranes that are unsuitable for a primitive cell, since they require complex highly evolved protein machinery to mediate both growth and the transport of nutrients and waste products into and out of the cell.
The properties of bilayer membranes composed of simple, single chain amphiphiles such as fatty acids provide a solution to the problem of early cell membrane composition. A component of phospholipids, fatty acids are considered prebiotically plausible due to their presence in meteorites (55, 89) and because of the possibility of abiotic terrestrial synthesis via Fischer-Tropsch-type reactions (50, 68). Early work demonstrated that fatty acids can form a variety of aggregate phases in buffered solutions, including bilayer membrane vesicles (21), much like phospholipids.
Fatty acid aggregation is dependent on the protonation state of the carboxyl head group and thus the pH of the solution. Fatty acids spontaneously assemble into bilayer membranes when the solution pH is near the pKa of the membrane-incorporated acid, due to hydrogen bonding between protonated and anionic monomers in the bilayer (14, 25). At higher pH, the deprotonated anions aggregate into micelles, while at lower pH the protonated acids condense into oil droplets. Fatty acid membrane assembly is an autocatalytic process, since pre-formed membrane increases the rate of de novo vesicle formation (4). In this autocatalytic assembly process existing vesicles may act as a surface on which newly formed bilayers can organize (12). What other surfaces could provide this same effect? In an intriguing example of prebiotic convergence, a variety of minerals including montmorillonite, the clay that catalyzes polymerization of nucleotides into RNA, also catalyze the formation of fatty acid vesicles (26, 27). Clay particles bearing adsorbed RNA oligomers can become encapsulated within fatty acid vesicles that they have helped to assemble. This could have provided a robust kinetic pathway for the encapsulation of newly-polymerized RNA within fatty acid vesicles, thereby assembling simple cell-like structures. These results demonstrate that physical processes could have exerted kinetic control over cellular self-assembly during the earliest stages of life.
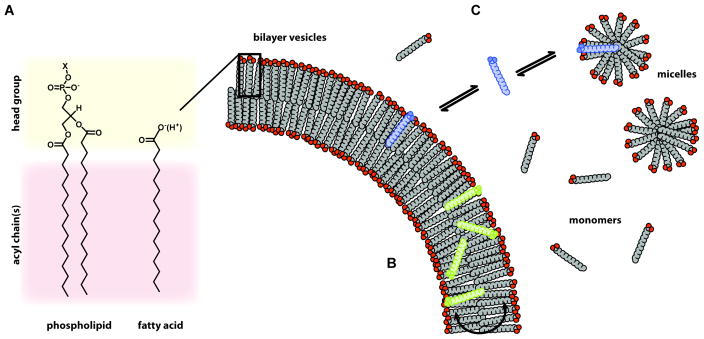
For a primitive cell to reproduce, its membrane would have had to be able to grow, divide, and the mediate the transport of chemical building blocks, all without the assistance of any complex protein machinery. Primitive cell membranes thus had to be both chemically simpler and functionally more complex than modern cell membranes. Though fatty acid membranes are structurally analogous to phospholipid membranes (Figure 4A), they have drastically different dynamic properties. In phospholipid membranes, lipids are largely restricted to lateral diffusion, as flip-flop occurs on a timescale of hours or more (46) because of the large, polar head group. Individual lipids are also kinetically trapped in their respective membrane vesicles due to their extremely low water-solubility. In fatty acid membranes, however, trans-bilayer flip-flop occurs very rapidly (milliseconds) (35) due to the small carboxyl head group, which is rapidly neutralized by protonation or salt (soap) formation (Figure 4B). The single acyl-chain lipids are also more water-soluble, and are constantly leaving and re-entering the bilayer. Thus, while a fatty acid vesicle is stable over indefinite timescales, individual monomers rapidly exchange between vesicles, as well as micelles in the solution (Figure 4C). The kinetics and exact modes of lipid exchange in these complex systems remains an area of active research. As we will see, these simple dynamic properties of fatty acid membranes have surprising consequences.
Figure 4.
Schematic of the dynamic processes intrinsic to fatty acid membrane systems. (A) Comparison of the chemical structures of phospholipids, the major constituent of modern cell membranes, and fatty acids, which have been proposed to serve an analogous role in primitive cell membranes. Phospholipids feature complex, charged head groups with a variety of possible functional groups (X) e.g. choline, glycerol, and serine. In contrast, fatty acids have a simple carboxylate as their hydrophilic head group. The smaller, easily neutralized carboxylate allows fatty acids to rapidly flip across the bilayer (B), which is crucial for the high permeability of fatty acid membranes. Fatty acids are also mono-acyl lipids, as opposed to di-acyl (di-alkyl for archaea) phospholipids, which increases their water solubility. Fatty acid bilayers are in equilibrium with significant concentrations of micelles and monomers in solution, with individual fatty acids rapidly transferring between these structures (C). This lipid phase polymorphism and dynamic exchange is essential for fatty acid vesicle growth and competition.
Membrane growth and division
In modern cells, phospholipids are constructed by membrane-localized enzymes, and are incorporated into the membrane at their site of synthesis. However, in the absence of internal, metabolic lipid synthesis, what thermodynamic forces would drive the process of membrane growth? Luisi and colleagues first demonstrated that the pH controlled phase transition of fatty acids can be used to drive vesicle growth by adding alkaline fatty acid micelles to a buffered suspension of pre-formed vesicles (4). Upon entering an environment of lower pH, micelles are destabilized and their component molecules either incorporate into existing bilayers or assemble into new ones. Vesicle growth has since been extensively studied using quantitative techniques, most notably light scattering (63) and Förster resonance energy transfer (FRET) (26). FRET studies have highlighted the kinetic competition between vesicle growth and de novo vesicle nucleation. The efficiency of the growth pathway is inversely dependent on the rate of micelle addition (12) and if the micelle feedstock is introduced very slowly, growth can approach 100% efficiency.
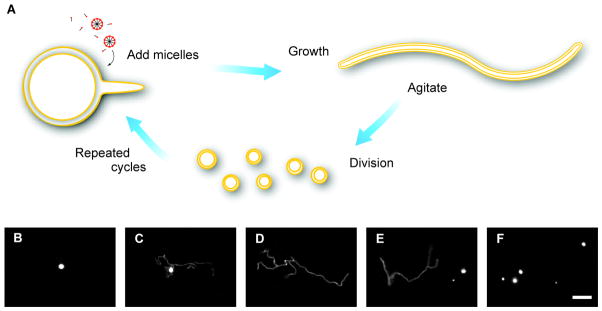
Division appears to be a more challenging task for a primitive cell, both because of the complexity of extant division machinery and the lack of an easily identifiable driving force. Early work on fatty acid vesicle division utilized pressure-driven extrusion through small pores as a model for primitive cellular division (26). However, extrusion is prebiotically implausible and the process results in significant loss of encapsulated contents. A novel pathway recently discovered in our laboratory provides a more robust and plausible option (91) (Figure 5). This pathway relies upon differing rates of membrane surface increase and vesicle volume change. Vesicle growth by micelle addition results in a rapid (minutes) increase in membrane surface area as new monomers are incorporated in the bilayer. However, the rate of the corresponding increase in volume is osmotically limited by the permeability of solutes in the media; if the solution contains highly charged molecules, for example, volume growth is slower (hours to days). When volume growth lags behind surface area expansion, the result is the transformation of the initially spherical vesicles into long, filamentous vesicles (Figure 5C, 5D). This is particularly dramatic for multilamellar vesicles (MLVs), which form spontaneously in lipid suspensions. Because the outermost bilayer grows first, and since the volume between adjacent bilayers in MLVs is minimal, the outermost bilayer emerges as a thin tubule that gradually elongates and progressively absorbs the remaining membranes and contents of the initially spherical vesicle. The resulting multilamellar filamentous structures are highly unstable and readily divide into daughter vesicles upon exposure to mild shear forces (Figure 5E, 5F), with negligible loss of encapsulated content. Given an environment rich in slowly permeating solutes such as amino acids and nucleotides, and in the presence of mild shear forces, such as those resulting from wind driven waves, growth and division become inherently coupled.
Figure 5.
Coupled growth and division of fatty acid vesicles. (A) Schematic and (B–F) microscopy images of large (~4 μm) fatty acid vesicles undergoing growth and division. (B) Alkaline fatty acid micelles are added to a solution of initially spherical vesicles. (C, D) Rapid incorporation of the micelles causes the pre-formed vesicles to grow into long, filamentous structures at 10 and 25 minutes, respectively, after micelle addition. (E, F) Mild shear forces, such as solution agitation, cause the filamentous vesicles to break apart into many daughter vesicles, which can then undergo further rounds of growth and division. Vesicles were labeled by encapsulated fluorescent dye, which stays entrapped throughout the cycle. Scale bar represents 10 μm. Figure modified, with permission, from Reference 91.
Although the physical mechanism of shear-induced division remains unclear, the process may be related to the previously reported “pearling instability” in tubular membrane vesicles (2, 3). When a surface tension is induced on a membrane, it drives vesicle shape fluctuations that minimize the membrane surface area. This energy-minimization causes initially tubular vesicles to pinch off into attached spherical structures (“beads on a string”). In these experiments surface tension was induced directly via optical trap pulling; shear force induction of membrane surface tension has not been thoroughly explored. An unexpected addendum to these physical models of vesicle division is the recent report of a similar process occurring in vivo (36, 37, 43). Mutant L-form (cell-wall less) bacterial strains have been shown to divide without the standard fission machinery but rather by a physical process that appears, at least visually, to involve the pearling instability. Before the evolution of cellular division machinery, cells may have depended on similar physical processes for their replication.
Genome replication in early cells
Replication of some kind of genetic polymer is a central requirement for the origin of life. Whether the first genetic material was RNA, or a simpler or easier to generate progenitor is unknown. Current experimental approaches to this problem include studies of the non-enzymatic (chemical) (29, 47) replication of a wide range of nucleic acid and other polymers, as well as studies of ribozyme-mediated RNA replication (30, 44). Since there is currently no informational replication system that does not rely on protein enzymes, the discovery of even one such system would be a great advance in our understanding of the chemical requirements for replication. Moreover, a fatty acid vesicle encapsulating catalytically active, self-replicating genetic polymers is an attractive minimal model for early cellular life. Such a simple cell would be inherently heterotrophic, obtaining the building blocks needed for replication from its environment. A key requirement would thus be the ability of its membrane to transport large, polar chemical components across its membrane without any protein machinery.
The phospholipid membranes of modern cells are generally impermeable to all but small, non-polar molecules, and it is thought that some extremophiles go to great lengths to minimize the permeability of their membranes (80). The low permeability of phospholipid membranes to polar solutes is consistent with the desolvation model of permeation: bound water has to dissociate before a solute molecule can diffuse into the hydrophobic core of the bilayer, and for large polar or charged molecules this is essentially impossible. However, studies of fatty acid-based membranes have shown surprisingly different permeation behavior. Early work demonstrated that fatty acids allow cations across membranes very rapidly because individual fatty acids carry complexed cations across during flip-flop (34). For larger molecules, fatty acid permeability is less well-understood, but is generally dependent on the flip-flop rate (which is inversely related to the acyl chain length) and packing constraints, such as the addition of minor membrane components with bulky head groups or branched alkyl chains. Membranes composed of fatty acids mixed with their associated glycerol monoesters and fatty alcohols have been shown to be both structurally stable, resisting aggregation from divalent cations, and highly permeable. Recent work by Mansy et. al. (47) demonstrated that such mixtures of lipids lead to vesicles that are permeable to nucleotides, while still permanently entrapping oligomers. When activated nucleotides were introduced into a solution of template-containing vesicles, spontaneous template-directed polymerization was observed, indicating that the charged nucleotides were able to enter the vesicles and then perform polymerization chemistry inside. Permeation of nucleotides is unlikely to be due to desolvation; rather a combination of interactions, including solute-lipid association, lipid flip-flop, and stabilization of transient defects are thought to contribute to transport. Such high permeability would be disastrous to modern cells, which must retain the building blocks that they have synthesized. The gradual transition from highly permeable, dynamic membranes to impermeable, sturdy membranes must have mirrored the evolution of both metabolism and transport machinery in early cells.
Primitive cell membranes occasionally show surprisingly selective permeability, which could have had important implications for the function of early cells lacking specific transporters. For example, fatty acid and phospholipid membranes are ~ 5-times more permeable to ribose than its diastereomers arabinose, lyxose and xylose (69). Recent molecular dynamics (MD) simulations indicate that these disparate permeabilities are a consequence of the favorable intramolecular hydrogen bonding between the ribose hydroxyls. The decrease in bound water corresponds to an increase in solubility in the hydrophobic interior of the bilayer membrane (86). Such selectivity could have imposed a kinetic preference on early cells for the use of specific building blocks (e.g. ribose for nucleotide synthesis) over others that might have been equally available in the environment but harder to take up spontaneously.
For a primitive cell genome to undergo continual replication, the template must separate from the newly synthesized complementary strand after each round of replication. The obvious physical solution for strand separation is thermal cycling, as used in the polymerase chain reaction (PCR). Experimental work shows that thermal cycling can easily occur as a result of the spontaneous generation of convective flow cells (6, 28). In these experiments, robust PCR (comparable to that in a commercial thermal cycler) was demonstrated in a variety of container geometries in the presence of a single heated wall or probe. Here the heat flux provides the temperature gradient needed to drive the convective flow so that duplex DNA melts and the strands separate during the transient exposure to high temperature, followed by strand copying at lower temperature. Analogous prebiotic situations, such as cold ponds with local geothermal heating, are easily conceivable and could provide a simple means by which a primitive cell cycle could be driven by environmental fluctuations.
The presumption of thermal strand separation has important implications for the chemical nature of the cellular components. Genetic polymers that feature high duplex stability, and thus high melting temperatures, such as peptide nucleic acid (PNA) (64), or even very long RNA oligomers, are hard to denature by thermal cycling at < 100° C. Without helicases or processive polymerases, such polymers would therefore not be suitable for a primitive cell. On the other hand, high thermostability would have been essential for the membranes of these cells to prevent the loss of genetic materials during cycling. Recent work has shown fatty acid membranes to be surprisingly thermostable (48), especially when fatty alcohol and fatty acid glycerol ester derivatives are incorporated into the membrane. This is presumably because of stabilizing head group interactions between these derivatives, which feature hydrogen bond donors and acceptors, and neighboring fatty acids in the bilayer. The seemingly ideal nature of these fatty acid mixtures for early cells is reassuring; abiotic modes of lipid synthesis would have certainly yielded similarly complex product mixtures. This is in contrast to nucleic acids, where high monomer purity is a seemingly strict requirement for template-copying.
Emergence of complexity in early cells
Biological systems are not defined as such by their chemical components but rather by their ability to undergo Darwinian evolution. Evolution by natural selection has resulted in the spread of unicellular life across the planet as cells have adapted to a wide range of environmental niches and evolved the ability to metabolize virtually any available nutrient or source of chemical energy. In contrast, the first simple heterotrophic cells depended on the environmental supply of pre-assembled nutrients. In such a precarious situation, evolution and adaptation could have taken hold in unexpected ways.
A striking example of this competition for limited resources is the ability of fatty acid vesicles to compete with one another through the preferential exchange of fatty acid monomers. Fatty acid vesicles that are hypertonic (osmotically swollen) because of a high concentration of internal nucleic acids or other impermeable solutes will grow at the expense of nearby isotonic, empty vesicles, which shrink (11). The growth of swollen vesicles occurs because osmotic swelling results in membrane tension, which can be relaxed by increasing the membrane surface area. This in turn occurs because the normal near-equilibrium state of monomer exchange between individual vesicles is altered as fatty acid desorption from the osmotically stressed vesicles is reduced, which causes a net influx of fatty acid monomers from the relaxed vesicles into the osmotically stressed vesicles. This process depends upon the rapid exchange of fatty acid monomers between individual vesicles in a population, and therefore does not occur with phospholipid vesicles. These observations imply that early cells could have competed for membrane components by evolving the ability to synthesize higher levels of impermeable solutes, such as the nucleic acid oligomers that made up their genome. Thus mutations that increased the efficiency of a ribozyme replicase or affected any other process that increased the rate of genomic replication would also drive cell growth. The importance of this osmotic competition effect may have been limited because of the high concentrations of encapsulated nucleic acid required to drive growth, and by the inherent difficulty of subsequently dividing osmotically pressurized vesicles. However, the preferential uptake of lipids is potentially a general route for the emergence of competition because any chemical component, such as e.g. more complex lipids, that reduces fatty acid desorption by stabilizing the bilayer could drive growth and be positively selected.
As the first cells experienced changing selective pressures and adapted to novel geochemical environments, Darwinian evolution would have fueled the development of greater diversity and complexity. There is obviously a nearly limitless set of plausible adaptations that would be beneficial for early cells. For example, it has been shown that fatty acid vesicle growth via micelles results in the generation of transient transmembrane pH gradients (13). If early cells evolved membranes that could slow rapid proton leakage and machinery that could harness a proton flux, such spontaneously generated pH gradients could have been an early source of chemical potential, fueling essential processes such as nucleotide activation. The ongoing initiative to recreate synthetic model cells (protocells) in the laboratory is an effort to experimentally observe such processes and discover novel mechanisms for the emergence of biological complexity (77).
Conclusions
The experiments that we have discussed paint a picture of an early biology that is dependent on its environment both as a source of chemical building blocks and as a facilitator of fundamental cellular processes. Evaluating scenarios for the origin of life requires experimental evaluation of the potential of abiotic physical and chemical processes to drive basic cellular functions. The evolution of increasingly complex enzymatic and structural machinery over the millions of years following the origin of life gradually freed cells from their restriction to highly specific physical niches and allowed the colonization of more of the planet. The further study of the phenomena described here as well as the discovery of novel physical mechanisms will be essential for a better understanding of how life could have began.
Summary Points.
Experimental models have emerged for the induction of chiral asymmetry in populations of molecules, and the subsequent amplification of a small initial asymmetry to a state approaching homochirality. Similar processes may have generated homochiral pools of biomolecular building blocks, thus paving the way for the emergence of the first biological systems.
Prebiotically relevant and geophysically reasonable methods for purifying and concentrating molecular building blocks have been proposed, leading to plausible pathways for the assembly of ordered, cell-like structures.
Membranes composed of mono-acyl lipids, such as fatty acids, seem to be well suited for a role as primitive biological membranes. The intrinsic physical properties of fatty acid-based membranes have been shown to allow for their growth and division, and the transport of polar building blocks.
The continuing effort to synthesize simple, synthetic cells in the laboratory is an informative approach to understanding how early biological systems could have functioned and evolved.
Acknowledgments
We acknowledge A. Ricardo, R. Bruckner, T. Zhu, J. Chen, and S. Trevino and many other members of the Szostak lab for helpful discussions. A. Ricardo proposed glyceraldehyde dimer formation as a potential mechanism for chiral enrichment. J.W.S is an Investigator of the Howard Hughes Medical Institute. This work was supported in part by grant EXB02-0031-0018 from the NASA Exobiology Program and and grant CHE 0434507 from the NSF.
Acronyms
- ee
enantiomeric excess
- CPL
circular polarized light
- LC
liquid crystal
- FRET
Förster resonance energy transfer
- MLV
multi-lamellar vesicle
- PCR
polymerase chain reaction
Terms/Definitions
- enantiomeric excess
the difference between the molar fractions (or percentages) of the two chiral enantiomers
- racemate
a mixture containing equal amounts (1:1 molar ratio) of both enantiomers of a chiral molecule
- conglomerate
a solid state in which each enantiomer of a chiral molecule forms separate crystals
- thermal diffusion
the tendency of molecules to migrate along a temperature gradient
- multi-lamellar vesicle
an enclosed membrane structure containing multiple, concentric bilayers
LITERATURE CITED
- 1.Baaske P, Weinert FM, Duhr S, Lemke KH, Russell MJ, Braun D. Extreme accumulation of nucleotides in simulated hydrothermal pore systems. Proc Natl Acad Sci U S A. 2007;104:9346. doi: 10.1073/pnas.0609592104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bar-Ziv R, Moses E. Instability and “pearling” states produced in tubular membranes by competition of curvature and tension. Phys Rev Lett. 1994;73:1392. doi: 10.1103/PhysRevLett.73.1392. [DOI] [PubMed] [Google Scholar]
- 3.Bar-Ziv R, Tlusty T, Moses E. Critical Dynamics in the Pearling Instability of Membranes. Physical Review Letters. 1997;79:1158. [Google Scholar]
- 4.Berclaz N, Muller M, Walde P, Luisi PL. Growth and Transformation of Vesicles Studied by Ferritin Labeling and Cryotransmission Electron Microscopy. The Journal of Physical Chemistry B. 2001;105:1056. [Google Scholar]
- 5.Blackmond DG, McMillan CR, Ramdeehul S, Schorm A, Brown JM. Origins of asymmetric amplification in autocatalytic alkylzinc additions. J Am Chem Soc. 2001;123:10103. doi: 10.1021/ja0165133. [DOI] [PubMed] [Google Scholar]
- 6.Braun D, Goddard NL, Libchaber A. Exponential DNA replication by laminar convection. Phys Rev Lett. 2003;91:158103. doi: 10.1103/PhysRevLett.91.158103. [DOI] [PubMed] [Google Scholar]
- 7.Breslow R, Cheng ZL. On the origin of terrestrial homochirality for nucleosides and amino acids. Proc Natl Acad Sci U S A. 2009;106:9144. doi: 10.1073/pnas.0904350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breslow R, Levine MS. Amplification of enantiomeric concentrations under credible prebiotic conditions. Proc Natl Acad Sci U S A. 2006;103:12979. doi: 10.1073/pnas.0605863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budin I, Bruckner RJ, Szostak JW. Formation of protocell-like vesicles in a thermal diffusion column. J Am Chem Soc. 2009;131:9628. doi: 10.1021/ja9029818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butlerow A. Formation synthetique d’une substance sucree. Compt Rend Acad Sci. 1861;53:145. [Google Scholar]
- 11.Chen IA, Roberts RW, Szostak JW. The emergence of competition between model protocells. Science. 2004;305:1474. doi: 10.1126/science.1100757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen IA, Szostak JW. A kinetic study of the growth of fatty acid vesicles. Biophys J. 2004;87:988. doi: 10.1529/biophysj.104.039875. A comprehensive FRET-based study of fatty acid vesicle growth by micelle addition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen IA, Szostak JW. Membrane growth can generate a transmembrane pH gradient in fatty acid vesicles. Proc Natl Acad Sci U S A. 2004;101:7965. doi: 10.1073/pnas.0308045101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cistola DP, Hamilton JA, Jackson D, Small DM. Ionization and phase behavior of fatty acids in water: application of the Gibbs phase rule. Biochemistry. 1988;27:1881. doi: 10.1021/bi00406a013. [DOI] [PubMed] [Google Scholar]
- 15.Cronin JR, Pizzarello S. Enantiomeric excesses in meteoritic amino acids. Science. 1997;275:951. doi: 10.1126/science.275.5302.951. [DOI] [PubMed] [Google Scholar]
- 16.Feeley TM, Hargreaves MK, Marshall DL. The circular dichroism of some [alpha]-hydroxyaldehydes. Tetrahedron Letters. 1968;9:4831. [Google Scholar]
- 17.Ferris JP, Ertem G. Oligomerization of ribonucleotides on montmorillonite: reaction of the 5′-phosphorimidazolide of adenosine. Science. 1992;257:1387. doi: 10.1126/science.1529338. [DOI] [PubMed] [Google Scholar]
- 18.Frank FC. On spontaneous asymmetric synthesis. Biochim Biophys Acta. 1953;11:459. doi: 10.1016/0006-3002(53)90082-1. [DOI] [PubMed] [Google Scholar]
- 19.Fuller WD, Sanchez RA, Orgel LE. Studies in prebiotic synthesis. VI. Synthesis of purine nucleosides. J Mol Biol. 1972;67:25. doi: 10.1016/0022-2836(72)90383-x. [DOI] [PubMed] [Google Scholar]
- 20.Gabel NW, Ponnamperuma C. Model for origin of monosaccharides. Nature. 1967;216:453. doi: 10.1038/216453a0. [DOI] [PubMed] [Google Scholar]
- 21.Gebicki JM, Hicks M. Ufasomes are stable particles surrounded by unsaturated fatty acid membranes. Nature. 1973;243:232. doi: 10.1038/243232a0. [DOI] [PubMed] [Google Scholar]
- 22.Glass JI, Assad-Garcia N, Alperovich N, Yooseph S, Lewis MR, et al. Essential genes of a minimal bacterium. Proc Natl Acad Sci U S A. 2006;103:425. doi: 10.1073/pnas.0510013103. A transposon-based screen identifying a potentially minimal set of genes in the simple bacterium Mycoplasma genitalium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glavin DP, Dworkin JP. Enrichment of the amino acid L-isovaline by aqueous alteration on CI and CM meteorite parent bodies. Proc Natl Acad Sci U S A. 2009;106:5487. doi: 10.1073/pnas.0811618106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guerrier-Takada C, Gardiner K, Marsh T, Pace N, Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983;35:849. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- 25.Haines TH. Anionic lipid headgroups as a proton-conducting pathway along the surface of membranes: a hypothesis. Proc Natl Acad Sci U S A. 1983;80:160. doi: 10.1073/pnas.80.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanczyc MM, Fujikawa SM, Szostak JW. Experimental models of primitive cellular compartments: encapsulation, growth, and division. Science. 2003;302:618. doi: 10.1126/science.1089904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanczyc MM, Mansy SS, Szostak JW. Mineral surface directed membrane assembly. Orig Life Evol Biosph. 2007;37:67. doi: 10.1007/s11084-006-9018-5. [DOI] [PubMed] [Google Scholar]
- 28.Hennig M, Braun D. Convective polymerase chain reaction around micro immersion heater. Applied Physics Letters. 2005;87:183901. [Google Scholar]
- 29.Inoue T, Orgel LE. A nonenzymatic RNA polymerase model. Science. 1983;219:859. doi: 10.1126/science.6186026. [DOI] [PubMed] [Google Scholar]
- 30.Johnston WK, Unrau PJ, Lawrence MS, Glasner ME, Bartel DP. RNA-catalyzed RNA polymerization: accurate and general RNA-templated primer extension. Science. 2001;292:1319. doi: 10.1126/science.1060786. The in-vitro selection of a ribozyme that catalyzes the template-directed extension of an RNA primer with nucleoside triphosphate substrates. [DOI] [PubMed] [Google Scholar]
- 31.Jones AL, Milberger EC. Separation of Organic Liquid Mixtures by Thermal Diffusion. Industrial & Engineering Chemistry. 1953;45:2689. [Google Scholar]
- 32.Joyce GF, Schwartz AW, Miller SL, Orgel LE. The case for an ancestral genetic system involving simple analogues of the nucleotides. Proc Natl Acad Sci U S A. 1987;84:4398. doi: 10.1073/pnas.84.13.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joyce GF, Visser GM, van Boeckel CA, van Boom JH, Orgel LE, van Westrenen J. Chiral selection in poly(C)-directed synthesis of oligo(G) Nature. 1984;310:602. doi: 10.1038/310602a0. [DOI] [PubMed] [Google Scholar]
- 34.Kamp F, Hamilton JA. pH gradients across phospholipid membranes caused by fast flip-flop of un-ionized fatty acids. Proc Natl Acad Sci U S A. 1992;89:11367. doi: 10.1073/pnas.89.23.11367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamp F, Zakim D, Zhang F, Noy N, Hamilton JA. Fatty acid flip-flop in phospholipid bilayers is extremely fast. Biochemistry. 1995;34:11928. doi: 10.1021/bi00037a034. [DOI] [PubMed] [Google Scholar]
- 36.Kandler G, Kandler O. Untersuchungen über die Morphologie und die Vermehrung der pleuropneumonie-ähnlichen Organismen und der L-Phase der Bakterien. Archives of Microbiology. 1954;21:178. [PubMed] [Google Scholar]
- 37.Kandler G, Kandler O, Huber O. Untersuchungen über die Morphologie und die Vermehrung der pleuropneumonieähnlichen Organismen und der L-Phase der Bakterien. Archives of Microbiology. 1954;21:202. [PubMed] [Google Scholar]
- 38.Kelley DS, Karson JA, Fruh-Green GL, Yoerger DR, Shank TM, et al. A serpentinite-hosted ecosystem: the Lost City hydrothermal field. Science. 2005;307:1428. doi: 10.1126/science.1102556. [DOI] [PubMed] [Google Scholar]
- 39.Klussmann M, Iwamura H, Mathew SP, Wells DH, Jr, Pandya U, et al. Thermodynamic control of asymmetric amplification in amino acid catalysis. Nature. 2006;441:621. doi: 10.1038/nature04780. [DOI] [PubMed] [Google Scholar]
- 40.Klussmann M, Izumi T, White AJ, Armstrong A, Blackmond DG. Emergence of solution-phase homochirality via crystal engineering of amino acids. J Am Chem Soc. 2007;129:7657. doi: 10.1021/ja0708870. [DOI] [PubMed] [Google Scholar]
- 41.Kruger K, Grabowski PJ, Zaug AJ, Sands J, Gottschling DE, Cech TR. Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell. 1982;31:147. doi: 10.1016/0092-8674(82)90414-7. [DOI] [PubMed] [Google Scholar]
- 42.Larralde R, Robertson MP, Miller SL. Rates of decomposition of ribose and other sugars: implications for chemical evolution. Proc Natl Acad Sci U S A. 1995;92:8158. doi: 10.1073/pnas.92.18.8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leaver M, Dominguez-Cuevas P, Coxhead JM, Daniel RA, Errington J. Life without a wall or division machine in Bacillus subtilis. Nature. 2009;457:849. doi: 10.1038/nature07742. [DOI] [PubMed] [Google Scholar]
- 44.Lincoln TA, Joyce GF. Self-sustained replication of an RNA enzyme. Science. 2009;323:1229. doi: 10.1126/science.1167856. A recent demonstration of ribozyme autocatalysis in which two RNA ligases catalyze each other’s assembly from oligonucleotide substrates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.List B, Lerner RA, Barbas CF. Proline-Catalyzed Direct Asymmetric Aldol Reactions. Journal of the American Chemical Society. 2000;122:2395. [Google Scholar]
- 46.Liu J, Conboy JC. 1,2-diacyl-phosphatidylcholine flip-flop measured directly by sum-frequency vibrational spectroscopy. Biophys J. 2005;89:2522. doi: 10.1529/biophysj.105.065672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mansy SS, Schrum JP, Krishnamurthy M, Tobe S, Treco DA, Szostak JW. Template-directed synthesis of a genetic polymer in a model protocell. Nature. 2008;454:122. doi: 10.1038/nature07018. Presents an experimental model for a simple, heterotrophic cell made possible by spontaneous template copying chemistry and the permeability of fatty acid-based membranes to activated nucleotides. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mansy SS, Szostak JW. Thermostability of model protocell membranes. Proc Natl Acad Sci U S A. 2008;105:13351. doi: 10.1073/pnas.0805086105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McBride JM, Tully JC. Physical chemistry: did life grind to a start? Nature. 2008;452:161. doi: 10.1038/452161a. [DOI] [PubMed] [Google Scholar]
- 50.McCollom TM, Ritter G, Simoneit BR. Lipid synthesis under hydrothermal conditions by Fischer-Tropsch-type reactions. Orig Life Evol Biosph. 1999;29:153. doi: 10.1023/a:1006592502746. [DOI] [PubMed] [Google Scholar]
- 51.Meierhenrich UJ, Nahon L, Alcaraz C, Bredehoft JH, Hoffmann SV, et al. Asymmetric vacuum UV photolysis of the amino acid leucine in the solid state. Angew Chem Int Ed Engl. 2005;44:5630. doi: 10.1002/anie.200501311. [DOI] [PubMed] [Google Scholar]
- 52.Miller SL. A production of amino acids under possible primitive earth conditions. Science. 1953;117:528. doi: 10.1126/science.117.3046.528. [DOI] [PubMed] [Google Scholar]
- 53.Monnard PA, Kanavarioti A, Deamer DW. Eutectic phase polymerization of activated ribonucleotide mixtures yields quasi-equimolar incorporation of purine and pyrimidine nucleobases. J Am Chem Soc. 2003;125:13734. doi: 10.1021/ja036465h. [DOI] [PubMed] [Google Scholar]
- 54.Nakata M, Zanchetta G, Chapman BD, Jones CD, Cross JO, et al. End-to-end stacking and liquid crystal condensation of 6 to 20 base pair DNA duplexes. Science. 2007;318:1276. doi: 10.1126/science.1143826. [DOI] [PubMed] [Google Scholar]
- 55.Naraoka H, Shimoyama A, Harada K. Molecular distribution of monocarboxylic acids in Asuka carbonaceous chondrites from Antarctica. Orig Life Evol Biosph. 1999;29:187. doi: 10.1023/a:1006547127028. [DOI] [PubMed] [Google Scholar]
- 56.Nelson KE, Robertson MP, Levy M, Miller SL. Concentration by evaporation and the prebiotic synthesis of cytosine. Orig Life Evol Biosph. 2001;31:221. doi: 10.1023/a:1010652418557. [DOI] [PubMed] [Google Scholar]
- 57.Noorduin WL, Izumi T, Millemaggi A, Leeman M, Meekes H, et al. Emergence of a single solid chiral state from a nearly racemic amino acid derivative. J Am Chem Soc. 2008;130:1158. doi: 10.1021/ja7106349. [DOI] [PubMed] [Google Scholar]
- 58.Noorduin WL, Meekes H, Bode AAC, van Enckevort WJP, Kaptein B, et al. Explanation for the Emergence of a Single Chiral Solid State during Attrition-Enhanced Ostwald Ripening: Survival of the Fittest. Crystal Growth & Design. 2008;8:1675. [Google Scholar]
- 59.Pitsch S, Eschenmoser A, Gedulin B, Hui S, Arrhenius G. Mineral induced formation of sugar phosphates. Orig Life Evol Biosph. 1995;25:297. doi: 10.1007/BF01581773. [DOI] [PubMed] [Google Scholar]
- 60.Pizzarello S, Weber AL. Prebiotic amino acids as asymmetric catalysts. Science. 2004;303:1151. doi: 10.1126/science.1093057. [DOI] [PubMed] [Google Scholar]
- 61.Powner MW, Gerland B, Sutherland JD. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature. 2009;459:239. doi: 10.1038/nature08013. [DOI] [PubMed] [Google Scholar]
- 62.Prabahar KJ, Ferris JP. Adenine derivatives as phosphate-activating groups for the regioselective formation of 3′,5′-linked oligoadenylates on montmorillonite: possible phosphate-activating groups for the prebiotic synthesis of RNA. J Am Chem Soc. 1997;119:4330. doi: 10.1021/ja9700764. [DOI] [PubMed] [Google Scholar]
- 63.Rasi S, Mavelli F, Luisi PL. Matrix effect in oleate micelles-vesicles transformation. Orig Life Evol Biosph. 2004;34:215. doi: 10.1023/b:orig.0000009841.20997.ac. [DOI] [PubMed] [Google Scholar]
- 64.Ratilainen T, Holmen A, Tuite E, Haaima G, Christensen L, et al. Hybridization of peptide nucleic acid. Biochemistry. 1998;37:12331. doi: 10.1021/bi9808722. [DOI] [PubMed] [Google Scholar]
- 65.Ricardo A, Carrigan MA, Olcott AN, Benner SA. Borate minerals stabilize ribose. Science. 2004;303:196. doi: 10.1126/science.1092464. [DOI] [PubMed] [Google Scholar]
- 66.Robertson MP, Miller SL. An efficient prebiotic synthesis of cytosine and uracil. Nature. 1995;375:772. doi: 10.1038/375772a0. [DOI] [PubMed] [Google Scholar]
- 67.Rosenberg RA, Abu Haija M, Ryan PJ. Chiral-selective chemistry induced by spin-polarized secondary electrons from a magnetic substrate. Phys Rev Lett. 2008;101:178301. doi: 10.1103/PhysRevLett.101.178301. [DOI] [PubMed] [Google Scholar]
- 68.Rushdi AI, Simoneit BR. Lipid formation by aqueous Fischer-Tropsch-type synthesis over a temperature range of 100 to 400 degrees C. Orig Life Evol Biosph. 2001;31:103. doi: 10.1023/a:1006702503954. [DOI] [PubMed] [Google Scholar]
- 69.Sacerdote MG, Szostak JW. Semipermeable lipid bilayers exhibit diastereoselectivity favoring ribose. Proc Natl Acad Sci U S A. 2005;102:6004. doi: 10.1073/pnas.0408440102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schoning K, Scholz P, Guntha S, Wu X, Krishnamurthy R, Eschenmoser A. Chemical etiology of nucleic acid structure: the alpha-threofuranosyl-(3′-->2′) oligonucleotide system. Science. 2000;290:1347. doi: 10.1126/science.290.5495.1347. [DOI] [PubMed] [Google Scholar]
- 71.Senma M, Taira Z, Osaki K, Taga T. Glyceraldehyde; an X-Ray Study of DL-Glyceraldehyde Dimer. Journal of the Chemical Society, Chemical Communications. 1973:880. [Google Scholar]
- 72.Shapiro R. Prebiotic ribose synthesis: a critical analysis. Orig Life Evol Biosph. 1988;18:71. doi: 10.1007/BF01808782. [DOI] [PubMed] [Google Scholar]
- 73.Soai K, Shibata T, Morioka H, Choji K. Asymmetric autocatalysis and amplification of enantiomeric excess of a chiral molecule. Nature. 1995;378:767. Provides the first example of enantioenrichment by chiral autocatalysis and cross-inhibition. [Google Scholar]
- 74.Springsteen G, Joyce GF. Selective derivatization and sequestration of ribose from a prebiotic mix. J Am Chem Soc. 2004;126:9578. doi: 10.1021/ja0483692. [DOI] [PubMed] [Google Scholar]
- 75.Steitz TA, Moore PB. RNA, the first macromolecular catalyst: the ribosome is a ribozyme. Trends Biochem Sci. 2003;28:411. doi: 10.1016/S0968-0004(03)00169-5. [DOI] [PubMed] [Google Scholar]
- 76.Stribling R, Miller SL. Template-directed synthesis of oligonucleotides under eutectic conditions. J Mol Evol. 1991;32:289. doi: 10.1007/BF02102186. [DOI] [PubMed] [Google Scholar]
- 77.Szostak JW, Bartel DP, Luisi PL. Synthesizing life. Nature. 2001;409:387. doi: 10.1038/35053176. [DOI] [PubMed] [Google Scholar]
- 78.Trinks H, Schroder W, Biebricher CK. Ice and the origin of life. Orig Life Evol Biosph. 2005;35:429. doi: 10.1007/s11084-005-5009-1. [DOI] [PubMed] [Google Scholar]
- 79.Tuinier R, Rieger J, de Kruif CG. Depletion-induced phase separation in colloid-polymer mixtures. Adv Colloid Interface Sci. 2003;103:1. doi: 10.1016/S0001-8686(02)00081-7. [DOI] [PubMed] [Google Scholar]
- 80.Valentine DL. Adaptations to energy stress dictate the ecology and evolution of the Archaea. Nat Rev Microbiol. 2007;5:316. doi: 10.1038/nrmicro1619. [DOI] [PubMed] [Google Scholar]
- 81.Verlander MS, Lohrmann R, Orgel LE. Catalysts for the self-polymerization of adenosine cyclic 2′, 3′-phosphate. J Mol Evol. 1973;2:303. doi: 10.1007/BF01654098. [DOI] [PubMed] [Google Scholar]
- 82.Viedma C. Chiral symmetry breaking during crystallization: complete chiral purity induced by nonlinear autocatalysis and recycling. Phys Rev Lett. 2005;94:065504. doi: 10.1103/PhysRevLett.94.065504. Provides the first example of enantioenrichment by cycles of crystal growth and dissolution. [DOI] [PubMed] [Google Scholar]
- 83.Viedma C, Ortiz JE, de Torres T, Izumi T, Blackmond DG. Evolution of solid phase homochirality for a proteinogenic amino acid. J Am Chem Soc. 2008;130:15274. doi: 10.1021/ja8074506. [DOI] [PubMed] [Google Scholar]
- 84.Wakamatsu H, Yamada Y, Saito T, Kumashiro I, Takenishi T. Synthesis of Adenine by Oligomerization of Hydrogen Cyanide. The Journal of Organic Chemistry. 1966;31:2035. [Google Scholar]
- 85.Weber AL, Pizzarello S. The peptide-catalyzed stereospecific synthesis of tetroses: a possible model for prebiotic molecular evolution. Proc Natl Acad Sci U S A. 2006;103:12713. doi: 10.1073/pnas.0602320103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wei C, Pohorille A. Permeation of membranes by ribose and its diastereomers. J Am Chem Soc. 2009;131:10237. doi: 10.1021/ja902531k. [DOI] [PubMed] [Google Scholar]
- 87.Wilson DS, Szostak JW. In vitro selection of functional nucleic acids. Annu Rev Biochem. 1999;68:611. doi: 10.1146/annurev.biochem.68.1.611. [DOI] [PubMed] [Google Scholar]
- 88.Woese CR. The genetic code : the molecular basis for genetic expression. New York: Harper & Row; 1967. p. viii. [Google Scholar]
- 89.Yuen GU, Kvenvolden KA. Monocarboxylic Acids in Murray and Murchison Carbonaceous Meteorites. Nature. 1973;246:301. [Google Scholar]
- 90.Zanchetta G, Nakata M, Buscaglia M, Bellini T, Clark NA. Phase separation and liquid crystallization of complementary sequences in mixtures of nanoDNA oligomers. Proc Natl Acad Sci U S A. 2008;105:1111. doi: 10.1073/pnas.0711319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu TF, Szostak JW. Coupled Growth and Division of Model Protocell Membranes. J Am Chem Soc. 2009;131:5705. doi: 10.1021/ja900919c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Related Resources
- Anastasi C, Buchet FF, Crowe MA, Parkes AL, Powner MW, et al. RNA: prebiotic product, or biotic invention? Chem Biodivers. 2007;4:721. doi: 10.1002/cbdv.200790060. [DOI] [PubMed] [Google Scholar]
- Mansy SS, Szostak JW. Reconstructing the emergence of cellular life through the synthesis of model protocells. Cold Spring Harb Symp Quant Biol. 2009 doi: 10.1101/sqb.2009.74.014. in press. [DOI] [PubMed] [Google Scholar]
- Orgel LE. Prebiotic chemistry and the origin of the RNA world. Crit Rev Biochem Mol Biol. 2004;39:99. doi: 10.1080/10409230490460765. [DOI] [PubMed] [Google Scholar]