Abstract
Flapping flight is energetically more costly than running, although it is less costly to fly a given body mass a given distance per unit time than it is for a similar mass to run the same distance per unit time. This is mainly because birds can fly faster than they can run. Oxygen transfer and transport are enhanced in migrating birds compared with those in non-migrators: at the gas-exchange regions of the lungs the effective area is greater and the diffusion distance smaller. Also, migrating birds have larger hearts and haemoglobin concentrations in the blood, and capillary density in the flight muscles tends to be higher. Species like bar-headed geese migrate at high altitudes, where the availability of oxygen is reduced and the energy cost of flapping flight increased compared with those at sea level. Physiological adaptations to these conditions include haemoglobin with a higher affinity for oxygen than that in lowland birds, a greater effective ventilation of the gas-exchange surface of the lungs and a greater capillary-to-muscle fibre ratio. Migrating birds use fatty acids as their source of energy, so they have to be transported at a sufficient rate to meet the high demand. Since fatty acids are insoluble in water, birds maintain high concentrations of fatty acid–binding proteins to transport fatty acids across the cell membrane and within the cytoplasm. The concentrations of these proteins, together with that of a key enzyme in the β-oxidation of fatty acids, increase before migration.
This article is part of the themed issue ‘Moving in a moving medium: new perspectives on flight’.
Keywords: flapping flight, energy costs, oxygen transport, metabolic substrates
1. Introduction
Any flight performed by an individual bird in its natural environment will probably include some or all of the following: take-off; climb; a combination of gliding, bounding, soaring or continuous forward flapping flight; manoeuvring, including tight turns, which may involve periods of burst flight. Gliding and soaring flight are energetically the least expensive in this list, whereas any form of flying that involves an explosive burst of activity, such as take-off, will be energetically the most expensive. Continuous forward flapping flight has an intermediate rate of energy expenditure [1].
These various levels of energy expenditure are provided by different proportions of anaerobic and aerobic metabolic pathways. Burst flight is almost exclusively anaerobic, using glycogen stored in the glycolytic muscle fibres, whereas continuous flapping flight can be exclusively aerobic using oxygen, glucose, proteins and, predominantly, fatty acids (particularly during long-duration flights). The early stages of flight can involve both aerobic and anaerobic pathways. Both the oxygen and, to a greater or lesser extent, the metabolic substrates are transported by the cardiovascular system, either from the lungs in the case of oxygen or from the gastrointestinal tract and stores elsewhere in the body in the case of metabolic substrates (figure 1). Much of this article will focus on these aspects of flight: the physiological processes involved in the maintenance of aerobic flight.
Figure 1.
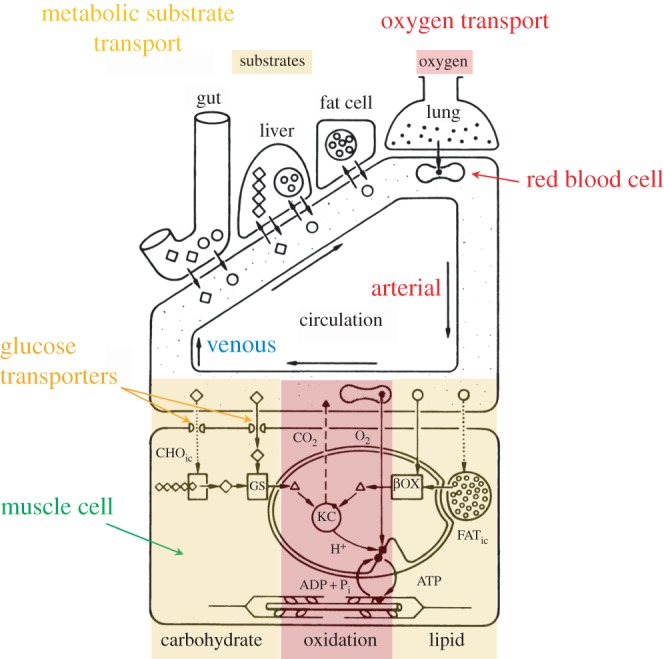
Role of the circulatory system in transporting the respiratory gases and metabolic substrates around the body. The diagram is based on the situation in mammals. As well as transporting the respiratory gases, oxygen and carbon dioxide, to and from the metabolizing cells, the circulatory system also transports carbohydrates (diamonds, CHO) and fatty acids (circles, FAT), both directly from the gut and from stores in the liver and fat cells. The transported carbohydrates and fatty acids go either directly into the glycolysis (GS) or β-oxidation (βOX) pathways, respectively, and then to the Krebs cycle (KC) or to replenish the intracellular stores (CHOic and FATic). (Adapted from Hoppeler & Weibel [2].)
2. Mitochondria: the powerhouses of oxidative muscle fibres
The flight muscles of one particular group of birds, the Galliformes (such as the Phasianidae: pheasants and grouse, jungle fowl, and the Numididae: guinea fowl) possess almost exclusively glycolytic (known as fast glycolytic) muscle fibres, so their flight pattern is limited to short bursts of activity before they fatigue. At the other extreme, the flight muscles of long-distance migrants have a predominance of oxidative fibres, while those of small passerines tend to have nothing but oxidative fibres [1]. These oxidative muscle fibres are designated fast oxidative glycolytic, but they are so oxidative that it has been suggested they should be called fast oxidative fibres [3].
The enzymes responsible for aerobic metabolism are located in the mitochondria. The total volume of the mitochondria in a set of muscles is determined by multiplying the volume density of mitochondria (percentage of a given volume of muscle occupied by mitochondria) by the volume of the muscle, and it determines the maximum rate of oxygen demand of those muscles. However, the metabolic rate in birds and mammals does not scale in direct proportion to body mass (M); in flying birds, it scales approximately as M0.72 [3]. This means that the metabolic rate per unit body mass scales approximately as M−0.28. In other words, metabolic rate per unit body mass is greater in smaller birds than in larger ones. Not surprisingly, it has been found in mammals that mitochondrial volume density scales in a similar fashion to body mass as does metabolic rate [4]. Thus, it would be expected that mitochondrial volume density (and capillary density, which is directly related to mitochondrial volume density [5]) would be greater in the muscle cells of smaller birds, and this must be borne in mind throughout the following discussion.
Mitochondrial volume density is around 20% in the flight muscles of tufted ducks (Aythya fuligula, body mass 638 g) and pigeons (Columba livia, body mass 304 g) [6,7], although the value is higher in wild pigeons (22%) than in sedentary ones (15.7%). However, in the flight muscles of rufous hummingbirds (Selaphorus rufus, body mass 3–4 g), pre-migratory dunlin (Calidris alpine, body mass 65–70 g) and sanderling (C. alba, 80–90 g), mitochondrial volume density is staggeringly 34% [8,9]. During winter, however, the mitochondrial volume densities were around 28% for both dunlin and sanderling and their body masses were 50–55 g and 55–60 g, respectively. In addition, the surface area of the inner membrane of the mitochondria (where the aerobic enzymes are located) per unit mitochondrial volume in the flight muscles of hummingbirds is 57 m2 cm−3 compared with around 35 m2 cm−3 in the locomotor muscles of cats [10,11]. These characteristics of the mitochondria in the flight muscle of hummingbirds make it the most aerobic tissue among vertebrates. It has an estimated maximum rate of oxygen consumption of 2 ml g−1 min−1 (89 μmmol g−1 min−1, [12]) compared with an estimated rate of 0.87 ml g−1 min−1 by the flight muscles of tufted ducks [6]. So, provided adequate oxygen and metabolic substrate are supplied to the flight muscles, migrating birds have the potential to consume oxygen at a high rate, but what are the energy demands of non-stop flapping flight?
3. The energy cost of sustained flight
The demonstration by Tucker in 1966 [13] that it is possible to measure the rate of oxygen consumption (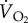 in ml min−1 or
in ml min−1 or 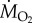 in mmol min−1; 1 mmol = 22.4 ml at standard temperature and atmospheric pressure. The nomenclature is given in table 1) of birds flying in a wind tunnel was a major breakthrough in the study of the physiology of flight. Note, the rate of oxygen uptake is an indication of metabolic power input, and data have been obtained from a number species of birds flying at a range of air speeds since Tucker's 1966 study (figure 2). If fat is the sole metabolic substrate then the measured respiratory exchange ratio (RER), which is equivalent to the respiratory quotient when the bird is in a steady state, will be 0.71 and 1 ml O2 s−1 = 19.6 W. If RER is unknown, then a value of 0.8, where 1 ml O2 s−1 = 20.1 W, is often used for calculations of power input during aerobic activity [14]. However, a note of caution, Walsberg & Hoffman [15] found that errors averaging around 17% over many hours of recording could result when using the conventional methods of conversion outlined above.
in mmol min−1; 1 mmol = 22.4 ml at standard temperature and atmospheric pressure. The nomenclature is given in table 1) of birds flying in a wind tunnel was a major breakthrough in the study of the physiology of flight. Note, the rate of oxygen uptake is an indication of metabolic power input, and data have been obtained from a number species of birds flying at a range of air speeds since Tucker's 1966 study (figure 2). If fat is the sole metabolic substrate then the measured respiratory exchange ratio (RER), which is equivalent to the respiratory quotient when the bird is in a steady state, will be 0.71 and 1 ml O2 s−1 = 19.6 W. If RER is unknown, then a value of 0.8, where 1 ml O2 s−1 = 20.1 W, is often used for calculations of power input during aerobic activity [14]. However, a note of caution, Walsberg & Hoffman [15] found that errors averaging around 17% over many hours of recording could result when using the conventional methods of conversion outlined above.
Table 1.
Nomenclature.
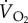 or or 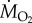
|
rate of oxygen consumption |
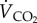 |
rate of carbon dioxide production |
| Pi | power input |
| Po | power output |
| fH | heart rate |
| VS | cardiac stroke volume |
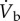 |
cardiac output |
| CIO2 | oxygen content in inspired air |
| CEO2 | oxygen content in expired air |
| CaO2 | oxygen content in arterial blood |
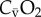 |
oxygen content in mixed venous blood |
| VT | respiratory tidal volume |
| fresp | respiratory frequency |
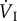 |
respiratory minute volume |
| PO2 | partial pressure of oxygen |
| PaO2 | partial pressure of oxygen in arterial blood |
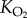 |
Krogh's diffusion constant for oxygen |
| A | total effective area of the gas exchange surface |
| D | mean diffusion distance over which the respiratory gases diffuse |
| Hb | haemoglobin |
| PaCO2 | partial pressure of carbon dioxide in arterial blood |
| P50 | partial pressure at which haemoglobin is 50% saturated |
Figure 2.
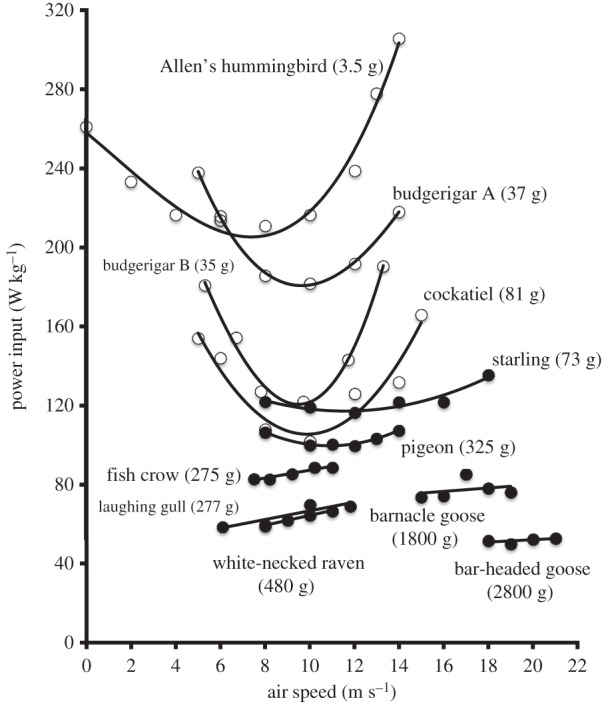
Power input of flapping flight by birds flying in a wind tunnel. Power input (W kg−1) converted from measurements of rates of oxygen consumption, measured at different air speeds for 10 species of birds during horizontal flapping flight in a wind tunnel. Note in this figure that mass-specific rate of oxygen consumption (ml kg−1 s−1) has been converted to mass-specific power input (W kg−1) [1].
The data in figure 2 show that five of the species have a U-shaped curve similar to that predicted by aerodynamic theory for power output [16], while the remainder have an almost flat or sloped relationship. Figure 3 illustrates that, on average, the minimum energetic cost of forward flapping flight in birds is 9.2 times the basal metabolic rate of non-passerine birds and that it is 2.2 times that of mammals of similar body mass running at their maximum sustainable speed. Thus, flight is an energetically costly form of transport. However, Tucker [17] calculated the amount of energy consumed (in Joules) to transport one unit of body mass (in grams) over one unit of distance (in kilometres), known as the energetic cost of transport (COT), for animals when swimming, running and flying. These, together with some more recent data, are shown in figure 4 and indicate that it is 2.7 times more costly for animals of similar mass to fly a given distance than it is for others to swim the same distance, but that it is 7.5 times more costly to run than it is to fly over the same distance. This difference in COT is, at least partly, because animals can fly much faster than they can run. For example, barnacle geese (Branta leucopsis) can run at about 1 m s−1 on a treadmill [20], but can fly in a wind tunnel at over 20 m s−1 [21]. Thus, flight is an ideal mode of transport for long-distance travel, such as migration. Note also that COT decreases as the mass of the animals increases, regardless of the mode of transport.
Figure 3.
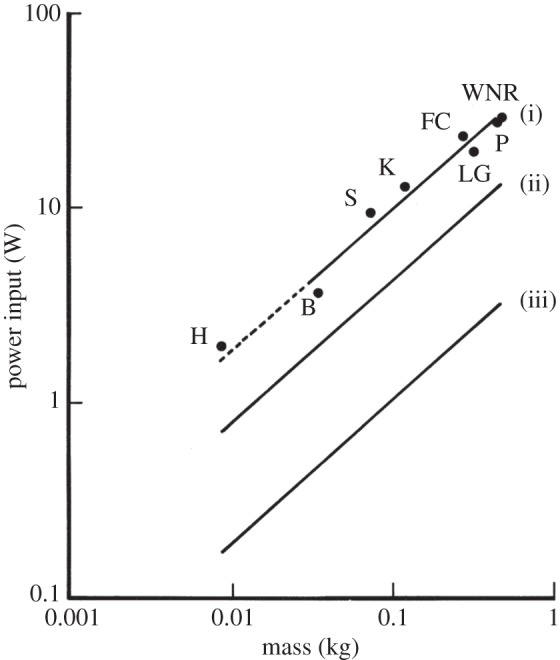
The relationships between power input (Pi) and body mass (Mb). Least-squares regression analysis was used for (i) minimum power input (Pim) of seven species of birds during forward flapping flight in a wind tunnel; H, hummingbird Colibri coruscans; B, budgerigar Melopsittacus undulatus; S, starling Sturnus vulgaris; K, American kestrel Falco sparverius; LG, laughing gull Larus atricilla; FC, fish crow Corvus ossifragus; P, pigeon Columba livia; WNR, white-necked raven Corvus cryptoleucus, where 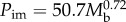 . (ii) Small mammals during maximum sustainable exercise where
. (ii) Small mammals during maximum sustainable exercise where 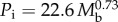 . (iii) Resting non-passerine birds where
. (iii) Resting non-passerine birds where 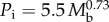 [1].
[1].
Figure 4.
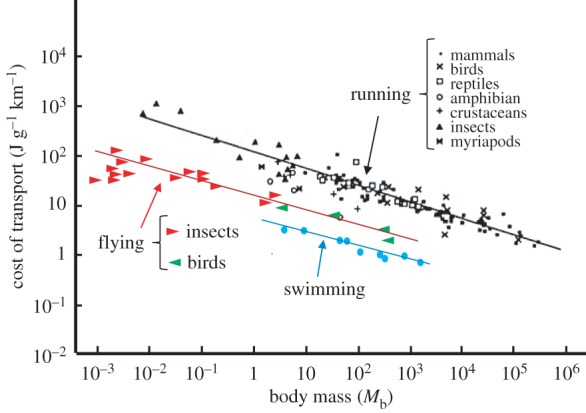
Minimum energy cost of transport (COT) for running, flying and swimming animals as a function of body mass. The data points plotted on double logarithmic scales were fitted by regression lines log COT = log a
+
b log(Mb), where a is the energy cost at Mb = 1 g and b is the scaling factor in the scaling equation COT 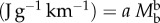 . COT for running, 120 (Mb)−0.32; for flying, 16 (Mb)−0.30 and for swimming, 6 (Mb)−0.32. With permission, D. G. Stephenson (Data points were adapted from Alexander [18] for running and from Schmidt-Nielsen [19] for flying and swimming).
. COT for running, 120 (Mb)−0.32; for flying, 16 (Mb)−0.30 and for swimming, 6 (Mb)−0.32. With permission, D. G. Stephenson (Data points were adapted from Alexander [18] for running and from Schmidt-Nielsen [19] for flying and swimming).
Power input (Pi) of any muscular activity (in this case, sustained bird flight) is not only determined by the energy required to produce that activity (the mechanical power, or power output—Po) but by the overall efficiency by which the flight muscles, in particular, and the whole animal, in general, can produce the required mechanical power from the chemical energy released during the oxidation of metabolic substrates in the mitochondria. The ratio between Po and Pi is the overall efficiency (e) of the bird. Efficiency has been determined in a number of ways. It has been suggested that e of the flight muscles should be around 0.2, but estimates made from birds flying in wind tunnels give a wide range of values. Power input is usually determined either by measuring 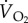 or rate of CO2 production,
or rate of CO2 production, 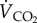 (either directly or indirectly, using allometric data), but Po during forward flapping flight has been estimated by a number of methods including the use of aerodynamic models, kinematics and by direct measurements of strain recordings from the humerus. The values of e obtained by these methods vary between 0.23 and 0.11 [1]. The remaining 77–89% of the energy consumed is dissipated as heat. Such studies also indicate that e does not have a constant value within a species; it may vary with flight speed and increase with increasing body mass. Hovering hummingbirds have an overall efficiency of around 0.08 if perfect elastic storage of energy occurs [22].
(either directly or indirectly, using allometric data), but Po during forward flapping flight has been estimated by a number of methods including the use of aerodynamic models, kinematics and by direct measurements of strain recordings from the humerus. The values of e obtained by these methods vary between 0.23 and 0.11 [1]. The remaining 77–89% of the energy consumed is dissipated as heat. Such studies also indicate that e does not have a constant value within a species; it may vary with flight speed and increase with increasing body mass. Hovering hummingbirds have an overall efficiency of around 0.08 if perfect elastic storage of energy occurs [22].
Many morphological and behavioural features of birds have evolved which reduce the energy cost of flight. Examples are wing shape, the use of thermals and tail winds, orographic lift and V-formation flight [23–27]. In fact, based on studies in which continually monitored heart rate was used as an indicator of Pi, migrating barnacle and bar-headed geese (B. leucopsis and Anser indicus, respectively) fly along the most energy-efficient routes available [28–30]. Even with such energy-conserving mechanisms, the respiratory and/or the circulatory systems still have to provide a continual supply of oxygen and metabolic substrates to match the high metabolic demands of the flight muscles.
4. The transport of oxygen during forward flapping flight
The functioning of the respiratory and cardiovascular systems in response to an increase in the demand for oxygen can be described by the Fick principle of convection: the rate of oxygen consumption is equal to the amount of air (respiratory minute volume) or blood (cardiac output) pumped per minute, multiplied by the amount of oxygen extracted by the gas exchange system from the respiratory medium (air, in the case of birds) or by the metabolizing cells from the blood. The ventilatory system and the heart control the amount of respiratory medium and blood they pump per minute by varying the rate at which they pump (respiratory frequency or heart rate) and the volume they pump during each cycle (respiratory tidal volume or cardiac stroke volume)
These relationships can be described by the following two equations—the first relates to the respiratory system and the second to the cardiovascular system:
| 4.1 |
and
| 4.2 |
where fresp is respiratory frequency; VT, respiratory tidal volume; (fresp × VT), respiratory minute volume (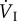 ); fH, heart rate; VS, cardiac stroke volume; (fH × VS), cardiac output (
); fH, heart rate; VS, cardiac stroke volume; (fH × VS), cardiac output (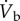 ); CIO2, oxygen content in inspired air; CEO2, oxygen content in expired air; CaO2, oxygen content in arterial blood;
); CIO2, oxygen content in inspired air; CEO2, oxygen content in expired air; CaO2, oxygen content in arterial blood; 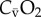 , oxygen content in mixed venous blood; (CIO2 – CEO2) and
, oxygen content in mixed venous blood; (CIO2 – CEO2) and 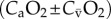 are the amounts of oxygen extracted (oxygen extraction) from inspired air and arterial blood, respectively.
are the amounts of oxygen extracted (oxygen extraction) from inspired air and arterial blood, respectively.
In air, the concentration of oxygen is directly related to its partial pressure (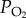 ), so at
), so at 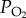 of 21 kPa (157 mmHg) at standard temperature (273 K or 0°C) and pressure (101.3 kPa or 760 mm Hg), the concentration of oxygen (
of 21 kPa (157 mmHg) at standard temperature (273 K or 0°C) and pressure (101.3 kPa or 760 mm Hg), the concentration of oxygen (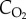 ) is 210 ml l−1. By contrast,
) is 210 ml l−1. By contrast, 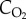 varies in blood in a sigmoid fashion as
varies in blood in a sigmoid fashion as 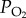 increases. Although the amount of oxygen in solution in the blood is low, the amount of oxygen carried in fully saturated blood can be at least as high as that in air because of the presence of haemoglobin (Hb) in the blood. The concentration of Hb in the blood determines the maximum amount of oxygen that the blood can carry, which is known as the oxygen carrying capacity. The
increases. Although the amount of oxygen in solution in the blood is low, the amount of oxygen carried in fully saturated blood can be at least as high as that in air because of the presence of haemoglobin (Hb) in the blood. The concentration of Hb in the blood determines the maximum amount of oxygen that the blood can carry, which is known as the oxygen carrying capacity. The 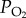 at which the Hb is 50% saturated is called the P50. A number of factors can affect the P50. If it is shifted to the left, there is an increase in affinity of Hb for oxygen, if it is shifted it to the right, there is a decrease in affinity.
at which the Hb is 50% saturated is called the P50. A number of factors can affect the P50. If it is shifted to the left, there is an increase in affinity of Hb for oxygen, if it is shifted it to the right, there is a decrease in affinity.
Oxygen combines reversibly with haemoglobin and the change in the concentration of oxygen in blood for a given change in 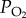 is called the capacitance coefficient (β). So, in arterial blood
is called the capacitance coefficient (β). So, in arterial blood
where CaO2, oxygen concentration in arterial blood and PaO2, partial pressure of oxygen in arterial blood.
The difference in the partial pressure of oxygen across the gas exchange regions of the lungs and within the muscles is extremely important, as this drives diffusion from the lungs into the blood and from the blood into the metabolizing tissue. Other factors that influence the rates of diffusion at these two surfaces are the total effective area (A) of the gas exchange surface, the mean diffusion distance (thickness, d) over which the respiratory gases diffuse and the diffusion constant for oxygen, 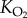 , of the tissues. So, the rate at which oxygen diffuses across a gas exchange surface (
, of the tissues. So, the rate at which oxygen diffuses across a gas exchange surface (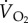 ) is described by Fick's first law of diffusion:
) is described by Fick's first law of diffusion:
| 4.3 |
(P1O2−P2O2), difference in average 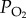 at either side of the gas exchanger;
at either side of the gas exchanger; 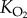 , the amount of oxygen diffusing per unit time through a unit cross-sectional area, and unit thickness of the gas exchange surface for a unit difference in partial pressure of oxygen across the exchange surface.
, the amount of oxygen diffusing per unit time through a unit cross-sectional area, and unit thickness of the gas exchange surface for a unit difference in partial pressure of oxygen across the exchange surface.
Those animals with a high maximum demand for oxygen need to have as large a surface area of the gas exchanger, as great a difference in partial pressure across the gas exchanger and as small a diffusion distance across the exchanger as possible.
Any of the variables on the right-hand side of equations (4.1) and (4.2) can vary in response to an increase in oxygen demand during flight, as shown in table 2. At relatively low ambient temperatures (<23°C), minute ventilation volume increases by a similar proportion as 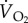 , which means that oxygen extraction from inspired air hardly changes from its value in resting birds. The relative contributions of fresp and VT to the increase in
, which means that oxygen extraction from inspired air hardly changes from its value in resting birds. The relative contributions of fresp and VT to the increase in 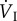 during flight vary between species. In white-necked ravens (Corvus leucocephalus), VT does not change at all, whereas in fish crows and blacked-billed magpies (Corvus ossifragus and Pica pica, respectively), it doubles, and in starlings (Sturnus vulgaris), it increases fourfold. Thus, in the first three of these species, fresp makes the greater contribution, but in the fourth species, VT predominates. The possible reasons for these differences are not clear.
during flight vary between species. In white-necked ravens (Corvus leucocephalus), VT does not change at all, whereas in fish crows and blacked-billed magpies (Corvus ossifragus and Pica pica, respectively), it doubles, and in starlings (Sturnus vulgaris), it increases fourfold. Thus, in the first three of these species, fresp makes the greater contribution, but in the fourth species, VT predominates. The possible reasons for these differences are not clear.
Table 2.
Mean values of respiratory frequency, tidal volume, minute ventilation volume, oxygen extraction, and rate of oxygen uptake. Values in parentheses indicate the factorial increase of the variable above the resting value. fresp, respiratory frequency (min−1); VT, tidal volume (ml); 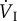 , minute ventilation volume (l min−1 BTPS, except for fish crow, where it is l min−1 STPD); O2ext, oxygen extraction;
, minute ventilation volume (l min−1 BTPS, except for fish crow, where it is l min−1 STPD); O2ext, oxygen extraction; 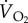 , rate of oxygen uptake (ml O2 min−1 STPD) during rest and while hovering in two species of hummingbirds, and while flying in a wind tunnel for five other species of birds. The values for
, rate of oxygen uptake (ml O2 min−1 STPD) during rest and while hovering in two species of hummingbirds, and while flying in a wind tunnel for five other species of birds. The values for 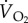 during flight in a wind tunnel are the minima that have been recorded. BTPS, body temperature and pressure, saturated; STPD, standard temperature and pressure, dry [1].
during flight in a wind tunnel are the minima that have been recorded. BTPS, body temperature and pressure, saturated; STPD, standard temperature and pressure, dry [1].
| mass (kg) | rest |
flight |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| fresp | VT | 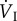 |
O2ext | 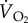 |
fresp | VT | 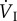 |
O2ext | 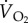 |
||
| hummingbird (>20°C) (Amazilia fimbriata fluviatilis) | 0.006 | — | — | — | — | — | 280 | 0.63 | 0.18 | 0.13 | 4.1 |
| hummingbird (36°C) (Colibri coruscans) | 0.008 | — | — | — | — | — | 330 | 0.38 | 0.12 | 0.24 | 5.0 |
| budgerigar (18–20°C) (Melopsittacus undulatus) | 0.035 | — | — | 0.047 | 0.27 | 2.62 | 199 | 1.15 | 0.232 (×4.9) | 0.26 | 10.9 (×4.2) |
| starling (10–14°C) (Sturnus vulgaris) | 0.073 | 92 | 0.67 | 0.061 | 0.28 | 3.16 | 180 | 2.8 | 0.504 (×8.3) | 0.31 | 28.1 (×8.9) |
| black-billed magpie (Pica pica) | 0.165 | 52.4 | 2.95 | 0.154 | — | — | 162 | 6.1 | 0.953 (×6.2) | — | — |
| fish crow (12–22°C) (Corvus ossifragus) | 0.275 | 27.3 | 8.2 | 0.223 | 0.19 | 8.5 | 120 | 14.9 | 1.79 (×8.0) | 0.19 | 68.0 (×8.0) |
| white-necked raven (14–22°C) (Corvus cryptoleucus) | 0.48 | 32.5 | 10.5 | 0.34 | 0.24 | 17.0 | 140 | 10.7 | 1.40 (×4.1) | 0.29 | 84.9 (×5.0) |
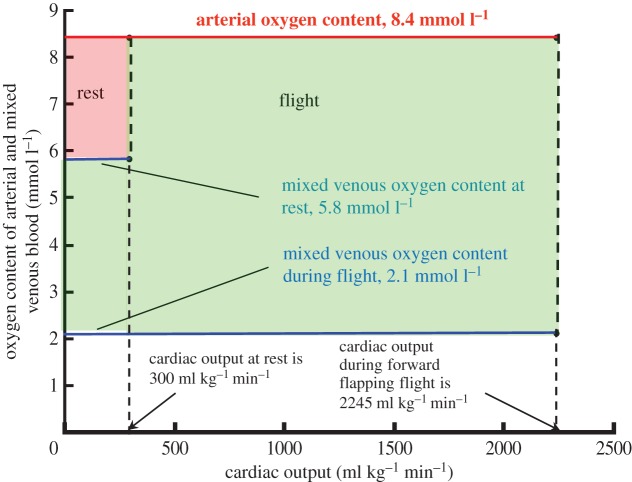
The role of the cardiovascular system in supplying oxygen to the flight muscles has been studied in only one species, the pigeon [31,32]. In the study by Peters et al. [32], there was a 17.4-fold increase in 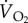 and this was supported mainly by a 7.4-fold increase in
and this was supported mainly by a 7.4-fold increase in 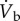 together with a 2.4-fold increase in oxygen extraction (figure 5). The large increase in
together with a 2.4-fold increase in oxygen extraction (figure 5). The large increase in 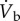 was predominantly the result of a sixfold increase in fH, with only a 23% increase in VS. So, an increase in the amount of oxygen extracted (from the blood) plays an important role in the delivery of oxygen to the flight muscles. The amount of oxygen stored in mixed venous blood in resting animals is known as the venous reserve, and the greater this amount the more oxygen is available to the animal during strenuous activity, such as flight.
was predominantly the result of a sixfold increase in fH, with only a 23% increase in VS. So, an increase in the amount of oxygen extracted (from the blood) plays an important role in the delivery of oxygen to the flight muscles. The amount of oxygen stored in mixed venous blood in resting animals is known as the venous reserve, and the greater this amount the more oxygen is available to the animal during strenuous activity, such as flight.
Figure 5.
Contributions of different components of the cardiovascular system to rate of oxygen consumption in resting and flying pigeons (Columba livia). The values for cardiac output and oxygen contents of arterial and mixed venous blood in the resting birds support a rate of oxygen consumption of 0.8 mmol O2 kg−1 min−1, which is represented by the area of the smaller (rest) quadrangle. The values for cardiac output and oxygen content of arterial mixed venous blood in flying birds support a rate of oxygen consumption of 13.8 mmol O2 kg−1 min−1, which is 17 times that during rest and this is represented by the total area of the larger (flight) quadrangle. (Adapted from Peters et al. [32].)
The maximum amount of oxygen that can be carried in the blood is related not only to the partial pressure of oxygen but also to the concentration of Hb, and some species of birds can vary the concentration of their Hb. When bar-tailed godwits (Limosa lapponica) arrive at their stopover point in the Netherlands after flying around 4500 km from their overwintering site in West Africa, their Hb concentration is 154 g l−1, whereas after refuelling and before they set off on the second leg of their migration, it reaches 176 g l−1 [33]. The authors suggest that the increase in Hb concentration is to raise the maximum oxygen carrying capacity of the blood in anticipation of the increased aerobic demands of the impending flight in conjunction with an increased body mass. There is also a tendency for the masses of the heart and flight muscles to increase during the pre-migratory period in some long-distance migrants such as red knot (Calidris canutus), and these changes relate directly to the overall increase in body mass, which is mainly the result of the accumulation of fat stores [34,35]. At the same time, other organs such as the gastrointestinal tract and liver tend to decrease in mass. The magnitude of these changes varies between species and is greater in those that migrate over oceans where there is little opportunity for feeding. All these changes are reversible and are examples of phenotypic flexibility.
It is the function of the respiratory system to maintain the partial pressure of oxygen in the air at the gas exchange surface in the lungs (P1O2 in equation (4.3)) as high a level as possible. The partial pressure of oxygen in the blood at the exchange surface (P2O2 in equation (4.3)) depends on the balance between the rate at which oxygen is being presented to the exchange surface by the ventilatory system and the rate at which it is being transported away from other side of the surface by the circulation. The other important factors in gas exchange are the thickness and effective surface area of the exchange surface (d and A, respectively, in equation (4.3)). These factors have been determined for a number of species of birds, and again we see a large variability depending on the maximum oxygen demand of the species. For example, in feral, and most likely non-migrating, mallard ducks (Anas platyrhynchos), effective surface area and thickness of the diffusion barrier in the lungs are 28.7 cm2 g−1 and 0.13 μm, respectively, whereas in wild African rock martins (Hirundo fuligula), the respective values are 86.5 cm2 g−1 and 0.09 μm [36]. These latter values are very similar to those from the lungs of violet-eared hummingbirds (Colibri coruscans): 87 cm2 g−1 and 0.10 μm [37].
Oxygen extraction by muscles depends to a large extent on the density of blood capillaries in those muscles; the greater the capillary density, the greater the surface area for gas exchange (equation (4.3)). In fact, the flight muscles of passerines that migrate long distances have a greater capillary density (1935 capillaries mm−2) than those of species that are only partially migratory or do not migrate at all (1604 capillaries mm−2, [38]). An extreme example is the flight muscle of rufous hummingbirds which has a capillary density of 7000 mm−2 [8]. Rufous hummingbirds migrate from the western edges of North America to the wooded areas of Mexico each year, a distance of approximately 3500 km. For comparison, the flight muscles of sedentary and wild pigeons have a capillary density of approximately 4000 mm−2 [7].
5. Flight at high altitude
Most species of birds fly below an altitude of 1 km above ground level during their migrations [1]. However, a number of species climb above this altitude to select favourable wind directions and/or air temperatures, or they have to negotiate large mountain barriers during their migration [1,27]. Those species that routinely fly to higher altitudes where air density, partial pressure of oxygen (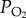 ) and air temperature are lower than those at sea level, are of particular physiological interest. One such species is the bar-headed goose (A. indicus) which for many years was thought to migrate over the top of Mt Everest, based on the anecdotal and auditory evidence presented by Swan [39,40]. Following these claims, bar-headed geese became the subjects of intense study to discover not only how they might survive at such high altitudes, but also how they manage to perform such a high level of aerobic exercise as flapping flight under such hypoxic conditions (reviewed by Butler [41]). At an altitude equivalent to that at the top of Mt Everest (8848 m),
) and air temperature are lower than those at sea level, are of particular physiological interest. One such species is the bar-headed goose (A. indicus) which for many years was thought to migrate over the top of Mt Everest, based on the anecdotal and auditory evidence presented by Swan [39,40]. Following these claims, bar-headed geese became the subjects of intense study to discover not only how they might survive at such high altitudes, but also how they manage to perform such a high level of aerobic exercise as flapping flight under such hypoxic conditions (reviewed by Butler [41]). At an altitude equivalent to that at the top of Mt Everest (8848 m), 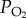 and air density are approximately one-third of those at sea level (6.7 kPa and 0.47 g m−3, respectively, as opposed to 21 kPa and 1.22 g m−3). This means that not only is there much less oxygen available, but lift generation becomes more difficult, although drag is proportionately reduced. Despite the reduction in drag, the minimum mechanical cost of flying (Po) is over 50% greater at the higher altitude [29]. So, how do bar-headed geese do it?
and air density are approximately one-third of those at sea level (6.7 kPa and 0.47 g m−3, respectively, as opposed to 21 kPa and 1.22 g m−3). This means that not only is there much less oxygen available, but lift generation becomes more difficult, although drag is proportionately reduced. Despite the reduction in drag, the minimum mechanical cost of flying (Po) is over 50% greater at the higher altitude [29]. So, how do bar-headed geese do it?
Perhaps one of the most important adaptations in bar-headed geese is the possession of haemoglobin (Hb) that has a higher affinity for oxygen than that of other species of birds [41]. This means that the oxygen equilibrium curve (OEC) is shifted to the left in bar-headed geese relative to that in other birds, and the PaO2 at which the Hb is 50% saturated (P50) is lower. For example, P50 for the blood of bar-headed geese at pH 7.5 is approximately 5 kPa compared with 7.5 kPa in Pekin ducks (A. platyrhynchos) and Canada geese (Branta canadensis). This adaptation means that bar-headed geese are able to maintain the oxygen content of their arterial blood (CaO2) when exposed to hypoxia at as high a level as possible without the need to produce more red blood cells (RBCs), as lowland birds do when exposed to hypoxia. The problem with increasing the number of RBCs is that it is accompanied by an increase in the viscosity of the blood, which could compromise its circulation at least partly by increasing the work load of the heart.
The difference in oxygen affinity between the Hb of bar-headed geese and those of lowland water fowl is the result of one particular amino acid substitution (α119 Pro →Ala). This substitution alters the contact between the α1 and β1 chains of the Hb, conferring a small increase in its oxygen affinity, which is then amplified by the interaction with inositol pentaphosphate [41,42]. Similar substitutions of different amino acids have similar effects in other species of high-altitude birds.
Although total ventilation (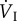 ) in bar-headed geese is similar to that in greylag geese (Anser anser) during severe hypoxia (less than 7% oxygen at sea level, that is equivalent to above about 8800 m), tidal volume (VT) increases proportionately more and respiratory frequency (fresp) proportionately less in bar-headed geese than in the other species studied [43]. The greater tidal volume reduces the contribution of dead space (that part of the upper respiratory system where there is no gas exchange, such as the trachea and bronchi) to total ventilation (
) in bar-headed geese is similar to that in greylag geese (Anser anser) during severe hypoxia (less than 7% oxygen at sea level, that is equivalent to above about 8800 m), tidal volume (VT) increases proportionately more and respiratory frequency (fresp) proportionately less in bar-headed geese than in the other species studied [43]. The greater tidal volume reduces the contribution of dead space (that part of the upper respiratory system where there is no gas exchange, such as the trachea and bronchi) to total ventilation (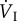 ) and, therefore, results in a greater effective ventilation of the gas exchange surface of the lungs. Related to this is the very small difference between PO2 in inspired air (PIO2) and that in the arterial blood (PaO2) of bar-headed geese when breathing hypoxic gas.
) and, therefore, results in a greater effective ventilation of the gas exchange surface of the lungs. Related to this is the very small difference between PO2 in inspired air (PIO2) and that in the arterial blood (PaO2) of bar-headed geese when breathing hypoxic gas.
When breathing air with 21% oxygen (i.e. the same as that at sea level), the difference between PIO2 and PaO2 is about 8 kPa, whereas at a PO2 approximately equivalent to that at the top of Mt Everest (breathing 7% oxygen at sea level), the difference is only 1.6 kPa in bar-headed geese but 2.1 kPa in the other species studied [43]. For comparison, in four human climbers close to the top of Mt Everest, the average difference was 3.0 kPa [44]. Thus, the large increase in 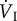 in response to hypoxia in birds serves to maintain PaO2 at as high a level as possible so as to enhance the transport of oxygen from the blood to the flight muscles.
in response to hypoxia in birds serves to maintain PaO2 at as high a level as possible so as to enhance the transport of oxygen from the blood to the flight muscles.
As well as the specific adaptations in high-altitude birds, early studies indicated that birds, in general, have a number of adaptations that enable them to tolerate the level of hypoxia that exists at the top of Mt Everest. The hyperventilation (level of ventilation of the gas exchange surfaces of the lungs that leads to excessive removal of CO2) in response to hypoxia not only maintains PaO2 as high as possible, it also reduces PaCO2 (hypocapnia) and hence increases pH in the blood (respiratory alkalosis). In a number of species of mammals (dog, monkey, rat, human), the hypocapnia caused by hyperventilation leads to a reduction in cerebral blood flow, but this is not the case in ducks and bar-headed geese [45,46]. Also, hypoxia causes a greater increase in cerebral blood flow and, therefore, supply of oxygen in ducks than it does in dogs, rats and humans [47]. Both of these responses are central to birds having a higher tolerance to the hypoxia of high altitude than mammals.
At high altitude, both hypocapnia and hypoxia occur together (hypocapnic hypoxia) and under these conditions, hypocapnia attenuates the increase in cerebral blood flow caused by hypoxia. However, both ducks and bar-headed geese are able to maintain cerebral and coronary oxygen supply during hypoxia, although in geese, this is the result of a higher CaO2 and smaller increases in blood flow than in ducks. There is a leftward shift of the OEC as a result of the hypocapnia and alkalosis (Bohr effect) and in bar-headed geese, the alkalosis during severe hypoxia is greater than that in Pekin ducks. This greater alkalosis together with the inherently higher affinity of their Hb for oxygen means that at a given (low) PaO2, CaO2 is at least twofold greater in the geese than it is in the ducks [48]. What is more, bar-headed geese are able to maintain or even increase perfusion of all tissues during severe hypoxia [49].
Also, the respiratory system of birds has a different structure from that of mammals; bulk air flow is at right angles to bulk blood flow. This arrangement is known as a cross-current system, which is theoretically more effective as a gas exchanger, particularly at high altitude, than the alveolar system of mammals [50,51]. Indeed, it has been concluded that the greater PaO2 of a bird (Pekin duck) than that of a mammal at the level of hypoxia equivalent to that at the top of Mt Everest would allow a bird to gain an additional 700 m in altitude [51].
The skeletal muscles of bar-headed geese also possess adaptations that enhance oxygen delivery to the mitochondria. Both capillary density and capillary-to-fibre ratio are greater in a leg muscle (gastrocnemius) of newly hatched bar-headed geese that were incubated in normoxic air than in that of Canada geese. However, if the eggs are exposed to hypoxia during incubation there are increases in capillary density and capillary-to-fibre ratio in muscle of newly hatched Canada geese, but not in that of bar-headed geese. So, a high level of capillarity is not dependent on exposure to hypoxia during development in bar-headed geese; it is genetically based [52]. Also, there is a 24% greater capillary : fibre ratio in the flight muscles of bar-headed geese compared with that in barnacle geese, but no significant difference in capillary density [53] and there is, on average across the flight muscles, a higher proportion of oxidative fibres in bar-headed geese than in those of barnacle geese (82.5% versus 76.8%).
Taken together, the adaptations of birds in general and of bar-headed geese in particular, have contributed to the proposal that they enable this species to ‘fly at up to 9000 m elevation during their migration over the Himalayas, sustaining high metabolic rates in the severe hypoxia at these altitudes’ [54]. Certainly, bar-headed geese can maintain their maximum sustainable running speed for as long when breathing 7% oxygen at sea level as when they are breathing air, whereas barnacle geese cannot [20]; but what is the scientific evidence that they routinely migrate, using powered flapping flight, at elevations over 8000 m? Until recently, there was none [29].
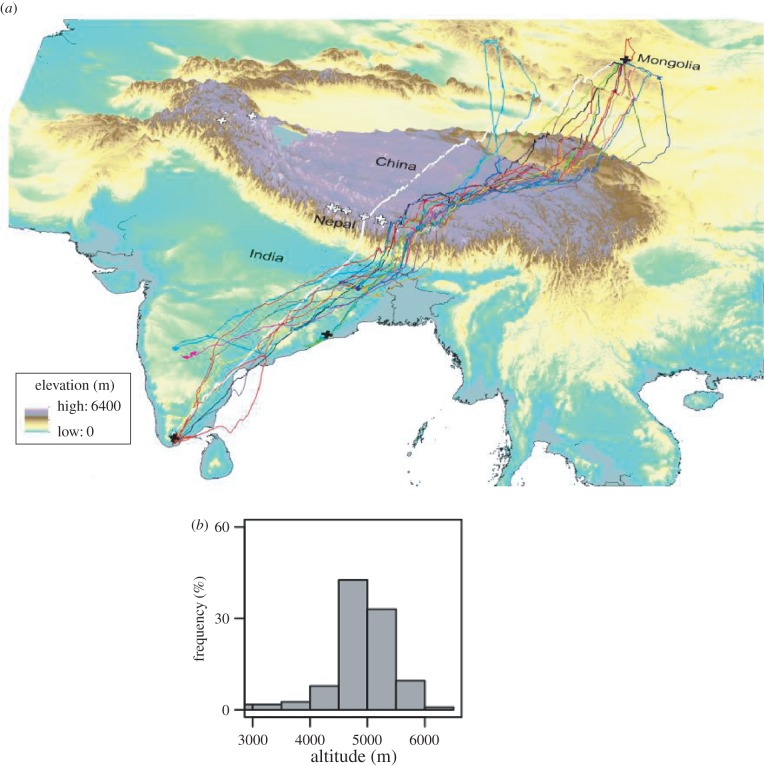
Tracking studies of a wild population of geese that breed in Mongolia and overwinter in India have shown that the maximum altitudes reached were 7290 m when going south and 6540 m going north (one goose in each case), but 95% of the locations from GPS trackers were at less than 5490 m. The birds typically travel along valleys and not over summits (figure 6) and mostly at less than 600 m above the ground [29]. Heart rate was recorded and stored by data loggers in migrating bar-headed geese and used as an indicator of metabolic rate [30]. Heart rate, and hence metabolic rate, was higher at higher altitudes (average of 364 beats min−1 above altitudes of 4800 m, but only 300 beats min−1 at altitudes below 2300 m). Generally, increased rates of ascent were associated with higher heart rates. However, on a few occasions, unusually high rates of ascent lasting for about 30 min were not associated with higher heart rates (or metabolic rates). These events were interpreted as evidence that the geese were obtaining assistance from updrafts due to orographic lift, presumably by flying along the windward side of a ridge. Such phenomena could explain why a few exceptionally high-altitude flights have been recorded or observed. It is possible, therefore, that weaker vertical updrafts could also provide assistance during other phases of migratory flights and could be comparable to the assistance geese might receive during V-formation flight. This would at least partly explain why average heart rate (and, presumably, metabolic rate) during migration above altitude of 4800 m is lower than that recorded from geese flying in a wind tunnel at sea level (435 beats min−1, [21]).
Figure 6.
Biannual migration of bar-headed geese (Anser indicus) between India and China or Mongolia. (a) Three-dimensional map showing release sites (black crosses) of geese in India (two sites) and Mongolia (one site). Dark lines represent 16 different birds and shaded background indicates elevation—see key next to map. Solid white line shows the shortest route, the great circle route, and the white crosses show locations of the world's highest mountains, those over 8000 m in altitude. (b) Frequency distribution of altitudes at which the geese fly; 95% of flights are below altitudes of 5800 m and the majority are less than 600 m above the ground (not shown). (Adapted or modified from: Hawkes et al. [29].)
So, there is no evidence as yet that bar-headed geese routinely fly at altitudes even approaching that at the top of Mt Everest. Nonetheless, forward flapping flight at an altitude of 5500 m, where barometric pressure is half that at sea level, is still an impressive feat made possible by all the adaptations discussed above.
6. Metabolic substrates and their transport
The oxygen transported in the blood to the mitochondria in the muscle cells is used to transform digested and absorbed foodstuffs into ATP via the tricarboxylic acid cycle (TCA) for carbohydrates. Fatty acids undergo β-oxidation before entering the TCA cycle, and amino acids are deaminated. So, the breakdown products of foodstuffs have to be transported to the mitochondria.
Before long-distant flights, migrating birds lay down a lot of fat which is used to fuel the high energy requirements of their journey ahead. Experiments on pigeons flying in a wind tunnel have shown that after take-off and an initial high RER around 1 (indicating carbohydrate metabolism), RER gradually declines over the next hour or so to reach a steady value of about 0.7, which indicates lipid metabolism [55]. The flight muscles of pigeons contain some glycolytic fibres, but for those species with almost 100% oxidative fibres, such as small passerines [38], the use of lipids most probably occurs much sooner after take-off.
The use of lipid as an energy store takes advantage of its much higher energy density than those of proteins or glycogen in the body, as shown in table 3. The values often quoted for these substrates are for dry mass, whereas in the body, there are variable amounts of water associated with them, with lipids being the least hydrated [56]. This means that in the body of an animal, adipose tissue provides 8–10 times more energy per gram than protein, such as muscles, or stored glycogen.
Table 3.
Energy densities (kilojoules per gram) of the three main sources of energy in birds [56].
| glycogen (mainly in liver, and also in muscle) | lipids (in adipose tissue) | protein (mainly in skeletal muscle and gastrointestinal tract) | |
|---|---|---|---|
| energy density when dry | 17.5 | 39.6 | 17.8 |
| water content when in body (%) | 75–80 | 5 | 70 |
| energy density when in body | 3.5–4.4 | 37.6 | 5.3 |
However, there are a number of potential disadvantages to using lipids as the major fuel source during long-distance flights:
First, in terms of the amount of energy produced, lipids produce 6–8.5 times less water than protein or glycogen. However, after non-stop flights of many hours by songbirds or even of several days by shorebirds, there is no sign of dehydration [57], which suggests that protein may be catabolized during such flights to maintain the birds in water-balance. Evidence for such a protein-for-water strategy has been provided by flying Swainson's thrushes (Catharus ustulatus) in high (80%) and low (13%) relative humidity (RH) for an average of 2.7 h in a wind tunnel. When flying in low RH, the birds lost 3.5 mg lean mass min−1, whereas in high RH, there was no relationship between lean mass loss and flight durations. So, lean body mass (gastrointestinal tract, muscles) may be increased prior to migration in order to provide the birds with sufficient water during long, non-stop flights.
Second, another potential problem is that fatty acids are oxidized in the TCA cycle and, during fasting (as during non-stop migration), intermediates of the cycle are continuously lost and have to be replaced from carbohydrates or from certain amino acids. Thus, migrating birds that have low stores of glycogen may have to rely on protein in the body to maintain the required level of fatty acid oxidation. Loss of protein could, therefore, limit flight range in some species of migrants [56].
The third potential disadvantage of relying on high flux rates of fatty acids from the adipose tissue to the muscles is that fatty acids are insoluble in water and are transported by a carrier protein, albumin. There is some debate as to how fatty acids cross the cell membrane, enter the cytosol (cytoplasm) of the cell and are then transferred to the mitochondria in both mammals and birds.
Fatty acids in migrating birds are stored predominantly in fat cells or adipocytes, or in the liver. In adipocytes, fatty acids are transformed into triacylglycerols, which are stored as anhydrous droplets and may occupy more than 95% of the volume of the adipocyte. Triacylglycerols synthesized in the liver may be combined with cholesterol and phospholipids to form a number of different types of lipoproteins, which are subsequently released into the circulation for uptake by tissues such as muscle.
Mammals transport non-esterified fatty acids in the blood by binding them to albumin. However, it would not be feasible for migrating birds to increase the concentration of plasma albumin drastically as this would lead to a large increase in plasma colloid osmotic pressure [58]. So, instead of transporting lipids in the blood solely by binding them to albumin, migratory birds may also use lipoproteins. For smaller migrants, such as a number of species of passerines, it has been suggested that fatty acids are taken up by the liver, thus increasing the availability of fatty acid binding sites on plasma albumin, as well as being transported to the muscles bound to albumin [58].
According to this suggestion, the increased availability of fatty acid binding sites improves the transport of fatty acids in the blood. Also, once in the liver, the fatty acids are transformed to triglycerides that are transported in the blood as very low density lipoproteins (VLDLs). The VLDL is converted back to fatty acids by lipoprotein lipase present in the endothelium of the capillaries. The fatty acids are then taken up by the muscles.
This mechanism enables the concentration of plasma protein in migrating passerine birds to be maintained at a physiological level. In addition, since the proportion of triglycerides in VLDL is 60–70% (compared with only 3% in albumin), there is a dramatic increase in the rate of fatty acid transport by the circulatory system of these birds. However, this suggested mechanism has not been completely supported by other studies, and further investigations are required in order to determine what role, if any, VLDLs play in the transport of fatty acids in the blood of migrating birds [59].
Fatty acids provide approximately 80% of the energy for locomotion in mammals at relatively low levels of activity, that is, at less than 40% of maximum oxygen consumption (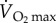 ). However, as a result of the transport limitations for fatty acids, they provide a decreasing proportion of the energy required above 40%
). However, as a result of the transport limitations for fatty acids, they provide a decreasing proportion of the energy required above 40% 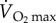 [60]. Indeed, at close to maximum rate of oxygen consumption, fatty acids supply only 10–20% of the energy being used. Carbohydrates, therefore, provide an increasing proportion of energy at increasing levels of exercise in mammals.
[60]. Indeed, at close to maximum rate of oxygen consumption, fatty acids supply only 10–20% of the energy being used. Carbohydrates, therefore, provide an increasing proportion of energy at increasing levels of exercise in mammals.
Even at low levels of exercise, 75–85% of the fatty acids in mammals are obtained from the fat droplets within the muscle cells themselves and not via the circulation, which is unable to provide metabolic substrates at the rate they are being used. The circulation does, however, replenish the intracellular stores at the end of the period of exercise.
As discussed above, the minimum metabolic rate of birds flying in a wind tunnel is approximately twice as great as the maximum metabolic rate (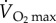 ) of similar sized mammals and birds running on a treadmill. During sustained flapping flight of birds, most of this huge energy demand is provided by fatty acids from stored fat. It has been estimated that migrating birds exercise at 70–90%
) of similar sized mammals and birds running on a treadmill. During sustained flapping flight of birds, most of this huge energy demand is provided by fatty acids from stored fat. It has been estimated that migrating birds exercise at 70–90% 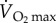 (although recent studies on bar-headed geese indicate that it may not be as high as this, see above) and that 90% of their energy demand comes from fatty acids, with the remaining 10% coming from proteins [56,59]. The inevitable conclusion is that migrating birds may have to transport fatty acids from body stores to the mitochondria of the working muscles up to 20 times faster than running mammals.
(although recent studies on bar-headed geese indicate that it may not be as high as this, see above) and that 90% of their energy demand comes from fatty acids, with the remaining 10% coming from proteins [56,59]. The inevitable conclusion is that migrating birds may have to transport fatty acids from body stores to the mitochondria of the working muscles up to 20 times faster than running mammals.
In addition to the circulation, another crucial step to the transport of fatty acids in mammals occurs across the plasma membrane of the muscle cell (sarcolemma). Recent evidence suggests that membrane-bound transport proteins play the major role in this process, so we might expect migratory birds to show differences from mammals in these locations. This is indeed the case.
The movement of fatty acids across the cell membrane of muscle fibres in birds appears to be similar to that in mammals. Fatty acid binding protein (FABP) bound to the plasma membrane (known as FABPpm) has recently been identified in the flight muscles of migratory white-throated sparrows (Zonotrichia albicollis), and it has been found to double in abundance during migration [61]. FABP within the cytoplasm (known as heart-type FABP or H-FABP) acts as a ‘sink’ for fatty acids, thereby increasing their solubility in the cytosol, their rate of removal from the inner surface of the plasma membrane, and their rate of intracellular diffusion. The flight muscles of birds have greater concentrations of H-FABP than the muscles of mammals; migratory western sandpipers (Calidris mauri) have about 10 times greater concentrations of H-FABP [62]. What is more, H-FABP increases by about 70% during migration in both western sandpipers and barnacle geese [63].
There is still one more hurdle before the fatty acids can enter the mitochondria for β-oxidation, as acyl-CoA esters cannot pass the inner membrane of the mitochondria directly. In mammals, this process involves the enzymes carnitine acyl transferase-1 (CAT 1), which is present on the outer mitochondrial membrane and carnitine acyl transferase-2 (CAT 2), which is located on the inner membrane of mitochondria. These enzymes are also known as carnitine palmitoyl transferase (CPT), and CPT1 doubles in activity during the migratory season in white-throated sparrows [61]. This whole transport process could provide a major rate-limiting step in the use of fatty acids by active muscles, with CAT 1 being an important factor, at least in mammals [64].
This short review has illustrated the adaptations that have evolved in birds which enable them to provide the necessary oxygen and metabolic substrates required for the high metabolic rate associated with non-stop migratory flight. Such adaptations include a larger effective area and smaller mean diffusion distance at the gas exchange regions of the lungs in migrating birds compared with those in non-migrators, and higher haemoglobin concentration in the blood and capillary density in the flight muscles. Physiological adaptations in species like bar-headed geese, which migrate at high altitudes where the availability of oxygen is reduced and the energy cost of flapping flight increased, include haemoglobin with a higher affinity for oxygen compared with that in lowland birds, a greater effective ventilation of the gas exchange surface of the lungs, and a greater capillary-to-fibre ratio. Migrating birds use fatty acids as their main source of energy, so they have to be transported at a sufficient rate to meet the high demand. However, fatty acids are insoluble in water, but increased concentrations of FABPs are involved in transporting fatty acids across the cell membrane and within the cytoplasm during migration.
Competing interests
I declare I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Bishop CM, Butler PJ. 2015. Flight. In Sturkie's avian physiology (ed. Scanes CG.), pp. 919–974, 6th edn New York, NY: Academic Press. [Google Scholar]
- 2.Hoppeler H, Weibel ER. 1998. Limits for oxygen and substrate transport in mammals. J. Exp. Biol. 201, 1051–1064. [DOI] [PubMed] [Google Scholar]
- 3.Butler PJ. 1991. Exercise in birds. J. Exp. Biol. 160, 233–262. [Google Scholar]
- 4.Matthieu O, Krauer R, Hoppeler H, Gehr P, Lindstedt SL, Alexander RMcN, Taylor CR, Weibel ER. 1981. Design of the mammalian respiratory system. VII. Scaling mitochondrial volume in skeletal muscle to body mass. Respir. Physiol. 44, 113–128. ( 10.1016/0034-5687(81)90079-7) [DOI] [PubMed] [Google Scholar]
- 5.Hoppeler H, Mathieu O, Weibel EW, Krauer R, Lindstedt SL, Taylor CR. 1981. Design of the mammalian respiratory system. VIII. Capillaries in skeletal muscles. Respir. Physiol. 44, 129–150. ( 10.1016/0034-5687(81)90080-3) [DOI] [PubMed] [Google Scholar]
- 6.Turner DL, Butler PJ. 1988. The aerobic capacity of locomotory muscles in the tufted duck, Aythya fuligula. J. Exp. Biol. 135, 445–460. [DOI] [PubMed] [Google Scholar]
- 7.Mathieu-Costello O, Agey PJ, Longemann RB, Florez-Duquet M, Bernstein MH. 1994. Effect of flying activity on capillary-fibre geometry in pigeon flight muscle. Tissue Cell 26, 57–73. ( 10.1016/0040-8166(94)90083-3) [DOI] [PubMed] [Google Scholar]
- 8.Mathieu-Costello O, Suarez RK, Hochachka PW. 1992. Capillary-to-fiber geometry and mitochondrial density in hummingbird flight muscle. Respir. Physiol. 89, 113–132. ( 10.1016/0034-5687(92)90075-8) [DOI] [PubMed] [Google Scholar]
- 9.Evans PR, Davidson NC, Uttley JD, Evans RD. 1992. Premigratory hypertrophy of flight muscles and ultrastructural study. Ornis Scand. 23, 238–243. ( 10.2307/3676644) [DOI] [Google Scholar]
- 10.Suarez RK, Lighton JRB, Brown GS, Mathieu-Costello O. 1991. Mitochondrial respiration in hummingbird flight muscles. Proc. Natl Acad. Sci. USA 88, 4870–4873. ( 10.1073/pnas.88.11.4870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwerzmann K, Hoppeler H, Kayar SR, Weibel ER. 1989. Oxidative capacity of muscle and mitochondria: Correlation of physiological, biochemical, and morphometric characteristics. Proc. Natl Acad. Sci. USA 86, 1583–1587. ( 10.1073/pnas.86.5.1583) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suarez RK. 1992. Hummingbird flight: sustaining the highest mass-specific metabolic rate among vertebrates. Experientia 48, 565–570. ( 10.1007/BF01920240) [DOI] [PubMed] [Google Scholar]
- 13.Tucker VA. 1966. Oxygen consumption of a flying bird. Science 154, 150–151. ( 10.1126/science.154.3745.150) [DOI] [PubMed] [Google Scholar]
- 14.Schmidt-Nielsen K. 1997. Animal physiology: adaptation and environment, 5th edn Cambridge, UK: Cambridge University Press. [Google Scholar]
- 15.Walsberg GF, Hoffman TCM. 2005. Direct calorimetry reveals large errors in respirometric estimates of energy expenditure. J. Exp. Biol. 208, 1035–1043. ( 10.1242/jeb.01477) [DOI] [PubMed] [Google Scholar]
- 16.Pennycuick CJ. 1969. The mechanics of bird migration. Ibis 111, 525–556. ( 10.1111/j.1474-919X.1969.tb02566.x) [DOI] [Google Scholar]
- 17.Tucker VA. 1970. Energetic cost of locomotion in animals. Comp. Biochem. Physiol. 34, 841–846. ( 10.1016/0010-406X(70)91006-6) [DOI] [PubMed] [Google Scholar]
- 18.Alexander RM. 2005. Models and the scaling of energy costs for locomotion. J. Exp. Biol. 208, 1645–1652. ( 10.1242/jeb.01484) [DOI] [PubMed] [Google Scholar]
- 19.Schmidt-Nielsen K. 1972. Locomotion: energy cost of swimming, flying and running. Science 177, 222–227. ( 10.1126/science.177.4045.222) [DOI] [PubMed] [Google Scholar]
- 20.Hawkes LA, Butler PJ, Frappell PB, Meir JU, Milsom WK, Scott GR, Bishop CM. 2014. Maximum running speed of captive bar-headed geese is unaffected by severe hypoxia. PLoS ONE 9, e94015 ( 10.1371/journal.pone.0094015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward S, Bishop CM, Woakes AJ, Butler PJ. 2002. Relationships between heart rate and rate of oxygen consumption in flying and walking barnacle geese (Branta leucopsis) and bar-headed geese (Anser indicus). J. Exp. Biol. 205, 3347–3356. [DOI] [PubMed] [Google Scholar]
- 22.Wells DJ. 1993. Ecological correlates of hovering flight of hummingbirds. J. Exp. Biol. 178, 59–70. [Google Scholar]
- 23.Bowlin MS, Wikelske M. 2008. Pointed wings, low wingloading and calm air reduce migratory flight costs in songbirds. PLoS ONE 3, e2154 ( 10.1371/journal.pone.0002154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duerr AE, et al. 2012. Testing an emerging paradigm in migratory ecology shows surprising differences in efficiency between flight modes. PLoS ONE 7, e35548 ( 10.1371/jornal.phone.0035548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weimerskirch H, Martin J, Clerquin Y, Alexandre P, Jiraskova S. 2001. Energy saving in flight formation. Nature 413, 697–698. ( 10.1038/35099670) [DOI] [PubMed] [Google Scholar]
- 26.Portugal S, Hubel TY, Fritz J, Heese S, Trobe D, Voelkl B, Hailes S, Wilson AM, Usherwood JR. 2014. Upwash exploitation and downwash avoidance by flap phasing in ibis formation flight. Nature 505, 399–404. (doi:101038/nature12939) [DOI] [PubMed] [Google Scholar]
- 27.Liechti F. 2006. Birds: blowin' in the wind? J. Ornithol. 147, 202–211. ( 10.1007/s10336-006-0061-9) [DOI] [Google Scholar]
- 28.Butler PJ, Woakes AJ, Bishop CM. 1998. Behaviour and physiology of Svalbard barnacle geese, Branta leucopsis, during their autumn migration. J. Avian Biol. 29, 536–545. ( 10.2307/3677173) [DOI] [Google Scholar]
- 29.Hawkes LA, et al. 2012. The paradox of extreme high altitude migration in bar-headed geese Anser indicus. Proc. R. Soc. B 280, 2012–2114. ( 10.1098/rsbb.2012.2114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bishop CM, et al. 2015. The roller coaster flight strategy of bar-headed geese conserves energy during Himalayan migrations. Science 347, 250–254. ( 10.1126/science.1258732) [DOI] [PubMed] [Google Scholar]
- 31.Butler PJ, West NH, Jones DR. 1977. Respiratory and cardiovascular responses of the pigeon to sustained, level flight a wind-tunnel. J. Exp. Biol. 71, 7–26. [Google Scholar]
- 32.Peters GW, Steiner DA, Rigoni JA, Mascilli AD, Schnepp RW, Thomas SP. 2005. Cardiorespiratory adjustments of homing pigeons to steady wind tunnel flight. J. Exp. Biol. 208, 3109–3120. ( 10.1242/jeb.01751) [DOI] [PubMed] [Google Scholar]
- 33.Landys-Ciannelli MM, Jukuma J, Piersma T. 2002. Blood parameter changes during stopover in a long-distance migratory shorebird, the bar-tailed godwit Limosa lapponica taymyrensis. J. Avian Biol. 33, 451–455. ( 10.1034/j.1600-048X.2002.03051.x) [DOI] [Google Scholar]
- 34.Piersma T. 1998. Phenotypic flexibility during migration: optimization of organ size contingent on risks and rewards of fueling and flight. J. Avian Biol. 29, 511–520. ( 10.2307/3677170) [DOI] [Google Scholar]
- 35.Piersma T, Gudmundsson GA, Lilliendahl K. 1999. Rapid changes in the size of different functional organ and muscle groups during refuelling in a long-distant migrating shorebird. Physiol. Biochem. Physiol. 72, 405–415. [DOI] [PubMed] [Google Scholar]
- 36.Maina JN, King AS, Settle G. 1989. An allometric study of pulmonary morphometric parameters in birds, with mammalian comparisons. Phil. Trans. R. Soc. B 326, 1–57. ( 10.1098/rstb.1989.0104) [DOI] [PubMed] [Google Scholar]
- 37.Dubach M. 1981. Quantitative analysis of the respiratory system of the house sparrow, budgerigar and violet-eared hummingbird. Resp. Physiol. 46, 43–60. ( 10.1016/0034-5687(81)90067-0) [DOI] [PubMed] [Google Scholar]
- 38.Lundgren BO, Keissling K-H. 1988. Comparative aspects of fibre types, areas and capillary supply in the pectoralis muscle of some passerine birds with different migratory behaviour. J. Comp. Physiol. B 138, 165–173. ( 10.1007/BF01075830) [DOI] [Google Scholar]
- 39.Swan LW. 1961. The ecology of the high himalayas. Sci. Am. 205, 68–78. ( 10.1038/scientificamerican1061-68) [DOI] [Google Scholar]
- 40.Swan LW. 1970. Goose of the Himalayas. Nat. Hist. 79, 68–75. [Google Scholar]
- 41.Butler PJ. 2010. High fliers: The physiology of bar-headed geese. Comp. Biochem. Physiol. A. 156, 325–329. ( 10.1016/j.cbpa.2010.01016) [DOI] [PubMed] [Google Scholar]
- 42.Weber RE, Jessen T-H, Malte H, Tame J. 1993. Mutant hemoglobins (α119-Ala and β55-Ser): Functions related to high-altitude respiration in geese. J. Appl. Physiol. 75, 2646–2655. [DOI] [PubMed] [Google Scholar]
- 43.Scott GR, Milsom WK. 2007. Control of breathing and adaptation to high altitude in the bar-headed goose. Am. J. Physiol. 293, R379–R391. ( 10.1152/ajpcell.00547.2006) [DOI] [PubMed] [Google Scholar]
- 44.Grocott MPW, Martin DS, Levett DZH, McMorrow R, Windsor J, Montgomery HE. 2009. Arterial blood gases and oxygen content in climbers on Mount Everest. N. Engl. J. Med. 360, 140–149. ( 10.1056/NEJMoa0801581) [DOI] [PubMed] [Google Scholar]
- 45.Grubb B, Mills CB, Colacino JM, Schmidt-Nielsen K. 1977. Effect of arterial carbon dioxide on cerebral blood flow in ducks. Am. J. Physiol. 232, H596–H601. [DOI] [PubMed] [Google Scholar]
- 46.Faraci FM, Fedde MR. 1986. Regional circulatory responses to hypocapnia and hypercapnia in bar-headed geese. Am. J. Physiol. 250, R499–R504. [DOI] [PubMed] [Google Scholar]
- 47.Grubb B, Colacino JM, Schmidt-Nielsen K. 1978. Cerebral blood flow in birds: effect of hypoxia. Am. J. Physiol. 234, H230–H234. [DOI] [PubMed] [Google Scholar]
- 48.Faraci FM, Kilgore DL Jr, Fedde MR. 1984. Oxygen delivery to the heart and brain during hypoxia: Pekin duck vs. bar-headed goose. Am. J. Physiol. 247, R69–R75. [DOI] [PubMed] [Google Scholar]
- 49.Faraci FM, Kilgore DL Jr, Fedde MR. 1985. Blood flow distribution during hypocapnic hypoxia in Pekin ducks and bar-headed geese. Respir. Physiol. 61, 21–30. ( 10.1016/0034-5687(85)90025-8) [DOI] [PubMed] [Google Scholar]
- 50.Piiper J, Scheid P. 1975. Gas transport efficacy of gills, lungs and skin: theory and experimental data. Respir. Physiol. 23, 209–221. ( 10.1016/0034-5687(75)90061-4) [DOI] [PubMed] [Google Scholar]
- 51.Shams H, Scheid P. 1989. Efficiency of parabronchial gas exchange in deep hypoxia: measurements in the resting duck. Respir. Physiol. 77, 135–146. ( 10.1152/ajpregu.00161.2007) [DOI] [PubMed] [Google Scholar]
- 52.Snyder GK, Byers RL, Kayar SR. 1884. Effects of hypoxia on tissue capillarity in geese. Respir. Physiol. 58, 151–160. ( 10.1016/0034-5687(84)90144-0) [DOI] [PubMed] [Google Scholar]
- 53.Scott GR, Egginton S, Richards JG, Milsom WK.2009. Evolution of muscle phenotype for extreme high altitude flight in the bar-headed goose. Proc. R. Soc. B. 276 , 3645–3653. ( ) [DOI]
- 54.Scott GR, Schulte PM, Egginton S, Scott ALM, Richards JG, Milsom WK. 2011. Molecular evolution of cytochrome c oxidase underlies high-altitude adaptation in the bar-headed goose. Mol. Biol. Evol. 28, 351–363. ( 10.1093/molbev/msq205) [DOI] [PubMed] [Google Scholar]
- 55.Rothe H-J, Biesel W, Nachtigall W. 1987. Pigeon flight in a wind tunnel. II. Gas exchange and power requirements. J. Comp. Physiol. B 157, 99–109. ( 10.1007/BF00702734) [DOI] [Google Scholar]
- 56.Jenni L, Jenni-Eiermann S. 1998. Fuel supply and metabolic constraints in migrating birds. J. Avian Biol. 29, 521–528. ( 10.2307/3677171) [DOI] [Google Scholar]
- 57.Gerson AR, Guglielmo CG. 2011. Flight at low ambient humidity increases protein catabolism in migratory birds. Science 333, 1434–1436. ( 10.1126/science.1210449) [DOI] [PubMed] [Google Scholar]
- 58.Jenni-Eiermann S, Jenni L. 1992. High plasma triglyceride levels in small birds during migratory flight: a new pathway for fuel supply during endurance locomotion at very high mass-specific metabolic rates? Physiol. Zool. 65, 112–123. ( 10.1086/physzool.65.1.30158242) [DOI] [Google Scholar]
- 59.Guglielmo CG. 2010. Move that fatty acid: fuel selection and transport in migratory birds and bats. Integr. Comp. Biol. 50, 336–345. ( 10.1093/icb/icq097) [DOI] [PubMed] [Google Scholar]
- 60.Weber J-M, Roberts TJ, Vock R, Weibel ER, Taylor CR. 1996. Design of the oxygen and substrate pathways III: Partitioning energy provision from carbohydrates. J. Exp. Biol. 199, 1659–1666. [DOI] [PubMed] [Google Scholar]
- 61.McFarlan JT, Bonen A, Guglielmo CG. 2009. Seasonal upregulation of fatty acid transporters in flight muscles of migratory white-throated sparrows (Zonotrichia albicollis). J. Exp. Biol. 212, 2934–2940. ( 10.1242/jeb.031682) [DOI] [PubMed] [Google Scholar]
- 62.Guglielmo CG, Haunerland NH, Hochachka PW, Williams TD. 2002. Seasonal dynamics of flight muscle fatty acid binding protein and catabolic enzymes in a migratory bird. Am. J. Physiol. 282, R1405–R1413. ( 10.1152/ajpregu.00267.2001) [DOI] [PubMed] [Google Scholar]
- 63.Pelsers MAL, Bishop CM, Butler PJ, Glatz JFC. 1999. Fatty acid-binding protein in heart and skeletal muscles of the migratory barnacle goose throughout development. Am. J. Physiol. 276, R637–R643. [DOI] [PubMed] [Google Scholar]
- 64.Kiens B. 2006. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol. Rev. 86, 205–243. ( 10.1152/physrev.00023.2004) [DOI] [PubMed] [Google Scholar]