Abstract
Hummingbirds are well known for their ability to sustain hovering flight, but many other remarkable features of manoeuvrability characterize the more than 330 species of trochilid. Most research on hummingbird flight has been focused on either forward flight or hovering in otherwise non-perturbed air. In nature, however, hummingbirds fly through and must compensate for substantial environmental perturbation, including heavy rain, unpredictable updraughts and turbulent eddies. Here, we review recent studies on hummingbirds flying within challenging aerial environments, and discuss both the direct and indirect effects of unsteady environmental flows such as rain and von Kármán vortex streets. Both perturbation intensity and the spatio-temporal scale of disturbance (expressed with respect to characteristic body size) will influence mechanical responses of volant taxa. Most features of hummingbird manoeuvrability remain undescribed, as do evolutionary patterns of flight-related adaptation within the lineage. Trochilid flight performance under natural conditions far exceeds that of microair vehicles at similar scales, and the group as a whole presents many research opportunities for understanding aerial manoeuvrability.
This article is part of the themed issue ‘Moving in a moving medium: new perspectives on flight’.
Keywords: flight, hummingbird, manoeuvrability, perturbation, rain, unsteady flow
1. Introduction
Birds, bats and insects in the wild often fly through a variety of perturbed aerial conditions, including turbulence and variable winds, rain and transit through vegetation which presents both obstacles and voids over a range of spatial frequencies. In the laboratory, such factors can either be eliminated or at least minimized, facilitating the study of ‘clean air’ flight performance. Although highly informative, many other features of animal flight derive from various compensatory kinematics and aerodynamics that can only be elicited via perturbations using either impulsive or sustained mechanical and sensory cues [1–3]. Such aspects of performance are much less studied relative to steady-state horizontal flight or hovering, but are essential to flight and survival in the natural world. Animal flight manoeuvrability, more generally, is a topic under intense current investigation [4–8], but specific aspects of axial and torsional agility used when flying in aforementioned environmental conditions remain largely unstudied. Although of obvious biological importance, such capacities can also be relevant to the survivability of microair vehicles in comparable environments.
To this end, the use of various nectar-feeding volant taxa provides numerous advantages for laboratory-based investigation of responses to naturally occurring aerial challenges. Station-keeping in either still air or in wind is required for these animals to extract nectar from flowers, and necessitates either bilaterally symmetric or, in some cases, asymmetric alteration of wing and body motions to yield stable orientations when aerodynamically challenged. Hummingbirds (family Trochilidae) are particularly good at maintaining essentially motionless head positions when feeding during flight, and are apparently oblivious to any aerodynamic turmoil that may occur around them. As a consequence, the air within which they fly can be experimentally altered, and full sets of biomechanical and metabolic measurements can be obtained. Although much initial work with hummingbirds studied hovering responses to hypodense, hypoxic and hyperoxic conditions [9–13], more recent emphasis has been placed on their forward flight performance [14–16] and manoeuvrability [1,17,18]. Here, we review recent findings for animal flight both in rain and under turbulent conditions, with an emphasis on hummingbirds, but also discuss emerging aspects of trochilid flight performance relative to vegetational transit, sexual selection [19,20] and territoriality [21].
2. Flight in turbulent air
All animal fliers move through the troposphere, a region characterized by meso-scale circulation and winds maintained by gradients of thermal convection and by the Coriolis effect. On smaller spatial scales, such currents produce turbulent flows through interaction with other air masses (e.g. via the Kelvin–Helmholtz instability), with topographical or water surfaces, and by moving around bluff bodies such as vegetation or geomorphological structures. Formally, turbulence is characterized by erratic velocity fluctuations in both space and time, giving rise to large eddies that translate and decompose into progressively smaller vortices until such a point as all kinetic energy is frictionally dissipated as heat and/or sound. Viscosity thus plays an important role in determining the spatial and temporal scales of these unsteady flows. Given that most animal fliers are relatively small (e.g. the mean adult insect body size is 4–5 mm [22]), major aerodynamic compensation for turbulent perturbations must be routine for the majority of volant taxa.
Nonetheless, in spite of the ubiquity of atmospheric turbulence, our knowledge of its consequences for animal flight performance is limited. Early work with birds found no effect on flight metabolic rates of low turbulence intensities (i.e. less than 2% r.m.s. fluctuation [23]), but identified an approximate doubling of metabolic rates for fast flight at high levels of turbulence (43% r.m.s. [24,25]). Thus, the costs of flight depend on turbulence intensity and can be substantial relative to free flight at the same mean airspeed in unperturbed air. High levels of turbulence may also influence overall stability and can elicit specific and sustained responses to effect control. For example, orchid bees flying in highly turbulent flows exhibit large roll oscillations, and extend their enlarged hindlegs laterally to increase the moment of inertia about this axis by as much as 50% [26]. Such leg extension results in an approximately 30% higher drag on the insect's body, indicating that associated power expenditure is also elevated in such flows. Not surprisingly, maximum flight speeds are also reduced for flight in high levels of homogeneous turbulence, possibly indicating a power limit associated with the increased costs of flight control [26].
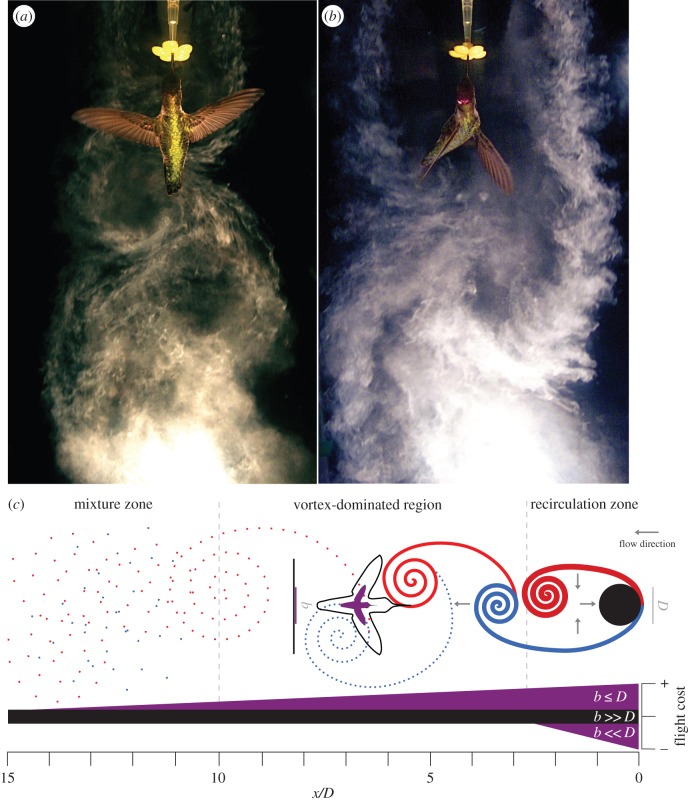
Recently, hummingbirds were studied flying within von Kármán vortex streets [27], a turbulent flow system characterized by periodic counter-rotating vortices shed from an upstream object. Such flow is laminar at Reynolds numbers (Re) below 10 or so, but with increasing Re becomes unstable and turbulent near Re of 2000, creating a vortex street (figure 1). Hummingbirds were studied as they fed from a small artificial flower within the working section of a wind tunnel, which was operated at three different airspeeds (3, 6 and 9 m s−1). Upstream of the feeder, vertical cylinders of variable diameters were placed to generate vortex streets at different shedding frequencies and sizes. Flight kinematics and bird metabolic rates at the feeder were measured using high-speed videography and mask respirometry, respectively. When flying through relatively small vortices, hummingbirds exhibited only small changes in wing and body kinematics, and showed no change in metabolic rate relative to flight at the same but unperturbed flow speed. By contrast, flight within the vortex street generated using the largest upstream cylinder, of diameter comparable to the bird's wingspan, resulted in dramatically increased levels of variance in wing and body kinematics. Concomitantly, rates of oxygen consumption increased by up to 25% under such conditions (and at all measured airspeeds) relative to flight in unperturbed air [27] (see also [29]).
Figure 1.
Adult male Anna's hummingbird flying at 6 m s−1 into the von Kármán wake generated by a vertical cylinder of diameter D. For a 4 cm diameter cylinder (a), two counter-rotating vortices interact near-simultaneously with the bird, whereas for a larger cylinder (b; 9 cm diameter), shed vortices interact more sequentially with the hummingbird, inducing greater asymmetries in kinematic variance. (c) Schematic indicating decay (i.e. decreasing line width) with downstream distance x of a turbulent wake generated by a stationary cylinder at Re ∼ 103 [28], and the expected additional flight costs as a function of position within the wake for an animal flier of wingspan b relative to the cylinder diameter. Zero-flight cost indicates the cost of flying in steady flow with no cylinder present.
These size-dependent effects of the von Kármán vortex street derive from the relative timing of contact of the bird with pairs of counter-rotating vortices. For the vortex street generated by the smaller cylinders in this study, two vortices of alternating sense present themselves more synchronously to the bird's body and wings, and tend at least partially to offset one another (figure 1a,c). By contrast, larger shed vortices interact individually and alternately with the flying hummingbird (figure 1b,c), resulting in strong bilaterally asymmetric local flows and requiring much more variable wingbeat kinematics to effect compensation. Intriguingly, intermittent tail spreading also was more frequent under such conditions, suggesting a role for damping of body oscillations [27]. Similar size-dependent effects of flight within vortex streets were also observed for hawkmoths while feeding at lower airpeeds, up to 2 m s−1 [30]. In this case, however, changes in wingbeat kinematics were shown to decrease with increasing downstream distance from the generating cylinder, in accordance with the well-known rapid decay of the von Kármán vortex street at higher Re [31] (figure 1c). As with orchid bees [26], moths flying in turbulent flows exhibited reductions in maximum flight speed, suggesting limiting effects on either flight control or power expenditure [30].
Overall, the effects of such unsteady flows on flight control and energetics correlate positively with turbulence intensity and with the size of vortical structures. Animals flying within turbulent flows characterized by length scales similar to a wingspan or more, or flying at downstream distances close to the physical source of perturbation (i.e. less than 10 times the bluff-body diameter; figure 1c) will need to implement chronic and potentially costly course corrections. Alternatively, benefits can sometimes derive from station-keeping within the immediate recirculation zone, a region where the mean streamwise velocity is directed upstream and where the transverse velocities are directed to flow's midline (figure 1c) [28]. Although well documented for fish [32], it is unclear if there are any ecological circumstances in which such advantages might pertain to volant taxa. For small insects, however, such recirculating flows can be high relative to typical airspeeds (see electronic supplementary material, Video S1). Flight near and through vegetation for these animals may be much more influenced by local flow fields and wind regimes than we currently appreciate.
In addition to sustained turbulence, natural atmospheric flows present to animal fliers a wide range of transient perturbations, such as sudden wind gusts. For example, large raptors rapidly curl both wings ventrally when flying through headwind gusts [33]. Perturbation studies using airpuffs with flying insects have similarly demonstrated fast but also bilaterally asymmetric responses in wingbeat kinematics, particularly in stroke amplitude [2]. In the spatial domain, sharp gradients in airflow can also present distinct challenges to flight control. For example, hawkmoths hover-feeding within tornado-like vortices (with transverse speeds up to 1.2 m s−1 and a swirl ratio of 0.11) asymmetrically alter their stroke plane angles and wing angles of attack to sustain continuous turns in yaw [34]. Nectar-feeders more generally must maintain position while hovering at flowers, and a broad spectrum of aerial disturbances (imposed either symmetrically or asymmetrically) must characterize their natural flight. Hovering hummingbirds, in particular, exhibit high spatial fidelity and millimetre-scale positioning of the head while feeding. They accordingly can serve as a productive experimental platform for future investigations of response to aerial disturbances on varying spatial and temporal scales.
3. Flight in rain
Physically, rainfall is a dispersed two-phase environmental flow consisting of water droplets moving mostly vertically through a turbulent atmosphere. Rain presents a challenge for animal fliers given the immediate mechanical effects of drop impact, possibly impaired vision, short-term effects of wetting on thermoregulation, and even long-term effects of enhanced pathogenic growth associated with wet skin or cuticle. The much higher thermal conductivity of water relative to air, for example, may explain a doubling of metabolic rate for tropical bats flying in rain [35]. Wetting can also augment the effective body mass, increasing the costs of flight and reducing manoeuvrability. In aquatic birds, the extra load produced by wetting can be as much as 10% of body mass [36]; wet alcids and storm petrels taking off with such extra loads reach significantly lower flight speeds and heights compared to takeoff in dry conditions [37].
Similarly, wet hummingbirds hovering in artificial rain for only 2 s experience mass increases of up to 4% and, in parallel with wingbeat kinematic changes, expend substantially more induced power [38]. The rate of wetting, not surprisingly, depends on exposure time and intensity of precipitation. Hummingbirds in light rain require 1 min to increase effective body mass by approximately 20%, but in heavy rain this requires only 20 s. To alleviate associated mass-loading, both perched (figure 2a) and hovering hummingbirds (figure 2b) rapidly rotate their heads and shake their wings to shed water. Perched hummingbirds shake their wings, head and body at accelerations of up to 30g, expelling nearly 90% of the water adhered to their plumage over a period of only several hundred milliseconds [39]. Insects, which are otherwise well known for their hydrophobic cuticles, also can become wet in rain, with a doubling of body mass under certain environmental conditions [40]. Even some vertebrates [39,41] as well as insects (e.g. craneflies; V.M.O.-J. 2011 personal observation) use the same behavioural response to reduce wetting, namely rapid counter-oscillation of their body parts.
Figure 2.
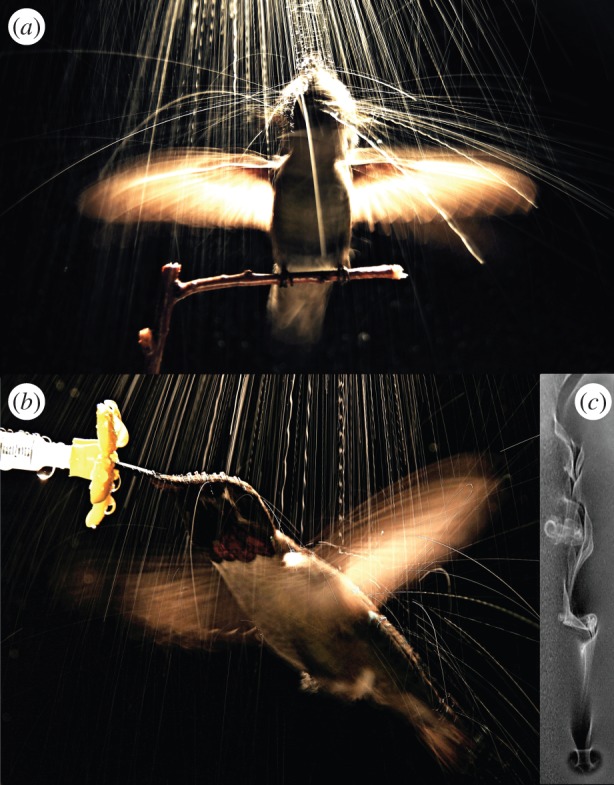
Long-duration exposure of an Anna's hummingbird shaking off water while perched (a) and when hovering (b) in moderate experimentally generated rain [38]. (c) Vortex street produced by a falling water droplet (approx. 2.5 mm) falling at approximately 2 m s−1.
Large raindrops generally fall faster than smaller drops, although under certain conditions (e.g. drop splitting), smaller raindrops can travel at unexpectedly high speeds [42]. Impacts of raindrops can generate significant downward forces on an animal's body. For example, millimetre-sized drops falling on hovering hummingbirds (figure 2b) can increase the effective downwards force by up to 2.5% of body weight, with significant effects on induced power expenditure [38]. Aerodynamic performance may also be impaired; aeroplanes flying in heavy rain experience a 50% increase in profile drag on the wings [43]. Furthermore, instantaneous pressures associated with drop impact can be high (of the order of approx. 102 kPa [44]), potentially causing damage to axial or appendicular structures of insects, in particular. The effects of raindrop impact will nonetheless depend strongly on animal size. Species with exposed surface area equal to or less than drop size can experience only partial momentum transfer during impacts. However, such reduced transfer can result in substantial roll, pitch and yaw torques, sometimes even causing impact with the ground [40] (electronic supplementary material, Videos S1 and S2; figure 3a).
Figure 3.
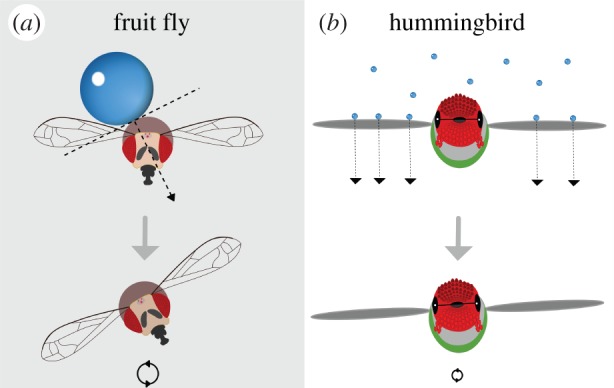
Schematic illustrating effects of millimetre-scale raindrop impacts on a small (a) and a large (b) flier. Impact force per drop transferred to small insects (here indicated as a fruit fly) is small but can produce a large torque. By contrast, larger animals (here indicated as a hummingbird) can be hit by multiple drops at the same time, but with a more balanced spatial distribution over their body and wings. Flight control in rain is thus more challenging for smaller animals than for large ones.
By contrast, larger animals will experience much higher levels of momentum transfer during drop impacts (figure 3b), albeit distributed much more evenly across the body and wings. For example, hummingbirds hovering in heavy rain exhibit large-scale compensatory responses in wing and body kinematics, but show no sign of perturbation in roll or loss of flight control [38]. Similarly, large seabirds fly placidly in heavy rain (V.M.O.-J. 2015, personal observation). Coincidentally, falling raindrops produce a characteristic wake in air that resembles a von Kármán vortex street, and given a Re typically greater than 1000 [45], generate substantial flow instabilities (electronic supplementary material, Video S3; figure 2c). For small insect fliers, the highly perturbed and interacting wakes of multiple raindrops will pose additional aerodynamic challenges. Many social insects produce nuptial flights during heavy rain, and these taxa will be particularly susceptible to such aerial disruption. Termite alates are remarkable in this regard as their flight capacities are weak, with seemingly flimsy wings and a strong tendency to dealate, yet nonetheless fly during torrential rainstorms (R.D. 1987–2014, personal observation, Panama). Given that nuptial flights also can involve numerous manoeuvres during the course of mate choice, selection for sustained flight capacity and control in rain is likely intense.
Vision can also be impaired during motion through rain; human drivers reduce vehicle speeds substantially during storms, and do so particularly at night [46]. We have noticed that hummingbirds hovering in heavy rain frequently close their eyes, possibly to avoid the direct impact of drops (electronic supplementary material, figure S1). Even more extreme examples of natural two-phase flows can be identified—some birds and insects (e.g. Arctic bumblebees) can fly in falling snow, hail or in sandstorms [47,48], and some swifts transit waterfalls to reach their nests. These remarkable feats are unstudied from behavioural and biomechanical perspectives. Aerial control during flight in rain is perhaps the most easily studied experimentally, as falling water drops can readily be generated in the laboratory at different sizes and intensities [38]. Given the increasing miniaturization of quadcopters and other flying vehicles, studies of animal flight in rain, snow and turbulent air will also be of technological relevance.
4. Into the real world
Animal fliers as well as aeroplanes can damp small-scale perturbations via the passive mechanism of static stability (e.g. dihedral wing positioning, restorative tail torques). By contrast, intense flow perturbations associated with heavy storms and sustained turbulent gusts will require active responses to effect translational and rotational control. Stable flight under such challenging environmental conditions must derive from general features of manoeuvrability (e.g. the capacities for axial and torsional agility [22]) used in other contexts of flight behaviour, including aperture negotiation and prey capture. Thus, capacities for maximum force production in translation, and for maximum torques in body rotation, will determine rapidity of responses to aerial challenges imposed by variable winds, climate and physical obstacles. For flight in turbulent air or rain, underlying dynamics of the kinematic responses summarized here have not yet been characterized, but may be expected to have allometric correlates, with more rapid rotational and translational accelerations characterizing smaller fliers [49]. In rain and for flight in the vortex streets described here, however, larger animals will also experience external forces and torques that are relatively smaller (figures 1 and 3), so some interplay between body size and stability might be expected. With hummingbirds in either hovering or forward flight at a feeder, externally imposed aerodynamic forces and torques can be systematically varied in space (e.g. via use of air jets positioned at different locations around the animal), and also in time (e.g. through variable-duration jet activation). Experimental assessment of the rapidity of responses relative to the timing and intensity of environmental challenge is therefore possible with these birds.
A day in the life of a hummingbird consists of numerous feats of aerobatic splendour, including precise takeoffs and landings in variable winds, high-speed vegetational transit, chases of conspecifics, hawking of small insects, and, of course, highly stable feeding at flowers. Engineers would be pleased to emulate any one of these feats using flapping-wing devices, and the full range of hummingbird flight performance remains largely undescribed. Although most biomechanical and physiological attention has been focused on only two common species (i.e. Anna's hummingbird and the ruby-throated hummingbird), an impressive range of intra- and interspecific variation characterizes the lineage. Since about 22 Ma, hummingbirds have rapidly diversified into more than 330 species, with concomitant variation in body size and other aspects of flight-related morphology and physiology [50,51]. Sex-specific consequences of wing and body morphology for flight performance have also been identified in some behavioural contexts (e.g. [11]), but merit further attention. Flight-related costs of sexually selected features such as elongated tails in male hummingbirds [20], as well as novel use of tail feathers in sonation [19], are also of interest given the high levels of dimorphism in some clades.
We continue to be surprised by features of hovering performance in hummingbirds derived from their obligate nectarivory. For example, substantial consequences of variable floral orientation for hover-feeding kinematics and energetics have only recently been described, as has the capacity of hummingbirds to sustain backward flight [52,53]. One major gap in our understanding of their flight biology, however, concerns daily time and energy budgets. The relatively small body sizes of hummingbirds have, to date, precluded attachment of accelerometers and data loggers, and patterns of movement ecology and associated costs remain obscure. Recent work on intraspecific aerial interactions, however, documents intense and energetically demanding manoeuvres [18,21]. Manoeuvring to capture small insect prey in mid-air is also an obligate feature of hummingbird biology, but neither this nor many other interesting flight behaviours (e.g. vertical ascent) have been studied.
Given that hummingbirds vary interspecifically in body mass from 1.8 to 22 g, the allometry of flight performance has attracted considerable attention. Maximum flight power, as assayed using load-lifting trials, is negatively allometric [17]; larger birds have relatively less mechanical power available from the flight muscle. Additional tasks, such as obstacle avoidance and aperture negotiation (as occur during vegetational transit) might similarly be expected to show strong size-dependence. For example, intraspecific size variation in bumblebees yields differences in transit performance through obstacle fields, with larger individuals flying more slowly and with greater sinuosity [54]. In hummingbirds, maximum load-lifting capacity also declines significantly with elevation [17]. Broader features of hummingbird manoeuvrability, particularly torsional agility (i.e. the capacity for rotational accelerations), should similarly be influenced by lower air densities, and may shape the outcome of competitive aerial interactions.
Overall, hummingbirds represent an emerging model system for the comparative study of animal flight mechanics under a variety of challenging aerial conditions. Their high species richness, relative ease of capture, and propensity to visit artificial flowers ensure successful experimental manipulations and the reliable acquisition of kinematic and aerodynamic data. As research agendas in animal locomotion progressively move away from steady-state behaviours (e.g. hovering and constant-velocity forward flight) towards manoeuvres and performance under more natural environmental conditions, we fully expect hummingbirds to be at the forefront of experimental taxa. Hummingbirds are, however, restricted geographically to the New World, so there will be an inevitable continental bias to this outcome. In the Old World, their ecological analogues are the sunbirds (Nectariniidae) and the honey-eaters (Meliphagidae), both of which can only transiently hover. Compared to our knowledge of hummingbird flight physiology and biomechanics, these two lineages of birds are much less studied, but presumably have solved similar problems associated with nectar extraction from flowers when under variable aerodynamic challenge. Nonetheless, hummingbirds represent the premier vertebrate example of hovering and manoeuvrability, and we anticipate continuing scientific as well as popular interest in their remarkable abilities.
Supplementary Material
Acknowledgements
We thank all members of the UC-Berkeley Animal Flight Laboratory for comments and support over many years on the various research projects described herein. Anand Varma contributed photographic advice.
Authors' contributions
All authors contributed to conception of this article and to its writing. Victor Ortega-Jimenez drafted the figures.
Competing interests
The authors declare no competing interests.
Funding
Much of the experimental work described here was supported by AFOSR Flow Interactions and Control grant no. 13RSA030 to R.D.
References
- 1.Altshuler DL, Quicazán-Rubio EM, Segre PS, Middleton KM. 2012. Wingbeat kinematics and motor control of yaw turns in Anna's hummingbirds (Calypte anna). J. Exp. Biol. 215, 4070–4084. ( 10.1242/jeb.075044) [DOI] [PubMed] [Google Scholar]
- 2.Vance JT, Faruque I, Humbert JS. 2013. Kinematic strategies for mitigating gust perturbations in insects. Bioinspir. Biomim. 8, 016004 ( 10.1088/1748-3182/8/1/016004) [DOI] [PubMed] [Google Scholar]
- 3.Muijires FT, Elzinga MJ, Melis JM, Dickinson MH. 2014. Flies evade looming targets by executing rapid visually directed banked turns. Science 344, 172–177. ( 10.1126/science.1248955) [DOI] [PubMed] [Google Scholar]
- 4.Sun M. 2014. Insect flight dynamics: stability and control. Rev. Mod. Phys. 86, 615–645. ( 10.1103/RevModPhys.86.615) [DOI] [Google Scholar]
- 5.Socha JJ, Jafari F, Munk Y, Byrnes G. 2015. How animals glide: from trajectory to morphology. Can. J. Zool. 93, 901–924. ( 10.1139/cjz-2014-0013) [DOI] [Google Scholar]
- 6.Altshuler DL, Bahlman JW, Dakin R, Gaede AH, Goller B, Lentink D, Segre PS, Skandalis DA. 2015. The biophysics of bird flight: functional relationships integrate aerodynamics, morphology, kinematics, muscles, and sensors. Can. J. Zool. 93, 961–975. ( 10.1139/cjz-2015-0103) [DOI] [Google Scholar]
- 7.Swartz SM, Konow N. 2015. Advances in the study of bat flight: the wing and the wind. Can. J. Zool. 93, 977–990. ( 10.1139/cjz-2015-0117) [DOI] [Google Scholar]
- 8.Hedrick TL, Combes SA, Miller LA. 2015. Recent developments in the study of insect flight. Can. J. Zool. 93, 925–943. ( 10.1139/cjz-2013-0196) [DOI] [Google Scholar]
- 9.Chai P, Dudley R. 1995. Limits to vertebrate locomotor energetics suggested by hummingbirds hovering in heliox. Nature 377, 722–725. ( 10.1038/377722a0) [DOI] [Google Scholar]
- 10.Dudley R, Chai P. 1996. Animal flight mechanics in physically variable gas mixtures. J. Exp. Biol. 199, 1881–1885. [DOI] [PubMed] [Google Scholar]
- 11.Chai P, Harrykissoon R, Dudley R. 1996. Hummingbird hovering performance in hyperoxic heliox: effects of body mass and sex. J. Exp. Biol. 199, 2745–2755. [DOI] [PubMed] [Google Scholar]
- 12.Altshuler DL, Dudley R. 2003. Kinematics of hummingbird hovering flight along simulated and natural elevational gradients. J. Exp. Biol. 206, 3139–3147. ( 10.1242/jeb.00540) [DOI] [PubMed] [Google Scholar]
- 13.Altshuler DL, Dudley R. 2006. The physiology and biomechanics of avian flight at high altitude. Integr. Comp. Biol. 46, 62–71. ( 10.1093/icb/icj008) [DOI] [PubMed] [Google Scholar]
- 14.Chai P, Dudley R. 1999. Maximum flight performance of hummingbirds: capacities, constraints, and trade-offs. Am. Nat. 153, 398–411. ( 10.1086/303179) [DOI] [Google Scholar]
- 15.Tobalske BW, Warrick DR, Clark CJ, Powers DR, Hedrick TL, Hyder GA, Biewener AA. 2007. Three-dimensional kinematics of hummingbird flight. J. Exp. Biol. 210, 2368–2382. ( 10.1242/jeb.005686) [DOI] [PubMed] [Google Scholar]
- 16.Clark CJ, Dudley R. 2010. Hovering and forward flight energetics in Anna's and Allen's Hummingbirds. Physiol. Biochem. Zool. 83, 654–662. ( 10.1086/653477) [DOI] [PubMed] [Google Scholar]
- 17.Altshuler DL, Dudley R, Heredia SM, McGuire JA. 2010. Allometry of hummingbird lifting performance. J. Exp. Biol. 213, 725–734. ( 10.1242/jeb.037002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segre PS, Dakin R, Zordan VB, Dickinson MH, Straw AD, Altshuler DL. 2015. Burst muscle performance predicts the speed, acceleration, and turning performance of Anna's Hummingbirds. eLife 4, e11159 ( 10.7554/eLife.11159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark CJ. 2009. Courtship dives of Anna's hummingbird offer insights into flight performance limits. Proc. R. Soc. B 276, 3047–3052. ( 10.1098/rspb.2009.0508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark CJ, Dudley R. 2009. Flight costs of long, sexually selected tails in hummingbirds. Proc. R. Soc. B 276, 2109–2115. ( 10.1098/rspb.2009.0090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sholtis KM, Shelton RM, Hedrick TL. 2015. Field flight dynamics of hummingbirds during territory encroachment and defense. PLoS ONE 10, e0125659 ( 10.1371/journal.pone.0125659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dudley R. 2000. The biomechanics of insect flight: form, function, evolution. Princeton, NJ: Princeton University Press. [Google Scholar]
- 23.Tucker VA. 1972. Metabolism during flight in the laughing gull, Larus atricilla. Am. J. Physiol. 222, 237–245. [DOI] [PubMed] [Google Scholar]
- 24.Tucker VA. 1966. Oxygen consumption of a flying bird. Science 154, 150–151. ( 10.1126/science.154.3745.150) [DOI] [PubMed] [Google Scholar]
- 25.Tucker VA. 1968. Respiratory exchange and evaporative water loss in the flying budgerigar. J. Exp. Biol. 48, 67–87. [Google Scholar]
- 26.Combes SA, Dudley R. 2009. Turbulence-driven instabilities limit insect flight performance. Proc. Natl Acad. Sci. USA 106, 9105–9108. ( 10.1073/pnas.0902186106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ortega-Jimenez VM, Sapir N, Wolf M, Variano EA, Dudley R. 2014. Into turbulent air: size-dependent effects of von Kármán vortex streets on hummingbird flight kinematics and energetics. Proc. R. Soc. B 281, 20140180 ( 10.1098/rspb.2014.0180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parnaudeau P, Carlier J, Heitz D, Lamballais E. 2008. Experimental and numerical studies of the flow over a circular cylinder at Reynolds number 3900. Phys. Fluids 20, 085101 ( 10.1063/1.2957018) [DOI] [Google Scholar]
- 29.Bowlin MS, Wikelski M. 2008. Pointed wings, low wingloading and calm air reduce migratory flight costs in songbirds. PLoS ONE 3, e2154 ( 10.1371/journal.pone.0002154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ortega-Jimenez VM, Greeter JS, Mittal R, Hedrick TL. 2013. Hawkmoth flight stability in turbulent vortex streets. J. Exp. Biol. 216, 4567–4579. ( 10.1242/jeb.089672) [DOI] [PubMed] [Google Scholar]
- 31.Prasad A, Williamson CHK. 1997. Three-dimensional effects in turbulent bluff-body wakes. J. Fluid Mech. 343, 235–265. ( 10.1017/S002211209700579X) [DOI] [Google Scholar]
- 32.Liao JC. 2004. Neuromuscular control of trout swimming in a vortex street: implications for energy economy during the Karman gait. J. Exp. Biol. 207, 3495–3506. ( 10.1242/jeb.01125) [DOI] [PubMed] [Google Scholar]
- 33.Reynolds KV, Thomas ALR, Taylor GK. 2014. Wing tucks are a response to atmospheric turbulence in the soaring flight of the steppe eagle Aquila nipalensis. J. R. Soc. Interface 11, 20140645 ( 10.1098/rsif.2014.0645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ortega-Jimenez VM, Mittal R, Hedrick TL. 2014. Hawkmoth flight performance in tornado-like whirlwind vortices. Bioinsp. Biomim. 9, 025003 ( 10.1088/1748-3182/9/2/025003) [DOI] [PubMed] [Google Scholar]
- 35.Voigt CC, Schneeberger K, Voigt-Heucke SL, Lewanzik D. 2011. Rain increases the energy cost of bat flight. Biol. Lett. 7, 793–795. ( 10.1098/rsbl.2011.0313) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahoney SA. 1984. Plumage wettability of aquatic birds. Auk 101, 181–185. [Google Scholar]
- 37.Ortega-Jimenez VM, Álvarez-Borrego S, Arriaga-Ramírez S, Renner M, Bridge ES. 2010. Takeoff flight performance and plumage wettability in Cassin's Auklet Ptychoramphus aleuticus, Xantus's Murrelet Synthliboramphus hypoleucus and Leach's Storm-petrel Oceanodroma leucorhoa. J. Ornithol. 151, 169–177. ( 10.1007/s10336-009-0441-z) [DOI] [Google Scholar]
- 38.Ortega-Jimenez VM, Dudley R. 2012. Flying in the rain: hovering performance of Anna's hummingbirds under varied precipitation. Proc. R. Soc. B 279, 3996–4002. ( 10.1098/rspb.2012.1285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ortega-Jimenez VM, Dudley R. 2012. Aerial shaking performance of wet Anna's hummingbirds. J. R. Soc. Interface 9, 1093–1099. ( 10.1098/rsif.2011.0608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dickerson AK, Shankles PG, Madhavan NM, Hu DL. 2012. Mosquitoes survive raindrop collisions by virtue of their low mass. Proc. Natl Acad. Sci. USA 109, 9822–9827. ( 10.1073/pnas.1205446109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dickerson AK, Mills ZG, Hu DL. 2012. Wet mammals shake at tuned frequencies to dry. J. R. Soc. Interface 9, 3208–3218. ( 10.1098/rsif.2012.0429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montero-Martínez G, Kostinski AB, Shaw RA, García-García F. 2009. Do all raindrops fall at terminal speed? Geophys. Res. Lett. 36, L11818 ( 10.1029/2008GL037111) [DOI] [Google Scholar]
- 43.Haines PA, Luers JK. 1983. Aerodynamic penalties of heavy rain on a landing aircraft. J. Aircraft 20, 111–119. ( 10.2514/3.44839) [DOI] [Google Scholar]
- 44.Nearing MA, Bradford JM, Holtz RD. 1987. Measurement of waterdrop impact pressures on soil surfaces. Soil Sci. Soc. Am. J. 51, 1302–1306. ( 10.2136/sssaj1987.03615995005100050038x) [DOI] [Google Scholar]
- 45.Sakamoto RH, Haniu H. 1990. A study on vortex shedding from spheres in a uniform flow. J. Fluids Eng. 112, 386–392. ( 10.1115/1.2909415) [DOI] [Google Scholar]
- 46.Unrau D, Andrey J. 2006. Driver response to rainfall on urban expressways. Transport. Res. Rec. 1980, 24–30. ( 10.3141/1980-06) [DOI] [Google Scholar]
- 47.Hume R. 1986. Reactions of birds to heavy rain. Br. Birds 79, 326–329. [Google Scholar]
- 48.Gates WH. 1933. Hailstone damage to birds. Science 78, 263–264. ( 10.1126/science.78.2021.263) [DOI] [PubMed] [Google Scholar]
- 49.Dudley R. 2002. Mechanisms and implications of animal flight maneuverability. Integr. Comp. Biol. 42, 135–140. ( 10.1093/icb/42.1.135) [DOI] [PubMed] [Google Scholar]
- 50.Altshuler DL, Dudley R. 2002. The ecological and evolutionary interface of hummingbird flight physiology. J. Exp. Biol. 205, 2325–2336. [DOI] [PubMed] [Google Scholar]
- 51.McGuire JA, Witt C, Van Remsen J, Corl A, Rabosky DL, Altshuler DL, Dudley R. 2014. Molecular phylogenetics and the diversification of hummingbirds (Apodiformes: Trochilidae). Curr. Biol. 24, 910–916. ( 10.1016/j.cub.2014.03.016) [DOI] [PubMed] [Google Scholar]
- 52.Sapir N, Dudley R. 2012. Backward flight in hummingbirds employs unique kinematic adjustments and entails low metabolic cost. J. Exp. Biol. 215, 3603–3611. ( 10.1242/jeb.073114) [DOI] [PubMed] [Google Scholar]
- 53.Sapir N, Dudley R. 2013. Implications of floral orientation for flight kinematics and metabolic expenditure of hover-feeding hummingbirds. Funct. Ecol. 27, 227–235. ( 10.1111/1365-2435.12024) [DOI] [Google Scholar]
- 54.Crall JD, Ravi S, Mountcastle AM, Combes SA. 2015. Bumblebee flight performance in cluttered environments: effects of obstacle orientation, body size and acceleration. J. Exp. Biol. 218, 2728–2737. ( 10.1242/jeb.121293) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.