Abstract
Background
Obstructive sleep apnoea is the most common form of sleep-disordered breathing in adults and children. It is associated with many adverse health consequences. The objectives this study were to determine the prevalence, awareness and reporting of symptoms of obstructive sleep apnoea among hospitalized adult patients in Nigeria.
Methods
This was a multicenter cross-sectional study involving 1420 adult patients admitted to general medical and surgical wards of selected hospitals from March to April 2013. A questionnaire embedded with Berlin questionnaire, Epworth sleepiness scale and questions on level of awareness and reporting of symptoms of sleep apnoea was used for data collection.
Results
One-third of the patients (33.4%) reported snoring, 16.3% had excessive daytime sleepiness, 10.0% experienced daytime fatigue, and 8.0% experienced drowsy driving. Approximately 5% reported witnessed apnoea and 18.0% had high risks for obstructive sleep apnoea. The frequency of high risk for sleep apnoea increased with age and declined after 65 years and also increased with the body mass index. Snoring, excessive daytime sleepiness and high risk for obstructive sleep apnoea were more common in patients with chronic medical conditions and who were admitted to the urban hospitals. The majority were not aware that snoring (77.3%) and excessive daytime sleepiness (65.8%) constitute a medical problem, and only 4.5% reported these symptoms to their doctors.
Conclusion
The level of awareness and under-reporting of sleep apnoea symptoms are poor. The high prevalence of obstructive sleep apnoea symptoms from this study should form the basis for screening hospitalized patients with chronic medical condition across the country.
Keywords: Obstructive sleep apnoea, Nigeria, Snoring, Prevalence, Awareness, Under-reporting
Introduction
Obstructive sleep apnoea (OSA) is one of the most important medical conditions identified in the past 50 years (1). Globally, it is by far the most common form of sleep-disordered breathing in adults and children and is associated with many other adverse health consequences, including increased risk of death (1–4). Untreated, sleep apnoea is known to cause a significant socio-economic burden as a result of its effects on employment, lost income and increased healthcare utilization (5–6). Obstructive sleep apnoea is defined by the occurrence of daytime sleepiness, loud snoring, witnessed breathing interruptions or awakening due to gasping or choking in the presence of five obstructive respiratory events (apnoeas, hypopneas and respiratory effort related arousal) per hours of sleep (7).
The predisposing factors for OSA include obesity, male gender, ethnicity and nasal obstruction; large tonsils (particularly in children). Other risk factors include an underactive thyroid gland; use of alcohol, tobacco, and sedatives and menopause in women (1–7).
Berlin questionnaire is a simple screening tool used to identify subjects who are at high risk for OSA and diagnosed by an overnight polysomnography (PSG) in a sleep laboratory or at home by a portable diagnostic monitoring and auto-adjusting therapeutic (1,7,8). The gold standard for management is continuous positive airway pressure (CPAP), in addition to behavioral therapy and physical and/or mechanical interventions (7).
OSA affects 2–4% of the middle-aged male population and two percent of children aged 8 to 11 years in the USA (7–11). Adewole, et al in a community-based study in Abuja, Nigeria, found that 22% of men and 16% of women were at high risk for sleep apnoea and were likely to have obesity, sleepiness and chronic medical conditions (12). Other studies in southwestern Nigeria have also found a high prevalence of snoring and risk of OSA among hypertensive and heart failure subjects attending an outpatient clinic (13–15).
Acute and chronic conditions may increase obstructive sleep apnea manifestation (1–2). The hospitalization of a patient with OSA can present an opportunity for screening, diagnosis and management of the condition. The majority of the individuals with the condition under-report their symptoms and remain under diagnosed even at presentation in the hospital (11). There is no study, to our knowledge, that has evaluated the level of prevalence, awareness, and reporting of symptoms of OSA among hospitalized patients in Nigeria. The objectives of this study were to determine the prevalence, awareness and reporting of obstructive sleep apnoea symptoms among hospitalized adult patients in medical and surgical wards in Nigeria.
Patients and Methods
Study Design: This was a multicenter cross-sectional study involving adult patients admitted into general medical and surgical wards of six selected Nigerian hospitals. It was conducted from March 2013 to April 2013.
Study setting: This study was conducted in Nigeria, a country located in West Africa and is divided into six geopolitical zones. The country has a population of about 145 million, with over 250 languages and an annual growth rate of 2 %. The setting of this survey was six tertiary hospitals in Nigeria. The investigators selected four university teaching hospitals and two federal medical centres which had estimated 800-bed spaces in the medical and surgical wards and average monthly bed occupancy rate of 80%. These hospitals were selected for reasons of geographical spread and easy coordination by the investigators.
Sample size and selection: The sample size was determined using the Cochran formula (16): n = z 2pq /d2 where n = sample size, prevalence of snoring which is 30% from previous study (12); the q = (1 − p), z = standard normal deviation usually set at 1.96 which corresponds to the 95% confidence interval. d = degree of accuracy was set at 0.05.The calculated sample size was 338 after adjusting for 5% non-response obtained by pretesting of the questionnaire. The estimated average patients' turnover over a period of two months was 1280 and less than 10000. Cochran's correction formula was used to calculate the minimum sample size. This calculation is as follows: n = 338/(1 + 338/1280) = 258. We used a design effect (DEFF) of 1.0, six age strata, and the final sample size was approximately 1600. The design effect (DEFF) of 1.0 was used because age strata distributions across the centres did not significantly differ from each other despite the variation in the size of the centres (17). The estimated sample size was distributed proportionally based on the total number of bed and occupancy rate in the participating centres.
Sample selection: A simple random sampling method was adopted because it provided less opportunity for errors caused due to confusion. We made a first sampling frame consisting of patients in each ward, and each patient was allocated number starting from 1 in ascending order on the first day of commencing the study. Subsequent patients who were admitted were also allocated their numbers which were quite different from the previous ones. All patients with allocated odd numbers were randomly selected until the sample size was attained. Eligible patients were approached by the resident, who provided patient information, consent form and verbal explanation of the study. The patients who provided consent and met the inclusion criteria were recruited as study participants.
Inclusion and exclusion criteria: The inclusion criteria were patients of at least 18 years of age, willingness to participate, admitted into medical and surgical wards, conscious and hemodynamically stable. Patients with language barriers to participation were also included whenever translation was available by the interpreter. The exclusion criteria were an unwillingness to participate in the study and cognitive impairment and unconsciousness. Anonymity and confidentiality of the participants and their information were guaranteed by the investigators.
Survey instrument: A questionnaire embedded with Berlin questionnaire, Epworth sleepiness scale and questions on patients' level of awareness and reporting of symptoms of OSA was used for data collection. Furthermore, the survey instrument was also used to obtain socio-demographic information, comorbidity and use of sedative and alcohol, tobacco smoking, family history of relatives with snoring/EDS.
Data collection: The questionnaire was administered by residents in the department of internal medicine who were trained by the supervisor in charge of each centre. They were made to complete a self-interview to become familiar with the survey instrument and also had scripted practice sessions with the supervisor, and the likely problems were discussed. The patients who were unable to read English were helped with the questionnaire translated into the three major Nigerian languages. We also made an arrangement for the translation into Fulani language for those who could not communicate in English and Hausa in the Yola study site in the North East. Fortunately, all the recruited subjects were able to speak and comprehend English or one of the three major languages. To reduce recall bias, an additional collection of information from persons who shared the same bedroom and sleep on the same bed was made.
Anthropometric indices in the form of waist/hip circumference, body weight (kg) and height (centimetre) were measured and body mass index calculated and expressed in Kg/m2. Obesity was defined as body mass index of ≥30 Kg/m2. Blood pressure was measured and hypertension was diagnosed by either a persistent blood pressure >140/90mmHg or the use of antihypertensive medications (18).
Frequency and risk of excessive sleepiness, OSA symptoms: The risk of OSA and excessive daytime sleepiness were established by validated Berlin questionnaire (8) and Epworth sleepiness scale (18). These two questionnaires have not been validated in any of the Nigerian languages. The Berlin questionnaire and Epworth sleepiness scale were tested for comprehensibility and translated, with back translation into English in the major three of the 250 Nigerian languages. In scoring the questions, answers were scored as a positive or negative response. Based on the scoring, they were sub-classified into three scoring categories: predetermination of a high risk and low risk of OSA using the Berlin Questionnaire was determined on the basis of the responses in 3 symptom categories. In category 1, high risk was defined as persistent symptoms (>3 to 4 times/week) for ≥2 questions about snoring. In category 2, high risk was defined as persistent (>3 to 4 times/week) daytime tiredness or fatigue. In category 3, high risk was defined as a history of high blood pressure or a body mass index >30 kg/m2. To be considered as a high risk for OSA, a patient had to qualify for ≥2 symptom categories. Those who reported not having persistent symptoms or who qualified for only one symptom category were placed in the lower risk group. The Epworth Scoring Scale (ESS) ranges from 0 to 24, and scores >10 were associated with excessive daytime sleepiness. ESS Score of 1–6 = Good, 7 – 10 = Okay and 11 or higher = high risk for excessive daytime sleepiness. Where possible, the interviewer notified the participant about the risk of OSA, excessive daytime sleepiness and the need for further evaluation.
Recognition and reporting of OSA symptoms: To determine the patients' level of awareness of the symptoms of OSA, they were asked the following questions: (a) Do you consider snoring to be a medical problem? (b) Do you consider excessive sleeping during the daytime to be a medical problem? (c) Have you reported or sought the advice of a doctor in relation to OSA symptoms during recent hospital visits. The responses to the questions were expressed as ‘yes /no’ or ‘don't know’.
Data analysis: The data were analyzed using Statistical Package for the Social Sciences (SPSS) software version 15 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were used to examine the general characteristics of the patients. The demographic variables, which were normally distributed, were described as a mean and standard deviation. A comparison between groups was made with the Student independent ‘t’ test and Mann-Whitney U. Categorical variables were reported as frequency distribution and compared using the chi-square test or Fisher's exact test. A P-value of < 0.05 was considered statistically significant.
Ethical approval: The study was approved by the ethics and research committee of the respective study institutions. All patients gave either verbal or written consent to participate in the study.
Results
Characteristics of the patients: A total of 1591 patients were admitted to the medical and surgical wards of the selected hospitals and they were informed about the study. Only 1420 patients met the inclusion criteria and were enrolled in the study with a completion rate of 89.3%.The median age of the patients was 45 years with inter-quartile range (IQR of 32–60 year); 747(52.6%) were females and 889(62.6%) had one or more co-morbidity with 589(40.1%) having hypertension which was the commonest comorbidity. Five hundred and thirty-seven (37.8%) had a first-degree relative with snoring or excessive sleepiness (Table 1).
Table 1.
Characteristics of the patients in the study
| Characteristics | N (%) |
| Median age (Q1–Q4) in years | 45(32–60) |
| Mean Body mass index (Kgm−2) | 24.4(4.5) |
| Sex | |
| Male | 673(47.4) |
| Female | 747(52.6) |
| Education | |
| None/primary | 320(22.5) |
| Secondary | 589(41.5) |
| Tertiary | 511(36.0) |
| Location of hospital | |
| Urban | 766(53.9) |
| Semi urban-Rural | 654(46.1) |
|
Presence of Chronic medical condition |
889(62.6) 589(40.1) |
| Hypertension | 181(12.7) |
| Type 2 DM | 160(11.3) |
| Obesity | 156(11.0) |
| Peptic Ulcer | 64(4.5) |
| Asthma | 64(4.5) |
| Insomnia | 46(3.2) |
| Stroke | 46(3.2) |
| Heart failure | 38(2.7) |
| COPD | 26(1.8) |
| Rhinitis | 16(1.1) |
| Metabolic syndrome X | |
| 202(14.2) | |
| Others | |
| Use of sedatives | 156(11.0) |
| Tobacco smoking | 164(11.5) |
| Alcohol consumption | 342(24.1) |
| First-degree relative snoring or excessive sleepiness |
537(37.8) |
(Q1–Q4)-interquartile range
Prevalence and Risk of OSA symptoms: A total 474(33.4%) patients reported snoring, 144(26.4) had loud snoring, 70(4.9%) reported witnessed apnoea, 142(10.0%) had daytime fatigue and 114(8.0%) reported drowsy driving. Two hundred and thirty-one patients (16.3%) had excessive daytime sleepiness (EDS), i.e. the Epworth Sleepiness Scale >10 and 256(18.0%) had a high risk for OSA. Men had a significantly higher frequency of persistent snoring, daytime fatigue and drowsy driving than women. The Berlin scores and Epworth scores for the risk of OSA and excessive daytime sleepiness respectively were also higher among men than among women (Table 2).
Table 2.
Symptoms and risk of obstructive sleep apnoea (OSA) by Gender.
| Symptoms | Male N = 673 |
Female N=747 |
All subjects N=1420 |
| Snoring | 248(36.8) | 226(30.3) | 474(33.4)* |
| Loud snoring | 78(27.9) | 66(24.8) | 144(26.4) |
| Persistent snoring | 134(19.9) | 116(15.5) | 250(17.6)* |
| Snoring bothering others | 140(20.8) | 122(16.3) | 262(18.5)* |
| Witnessed apnoea | 38(5.6) | 32(4.3) | 70(4.9) |
| Daytime Fatigue | 78(11.6) | 64(8.6) | 142(10.0) |
| Unrefreshing sleep | 80(11.9) | 58(7.8) | 138(9.7)* |
| Drowsiness on steering | 80(11.9) | 34(4.6) | 114(8.0)* |
| Risk for sleep apnoea | |||
| Low risk | 547(81.3) | 617(82.6) | 1164(82.0) |
| High risk | 126(18.7) | 130(17.4) | 256(18.0) |
| Excessive daytime sleepiness(ESS>10) | 113(16.8) | 118(15.8) | 231(16.3) |
Data are presented as n (%), ESS-Epworth Sleepiness Scale,
p values<0.05
Patients with chronic medical diseases (CMD) significantly had higher frequency of snoring (43.6% vs.16.2%), high risk for OSA (27.9% vs. 1.5%) and EDS (18.3% vs.5.8 %) than those without CMD (Table 3).
Table 3.
Symptoms and risk of obstructive sleep apnoea (OSA) by CMD
| Symptoms | CMD N = 889 |
No CMD N=531 |
All subjects N=1420 |
| Snoring | 388(43.6) | 86(16.2) | 474(33.4)* |
| Loud snoring | 112(12.6) | 32(6.0) | 144(26.4) |
| Persistent snoring | 222(25.0) | 28(5.3) | 250(17.6)* |
| Snoring bothering others | 230(25.9) | 32(6.0) | 262(18.5)* |
| Witnessed apnoea | 58(6.5) | 12(2.3) | 70(4.9)* |
| Daytime Fatigue | 114(12.8) | 28(5.3) | 142(10.0)* |
| Unrefreshing sleep | 118(13.3) | 20(3.8) | 138(9.7)* |
| Drowsiness on steering | 84(9.4) | 30(5.6) | 114(8.0)* |
| Risk for sleep apnoea | |||
| Low risk | 641(72.1) | 523(98.5) | 1164(82.0) |
| High risk | 248(27.9) | 9(1.5) | 256(18.0)* |
| Excessive daytime sleepiness(ESS>10) | 132(18.3) | 72(5.8) | 231(16.3)* |
Data are presented as n (%), ESS-Epworth Sleepiness Scale,
p values <0.05
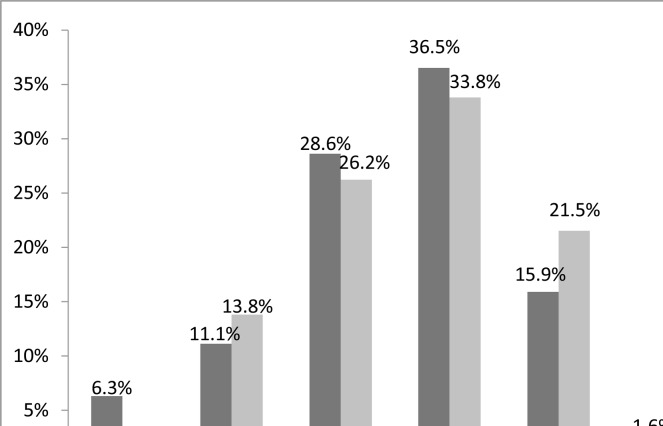
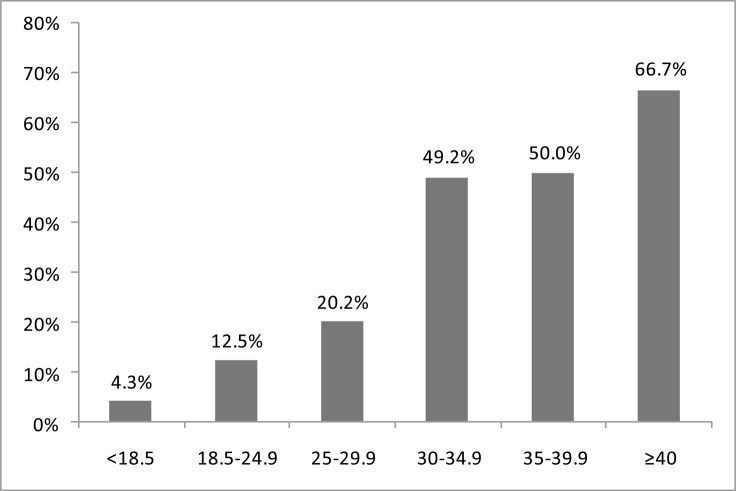
The frequency of high risk for OSA increased from the age of 18 years and peaked within the age range of 40–64 years, then declined after 65 years (Figure 1). Similarly, high risk for sleep apnoea also increased with the body mass index. The frequency of high risk for OSA was 66.7% in patients with morbid obesity and 4.3% in those who were underweight (Figure 2).
Figure 1.
High Risk for OSA by age for men and women
Figure 2.
High Risk for OSA by Body mass index
By hospital location, out of the 766 subjects recruited in the urban hospitals, 280(36.6%) had snoring, 126(16.5%) had excessive daytime sleepiness and 150(19.6%) had a high risk for OSA. Out of 654 subjects in the semi-urban and rural hospitals, 194(29.7%) had snoring, 104(16.0%) had excessive daytime sleepiness and 106(16.2%) had a high risk for OSA (Table 4).
Table 4.
Symptoms and risk of OSA by Hospital location.
| Symptoms | Urban N = 766 |
Semi urban/Rural N=654 |
All subjects N=1420 |
| Snoring | 280(36.6) | 194(29.7) | 474(33.4)* |
| Excessive daytime sleepiness(ESS>10) | 126(16.5) | 105(16.0) | 231(16.3) |
| High risk for sleep apnoea | 150(19.6) | 10616.2) | 256(18.0)* |
Data are presented as n (%), ESS-Epworth Sleepiness Scale
p values<0.05
Awareness and Reporting of OSA symptoms
The majority of the patients were not aware that snoring (77.3%) and excessive daytime sleepiness (65.8%) constitute a medical problem. Only 4.5% of them sought their doctor's advice during hospital visits for sleep apnoea symptom. The patients at high risk for OSA were more likely to be aware of the medical importance of the condition and report their sleep apnoea symptoms to their doctors than the low-risk patients (Table 5).
Table 5.
Awareness and reporting symptoms of OSA.
| Awareness statement | Low risk | High risk | Total | P value |
| Snoring is a medical problem | ||||
| Yes | 21.1 | 29.7 | 22.7 | 0.001 |
| No | 72.9 | 70.3 | 77.3 | |
| Excessive sleeping during the daytime is a medical problem |
||||
| Yes | 33.3 | 38.3 | 34.2 | |
| No | 66.7 | 61.7 | 65.8 | 0.043 |
| Reporting sleep related problem to doctors during hospital visits |
3.3 | 10.2 | 4.5 | |
| 96.7 | 89.8 | 95.5 | <0.001 |
Data presented as %
Discussion
The findings of this study revealed that one-third of the patients reported snoring, 16.3% had excessive daytime sleepiness, 10.0% experienced daytime fatigue, and 8.0% experienced drowsy driving. Approximately 5 % reported witnessed apnoea and 18.0% had high risks for obstructive sleep apnoea. Sixteen percent had excessive daytime sleepiness (EDS) and 18% had a high risk for OSA. Persistent snoring, daytime fatigue, drowsiness on the steering wheel, excessive daytime sleepiness and high risk for OSA were frequently higher in men than in women in this study. Patients with chronic medical conditions (CMD) had significantly higher frequency of OSA symptoms, high risk of OSA and excessive daytime sleepiness than those without chronic medical condition. The majority of the patients and especially the females and low-risk patients were not aware or fail to recognize that snoring and EDS are medical problems. Only 4.5% had reported their sleep apnoea symptoms to their doctors.
The prevalence of snoring obtained in this study is comparable to 36% from primary care facilities in the USA but lower than 40–52% reported in studies conducted in Saudi Arabia (21–23). In addition, it is higher than 22% in Turkey, 24.9% in Pakistan, 26% in Europe and 28.7% in Jordan (21, 24–26).
Our study revealed that 16% had EDS, 10% experienced daytime fatigue and 8% reported drowsiness while driving. This observation is similar to 10% drowsy driving reported from a study in Pakistan (25), while it is lower than 12–23% in the USA and 4–14% in Europe (21). Drowsiness while driving is a major public health hazard and is associated with increased risk of motor vehicle accident and poor occupational performance. Therefore, early diagnosis and treatment programs are needed for people who are at risk. The variations in results of symptoms in the different studies might be due to the diagnostic criteria and methodologies adopted in conducting these studies, variation in age of study population, cephalometric variations influenced by genetic factors and burdens of chronic medical conditions.
We also observed the predominance of these symptoms in male patients than in female patients. Our finding is in agreement with other studies (21–26), whereas studies from Saudi Arabia reported a higher frequency of witnessed apnea among female patients (23–24).
Prevalence of high risk for OSA using Berlin questionnaire was 18%, and is higher than 10% reported from Pakistan, but it is comparable to 17.4% found in Southwestern Nigeria, 16.8% in Jordan and 20.9% in the United Arab Emirates (13,26,27). The prevalence of high risk for OSA is higher in the western world, as 26–36% was observed in the USA and Europe and 33–39% in Saudi Arabia (21, 23–24).
Furthermore, male patients had a higher risk of sleep apnoea than female patients. This result is in agreement with other studies (13, 21, 26). This male predominance can be due to gender differences in the tobacco smoking, alcohol use, adipose tissue distribution, upper airway anatomy and muscle function, control of ventilation, and the effects of sex hormones and leptin (10). In contrast to our study, three studies in conducted in the Middle East reported that female patients were more prone to sleep apnea (23–24, 27). These findings can be explained by the high prevalence of female obesity in that population (1, 2). The female populations in the Middle East are mostly in purdah because of their religious beliefs. This often leads to limited outdoor activities, inactivity and excessive calorie retention resulting in weight gain.
Findings from this study also showed that chronic medical conditions were associated with high of risk sleep apnea. This finding is in concordance with other studies (12–14, 28, 29).
The result of this study also revealed that frequency of snoring and high risk for OSA were significantly higher in subjects who attended urban hospitals than the ones in semi-urban and rural hospitals. This result is similar to studies in China and built up areas in the United Kingdom (30–31). Health care utilization of facilities are a direct representation of the local population and therefore it can be concluded that snoring, EDS and the risk of sleep apnea were more prevalent among urban than rural residents. This result suggests that risk factors for sleep apnoea and lifestyle are different across urban and semi-urban/rural areas. Sedentary lifestyle and lack of exercise which are common in urban dwelling could lead to obesity and elevated body mass index and hypertension which are risk factors for sleep apnoea. In the rural areas, there is a lack of transport and most rural dwellers have to walk long distance daily to get water and carry out other daily chores. This daily activity prevents them from becoming obese and developing high blood pressure. In Africa, diabetes which is risk factors for OSA is more common among the wealthy ones, hence its designation as the “disease of opulence,” and remains more pronounced in urban areas where people tend to be less physically active, eating diets rich in saturated fats and refined sugars (32). Hypertension, Obesity and Diabetic Mellitus are found to be more common in urban than in the rural areas (33).
The majority of the patients were not aware that snoring and daytime sleepiness constitute medical problems. The awareness of snoring and excessive daytime sleepiness as medical problems, reporting to their doctors during hospital visits was higher among male patients and those at high risk for sleep apnoea.
Interestingly, our study also showed that reporting of symptoms was very low. Under-reporting and under-recognition were reported in other studies (34–35). The under-reporting of OSA symptoms may be attributed to poor awareness of the condition and poor patients' recall. It may also be due to poor knowledge and awareness on the part of the patients regarding the risks and consequences of sleep apnoea. These results may suggest the need for a future study on knowledge and awareness of the physicians and the patients regarding the risks, consequences and health economics of sleep.
The strength of our study that it is based on appreciable sample size of the respondents, widespread recruitment of patients from the urban, semi-urban and rural areas with varying socio-demographic profiles and lifestyles. Despite the aforementioned strengths, the study has some potential limitations which include the possible recall bias on the part of the patients and their relatives which affect their level of reporting of symptoms and actual prevalence. This problem was addressed with an additional collection of information from persons who shared the same bedroom and sleep on the same bed. This study did not capture all the seasons of the year; consequently, some seasonal conditions may have been underrepresented and others overrepresented. The adoption of hospital setting may have created some selection bias. However, this sampling technique provided less opportunity for errors due to confusion. This sampling technique also allows for studying patients available, giving a good representation of the overall patient population within in a reasonable period of time.
The prevalence of symptoms of OSA is high among hospitalized patients with CMD. The level of awareness and under-reporting of sleep apnoea symptoms are poor. Also, the high prevalence of OSA symptoms from this study should form the basis for screening hospitalized patients with CMD across the country.
References
- 1.Dauglas NJ. Sleep Apnoea. In: Fausi AS, Kasper DL, Longo LD, Braunwald E, Hauser SL, Jameson JL, et al., editors. Harrisons Principles of Internal Medicine. New York: McGraw-Hill; 2008. pp. 1665–1667. [Google Scholar]
- 2.Al Lawati NM, Patel SR, Ayas NT. Epidemiology, risk factors, and consequences of obstructive sleep apnea and short sleep duration. Prog Cardiovasc Dis. 2009;51:285–293. doi: 10.1016/j.pcad.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Caples SM, Garcia-Touchard A, Somers VK. Sleep-disordered breathing and cardiovascular risk. Sleep. 2007;30:291–303. doi: 10.1093/sleep/30.3.291. [DOI] [PubMed] [Google Scholar]
- 4.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 5.AlGhanim N, Comondore VR, Fleetham J, Marra CA, Ayas NT. The economic impact of obstructive sleep apnea. Lung. 2008;186:7–12. doi: 10.1007/s00408-007-9055-5. [DOI] [PubMed] [Google Scholar]
- 6.Albarrak M, Banno K, Sabbagh AA, Delaive K, Walld R, Manfreda J, et al. Utilization of healthcare resources in obstructive sleep apnea syndrome: a 5-year follow-up study in men using CPAP. Sleep. 2005;28:1306–1311. doi: 10.1093/sleep/28.10.1306. [DOI] [PubMed] [Google Scholar]
- 7.Collop NA, Anderson WM, Boehlecke B, et al. Clinical Guidelines for the Use of Unattended Portable Monitors in the Diagnosis of Obstructive Sleep Apnea in Adult Patients. J Clin Sleep Med. 2007;3:737–747. [PMC free article] [PubMed] [Google Scholar]
- 8.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 9.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 10.Rosen CL, Larkin EK, Kirchner HL, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003;142:383–389. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 11.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 12.Adewole OO, Hakeem A, Erhabor G, Fola A, Ajonwon Z. Obstructive sleep apnoea among adults in Nigeria. J Niger Med Assoc. 2009;101:720–725. doi: 10.1016/s0027-9684(15)30983-4. [DOI] [PubMed] [Google Scholar]
- 13.Sogebi OA, Ogunwale Risk factors of obstructive sleep apnea among Nigerian outpatients. Braz J Otorhinolaryngol. 2012;78:27–33. doi: 10.5935/1808-8694.20120029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akintunde AA, Okunola OO, Oluyombo R, Oladosu YO, Opadijo OG. Snoring and risk for obstructive sleep apnea among Nigerians with hypertensive: Prevalence and clinical correlates. Pan Afr Med J. 2012;11:75. [PMC free article] [PubMed] [Google Scholar]
- 15.Akintunde AA. Snoring and risk for obstructive sleep apnea among Nigerians with heart failure: Prevalence and clinical correlates. Heart Views. 2013;14:17–21. doi: 10.4103/1995-705X.107115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naing L, Winn T, Rusli BN. Practical issues in calculating the sample size for prevalence studies. Arch Orofac Sci. 2006;1:9–14. [Google Scholar]
- 17.Vierron E, Giraudeau B. Design effect in multicenter studies: gain or loss of power? BMC Medical Research Methodology. 2009;9:39. doi: 10.1186/1471-2288-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 19.Bartlett JE, Kotrlik JW, Higgins C. Organizational research: Determining appropriate sample size for survey research. Inf Technol Learn Perform J. 2001;19:43–50. [Google Scholar]
- 20.Chobanian AV, Bakris GL, Black HR, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 21.Netzer NC, Hoegel JJ, Loube D, et al. Prevalence of symptoms and risk of sleep apnea in primary care. Chest. 2003;124:1406–1414. doi: 10.1378/chest.124.4.1406. [DOI] [PubMed] [Google Scholar]
- 22.BaHammam AS, Alrajeh MS, Al-Jahdali HH, BinSaeed AA. Prevalence of symptoms and risk of sleep apnea in middle-aged Saudi males in primary care. Saudi Med J. 2008;29:423–426. [PubMed] [Google Scholar]
- 23.BaHammam AS, Alrajeh MS, Al-Jahdali HH, BinSaeed AA. Prevalence of symptoms and risk of sleep apnea in middle-aged Saudi women in primary care. Saudi Med J. 2009;30:1572–1576. [PubMed] [Google Scholar]
- 24.Doğan OT, Berk S, Ozşahin SL, Arslan S, Düzenli H, Akkurt I. Symptom prevalence of obstructive sleep apnea-hypopnea syndrome in health-care providers in central Sivas. Tuberk Toraks. 2008;56:405–413. [PubMed] [Google Scholar]
- 25.Taj F, Aly Z, Kassi M, Ahmed M. Identifying people at high risk for developing sleep apnea syndrome (SAS): a cross-sectional study in a Pakistani population. BMC Neurol. 2008;17(8):50. doi: 10.1186/1471-2377-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khassawneh B, Ghazzawi M, Khader Y, et al. Symptoms and risk of obstructive sleep apnea in primary care patients in Jordan. Sleep Breath. 2009;13:227–232. doi: 10.1007/s11325-008-0240-4. [DOI] [PubMed] [Google Scholar]
- 27.Mahboub B, Afzal S, Alhariri H, Alzaabi A, Vats M, Soans A. Prevalence of symptoms and risk of sleep apnea in Dubai, UAE. Int J Gen Med. 2013;6:109–114. doi: 10.2147/IJGM.S40001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozoh OB, Okubadejo NU, Akinkugbe AO, et al. Prospective assessment of the risk of obstructive sleep apnea in patients attending a tertiary health facility in Sub-Saharan Africa. The Pan African Medical Journal. 2014;17:302. doi: 10.11604/pamj.2014.17.302.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obaseki DO, Kolawole BA, Gomerep SS, Obaseki JE, Abidoye IA, Ikem RT, Erhabor GE. Prevalence and predictors of obstructive sleep apnea syndrome in a sample of patients with type 2 Diabetes Mellitus in Nigeria. Niger Med J. 2014;55:24–28. doi: 10.4103/0300-1652.128154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J1, Wei C, Huang L, Wang W, Liang D, Lei Z, et al. Prevalence of signs and symptoms suggestive of obstructive sleep apnea syndrome in Guangxi, China. Sleep Breath. 2014;18(2):375–382. doi: 10.1007/s1132.5-013-0896-2. [DOI] [PubMed] [Google Scholar]
- 31.Steier J, Martin A, Harris J, Jarrold I, Pugh D, Williams A. Predicted relative prevalence estimates for obstructive sleep apnoea and the associated healthcare provision across the UK. Thorax. 2014;69(4):390–392. doi: 10.1136/thoraxjnl-2013-203887. [DOI] [PubMed] [Google Scholar]
- 32.Cooper R, Rotimi C, Ataman S, McGee D, Osotmehin B, Kadiri S, et al. The prevalence of hypertension in seven populations of West African origin. Am J Public Health. 1997;87:160–168. doi: 10.2105/ajph.87.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.International Diabetes Federation, author. Diabetes Atlas. 3rd ed. Belgium: IDF, Brussels; 2007. [PubMed] [Google Scholar]
- 34.Stein MA, Mendelsohn J, Obermeyer WH, Amromin J, Benca R. Sleep and behavior problems in school-aged children. Pediatrics. 2001;107:E60. doi: 10.1542/peds.107.4.e60. [DOI] [PubMed] [Google Scholar]
- 35.Blunden SL, Lushington K, Lorenzen B, Ooi T, Fung F, Kennedy D. Are sleep problems under-recognised in general practice? Arch Dis Child. 2004;89:708–712. doi: 10.1136/adc.2003.027011. [DOI] [PMC free article] [PubMed] [Google Scholar]