Abstract
Purpose/Objectives
To review common tyrosine kinase inhibitors, as well as their ocular side effects and management.
Data Sources
A comprehensive literature search was conducted using cINahl®, Pubmed, and cochrane databases for articles published since 2004 with the following search terms: ocular toxicities, tyrosine kinase inhibitors, ophthalmology, adverse events, eye, and vision.
Data Synthesis
Tyrosine kinase inhibitors can cause significant eye toxicity.
Conclusions
Given the prevalence of new tyrosine kinase inhibitor therapies and the complexity of possible pathogenesis of ocular pathology, oncology nurses can appreciate the occurrence of ocular toxicities and the role of nursing in the management of these problems.
Implications for Nursing
Knowledge of the risk factors and etiology of ocular toxicity of targeted cancer therapies can guide nursing assessment, enhance patient education, and improve care management. Including a review of eye symptoms and vision issues in nursing assessment can enhance early detection and treatment of ocular toxicity.
Keywords: targeted therapy, ocular, toxicity, ophthalmology, tyrosine kinase inhibitors
Systemic anticancer therapies can cause acute and chronic damage to the eye. Ocular toxicity is generally underestimated and under-reported, and many may consider it to be a minor side effect. However, for the patient, blurred or loss of vision and other ocular symptoms can be troublesome and negatively affect quality of life. Damage to the ocular surfaces, retina, cornea, and optic nerve can be temporary or permanent. Ocular side effects are not uncommon with cytotoxic chemotherapy, but they are rarely severe or dose limiting (Huillard et al., 2014). However, serious toxicity has been associated with molecularly targeted cancer therapies, particularly tyrosine kinase inhibitors (TKIs). This class of drug targets receptors of tyrosine kinase, an enzyme responsible for the activation of proteins involved in the growth, progression, and spread of cancer. This targeted approach is expected to have fewer side effects than more traditional cytotoxic drugs that act nonspecifically on all dividing cells of the body. Ophthalmic side effects of targeted therapies differ depending on the specific molecular targets and the area of the eye involved.
Eye Anatomy and Function
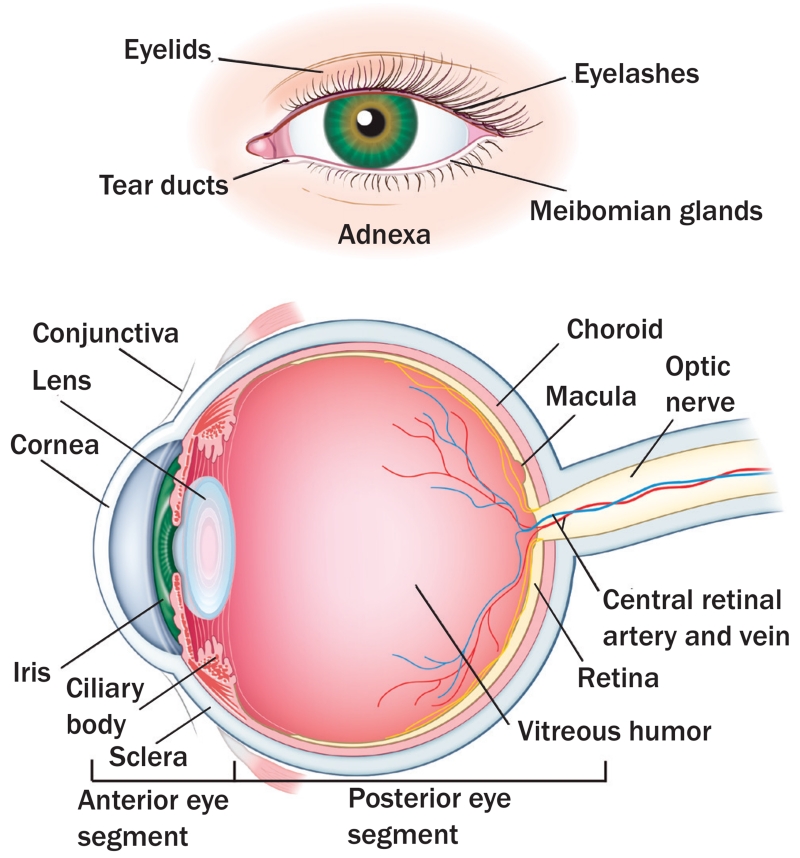
The eye can be regarded in sections, including the ocular adnexa, anterior segment of the eye, and the posterior segment (see Figure 1). The adnexa of the eye includes the eyelids, eyelashes, lacrimal system, and meibomian glands. The eyelids are folds of muscular tissue that serve to protect the eyeball. The skin on the outer surface of the eyelids is very thin, with little subcutaneous fat, allowing allergic reaction or inflammation to quickly manifest as swelling and edema (Kligman, Francis, & Abramson, 2015; Lang, 2000). The eyelashes protrude from the anterior aspect of the eyelid margin and aid in protecting the eye. Three or four rows of eyelashes contain about 150 lashes on the upper eyelid, and two rows contain about 75 lashes on the lower eyelid. The lacrimal system is comprised of tear-producing glands and a system to drain the tears. With each blink, the eyelid spreads the tears in a film over the surface of the eye, and the tears drain into the nose via the nasolacrimal duct. Goblet cells, which are scattered throughout the conjunctiva, secrete glycoprotein in the form of mucin that forms one layer of the tear film (Vagefi, Sullivan, Corrêa, & Augsburger, 2011). The tears are an important source of moisture, oxygen, and nutrients for the eye. Tears contain antimicrobial substances, including lysozyme and immunoglobulin (Ig) A and IgG antibodies. The aqueous component of the tear film dilutes infectious material, and mucus traps debris (Nijm, Garcia-Ferrer, Schwab, Augsburger, & Corrêa, 2011). Decreases in the production of tears or disruption of the tear film can result in dryness, causing irritation and ulceration (Hazin, Abuzetun, Daoud, & Abu-Khalaf, 2009). Meibomian glands, present on the eyelids, make sebum, an oily substance that is discharged through tiny holes to prevent evaporation of the tear film. About 50 meibomian glands are present on the upper eyelids and 25 on the lower eyelids.
FIGURE 1. Eye Anatomy.
Note. copyright 2015 by memorial Sloan Kettering cancer center. Used with permission.
The anterior segment of the eye contains the conjunctiva, cornea, sclera, iris, and lens. The conjunctiva is a thin vascular membrane that lines the eyelids and the anterior surface of the eye. Together with the surface of the cornea, the conjunctiva forms the conjunctival sac. Along with allowing the eyeball to move freely, the conjunctiva sac acts as a physical barrier and serves a protective function, hosting immune cells that aid in colonizing bacteria. The cornea is a sensitive structure of the eye and receives sensory signals from the trigeminal nerve. Injury to the cornea can cause severe pain, reflexive tearing, and involuntary closing of the eye (Lang, 2000). The pain is worsened by movement of the lids (particularly the upper lid) over the cornea and usually persists until healing occurs. Because the cornea serves as the “window” of the eye and refracts light rays, corneal damage usually blurs vision, particularly if it is centrally located (Biswell, 2011). The corneal epithelium layer acts as a barrier to microorganisms into the cornea. If the epithelium becomes damaged, infection may ensue. The sclera is a fibrous connective tissue that helps form the rigid outer covering of the eye (all the way around except for the cornea); fibers from the optic nerves and all six ocular muscles enter into the sclera. The iris is a thin, circular structure in the eye and is responsible for controlling the diameter and size of the pupil and, therefore, the amount of light reaching the retina. The amount of pigment in the iris determines eye color. In the center of the iris is the pupil. The lens is present behind the iris and focuses light onto the retina. The lens is an epithelial structure containing no blood vessels or nerves, but it allows transportation of water, nutrients, and minerals. With age, the water content decreases, causing the lens to become harder, less elastic, and less transparent. A cataract occurs when the lens becomes cloudy, affecting vision. The ciliary body includes the ciliary muscle, which controls the shape of the lens, and the ciliary epithelium, which produces the aqueous humor. The ciliary body is part of the uvea, the layer of tissue that delivers oxygen and nutrients to the eye tissues.
Finally, the posterior segment of the eye contains the choroid, retina, macula, and vitreous humor. The choroid is a dark brown membrane and the vascular layer of the eye between the retina and the sclera. The choroid is rich with blood vessels, supplies blood and nutrients to the retina, and conducts arteries and nerves to other structures within the eye. The retina is a light-sensitive structure that lines the inside surface of the eye. It contains 10 layers, including 3 layers of nerve cells. These cells convert incoming light into electrical impulses, transmitted to the brain via the optic nerve and interpreted as visual images. The retinal pigment epithelium (RPE) is a single layer of cells attached to the choroid; along with the vascular epithelium, the RPE forms the blood–retinal barrier and provides nutrients for the photoreceptor cells (van der Noll, Leijen, Neuteboom, Beijnen, & Schellens, 2013). The macula is an oval-shaped area near the center of the retina and contains the structures essential for central vision. The vitreous humor is a jelly-like substance that occupies the space behind the lens and in front of the retina. It contains no blood vessels, and the majority of its volume is water.
Molecular Targets and Ocular Effects
The eye is a highly differentiated organ and has had a pivotal role in the evolution of human genomics, with at least 90% of the genes in the human genome expressed in one or more of the eye’s many tissues and cells at some point during a person’s life (Sheffield & Stone, 2011). The complex functioning of the eye combines the neural network system with blood vessels, muscles, and skin that can be affected by targeted agents, often with different receptor-specific patterns that do not occur in this form elsewhere in the body (Hager & Seitz, 2014). Table 1 lists common TKIs, as well as their targets, specific cancers, and associated ocular effects.
TABLE 1. Selected TKIs and Ocular Effects.
| TKI | PMT | Cancer Indication |
Adverse Ocular event |
|---|---|---|---|
| Afatinib (Gilotrif®) Erlotinib (Tarceva®) Gefitinib (Iressa®) |
|
|
|
| Crizotinib (Xalkori®) |
|
|
|
| Dabrafenib (Tafinlar®) Vemurafenib (Zelboraf®) |
|
|
|
| Dasatanib (Sprycel®) Imatinib (Gleevec®) Nilotinib (Tasigna®) |
|
|
|
| Trametinib (Mekinist®) |
|
|
|
| Sunitinib (Sutent®) |
|
|
|
| Vandetanib (Caprelsa®) |
|
|
|
ALK–anaplastic lymphoma kinase; EGFR–epidermal growth factor receptor; MEK–mitogen-activated protein kinase inhibitor; PDGFR–platelet-derived growth factor receptor; PMT–primary molecular target; TKI–tyrosine kinase inhibitor; VEGF–vascular endothelial growth factor
Note. Based on information from Huillard et al., 2014; Kheir et al., 2014; Renouf et al., 2012.
Epidermal growth factor receptor (EGFR) is present in ocular and periocular tissue, including the eyelids, eyelash follicles, tear glands, conjunctiva, and cornea. EGFR-mediated processes are essential for eyelash growth, wound healing, and proliferation of corneal epithelial cells (Kheir, Sniegowski, El-Sawy, Li, & Esmaeli, 2014). Treatment with EGFR inhibitors can cause corneal thinning and erosion, and inhibition of EGF on the eyelashes can cause significant growth and trichomegaly. EGFR stimulates proliferation of the epithelial cells of the meibomian glands in the eyelids; with inhibition, the eyelids and meibomian glands can become inflamed, causing blepharitis and meibomitis. Typically, inflammation, conjunctivitis, and blepharitis occur early in therapy (around week 3), and trichomegaly rarely occurs before weeks 8–12 of therapy (Fraunhelder & Fraunhelder, 2012). EGFR TKIs responsible for this toxicity include erlotinib (Tarceva®) and gefitinib (Iressa®); lapatinib (Tykerb®), also an EGFR inhibitor, has not been associated with ocular adverse events, which is potentially related to less selective targeting or underreporting of toxicity (Huillard et al., 2014).
The platelet-derived growth factor receptor (PDGFR) pathway is involved with maintenance of interstitial pressure within the dermis. Dermal dendrocytes in the periocular tissue can express PDGF, as well as c-kit (also known as mast or stem cell growth factor receptor); inhibition of theses receptors leads to increased capillary permeability and fluid extravasation (Kheir et al., 2014; Renouf, Velazquez, Simpron, Siu, & Bedard, 2012). Drugs that target these receptors can cause periorbital edema and epiphora in as many as 70% of patients (Fraunfelder, Solomon, Druker, Esmaeli, & Kuyl, 2004). The edema usually occurs from five to eight weeks after initiation of treatment but can occur as early as within 24 hours or as late as after one year, depending on the dose (Hager & Seitz, 2014). Imatinib (Gleevec®) may also cause subconjunctival hemorrhages, thought to be related to targeting c-kit–positive mast cells on the conjunctiva (Kheir et al., 2014).
The mitogen-activated protein kinase (MEK) pathway plays a role in transduction of cellular signals, regulating proliferation, survival, and malignant transformation (Kheir et al., 2014). Agents targeting this pathway are rapidly becoming promising therapies for metastatic disease, particularly melanomas. However, MEK inhibitors can cause sight-threatening adverse events. The retinal epithelial pigment cells are responsible for maintaining the outer blood–retinal barrier and preventing fluid accumulation within the retinal layers. Inhibition of the MEK pathway may alter permeability of these cells and disrupt the ability to prevent fluid accumulation (Duncan, Chang, & Patronas, 2015). The result is subretinal fluid accumulation, causing serous retinal detachment and sight issues. Retinal vein occlusion (RVO) may occur when fluid blocks the vasculature that drains the retina. With blockage, pressure builds up in the capillaries, leading to hemorrhage and leakage of fluid and blood, which can lead to macular edema and ischemia. Inhibition of MEK may result in inflammatory responses and, combined with activation of a coagulation cascade, increases the risk of RVO (Duncan et al., 2015; Huang et al., 2009). MEK inhibition may also lead to the development of uveitis; dysregulation of the tight junctions of the endothelial cells of the ciliary body may contribute to inflammation, leading to uveitis (Niro et al., 2015).
BRAF is a member of the Raf kinase family of growth signal transduction protein kinases. This protein plays a role in regulating the MAP kinase/extracellular signal–regulated kinases signaling pathway, which affects cell division and differentiation. The BRAF gene is mutated in 50% of melanomas and in smaller percentages of papillary thyroid cancer and other malignancies (Kheir et al., 2014). Vemurafenib (Zelboraf®) and dabrafenib (Tafinlar®) are two approved BRAF inhibitors that target a mutated form of the BRAF gene (V600E). Uveitis and macular edema may occur after taking these drugs (Liu et al., 2014). A serious adverse event associated with BRAF inhibitors is the development of keratoacanthomas and squamous cell carcinomas, which can occur on the skin around the eyes and on the eyelids (Kheir et al., 2014).
Anaplastic lymphoma kinase (ALK) is the target for the tyrosine kinase inhibitor crizotinib (Xalkori®). Along with c-Met receptor inhibition, crizotinib targets a specific genetic alteration in ALK. Although uncommon, occurring in only 2%–7% of all non-small cell lung cancers, ALK mutations are more prevalent in patients who have never smoked or who have a history of light smoking and in patients with adenocarcinomas (Kwak et al., 2010). Ocular problems, usually mild light/dark adjustment deficits, occur in as many as 42%–62% of patients taking crizitonib (Hager & Seitz, 2014; Liu et al., 2014). Visual effects are described as photopsia (flashes), light trails, or brief image persistence (i.e., post-flashbulb effect) occurring particularly when transferring from low to bright light conditions. This condition often improves with length of time receiving therapy (Kwak et al., 2010).
Vascular endothelial growth factor receptor (VEGF) is a target of the TKIs vandetanib and sunitinib; their action blocks VEGF-stimulated endothelial cell proliferation and migration and reduces tumor vessel permeability. Although direct ocular toxicity with VEGF TKIs are rare, they are known to be associated with toxicities that lead to visual symptoms, such as hypertension and posterior reverse encephalopathy syndrome, causing blurred vision and retinopathy (Renouf et al., 2012). Intravitreal injection of anti-VEGF agents are now used to treat common retinal diseases, including neovascular age-related macular degeneration, diabetic retinopathy, and retinal vein occlusions (Falavarjani & Nguyen, 2013).
Effects on the Eye
Keratitis
Keratitis is an inflammation of the cornea, often beginning with the erosion of the epithelial surface (Huillard et al., 2014). The epithelial cells control corneal hydration; targeted agents can decrease proliferation of these corneal epithelial cells, which can lead to delay in wound healing and scarring. Corneal opacity occurs when the cornea becomes scarred. Symptoms of keratitis may include blurred vision, photophobia, periocular pain, and foreign body sensation.
Fluorescein 2% may be used by the ophthalmologist to detect corneal surface changes and epithelial defects from keratitis. A small amount of fluorescein from a single-use sterile paper strip is placed on the corner of the eye, and the dye is distributed across the surface of the eye. Fluorescein stains any erosions or microscopic defects of the corneal epithelium, allowing for enhanced visualization during slit lamp examination.
Treatment of keratitis includes many of the interventions used to treat dry eye; artificial tears (ATs) and lubricants are important to promote hydration of the cornea. Topical corticosteroids, such as fluorometholone or prednisolone drops, may be prescribed to disrupt the cycle of inflammation and epithelial damage. Steroids can quickly provide relief from discomfort but should only be used in short courses because use has been associated with acute increased intraocular pressure and glaucoma (Caparas, 2015). Topical antibiotic drops may also be prescribed if infection is present. For severe epithelial defects, bandage contact lenses may be used (Kligman et al., 2015). Bandage lenses protect the cornea from potential exterior sources of injury and from the possible shearing effect created by the lids during blinking, which can inhibit re-epithelialization and cause more pain.
Uveitis
Uveitis, which can occur from MEK and BRAF inhibitor use, is the inflammation of the iris, ciliary body, or choroid, and it can be classified depending on where the inflammation arises (Huillard et al., 2014). Symptoms include periocular pain, blurred vision, and presence of floaters. Photophobia and redness may also be present. With persistent uveitis, macular edema may occur and can lead to loss of vision. Synechia, a condition when the iris adheres to either the cornea or lens, can develop, impeding aqueous outflow and causing ocular hypertension or glaucoma. Corticosteroid drops are prescribed to decrease inflammation and cycloplegic or mydriatic dilating agents to help pull the iris away from the lens, preventing synechia formation and reducing discomfort. If intraocular pressure is increased, beta blockers or alpha antagonists can be prescribed. Close ophthalmic monitoring is required to ensure that uveitis is resolving because the offending drug may need to be held during healing.
Conjunctivitis
Conjunctivitis occurs when damage to the epithelial layer lining the sclera and the inside of the eyelids becomes inflamed (Hazin et al., 2009). Damage to the conjunctival epithelium causes hypertrophy, edema, cellular death, and exfoliation. Inflammatory cells migrate through the epithelium to the surface and combine with fibrin and mucus exudate, which is responsible for the “matting” on the lid margins, particularly in the morning (Nijm et al., 2011). Symptoms of conjunctivitis include hyperemia (redness of the sclera), irritation, discharge, epiphora (watery eyes), and itching. Symptoms are generally relieved when the offending drug is discontinued. ATs should be encouraged to provide relief of dryness and, if itching is severe, use of antihistamine drops should be considered. A short course of steroid drops may also be prescribed. Cool compresses several times per day may be effective to soothe irritation.
Epiphora
Epiphora, or watery eyes, can be caused by excessive tear production or the deficient drainage of tears from the eyes. Toxic effects from drugs can irritate the conjunctiva and corneal epithelium, leading to irritation and hypersecretion of tears (Hazin et al., 2009). Occasionally, stricture or stenosis of the lacrimal drainage may occur. Treatment includes the administration of ATs and antihistamine drops. If stenosis or stricture is present, lacrimal irrigation or dilatation with a small probe may be performed by the ophthalmologist to improve flow through the tear ducts.
Photophobia
Photophobia is a condition in which the eyes are overly sensitive to light. It has been linked to BRAF and MEK inhibitor therapy. Patients may complain of discomfort in bright light and may need to squint or close eyes. Headache may accompany the photophobia. Treatment involves correcting the underlying condition, which is usually uveitis or corneal abrasion (Hazin et al., 2009). Patients should be encouraged to keep lights as dim as possible and use sunglasses when in bright light.
Periorbital and Eyelid Edema
Periorbital and eyelid edema occurs when inflammation and accumulation of fluid of the interstitial tissues within the skin around the eyes and lids. PDGFR inhibitors, such as imatinib, can cause this ocular toxicity, which usually occurs in association with lower extremity peripheral edema (Quintás-Cardama, Cortés, & Kantarjian, 2008). This edema typically resolves with conservative management, such as low-sodium diet, restricting fluid intake, elevation of the head during sleep, and diuretics (Larson, Bergstrom, Cameron, Erickson, & Grimm, 2007; Quintás-Cardama et al., 2008). Topical or systemic steroids may also be prescribed. More severe cases of periorbital edema that result in visual impairment might require interruption of drug therapy and, in some cases, surgical excision of excess periocular skin (Kheir et al., 2014). Cool compresses may provide comfort.
Blepharitis and Meibomitis
Blepharitis (inflammation of the eyelids) and meibomitis (inflammation of the meibomian glands on the eyelids) can occur from EGFR-targeted therapy. Scales or scurf can cling to the lashes of the upper and lower lids. Symptoms include blurred vision with eye irritation, itching and burning of the lid margins, and discharge. The eyes may appear to be red-rimmed. Treatment includes eyelid hygiene; the patient should be educated to apply warm compresses for five minutes to the eyelids twice daily and gently scrub the eyelids daily with a moistened gauze or applicator to mechanically remove the scales. Diluted baby shampoo can be used on applicators to assist with scale removal if desired. In some cases, application of an antibiotic or sulfacetamide ointment with a cotton applicator to the lid margins may be prescribed. Proper handwashing and hygiene should also be emphasized.
Trichomegaly
Trichomegaly is theabnormal growth of the eyelashes. It has been associated with the EGFR TKIs erlotinib and gefitinib. These agents block the receptors involved in the development of hair follicles and allow for continued lash growth, which can lead to trichiasis. Trichiasis occurs when the lashes grow back into the conjunctiva and cornea, causing irritation and potentially ulceration. Patients should be instructed not to trim their own lashes; this should be done by an ophthalmologist who can determine if lashes need to be removed.
Serous Retinal Detachment
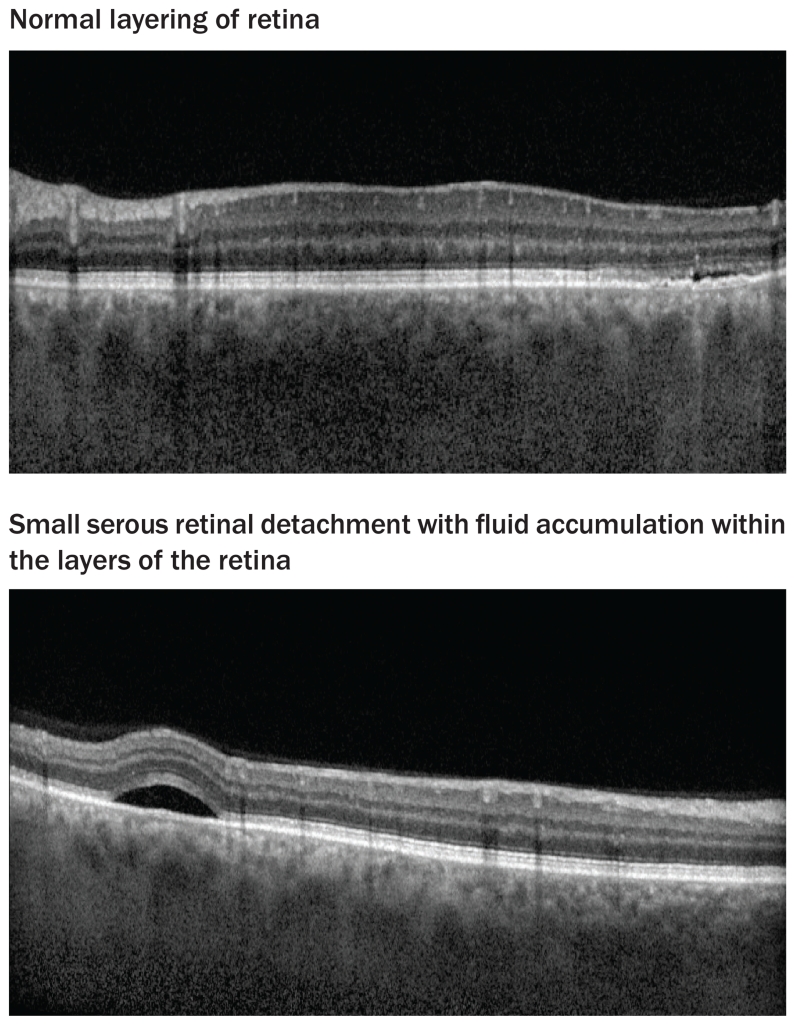
Serous retinal detachment occurs when fluid accumulates under the layers of the retina; symptoms include blurred vision, often in both eyes. Diagnosis can be confirmed by optical coherence tomography (OCT). OCT is a noninvasive imaging test that uses light waves to take cross-sectional images of the retina. Figure 2 shows an OCT image of a normal retina and a retina with the accumulation of fluid under the retinal layers. This retinopathy is usually dependent on dose and time and is often self-limiting and resolves without local treatment or alteration of drug therapy. For retinal changes accompanied by visual acuity changes or retinal fluid that has persisted longer than three months, dose reduction or temporary discontinuation should be considered (Duncan et al., 2015; Niro et al., 2015; van der Noll et al., 2013). The Amsler Grid is a grid of horizontal and vertical lines used to monitor a person’s central visual field. It can be useful for patients with serous detachment to monitor vision at home. The patient, with one eye at a time, looks at the small dot in the center of the grid. If the grid lines are wavy, broken, or distorted, or if there are blurred or missing patches, this is called metamorphopsia, and they are instructed to follow up with ophthalmologist.
FIGURE 2. Ocular Coherence Tomography.
Note. Images courtesy of Jasmin Francis, mD. Used with permission.
Retinal Vein Occlusion
Retinal vein occlusion is a sight-threatening condition linked to the MEK inhibitors. Patients often present with sudden, painless, unilateral loss or distortion of vision. The degree of vision loss depends on the extent of retinal involvement and macular perfusion status; macular edema is most frequently the cause of the vision loss (Wong & Scott, 2010). Visual acuity testing and confrontational visual field testing is done to evaluate for defects and fluorescein angiography to determine the extent of vasculopathy. Treatment of vein occlusion with anti-VEGF and steroid injection has proven useful in decreasing macular edema and helping improve vision (Duncan et al., 2015).
Management of Ocular Toxicity
Many targeted agents cause dryness of the eyes, which is one of the most common conditions treated by ophthalmologists (Hazin et al., 2009). Dry eye syndrome (DES) is also known as keratoconjunctiva sicca or dysfunctional tear syndrome (Borkar, Lacouture, & Basti, 2013). Symptoms of DES are usually bilateral and include mildly to moderately decreased vision, burning, and the sensation of a foreign body in the eye. Excess reflexive tearing may also be seen to counteract the irritation from dryness. This symptom is often exacerbated by environmental factors, such as wind, smoke, heat, and low humidity, or from prolonged use of the eye, such as working for long periods on a computer. Targeted drugs may cause changes in the composition of the tear film, causing the film to break quickly and allowing the cornea and eyes to dry (Lang, 2000). A Schirmer test can be performed to measure the volume of tears as a surrogate for tear production. Small strips of blotting paper are placed under the lower eyelids and later measured for absorption of tears. The nurse administers an anesthetic drop in each eye to prevent irritation prior to inserting the strips because irritation may generate reflex tearing and affect results. Proparacaine is commonly the anesthetic drop used because it is the least irritating of the topical ophthalmic anesthetics (Flach & Fraunfelder, 2011). The tip of one end of the strip is folded at the notch on the paper and is inserted. The patient is then asked to sit quietly for five minutes, and the strips are removed and measured for wetness. Normal tear production creates about 10–15 mm of wetness on each paper strip. Older adult patients may wet only about 10 mm because hypolacrimation occurs with aging (Sharma & Hindman, 2014). A value of less than 5 mm is suggestive of a dry eye state.
The mainstay of DES treatment includes replacement of tears with over-the-counter ATs. These ocular lubrication products usually contain electrolytes, surfactants, and viscosity agents in a hypotonic or isotonic buffered solution; ATs vary mostly in electrolyte composition, osmolarity, viscous agent, and the presence or absence of a preservative (Caparas, 2015). Use of preservative-free ATs is often preferred because the preservative is commonly the cause of allergic reactions (Flach & Fraunfelder, 2011) and can be irritating to the cornea, conjunctiva, and eyelids (Kligman et al., 2015). The addition of a lubricating ointment or gel at bedtime can provide palliation of dryness. If DES is severe, cyclosporine A (Restasis®) can be prescribed and can address the cause of dry eye rather than just palliate. Topical ophthalmic cyclosporine acts as an immunomodulatory with anti-inflammatory effects. It has been shown to inhibit T-cell activation, decrease and downregulate activated lymphocytes and inflammatory cytokines (interleukin 6) in the conjunctiva, reduce conjunctival inflammatory and apoptotic markers, and increase goblet cell numbers (Caparas, 2015; Kligman et al., 2015). The most common side effect of cyclosporine is redness and stinging, with as many as 17% of patients complaining of burning with application (Allergan, Inc., 2013). Patients should be educated that clinical improvement may take time (one to two months), and the recommended duration of treatment is at least six months (Caparas, 2015).
Punctal occlusion with the placement of punctal plugs into the tear ducts to retain lacrimal secretions may also be a consideration for treatment of severe DES. For patient with continued symptoms despite the previously stated interventions, autologous serum drops may be used. As a type of anti-inflammatory therapy, drops are prepared from the patient’s own serum and then further diluted in saline. The serum contains high concentrations of essential tear components, including epidermal growth factor, fibronectin, neurotrophic growth factor, and vitamin A, and it has been shown to stabilize tear film and improve ocular surface disease (Coursey & de Paiva, 2014; Kojima et al., 2008).
Some evidence exists that the use of dietary supplements containing omega-3 and omega-6 fatty acids may improve DES symptoms (Al Mahmood & Al-Swailem, 2014; Bhargava, Kumar, Kumar, Mehra, & Mishra, 2013; Pinazo-Durán et al., 2013). Larger randomized multicenter trials and trials with patients with cancer undergoing chemotherapy are needed before a general recommendation to use antioxidants for DES is reached. Individual consideration warrants discussion between the oncologist and ophthalmologist regarding the risk–benefit ratio related to the possibility that antioxidants may protect tumor cells (Lawenda et al., 2008).
The nurse should perform a medication review to evaluate other medications that may be contributing to dry eye. Phenothiazines, nasal decongestants, anticholinergics, antiulcer drugs, and retinoids can cause hyposecretion of tears (Hazin et al., 2009). All patients with dry eye should be educated on making lifestyle modifications, such as the use of humidifiers and smoking cessation. Patients with significant dry eye should be discouraged from wearing contact lenses because dryness is a risk factor for ulceration and keratitis (Huillard et al., 2014). The incidence of keratitis is particularly high with soft lenses, predominantly with extended wear; overnight wear increases the risk by five times compared to daily wear with regular replacement (Flach & Fraunfelder, 2011). Patients should be advised to have eyeglasses available and to remove contact lenses whenever an eye becomes uncomfortable or inflamed. Prompt referral to an ophthalmologist is warranted to rule out keratitis if dryness, discomfort, or inflammation persists.
Implications for Nursing
Although no consensus or recommendation exists for routine ophthalmic monitoring and management of ocular toxicities from TKIs (Agustoni et al., 2014), the oncology nurse can still do a lot to help preserve patient sight and quality of life. Promotion of hand hygiene before any eye care is an easy intervention because infections are a major cause of preventable ocular toxicity (Pirbhai, Kent, & Hodge, 2011). Patients who wear contact lenses should be advised to be meticulous about eye hydration, lens hygiene, and not using lenses beyond their disposal time. Careful, regular skin assessments of patients using BRAF inhibitors should be performed and, if lesions are found, referrals should be made to dermatologists or ophthalmologists because surgical excision, dose reduction, or discontinuation may be necessary (Kheir et al., 2014). Including a review of eye symptoms and vision issues in daily nursing assessment can enhance early detection and treatment. Promotion of healthy lifestyles with regard to diet, smoking cessation, and control of comorbidities is vital to reduce risk factors for enhanced toxicity. The use of sunglasses should be encouraged for all patients.
Although some ocular toxicity can easily be identified by the oncology nurses, asymptomatic nerve or retinal injury requires a specialized ophthalmic examination. A baseline ophthalmic examination should be performed prior to beginning TKI therapy. Patients need to be educated about potential TKI-specific eye issues and when to contact the clinician because prompt recognition, early diagnosis, and immediate treatment markedly improves the visual outcome of many ophthalmic conditions (Flach & Fraunfelder, 2011). When toxicities develop and patients are prescribed eye medications, nurses should not assume that patients understand how to properly administer them. Education and return demonstrations can enhance learning with confirmation of medication indication, dosage, and schedule. The decision to reduce dose or hold or discontinue a drug is complex and should be made after consideration of risk–benefit analysis and discussion with the oncologist and ophthalmologist.
Conclusion
As new TKIs and other targeted therapies move from clinical trials into daily clinical practice, education and awareness of ocular events is essential to optimize patient outcomes. Nurses understand the impact of vision limitations on quality of life, including a loss of independence and feelings of social isolation, which may be profound in patients with advanced cancer (Niro et al., 2015). With knowledge of the potential ocular effects of these modalities, the nurse can closely monitor and educate at-risk patients to promote well-being and preserve vision. Anticipation of treatment-related toxicity can provide opportunity to intervene early to prevent morbidity and keep patients on life-prolonging therapy.
Knowledge Translation.
Tyrosine kinase inhibitors (TKIs) have unique side effect profiles, differing widely from cytotoxic chemotherapy, and nurses should encourage an ophthalmic baseline assessment and for the patient to immediately report any vision issues.
ocular toxicities are often unrecognized and unreported because priority is given to other life-threatening effects.
Anticipation of ocular toxicities from TKIs may provide the opportunity to develop intervention strategies to minimize or eliminate adverse events and allow patients to remain on therapy.
Footnotes
No financial relationships to disclose.
Mention of specific products and opinions related to those products do not indicate or imply endorsement by the Oncology Nursing Forum or the Oncology Nursing Society.
Davis can be reached at davism@mskcc.org, with copy to editor at oNFEditor@ons.org.
References
- Agustoni F, Platania M, Vitali M, Zilembo N, Haspinger E, Sinno V, Garassino MC. Emerging toxicities in the treatment of non-small cell lung cancer: Ocular disorders. Cancer Treatment Reviews. 2014;40:197–203. doi: 10.1016/j.ctrv.2013.05.005. doi:10.1016/j.ctrv.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Allergan, Inc. Restasis®(cyclosporine ophthalmic emulsion) [Package insert] 2013 Retrieved from http://www.allergan.com/assets/pdf/restasis_pi.pdf.
- Al Mahmood AM, Al-Swailem SA. Essential fatty acids in the treatment of dry eye syndrome: A myth or reality? Saudi Journal of Ophthalmology. 2014;28:195–197. doi: 10.1016/j.sjopt.2014.06.004. doi:10.1016/j.sjopt.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava R, Kumar P, Kumar M, Mehra N, Mishra A. A randomized controlled trial of omega-3 fatty acids in dry eye syndrome. International Journal of Ophthalmology. 2013;6:811–816. doi: 10.3980/j.issn.2222-3959.2013.06.13. doi:10.3980/j.issn.2222-3959.2013.06.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswell R. Cornea. In: Riordan-Eva P, Cunningham ET Jr., editors. Vaughan and Asbury’s general ophthalmology. 18th ed. McGraw-Hill Medical; New York, NY: 2011. [Google Scholar]
- Borkar DS, Lacouture ME, Basti S. Spectrum of ocular toxicities from epidermal growth factor receptor inhibitors and their intermediate-term follow-up: A five-year review. Supportive Care in Cancer. 2013;21:1167–1174. doi: 10.1007/s00520-012-1645-y. doi:10.1007/s00520-012-1645-y. [DOI] [PubMed] [Google Scholar]
- Caparas VL. Medical management of dry eye. In: Chan C, editor. Dry eye: A practical approach. Springer-Verlag Berlin Heidelberg; Berlin, Germany: 2015. pp. 55–61. [Google Scholar]
- Coursey TG, de Paiva CS. Managing Sjögren’s syndrome and non-Sjögrens syndrome dry eye with antiflammatory therapy. Clinical Ophthalmology. 2014;8:1447–1458. doi: 10.2147/OPTH.S35685. doi:10.2147/OPTH .S35685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan KE, Chang LY, Patronas M. MEK inhibitors: A new class of chemotherapeutic agents with ocular toxicity. Eye. 2015;29:1003–1012. doi: 10.1038/eye.2015.82. doi:10.1038/eye.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falavarjani KG, Nguyen QD. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: A review of literature. Eye. 2013;27:787–794. doi: 10.1038/eye.2013.107. doi:10.1038/eye.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flach AJ, Fraunfelder FW. Ophthalmic therapeutics. In: Riordan-Eva P, Cunningham ET Jr., editors. Vaughan and Asbury’s general ophthalmology. 18th ed. McGraw-Hill Medical; New York, NY: 2011. [Google Scholar]
- Fraunhelder FT, Fraunhelder FW. Trichomegaly and other external eye side effects associated with epidermal growth factor. Cutaneous and Ocular Toxicology. 2012;31:195–197. doi: 10.3109/15569527.2011.636118. doi:10.310 9/15569527.2011.636118. [DOI] [PubMed] [Google Scholar]
- Fraunfelder FW, Solomon J, Druker BJ, Esmaeli B, Kuyl J. Ocular side-effects associated with imatinib mesylate (Gleevac®) Journal of Ocular Pharmacology and Therapeutics. 2004;19:371–375. doi: 10.1089/108076803322279426. doi:10.1089/1080768033222279426. [DOI] [PubMed] [Google Scholar]
- Hager T, Seitz B. Ocular side effects of biological agents in oncology: What should clinicians be aware of? OncoTargets and Therapy. 2014;7:69–77. doi: 10.2147/OTT.S54606. doi:10.2147/OTT.S4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazin R, Abuzetun JY, Daoud YJ, Abu-Khalaf MM. Ocular complications of cancer therapy: A primer for the ophthalmologist treating cancer patients. Current Opinion in Ophthalmology. 2009;20:308–317. doi: 10.1097/ICU.0b013e32832c9007. doi:10.1097/ICU.Ob013e32832c9007. [DOI] [PubMed] [Google Scholar]
- Huang W, Yang AH, Matsumoto D, Collette W, Morroquin L, Ko M, Younis HS. PD0325901, a mitogen-activated protein kinase inhibitor, produces ocular toxicity in rabbit animal model of retinal vein occlusion. Journal of Ocular Pharmacology and Therapeutics. 2009;25:519–530. doi: 10.1089/jop.2009.0060. [DOI] [PubMed] [Google Scholar]
- Huillard O, Bakalian S, Levy C, Desjardins L, Lumbroso-Le Rouic L, Pop S, Le Tourneau C. Ocular adverse events of molecularly targeted agents approved in solid tumours: A systematic review. European Journal of Cancer. 2014;50:638–648. doi: 10.1016/j.ejca.2013.10.016. doi:10.1016/j.ecja.2013.10.016. [DOI] [PubMed] [Google Scholar]
- Kheir WJ,, Sniegowski MC, El-Sawy T, Li A, Esmaeli B. Ophthalmic complications of targeted cancer therapy and recently recognized ophthalmic complications of traditional chemotherapy. Survey of Ophthalmology. 2014;59:498–502. doi: 10.1016/j.survophthal.2014.02.004. doi:10.1016/j.survophthal.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Kligman BE, Francis JH, Abramson DH. Ocular complications due to cancer treatment. In: Schwartz CL, Hobbie WL, Constine LS, Ruccione KS, editors. Survivors of childhood and adolescent cancer: A multidisciplinary approach. 3rd ed. Springer; New York, NY: 2015. pp. 96–111. [Google Scholar]
- Kojima T, Higuchi A, Goto E, Matsumoto Y, Dogru M, Tsubota K. Autologous serum eye drops for the treatment of dry eye diseases. Cornea. 2008;27(Suppl.):S25–S30. doi: 10.1097/ICO.0b013e31817f3a0e. doi:10.1097/ICO.0b013e31817f3a0e. [DOI] [PubMed] [Google Scholar]
- Kwak LE, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, Iafrate AJ. Anaplastic lymphoma kinase inhibition in non–small-cell lung cancer. New England Journal of Medicine. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. doi:10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang GL. Ophthalmology: A pocket textbook atlas. Thieme; New York, NY: 2000. [Google Scholar]
- Larson JS, Bergstrom LK, Cameron JD, Erickson LA, Grimm TE. Severe periorbital edema secondary to imatinib mesylate for chronic myelogenous leukemia. Archives of Ophthalmology. 2007;125:985–986. doi: 10.1001/archopht.125.7.985. [DOI] [PubMed] [Google Scholar]
- Lawenda BD, Kelly KM, Ladas EJ, Sagar SM, Vickers A, Blumberg JB. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? Journal of the National Cancer Institute. 2008;11:773–783. doi: 10.1093/jnci/djn148. doi:10.1093/jnci/djn148. [DOI] [PubMed] [Google Scholar]
- Liu CY, Francis JH, Brodie SE, Marr B, Pulido JS, Marmor MF, Abramson DH. Retinal toxicities of cancer therapy drugs: Biologics, small molecule inhibitors and chemotherapies. Retina. 2014;34:1261–1280. doi: 10.1097/IAE.0000000000000242. [DOI] [PubMed] [Google Scholar]
- Nijm LM, Garcia-Ferrer FJ, Schwab IR, Augsburger JJ, Corrêa ZM. Conjunctiva and tears. In: Riordan-Eva P, Cunningham ET Jr., editors. Vaughan and Asbury’s general ophthalmology. 18th ed. McGraw-Hill Medical; New York, NY: 2011. [Google Scholar]
- Niro A, Strippoli S, Alessio G, Sborgia L, Recchimurzo N, Guida M. Ocular toxicity in metastatic melanoma patients treated with mitogen-activated protein kinase inhibitors: A case series. American Journal of Ophthalmology. 2015;160:959–967. doi: 10.1016/j.ajo.2015.07.035. doi:10.1016/j.ajo.2015.07.035. [DOI] [PubMed] [Google Scholar]
- Pinazo-Durán MD, Galbis-Estrada C, Pons-Vázquez S, Cantú-Dibildox J, Marco-Ramírez C, Benítez-del-Castillo J. Effects of a nutraceutical formulation based on the combination of antioxidants and omega-3 essential fatty acids in the expression of inflammation and immune response mediators in tears from patients with dry eye disorders. Clinical Interventions in Aging. 2013;8:139–148. doi: 10.2147/CIA.S40640. doi:10.2147/CIA.S4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirbhai A, Kent SS, Hodge WG. Causes and prevention of vision loss. In: Riordan-Eva P, Cunningham ET Jr., editors. Vaughan and Asbury’s general ophthalmology. 18th ed. McGraw-Hill Medical; New York, NY: 2011. [Google Scholar]
- Quintás-Cardama A, Cortés JE, Kantarjian H. Practical management of toxicities associated with tyrosine kinase inhibitors in chronic myeloid leukemia. Clinical Lymphoma and Myeloma. 2008;8(Suppl. 3):S82–S88. doi: 10.3816/CLM.2008.s.003. doi:10.3816/CLM.2008.s.003. [DOI] [PubMed] [Google Scholar]
- Renouf DJ, Velazquez JP, Simpron, Siu LL, Bedard PL. Ocular toxicity of targeted therapies. Journal of Clinical Oncology. 2012;30:3277–3286. doi: 10.1200/JCO.2011.41.5851. [DOI] [PubMed] [Google Scholar]
- Sharma A, Hindman HB. Aging: A predisposition to dry eyes. Journal of Ophthalmology. 2014;2014:1–8. doi: 10.1155/2014/781683. doi:10.1155/2014/781683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield VC, Stone EM. Genomics and the eye. New England Journal of Medicine. 2011;364:1932–1942. doi: 10.1056/NEJMra1012354. doi:10.1056/NEJMra1012354. [DOI] [PubMed] [Google Scholar]
- Vagefi MR, Sullivan JH, Corrêa ZM,, Augsburger JJ. Lids and lacrimal apparatus. In: Riordan-Eva P, Cunningham ET Jr., editors. Vaughan and Asbury’s general ophthalmology. 18th ed. McGraw-Hill Medical; New York, NY: 2011. [Google Scholar]
- van der Noll R, Leijen S, Neuteboom GH, Beijnen JH, Schellens JH. Effect of inhibition of the FGFR-MAPK signaling pathway on the development of ocular toxicities. Cancer Treatment Reviews. 2013;39:664–672. doi: 10.1016/j.ctrv.2013.01.003. doi:10.1016/j.ctrv.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Wong TY, Scott IU. Retinal-vein occlusion. New England Journal of Medicine. 2010;363:2135–2144. doi: 10.1056/NEJMcp1003934. doi:10.1056/NEJMcP1003934. [DOI] [PubMed] [Google Scholar]