Abstract
This study examined the link between circadian rhythm changes due to bright light exposure and subthreshold bipolarity. Molecular circadian rhythms, polysomnography, and actigraphy data were studied in 25 young, healthy male subjects, divided into high and low mood disorder questionnaire (MDQ) score groups. During the first 2 days of the study, the subjects were exposed to daily-living light (150 lux) for 4 hours before bedtime. Saliva and buccal cells were collected 5 times a day for 2 consecutive days. During the subsequent 5 days, the subjects were exposed to bright light (1,000 lux), and saliva and buccal cell samples were collected in the same way. Molecular circadian rhythms were analyzed using sine regression. Circadian rhythms of cortisol (F = 16.956, p < 0.001) and relative PER1/ARNTL gene expression (F = 122.1, p < 0.001) showed a delayed acrophase in both groups after bright light exposure. The high MDQ score group showed a significant delay in acrophase compared to the low MDQ score group only in salivary cortisol (F = 8.528, p = 0.008). The high MDQ score group showed hypersensitivity in cortisol rhythm shift after bright light exposure, suggesting characteristic molecular circadian rhythm changes in the high MDQ score group may be related to biological processes downstream from core circadian clock gene expression.
Bipolar disorder (BD) is a chronic mental illness characterized by alternations in mood, activity, sleep, and energy. Patients with BD suffer from marked functional impairments and a reduced quality of life1. As a result, BD is considered to be a major social burden2. In order to ameliorate this social burden, it is important to develop methods for the accurate diagnosis of BD, to prevent its onset, to provide more appropriate therapeutic strategies for its treatment, to increase the duration of inter-episode periods, and to improve its prognosis.
Research on the association between BD and circadian rhythms is an important key to understanding BD. Disturbances in circadian rhythms have been suggested to be core features of BD and are thought to play a fundamental role in BD pathophysiology3. A number of studies conclude that disruptions4,5, phase shift6,7, or instability8 of circadian rhythms are related to BD. Moreover, genetic studies have suggested an association between circadian genes and BD9,10,11,12. The therapeutic effects of mood stabilizers such as lithium or valproate in treating BD are believed to be due to effects on the rhythmic properties of circadian genes and molecular clocks via the inhibition of glycogen synthase kinase-3β13,14.
Light plays a primary role in the regulation of circadian rhythms, and light exposure is the main environmental cue for the synchronization of circadian rhythms in humans15. Excessive exposure to light at inappropriate times or a lack of light exposure at the required time may be involved in the pathophysiology of some mood disorders. Controlled therapeutic light exposure is usually used to treat certain mood disorders15. A previous study concluded that some subjects may be more sensitive to variations in natural bright light and that an increased sensitivity to light may be related to the development of affective disorders16. Seasonal variations in manic episodes have been reported to be related to the number of hours of daily sunshine17. Therefore, sensitivity to light exposure may be a characteristic feature of mood disorders.
A number of studies have investigated a broad range of variables, such as genetic factors, phenotypic manifestations, biomarkers, and neuroimaging patterns, in normal subjects at high risk for developing BD18. There are various methods used for the selection of subjects at risk of developing BD, including familial history of BD19 or clinical features such as early onset and severity of depression, recurrent and atypical depression, subclinical mania, and psychosis20. Another method for selecting at-risk subjects is the use of clinical scales such as the childhood bipolar questionnaire21, the bipolar-at-risk criteria22, or the mood disorder questionnaire (MDQ)23.
We hypothesized that normal subjects with high MDQ scores would be more sensitive to bright light exposure before bedtime and thus show more dramatic changes in molecular circadian rhythms, sleep parameters, and behavioral rhythms compared to subjects with low MDQ scores. We thus designed and performed a controlled experimental study to investigate this potential characteristic marker of subthreshold bipolarity in normal subjects.
Results
Circadian rhythm of cortisol concentration in saliva
To investigate the impact of bright artificial light at night (ALAN) exposure before bedtime on circadian rhythm of cortisol concentration, we analyzed salivary cortisol concentrations of all 25 subjects using paired t-tests (Table 1 and Fig. 1). A significantly delayed acrophase in salivary cortisol concentration (t = −3.927, p = 0.001) was found in subjects after bright ALAN exposure. The mesor of cortisol concentration also significantly increased between nights (t = −2.494, p = 0.02). We found that bright ALAN exposure before bedtime in healthy adults significantly delayed the acrophase and increased the mesor of the circadian rhythm of cortisol concentration.
Table 1. Results of paired t-tests or Wilcoxon signed-rank tests on the circadian rhythm variables of salivary cortisol concentration and relative PER1/ARNTL gene expression levels between Night 1 and Night 2.
| Type of sample | Variables | Night 1 | Night 2 | t or Z | df | p-value(two tailed) |
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||||
| Cortisol concentration (n = 25) | Period (hr) | 24.813 ± 0.777 | 25.175 ± 0.985 | −1.523 | 24 | 0.141 |
| Amplitude (μg/dL) | 0.217 ± 0.033 | 0.205 ± 0.064 | 0.807 | 24 | 0.428 | |
| Acrophase (time) | 9.935 ± 0.964 | 11.646 ± 1.936 | −3.927 | 24 | 0.001† | |
| Mesor (μg/dL) | 0.272 ± 0.036 | 0.307 ± 0.048 | −2.494 | 24 | 0.020† | |
| Relative PER1/ARNTL gene expression (n = 25) | Period (hr)* | 24.237 ± 0.747 | 24.215 ± 0.880 | 0.040 | — | 0.968 |
| Amplitude | 28.186 ± 3.487 | 22.103 ± 4.993 | 6.555 | 24 | <0.001‡ | |
| Acrophase (time) | 15.886 ± 0.592 | 18.504 ± 0.965 | −11.146 | 24 | <0.001‡ | |
| Mesor | 25.919 ± 3.197 | 22.983 ± 4.698 | 2.638 | 24 | 0.014† |
†p-value < 0.05.
‡p-value < 0.001.
*Wilcoxon signed-rank test.
Night 1, exposure to artificial light of 150-lux intensity at night.
Night 2, exposure to artificial light of 1,000-lux intensity at night.
Amplitude and Mesor of Relative PER1/ARNTL gene expression indicate the relative PER1/ARNTL gene expression ratio.
All variables except those tested using the Wilcoxon signed-rank test were analyzed by a paired t-test.
hr, hour; time, time of the day; TST, total sleep time; min, minutes; n, number of subjects; SD, standard deviation; and df, degrees of freedom.
Figure 1. Altered molecular circadian rhythms of salivary cortisol and relative PER1/ARNTL expression levels following bright light exposure before bedtime.
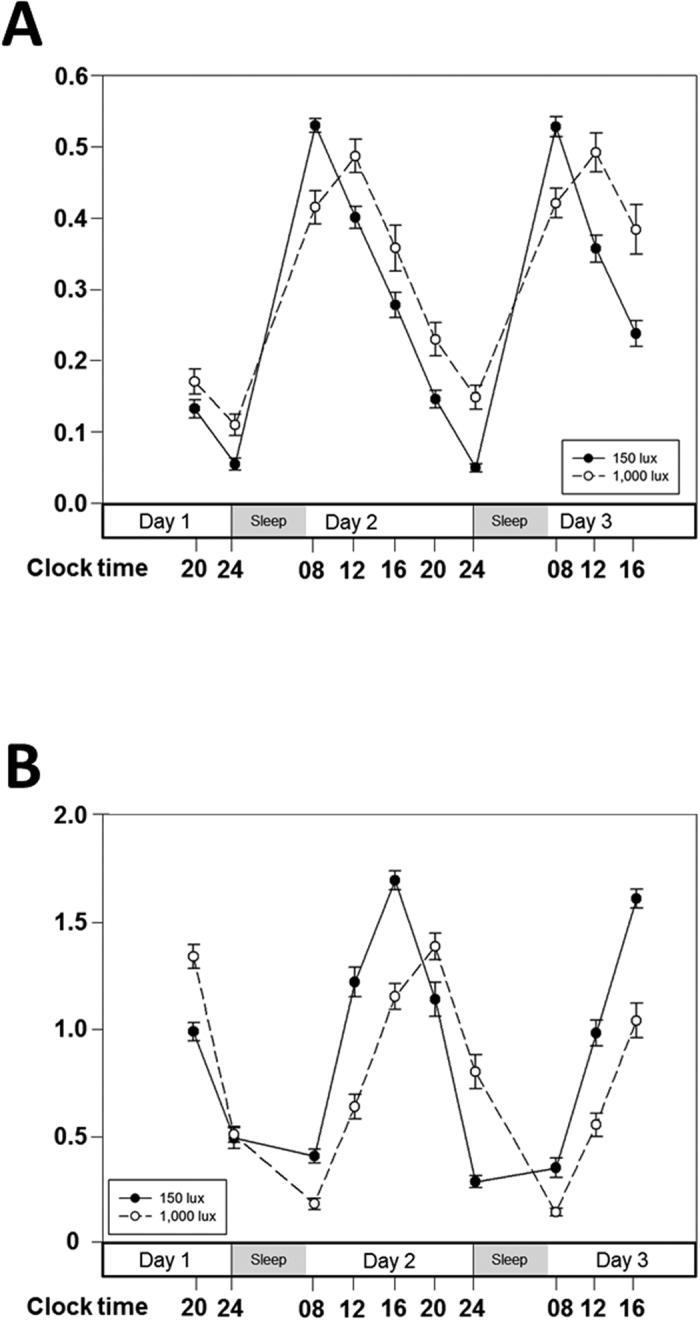
Twenty-five young, healthy male subjects were exposed to 150 lux artificial light at night (ALAN) before bedtime for 2 consecutive days. The circadian rhythms of salivary cortisol concentration (A) and relative PER1/ARNTL expression levels (B) are indicated as black circles connected with a continuous black line. After the 150 lux ALAN exposure, the same 25 subjects were exposed to 1,000 lux ALAN for 5 consecutive nights. The circadian rhythms of salivary cortisol concentration (A) and relative PER1/ARNTL expression levels (B) are indicated by white closed circles connected with a dashed black line. Data are expressed as mean ± standard error of the mean (SEM).
Next, we analyzed the changes in circadian rhythm of cortisol concentration over the course of the study between the two groups, namely the high MDQ score group and the low MDQ score group, after bright ALAN exposure using a repeated measures ANOVA (Table 2 and Fig. 2). Both groups showed a significantly delayed acrophase (F = 16.956, p < 0.001) and a significant increase in mesor (F = 5.678, p = 0.026) of salivary cortisol concentration over the course of the study. The most noteworthy finding of the present study is the night—group interaction observed in the acrophase of the salivary cortisol concentration. We observed that the acrophase in the high MDQ score group was significantly delayed compared with that in the low MDQ score group (F = 8.528, p = 0.008). Altogether, bright ALAN exposure before bedtime caused a significantly delayed acrophase in cortisol concentrations in both groups. Moreover, the high MDQ score group was more sensitive to bright ALAN exposure, showing a significant increase in acrophase delay in cortisol concentration compared with the low MDQ score group.
Table 2. Results of a repeated measures ANOVA of the circadian rhythm variables of salivary cortisol concentration and relative PER1/ARNTL gene expression levels between Nights 1 and 2 and high and low mood disorder questionnaire (MDQ) score groups.
| Type of sample | Variables | Nights | High MDQ score group (n = 14 or 13) | Low MDQ score group (n = 11 or 8) | Source | F | p |
|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ||||||
| Cortisol concentration (n = 25) | Period (hr) | Night 1 | 24.847 ± 0.838 | 24.769 ± 0.729 | Nights | 2.518 | 0.126 |
| Night 2 | 25.051 ± 0.913 | 25.333 ± 1.093 | Group | 0.142 | 0.710 | ||
| Night*Group | 0.554 | 0.464 | |||||
| Amplitude (μg/dL) | Night 1 | 0.223 ± 0.031 | 0.209 ± 0.036 | Nights | 0.809 | 0.378 | |
| Night 2 | 0.222 ± 0.064 | 0.182 ± 0.06 | Group | 4.248 | 0.051 | ||
| Night*Group | 0.033 | 0.400 | |||||
| Acrophase (time) | Night 1 | 9.866 ± 1.114 | 10.021 ± 0.775 | Nights | 16.956 | <0.001‡ | |
| Night 2 | 12.562 ± 1.668 | 10.481 ± 1.647 | Group | 5.995 | 0.022† | ||
| Night*Group | 8.528 | 0.008† | |||||
| Mesor (μg/dL) | Night 1 | 0.265 ± 0.035 | 0.283 ± 0.036 | Nights | 5.678 | 0.026† | |
| Night 2 | 0.321 ± 0.039 | 0.288 ± 0.054 | Group | 0.531 | 0.473 | ||
| Night*Group | 3.792 | 0.064 | |||||
| Relative PER1/ARNTL gene expression (n = 25) | Period (hr) | Night 1 | 24.225 ± 0.724 | 24.252 ± 0.81 | Nights | 0.01 | 0.920 |
| Night 2 | 24.226 ± 0.917 | 24.2 ± 0.875 | Group | <0.001 | 0.999 | ||
| Night*Group | 0.011 | 0.918 | |||||
| Amplitude | Night 1 | 27.383 ± 3.182 | 29.209 ± 3.737 | Nights | 44.774 | <0.001‡ | |
| Night 2 | 22.243 ± 5.848 | 21.925 ± 3.913 | Group | 0.258 | 0.617 | ||
| Night*Group | 1.335 | 0.260 | |||||
| Acrophase (time) | Night 1 | 16.118 ± 0.549 | 15.591 ± 0.529 | Nights | 122.1 | <0.001‡ | |
| Night 2 | 18.58 ± 0.906 | 18.408 ± 1.072 | Group | 2.728 | 0.112 | ||
| Night*Group | 0.549 | 0.466 | |||||
| Mesor | Night 1 | 25.799 ± 3.38 | 26.072 ± 3.105 | Nights | 7.338 | 0.013† | |
| Night 2 | 23.722 ± 5.321 | 22.044 ± 3.798 | Group | 0.352 | 0.559 | ||
| Night*Group | 0.750 | 0.396 |
†p-value < 0.05.
‡p-value < 0.001.
Night 1, exposure to artificial light of 150-lux intensity at night.
Night 2, exposure to artificial light of 1000-lux intensity at night.
Amplitude and Mesor of Relative PER1/ARNTL gene expression indicate the relative ratio of PER1/ARNTL gene expression.
hr, hour; time, time of the day; min, minutes; n, number of subjects; and SD, standard deviation.
Figure 2. Altered molecular circadian rhythms of salivary cortisol concentration and relative PER1/ARNTL expression levels following bright light exposure before bedtime in high and low mood disorder questionnaire (MDQ) score groups.
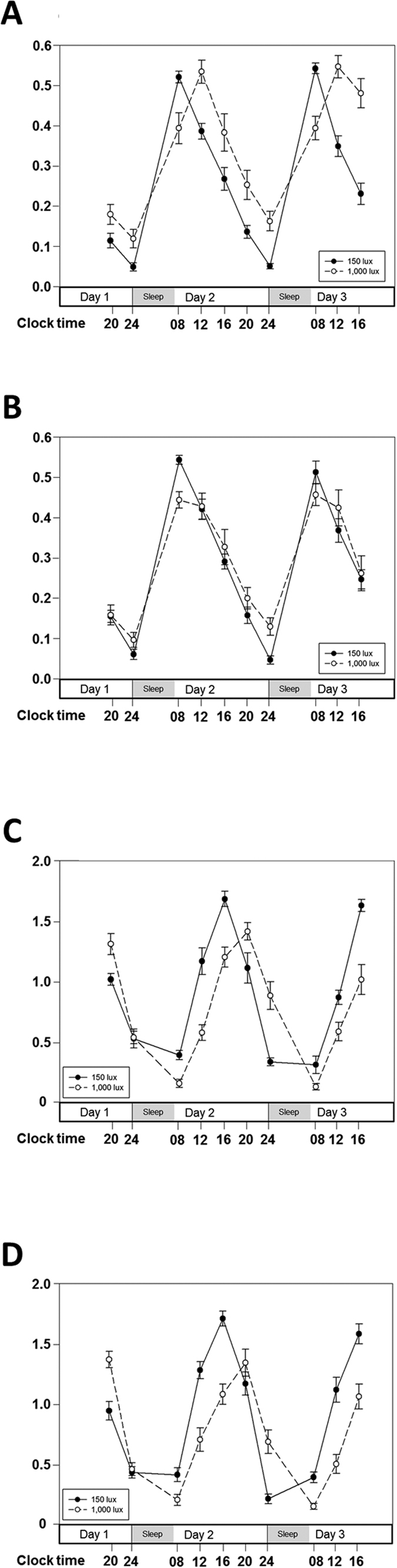
Fourteen young, healthy male subjects with subthreshold bipolarity (high MDQ score group) were exposed to 150 lux artificial light at night (ALAN) for 2 consecutive days. The circadian rhythms of salivary cortisol concentration (A) and relative PER1/ARNTL expression levels (C) are indicated by black circles connected with a continuous black line. After exposure to 150 lux ALAN, the same 14 subjects in the high MDQ score group were exposed to 1,000 lux ALAN for 5 consecutive days. The circadian rhythms of salivary cortisol concentration (A) and relative PER1/ARNTL expression levels (C) are indicated by white closed circles connected with a dashed black line. Eleven young, healthy male subjects with low risk of bipolar disorder (low MDQ score group) were exposed to 150 lux ALAN for 2 consecutive days. The circadian rhythms of salivary cortisol concentration (B) and relative PER1/ARNTL expression levels (D) are indicated by black circles connected with a continuous black line. After exposure to 150 lux ALAN, the same 11 subjects in the low MDQ score group were exposed to 1,000 lux ALAN for 5 consecutive days. The circadian rhythms of salivary cortisol concentration (B) and relative PER1/ARNTL expression levels (D) are indicated by white closed circles connected with a dashed black line. Data are expressed as mean ± standard error of the mean (SEM).
Circadian rhythm of relative PER1/ARNTL gene expression levels in buccal epithelial cells
To study the impact of bright ALAN exposure before bedtime on circadian rhythm of relative PER1/ARNTL gene expression, we used the same analysis as performed on cortisol concentration (Table 1 and Fig. 1). We found a significant delay in acrophase (t = −11.146, p < 0.001) and a significant decrease in mesor of the relative PER1/ARNTL gene expression ratio (t = 2.638, p = 0.014) in subjects after bright ALAN exposure. The amplitude of relative PER1/ARNTL gene expression levels was significantly suppressed between nights after bright ALAN exposure (t = 6.555, p < 0.001). We confirmed that bright ALAN exposure before bedtime in healthy adults significantly delayed the acrophase, decreased the mesor, and suppressed the amplitude of the circadian rhythm of the relative PER1/ARNTL gene expression ratio.
We investigated the changes in circadian rhythm of relative PER1/ARNTL gene expression ratio over the course of the study in the two groups after bright ALAN exposure using a repeated measures ANOVA (Table 2 and Fig. 2). Both groups exhibited a significantly delayed acrophase (F = 122.1, p < 0.001), a reduced amplitude (F = 44.774, p < 0.001), and a decrease in mesor (F = 7.338, p = 0.013) of relative PER1/ARNTL gene expression levels during the course of the study. However, there was no night—group interaction of the PER1/ARNTL gene expression ratio and no difference between groups.
Behavioral rhythm of actigraphy data
There was no significant change between nights in the behavioral rhythm of actigraphy data after bright ALAN exposure before bedtime in subjects as a whole (Table 3). A repeated measures ANOVA on the behavioral rhythm showed no statistically significant differences between nights of the study in both groups. However, we found significant night—group interactions for the period of the behavioral rhythm (F = 7.844, p = 0.01), as shown in Table 4. The period of the behavioral rhythm in the high MDQ score group increased, whereas that in the low MDQ score group decreased between nights.
Table 3. Results of paired t-tests or Wilcoxon signed-rank tests of the behavioral rhythm and sleep parameters between Night 1 and Night 2.
| Type of sample | Variables | Night 1 | Night 2 | t or Z | df | p-value (two tailed) |
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||||
| Behavioral rhythm (n = 25) | Period (hr) | 24.064 ± 0.617 | 23.900 ± 0.585 | 1.071 | 24 | 0.295 |
| Amplitude | 168.331 ± 53.029 | 184.816 ± 53.643 | −1.085 | 24 | 0.289 | |
| Acrophase (time) | 15.649 ± 1.714 | 15.332 ± 1.436 | 0.662 | 24 | 0.515 | |
| Mesor | 243.126 ± 71.803 | 244.961 ± 59.853 | −0.098 | 24 | 0.923 | |
| Robustness (%) | 24.008 ± 7.854 | 23.476 ± 5.277 | 0.301 | 24 | 0.766 | |
| Sleep parameters (n = 21) | TST (min)* | 384.380 ± 18.202 | 385.548 ± 16.381 | −0.818 | — | 0.413 |
| SE (%) | 97.433 ± 1.580 | 97.405 ± 1.775 | 0.099 | 20 | 0.922 | |
| WASO (min)* | 6.024 ± 5.278 | 5.74 ± 5.449 | −1.252 | — | 0.211 | |
| SL (min) | 4.000 ± 2.617 | 4.520 ± 3.120 | −0.732 | 20 | 0.473 | |
| Stage N1 (%) | 12.262 ± 4.923 | 13.395 ± 6.688 | −1.213 | 20 | 0.239 | |
| Stage N2 (%) | 45.267 ± 5.791 | 44.467 ± 5.865 | 0.535 | 20 | 0.598 | |
| Stage N3 (%) | 18.638 ± 7.386 | 17.557 ± 8.642 | 0.994 | 20 | 0.332 | |
| Stage R (%) | 23.848 ± 4.777 | 24.571 ± 3.761 | −0.818 | 20 | 0.423 | |
| Stage R latency (min)* | 71.430 ± 42.432 | 71.762 ± 40.937 | −0.709 | — | 0.478 | |
| RDI* | 4.067 ± 2.923 | 4.090 ± 3.470 | −0.348 | — | 0.728 | |
| AHI | 2.433 ± 0.382 | 2.400 ± 2.399 | 0.117 | 20 | 0.908 | |
| RERAI | 1.633 ± 1.451 | 1.720 ± 1.591 | −0.633 | 20 | 0.534 | |
| PLMI* | 2.150 ± 5.899 | 2.929 ± 8.166 | −0.691 | — | 0.490 | |
| LMI* | 5.676 ± 6.419 | 6.933 ± 9,168 | −0.869 | — | 0.385 | |
| TA (min) | 12.952 ± 4.249 | 13.410 ± 5.311 | −0.718 | 20 | 0.481 | |
| SA (min) | 8.052 ± 3.800 | 8.424 ± 4.537 | −0.688 | 20 | 0.499 | |
| Supine position (%)* | 77.790 ± 18.453 | 80.252 ± 20.704 | −0.262 | — | 0.793 |
†p-value < 0.05.
*Wilcoxon signed-rank test.
Night 1, exposure to artificial light of 150-lux intensity at night.
Night 2, exposure to artificial light of 1,000-lux intensity at night.
All variables except those tested using the Wilcoxon signed-rank test were analyzed by a paired t-test.
hr, hour; time, time of the day; TST, total sleep time; SE, sleep efficiency; WASO, wake time after sleep onset; SL, sleep latency; AHI, apnea + hypopnea index; RDI, respiratory disturbance index; RERAI, respiratory effort-related arousal index; PLMI, periodic limb movement during sleep index; LMI, limb movement index; TA, total arousal; SA, spontaneous arousal; min, minutes; n, number of subjects; SD, standard deviation; and df, degrees of freedom.
Table 4. Results of a repeated measures ANOVA of the behavioral rhythm and sleep parameters between Nights 1 and 2 and high and low mood disorder questionnaire (MDQ) score groups.
| Type of sample | Variables | Nights | High MDQ score group (n = 14 or 13) | Low MDQ score group (n = 11 or 8) | Source | F | p |
|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ||||||
| Behavioral rhythm (n = 25) | Period (hr) | Night 1 | 23.914 ± 0.728 | 24.255 ± 0.391 | Nights | 2.375 | 0.137 |
| (n = 25) | Night 2 | 24.086 ± 0.429 | 23.664 ± 0.687 | Group | 0.046 | 0.832 | |
| Night*Group | 7.844 | 0.010† | |||||
| Amplitude | Night 1 | 157.870 ± 37.402 | 181.644 ± 67.692 | Nights | 0.839 | 0.369 | |
| Night 2 | 196.098 ± 47.618 | 170.456 ± 59.610 | Group | 0.004 | 0.952 | ||
| Night*Group | 2.804 | 0.108 | |||||
| Acrophase (time) | Night 1 | 15.339 ± 1.695 | 16.045 ± 1.736 | Nights | 0.518 | 0.479 | |
| Night 2 | 15.279 ± 1.359 | 15.399 ± 1.593 | Group | 0.987 | 0.331 | ||
| Night*Group | 0.358 | 0.555 | |||||
| Mesor | Night 1 | 218.217 ± 74.838 | 274.828 ± 55.845 | Nights | 0.013 | 0.911 | |
| Night 2 | 248.568 ± 66.164 | 240.369 ± 53.528 | Group | 1.713 | 0.204 | ||
| Night*Group | 3.212 | 0.086 | |||||
| Robustness (%) | Night 1 | 24.921 ± 9.231 | 22.845 ± 5.884 | Nights | 0.047 | 0.830 | |
| Night 2 | 23.364 ± 4.939 | 23.618 ± 5.922 | Group | 0.196 | 0.662 | ||
| Night*Group | 0.417 | 0.525 | |||||
| Sleep parameters | TST (min) | Night 1 | 387.12 ± 19.777 | 379.94 ± 15.481 | Nights | 0.658 | 0.427 |
| (n = 21) | Night 2 | 387.038 ± 17.578 | 383.125 ± 15.038 | Group | 0.528 | 0.476 | |
| Night*Group | 0.725 | 0.405 | |||||
| SE (%) | Night 1 | 97.246 ± 1.624 | 97.738 ± 1.561 | Nights | 0.006 | 0.940 | |
| Night 2 | 97.200 ± 2.010 | 97.738 ± 1.369 | Group | 0.536 | 0.473 | ||
| Night*Group | 0.006 | 0.940 | |||||
| WASO (min) | Night 1 | 6.462 ± 4.850 | 5.313 ± 6.193 | Nights | 0.255 | 0.619 | |
| Night 2 | 6.500 ± 5.583 | 4.500 ± 5.345 | Group | 0.460 | 0.506 | ||
| Night*Group | 0.309 | 0.585 | |||||
| SL (min) | Night 1 | 4.308 ± 2.905 | 3.500 ± 2.155 | Nights | 0.643 | 0.432 | |
| Night 2 | 4.58 ± 2.907 | 4.44 ± 3.649 | Group | 0.190 | 0.668 | ||
| Night*Group | 0.197 | 0.662 | |||||
| Stage N1 (%) | Night 1 | 13.046 ± 5.067 | 10.988 ± 4.716 | Nights | 0.729 | 0.404 | |
| Night 2 | 15.262 ± 7.280 | 10.363 ± 4.469 | Group | 2.116 | 0.162 | ||
| Night*Group | 2.325 | 0.144 | |||||
| Stage N2 (%) | Night 1 | 45.708 ± 6.552 | 44.550 ± 4.612 | Nights | 0.579 | 0.456 | |
| Night 2 | 46.092 ± 6.024 | 41.825 ± 4.815 | Group | 1.696 | 0.208 | ||
| Night*Group | 1.022 | 0.325 | |||||
| Stage N3 (%) | Night 1 | 17.738 ± 8.495 | 20.100 ± 5.310 | Nights | 0.283 | 0.601 | |
| Night 2 | 14.946 ± 9.784 | 21.800 ± 4.009 | Group | 1.879 | 0.186 | ||
| Night*Group | 4.785 | 0.041† | |||||
| Stage R (%) | Night 1 | 23.508 ± 5.740 | 24.400 ± 2.852 | Nights | 0.946 | 0.343 | |
| Night 2 | 23.685 ± 3.926 | 26.013 ± 3.186 | Group | 0.888 | 0.358 | ||
| Night*Group | 0.609 | 0.445 | |||||
| Stage R latency (min) | Night 1 | 77.88 ± 45.513 | 60.94 ± 37.282 | Nights | 0.017 | 0.897 | |
| Night 2 | 76.346 ± 46.806 | 64.313 ± 30.495 | Group | 0.679 | 0.420 | ||
| Night*Group | 0.124 | 0.729 | |||||
| RDI | Night 1 | 4.646 ± 3.096 | 3.125 ± 2.518 | Nights | 0.011 | 0.918 | |
| Night 2 | 4.62 ± 3.655 | 3.23 ± 3.179 | Group | 1.099 | 0.308 | ||
| Night*Group | 0.028 | 0.869 | |||||
| AHI | Night 1 | 2.823 ± 1.836 | 1.800 ± 1.489 | Nights | 0.001 | 0.971 | |
| Night 2 | 2.62 ± 2.690 | 2.03 ± 1.946 | Group | 0.838 | 0.371 | ||
| Night*Group | 0.391 | 0.539 | |||||
| RERAI | Night 1 | 2.07 ± 2.53 | 1.51 ± 1.34 | Nights | 2.246 | 0.149 | |
| Night 2 | 1.63 ± 1.96 | 1.28 ± 1.17 | Group | 1.027 | 0.324 | ||
| Night*Group | 0.198 | 0.661 | |||||
| PLMI | Night 1 | 3.28 ± 7.356 | 0.33 ± 0.709 | Nights | 0.662 | 0.426 | |
| Night 2 | 4.223 ± 10.2807 | 0.825 ± 1.086 | Group | 1.065 | 0.315 | ||
| Night*Group | 0.063 | 0.805 | |||||
| LMI | Night 1 | 6.892 ± 7.894 | 3.700 ± 1.922 | Nights | 1.505 | 0.235 | |
| Night 2 | 8.169 ± 11.440 | 4.925 ± 2.895 | Group | 0.883 | 0.359 | ||
| Night*Group | 0.001 | 0.980 | |||||
| TA (min) | Night 1 | 13.638 ± 4.409 | 11.838 ± 3.996 | Nights | 0.054 | 0.820 | |
| Night 2 | 15.115 ± 5.842 | 10.638 ± 2.811 | Group | 2.498 | 0.130 | ||
| Night*Group | 5.000 | 0.038† | |||||
| SA (min) | Night 1 | 8.046 ± 3.649 | 8.063 ± 4.294 | Nights | 0.036 | 0.852 | |
| Night 2 | 9.300 ± 5.302 | 7.000 ± 2.622 | Group | 0.392 | 0.539 | ||
| Night*Group | 5.266 | 0.033 | |||||
| Supine position (%) | Night 1 | 76.823 ± 21.330 | 79.363 ± 13.726 | Nights | 0.882 | 0.359 | |
| Night 2 | 78.177 ± 21.308 | 83.625 ± 20.624 | Group | 0.222 | 0.643 | ||
| Night*Group | 0.237 | 0.632 |
†p-value < 0.05.
Night 1, exposure to artificial light of 150-lux intensity at night.
Night 2, exposure to artificial light of 1000-lux intensity at night.
The numbers of subjects in the high MDQ score group (n = 14) and the low MDQ score group (n = 11) are for the variables of behavioral rhythm. However, sleep parameters have different numbers of subjects in each group: high MDQ score group (n = 13) and low MDQ score group (n = 8).
hr, hour; time, time of the day; TST, total sleep time; SE, sleep efficiency; WASO, wake time after sleep onset; SL, sleep latency; AHI, apnea + hypopnea index; RDI, respiratory disturbance index; RERAI, respiratory effort-related arousal index; PLMI, periodic limb movement during sleep index; LMI, limb movement index; TA, total arousal; SA, spontaneous arousal; min, minutes; n, number of subjects; and SD, standard deviation.
Sleep parameters of polysomnography
We analyzed sleep parameters of polysomnography in 21 subjects using the same method as above and found no significant differences between nights in subjects as a whole (Table 3). As shown in Table 4, a repeated measures ANOVA on sleep parameters showed no statistically significant differences between nights in both groups. However, there were significant night—group interactions for sleep parameters in stage N3 (F = 4.785, p = 0.041) and TA (F = 5.0, p = 0.038). Stage N3 showed a decreasing tendency in the high MDQ score group and an increasing tendency in the low MDQ score group. Stage TA showed an increasing tendency in the high MDQ score group and a decreasing tendency in the low MDQ score group.
Discussion
We performed a controlled experimental study on young, healthy male adults divided into high and low MDQ score groups to investigate whether bright ALAN exposure affects molecular circadian rhythms, sleep parameters, and behavioral rhythms.
Both circadian gene rhythms and hormonal rhythms were shown to have a significant delay of acrophase after bright ALAN exposure when groups were considered separately and when all subjects were grouped together. Almost 99% of the populations of the U.S. and European countries experience significant ALAN. As a result, exposure to ALAN has become a part of modern everyday life24,25,26. Various studies have reported negative impacts of ALAN exposure on humans15,25,27. Bright light is a powerful factor in resetting the human circadian pacemaker independent of the timing of the sleep-wake cycle28,29. Therefore, bright light exposure may have broad effects on humans resulting from disturbance of the circadian system. Research on the effects of bright ALAN exposure on humans is limited30,31. Our study provides evidence that bright ALAN exposure just before bedtime significantly changes molecular circadian rhythms. Since circadian rhythms are closely related to a wide range of endocrine32, immune33, physical34, and mental35,36 states, it is very important to limit bright ALAN exposure before bedtime.
The most noteworthy finding of the present study is the observation of significant molecular circadian rhythm changes after bright ALAN exposure between the two different groups over the course of the study. Specifically, we observed a significantly delayed acrophase in the cortisol circadian rhythm after bright ALAN exposure in the high MDQ score group when compared to the low MDQ score group. This remarkable finding leads us to suggest that the hypersensitivity of the cortisol circadian rhythm to bright light exposure may be used as a possible biological marker for determining the risk of BD. Cortisol, an adrenal hormone essential for lipid and glucose metabolism, has been used as an important tool for studying circadian rhythms37,38,39,40 as its circadian rhythm is synchronized with light exposure41. Because cortisol and its circadian rhythms are closely related to various mental states such as stress42, major depression43,44, post-traumatic stress disorder45,46, and BD47,48, it is important to study the relationship between the circadian rhythm of cortisol and the risk of BD. Interestingly, studies on plasma melatonin levels suggest that hypersensitivity to light may be a possible marker for manic-depressive illness49,50. Numberger et al. reported a hypersensitivity to melatonin suppression by light in young, healthy people at high risk for major affective disorders51. Karen et al. reported that melatonin secretion and sensitivity to bright nocturnal light are highly heritable52. The present study on multi-dimensional variables including cortisol levels is especially meaningful, as research on cortisol has been somewhat lacking in comparison to research focused on melatonin48,53. Several studies have examined cortisol in unaffected offspring of parents with BD, revealing a putative relationship to BD risk54,55. However, these studies provide limited information because cortisol levels were checked not by its circadian rhythm but by its simple level for measurement. To further investigate the link between cortisol levels and BD risk, the present study measured and analyzed the changes in the circadian rhythm of cortisol after bright ALAN exposure.
In addition to the circadian rhythms of cortisol concentration in saliva, this study also observed circadian rhythms of gene expression in buccal mucosa. The regulation of circadian gene expression may reflect the circadian systems of peripheral cells as well as the influence of cortisol rhythms and melatonin reflecting a central pacemaker localized in the suprachiasmatic nucleus (SCN)56. Several previous studies have shown that circadian rhythms of circadian gene expression occur not only in the SCN but also in peripheral organs57,58,59,60,61. In this study, to observe circadian rhythms of gene expression, we used mRNA extracted from buccal epithelial cells. In the preliminary research of this study, we tested five circadian genes (ARNTL, PER1, PER2, PER3, and NR1D1) extracted from buccal epithelial cells of five healthy people (independent from the present main study) who had been confirmed to show regular circadian rhythms by actigraphy and salivary cortisol concentration. However, the preliminary result did not show distinct circadian rhythms of gene expressions when each gene was observed alone (Supplementary Figure S1A). It was because of several characteristics of buccal epithelial cells. First, buccal epithelial cells are necessarily collected together with saliva, which destroys RNA to protect the host from viral infection. Second, it is impossible to obtain the same number of buccal epithelial cells in each collection (performed by gently scraping the inner cheek using a cytological brush), leading to varying amounts of mRNA. Therefore, we decided to revise mRNA expression values by calculating their expression by ratio, as performed and validated in a previous study62. Among observed mRNA expression of five circadian genes, we observed more relevant circadian rhythms of ARNTL and PER1 than in the others. The circadian rhythms of ARNTL and PER1 were inverse in phase to each other (Supplementary Figures S1A and S2), in agreement with previous studies12,62,63,64,65. Accordingly, to obtain the most distinguishable circadian rhythms, we investigated the relative gene expression of ARNTL and PER1 and showed that the ratio of PER1/ARNTL in buccal epithelial cells is a reliable method for measuring circadian rhythms of peripheral circadian gene expression (Supplementary Figures S1B and S2). Circadian gene expression patterns are known to directly reflect circadian rhythms in humans66. For this reason, the PER1/ARNTL gene expression ratio is thought to show the effects of bright ALAN exposure more directly and sensitively than other measures. However, we observed that the cortisol circadian rhythm was much more delayed than the PER1/ARNTL expression rhythm in the high MDQ score group compared to the low MDQ score group. We believe that this difference between the cortisol rhythm and the circadian gene rhythm suggests that BD risk is not related to circadian genes themselves but to the regulation of the circadian rhythm process after changes in the expression of core circadian genes.
Gaspar et al. reported that human genetic differences in major signaling pathways may be related to the light-dependent suppression of melatonin in BD67. Cortisol expression, which exhibits circadian rhythms reminiscent of those of melatonin, may be differentially affected by genetic differences in signaling pathways related to light-dependent changes based on the MDQ scores of subjects. On the other hand, expression levels of circadian genes are not considered suitable markers for predicting subthreshold bipolarity, as these genes are more directly affected by and more sensitive to bright ALAN exposure. The changes in these markers may thus predominantly reflect the effects of bright ALAN exposure.
We found significant night—group interactions for stage N3 and TA sleep parameters, as well as for the period of the behavioral rhythm. The night—group interactions for stage N3 and TA suggest that subjects with high MDQ scores may be more negatively affected by bright ALAN exposure before bedtime resulting in decreased deep sleep and increased arousal during sleep. However, interpretation of the results from this study is limited by the fact that parameters of stage N3 and TA and the period of the behavioral rhythm did not show significant differences between nights in either group.
We aimed to investigate the impact of bright ALAN exposure on a number of genetic, hormonal, and behavioral measures in normal subjects with high MDQ scores. The acrophase of the cortisol circadian rhythm was the only variable found to predict subthreshold bipolarity. This finding suggests that there may be a critical step related to BD along the pathway from gene expression to hormone production. This study was limited by a relatively small number of subjects and the fact that we were not able to specifically identify the pathway that determines BD risk. However, this study provides a significant advance in the field because it describes an experimental approach to examine our hypothesis, thereby introducing a meaningful way to elucidate causality of BD onset and recurrence in addition to identifying a putative method for predicting the risk of BD in normal subjects. In the future, we hope to verify the results of the present study and to advance our understanding further by using intensive molecular techniques on a larger number of subjects.
Methods
Subjects
From September 2013 to August 2014, a total of 29 healthy male adults ranging from 20 to 30 years of age (mean ± SD: 26.00 ± 2.89) were recruited for this study. For purposes of homogeneity, we decided to use only male subjects because data from female subjects may be more strongly influenced by hormonal changes such as the menstrual cycle. Advertisements for volunteers who “sleep like a baby” and “keep regular sleep-wake cycles” were posted on the internet bulletin boards of the Korea University to recruit these subjects. All volunteers were interviewed by psychiatrists specializing in sleep (authors HJL and CHC) in order to exclude volunteers who were overweight and suspected of snoring. Through in-depth interviews by psychiatrists with all volunteers, we confirmed that subjects had no personal or familial psychiatric history. All participants completed questionnaires regarding their sleep conditions and physical and psychiatric health. In particular, we gathered information about typical sleep patterns during weekdays and weekends as well as sleep environment and hygiene, including patterns of caffeine, alcohol, cigarette, and drug use. The MDQ, a subjective self-report screening tool, was used to assess subthreshold bipolarity. A high MDQ score (usually  7) is associated with an increased probability of BD23. The MDQ has been used as an assessment tool not only for the study of BD23,68 but also for study of the risk of BD in the general population69,70 or in subjects with major depressive disorder71,72. All participants provided informed written consent prior to enrollment after a full explanation and understanding of this study. The study protocol was approved by the Institutional Review Board of Korea University Anam Hospital (AN12261-010) and was conducted in accordance with the Declaration of Helsinki.
7) is associated with an increased probability of BD23. The MDQ has been used as an assessment tool not only for the study of BD23,68 but also for study of the risk of BD in the general population69,70 or in subjects with major depressive disorder71,72. All participants provided informed written consent prior to enrollment after a full explanation and understanding of this study. The study protocol was approved by the Institutional Review Board of Korea University Anam Hospital (AN12261-010) and was conducted in accordance with the Declaration of Helsinki.
During the week prior to the main experiment, all participants were asked to wear a wrist actigraph (Actiwatch-L®, Mini Mitter) to verify that they were keeping a regular sleep-wake cycle. After reviewing the actigraphy data for each participant, we found one participant who showed a disturbed sleep-wake cycle characterized by excessive napping. Three participants dropped out of the study for personal reasons. The remaining 25 participants completed the entire experimental process. These participants were divided into two groups based on their MDQ mood scores: the high MDQ score group (MDQ score ≥ 7; mean ± SD: 9.571 ± 1.697; 14 subjects), and the low MDQ score group (MDQ score < 7; mean ± SD: 2.091 ± 2.212; 11 subjects). Student’s t-test or the Mann-Whitney test was performed to compare demographic characteristics between the high and low MDQ score groups according to a normality assumption. There were no differences in age (mean ± SD: 25.64 ± 2.53 vs. 24.91 ± 1.81, p = 0.43), weight (kg; 68.31 ± 10.43 vs. 70.10 ± 4.11, p = 0.60), or height (cm; 174.07 ± 5.55 vs. 176.45 ± 5.65, p = 0.30) as determined by Student’s t-tests between high and low MDQ score groups, respectively. Similarly, there were no differences in education between the two groups (p = 0.168) as determined by a Mann-Whitney test.
Protocol
Figure 3 shows an overview of the protocol used in this study. Beginning at one week prior to the main experiment, all participants were prohibited from napping during the daytime and permitted to sleep only at night in order to keep regular sleep-wake cycles. Sleep logs and wrist actigraph records were used to monitor adherence to these guidelines. All participants were prohibited from consuming medicine, coffee, or alcohol, because of potential effects on sleep-wake regularity. Moreover, they were instructed to limit light exposure to daily-living light after 20:00 h and were required to sleep at midnight and to wake up at 07:00 h. All participants maintained their normal daily living and slept in their own beds at home.
Figure 3. Protocol design.
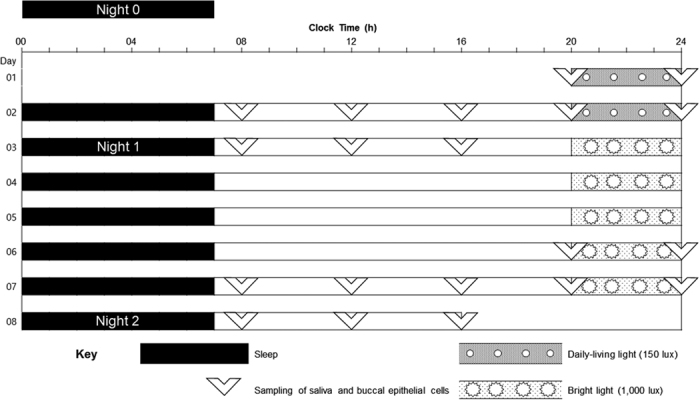
Sleep was scheduled from 00:00 to 07:00 during the 8 days of the experiment. On study days 1 and 2, the participants were exposed to 150 lux daily-living artificial light at night (ALAN) before bedtime. On study days 3, 4, 5, 6, and 7, the participants were exposed to 1,000 lux bright ALAN before bedtime. All daily-living and bright ALAN exposures were scheduled from 20:00 to 24:00. Samplings of salivary and buccal epithelial cells were scheduled with 4-hour intervals starting at 20:00 on study day 1 to 16:00 on study day 3 and from 20:00 on study day 6 to 16:00 on study day 8. No samples were collected during sleep times. Nocturnal polysomnography (NPSG) was conducted at three time points (Night 0, Night 1, and Night 2). Night 0 was in fact 3 nights before the main experimental study, during the regular sleep-wake cycle and daily-living ALAN exposure before sleep in order to reduce interference from the first-night effect. Nights 1 and 2 were the last nights of the 150 lux and the 1,000 lux ALAN exposure, respectively.
The main experimental study was carried out for 8 consecutive days and 7 nights. The study was divided into two phases based on differences in the intensity of ALAN: 2 nights at 150 lux and 5 nights at 1,000 lux. We designed and installed a controllable light box system in the experimental room to create the differences in light exposure. The system was installed by an illumination expert affiliated with the Korea Institute of Lighting Technology. The light box was installed in the ceiling of the experimental room so that the light illuminated the whole room. The experimental room was 30 m2 in size and had seven light-emitting diode (LED) lights, each with a size of 120 cm × 30 m, evenly distributed along the ceiling. The light illuminated the entire room and the light intensity was controlled using a dimmer. Only the staff were permitted to use the dimmer and control the intensity of lighting; all participants were prohibited from using the dimmer.
The subjects maintained their usual daily routine activities outside the unit during the daytime while wearing wrist actigraphs for monitoring their daily routine. Subjects were exposed to daily-living light (150 lux) for 4 hours (from 20:00 h to 24:00 h) before bedtime during the first 2 consecutive nights of the study. They were then exposed to bright light (1,000 lux) for 4 hours (from 20:00 h to 24:00 h) before bedtime during the next 5 consecutive nights of the study. The light intensity in the experimental room was checked on a nightly basis by an illuminometer (ANA-F11, Tokyo Photo, Japan) placed at horizontal eye level when sitting upright in a chair. Subjects read books or studied independently but were prohibited from watching television or using smartphones or tablets in the experimental room. All participants went to bed at the clinical trial center in the Korea University Anam Hospital every night of the study period, except during the nocturnal polysomnography (NPSG) session, which was performed in the sleep lab of the Sleep-Wake Disorders Center in the Korea University Anam Hospital. They were required to sleep at midnight and to wake up at 07:00 h during the study period in order to keep a consistent time-in-bed.
Saliva and buccal epithelial cells were collected from the subjects in order to analyze the circadian rhythms of cortisol and circadian gene expression. Sample collection was performed at 08:00 h, 12:00 h, 16:00 h, 20:00 h, and 24:00 h for the last 2 consecutive days of the experiment and was carried out by trained staff in the monitoring unit of the Sleep-Wake Disorders Center. The subjects were instructed to always be in the monitoring unit on time at every sampling point, so subjects maintained their daily routines during the 2 consecutive sampling days in the monitoring room. The subjects provided saliva samples directly into Salivettes (Sarstedt AG & Co., Nümbrecht, Germany) at each of the specified time points. The Salivette device was used according to the manufacturer’s instructions and stored at −20 °C until the time of the assay. Immediately after saliva sample collection, buccal epithelial cell samples were collected by gently scraping both inner cheeks using a cytological brush. The buccal epithelial cell samples were immediately placed into RNAlater reagent (Sigma-Aldrich, St. Louis, MO, USA) and were then maintained at −20 °C until the time of analysis. The saliva samples were used to for the cortisol assay, and each participant’s cortisol circadian rhythm was determined. cDNA was synthesized from total RNA of collected buccal epithelial cells, and circadian gene expression rhythms were determined using the PER1/ARNTL expression ratio.
NPSG was conducted on 3 different nights during the study period. Three nights before the main experiment, each participant underwent an initial NPSG session (Night 0) while maintaining a regular sleep-wake cycle and normal ALAN exposure before bedtime to reduce interference from the first-night effect. The second and third NPSG sessions (Nights 1 and 2) were conducted on the last night of each ALAN schedule.
Measurements
Measurement of cortisol concentration in saliva
The Coat-A-Count Cortisol assay (Siemens Healthcare Diagnostics, Inc., Los Angeles, USA), a direct double-antibody radioimmunoassay, was used for the determination of salivary cortisol levels. The kit was used according to the manufacturer’s instructions. The analytical sensitivity of the assay was 0.01 μg/dL and the intra-assay coefficient of variation was 3% for samples with a mean concentration of 0.19 ± 0.10 μg/dL and 4% for samples with a mean concentration of 0.24 ± 0.02 μg/dL. The inter-assay coefficient of variation was 12% for samples with a mean concentration of 1.85 ± 0.10 μg/dL and 14% for samples with a mean concentration of 0.24 ± 0.02 μg/dL.
Measurement of circadian gene expression level in buccal epithelial cells
The expression levels of circadian rhythm-related genes PER1 and ARNTL were determined by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and the PER1/ARNTL ratio at each sampling time point was used to measure circadian rhythm. Total RNA was isolated from buccal epithelial cells using the RNeasy Micro Kit (Qiagen Inc., Valencia, CA, USA). The final elution of RNA was performed using 20 μL of RNase-free water. To synthesize cDNA, the whole RNA sample was reverse-transcribed in 40 μL reactions using the Sensiscript Reverse Transcription Kit (Qiagen Inc., Valencia, CA, USA) according to the manufacturer’s protocol. Two-microliter aliquots of cDNA were amplified using Taqman PCR reactions in an Applied Biosystems StepOnePlus Real-Time PCR System (ThermoFisher Scientific, Foster City, CA, USA). The primers and Taqman probes used in this experiment are as follows; PER1 (NM_002616): forward 5′-CTCACACAGCTCCTCCTCAG-3′, reverse 5′-TTTGTGCTCTTGCTGCTCTC-3′, probe 5′FAM-CGGCAAGGACTCAGCCCTGC-3′BHQ1; ARNTL (NM_001030272): forward 5′-TGCCTCGTCGCAATTGG-3′, reverse 5′-ACCCTGATTTCCCCGTTCA-3′, probe 5′FAM-CGACTGCATTCTCATGTAGTTCCACAACCA-3′BHQ1.
Measurement of actigraphy data
We measured activity, sleep, and light exposure for each participant using the wrist actigraph and the Philips Respironics Actiware software Version 6.0.4 (Philips Respironics, Bend, OR, USA). Participants continuously wore the wrist actigraph on the non-dominant hand and only removed it during times when it might have got wet or damaged. All participants also completed a standardized diary to provide daily records of bedtime, wake-up time, naps, daytime activities, and any periods during which the wrist actigraph was removed. Actigraphy data collected from the wrist actigraph were compared with the self-reported standardized diaries to verify their accuracy.
Measurement of sleep parameters
Sleep parameters from NPSG were scored manually according to the standard criteria of the American Academy of Sleep Medicine manual for the scoring of sleep and associated events73. Sleep parameters such as total sleep time (TST); sleep efficiency (SE); wake after sleep onset (WASO); sleep latency (SL); stages N1, N2, N3, and R, stage R latency; REM density; apnea hypopnea index (AHI); respiratory effort-related arousal index (RERA index); respiratory disturbance index (RDI; AHI + RERA index); percentage of supine position (Supine); periodic limb movement during sleep index (PLMS index); limb movement index (LM index); total arousal time (TA); and spontaneous arousal time (SA) were extracted from the scored results of each NPSG session. All sleep stages (N1, N2, N3, and R) were converted to percentages before analysis to adjust for the difference in TST of each subject on each night. We encountered technical problems, which led to incomplete data collection during the NPSG session for one case in the high MDQ score group and for three cases in the low MDQ score group. In total, NPSG data from 21 subjects (13 subjects in the high MDQ score group and 8 subjects in the low MDQ score group) were analyzed.
Data Analysis
Salivary cortisol concentrations and relative PER1/ARNTL gene expression levels at 8:00 h, 12:00 h, 16:00 h, 20:00 h, and 24:00 h from the last two consecutive days of each of the two different ALAN schedules were fitted with sine curves for sine regression analysis. Variables such as period, amplitude, acrophase, and mesor for each of the molecular circadian rhythms were then extracted for analysis. Sine regression analysis was performed using SigmaPlot software Version 10.0 (Systat Software, Inc., San Jose, CA, USA). The period, robustness, mesor, amplitude, and acrophase variables for behavioral rhythm were calculated from the physical activity data and analyzed using Cosinor software (Circadian Rhythm Laboratory of Boise State University, Boise, ID, USA).
In order to determine the impact of bright ALAN exposure before bedtime on all of the subjects, the circadian rhythm variables of salivary cortisol concentration, relative PER1/ARNTL gene expression levels, and behavioral rhythm and sleep parameters for each night were analyzed using paired t-tests or Wilcoxon signed-rank tests. The appropriate statistical test was chosen based on the results of normality tests performed for each of the variables using both the Kolmogorov-Smirnov and the Shapiro-Wilk methods.
To find differential effects of bright ALAN exposure before bedtime on the different groups on different nights, a repeated measures analysis of variance (ANOVA) was performed for each variable.
Additional Information
How to cite this article: Cho, C.-H. et al. Molecular circadian rhythm shift due to bright light exposure before bedtime is related to subthreshold bipolarity. Sci. Rep. 6, 31846; doi: 10.1038/srep31846 (2016).
Supplementary Material
Acknowledgments
This study was supported by the Future Environmental R&D grant funded by the Korea Environmental Industry and Technology Institute (No. RE201206020) and by the Korea Health 21 R&D Project funded by the Ministry of Health & Welfare, Republic of Korea (HI14C3212).
Footnotes
Author Contributions C.-H.C., J.-H.M., H.-K.Y., S.-G.K., D.G., G.-H.S., L.K., E.-I.L. and H.-J.L. designed the experiment; C.-H.C., J.-H.M., J.-M.L. and H.-J.L. performed the experiment; C.-H.C., J.-H.M. and H.-J.L. analyzed the data; C.-H.C., J.-H.M. and H.-J.L. wrote the manuscript.
References
- Miller S., Dell’Osso B. & Ketter T. A. The prevalence and burden of bipolar depression. J Affect Disord 169 Suppl 1, S3–11, doi: 10.1016/s0165-0327(14)70003-5 (2014). [DOI] [PubMed] [Google Scholar]
- Abreu T. & Braganca M. The bipolarity of light and dark: A review on Bipolar Disorder and circadian cycles. J Affect Disord 185, 219–229, doi: 10.1016/j.jad.2015.07.017 (2015). [DOI] [PubMed] [Google Scholar]
- Gonzalez R. The relationship between bipolar disorder and biological rhythms. J Clin Psychiatry 75, e323–e331, doi: 10.4088/JCP.13r08507 (2014). [DOI] [PubMed] [Google Scholar]
- Wehr T. A. et al. Sleep and circadian rhythms in affective patients isolated from external time cues. Psychiatry Res 15, 327–339 (1985). [DOI] [PubMed] [Google Scholar]
- Kripke D. F., Mullaney D. J., Atkinson M. & Wolf S. Circadian rhythm disorders in manic-depressives. Biol Psychiatry 13, 335–351 (1978). [PubMed] [Google Scholar]
- Wood J. et al. Replicable differences in preferred circadian phase between bipolar disorder patients and control individuals. Psychiatry Res 166, 201–209, doi: 10.1016/j.psychres.2008.03.003 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr T. A., Muscettola G. & Goodwin F. K. Urinary 3-methoxy-4-hydroxyphenylglycol circadian rhythm. Early timing (phase-advance) in manic-depressives compared with normal subjects. Arch Gen Psychiatry 37, 257–263 (1980). [DOI] [PubMed] [Google Scholar]
- Jones S. H., Hare D. J. & Evershed K. Actigraphic assessment of circadian activity and sleep patterns in bipolar disorder. Bipolar Disord 7, 176–186, doi: 10.1111/j.1399-5618.2005.00187.x (2005). [DOI] [PubMed] [Google Scholar]
- Lee H. J., Son G. H. & Geum D. Circadian rhythm hypotheses of mixed features, antidepressant treatment resistance, and manic switching in bipolar disorder. Psychiatry investigation 10, 225–232, doi: 10.4306/pi.2013.10.3.225 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciukiewicz M. et al. Analysis of genetic association and epistasis interactions between circadian clock genes and symptom dimensions of bipolar affective disorder. Chronobiol Int 31, 770–778, doi: 10.3109/07420528.2014.899244 (2014). [DOI] [PubMed] [Google Scholar]
- Shi J. et al. Clock genes may influence bipolar disorder susceptibility and dysfunctional circadian rhythm. Am J Med Genet B Neuropsychiatr Genet 147B, 1047–1055, doi: 10.1002/ajmg.b.30714 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. et al. Impact of circadian nuclear receptor REV-ERBalpha on midbrain dopamine production and mood regulation. Cell 157, 858–868, doi: 10.1016/j.cell.2014.03.039 (2014). [DOI] [PubMed] [Google Scholar]
- Padiath Q. S., Paranjpe D., Jain S. & Sharma V. K. Glycogen synthase kinase 3beta as a likely target for the action of lithium on circadian clocks. Chronobiol Int 21, 43–55 (2004). [DOI] [PubMed] [Google Scholar]
- Li X., Bijur G. N. & Jope R. S. Glycogen synthase kinase-3beta, mood stabilizers, and neuroprotection. Bipolar Disord 4, 137–144 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont M. & Beaulieu C. Light exposure in the natural environment: relevance to mood and sleep disorders. Sleep Med 8, 557–565, doi: 10.1016/j.sleep.2006.11.008 (2007). [DOI] [PubMed] [Google Scholar]
- Guillemette J., Hebert M., Paquet J. & Dumont M. Natural bright light exposure in the summer and winter in subjects with and without complaints of seasonal mood variations. Biol Psychiatry 44, 622–628 (1998). [DOI] [PubMed] [Google Scholar]
- Lee H. J., Kim L., Joe S. H. & Suh K. Y. Effects of season and climate on the first manic episode of bipolar affective disorder in Korea. Psychiatry Res 113, 151–159 (2002). [DOI] [PubMed] [Google Scholar]
- Maletic V. & Raison C. Integrated neurobiology of bipolar disorder. Front Psychiatry 5, 98, doi: 10.3389/fpsyt.2014.00098 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgi B. et al. Genomic view of bipolar disorder revealed by whole genome sequencing in a genetic isolate. PLoS genetics 10, e1004229, doi: 10.1371/journal.pgen.1004229 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiste A. et al. Bipolar polygenic loading and bipolar spectrum features in major depressive disorder. Bipolar Disord 16, 608–616, doi: 10.1111/bdi.12201 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papolos D., Mattis S., Golshan S. & Molay F. Fear of harm, a possible phenotype of pediatric bipolar disorder: a dimensional approach to diagnosis for genotyping psychiatric syndromes. J Affect Disord 118, 28–38, doi: 10.1016/j.jad.2009.06.016 (2009). [DOI] [PubMed] [Google Scholar]
- Bechdolf A. et al. The predictive validity of bipolar at-risk (prodromal) criteria in help-seeking adolescents and young adults: a prospective study. Bipolar Disord 16, 493–504, doi: 10.1111/bdi.12205 (2014). [DOI] [PubMed] [Google Scholar]
- Hirschfeld R. M. et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry 157, 1873–1875, doi: 10.1176/appi.ajp.157.11.1873 (2000). [DOI] [PubMed] [Google Scholar]
- Fonken L. K. & Nelson R. J. Illuminating the deleterious effects of light at night. F1000 medicine reports 3, 18, doi: 10.3410/m3-18 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navara K. J. & Nelson R. J. The dark side of light at night: physiological, epidemiological, and ecological consequences. J Pineal Res 43, 215–224, doi: 10.1111/j.1600-079X.2007.00473.x (2007). [DOI] [PubMed] [Google Scholar]
- Kyba C. C. et al. Citizen science provides valuable data for monitoring global night sky luminance. Scientific reports 3, 1835, doi: 10.1038/srep01835 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho C. H. et al. Exposure to dim artificial light at night increases REM sleep and awakenings in humans. Chronobiol Int 1–7, doi: 10.3109/07420528.2015.1108980 (2015). [DOI] [PubMed] [Google Scholar]
- Czeisler C. et al. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science 244, 1328–1333, doi: 10.1126/science.2734611 (1989). [DOI] [PubMed] [Google Scholar]
- Czeisler C. et al. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science 233, 667–671, doi: 10.1126/science.3726555 (1986). [DOI] [PubMed] [Google Scholar]
- Badia P., Myers B., Boecker M., Culpepper J. & Harsh J. R. Bright light effects on body temperature, alertness, EEG and behavior. Physiology & Behavior 50, 583–588, doi: 10.1016/0031-9384(91)90549-4 (1991). [DOI] [PubMed] [Google Scholar]
- Rüger M., Gordijn M. C. M., Beersma D. G. M., de Vries B. & Daan S. Time-of-day-dependent effects of bright light exposure on human psychophysiology: comparison of daytime and nighttime exposure. American Journal of Physiology–Regulatory, Integrative and Comparative Physiology 290, R1413–R1420, doi: 10.1152/ajpregu.00121.2005 (2006). [DOI] [PubMed] [Google Scholar]
- Czeisler C. A. & Klerman E. B. Circadian and sleep-dependent regulation of hormone release in humans. Recent progress in hormone research 54, 97–130; discussion 130–132 (1999). [PubMed] [Google Scholar]
- Born J., Lange T., Hansen K., Mölle M. & Fehm H.-L. Effects of sleep and circadian rhythm on human circulating immune cells. The Journal of Immunology 158, 4454–4464 (1997). [PubMed] [Google Scholar]
- Stevens R. G. Light-at-night, circadian disruption and breast cancer: assessment of existing evidence. International journal of epidemiology 38, 963–970, doi: 10.1093/ije/dyp178 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrosian T. A. & Nelson R. J. Influence of the modern light environment on mood. Molecular psychiatry 18, 751–757, doi: 10.1038/mp.2013.70 (2013). [DOI] [PubMed] [Google Scholar]
- Karatsoreos I. N. Links between Circadian Rhythms and Psychiatric Disease. Front Behav Neurosci 8, 162, doi: 10.3389/fnbeh.2014.00162 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcheva B. et al. Circadian clocks and metabolism. Handbook of experimental pharmacology, 127–155, doi: 10.1007/978-3-642-25950-0_6 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groschl M., Rauh M. & Dorr H. G. Circadian rhythm of salivary cortisol, 17alpha-hydroxyprogesterone, and progesterone in healthy children. Clinical chemistry 49, 1688–1691 (2003). [DOI] [PubMed] [Google Scholar]
- Bailey S. L. & Heitkemper M. M. Circadian rhythmicity of cortisol and body temperature: morningness-eveningness effects. Chronobiol Int 18, 249–261 (2001). [DOI] [PubMed] [Google Scholar]
- Shinkai S., Watanabe S., Kurokawa Y. & Torii J. Salivary cortisol for monitoring circadian rhythm variation in adrenal activity during shiftwork. International archives of occupational and environmental health 64, 499–502 (1993). [DOI] [PubMed] [Google Scholar]
- Orth D. N. & Island D. P. Light synchronization of the circadian rhythm in plasma cortisol (17-OHCS) concentration in man. J Clin Endocrinol Metab 29, 479–486, doi: 10.1210/jcem-29-4-479 (1969). [DOI] [PubMed] [Google Scholar]
- Ockenfels M. C. et al. Effect of chronic stress associated with unemployment on salivary cortisol: overall cortisol levels, diurnal rhythm, and acute stress reactivity. Psychosomatic medicine 57, 460–467 (1995). [DOI] [PubMed] [Google Scholar]
- Souetre E. et al. Effect of recovery on the cortisol circadian rhythm of depressed patients. Biol Psychiatry 24, 336–340 (1988). [DOI] [PubMed] [Google Scholar]
- Voderholzer U. et al. Impact of sleep deprivation and subsequent recovery sleep on cortisol in unmedicated depressed patients. Am J Psychiatry 161, 1404–1410, doi: 10.1176/appi.ajp.161.8.1404 (2004). [DOI] [PubMed] [Google Scholar]
- Yehuda R., Teicher M. H., Trestman R. L., Levengood R. A. & Siever L. J. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol Psychiatry 40, 79–88, doi: 10.1016/0006-3223(95)00451-3 (1996). [DOI] [PubMed] [Google Scholar]
- Yehuda R. et al. Low urinary cortisol excretion in patients with posttraumatic stress disorder. The Journal of nervous and mental disease 178, 366–369 (1990). [DOI] [PubMed] [Google Scholar]
- Cervantes P., Gelber S., Kin F. N., Nair V. N. & Schwartz G. Circadian secretion of cortisol in bipolar disorder. J Psychiatry Neurosci 26, 411–416 (2001). [PMC free article] [PubMed] [Google Scholar]
- Linkowski P. et al. The 24-hour profiles of cortisol, prolactin, and growth hormone secretion in mania. Arch Gen Psychiatry 51, 616–624 (1994). [DOI] [PubMed] [Google Scholar]
- Lewy A. J. et al. Supersensitivity to light: possible trait marker for manic-depressive illness. Am J Psychiatry 142, 725–727 (1985). [DOI] [PubMed] [Google Scholar]
- Nathan P. J., Burrows G. D. & Norman T. R. Melatonin sensitivity to dim white light in affective disorders. Neuropsychopharmacology 21, 408–413, doi: 10.1016/s0893-133x(99)00018-4 (1999). [DOI] [PubMed] [Google Scholar]
- Nurnberger J. I. Jr. et al. Supersensitivity to melatonin suppression by light in young people at high risk for affective disorder. A preliminary report. Neuropsychopharmacology 1, 217–223 (1988). [DOI] [PubMed] [Google Scholar]
- Hallam K. T. et al. The heritability of melatonin secretion and sensitivity to bright nocturnal light in twins. Psychoneuroendocrinology 31, 867–875, doi: 10.1016/j.psyneuen.2006.04.004 (2006). [DOI] [PubMed] [Google Scholar]
- Joyce P. R., Sellman J. D., Donald R. A., Livesey J. H. & Elder P. A. The unipolar-bipolar depressive dichotomy and the relationship between afternoon prolactin and cortisol levels. J Affect Disord 14, 189–193 (1988). [DOI] [PubMed] [Google Scholar]
- Ellenbogen M. A., Hodgins S., Walker C. D., Couture S. & Adam S. Daytime cortisol and stress reactivity in the offspring of parents with bipolar disorder. Psychoneuroendocrinology 31, 1164–1180, doi: 10.1016/j.psyneuen.2006.08.004 (2006). [DOI] [PubMed] [Google Scholar]
- Ellenbogen M. A., Santo J. B., Linnen A. M., Walker C. D. & Hodgins S. High cortisol levels in the offspring of parents with bipolar disorder during two weeks of daily sampling. Bipolar Disord 12, 77–86, doi: 10.1111/j.1399-5618.2009.00770.x (2010). [DOI] [PubMed] [Google Scholar]
- Hastings M. H., Reddy A. B. & Maywood E. S. A clockwork web: circadian timing in brain and periphery, in health and disease. Nature reviews. Neuroscience 4, 649–661, doi: 10.1038/nrn1177 (2003). [DOI] [PubMed] [Google Scholar]
- Oishi K., Sakamoto K., Okada T., Nagase T. & Ishida N. Antiphase circadian expression between BMAL1 and period homologue mRNA in the suprachiasmatic nucleus and peripheral tissues of rats. Biochemical and biophysical research communications 253, 199–203, doi: 10.1006/bbrc.1998.9779 (1998). [DOI] [PubMed] [Google Scholar]
- Zylka M. J., Shearman L. P., Weaver D. R. & Reppert S. M. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron 20, 1103–1110 (1998). [DOI] [PubMed] [Google Scholar]
- Bittman E. L., Doherty L., Huang L. & Paroskie A. Period gene expression in mouse endocrine tissues. American journal of physiology. Regulatory, integrative and comparative physiology 285, R561–R569, doi: 10.1152/ajpregu.00783.2002 (2003). [DOI] [PubMed] [Google Scholar]
- Carr A. J. et al. Photoperiod differentially regulates circadian oscillators in central and peripheral tissues of the Syrian hamster. Current biology: CB 13, 1543–1548 (2003). [DOI] [PubMed] [Google Scholar]
- Tong Y. et al. Expression of haPer1 and haBmal1 in Syrian hamsters: heterogeneity of transcripts and oscillations in the periphery. J Biol Rhythms 19, 113–125, doi: 10.1177/0748730403262871 (2004). [DOI] [PubMed] [Google Scholar]
- Guo H., Brewer J. M., Lehman M. N. & Bittman E. L. Suprachiasmatic regulation of circadian rhythms of gene expression in hamster peripheral organs: effects of transplanting the pacemaker. The Journal of neuroscience: the official journal of the Society for Neuroscience 26, 6406–6412, doi: 10.1523/jneurosci.4676-05.2006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son G. H. et al. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proceedings of the National Academy of Sciences of the United States of America 105, 20970–20975, doi: 10.1073/pnas.0806962106 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakova M., Prasko J., Latalova K., Sladek M. & Sumova A. The circadian system of patients with bipolar disorder differs in episodes of mania and depression. Bipolar Disord 17, 303–314, doi: 10.1111/bdi.12270 (2015). [DOI] [PubMed] [Google Scholar]
- Akashi M. et al. Noninvasive method for assessing the human circadian clock using hair follicle cells. Proceedings of the National Academy of Sciences of the United States of America 107, 15643–15648, doi: 10.1073/pnas.1003878107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran S., Almon R. R., DuBois D. C. & Jusko W. J. Circadian rhythms in gene expression: Relationship to physiology, disease, drug disposition and drug action. Advanced drug delivery reviews 62, 904–917, doi: 10.1016/j.addr.2010.05.009 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar L. et al. Human cellular differences in cAMP–CREB signaling correlate with light-dependent melatonin suppression and bipolar disorder. The European journal of neuroscience 40, 2206–2215, doi: 10.1111/ejn.12602 (2014). [DOI] [PubMed] [Google Scholar]
- Boschloo L. et al. The Mood Disorder Questionnaire (MDQ) for detecting (hypo)manic episodes: its validity and impact of recall bias. J Affect Disord 151, 203–208, doi: 10.1016/j.jad.2013.05.078 (2013). [DOI] [PubMed] [Google Scholar]
- Hirschfeld R. M. et al. Validity of the mood disorder questionnaire: a general population study. Am J Psychiatry 160, 178–180, doi: 10.1176/appi.ajp.160.1.178 (2003). [DOI] [PubMed] [Google Scholar]
- Carta M. G. et al. Does Mood Disorder Questionnaire identify sub-threshold bipolarity? Evidence studying worsening of quality of life. J Affect Disord 183, 173–178, doi: 10.1016/j.jad.2015.04.058 (2015). [DOI] [PubMed] [Google Scholar]
- Rybakowski J. K. et al. Use of the Hypomania Checklist-32 and the Mood Disorder Questionnaire for detecting bipolarity in 1051 patients with major depressive disorder. Eur Psychiatry 27, 577–581, doi: 10.1016/j.eurpsy.2010.12.001 (2012). [DOI] [PubMed] [Google Scholar]
- Kim B., Wang H. R., Son J. I., Kim C. Y. & Joo Y. H. Bipolarity in depressive patients without histories of diagnosis of bipolar disorder and the use of the Mood Disorder Questionnaire for detecting bipolarity. Compr Psychiatry 49, 469–475, doi: 10.1016/j.comppsych.2008.01.002 (2008). [DOI] [PubMed] [Google Scholar]
- RB B. et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.0., (American Academy of Sleep Medicine, 2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


