Abstract
Objectives
The objectives of this study were to elucidate the genetic context of a novel plasmid-mediated fosA variant, fosA6, conferring fosfomycin resistance and to characterize the kinetic properties of FosA6.
Methods
The genome of fosfomycin-resistant Escherichia coli strain YD786 was sequenced. Homologues of FosA6 were identified through BLAST searches. FosA6 and FosAST258 were purified and characterized using a steady-state kinetic approach. Inhibition of FosA activity was examined with sodium phosphonoformate.
Results
Plasmid-encoded glutathione-S-transferase (GST) FosA6 conferring high-level fosfomycin resistance was identified in a CTX-M-2-producing E. coli clinical strain at a US hospital. fosA6 was carried on a self-conjugative, 69 kb IncFII plasmid. The ΔlysR-fosA6-ΔyjiR_1 fragment, located between IS10R and ΔIS26, was nearly identical to those on the chromosomes of some Klebsiella pneumoniae strains (MGH78578, PMK1 and KPPR1). FosA6 shared >99% identity with chromosomally encoded FosAPMK1 in K. pneumoniae of various STs and 98% identity with FosAST258, which is commonly found in K. pneumoniae clonal complex (CC) 258 including ST258. FosA6 and FosAST258 demonstrated robust GST activities that were comparable to each other. Sodium phosphonoformate, a GST inhibitor, reduced the fosfomycin MICs by 6- to 24-fold for K. pneumoniae and E. coli strains carrying fosA genes on the chromosomes and plasmids, respectively.
Conclusions
fosA6, probably captured from the chromosome of K. pneumoniae, conferred high-level fosfomycin resistance in E. coli. FosA6 functioned as a GST and inactivated fosfomycin efficiently. K. pneumoniae may serve as a reservoir of fosfomycin resistance for E. coli.
Introduction
Escherichia coli accounts for the majority of urinary tract infections. Recent surveillance studies indicate very low rates of fosfomycin resistance in this species.1,2 As such, fosfomycin was included as one of the first-line treatment options for uncomplicated urinary tract infections in the most recent treatment guidelines published by the IDSA and ESCMID.3 Fosfomycin belongs to an antimicrobial class of its own and functions by inactivating the cytosolic N-acetylglucosamine enolpyruvyl transferase (MurA), which prevents the formation of N-acetylmuramic acid from N-acetylglucosamine and phosphoenolpyruvate, the initial step in peptidoglycan chain formation of the bacterial wall.4 However, E. coli can acquire resistance to fosfomycin through several mechanisms, including impaired transport, target modification or overexpression, and inactivation of fosfomycin itself.5 Fosfomycin-modifying enzymes can confer fosfomycin resistance by breaking its epoxide ring and inactivating the agent.6 Of the three major classes of fosfomycin resistance enzymes (FosA, FosB and FosX), FosA is the group of enzymes most frequently reported among Gram-negative pathogens including E. coli.7–11 FosA enzymes can catalyse the nucleophilic addition of glutathione to carbon-1 of fosfomycin.6 An increasing number of studies report identification of ESBL-producing E. coli isolates that are resistant to fosfomycin due to plasmid-mediated production of FosA3 from both animal and human sources in East Asian countries.7–11 We recently reported a case of FosA3-producing E. coli identified in a hospital in Pennsylvania.12 In addition, plasmid-mediated production of FosA5, also termed FosKP96, has been reported in E. coli and Klebsiella pneumoniae from China and Hong Kong.11,13,14 Here, we report the identification of a novel plasmid-mediated FosA variant, FosA6, in an ESBL-producing E. coli strain and characterize its kinetic properties as well as genetic context.
Materials and methods
Strains
Fosfomycin-resistant E. coli strain YD786 was identified from the urine of a female inpatient who had recurrent urinary tract infections, but did not have a documented history of prior fosfomycin therapy. K. pneumoniae clinical strains NDM01,15 CRKpE6 and CRKpC1, available in our research laboratory, were used as strains producing FosAPMK1, FosAST37 and FosAST258, respectively. FosAPMK1, FosAST37 and FosAST258 are some of the most commonly observed chromosomally encoded FosA in K. pneumoniae (GenBank accession numbers WP_004146118, WP_004182826 and WP_002887377) and are closely related to FosA6 described in this study.
Susceptibility testing
MICs of fosfomycin and other commonly used agents were determined by Etest (bioMérieux, Durham, NC, USA) and commercially available broth microdilution testing plates (Sensititre GNX2F), respectively, and interpreted according to CLSI guidelines.16 E. coli ATCC 25922 (susceptible to fosfomycin) was used as the quality control strain. Inhibition of the glutathione-S-transferase activity of FosA was examined with sodium phosphonoformate as reported previously17 with the following modification, where fosfomycin Etest was placed on Mueller–Hinton agar plates with or without 500 mg/L sodium phosphonoformate. E. coli 55B8 was used as the fosfomycin-resistant, fosA-negative control strain. This clinical strain does not possess any fosA gene, but rather lacks the hexose phosphate transporter gene uhpT as the fosfomycin resistance mechanism, as evidenced by PCR and RT–PCR.
PCR and cloning
PCR for fosA3 was conducted as previously described.12 The chromosome of YD786 was extracted, digested with restriction enzyme Sau3AI and ligated with cloning vector pUC19 (Thermo Scientific, Waltham, MA, USA) which was digested with BamHI. E. coli TOP10 (Thermo Scientific) was transformed with this ligated product and transformants were identified by growth on LB agar plates containing 50 mg/L ampicillin, 50 mg/L fosfomycin and 25 mg/L glucose-6-phosphate. Nucleotide and protein BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) were used to find homologues of fosA6 and FosA6.
fosA6, fosAPMK1, fosAST37 and fosAST258 were cloned into pBCSK− (Agilent Technologies, Santa Clara, CA, USA) using the following primers: FosA-XbaI-F, 5′-TGCTCTAGATGCTGAGTGGACTGAATCAC-3′; FosA-HindIII-R, 5′-TCCAAGCTTCACTGATCAAAAAACACCATCC-3′; and FosA258-HindIII-R, 5′-TCCAAGCTTCACTGTTCAAAAAACACCATCC-3′.
Transferability of plasmids
Transformation and conjugation were performed as described previously,18 using E. coli TOP10 and azide-resistant E. coli J53 as recipients, respectively. Transformants and transconjugants were selected on LB agar plates containing fosfomycin and glucose-6-phosphate as above, whereas 100 mg/L sodium azide was also added for selection of the transconjugants.
WGS
The YD786 genome was sequenced by HiSeq 2500 (Illumina, San Diego, CA, USA) and PacBio RS II (Pacific Biosciences, Menlo Park, CA, USA) as previously described,19 resulting in full assembly of the chromosome and two plasmids (pYD786-1 and pYD786-2) and partial assembly of pYD786-3 and pYD786-4. Gaps in pYD786-3 and pYD786-4 were filled with HiSeq reads and verified by PCR and sequencing (data not shown). The chromosomal and plasmid sequences were submitted under accession numbers CP013112.1 and KU254578–81, respectively.
Purification of FosA6 and FosAST258 and steady-state kinetic assays
fosA6 and fosAST258 were synthesized by GenScript (Piscataway, NJ, USA) and cloned into the pE-SUMOstar prokaryotic expression vector (LifeSensors, Malvern, PA, USA) according to the manufacturer's instructions. Fosfomycin-dependent glutathione conjugation was detected spectrophotometrically using monochlorobimane (Sigma–Aldrich). A standard curve was prepared using 0–750 μM glutathione. Data were fitted to Michaelis–Menten equations using SigmaPlot (Systat Software, San Jose, CA, USA). Details of the purification and kinetic assays are available as Supplementary data at JAC Online.
Results and discussion
Antimicrobial susceptibility of E. coli YD786
E. coli YD786 was resistant to cephalosporins, aztreonam, fluoroquinolones and doxycycline and intermediate to minocycline (Table S1). Notably, it showed high-level resistance to fosfomycin with an MIC of 512 mg/L (susceptibility breakpoint, 64 mg/L).16 The MIC was reduced by 16-fold to 32 mg/L in the presence of 500 mg/L sodium phosphonoformate, which suggested the presence of FosA-group glutathione-S-transferase activity. However, PCR was negative for fosA3, which is the most commonly reported fosA gene in E. coli worldwide. Sequences of murA, glpT and uhpT, the three genes commonly implicated in fosfomycin resistance,5 were identical to those of fosfomycin-susceptible reference strain ATCC 25922. In comparison, fosfomycin resistance could not be reversed by sodium phosphonoformate in the fosA-negative, fosfomycin-resistant control strain 55B8 (Table 1).
Table 1.
MICs for E. coli clones carrying various fosA genes and K. pneumoniae clinical isolates with chromosomal fosA genes
| Strain | MIC of fosfomycin (mg/L) | MIC of fosfomycin in the presence of PPF (mg/L) | Amino acid alteration compared with FosAPMK1 |
||
|---|---|---|---|---|---|
| Ile91 | Pro130 | Asp138 | |||
| E. coli TOP10 (pBCSK−) | 0.38 | 0.50 | |||
| E. coli TOP10 (pFosAPMK1) | 16 | 0.75 | |||
| E. coli TOP10 (pFosA6) | 12 | 0.75 | Gln | ||
| E. coli TOP10 (pFosAST37) | 16 | 1 | Val | ||
| E. coli TOP10 (pFosAST258) | 12 | 0.75 | Val | Glu | |
| K. pneumoniae NDM01(FosAPMK1) | 24 | 4 | |||
| K. pneumoniae CRKpE6 (FosAST37) | 16 | 2 | Val | ||
| K. pneumoniae CRKpC1(FosAST258) | 24 | 1 | Val | Glu | |
| E. coli 55B8 (fosA negative) | >1024 | >1024 | |||
MICs were determined by Etest. Sodium phosphonoformate (PPF) was added to Mueller–Hinton agar at 500 mg/L. The E. coli clones harbour fosA genes carried on vector pBCSK−. E. coli 55B8 was included as a fosfomycin-resistant, fosA-negative control strain, which has a defective uhpT gene.
Cloning and sequencing of fosA6
Genomic cloning of the fosfomycin resistance determinant from strain YD786 yielded E. coli TOP10 harbouring recombinant plasmid pYD786S14, which was highly resistant to fosfomycin with an MIC of >1024 mg/L. Sequencing of pYD786S14 revealed an 815 bp insert, which shared 99% nucleotide identity with multiple chromosomal sequences of K. pneumoniae, including those of the epidemic carbapenem-resistant ST258 strains and K. pneumoniae PMK1 (ST15, CP008929.1), which is an NDM-producing strain that caused an outbreak of neonatal infections in a Nepali hospital.20 The insert contained a single 420 bp ORF encoding FosA, hereafter referred to as FosA6 since it was located on a plasmid and shared 96% and 79% identity with FosA5 and FosA3 at the amino acid level, respectively. BLAST searches identified FosA6 homologues to be widely encoded on the chromosomes of K. pneumoniae (∼700 K. pneumoniae out of 800 Enterobacteriaceae sequences found in GenBank; data not shown). The amino acid sequence of FosA6 shared >99% identity with FosAPMK1 with one amino acid substitution (Pro130Gln) and 98% identity with FosAST258 differing by only three amino acids (Val91Ile, Pro130Gln and Glu138Asp; Table 1). FosAPMK1 is distributed in 84 K. pneumoniae strains, represented by 33 STs in different clonal complexes (Table S2 and Figure S1), including reference strains MGH78578 (ST38, CP000647.1), NTUH-K2044 (ST23, AP006725.1) and an NDM-producing strain reported from our hospital previously (K. pneumoniae NDM01; ST14, CP006798.1).15 FosAST258 is identified in 427 K. pneumoniae strains (data not shown), of which 119 were assigned STs based on the MLST scheme (https://cge.cbs.dtu.dk/services/MLST/), including 111 ST258 and 5 ST11 strains (Table S3 and Figure S1). FosAST258 appears to be common in the epidemic CC258 strains, including ST258, ST11 and ST512 among others, suggesting a wide distribution of closely related homologues of FosA6 in this species (Table S3 and Figure S1).
Genome analysis of E. coli YD786 and transferability of fosA6
E. coli YD786 had a genome of 4.9 Mb in length and belonged to ST410. It contained typical quinolone resistance-determining region substitutions Ser83Leu and Asp87Asn in GyrA and Ser80Ile in ParC. The plasmids were assembled as 227 kb blaCTX-M-2-carrying IncHI2 plasmid pYD786-1, 69 kb fosA6-carrying IncFII plasmid pYD786-2, 45 kb IncX1 plasmid pYD786-3 and 26 kb IncX2 plasmid pYD786-4.
pYD786-2 carried fosA6 as well as an IncFII replicon region, conjugative transfer operon (tra-trb), toxin/antitoxin addiction system (hok-mok) and plasmid stability and partition system (parB, parM). This overall structure was similar to blaNDM-1-carrying plasmids pGUE-NDM (JQ364967.1) and pMC-NDM (HG003695.1), identified in E. coli from India and Poland, respectively,21,22 except that pYD786-2 contained only fosA6 and floR (encoding chloramphenicol efflux protein) as resistance genes (Figure S2).
pYD786-2, the native fosA6-carrying plasmid in YD786, was transferable by transformation and broth mating. E. coli TOP10 (pYD786-2) had a fosfomycin MIC of 128 mg/L, which could be explained by the presence of fosA6. E. coli TOP10 (pYD786-2) was also resistant to chloramphenicol, but otherwise remained susceptible to other classes of antimicrobial agents (Table S1). The transconjugant had an identical resistance phenotype and plasmid profile to the transformant (data not shown).
Genetic environments of fosA6
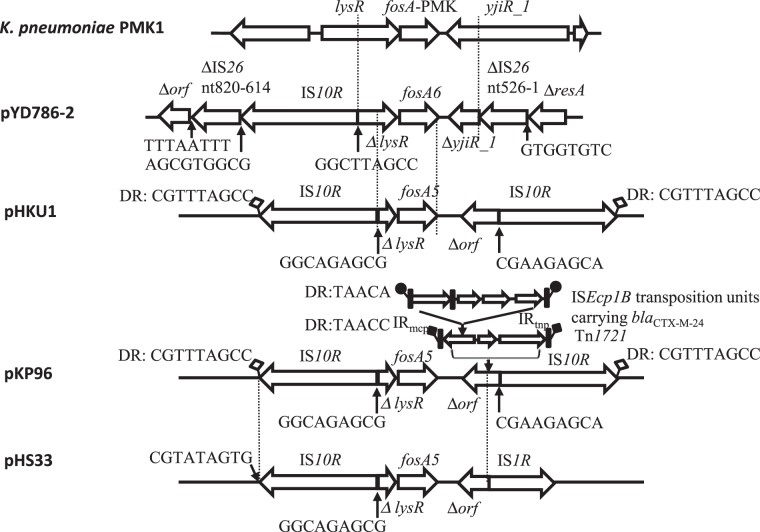
A transcriptional regulator gene lysR truncated by IS10R was located upstream of fosA6 and an aminotransferase truncated by IS26 was located downstream of fosA6 (Figure 1). This 1183 bp ΔlysR-fosA6-ΔyjiR_1 region was nearly identical to those in K. pneumoniae MGH78578, PMK1 and KPPR1 (ST493, CP009208.1) with only three or four nucleotide differences, suggesting its mobilization from the chromosome of this species. K. pneumoniae MGH78578 is also known as ATCC 700721 and was isolated from the sputum of a 66-year-old ICU patient in 1994. KPPR1 is a rifampicin-resistant derivative of ATCC 43816 commonly used in animal studies.
Figure 1.
Genetic environment of fosA6, fosA5 and the corresponding region on the chromosome of K. pneumoniae PMK1. Fragments between the dotted lines share >99% identity. The predicted ORFs and ISs are indicated by bold arrows and annotated above or below, with arrowheads indicating the direction of transcription. Putative DR sequences are given to indicate their boundaries. Paired filled/unfilled squares or circles represent DRs of transposition units of transposons or ISs. K. pneumoniae PMK1, CP008929.1; pHKU1, KC960485.1; pKP96, EU195449.1; and pHS33, KP143090.1.
This genetic context was very similar to that of fosA5, which was discovered in plasmids pHKU1 (KC960485.1),11 pKP96 (EU195449.1)14 and pHS33 (KP143090.1).13 However, the ΔlysR located upstream of fosA5 in these plasmids was shorter (143 versus 354 bp) than that upstream of fosA6 in pYD786-2 as a result of more substantial truncation by IS10R. In addition, downstream of fosA5 there was a gene of unknown function truncated by IS10R in pHKU1 and pKP96 or by IS1R in pHS33. The IS10R-flanked transposition unit bounded by direct repeats (DRs) in pHKU1 was identical to that in pKP96 except that in pKP96 subsequent transposition of Tn1721 and ISEcp1B resulted in the acquisition of blaCTX-M-24 (Figure 1). In contrast to these fosA5-containing plasmids, in pYD786-2, IS10R-ΔlysR-fosA6-ΔyjiR_1 interrupted IS26. The 5′ end of IS26 truncated resA while the 3′ end truncated an ORF of unknown function, a structure that, together with adjacent stbAB, is observed in some non-fosA-carrying IncFII plasmids (pEQ011, KF582523.1; pEC_B24, GU371926.1; p12-4374_62, CP012928.1).
Overall, it appeared likely that fosA6 was mobilized from the chromosome of K. pneumoniae closely related to PMK1 by IS10, but in a separate mobilization event compared with fosA5, given its distinct insertion site and mobilization onto an IncFII plasmid, as opposed to IncN or IncA/C plasmid for fosA5, and also the geographical separation where fosA5 was found in China and Hong Kong whereas fosA6 was identified in the USA.
Also of note is the fact that fosA6 is located on a plasmid that only carries one other resistance gene, floR, encoding a chloramphenicol efflux pump. In contrast, fosA3 has so far been identified exclusively on plasmids carrying a broad-spectrum β-lactamase gene (CTX-M-group ESBL,8,10,23,24 CMY-2-group plasmid-mediated AmpC β-lactamase23,25 or KPC and NDM carbapenemases).24,25 However, the fact that fosA6 is located on a non-MDR plasmid suggests that these emerging fosfomycin resistance genes may have been overlooked. It is also possible that the acquisition of fosA6 was a relatively recent event and fosA6 may find other resistance genes as partners on the same plasmids in the future. Nevertheless, K. pneumoniae serving as a ubiquitous reservoir of fosA for E. coli is a concerning phenomenon given the selective pressure exerted by increasing use of fosfomycin.
Functionality of FosA6 and FosAST258
The steady-state kinetic parameters for fosfomycin (Table 2) were largely comparable for FosA6 and FosAST258, suggesting that the three amino acid differences between these genes do not impact fosfomycin binding. In contrast, the catalytic efficiency for glutathione for FosA6 was ∼2-fold higher than the value determined for FosAST258, which was predominantly driven by a change in KM.
Table 2.
Steady-state kinetic parameters determined for FosA6 and FosAST258
| Enzyme | Fosfomycina |
Glutathioneb |
||||||
|---|---|---|---|---|---|---|---|---|
| KM (mM) | Vmax (mM. min−1) | kcat (min−1) | kcat/KM (mM−1. min−1) | KM (mM) | Vmax (mM. min−1) | kcat (min−1) | kcat/KM (mM−1. min−1) | |
| FosA6 | 2.5 ± 1.5 | 0.1 ± 0.0 | 500.3 ± 70.4 | 200.1 | 5.4 ± 4.5 | 0.2 ± 0.1 | 937.9 ± 350.7 | 173.6 |
| FosAST258 | 2.1 ± 1.1 | 0.1 ± 0.0 | 438.9 ± 49.5 | 209.0 | 21.4 ± 13.1 | 0.5 ± 0.2 | 2018.2 ± 948.2 | 94.3 |
aMeasured at 20 mM glutathione.
bMeasured at 20 mM fosfomycin.
These kinetic data showed that FosAST258, the chromosomally encoded FosA produced by the epidemic KPC-producing ST258 strains, is able to inactivate fosfomycin as robustly as FosA6. Median fosfomycin MICs for KPC-producing K. pneumoniae strains are 16–64 mg/L,26,27 which are substantially higher than those for E. coli, which are typically in the 1–2 mg/L range.1,26 While further studies are needed, these findings suggest that fosA probably contributes to the higher baseline fosfomycin MICs for K. pneumoniae compared with E. coli that lacks chromosomal fosA as a species.28,29 The functionality of various FosA enzymes was also supported by reduction of fosfomycin MICs by 6- to 24-fold in the presence of sodium phosphonoformate for fosA-positive K. pneumoniae strains as well as E. coli clones carrying these fosA genes of K. pneumoniae origin (Table 1).
Sodium phosphonoformate behaves as a competitive inhibitor of fosfomycin by binding to FosA in the active site including conserved Thr9 and Mn(II).30 It has been successfully used as a diagnostic tool to detect production of FosA3, FosA4 and FosC217 and, in our study, FosA6 and chromosomal FosA of K. pneumoniae. It is worth noting that sodium phosphonoformate is approved for clinical use in the treatment of herpes virus infections as the antiviral compound foscarnet.
Conclusions
We report a novel glutathione-S-transferase FosA6 that confers high-level fosfomycin resistance in an E. coli clinical strain identified in Pennsylvania, USA. The gene was probably mobilized from K. pneumoniae chromosome to E. coli plasmid through an IS10-mediated mobilization event. K. pneumoniae may serve as a significant reservoir of fosfomycin resistance in E. coli as the use of this agent increases.
Funding
The study was conducted through internal funding. Q. G. was supported by grant no. 81102509 and grant no. 81120108024 from the National Natural Science Foundation of China, by grant no. 20124052 from the Shanghai Municipal Commission of Health and Family Planning and by grant no. 2014ZX09507-009 from the National Major Scientific and Technological Special Project for ‘Significant New Drugs Development’. The effort of Y. D. was supported by research grants from the National Institutes of Health (R01AI104895, R21AI107302 and R21AI123747).
Transparency declarations
Y. D. has served on advisor boards for Shionogi, Meiji Seika Pharma, Tetraphase Pharmaceuticals, Achaogen and Merck, has consulted for Melinta Therapeutics and has received research funding from Merck and The Medicines Company for studies unrelated to this work. All other authors: none to declare.
Supplementary data
Acknowledgements
Part of the study was presented at the Fifty-fifth Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, CA, USA, 2015 (Control Number 3054).
References
- 1.Karlowsky JA, Denisuik AJ, Lagace-Wiens PR et al. In vitro activity of fosfomycin against Escherichia coli isolated from patients with urinary tract infections in Canada as part of the CANWARD surveillance study. Antimicrob Agents Chemother 2014; 58: 1252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson JR, Drawz SM, Porter S et al. Susceptibility to alternative oral antimicrobial agents in relation to sequence type ST131 status and coresistance phenotype among recent Escherichia coli isolates from US veterans. Antimicrob Agents Chemother 2013; 57: 4856–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta K, Hooton TM, Naber KG et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52: e103–20. [DOI] [PubMed] [Google Scholar]
- 4.Kahan FM, Kahan JS, Cassidy PJ et al. The mechanism of action of fosfomycin (phosphonomycin). Ann N Y Acad Sci 1974; 235: 364–86. [DOI] [PubMed] [Google Scholar]
- 5.Castañeda-García A, Blázquez J, Rodríguez-Rojas A. Molecular mechanisms and clinical impact of acquired and intrinsic fosfomycin resistance. Antibiotics 2013; 2: 217–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson MK, Keithly ME, Sulikowski GA et al. Diversity in fosfomycin resistance proteins. Perspect Sci 2015; 4: 17–23. [Google Scholar]
- 7.Wachino J, Yamane K, Suzuki S et al. Prevalence of fosfomycin resistance among CTX-M-producing Escherichia coli clinical isolates in Japan and identification of novel plasmid-mediated fosfomycin-modifying enzymes. Antimicrob Agents Chemother 2010; 54: 3061–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hou J, Huang X, Deng Y et al. Dissemination of the fosfomycin resistance gene fosA3 with CTX-M β-lactamase genes and rmtB carried on IncFII plasmids among Escherichia coli isolates from pets in China. Antimicrob Agents Chemother 2012; 56: 2135–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Zheng B, Li Y et al. Antimicrobial susceptibility and molecular mechanisms of fosfomycin resistance in clinical Escherichia coli isolates in mainland China. PLoS One 2015; 10: e0135269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SY, Park YJ, Yu JK et al. Prevalence of acquired fosfomycin resistance among extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae clinical isolates in Korea and IS26-composite transposon surrounding fosA3. J Antimicrob Chemother 2012; 67: 2843–7. [DOI] [PubMed] [Google Scholar]
- 11.Ho PL, Chan J, Lo WU et al. Prevalence and molecular epidemiology of plasmid-mediated fosfomycin resistance genes among blood and urinary Escherichia coli isolates. J Med Microbiol 2013; 62: 1707–13. [DOI] [PubMed] [Google Scholar]
- 12.Alrowais H, McElheny CL, Spychala CN et al. Fosfomycin resistance in Escherichia coli, Pennsylvania, USA. Emerg Infect Dis 2015; 21: 2045–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Y, Xu X, Guo Q et al. Characterization of fosA5, a new plasmid-mediated fosfomycin resistance gene in Escherichia coli. Lett Appl Microbiol 2015; 60: 259–64. [DOI] [PubMed] [Google Scholar]
- 14.Shen P, Jiang Y, Zhou Z et al. Complete nucleotide sequence of pKP96, a 67 850 bp multiresistance plasmid encoding qnrA1, aac(6′)-Ib-cr and blaCTX-M-24 from Klebsiella pneumoniae. J Antimicrob Chemother 2008; 62: 1252–6. [DOI] [PubMed] [Google Scholar]
- 15.Doi Y, Hazen TH, Boitano M et al. Whole-genome assembly of Klebsiella pneumoniae coproducing NDM-1 and OXA-232 carbapenemases using single-molecule, real-time sequencing. Antimicrob Agents Chemother 2014; 58: 5947–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-fourth Informational Supplement M100-S24. CLSI, Wayne, PA, USA, 2014. [Google Scholar]
- 17.Nakamura G, Wachino J, Sato N et al. Practical agar-based disk potentiation test for detection of fosfomycin-nonsusceptible Escherichia coli clinical isolates producing glutathione S-transferases. J Clin Microbiol 2014; 52: 3175–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sidjabat HE, Paterson DL, Adams-Haduch JM et al. Molecular epidemiology of CTX-M-producing Escherichia coli isolates at a tertiary medical center in western Pennsylvania. Antimicrob Agents Chemother 2009; 53: 4733–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li JJ, Spychala CN, Hu F et al. Complete nucleotide sequences of blaCTX-M-harboring IncF plasmids from community-associated Escherichia coli strains in the United States. Antimicrob Agents Chemother 2015; 59: 3002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoesser N, Giess A, Batty EM et al. Genome sequencing of an extended series of NDM-producing Klebsiella pneumoniae isolates from neonatal infections in a Nepali hospital characterizes the extent of community- versus hospital-associated transmission in an endemic setting. Antimicrob Agents Chemother 2014; 58: 7347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonnin RA, Poirel L, Carattoli A et al. Characterization of an IncFII plasmid encoding NDM-1 from Escherichia coli ST131. PLoS One 2012; 7: e34752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiett J, Baraniak A, Izdebski R et al. The first NDM metallo-β-lactamase-producing Enterobacteriaceae isolate in Poland: evolution of IncFII-type plasmids carrying the blaNDM-1 gene. Antimicrob Agents Chemother 2014; 58: 1203–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tseng SP, Wang SF, Kuo CY et al. Characterization of fosfomycin resistant extended-spectrum β-lactamase-producing Escherichia coli isolates from human and pig in Taiwan. PLoS One 2015; 10: e0135864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang Y, Shen P, Wei Z et al. Dissemination of a clone carrying a fosA3-harbouring plasmid mediates high fosfomycin resistance rate of KPC-producing Klebsiella pneumoniae in China. Int J Antimicrob Agents 2015; 45: 66–70. [DOI] [PubMed] [Google Scholar]
- 25.Villa L, Guerra B, Schmoger S et al. IncA/C plasmid carrying blaNDM-1, blaCMY-16, and fosA3 in a Salmonella enterica serovar Corvallis strain isolated from a migratory wild bird in Germany. Antimicrob Agents Chemother 2015; 59: 6597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livermore DM, Warner M, Mushtaq S et al. What remains against carbapenem-resistant Enterobacteriaceae? Evaluation of chloramphenicol, ciprofloxacin, colistin, fosfomycin, minocycline, nitrofurantoin, temocillin and tigecycline. Int J Antimicrob Agents 2011; 37: 415–9. [DOI] [PubMed] [Google Scholar]
- 27.Endimiani A, Patel G, Hujer KM et al. In vitro activity of fosfomycin against blaKPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob Agents Chemother 2010; 54: 526–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu CL, Liu CY, Huang YT et al. Antimicrobial susceptibilities of commonly encountered bacterial isolates to fosfomycin determined by agar dilution and disk diffusion methods. Antimicrob Agents Chemother 2011; 55: 4295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samonis G, Maraki S, Rafailidis PI et al. Antimicrobial susceptibility of Gram-negative nonurinary bacteria to fosfomycin and other antimicrobials. Future Microbiol 2010; 5: 961–70. [DOI] [PubMed] [Google Scholar]
- 30.Rigsby RE, Rife CL, Fillgrove KL et al. Phosphonoformate: a minimal transition state analogue inhibitor of the fosfomycin resistance protein, FosA. Biochemistry 2004; 43: 13666–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.