Abstract
Understanding how temperature affects fitness is important for conservation and pest management, especially in the era of global climate change. Rhynchophorus ferrugineus (Oliver) (Coleoptera: Curculionidae) is a worldwide pest of many economically important crops. Although much is known about this pest’s life cycle, its adaptability to different temperatures is not fully understood. Here, we used age- and stage-specific life tables to investigate the effects of temperature on fitness-related traits and demographic parameters of R. ferrugineus under eight constant temperature regimens in the laboratory. The growth potential of these populations was also evaluated. The greatest longevity for males and females was 158.0 d at 24 °C and 144.5 d at 21 °C, respectively, but mean total fecundity was the highest at 27 °C. The intrinsic rate of increase (r), finite rate of increase (λ), and net reproductive rate (R0) increased initially at low temperatures and then decreased. All metrics reached a maximum at 27 °C and a minimum at 36 °C. Mean generation times (T ) decreased across the temperature range with a minimum at 36 °C. Our results indicate that the optimum temperature for growth of R. ferrugineus was approximately 27 °C. Our work will be of value for developing strategies for control management of this pest species.
The red palm weevil, Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae), is an extremely invasive and primary pest of the palm family. It attacks more than 20 palm species belonging to 16 different genera1. R. ferrugineus originated from South and Southeast Asia but has now spread to most palm-growing regions in Asia, Africa, Europe, and Oceania2. In China, the first reported infestation was identified in Cocos nucifera in Guangdong province in 1997 3. Subsequently, owing to transportation of infested plants, R. ferrugineus invaded and caused serious damage in many areas of China, including Hainan, Guangdong, Guangxi, Yunnan, Fujian, Hong Kong, Taiwan, Zhejiang, Jiangxi, and Shanghai4. Since R. ferrugineus larvae feed within the palm trunks and the resulting damage is generally only visible after long-term infection and considerable damage has occurred5, early detection is usually difficult. In addition, this behaviour provides the larvae with considerable protection against chemical insecticides, natural enemies, and pathogens. As a result, there is considerable current interest in developing an integrated pest management strategy based on pheromone traps and biological control6. Hence, knowledge of the life history and ecological adaptability of R. ferrugineus is important for identifying the optimal times for intervention to achieve more effective control management.
Environmental temperature is a significant factor influencing behaviour, distribution, development, survival, and reproduction in ectothermic organisms such as insects7,8. Knowledge of the temperature-dependent population growth potential of insect pest species is essential for predicting potential changes in population dynamics and for implementing efficient, economic, and ecological pest control strategies, especially in the context of predicted global climate warming9.
Analysis of the life history of R. ferrugineus will provide the data for predicting population peaks, establishing the timing for sampling operations and ecological zoning10, and developing integrated control programmes11. To date, the thermal requirements and the lower temperature thresholds for development, oviposition, and egg hatching in R. ferrugineus have been described5,12 along with effects on emergence13 and population growth14. However, there is limited information on how temperature affects population demographics and age- and stage-specific traits, which are important for metamorphic insects15. The traditional female age-specific life table ignores some important factors such as male individuals and stage differentiation, which may result in some problems. As both males and females are economically important and affect population dynamics, it is important to have information on both sexes. In addition, variations in developmental rates among individuals may help a population survive in unpredictable and harsh environmental conditions16.
The age- and stage-specific life table approach is a useful tool for conservation and pest management17, and is commonly employed to determine the growth parameters and the maximal growth potential of populations of insect pests18,19,20,21, mites17, and predators22 under different environmental conditions. This study was designed to quantify the manifold effects of temperature on the population fitness of R. ferrugineus. We used the age-stage, two-sex life table approach23 to investigate life-history traits, and to evaluate their impacts on the population demography of R. ferrugineus under different temperatures. Furthermore, the population growth potential of the weevil was estimated. This is the first comprehensive study of the effects of temperature on R. ferrugineus populations based on age stage-specific traits15. The results of our study showed that life history traits and demographic parameters were altered by changes in environmental temperatures; moreover, these effects were developmental stage- and age-specific. Our findings offer valuable insights into the establishment potential of R. ferrugineus in new environments with diverse temperature regimens and will be of value for the management of this pest species.
Results
Age stage-specific survival rate
At 15 and 18 °C, R. ferrugineus failed to complete development and reproduction. Data from these two temperatures were therefore excluded from all analyses. The age-stage survival curve, sxj, depicts the probability that a newly laid egg will survive to age x and stage j (Fig. 1a–f). The overlaps between different stages occur as a result of inter-individual variation in development rates. We found that the curve for survival of larvae had the slowest increase at 21 °C until the 10th day with a maximum survival rate of 65.3% (Fig. 1a). The fastest increasing curves were seen at 33 and 36 °C, but reach to the maximal survival rates of only 50% and 58%, respectively (Fig. 1e,f). At 27 °C, the curve took 4 d (one day more than at 33 and 36 °C) to reach the maximum survival rate of 85.3% (Fig. 1c); this survival rate was greater than at other temperatures. In addition, the curves of female and male emerged earliest at 33 °C, with 99 and 87 d, respectively (Fig. 1e).
Figure 1. Age-stage specific survival rates (sxj) of R. ferrugineus reared on sugarcane at different constant temperatures.
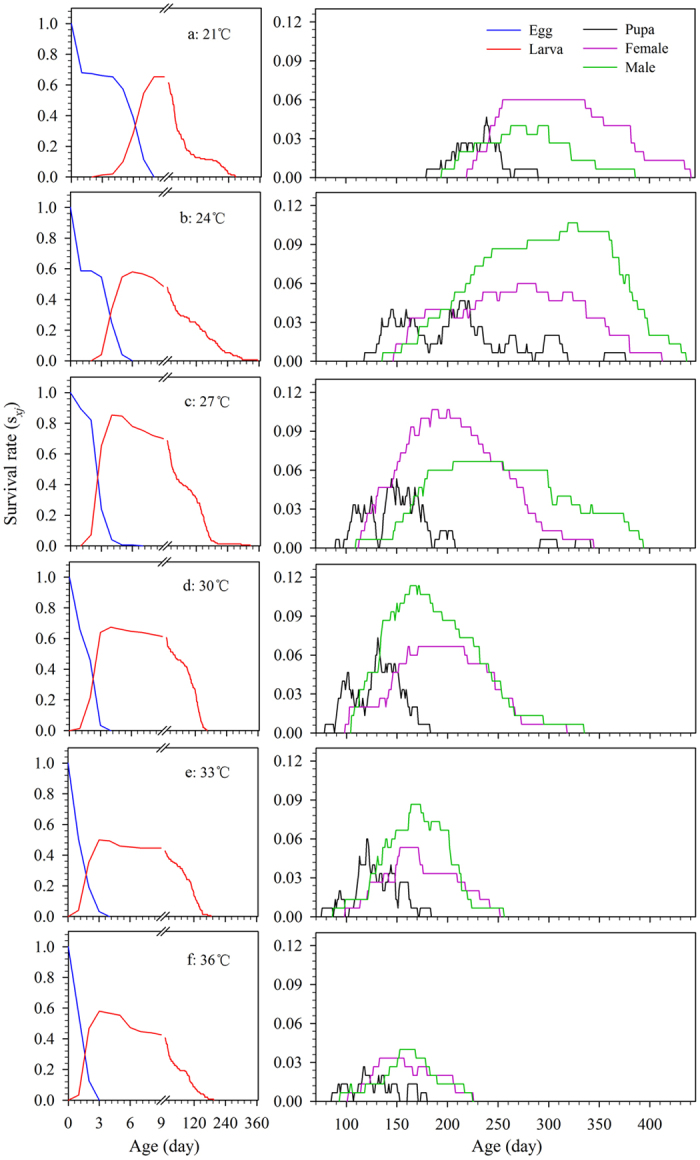
sxj, the probability that a newly laid egg will survive to age x and stage j.
Age stage-specific fecundity
The number of offspring produced by an individual weevil of age x and stage j is shown in Fig. 2a–f. In this species, females produce eggs and therefore there is only a single curve ( fx4) representing the females (stage 4). Different dynamic patterns for fx4, mx, and lxmx were observed at the six temperatures. The starting time of reproduction ( fx4, mx, and lxmx) was earlier with increasing temperature: 233 d at 21 °C, but 105 and 106 d at 33 and 36 °C, respectively. A similar advancing trend was observed for the timing of the first reproductive peak (Fig. 2a–f). The fx4 peaks appeared at 260, 179, 144, 115, 108, and 114 d at 21, 24, 27, 30, 33 and 36 °C, respectively. The curve for mx was lower than that for fx4 because it is a parameter of age-specific averaged fecundity that takes into account concurrent stages (Fig. 2a–f). The ranges of fx4, mx, and lxmx were largest at 186 d at 27 °C and shortest at 71 d at 36 °C (Fig. 2c,f). Thus, the egg laying performance of R. ferrugineus females was more stable and durable at 27 °C.
Figure 2. Age-specific survival rate (lx), female age-specific fecundity ( fx4), age-specific fecundity (mx), and age-specific maternity (lxmx) of R. ferrugineus reared on sugarcane at different constant temperatures.
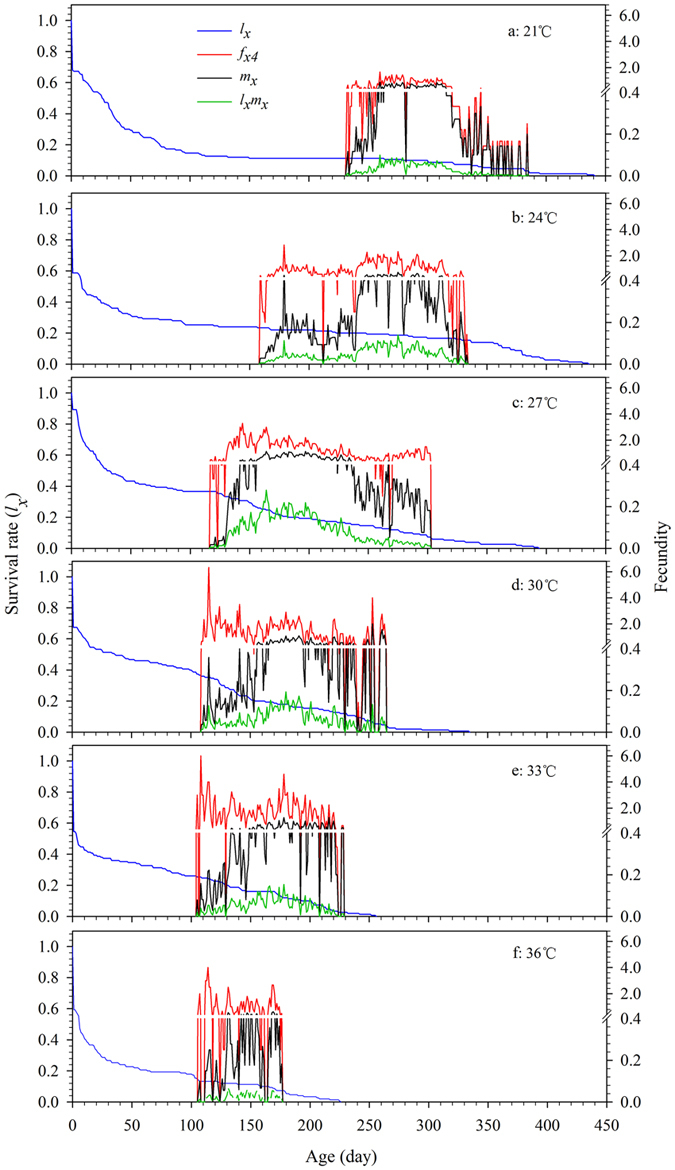
fx4, the mean fecundity of females at age x and stage 4 (female adult stage); lx, the probability of a newly laid egg surviving to age x; mx, the mean fecundity of individuals at age x.
Ignoring stage differentiation, the single age-specific survival rate (lx) gives the probability that an egg will survive to age x. We found that the curve of lx fell slowest at 24 °C with 16.7% of individuals surviving longer than 300 d (Fig. 2b); lx was relatively constant at 21 and 27 °C with 10.0% and 7.3% of individuals surviving longer than 300 d (Fig. 2a,c). However, there was a sharp fall in age-specific survival rate at 36 °C with no individual surviving longer than 250 d (Fig. 2f).
Development, longevity, and fecundity
The durations of developmental stages varied significantly at the different temperatures throughout the life cycle (Table 1). The duration of the egg stage steadily decreased from 6.6 d at 21 °C to 2.2 d at 36 °C (P < 0.05), suggesting that it was sensitive to temperature variation (Table 1). The pre-adult stage did not differ in duration between 21 and 24 °C (P > 0.05), but decreased significantly at 27 and 30 °C (P < 0.05). However, no further significant decrease was observed above 30 °C (P > 0.05) (Table 1).
Table 1. Means and standard errors of development time, adult longevity, adult preoviposition period (APOP), total preoviposition period (TPOP), oviposition days, fecundity of R. ferrugineus at six constant temperatures.
| Stage | Temperature (°C) |
|||||
|---|---|---|---|---|---|---|
| 21 | 24 | 27 | 30 | 33 | 36 | |
| Egg (d) | 6.6 ± 0.1 a | 4.4 ± 0.1 b | 3.3 ± 0.1 c | 2.7 ± 0.1 d | 2.3 ± 0.1 e | 2.2 ± 0.1 f |
| Larva (d) | 215.4 ± 5.6 a | 197.7 ± 9.8 a | 144.1 ± 8.1 b | 120.2 ± 3.5 c | 120.1 ± 6.3 c | 122.7 ± 5.8 c |
| Pupa (d) | 16.1 ± 0.8 b | 19.3 ± 0.8 a | 12.3 ± 0.3 c | 11.9 ± 0.3 c | 10.7 ± 0.4 d | 8.8 ± 0.3 e |
| Preadult (d) | 237.8 ± 5.9 a | 218.2 ± 10.7 a | 165.1 ± 8.4 b | 135.3 ± 3.7 c | 134.9 ± 4.4 c | 131.8 ± 6.5 c |
| Female adult (d) | 144.5 ± 10.8 a | 112.0 ± 18.4 ab | 94.7 ± 10.1 bcd | 99.4 ± 12.6 bc | 69.0 ± 10.3 cd | 62.2 ± 14.1 d |
| Male adult (d) | 87.1 ± 13.8 b | 158.0 ± 11.7 a | 132.2 ± 15.9 a | 89.5 ± 9.6 b | 56.3 ± 7.0 c | 45.9 ± 12.2 c |
| APOP (d) | 10.1 ± 1.0 a | 8.2 ± 1.2 ab | 4.7 ± 0.5 d | 7.0 ± 1.0 bc | 6.1 ± 0.5 bcd | 4.8 ± 0.9 cd |
| TPOP (d) | 249.7 ± 3.8 a | 204.9 ± 13.5 b | 151.5 ± 5.4 c | 144.3 ± 7.5 c | 139.0 ± 6.7 cd | 125.0 ± 6.0 d |
| Oviposition days | 53.6 ± 4.1 a | 59.9 ± 11.4 a | 56.2 ± 6.3 a | 48.0 ± 5.7 ab | 31.8 ± 6.9 bc | 23.6 ± 3.2 c |
| Fecundity (egg) | 75.2 ± 7.9 bc | 97.0 ± 21.1 ab | 125.0 ± 15.4 a | 124.1 ± 17.9 a | 108.1 ± 25.9 ab | 42.1 ± 10.3 d |
Means in the same row followed by the same letter are not significantly different. The SEs were estimated using 100,000 bootstraps and compared using a paired bootstrap test based on CI of differences.
Longevity of adult females varied significantly at different temperatures (Table 1). The maximum longevity, 144.5 ± 10.8 d, was observed at 21 °C, but decreased at higher temperatures and fell to a minimum of 62.2 ± 14.4 d at 36 °C (P < 0.05) (Table 1). However, longevity in adult males showed a different response to temperature variation compared to female adults (Table 1). The maximum longevity occurred at 24 °C, which was significantly greater than that at 21 °C (P < 0.05), but did not differ significantly from that at 27 °C (P > 0.05). At temperatures above 27 °C, male longevity decreased significantly (Table 1). These changes suggest that males may be more sensitive to temperature variation.
The longest adult preoviposition period (APOP) occurred at 21 °C, while the shortest occurred at 27 °C and was only 4.7 ± 0.5 d; this period was significantly shorter than at 21, 24, and 30 °C (P < 0.05), but was similar to that at 33 and 36 °C (P > 0.05) (Table 1). The duration of the total preoviposition period (TPOP) fell from 249.7 ± 3.8 d at 21 °C to 125.0 ± 6.0 d at 36 °C (P < 0.05), similarly to the changes seen for pre-adult stage duration (Table 1). High temperatures greatly reduced the preoviposition period, suggesting an energy trade-off in the extreme environment.
The longest reproductive period was at 24 °C, but there was no significant difference across the range 21 to 30 °C (P > 0.05); the minimum period was observed at 36 °C (P < 0.05) (Table 1). Female fecundity initially increased as temperatures rose but then decreased at the highest temperatures. The highest female fecundity (125.0 ± 15.4) was observed at 27 °C, but did not vary significantly across the temperature range of 24 to 33 °C (P > 0.05) (Table 1). There was a sharp decline in fecundity at 36 °C with a mean of only 42.1 offspring, which was significantly lower than that at all other temperatures (P < 0.05).
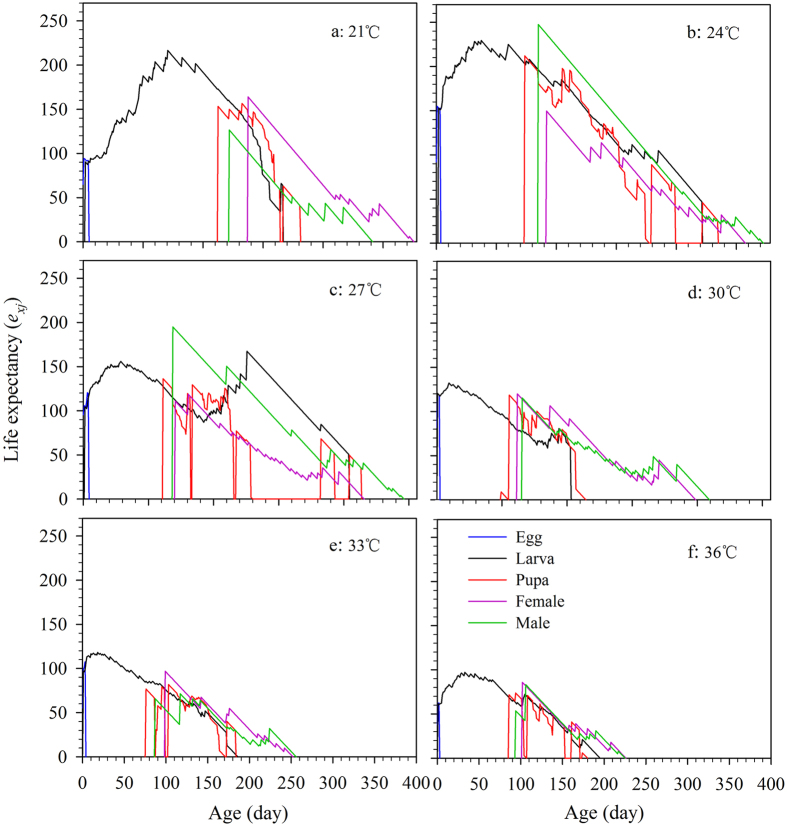
Age stage-specific life expectancy
The age stage-specific life expectancy (exj) describes the future expected life span of an individual of age x and stage j (Fig. 3a–f). The life expectancies of newborn weevils (e01) were 90.4, 153.0, 104.4, 120.4, 97.2, and 61.6 d at 21, 24, 27, 30, 33, and 36 °C, respectively (Fig. 3a–f), showing that both low and high temperatures could shorten life expectancies. The ex4 of females fell from 164.0 d at 21 °C to 85.8 at 36 °C (Fig. 3a–f), while the maximum ex4 of males, 247.6 d, was observed at 24 °C (Fig. 3b), but decreased at higher temperatures and fell to a minimum of 53.6 d at 36 °C (Fig. 3f).
Figure 3. Age-stage- and sex-specific life expectancy (exj) of R. ferrugineus reared on sugarcane at different constant temperatures.
exj, the future expected life span of an individual at age x and stage j.
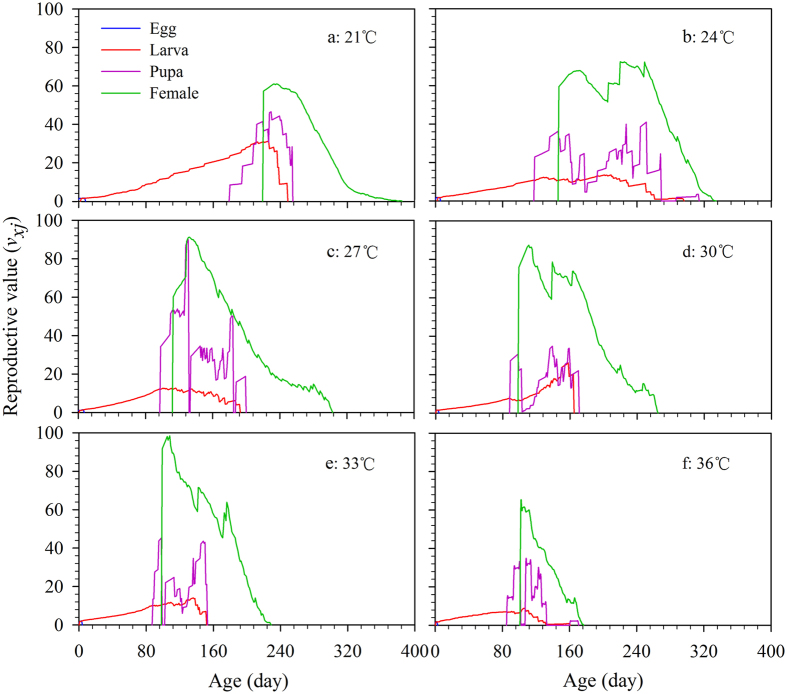
Age stage-specific reproductive value
The reproductive value (vxj) is the contribution of individuals of age x and stage j to the future population (Fig. 4a–f). After emergence of adult females at 220, 147, 113, 99, 99, and 102 d under temperature conditions from 21 to 36 °C, the vxj jumped to 57.3, 59.5, 60.1, 75.9, 91.6, and 60.8 eggs, respectively with increasing temperature, while the peak vxj occurred at 232 d (61.0 eggs), 220 d (72.5 eggs), 132 d (91.3 eggs), 111 d (87.4 eggs), 108 d (98.5 eggs), and 106 d (61.7 eggs) at the different temperatures (Fig. 4a–f). The longest duration of vx4 of female adults was 190 d at 27 °C, whereas it was only 75 d at 36 °C.
Figure 4. Age-stage reproductive value (vxj) of R. ferrugineus reared on sugarcane at different constant temperatures.
vxj, the contribution of individuals at age x and stage j to the future population quantity.
Population parameters
All three parameters showed an initial increase and a maximum at 27 °C with 0.0152 d−1 for r, 1.0153 d−1 for λ, and 16.67 offspring for R0; they then fell significantly to 0.0027 d−1 for r, 1.0027 d−1 for λ, and 1.69 offspring for R0 at 36 °C, which were similar to those at 21 °C (P > 0.05) (Table 2). This suggests that the optimum temperature for R. ferrugineus population among those tested was 27 °C, and that low or high temperatures had a clear negative effect on population growth. On the other hand, the mean generation time (T) was significantly shortened as temperatures increased (Table 2).
Table 2. Means and standard errors of the intrinsic rate of increase (r), finite rate of increase (λ), net reproductive rate (R 0), mean generation time (T ) of R. ferrugineus at six constant temperatures.
| Population parameters | Temperature (°C) |
|||||
|---|---|---|---|---|---|---|
| 21 | 24 | 27 | 30 | 33 | 36 | |
| r (d−1) | 0.0050 ± 0.0013 c | 0.0083 ± 0.0015 bc | 0.0152 ± 0.0015 a | 0.0128 ± 0.0021 ab | 0.0109 ± 0.0030 abc | 0.0027 ± 0.0037 c |
| λ (d−1) | 1.0050 ± 0.0013 c | 1.0084 ± 0.0016 bc | 1.0153 ± 0.0015 a | 1.0129 ± 0.0021 ab | 1.0109 ± 0.0031 abc | 1.0027 ± 0.0037 c |
| R0 (offspring/individual) | 4.51 ± 1.52 bc | 8.41 ± 2.84 ab | 16.67 ± 4.02 a | 9.93 ± 3.08 ab | 6.49 ± 2.56 bc | 1.69 ± 0.76 c |
| T (d) | 287.90 ± 5.32 a | 248.99 ± 9.89 b | 183.30 ± 6.76 c | 176.16 ± 9.62 c | 164.08 ± 9.85 cd | 144.89 ± 7.57 d |
Means in the same row followed by the same letter are not significantly different. The SEs were estimated using 100,000 bootstraps and compared using a paired bootstrap test based on the CI of difference.
Population projection
From an initial 10 eggs, the fastest growing population was that at 27 °C; this population was predicted to exceed 17,057 individuals after 600 d (Fig. 5). Population size increases were slowest at 21 and 36 °C, with a final size estimate of 65 and 21 individuals after 600 d, respectively (Fig. 5). At 27, 30, and 33 °C, population growth followed a straight line after 350, 400, and 430 d, respectively, showing that these populations were approaching a stable stage distribution at the three temperatures (Fig. 6).
Figure 5. Population projection (total population size) of R. ferrugineus reared on sugarcane at different constant temperatures.
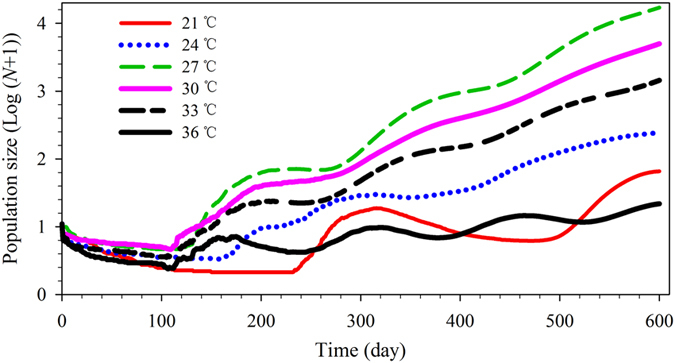
Population projection started with 10 eggs of R. ferrugineus. The total population quantities of R. ferrugineus were assessed at different times, which reflected population growth potential under different constant temperatures.
Figure 6. Population projection (population stage size) of R. ferrugineus reared on sugarcane at different constant temperatures.
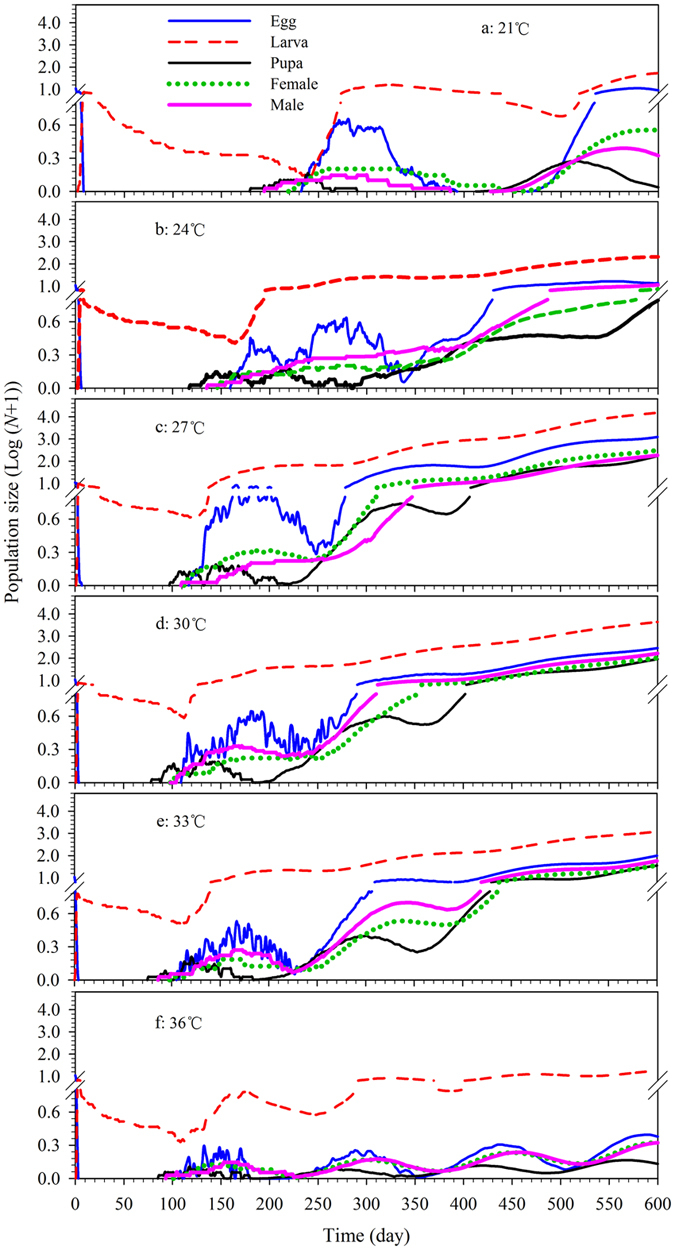
Population projection started with 10 eggs of R. ferrugineus. The population dynamics of R. ferrugineus in different stages were assessed at different times, which reflected the stable stage distribution under different constant temperatures.
Discussion
In ectothermic organisms such as insects, temperature is one of the most important environmental factors that regulate survival, development, reproduction, and seasonal occurrence24,25,26. Insects have an optimal temperature range for population growth and show significant restrictions on development at temperatures above or below the preferred range27,28. This temperature response has been found here in R. ferrugineus, where we found that R. ferrugineus populations survived under constant temperature conditions in the range 21 to 36 °C. The optimal temperature was 27 °C, which is consistent with the principal distributions of R. ferrugineus in China. We also found that R. ferrugineus was unable to complete development at 15 and 18 °C; this observation is consistent with the report by Li et al.14 that this species cannot complete development and reproduction at 16 °C (or 40 °C) on sugarcane and that the threshold temperature for egg hatching is 18.28 °C. Similarly, Zhao and Ju29 reported a survival rate for the generation of R. ferrugineus of only 10.0% at 19 °C. However, there are some contradictory data from other studies: Dembilio & Jacas5 and Dembilio et al.12 found that the threshold temperature for egg hatching was less than 14 °C on apple slices. These apparently contradictory differences may be attributable to geographical conditions, host plants, or other factors26,30.
In the present study, the durations of the immature stages of R. ferrugineus were temperature-dependent: the pre-adult stage fell from 237.8 ± 5.9 d to 131.8 ± 6.5 d as the temperature increased from 21 to 36 °C. These results are similar to those reported by Li et al.14 and Al-Nujiban et al.31, but are higher than the estimate obtained by Zhao & Ju29. These differences may be attributable to the different host plants31,32,33. Many studies have confirmed that the longest period for adult emergence occurs on sugarcane compared to date palm cultivars31,33. In addition, the prolongation of development times with decreasing temperatures may be due to the reduction in insect metabolism at lower temperatures, which has been reported for many insects including Octodonta nipae18, Lemnia biplagiata34, Bradysia odoriphaga24, and Corythucha ciliata35.
In insects, temperature is one of the most important determinants of reproduction to maintain populations36. This is especially the case for insect species that typically produce most offspring at an early age and have no parental care, such as Callosobruchus chinensis37, Cylas formicarius elegantulus38, and Drosophila melanogaster39. Our study showed the highest level of female fecundity was observed at 27 °C; fecundity at this temperature was significantly higher than at 21 and 36 °C. This trend in temperature-dependent fecundity has already been reported in R. ferrugineus14,29, and has also been found in Ophraella communa27, Anabrus simplex40, and Bradysia odoriphaga24. Some researchers argue that lower metabolic efficiency and more rapid energy consumption at low and high temperatures lead to a lower energy allocation to reproduction41. Thus, to ensure high-quality egg production, insects likely need to reduce their fecundity due to physiological trade-offs42. Fand et al.26 also reported reduced longevities in adults at high temperatures and a consequent shortening of the reproductive phase with decreased oviposition. Additionally, temperatures deviating from the normal range can alter reproductive physiology causing anomalous gonad development43, endocrine dyscrasia44, and abnormal sperm maturation and transfer45.
In general, the longevity of adult R. ferrugineus decreased with increasing temperature. This observation is consistent with the report by Zhao and Ju29 and in other insect species. We found a maximum female longevity at 21 °C and a decline to a minimum at 36 °C. Male longevity also sharply declined at 36 °C and was lower than that of female longevity. Sexual dimorphism in lifespan might result from sex-specific selection due to fundamental differences in how males and females optimize their fitness by allocating resources into current and future reproduction45,46. The reproductive strategies of males are more complicated than those of females and may involve higher energy requirements for successful mating (e.g., search for mates, competition, and courtship). As a result, limited energy resource availability at higher temperatures may make it necessary to sacrifice longevity for successful mating behaviour in males45,46. Liao et al.47 suggested that high temperatures suppress mating frequency and sperm transfer, indicating that substantial energy is required by the male for successful fecundity, resulting in an increased risk of death. In addition, many studies, especially in Drosophila, have found that the expression of genes affecting adult lifespan is temperature-dependent and that their relative sensitivity differs between the sexes48.
The curves for sxj, fxj, exj, and vxj, which take into account the variable development rates, provide a comprehensive reflection of population dynamics21. Our results showed the occurrence time of the maximum survive rate of R. ferrugineus in the specific stage and the fecundity reach to the maximum at a specific age under different temperature regimes (Figs 1 and 2). These data will be of value for choosing the optimum time and strategy for pest control49,50. In addition, we found that both low and high temperatures have negative effects on the contribution of individuals of R. ferrugineus to the future population (Figs 3 and 4); the life expectancies of newborn weevils (e01) were relatively low at 21 and 36 °C, respectively (Fig. 3a–f). Similarly, the peak of vxj was lowest (61.0 eggs) and occurred latest (232 d) at 21 °C, however, the peak value came earlier as temperatures increasing, but fell to a minimum of 61.7 eggs at 36 °C; this tendency is consistent with the report by Li et al.25, which is an important feature for evaluating the population growth potential20,21.
In this study, the intrinsic rate of increase (r), the finite rate of increase (λ), and the net reproductive rate (R0) of R. ferrugineus were at their highest at 27 °C (Table 2), which indicate that R. ferrugineus numbers may increase most rapidly at 27 °C. Interestingly, the emergence peaks of R. ferrugineus occur from May to June and from September to October in Fuzhou (Fujian), where the experimental population was initially collected, when the average temperature is about 27 °C; thus, our results may provide an important reference for risk assessment and management of R. ferrugineus. However, the population parameters were slightly lower than those reported by Li et al.14 and Zhao & Ju29 at the corresponding temperature, which might be attributable to differences in nutritional conditions or geographical populations26,30.
The fastest growing R. ferrugineus populations appeared at 27 and 30 °C, which may explain the suitability of Fujian province, and possibly the whole of subtropical and tropical zones, for R. ferrugineus (Fig. 5). In addition, change of stage structure during population growth can also be identified by population projection; stage structure is important for pest management because the dispersal and damage capability of the insects vary with stage21,25. As shown in Fig. 6, the R. ferrugineus populations reach the “stable age” or “stable age-stage” distribution after 350, 400, and 430 d, at 27, 30, and 33 °C, respectively. Furthermore, population projection offers a comprehensive understanding of the age and stage composition of a population during its growth. A population projection based on an age-stage, two-sex life table offers a comprehensive understanding of the age and stage composition of a population during its growth21,25.
In summary, we found that the growth rate and potential of R. ferrugineus populations was highest at 27 °C in an artificial environment. Using the age- and stage-specific life table approach, our study firstly simulated the population dynamics and evaluated the population growth potential of R. ferrugineus under different temperatures; this provide a vital foundation for determining the correct timing and strategies of chemical and biological control activities51, which often target pests at a specific age-stage, and for carrying out risk assessments. Here, we performed an experiment based only on the effects of temperature, a key abiotic factor for survival, development, and reproduction. Other environmental factors such as humidity, light, and rainfall that can influence pest population sizes were not considered. Humidity, for example, is especially important for rain-driven pests like Apolygus lucorum52 and Lygus lineolaris53. Although we speculate that temperature is the dominant abiotic factor affecting R. ferrugineus, an overall analysis of R. ferrugineus population dynamics needs to take a more full consideration of other environmental effects. Therefore, further studies are required to extend our results either by adding different abiotic factors or even applying abiotic-biotic interactions for R. ferrugineus based on temperature-dependent phenology.
Materials and Methods
Insect rearing
The R. ferrugineus adults used here were derived from insects collected in 2008 from a Canary Island date palm (Phoenix canariensis Hort. ex Chabaud) on the campus of Fujian Agriculture and Forestry University (FAFU), Fujian, China. A colony of the red palm weevils was maintained at 25 ± 0.5 °C and 75 ± 5% relative humidity with a 12 h light/ 12 h dark schedule in a growth chamber (PRX-250B-30, Haishu Saifu Experimental Instrument Factory, Ningbo, China) in our laboratory. As described previously54, the weevils were reared on fresh sugarcane stem tissues in clean plastic bottles (70 mm diameter, 105 mm height; Jiafeng Horticultural Products Co. Ltd., Shanghai, China) with moist filter paper to maintain humidity. The bottle neck was covered with fine mesh gauze to allow air ventilation. All sugarcane materials were bought from the fruit shop on campus. Every 2 d, the bottles were cleaned and fresh sugarcanes were added as necessary. To obtain a large breeding population, five pairs of R. ferrugineus adults per bottle and a total of 100 bottles were used to initiate the colony, and insects from the field populations were added to the laboratory colony every six months to maintain high genetic diversity. After two generations, the offspring were used for experimental studies.
Constant temperature experiment
The effects of different temperatures on population growth were tested by collecting newly-laid eggs from the laboratory colony and incubating them on petri dishes in growth chambers running at eight different temperatures (15, 18, 21, 24, 27, 30, 33, and 36 ± 0.5 °C); humidity and day length conditions were as described above. Fifty eggs, laid within a 24 h period, were placed on filter paper in a Petri dish (9 cm diameter) with a moist cotton wick to maintain humidity. Each petri dish was considered as one replicate, and three replicates were used at each temperature. The development time and hatching rate of eggs at each temperature were recorded and the moist cotton wick was changed daily. Each hatched larva was transferred individually to a new plastic bottle as described above. Fresh sugarcanes cut into small pieces (35 mm × 35 mm × 20 mm) with knife were provided as food and changed every two days until the larvae pupated. The moist filter paper was checked daily and renewed as needed. Development time and survival individual number were recorded daily. Pupae were collected and kept individually in plastic bottles for emergence and sex determination.
After emergence, male and female pairs were placed in individual plastic bottles with a piece of sugarcane as food and egg laying substrate. The sugarcane was also changed every two days. The number of eggs laid was monitored daily and the longevity of the adults was recorded.
Statistical analysis
The data from the different temperatures were analysed using an age-stage and two-sex life table approach23. The life history parameters, including age stage-specific survival rate (sxj, the probability that a newly laid egg will survive to age x and stage j), age stage-specific fecundity (fx4, the mean fecundity of females at age x), age-specific survival rates (lx, the probability of a newly laid egg surviving to age x), age-specific fecundity (mx, the mean fecundity of individuals at age x), age-specific maternity (lxmx), age-stage life expectancy (exj), age-stage reproductive value (vxj) and the demographic parameters of intrinsic rate of increase (r), finite rate of increase (λ), net reproductive rate (R0), mean generation time (T) were estimated and calculated using the computer program TWOSEX-MSChart55 (http://140.20.197.173/Ecology/Download/Twosex-MSChart.rar, last accessed 25 June 2015), which is designed in Visual BASIC for the Windows operating system and is available at http://nhsbig.inhs.uiuc.edu/wes/chi.html (Illinois Natural History Survey, Champaign-Urbana, IL). For population demographic variables, the bootstrap technique included in the TWOSEX-MSChart programme was used to estimate the means and standard errors for development time, longevity, adult preoviposition period (APOP), total preoviposition period (TPOP), oviposition days, fecundity, and the population parameters (r, λ, R0, and T)21,56,57 with 100,000 bootstrap replicates (B = 100,000). Differences among the different temperatures were compared by paired bootstrap tests based on the confidence interval of the difference between means21,56,57.
Projections of population growth of R. ferrugineus at different temperatures were based on the age-stage, two-sex life table theory23,51 and obtained using the TIMING-MSChart programme55. For comparison, an initial population of 10 eggs was used for the simulation at each temperature.
Additional Information
How to cite this article: Peng, L. et al. Demographic comparison and population projection of Rhynchophorus ferrugineus (Coleoptera: Curculionidae) reared on sugarcane at different temperatures. Sci. Rep. 6, 31659; doi: 10.1038/srep31659 (2016).
Acknowledgments
We are very grateful to Prof. Hsin Chi for his helpful comments on earlier versions of this manuscript and supplying the software for the life table study. This work was supported by the National Natural Science Foundation of China (31470656 and 31401744) and the Natural Science Foundation of Fujian Province (2015J01088).
Footnotes
Author Contributions L.P., Y.M. and Y.H. wrote the main manuscript text and prepared Figures 1–6. All authors reviewed the manuscript.
References
- Giblin-Davis R. M., Faleiro J. R., Jacas J. A., Peña J. E. & Vidyasagar P. S. P. V. Biology and management of the red palm weevil, Rhynchophorus ferrugineus. In: Potential Invasive Pests of Agricultural Crops (ed Peña J. E.), 1–34. CAB International, University of Florida, USA (2013). [Google Scholar]
- CAB International. Invasive species compendium: Rhynchophorus ferrugineus (red palm weevil) (2016).
- Qin W. Q., Zhao H. & Han C. W. The working rule of Rhynchophorus ferrugineus and the control. J. Yunnan Trop. Crops Sci. Technol. 25, 29–30 (2002). [Google Scholar]
- Wang G. H., Zhang X., Hou Y. M. & Tang B. Z. Analysis of the population genetic structure of Rhynchophorus ferrugineus in Fujian, China, revealed by microsatellite loci and mitochondrial COI sequences. Entomol. Exp. Appl. 155, 28–38 (2015). [Google Scholar]
- Dembilio Ó. & Jacas J. A. Basic bio-ecological parameters of the invasive red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae), in Phoenix canariensis under Mediterranean climate. Bull. Entomol. Res. 101, 153–163 (2011). [DOI] [PubMed] [Google Scholar]
- Shi Z. H., Lin Y. T. & Hou Y. M. Mother-derived trans-generational immune priming in the red palm weevil, Rhynchophorus ferrugineus Olivier (Coleoptera, Dryophthoridae). Bull. Entomol. Res. 104, 742–750 (2014). [DOI] [PubMed] [Google Scholar]
- van Lenteren J. C., Bale J., Bigler F., Hokkanen H. M. T. & Loomans A. J. M. Assession risks of releasing exotic biological control agents of arthropod pests. Annu. Rev. Entomol. 51, 609–643 (2006). [DOI] [PubMed] [Google Scholar]
- Bale J. et al. Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Global Change Biol. 8, 1–16 (2002). [Google Scholar]
- Kroschel J. et al. Predicting climate-change-caused changes in global temperature on potato tuber moth Phthorimaea operculella (Zeller) distribution and abundance using phenology modeling and GIS mapping. Agric. For. Meteorol. 15, 228–241 (2013). [Google Scholar]
- Wilson L. T. & Barnett W. W. Degree-days: an aid in crop and pest management. Calif. Agr. 37, 4–7 (1983). [Google Scholar]
- Day R. K. & Knight J. D. Operational aspects of forecasting migrant insect pests. In: Insect Migration: Tracking Resources Through Space and Time (eds. Drake V. A. & Gatehouse A. G.), 323–334. Cambridge University, Cambridge (1995). [Google Scholar]
- Dembilio Ó., Tapia G. V., Téllez M. M. & Jacas J. A. Lower temperature thresholds for oviposition and egg hatching of the red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae), in a Mediterranean climate. Bull. Entomol. Res. 102, 97–102 (2012). [DOI] [PubMed] [Google Scholar]
- Salama H. S., Hamdy M. K. & El-Din M. M. The thermal constant for timing the emergence of the red palm weevil, Rhynchophorus ferrugineus (Oliv.) (Coleoptera, Curculionidae). J. Pest Sci. 75, 26–29 (2002). [Google Scholar]
- Li L. et al. Effect of temperature on the population growth of Rhynchophorus ferrugineus (Coleoptera: Curculionidae) on sugarcane. Environ. Entomol. 39, 999–1003 (2010). [DOI] [PubMed] [Google Scholar]
- Kaufman L. V. & Wright M. G. Life history, seasonal phenology and parasitoids of the Hawaiian endemic moth Udea stellate (Lepidoptera: Crambidae). Ann. Entomol. Soc. Am. 102, 104–111 (2009). [Google Scholar]
- Huang Y. B. & Chi H. Age-stage, two-sex life table of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a discussion on the problem of applying females age-specific life table to insect populations. Insect Sci. 19, 263–273 (2012). [Google Scholar]
- Kavousi A. et al. Demographic traits of Tetranychus urticae (Acari: Tetranychidae) on leaf discs and whole leaves. J. Econ. Entomol. 102, 595–601 (2009). [DOI] [PubMed] [Google Scholar]
- Hou Y. M. & Weng Z. Q. Temperature-dependent development and life table parameters of Octodonta nipae (Coleoptera: Chrysomelidae). Environ. Entomol. 39, 1676–1684 (2010). [DOI] [PubMed] [Google Scholar]
- Hou Y. M., Miao Y. X. & Zhang Z. Y. Study on life parameters of the invasive species Octodonta nipae (Coleoptera: Chrysomelidae) on different palm species, under laboratory conditions. J. Econ. Entomol. 107, 1486–1495 (2014). [DOI] [PubMed] [Google Scholar]
- Peng L. et al. Generation-based life table analysis reveals manifold effects of inbreeding on the population fitness in Plutella xylostella. Sci. Rep. 5, 12749 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy G. V. P. & Chi H. Demographic comparison of sweetpotato weevil reared on a major host, Ipomoea batatas, and an alternative host, I. triloba. Sci. Rep. 5, 11871 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo T. L. & Liu T. X. Biology, life table and predation of Feltiella acarisuga (Diptera: Cecidomyiidae) feeding on Tetranychus cinnabarinus eggs (Acari: Tetranchidae). Biol. Control 39, 418–426 (2006). [Google Scholar]
- Chi H. & Liu H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 24, 225–240 (1985). [Google Scholar]
- Li W. X. et al. Effects of temperature on the age-stage, two-sex life table of Bradysia odoriphaga (Diptera: Sciaridae). J. Econ. Entomol. 108, 126–134 (2015). [DOI] [PubMed] [Google Scholar]
- Akca I., Ayvaz T., Yazici E., Smith C. L. & Chi H. Demography and population projection of Aphis fabae (Hemiptera: Aphididae): with additional comments on life table research criteria. J. Econ. Entomol. 108, 1466–1478 (2015). [DOI] [PubMed] [Google Scholar]
- Fand B. B., Sul N. T., Bal S. K. & Minhas P. S. Temperature impacts the development and survival of common cutworm (Spodoptera litura): simulation and visualization of potential population growth in India under warmer temperatures through life cycle modelling and spatial mapping. PLoS ONE 10, e0124682 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z. S., Guo J. Y., Chen H. S. & Wan F. H. Effects of temperature on survival, development, longevity and fecundity of Ophraella communa (Coleoptera: Chrysomelidae), a biological control agent against invasive ragweed, Ambrosia artemisiifolia L. (Asterales: Asteraceae). Environ. Entomol. 39, 1021–1027 (2010). [DOI] [PubMed] [Google Scholar]
- Olson J. F., Eaton M., Kells S. A., Morin V. & Wang C. Cold tolerance of bed bugs and practical recommendations for control. J. Econ. Entomol. 106, 2433–2441 (2013). [DOI] [PubMed] [Google Scholar]
- Zhao M. & Ju R. T. Effects of temperature on the development and fecundity of experimental population of Rhynchophorus ferrugineus. Acta Phytophy. Sin. 37, 517–521 (2010). [Google Scholar]
- De Barro P. J., Liu S. S., Boykin L. M. & Dinsdale A. B. Bemisia tabaci: a statement of species status. Annu. Rev. Entomol. 56, 1–19 (2011). [DOI] [PubMed] [Google Scholar]
- Al-Nujiban A. A. et al. Effect of date palm cultivar on fecundity and development of Rhynchophorus ferrugineus. B. Insectol. 68, 199–206 (2015). [Google Scholar]
- Salama H. S., Zaki F. N. & Abdel-Razek A. S. Ecological and biological studies on the red palm weevil Rhynchophorus ferrugineus (Olivier). Arch. Phytopathol. Plant Prot. 42, 392–399 (2009). [Google Scholar]
- Shahina F., Salma J., Mehreen G., Bhatti M. I. & Tabassum K. A. Rearing of Rhynchophorus ferrugineus in laboratory and field conditions for carrying out various efficacy studies using EPNs. Pak. J. Nematol. 27, 219–228 (2009). [Google Scholar]
- Yu J. Z., Chi H. & Chen B. H. Comparison of the life tables and predation rates of Harmonia dimidiate (F.) (Coleoptera: Coccinellidae) fed on Aphis gossypii Glover (Hemiptera: Aphididae) at different temperatures. Biol. Control 64, 1–9 (2013). [Google Scholar]
- Ju R. T., Zhu H. Y., Gao L., Zhou X. H. & Li B. Increases in both temperature means and extremes likely facilitate invasive herbivore outbreaks. Sci. Rep. 5, 15715 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowitz S. A. & Fischer K. Opposing effects of heat stress on male versus female reproductive success in Bicyclus anynana butterflies. J. Therm. Biol. 36, 283–287 (2011). [Google Scholar]
- Harano T. Inbreeding depression in development, survival, and reproduction in the adzuki bean beetle (Callosobruchus chinensis). Ecol. Res. 26, 327–332 (2011). [Google Scholar]
- Kuriwada T., Kumano N., Shiromoto K. & Haraguchi D. Effect of mass rearing on life history traits and inbreeding depression in the sweet potato weevil (Coleoptera: Brentidae). J. Econ. Entomol. 103, 1144–1148 (2010). [DOI] [PubMed] [Google Scholar]
- Valtonen T. M., Roff D. A. & Rantala M. J. Analysis of the effects of early nutritional environment on inbreeding depression in Drosophila melanogaster. J. Evol. Biol. 24, 196–205 (2011). [DOI] [PubMed] [Google Scholar]
- Srygley R. B. Effects of temperature and moisture on Mormon cricket reproduction with implications for responses to climate change. J. Insect Physiol. 65, 57–62 (2014). [DOI] [PubMed] [Google Scholar]
- Geister T. L. et al. Effects of temperature on reproductive output, egg provisioning, juvenile hormone and vitellogenin titres in the butterfly Bicyclus anynana. J. Insect Physiol. 54, 1253–1260 (2008). [DOI] [PubMed] [Google Scholar]
- Berger D., Walters R. & Gotthard K. What limits insect fecundity? Body size and temperature-dependent egg maturation and oviposition in a butterfly. Funct. Ecol. 22, 523–529 (2008). [Google Scholar]
- Gilbert L. I. et al. Ecdysteroids regulate yolk protein uptake by Drosophila melanogaster oocytes. J. Insect Physiol. 44, 637–644 (1998). [DOI] [PubMed] [Google Scholar]
- Shen Y. et al. Physiological effect of mild thermal stress and its induction of gene expression in the common cutworm, Spodoptera litura. J. Insect Physiol. 61, 34–41 (2014). [DOI] [PubMed] [Google Scholar]
- Fox C. W. & Stillwell R. C. Environmental effects on sex differences in the genetic load for adult lifespan in a seed-feeding beetle. Heredity 103, 62–72 (2009). [DOI] [PubMed] [Google Scholar]
- Robinson M. R., Mar K. U. & Lummaa V. Senescence and age-specific trade-offs between reproduction and survival in female Asian elephants. Ecol. Lett. 15, 260–266 (2012). [DOI] [PubMed] [Google Scholar]
- Liao H. J., Qian Q. & Liu X. D. Heat shock suppresses mating and sperm transfer in the rice leaf folder Cnaphalocrocis medinalis. Bull. Entomol. Res. 104, 383–392 (2014). [DOI] [PubMed] [Google Scholar]
- Vieira C. et al. Genotype-environment interaction for quantitative trait loci affecting lifespan in Drosophila melanogaster. Genetics 154, 213–227 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiaboe K. K. M., Peterson A. T., Kairo M. T. K. & Roda A. L. Predicting the potential worldwide distribution of the red palm weevil Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) using ecological niche modeling. Fla. Entomol. 95, 659–673 (2012). [Google Scholar]
- Al-Dosary N. M. N., Al-Dobai S. & Faleiro J. M. Review on the management of red palm weevil Rhynchophorus ferrugineus Olivier in date palm Phoenix dactylifera L. Emir. J. Food Agric. 28, 34–44 (2016). [Google Scholar]
- Chi H. Timing of control based on the stage structure of pest populations: A simulation approach. J. Econ. Entomol. 83, 1143–1150 (1990). [Google Scholar]
- Lu Y. H. & Wu K. M. Effect of relative humidity on population growth of Apolygus lucorum (Heteroptera: Miridae). Appl. Entomol. Zool. 46, 421–427 (2011). [Google Scholar]
- Day W. H. The effect of rainfall on the abundance of tarnished plant bug nymphs [Lygus lineolaris (Palisot)] in alfalfa fields. Trans. Am. Entomol. Soc. 132, 445–450 (2006). [Google Scholar]
- Wang F. et al. Technique for rearing Rhynchophorus ferrugineus on sugarcane. Chinese Bull. Entomol. 46, 967–969 (2009). [Google Scholar]
- Chi H. TWOSEX-MSChart: A computer program for the age-stage, two-sex life table analysis (2015). (http://140.120.197.173/ Ecology/Download/TWOSEX-MSChart.zip) (accessed 25 June 2015).
- Efron B. & Tibshirani R. J. An Introduction to the Bootstrap. Chapman & Hall, New York (1993). [Google Scholar]
- Akköprü E. P., Atlıhan R., Okut H. & Chi H. Demographic assessment of plant cultivar resistance to insect pests: A case study of the dusky-veined walnut aphid (Hemiptera: Callaphididae) on five walnut cultivars. J. Econ. Entomol. 108, 378–387 (2015). [DOI] [PubMed] [Google Scholar]