The crystal structure of disodium hydrogen citrate sesquihydrate has been solved and refined using laboratory X-ray single-crystal diffraction data, and optimized using density functional techniques.
Keywords: crystal structure, density functional theory, citrate, sodium
Abstract
The crystal structure of disodium hydrogen citrate sesquihydrate, 2Na2 +·C6H6O7 2−·1.5H2O, has been solved and refined using laboratory X-ray single-crystal diffraction data, and optimized using density functional techniques. The asymmetric unit contains two independent hydrogen citrate anions, four sodium cations and three water molecules. The coordination polyhedra of the cations (three with a coordination number of six, one with seven) share edges to form isolated 8-rings. The un-ionized terminal carboxylic acid groups form very strong hydrogen bonds to non-coordinating O atoms, with O⋯O distances of 2.46 Å.
Chemical context
In the course of a systematic study of the crystal structures of Group 1 (alkali metal) citrate salts to understand the anion’s conformational flexibility, ionization, coordination tendencies, and hydrogen bonding, we have determined several new crystal structures. Most of the new structures were solved using powder diffraction data (laboratory and/or synchrotron), but single crystals were used where available. The general trends and conclusions about the 16 new compounds and 12 previously characterized structures are being reported separately (Rammohan & Kaduk, 2016a
▸). Four of the new structures – NaKHC6H5O7, NaK2C6H5O7, Na3C6H5O7, and a second polymorph of NaH2C6H5O7 – have been published recently (Rammohan & Kaduk, 2016b
▸,c
▸,d
▸,e
▸) and two additional structures – KH2C6H5O7 and KH2C6H5O7(H2O)2 – have been communicated to the CSD (Kaduk & Stern, 2016a
▸,b
▸).
Structural commentary
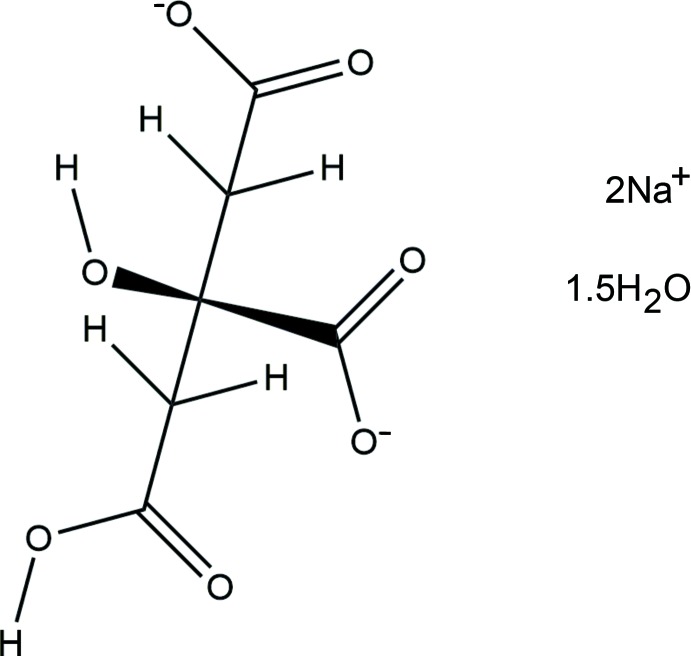
The asymmetric unit of the title compound is shown in Fig. 1 ▸. The root-mean-square deviation of the non-hydrogen atoms in the refined and DFT-optimized structures is only 0.048 Å. The excellent agreement between the two structures (Fig. 2 ▸) is strong evidence that the experimental structure is correct (van de Streek & Neumann, 2014 ▸). This discussion uses the DFT-optimized structure. Almost all of the bond lengths, bond angles, and torsion angles fall within the normal ranges indicated by a Mercury Mogul geometry check (Macrae et al., 2008 ▸). Only the C3—O13 bond length [observed = 1.416 (2), optimized = 1.410, Mogul average = 1.445 (11) Å, Z-score = 3.3] and the C2—C3—C4—C5 torsion angle [observed = −55.7 (1), optimized = −50.6°] are flagged as unusual. The standard deviation on the Mogul average for the C3—O13 distance is exceptionally low, resulting in the elevated Z-score. The C2—C3—C4—C5 torsion angle lies in the tail of a minority gauche conformation. None of the experimental quantities are flagged as unusual.
Figure 1.
The asymmetric unit of the DFT-optimized structure, with the atom numbering. The atoms are represented by 50% probability spheroids.
Figure 2.
Comparison of the refined and optimized structures of disodium hydrogen citrate sesquihydrate. The refined structure is in red, and the DFT-optimized structure is in blue.
The two independent citrate ions in the optimized structure are very similar; the root-mean-square displacement of the non-hydrogen atoms is 0.10 Å. Both anions occur in a gauche,trans conformation, which is one of the two low-energy conformations of an isolated citrate. The central carboxylate and hydroxyl groups are in the normal planar arrangement. The central and one terminal carboxylate groups in each hydrogen citrate anion are deprotonated. Both citrates chelate to Na2 atom through the end carboxylate atom O8, the central carboxylate atom O10, and the hydroxyl group O13.
The four independent Na1, Na2, Na3, and Na4 cations are 6-, 7-, 6-, and 6-coordinate. The 6-coordinate Na+ cations are in an approximately octahedral environment. The bond-valence sums are 1.12, 1.26, 1.16, and 1.20, respectively. Only the oxygen atoms O12 and O12A do not coordinate to an Na atom; these are part of central carboxylate groups, and the Na—O distances are very long at 2.76 Å. There are one, one, one, and three water molecules in the coordination spheres of atoms Na1, Na2, Na3, and Na4.
Supramolecular features
The [NaOx coordination polyhedra (x = 6, 7) share edges to form 8-ring units (Fig. 3 ▸), which are isolated from each other in the crystal structure (Fig. 4 ▸).
Figure 3.
The 8-rings formed by edge sharing of the Na coordination polyhedra.
Figure 4.
The crystal structure of Na2HC6H5O7(H2O)1.5, viewed down the a axis.
The OH functions of the carboxy groups, O7—H19 and O17A—H19A, form very strong hydrogen bonds to the non-coordinating atoms O12A and O12, respectively (Table 1 ▸). The experimental donor–hydrogen distances are significantly longer than the DFT-optimized ones. The refined O7—H19 and O7A—H19A distances are both 1.20 (3) Å, and the optimized distances are both 1.079 Å. The other hydrogen bonds participate in a variety of rings.
Table 1. Hydrogen-bond geometry (Å, °) .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O7A—H19A⋯O12 | 1.079 | 1.393 | 2.465 | 171.1 |
| O7—H19⋯O12A | 1.079 | 1.382 | 2.456 | 172.5 |
| O13A—H16A⋯O11A | 0.986 | 1.725 | 2.698 | 168.3 |
| O13—H16⋯O11 | 0.987 | 1.760 | 2.743 | 173.4 |
| O1W—H1W⋯O10 | 0.988 | 1.806 | 2.772 | 165.0 |
| O3W—H5W⋯O12A | 0.981 | 1.751 | 2.714 | 165.9 |
| O3W—H6W⋯O9A | 0.979 | 1.945 | 2.881 | 159.0 |
| O1W—H2W⋯O10A | 0.980 | 2.122 | 3.067 | 161.4 |
| O2W—H4W⋯O12 | 0.971 | 2.171 | 2.877 | 128.5 |
| O2W—H3W⋯O8 | 0.972 | 2.146 | 2.946 | 138.6 |
| O2W—H3W⋯O1W | 0.972 | 2.503 | 3.166 | 125.3 |
Database survey
Details of the comprehensive literature search for citrate structures are presented in Rammohan & Kaduk (2016a ▸). The observed powder pattern matched that of Na2HC6H5O7(H2O)2 in PDF entry 00-016-1182 (de Wolff et al., 1966 ▸) A reduced-cell search in the Cambridge Structural Database (Groom et al., 2016 ▸) yielded 104 hits, but limiting the chemistry to C, H, Na, and O only resulted in no hits.
Synthesis and crystallization
The sample was purchased from Sigma–Aldrich (lot #BCBC6031V). Single crystals were isolated from the as-received material.
Refinement details
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. All hydrogen-atom parameters were refined.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | 2Na2 +·C6H6O7 2−·1.5H2O |
| M r | 263.11 |
| Crystal system, space group | Triclinic, P

|
| Temperature (K) | 100 |
| a, b, c (Å) | 8.6713 (3), 10.6475 (4), 10.9961 (4) |
| α, β, γ (°) | 68.461 (1), 79.617 (2), 81.799 (2) |
| V (Å3) | 925.63 (6) |
| Z | 4 |
| Radiation type | Cu Kα |
| μ (mm−1) | 2.34 |
| Crystal size (mm) | 0.24 × 0.14 × 0.06 |
| Data collection | |
| Diffractometer | Bruker Kappa APEX CCD area detector |
| Absorption correction | Multi-scan (SADABS; Bruker, 2006 ▸) |
| T min, T max | 0.652, 0.753 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 9177, 3235, 3137 |
| R int | 0.021 |
| (sin θ/λ)max (Å−1) | 0.599 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.026, 0.070, 1.10 |
| No. of reflections | 3235 |
| No. of parameters | 370 |
| H-atom treatment | All H-atom parameters refined |
| Δρmax, Δρmin (e Å−3) | 0.36, −0.31 |
DFT Calculations
A density functional geometry optimization (fixed experimental unit cell) was carried out using CRYSTAL09 (Dovesi et al., 2005 ▸). The basis sets for the C, H, and O atoms were those of Gatti et al. (1994 ▸), and the basis set for Na was that of Dovesi et al. (1991 ▸). The calculation used 8 k-points and the B3LYP functional, and took about 10 days on a 2.4 GHz PC. U iso values were assigned to the optimized fractional coordinates based on the U eq values from the refined structure.
Supplementary Material
Crystal structure: contains datablock(s) na2c, na2c_DFT. DOI: 10.1107/S2056989016009014/vn2112sup1.cif
Structure factors: contains datablock(s) na2c. DOI: 10.1107/S2056989016009014/vn2112na2csup2.hkl
Structure factors: contains datablock(s) na2c_DFT. DOI: 10.1107/S2056989016009014/vn2112na2c_DFTsup3.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
(na2c) Disodium hydrogen citrate sesquihydrate. Crystal data
| 2Na+·C6H6O72−·1.5H2O | Z = 4 |
| Mr = 263.11 | F(000) = 540 |
| Triclinic, P1 | Dx = 1.888 Mg m−3 |
| a = 8.6713 (3) Å | Cu Kα radiation, λ = 1.54184 Å |
| b = 10.6475 (4) Å | Cell parameters from 7113 reflections |
| c = 10.9961 (4) Å | θ = 4.4–67.1° |
| α = 68.461 (1)° | µ = 2.34 mm−1 |
| β = 79.617 (2)° | T = 100 K |
| γ = 81.799 (2)° | Rod, colourless |
| V = 925.63 (6) Å3 | 0.24 × 0.14 × 0.06 mm |
(na2c) Disodium hydrogen citrate sesquihydrate. Data collection
| Bruker Kappa APEX CCD area detector diffractometer | 3235 independent reflections |
| Radiation source: microsource | 3137 reflections with I > 2σ(I) |
| MX optics monochromator | Rint = 0.021 |
| Detector resolution: 8 pixels mm-1 | θmax = 67.6°, θmin = 4.4° |
| ω and φ scans | h = −7→10 |
| Absorption correction: multi-scan (SADABS; Bruker, 2006) | k = −12→12 |
| Tmin = 0.652, Tmax = 0.753 | l = −13→12 |
| 9177 measured reflections |
(na2c) Disodium hydrogen citrate sesquihydrate. Refinement
| Refinement on F2 | Primary atom site location: dual |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.026 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.070 | All H-atom parameters refined |
| S = 1.10 | w = 1/[σ2(Fo2) + (0.0351P)2 + 0.4756P] where P = (Fo2 + 2Fc2)/3 |
| 3235 reflections | (Δ/σ)max = 0.002 |
| 370 parameters | Δρmax = 0.36 e Å−3 |
| 0 restraints | Δρmin = −0.31 e Å−3 |
(na2c) Disodium hydrogen citrate sesquihydrate. Special details
| Experimental. SADABS (Bruker,2006) was used for absorption correction. R(int) was 0.0787 before and 0.0318 after correction. The Ratio of minimum to maximum transmission is 0.8655. The λ/2 correction factor is 0.0015. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2sigma(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
(na2c) Disodium hydrogen citrate sesquihydrate. Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Na1 | −0.36905 (6) | 0.67174 (5) | 1.09406 (5) | 0.01145 (13) | |
| Na2 | −0.26692 (6) | 0.20337 (5) | 0.79550 (5) | 0.01118 (14) | |
| Na3 | 0.08801 (6) | 1.08405 (5) | 0.67885 (5) | 0.01112 (13) | |
| Na4 | 0.34928 (6) | 0.86038 (5) | 0.85665 (5) | 0.01460 (14) | |
| O1W | 0.26747 (14) | 0.99605 (11) | 0.99770 (10) | 0.0155 (2) | |
| H1W | 0.333 (3) | 1.053 (3) | 0.943 (3) | 0.047 (7)* | |
| H2W | 0.181 (3) | 1.034 (2) | 0.983 (2) | 0.037 (6)* | |
| O2W | 0.62348 (12) | 0.82465 (11) | 0.87230 (11) | 0.0161 (2) | |
| H3W | 0.679 (3) | 0.886 (3) | 0.849 (3) | 0.047 (7)* | |
| H4W | 0.647 (4) | 0.789 (3) | 0.814 (3) | 0.067 (9)* | |
| O3W | 0.09169 (12) | 0.84523 (10) | 0.82906 (11) | 0.0134 (2) | |
| H5W | 0.065 (3) | 0.813 (2) | 0.779 (2) | 0.036 (6)* | |
| H6W | 0.029 (3) | 0.813 (2) | 0.901 (2) | 0.029 (5)* | |
| O7 | −0.16831 (11) | 0.95459 (10) | 0.52380 (9) | 0.0127 (2) | |
| O8 | −0.20033 (11) | 1.04882 (10) | 0.67830 (9) | 0.0123 (2) | |
| O9 | −0.63550 (11) | 1.06594 (9) | 0.68976 (9) | 0.0109 (2) | |
| O10 | −0.53711 (11) | 1.17365 (9) | 0.79545 (9) | 0.0107 (2) | |
| O11 | −0.46736 (11) | 1.50841 (9) | 0.30164 (9) | 0.0108 (2) | |
| O12 | −0.49461 (11) | 1.33279 (9) | 0.24551 (9) | 0.0114 (2) | |
| O13 | −0.36210 (11) | 1.32227 (10) | 0.57450 (9) | 0.0098 (2) | |
| H16 | −0.419 (2) | 1.378 (2) | 0.603 (2) | 0.023 (5)* | |
| C1 | −0.23366 (15) | 1.04395 (13) | 0.57558 (13) | 0.0091 (3) | |
| C2 | −0.35405 (16) | 1.14161 (13) | 0.49599 (13) | 0.0092 (3) | |
| H14 | −0.413 (2) | 1.0882 (17) | 0.4733 (16) | 0.009 (4)* | |
| H15 | −0.291 (2) | 1.1960 (18) | 0.4148 (18) | 0.015 (4)* | |
| C3 | −0.46014 (15) | 1.23514 (13) | 0.55909 (13) | 0.0087 (3) | |
| C4 | −0.58579 (15) | 1.31651 (13) | 0.46879 (13) | 0.0094 (3) | |
| H17 | −0.648 (2) | 1.3845 (17) | 0.5050 (17) | 0.011 (4)* | |
| H18 | −0.6557 (19) | 1.2561 (16) | 0.4648 (15) | 0.006 (4)* | |
| C5 | −0.51150 (15) | 1.39448 (13) | 0.33037 (13) | 0.0090 (3) | |
| C6 | −0.55179 (15) | 1.15103 (13) | 0.69346 (13) | 0.0087 (3) | |
| O7A | −0.33313 (12) | 0.47238 (10) | 1.04866 (9) | 0.0137 (2) | |
| O8A | −0.32692 (11) | 0.32406 (9) | 0.94823 (9) | 0.0112 (2) | |
| O9A | 0.10082 (11) | 0.29981 (10) | 0.93250 (9) | 0.0125 (2) | |
| O10A | 0.00229 (11) | 0.19514 (9) | 0.82508 (10) | 0.0118 (2) | |
| O11A | −0.01900 (11) | 0.69477 (9) | 0.48444 (9) | 0.0112 (2) | |
| O12A | −0.01213 (11) | 0.75754 (9) | 0.65463 (9) | 0.0114 (2) | |
| O13A | −0.15562 (11) | 0.42424 (9) | 0.68263 (9) | 0.0099 (2) | |
| H16A | −0.101 (3) | 0.395 (2) | 0.626 (2) | 0.029 (5)* | |
| C1A | −0.27937 (15) | 0.42486 (13) | 0.95554 (13) | 0.0095 (3) | |
| C2A | −0.15323 (16) | 0.50579 (13) | 0.85584 (13) | 0.0099 (3) | |
| H14A | −0.088 (2) | 0.5291 (17) | 0.9052 (17) | 0.011 (4)* | |
| H15A | −0.209 (2) | 0.5897 (19) | 0.8023 (18) | 0.019 (4)* | |
| C3A | −0.05408 (15) | 0.43861 (13) | 0.76326 (13) | 0.0087 (3) | |
| C4A | 0.07926 (16) | 0.52892 (13) | 0.67715 (13) | 0.0097 (3) | |
| H17A | 0.138 (2) | 0.4872 (18) | 0.6159 (18) | 0.014 (4)* | |
| H18A | 0.148 (2) | 0.5361 (16) | 0.7318 (17) | 0.010 (4)* | |
| C5A | 0.01286 (15) | 0.66847 (13) | 0.59661 (13) | 0.0088 (3) | |
| C6A | 0.02354 (15) | 0.29878 (13) | 0.84637 (13) | 0.0091 (3) | |
| H19A | −0.415 (4) | 0.402 (3) | 1.143 (3) | 0.078 (10)* | |
| H19 | −0.090 (4) | 0.861 (3) | 0.591 (3) | 0.069 (9)* |
(na2c) Disodium hydrogen citrate sesquihydrate. Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Na1 | 0.0130 (3) | 0.0106 (3) | 0.0106 (3) | −0.0013 (2) | −0.0011 (2) | −0.0038 (2) |
| Na2 | 0.0106 (3) | 0.0124 (3) | 0.0117 (3) | −0.0022 (2) | −0.0012 (2) | −0.0053 (2) |
| Na3 | 0.0120 (3) | 0.0112 (3) | 0.0102 (3) | −0.0002 (2) | −0.0019 (2) | −0.0040 (2) |
| Na4 | 0.0160 (3) | 0.0117 (3) | 0.0137 (3) | −0.0042 (2) | −0.0051 (2) | 0.0009 (2) |
| O1W | 0.0162 (5) | 0.0143 (5) | 0.0132 (5) | −0.0023 (4) | −0.0003 (4) | −0.0020 (4) |
| O2W | 0.0163 (5) | 0.0145 (5) | 0.0150 (5) | −0.0029 (4) | −0.0018 (4) | −0.0021 (4) |
| O3W | 0.0150 (5) | 0.0154 (5) | 0.0105 (5) | −0.0042 (4) | −0.0025 (4) | −0.0040 (4) |
| O7 | 0.0153 (5) | 0.0116 (5) | 0.0123 (5) | 0.0038 (4) | −0.0041 (4) | −0.0064 (4) |
| O8 | 0.0144 (5) | 0.0127 (5) | 0.0108 (5) | 0.0010 (4) | −0.0039 (4) | −0.0052 (4) |
| O9 | 0.0118 (5) | 0.0101 (5) | 0.0105 (5) | −0.0037 (4) | −0.0024 (4) | −0.0018 (4) |
| O10 | 0.0120 (5) | 0.0118 (5) | 0.0088 (5) | −0.0023 (4) | −0.0003 (4) | −0.0043 (4) |
| O11 | 0.0120 (5) | 0.0091 (5) | 0.0105 (5) | −0.0018 (4) | −0.0007 (4) | −0.0025 (4) |
| O12 | 0.0146 (5) | 0.0112 (5) | 0.0088 (5) | −0.0031 (4) | −0.0003 (4) | −0.0035 (4) |
| O13 | 0.0094 (5) | 0.0096 (5) | 0.0117 (5) | −0.0024 (4) | −0.0008 (4) | −0.0050 (4) |
| C1 | 0.0082 (6) | 0.0084 (6) | 0.0094 (6) | −0.0032 (5) | 0.0011 (5) | −0.0018 (5) |
| C2 | 0.0100 (6) | 0.0096 (6) | 0.0085 (6) | −0.0012 (5) | −0.0018 (5) | −0.0033 (5) |
| C3 | 0.0083 (6) | 0.0081 (6) | 0.0099 (6) | −0.0011 (5) | −0.0017 (5) | −0.0031 (5) |
| C4 | 0.0088 (6) | 0.0092 (6) | 0.0089 (6) | −0.0011 (5) | −0.0006 (5) | −0.0020 (5) |
| C5 | 0.0059 (6) | 0.0105 (6) | 0.0095 (6) | 0.0019 (5) | −0.0025 (5) | −0.0025 (5) |
| C6 | 0.0064 (6) | 0.0077 (6) | 0.0103 (7) | 0.0026 (5) | −0.0020 (5) | −0.0021 (5) |
| O7A | 0.0178 (5) | 0.0129 (5) | 0.0108 (5) | −0.0048 (4) | 0.0044 (4) | −0.0063 (4) |
| O8A | 0.0139 (5) | 0.0090 (5) | 0.0102 (5) | −0.0024 (4) | −0.0004 (4) | −0.0029 (4) |
| O9A | 0.0122 (5) | 0.0140 (5) | 0.0111 (5) | −0.0017 (4) | −0.0043 (4) | −0.0027 (4) |
| O10A | 0.0128 (5) | 0.0098 (5) | 0.0136 (5) | −0.0006 (4) | −0.0014 (4) | −0.0051 (4) |
| O11A | 0.0126 (5) | 0.0116 (5) | 0.0085 (5) | −0.0005 (4) | −0.0013 (4) | −0.0026 (4) |
| O12A | 0.0148 (5) | 0.0090 (5) | 0.0111 (5) | −0.0003 (4) | −0.0034 (4) | −0.0037 (4) |
| O13A | 0.0096 (5) | 0.0124 (5) | 0.0090 (5) | −0.0007 (4) | −0.0022 (4) | −0.0049 (4) |
| C1A | 0.0101 (6) | 0.0088 (6) | 0.0083 (6) | 0.0018 (5) | −0.0032 (5) | −0.0017 (5) |
| C2A | 0.0105 (6) | 0.0097 (6) | 0.0094 (6) | −0.0016 (5) | −0.0003 (5) | −0.0035 (5) |
| C3A | 0.0085 (6) | 0.0090 (6) | 0.0088 (6) | −0.0012 (5) | −0.0022 (5) | −0.0029 (5) |
| C4A | 0.0089 (6) | 0.0096 (6) | 0.0098 (6) | −0.0004 (5) | −0.0010 (5) | −0.0027 (5) |
| C5A | 0.0053 (6) | 0.0101 (6) | 0.0102 (6) | −0.0033 (5) | 0.0019 (5) | −0.0029 (5) |
| C6A | 0.0063 (6) | 0.0111 (6) | 0.0077 (6) | −0.0012 (5) | 0.0024 (5) | −0.0021 (5) |
(na2c) Disodium hydrogen citrate sesquihydrate. Geometric parameters (Å, º)
| Na1—O2Wi | 2.3901 (11) | O10—Na2ix | 2.4085 (10) |
| Na1—O10ii | 2.3618 (11) | O10—C6 | 1.2612 (17) |
| Na1—O11iii | 2.4017 (10) | O11—Na1x | 2.4017 (10) |
| Na1—O7A | 2.3207 (11) | O11—C5 | 1.2351 (17) |
| Na1—O8Aiv | 2.7499 (11) | O12—Na4vii | 2.7435 (11) |
| Na1—O9Av | 2.3405 (11) | O12—C5 | 1.3027 (17) |
| Na2—O1Wv | 2.4765 (11) | O13—Na2ix | 2.5209 (11) |
| Na2—O8vi | 2.3917 (11) | O13—H16 | 0.83 (2) |
| Na2—O10vi | 2.4085 (10) | O13—C3 | 1.4158 (16) |
| Na2—O13vi | 2.5209 (11) | C1—C2 | 1.5127 (18) |
| Na2—O8A | 2.4128 (11) | C2—H14 | 0.940 (18) |
| Na2—O10A | 2.3995 (11) | C2—H15 | 0.982 (19) |
| Na2—O13A | 2.4647 (11) | C2—C3 | 1.5282 (18) |
| Na3—O3W | 2.4713 (11) | C3—C4 | 1.5533 (18) |
| Na3—O7vii | 2.3774 (11) | C3—C6 | 1.5572 (18) |
| Na3—O8 | 2.5820 (11) | C4—H17 | 0.997 (18) |
| Na3—O9viii | 2.3989 (10) | C4—H18 | 0.962 (17) |
| Na3—O10Aix | 2.2918 (11) | C4—C5 | 1.5139 (18) |
| Na3—O11Avii | 2.4512 (10) | O7A—C1A | 1.2896 (17) |
| Na4—O1W | 2.4397 (12) | O7A—H19A | 1.20 (3) |
| Na4—O2W | 2.3810 (12) | O8A—Na1iv | 2.7499 (11) |
| Na4—O3W | 2.3430 (11) | O8A—Na4v | 2.3157 (10) |
| Na4—O9viii | 2.2851 (10) | O8A—C1A | 1.2368 (17) |
| Na4—O12vii | 2.7435 (11) | O9A—Na1v | 2.3405 (11) |
| Na4—O8Av | 2.3157 (10) | O9A—C6A | 1.2588 (17) |
| O1W—Na2v | 2.4765 (11) | O10A—Na3vi | 2.2918 (11) |
| O1W—H1W | 0.87 (3) | O10A—C6A | 1.2528 (17) |
| O1W—H2W | 0.81 (3) | O11A—Na3vii | 2.4512 (10) |
| O2W—Na1viii | 2.3902 (11) | O11A—C5A | 1.2342 (17) |
| O2W—H3W | 0.80 (3) | O12A—C5A | 1.2997 (17) |
| O2W—H4W | 0.84 (3) | O12A—H19 | 1.25 (3) |
| O3W—H5W | 0.82 (3) | O13A—H16A | 0.84 (2) |
| O3W—H6W | 0.86 (2) | O13A—C3A | 1.4157 (16) |
| O7—Na3vii | 2.3773 (11) | C1A—C2A | 1.5112 (18) |
| O7—C1 | 1.2955 (17) | C2A—H14A | 0.960 (18) |
| O7—H19 | 1.20 (3) | C2A—H15A | 0.99 (2) |
| O8—Na2ix | 2.3917 (11) | C2A—C3A | 1.5268 (18) |
| O8—C1 | 1.2357 (17) | C3A—C4A | 1.5530 (18) |
| O9—Na3i | 2.3988 (10) | C3A—C6A | 1.5626 (18) |
| O9—Na4i | 2.2851 (10) | C4A—H17A | 0.971 (19) |
| O9—C6 | 1.2549 (17) | C4A—H18A | 0.946 (18) |
| O10—Na1ii | 2.3618 (11) | C4A—C5A | 1.5156 (18) |
| O2Wi—Na1—O11iii | 156.26 (4) | C5—O11—Na1x | 131.75 (9) |
| O2Wi—Na1—O8Aiv | 75.81 (4) | C5—O12—Na4vii | 152.04 (8) |
| O10ii—Na1—O2Wi | 98.54 (4) | Na2ix—O13—H16 | 90.8 (14) |
| O10ii—Na1—O11iii | 83.03 (4) | C3—O13—Na2ix | 106.96 (7) |
| O10ii—Na1—O8Aiv | 87.71 (3) | C3—O13—H16 | 108.1 (14) |
| O11iii—Na1—O8Aiv | 80.60 (3) | O7—C1—C2 | 112.53 (11) |
| O7A—Na1—O2Wi | 97.21 (4) | O8—C1—O7 | 123.49 (12) |
| O7A—Na1—O10ii | 159.74 (4) | O8—C1—C2 | 123.98 (12) |
| O7A—Na1—O11iii | 77.44 (4) | C1—C2—H14 | 105.8 (10) |
| O7A—Na1—O8Aiv | 83.87 (4) | C1—C2—H15 | 104.2 (11) |
| O7A—Na1—O9Av | 95.46 (4) | C1—C2—C3 | 117.38 (11) |
| O9Av—Na1—O2Wi | 88.70 (4) | H14—C2—H15 | 107.8 (14) |
| O9Av—Na1—O10ii | 97.56 (4) | C3—C2—H14 | 111.4 (10) |
| O9Av—Na1—O11iii | 114.69 (4) | C3—C2—H15 | 109.7 (10) |
| O9Av—Na1—O8Aiv | 164.25 (4) | O13—C3—C2 | 107.07 (10) |
| O8vi—Na2—O1Wv | 87.53 (4) | O13—C3—C4 | 111.32 (10) |
| O8vi—Na2—O10vi | 86.15 (4) | O13—C3—C6 | 111.84 (11) |
| O8vi—Na2—O13vi | 73.84 (4) | C2—C3—C4 | 109.53 (11) |
| O8vi—Na2—O8A | 169.75 (4) | C2—C3—C6 | 110.59 (10) |
| O8vi—Na2—O10A | 91.46 (4) | C4—C3—C6 | 106.52 (10) |
| O8vi—Na2—O13A | 114.69 (4) | C3—C4—H17 | 109.6 (10) |
| O10vi—Na2—O1Wv | 88.39 (4) | C3—C4—H18 | 109.9 (9) |
| O10vi—Na2—O13vi | 66.13 (3) | H17—C4—H18 | 109.3 (14) |
| O10vi—Na2—O8A | 94.93 (4) | C5—C4—C3 | 111.88 (11) |
| O10vi—Na2—O13A | 122.23 (4) | C5—C4—H17 | 106.8 (10) |
| O8A—Na2—O1Wv | 82.32 (4) | C5—C4—H18 | 109.3 (9) |
| O8A—Na2—O13vi | 115.91 (4) | O11—C5—O12 | 122.67 (12) |
| O8A—Na2—O13A | 73.28 (3) | O11—C5—C4 | 121.45 (12) |
| O10A—Na2—O1Wv | 81.90 (4) | O12—C5—C4 | 115.87 (11) |
| O10A—Na2—O10vi | 170.09 (4) | O9—C6—O10 | 125.63 (12) |
| O10A—Na2—O13vi | 122.36 (4) | O9—C6—C3 | 116.04 (11) |
| O10A—Na2—O8A | 85.75 (4) | O10—C6—C3 | 118.32 (11) |
| O10A—Na2—O13A | 67.43 (3) | C1A—O7A—Na1 | 142.00 (9) |
| O13A—Na2—O1Wv | 141.65 (4) | C1A—O7A—H19A | 117.2 (15) |
| O13A—Na2—O13vi | 69.45 (3) | Na2—O8A—Na1iv | 83.21 (3) |
| O3W—Na3—O8 | 82.77 (3) | Na4v—O8A—Na1iv | 94.23 (3) |
| O7vii—Na3—O3W | 98.24 (4) | Na4v—O8A—Na2 | 98.57 (4) |
| O7vii—Na3—O8 | 91.42 (4) | C1A—O8A—Na1iv | 120.62 (8) |
| O7vii—Na3—O9viii | 84.83 (4) | C1A—O8A—Na2 | 135.06 (9) |
| O7vii—Na3—O11Avii | 77.67 (4) | C1A—O8A—Na4v | 115.13 (8) |
| O9viii—Na3—O3W | 86.33 (4) | C6A—O9A—Na1v | 127.26 (9) |
| O9viii—Na3—O8 | 167.85 (4) | Na3vi—O10A—Na2 | 91.98 (4) |
| O9viii—Na3—O11Avii | 108.24 (4) | C6A—O10A—Na2 | 109.60 (8) |
| O10Aix—Na3—O3W | 101.16 (4) | C6A—O10A—Na3vi | 141.08 (9) |
| O10Aix—Na3—O7vii | 160.52 (4) | C5A—O11A—Na3vii | 128.77 (9) |
| O10Aix—Na3—O8 | 89.27 (4) | C5A—O12A—H19 | 111.2 (13) |
| O10Aix—Na3—O9viii | 98.11 (4) | Na2—O13A—H16A | 90.1 (14) |
| O10Aix—Na3—O11Avii | 83.15 (4) | C3A—O13A—Na2 | 108.01 (7) |
| O11Avii—Na3—O3W | 164.23 (4) | C3A—O13A—H16A | 108.3 (15) |
| O11Avii—Na3—O8 | 82.12 (3) | O7A—C1A—Na4v | 90.36 (8) |
| O1W—Na4—O12vii | 163.29 (4) | O7A—C1A—C2A | 112.97 (11) |
| O2W—Na4—O1W | 99.15 (4) | O8A—C1A—O7A | 123.45 (12) |
| O2W—Na4—O12vii | 68.41 (4) | O8A—C1A—C2A | 123.57 (12) |
| O3W—Na4—O1W | 94.02 (4) | C2A—C1A—Na4v | 143.09 (9) |
| O3W—Na4—O2W | 165.55 (5) | C1A—C2A—H14A | 106.4 (10) |
| O3W—Na4—O12vii | 97.48 (4) | C1A—C2A—H15A | 105.5 (11) |
| O9viii—Na4—O1W | 84.11 (4) | C1A—C2A—C3A | 116.50 (11) |
| O9viii—Na4—O2W | 95.14 (4) | H14A—C2A—H15A | 108.4 (15) |
| O9viii—Na4—O3W | 92.09 (4) | C3A—C2A—H14A | 111.1 (10) |
| O9viii—Na4—O12vii | 107.45 (4) | C3A—C2A—H15A | 108.6 (11) |
| O9viii—Na4—O8Av | 169.12 (4) | O13A—C3A—C2A | 107.23 (10) |
| O8Av—Na4—O1W | 85.14 (4) | O13A—C3A—C4A | 110.39 (10) |
| O8Av—Na4—O2W | 84.91 (4) | O13A—C3A—C6A | 111.81 (10) |
| O8Av—Na4—O3W | 90.34 (4) | C2A—C3A—C4A | 109.74 (11) |
| O8Av—Na4—O12vii | 82.72 (3) | C2A—C3A—C6A | 109.50 (11) |
| Na4—O1W—Na2v | 93.62 (4) | C4A—C3A—C6A | 108.16 (10) |
| Na4—O2W—Na1viii | 102.60 (4) | C3A—C4A—H17A | 108.9 (10) |
| Na4—O3W—Na3 | 88.36 (4) | C3A—C4A—H18A | 109.7 (10) |
| C1—O7—Na3vii | 144.02 (9) | H17A—C4A—H18A | 109.0 (14) |
| C1—O7—H19 | 116.4 (14) | C5A—C4A—C3A | 111.23 (11) |
| Na2ix—O8—Na3 | 85.37 (3) | C5A—C4A—H17A | 107.7 (10) |
| C1—O8—Na2ix | 135.12 (9) | C5A—C4A—H18A | 110.2 (10) |
| C1—O8—Na3 | 116.99 (8) | O11A—C5A—O12A | 122.21 (12) |
| Na4i—O9—Na3i | 91.51 (4) | O11A—C5A—C4A | 122.11 (12) |
| C6—O9—Na3i | 129.89 (8) | O12A—C5A—C4A | 115.66 (12) |
| C6—O9—Na4i | 119.94 (8) | O9A—C6A—C3A | 116.26 (11) |
| Na1ii—O10—Na2ix | 92.19 (4) | O10A—C6A—O9A | 125.12 (12) |
| C6—O10—Na1ii | 140.73 (8) | O10A—C6A—C3A | 118.60 (12) |
| C6—O10—Na2ix | 110.53 (8) | ||
| Na1iv—Na2—O8A—Na4v | 93.28 (4) | O8vi—Na2—O10A—C6A | 158.19 (9) |
| Na1iv—Na2—O8A—C1A | −127.35 (13) | O8vi—Na2—O13A—C3A | −117.77 (8) |
| Na1iv—Na2—O10A—Na3vi | −178.42 (3) | O8—Na3—O3W—Na4 | −175.84 (4) |
| Na1iv—Na2—O10A—C6A | −31.42 (10) | O8—C1—C2—C3 | −11.14 (19) |
| Na1iv—Na2—O13A—C3A | 103.13 (8) | O9viii—Na3—O3W—Na4 | 9.53 (4) |
| Na1viii—Na4—O1W—Na2v | −43.16 (3) | O9viii—Na3—O8—Na2ix | 138.10 (17) |
| Na1viii—Na4—O3W—Na3 | 161.99 (4) | O9viii—Na3—O8—C1 | −82.7 (2) |
| Na1ii—O10—C6—Na2ix | 121.50 (14) | O9viii—Na4—O1W—Na2v | −173.86 (4) |
| Na1ii—O10—C6—Na4i | −123.34 (11) | O9viii—Na4—O2W—Na1viii | 155.76 (5) |
| Na1ii—O10—C6—O9 | −99.84 (16) | O9viii—Na4—O3W—Na3 | −9.99 (4) |
| Na1ii—O10—C6—C3 | 79.80 (16) | O10ii—Na1—O7A—C1A | 165.62 (14) |
| Na1x—O11—C5—O12 | 8.17 (19) | O10vi—Na2—O8A—Na1iv | −10.14 (3) |
| Na1x—O11—C5—C4 | −173.27 (8) | O10vi—Na2—O8A—Na4v | 83.14 (4) |
| Na1—O7A—C1A—Na4v | 177.29 (11) | O10vi—Na2—O8A—C1A | −137.49 (12) |
| Na1—O7A—C1A—O8A | −152.86 (11) | O10vi—Na2—O13A—C3A | 140.54 (7) |
| Na1—O7A—C1A—C2A | 26.7 (2) | O11iii—Na1—O7A—C1A | −178.65 (15) |
| Na1iv—O8A—C1A—Na4v | 111.85 (10) | O12vii—Na4—O1W—Na2v | −39.06 (15) |
| Na1iv—O8A—C1A—O7A | 65.38 (15) | O12vii—Na4—O2W—Na1viii | −97.49 (5) |
| Na1iv—O8A—C1A—C2A | −114.13 (12) | O12vii—Na4—O3W—Na3 | −117.89 (4) |
| Na1v—O9A—C6A—O10A | 91.30 (15) | O13vi—Na2—O8A—Na1iv | 55.68 (4) |
| Na1v—O9A—C6A—C3A | −90.26 (13) | O13vi—Na2—O8A—Na4v | 148.96 (4) |
| Na2iv—Na1—O7A—C1A | 122.96 (14) | O13vi—Na2—O8A—C1A | −71.67 (13) |
| Na2ix—Na3—O3W—Na4 | −134.10 (3) | O13vi—Na2—O10A—Na3vi | −60.54 (5) |
| Na2ix—Na3—O8—C1 | 139.16 (10) | O13vi—Na2—O10A—C6A | 86.46 (9) |
| Na2v—Na4—O2W—Na1viii | 28.21 (4) | O13vi—Na2—O13A—C3A | −177.81 (8) |
| Na2v—Na4—O3W—Na3 | 117.97 (3) | O13—C3—C4—C5 | 62.49 (14) |
| Na2ix—O8—C1—O7 | 172.37 (9) | O13—C3—C6—Na2ix | −25.63 (9) |
| Na2ix—O8—C1—C2 | −7.3 (2) | O13—C3—C6—Na4i | −136.59 (11) |
| Na2ix—O10—C6—Na4i | 115.17 (5) | O13—C3—C6—O9 | −176.47 (11) |
| Na2ix—O10—C6—O9 | 138.67 (11) | O13—C3—C6—O10 | 3.86 (16) |
| Na2ix—O10—C6—C3 | −41.70 (13) | C1—C2—C3—O13 | 64.88 (14) |
| Na2ix—O13—C3—C2 | −88.15 (10) | C1—C2—C3—C4 | −174.29 (11) |
| Na2ix—O13—C3—C4 | 152.17 (8) | C1—C2—C3—C6 | −57.19 (15) |
| Na2ix—O13—C3—C6 | 33.14 (11) | C2—C3—C4—C5 | −55.72 (14) |
| Na2—O8A—C1A—Na4v | −134.67 (14) | C2—C3—C6—Na2ix | 93.60 (9) |
| Na2—O8A—C1A—O7A | 178.86 (9) | C2—C3—C6—Na4i | −17.36 (18) |
| Na2—O8A—C1A—C2A | −0.6 (2) | C2—C3—C6—O9 | −57.24 (15) |
| Na2—O10A—C6A—O9A | 136.65 (11) | C2—C3—C6—O10 | 123.09 (12) |
| Na2—O10A—C6A—C3A | −41.76 (13) | C3—C4—C5—O11 | −85.63 (15) |
| Na2—O13A—C3A—C2A | −89.61 (10) | C3—C4—C5—O12 | 93.01 (14) |
| Na2—O13A—C3A—C4A | 150.89 (8) | C4—C3—C6—Na2ix | −147.46 (9) |
| Na2—O13A—C3A—C6A | 30.44 (11) | C4—C3—C6—Na4i | 101.58 (14) |
| Na3vi—Na2—O8A—Na1iv | 178.64 (3) | C4—C3—C6—O9 | 61.70 (14) |
| Na3vi—Na2—O8A—Na4v | −88.08 (4) | C4—C3—C6—O10 | −117.96 (12) |
| Na3vi—Na2—O8A—C1A | 51.29 (13) | C6vi—Na2—O8A—Na1iv | −1.39 (4) |
| Na3vi—Na2—O10A—C6A | 147.00 (10) | C6vi—Na2—O8A—Na4v | 91.88 (4) |
| Na3vi—Na2—O13A—C3A | −78.33 (8) | C6vi—Na2—O8A—C1A | −128.75 (12) |
| Na3vii—O7—C1—O8 | −148.29 (11) | C6vi—Na2—O10A—Na3vi | 4.19 (12) |
| Na3vii—O7—C1—C2 | 31.4 (2) | C6vi—Na2—O10A—C6A | 151.19 (11) |
| Na3—O8—C1—O7 | 59.85 (15) | C6vi—Na2—O13A—C3A | 166.14 (7) |
| Na3—O8—C1—C2 | −119.78 (11) | C6viii—Na4—O1W—Na2v | −156.30 (5) |
| Na3i—O9—C6—Na2ix | 146.76 (11) | C6viii—Na4—O2W—Na1viii | 141.70 (5) |
| Na3i—O9—C6—Na4i | 121.40 (13) | C6viii—Na4—O3W—Na3 | 0.92 (5) |
| Na3i—O9—C6—O10 | 82.71 (16) | C6—C3—C4—C5 | −175.35 (11) |
| Na3i—O9—C6—C3 | −96.93 (12) | O7A—C1A—C2A—C3A | 164.94 (11) |
| Na3vi—O10A—C6A—O9A | −103.39 (16) | O8Aiv—Na1—O7A—C1A | 99.65 (15) |
| Na3vi—O10A—C6A—C3A | 78.20 (17) | O8A—Na2—O10A—Na3vi | −178.68 (4) |
| Na3vii—O11A—C5A—O12A | 12.16 (18) | O8A—Na2—O10A—C6A | −31.68 (9) |
| Na3vii—O11A—C5A—C4A | −169.68 (9) | O8A—Na2—O13A—C3A | 55.33 (8) |
| Na4i—Na1—O7A—C1A | 63.79 (15) | O8Av—Na4—O1W—Na2v | 4.45 (4) |
| Na4v—Na2—O8A—Na1iv | −93.28 (4) | O8Av—Na4—O2W—Na1viii | −13.32 (5) |
| Na4v—Na2—O8A—C1A | 139.37 (14) | O8Av—Na4—O3W—Na3 | 159.40 (4) |
| Na4v—Na2—O10A—Na3vi | 141.48 (3) | O8A—C1A—C2A—C3A | −15.50 (19) |
| Na4v—Na2—O10A—C6A | −71.52 (8) | O9Av—Na1—O7A—C1A | −64.53 (15) |
| Na4v—Na2—O13A—C3A | 38.47 (8) | O10A—Na2—O8A—Na1iv | 179.78 (3) |
| Na4i—O9—C6—Na2ix | 25.4 (2) | O10A—Na2—O8A—Na4v | −86.94 (4) |
| Na4i—O9—C6—O10 | −38.70 (17) | O10A—Na2—O8A—C1A | 52.43 (12) |
| Na4i—O9—C6—C3 | 141.66 (9) | O10A—Na2—O13A—C3A | −37.02 (7) |
| Na4vii—O12—C5—O11 | 105.06 (18) | O10Aix—Na3—O3W—Na4 | −88.02 (4) |
| Na4vii—O12—C5—C4 | −73.6 (2) | O10Aix—Na3—O8—Na2ix | 10.42 (4) |
| Na4v—O8A—C1A—O7A | −46.47 (16) | O10Aix—Na3—O8—C1 | 149.57 (9) |
| Na4v—O8A—C1A—C2A | 134.02 (11) | O11Avii—Na3—O3W—Na4 | 167.43 (13) |
| Na4v—C1A—C2A—C3A | 39.8 (2) | O11Avii—Na3—O8—Na2ix | −72.77 (3) |
| O1Wv—Na2—O8A—Na1iv | −97.84 (4) | O11Avii—Na3—O8—C1 | 66.39 (9) |
| O1Wv—Na2—O8A—Na4v | −4.56 (4) | O13A—Na2—O8A—Na1iv | 112.08 (3) |
| O1Wv—Na2—O8A—C1A | 134.81 (12) | O13A—Na2—O8A—Na4v | −154.64 (4) |
| O1Wv—Na2—O10A—Na3vi | 98.48 (4) | O13A—Na2—O8A—C1A | −15.27 (12) |
| O1Wv—Na2—O10A—C6A | −114.52 (9) | O13A—Na2—O10A—Na3vi | −105.03 (4) |
| O1Wv—Na2—O13A—C3A | 2.52 (11) | O13A—Na2—O10A—C6A | 41.97 (8) |
| O1W—Na4—O2W—Na1viii | 70.91 (5) | O13A—C3A—C4A—C5A | 59.20 (14) |
| O1W—Na4—O3W—Na3 | 74.25 (4) | O13A—C3A—C6A—O9A | −171.79 (11) |
| O2Wi—Na1—O7A—C1A | 24.84 (15) | O13A—C3A—C6A—O10A | 6.76 (16) |
| O2W—Na4—O1W—Na2v | −79.59 (4) | C1Av—Na4—O1W—Na2v | 16.94 (5) |
| O2W—Na4—O3W—Na3 | −130.03 (17) | C1Av—Na4—O2W—Na1viii | −32.71 (5) |
| O3W—Na3—O8—Na2ix | 111.76 (4) | C1Av—Na4—O3W—Na3 | 176.38 (4) |
| O3W—Na3—O8—C1 | −109.08 (10) | C1A—C2A—C3A—O13A | 65.52 (14) |
| O3W—Na4—O1W—Na2v | 94.45 (4) | C1A—C2A—C3A—C4A | −174.55 (11) |
| O3W—Na4—O2W—Na1viii | −84.54 (18) | C1A—C2A—C3A—C6A | −55.98 (15) |
| O7vii—Na3—O3W—Na4 | 93.76 (4) | C2A—C3A—C4A—C5A | −58.78 (14) |
| O7vii—Na3—O8—Na2ix | −150.11 (4) | C2A—C3A—C6A—O9A | −53.09 (15) |
| O7vii—Na3—O8—C1 | −10.96 (9) | C2A—C3A—C6A—O10A | 125.46 (12) |
| O7—C1—C2—C3 | 169.19 (11) | C3A—C4A—C5A—O11A | −90.55 (15) |
| O8vi—Na2—O8A—Na1iv | −105.8 (2) | C3A—C4A—C5A—O12A | 87.73 (14) |
| O8vi—Na2—O8A—Na4v | −12.5 (2) | C4A—C3A—C6A—O9A | 66.46 (14) |
| O8vi—Na2—O8A—C1A | 126.9 (2) | C4A—C3A—C6A—O10A | −114.99 (13) |
| O8vi—Na2—O10A—Na3vi | 11.19 (4) | C6A—C3A—C4A—C5A | −178.18 (11) |
Symmetry codes: (i) x−1, y, z; (ii) −x−1, −y+2, −z+2; (iii) x, y−1, z+1; (iv) −x−1, −y+1, −z+2; (v) −x, −y+1, −z+2; (vi) x, y−1, z; (vii) −x, −y+2, −z+1; (viii) x+1, y, z; (ix) x, y+1, z; (x) x, y+1, z−1.
(na2c_DFT). Crystal data
| C12H12Na4O14(H2O)3 | α = 68.4610° |
| Mr = 526.22 | β = 79.6170° |
| Triclinic, P1 | γ = 81.7990° |
| a = 8.6713 Å | V = 925.63 Å3 |
| b = 10.6475 Å | Z = 2 |
| c = 10.9961 Å | T = 100 K |
(na2c_DFT). Data collection
| h = → | l = → |
| k = → |
(na2c_DFT). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Na1 | −0.37343 | 0.67657 | 1.09352 | 0.01145* | |
| Na2 | −0.26397 | 0.20115 | 0.79858 | 0.01118* | |
| Na3 | 0.08732 | 1.08255 | 0.68250 | 0.01112* | |
| Na4 | 0.34457 | 0.86390 | 0.85431 | 0.01460* | |
| O7 | −0.16782 | 0.95531 | 0.52502 | 0.01270* | |
| O8 | −0.20053 | 1.04584 | 0.68290 | 0.01230* | |
| O9 | −0.64112 | 1.06940 | 0.68737 | 0.01090* | |
| O10 | −0.53712 | 1.16842 | 0.79941 | 0.01070* | |
| O11 | −0.47290 | 1.51265 | 0.29363 | 0.01080* | |
| O12 | −0.49766 | 1.32881 | 0.24827 | 0.01140* | |
| O13 | −0.36072 | 1.31975 | 0.58091 | 0.00980* | |
| H16 | −0.42399 | 1.38378 | 0.62003 | 0.02300* | |
| C1 | −0.23465 | 1.04470 | 0.57867 | 0.00910* | |
| C2 | −0.35390 | 1.14280 | 0.49792 | 0.00920* | |
| H14 | −0.42355 | 1.08420 | 0.46821 | 0.00900* | |
| H15 | −0.28636 | 1.20526 | 0.40698 | 0.01500* | |
| C3 | −0.46003 | 1.23681 | 0.56155 | 0.00870* | |
| C4 | −0.58433 | 1.32299 | 0.47061 | 0.00940* | |
| H17 | −0.64219 | 1.39939 | 0.51186 | 0.01100* | |
| H18 | −0.67129 | 1.25721 | 0.47345 | 0.00600* | |
| C5 | −0.51420 | 1.39486 | 0.32859 | 0.00900* | |
| C6 | −0.55479 | 1.15096 | 0.69442 | 0.00870* | |
| O7A | −0.33595 | 0.47056 | 1.05018 | 0.01370* | |
| O8A | −0.32484 | 0.31834 | 0.95223 | 0.01120* | |
| O9A | 0.10266 | 0.30045 | 0.93971 | 0.01250* | |
| O10A | 0.00830 | 0.19041 | 0.83347 | 0.01180* | |
| O11A | −0.01570 | 0.69714 | 0.48311 | 0.01120* | |
| O12A | −0.01724 | 0.75439 | 0.65877 | 0.01140* | |
| O13A | −0.15225 | 0.41596 | 0.68785 | 0.00990* | |
| H16A | −0.08773 | 0.38671 | 0.61789 | 0.02900* | |
| C1A | −0.27827 | 0.42098 | 0.95692 | 0.00950* | |
| C2A | −0.15358 | 0.50329 | 0.85782 | 0.00990* | |
| H14A | −0.08261 | 0.53524 | 0.91194 | 0.01100* | |
| H15A | −0.21626 | 0.59489 | 0.79720 | 0.01900* | |
| C3A | −0.05159 | 0.43504 | 0.76628 | 0.00870* | |
| C4A | 0.07944 | 0.52678 | 0.67668 | 0.00970* | |
| H17A | 0.14347 | 0.47918 | 0.60764 | 0.01400* | |
| H18A | 0.16142 | 0.53410 | 0.73772 | 0.01000* | |
| C5A | 0.01259 | 0.66803 | 0.59841 | 0.00880* | |
| C6A | 0.02761 | 0.29683 | 0.85249 | 0.00910* | |
| H19A | −0.41226 | 0.40666 | 1.13119 | 0.07800* | |
| H19 | −0.09903 | 0.87170 | 0.58711 | 0.06900* | |
| O1W | 0.25806 | 0.99725 | 0.99158 | 0.01550* | |
| H1W | 0.34046 | 1.05682 | 0.93555 | 0.04700* | |
| H2W | 0.16299 | 1.05211 | 0.95978 | 0.03700* | |
| O2W | 0.61368 | 0.82806 | 0.87514 | 0.01610* | |
| H3W | 0.68826 | 0.89557 | 0.85395 | 0.04700* | |
| H4W | 0.64281 | 0.78276 | 0.81112 | 0.06700* | |
| O3W | 0.08969 | 0.85012 | 0.82260 | 0.01340* | |
| H5W | 0.05984 | 0.80182 | 0.77073 | 0.03600* | |
| H6W | 0.01544 | 0.82092 | 0.90413 | 0.02900* |
(na2c_DFT). Bond lengths (Å)
| Na1—O10i | 2.333 | C1—C2 | 1.513 |
| Na1—O11ii | 2.357 | C2—H14 | 1.090 |
| Na1—O7A | 2.377 | C2—H15 | 1.094 |
| Na1—O8Aiii | 2.741 | C2—C3 | 1.536 |
| Na1—O9Aiv | 2.344 | C3—C4 | 1.561 |
| Na1—O2Wv | 2.363 | C3—C6 | 1.564 |
| Na2—O8vi | 2.385 | C4—H17 | 1.092 |
| Na2—O10vi | 2.441 | C4—H18 | 1.089 |
| Na2—O13vi | 2.496 | C4—C5 | 1.519 |
| Na2—O8A | 2.393 | O7A—C1A | 1.311 |
| Na2—O10A | 2.440 | O7A—H19A | 1.079 |
| Na2—O13A | 2.411 | O8A—Na1iii | 2.741 |
| Na2—O1Wiv | 2.494 | O8A—Na4iv | 2.292 |
| Na3—O7vii | 2.424 | O8A—C1A | 1.239 |
| Na3—O8 | 2.580 | O9A—Na1iv | 2.344 |
| Na3—O9viii | 2.348 | O9A—C6A | 1.267 |
| Na3—O10Aix | 2.304 | O10A—Na3vi | 2.304 |
| Na3—O11Avii | 2.462 | O10A—C6A | 1.263 |
| Na3—O3W | 2.387 | O11A—Na3vii | 2.462 |
| Na4—O9viii | 2.284 | O11A—C5A | 1.251 |
| Na4—O8Aiv | 2.292 | O12A—C5A | 1.293 |
| Na4—O1W | 2.391 | O13A—H16A | 0.986 |
| Na4—O2W | 2.349 | O13A—C3A | 1.413 |
| Na4—O3W | 2.331 | C1A—C2A | 1.506 |
| O7—Na3vii | 2.424 | C2A—H14A | 1.090 |
| O7—C1 | 1.312 | C2A—H15A | 1.092 |
| O7—H19 | 1.079 | C2A—C3A | 1.535 |
| O8—Na2ix | 2.385 | C3A—C4A | 1.556 |
| O8—C1 | 1.239 | C3A—C6A | 1.568 |
| O9—Na4v | 2.284 | C4A—H17A | 1.092 |
| O9—Na3v | 2.348 | C4A—H18A | 1.090 |
| O9—C6 | 1.255 | C4A—C5A | 1.523 |
| O10—Na2ix | 2.441 | O1W—Na2iv | 2.494 |
| O10—Na1i | 2.333 | O1W—H1W | 0.988 |
| O10—C6 | 1.273 | O1W—H2W | 0.980 |
| O11—Na1x | 2.357 | O2W—Na1viii | 2.363 |
| O11—C5 | 1.254 | O2W—H3W | 0.972 |
| O12—C5 | 1.295 | O2W—H4W | 0.971 |
| O13—Na2ix | 2.496 | O3W—H5W | 0.981 |
| O13—H16 | 0.987 | O3W—H6W | 0.979 |
| O13—C3 | 1.410 |
Symmetry codes: (i) −x−1, −y+2, −z+2; (ii) x, y−1, z+1; (iii) −x−1, −y+1, −z+2; (iv) −x, −y+1, −z+2; (v) x−1, y, z; (vi) x, y−1, z; (vii) −x, −y+2, −z+1; (viii) x+1, y, z; (ix) x, y+1, z; (x) x, y+1, z−1.
(na2c_DFT). Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O7A—H19A···O12 | 1.079 | 1.393 | 2.465 | 171.1 |
| O7—H19···O12A | 1.079 | 1.382 | 2.456 | 172.5 |
| O13A—H16A···O11A | 0.986 | 1.725 | 2.698 | 168.3 |
| O13—H16···O11 | 0.987 | 1.760 | 2.743 | 173.4 |
| O1W—H1W···O10 | 0.988 | 1.806 | 2.772 | 165.0 |
| O3W—H5W···O12A | 0.981 | 1.751 | 2.714 | 165.9 |
| O3W—H6W···O9A | 0.979 | 1.945 | 2.881 | 159.0 |
| O1W—H2W···O10A | 0.980 | 2.122 | 3.067 | 161.4 |
| O2W—H4W···O12 | 0.971 | 2.171 | 2.877 | 128.5 |
| O2W—H3W···O8 | 0.972 | 2.146 | 2.946 | 138.6 |
| O2W—H3W···O1W | 0.972 | 2.503 | 3.166 | 125.3 |
References
- Brandenburg, K. (2006). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Bruker (2004). XL and XM. Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2006). APEX2, SADABS and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Dassault Systemes (2014). Materials Studio. BIOVIA, San Diego CA.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Dovesi, R., Orlando, R., Civalleri, B., Roetti, C., Saunders, V. R. & Zicovich-Wilson, C. M. (2005). Z. Kristallogr. 220, 571–573.
- Dovesi, R., Roetti, C., Freyria-Fava, C., Prencipe, M. & Saunders, V. R. (1991). Chem. Phys. 156, 11–19.
- Gatti, C., Saunders, V. R. & Roetti, C. (1994). J. Chem. Phys. 101, 10686–10696.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Kaduk, J. A. & Stern, C. (2016a). Private Communication (refcodes 1446457 and 1446458). CCDC, Cambridge, England.
- Kaduk, J. A. & Stern, C. (2016b). Private Communication (refcodes 1446460 and 1446461). CCDC, Cambridge, England.
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Rammohan, A. & Kaduk, J. A. (2016a). Acta Cryst. B. Submitted.
- Rammohan, A. & Kaduk, J. A. (2016b). Acta Cryst. E72, 170–173. [DOI] [PMC free article] [PubMed]
- Rammohan, A. & Kaduk, J. A. (2016c). Acta Cryst. E72, 403–406. [DOI] [PMC free article] [PubMed]
- Rammohan, A. & Kaduk, J. A. (2016d). Acta Cryst. E72, 793–796. [DOI] [PMC free article] [PubMed]
- Rammohan, A. & Kaduk, J. A. (2016e). Acta Cryst. E72, 854–857. [DOI] [PMC free article] [PubMed]
- Streek, J. van de & Neumann, M. A. (2014). Acta Cryst. B70, 1020–1032. [DOI] [PMC free article] [PubMed]
- Wolff, P. de (1966). ICDD Grant-in-Aid, PDF entry 00-016-1182.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) na2c, na2c_DFT. DOI: 10.1107/S2056989016009014/vn2112sup1.cif
Structure factors: contains datablock(s) na2c. DOI: 10.1107/S2056989016009014/vn2112na2csup2.hkl
Structure factors: contains datablock(s) na2c_DFT. DOI: 10.1107/S2056989016009014/vn2112na2c_DFTsup3.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report






