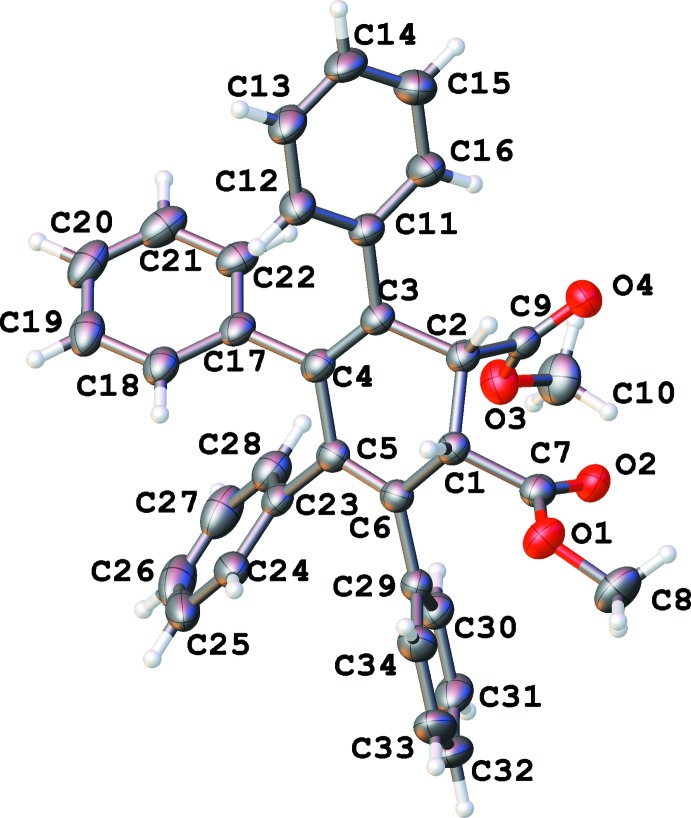
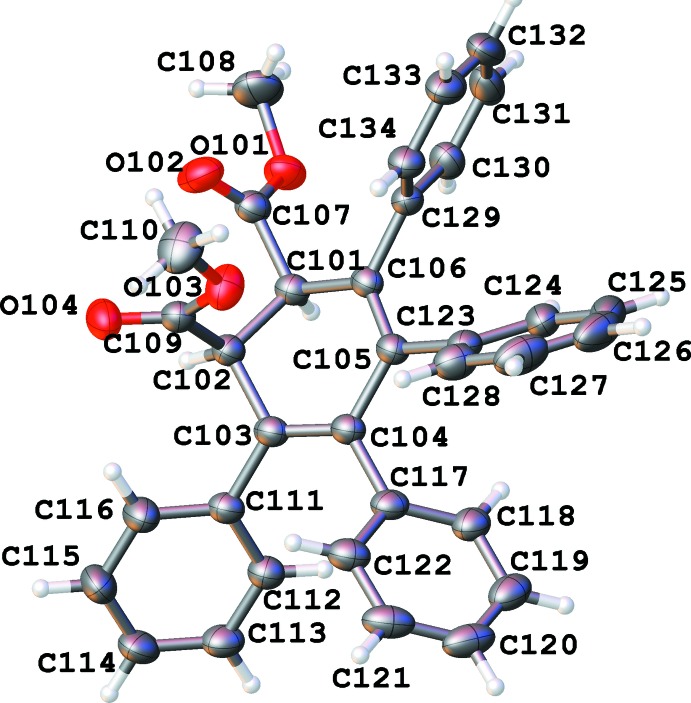
In the title compound, C34H28O4, the cyclohexadiene ring has a screw-boat conformation. All four phenyl rings in the two independent molecules are arranged in a propeller-like conformation. The two molecules exhibit S,R- and R,S- chirality and are connected via C—H⋯O intermolecular interactions.
Keywords: crystal structure; 1,3-cyclohexadiene; conformation
Abstract
In the title compound, C34H28O4, the cyclohexadiene ring has a screw-boat conformation with a torsion angle between the double bonds being on average ca 15° [15.2 (3) and −15.3 (3) in the two independent molecules]. All four phenyl rings in both molecules are arranged in a propeller-like conformation. The two molecules exhibit S,R- and R,S- chirality, respectively, and are connected via C—H⋯O intermolecular interactions. In turn, these weakly bound dimers form the molecular crystal.
Chemical context
Addition reactions of tetraphenylcyclopentadienone, often abbreviated to ‘tetracyclone’, were reviewed by Allen (1945 ▸, 1962 ▸). Tetracyclone reacts with unsaturated anhydrides, acids and esters, forming a number of polyfunctional carbonyl-bridge compounds. These species easily loose carbon monoxide to form dihydrobenzene (cyclohexadiene) derivatives. It was found that the use of maleic and fumaric esters yields various stereoisomers. The photochemical behavior of these compounds was studied (Fuchs & Yankelievich, 1968 ▸), showing a number of products including dimethyl tetraphenylphthalate. The relative simplicity of these reactions and the rich organic chemistry and spectroscopy of appropriate products make them attractive for use in undergraduate organic chemistry teaching laboratories.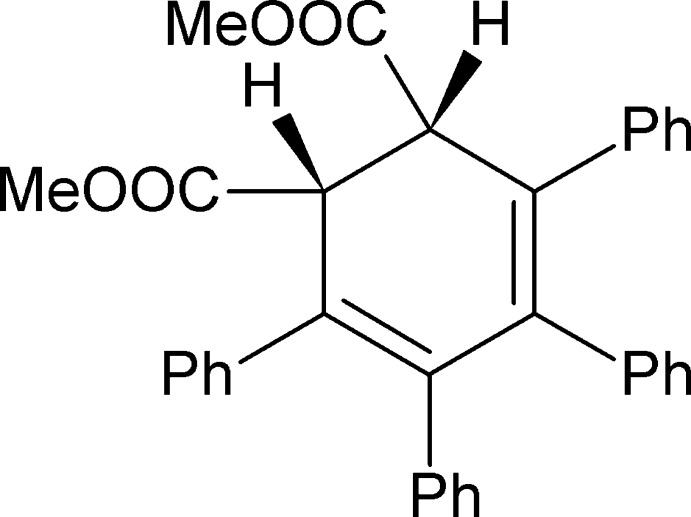
This study provides an opportunity to investigate the geometry of 1,3-cyclohexadiene rings surrounded by bulky substituents with no strong intermolecular interactions.
Database survey
Conjugation of two double bonds favors a coplanar π-system with a dihedral angle close to zero. However, in cyclic 1,3-cyclohexadiene molecules angle strain and steric effects promote a non-planar structure (Rabideau & Sygula, 1989 ▸). Even for non-cyclic systems, because of steric effects, the geometry of the higher energy non-trans conformer of 1,3-butadiene in the gas phase is non-planar s-gauche (De Maré et al., 1997 ▸). Addition of bulky substituents to the 1,3-butadiene molecule changes the conformational preference from trans to gauche even in the ground state.
The geometry of unsubstituted 1,3-cyclohexadiene was studied using electron diffraction in the gas phase (Traetteberg, 1968 ▸; Rabideau & Sygula, 1989 ▸) showing a dihedral angle of around 18°. The crystal structure of solid unsubstituted 1,3-cyclohexadiene is not reported. However, the 1,3-cyclohexadiene molecule has been incorporated into microporous vanadium benzenedicarboxylate (Wang et al., 2011 ▸) showing an almost flat conformation with a dihedral angle of 3.9° (refcode IXODUV). There are a large number of known 1,3-cyclohexadiene complexes with various metals, all with a mostly planar diene fragment. There are seventeen reported hexasubstituted 1,3-cyclohexadiene structures deposited in the Cambridge Structural Database (CSD Version 5.37; Groom et al., 2016 ▸). Of these structures, nine show a practically flat butadiene fragment with dihedral angles less than 3°. Two more (refcodes ONIWUE and TESNIT) show dihedral angles of 4.5 and 4.7°, respectively. Only four structures demonstrate dihedral angles similar to that of free 1,3-cyclohexadiene in the gas phase: GABGEQ (18.8°), HEUZOX (22.5°), JEKFUB (18.6°) and PUBMEG (20.1°). This last structure of trans-dimethyl 3,4,5,6-tetramethylcyclohexa-3,5-diene-1,2-dicarboxylate (Takahashi et al., 1998 ▸) is the closest to the title compound, with a cis conformation as for the title compound.
Structural commentary
There are two independent molecules (Figs. 1 ▸ and 2 ▸) in the asymmetric unit of the title compound, with S,R-chirality and R,S-chirality, respectively (Figs. 1 ▸, 2 ▸). After inversion they demonstrate a good overlay (Fig. 3 ▸) with an average deviation of 0.14 Å.
Figure 1.
Numbering scheme of the title compound with 50% probability elipsoids (S,R-isomer).
Figure 2.
Numbering scheme of the title compound with 50% probability elipsoids (R,S-isomer).
Figure 3.
Overlay of the two independent molecules, after inversion.
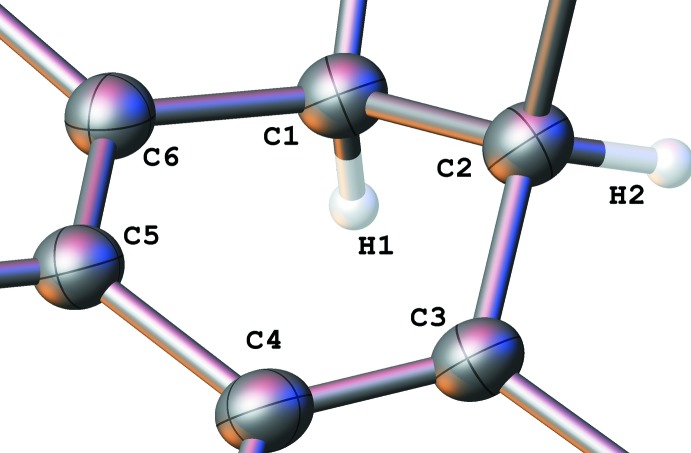
The cyclohexadiene rings (see Fig. 4 ▸, Table 1 ▸) are non-planar in a screw-boat conformation (Boeyens, 1978 ▸) with puckering parameters (C1–C6) Q = 0.437 (2) Å, θ = 115.8 (3)° and φ = 213.1 (3); (C101–C106) Q = 0.463 (2) Å, θ = 63.7 (2)° and φ = 33.5 (3)°.
Figure 4.
Cyclohexadiene ring with 50% probability elipsoids.
Table 1. Deviation from the mean plane of cyclohexadiene ring (Å).
| C1 | −0.269 (2) | C101 | −0.286 (2) |
|---|---|---|---|
| C2 | +0.280 (2) | C102 | +0.298 (2) |
| C3 | −0.089 (2) | C103 | −0.096 (2) |
| C4 | −0.112 (2) | C104 | −0.114 (2) |
| C5 | +0.126 (2) | C105 | +0.131 (2) |
| C6 | +0.064 (2) | C106 | +0.067 (2) |
Torsion angles between Csp3 atoms indicate a gauche conformation; the dihedral angles between the two double bonds are 15.2 (3) and −15.3 (3) for the two independent molecules (see Table 2 ▸). These values are practically the same as observed for free 1,3-cyclohexadiene in the gas phase: one can argue that the much lower values reported for 1,3-cyclohexadienes in the crystal state are caused by intermolecular interactions which may favor a flat butadiene fragment.
Table 2. Selected torsion angles (°).
| C4—C3—C2—C1 | −35.7 (3) | C105—C104—C103—C102 | −5.2 (3) |
| C4—C5—C6—C1 | 0.7 (3) | C5—C4—C3—C2 | 4.3 (3) |
| C3—C4—C5—C6 | 15.2 (3) | C5—C6—C1—C2 | −32.9 (3) |
| C3—C2—C1—C6 | 48.2 (2) | C106—C101—C102—C103 | −51.3 (2) |
| C101—C102—C103—C104 | 38.2 (3) | C102—C101—C106—C105 | 35.2 (3) |
| C104—C105—C106—C101 | −1.3 (3) | C103—C104—C105—C106 | −15.3 (3) |
All six substituents are practically flat. Both ester fragments are almost perpendicular to the mean plane of the cyclohexadiene ring (Table 3 ▸). All four phenyl rings in both molecules are arranged in a propeller-like formation with angles between 46 and 74° (see Table 3 ▸ for exact numbers) from the mean plane of the cyclohexadiene ring. This propeller-like formation is probably inherited from the precursor tetracyclone molecule (refcode KIKTUT02; Pal et al., 2014 ▸). Because of the large angles between the planes of the double bonds and each phenyl ring, very little conjugation may be expected. Therefore, substituents serve mainly as bulky decoration, protecting the cyclohexadiene ring from external steric influences.
Table 3. Dihedral angles between cyclohexadiene mean plane and substituent mean planes (°) .
| Atoms | angle | atoms | angle |
|---|---|---|---|
| C8/O2/C7/O1 | 79.35 (9) | C108–O101 | 71.07 (10) |
| C10/O4/C9/O3 | 97.38 (13) | C110–O104 | 97.82 (14) |
| C11–C16 | 59.72 (8) | C111–C116 | 57.22 (8) |
| C17–C22 | 46.53 (7) | C117–C122 | 46.12 (8) |
| C23–C28 | 56.38 (8) | C123–C128 | 56.89 (8) |
| C29–C34 | 69.88 (8) | C129–C134 | 73.46 (8) |
Supramolecular features
There are no usual hydrogen-bonding or stacking interactions in this structure.
Two hydrogen atoms of the cyclohexadiene group (H101 and H102) form short contacts (Desiraju & Steiner, 1999 ▸) with carbonyl oxygen atoms of another molecule (Table 4 ▸, Fig. 5 ▸). The corresponding hydrogen atoms of the other molecule (H1 and H2) do not have acceptors available for such bonding. These intermolecular interactions, however weak they are, keep together a pair of molecules with opposite chirality. Two short intramolecular C—H⋯O contacts within each molecule are also observed and may influence the molecular conformation. There are no other bonding short contacts between the weakly bound dimers and they form a usual molecular crystal.
Table 4. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C101—H101⋯O2 | 0.99 (3) | 2.39 (3) | 3.384 (3) | 176 (2) |
| C102—H102⋯O4 | 0.96 (3) | 2.48 (3) | 3.242 (3) | 136 (2) |
| C16—H16⋯O4 | 0.95 | 2.59 | 3.407 (3) | 145 |
| C116—H116⋯O104 | 0.95 | 2.54 | 3.388 (3) | 148 |
Figure 5.
Short C—H⋯O contacts connecting two molecules into a weakly bonded dimer in the crystal.
Synthesis and crystallization
The title compound was obtained by reaction of tetraphenylcyclopentadienone (common name tetracyclone) with dimethylmaleate following Allen & Sheps (1934 ▸). GC–MS analysis of the colorless crystalline product dissolved in dichloromethane shows one main compound with a parent peak at 500 which is consistent with the formula weight of the title compound. Because all precursor compounds were non-chiral and synthetic conditions should not induce chirality, we expected to see a racemic product. Crystallization from acetonitrile resulted in several hexagonal flakes, mostly with intergrown smaller crystals. Several crystals were tested, all resulting in essentially the same chiral trigonal structure. The highest quality structure, from a partial racemically twinned crystal, is reported here.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 5 ▸. The structure was refined as a two-component inversion twin. Cyclohexadiene hydrogen atoms H1, H2, H101 and H102 were refined in isotropic approximation with U iso = 1.2Ui so(C). All aromatic hydrogen atoms were refined with riding coordinates with C—H = 0.95–0.98 Å and U iso = 1.2U iso(C). Idealized methyl groups were refined as rotating groups with U iso = 1.5Uiso(C).
Table 5. Experimental details.
| Crystal data | |
| Chemical formula | C34H28O4 |
| M r | 500.56 |
| Crystal system, space group | Trigonal, P32 |
| Temperature (K) | 173 |
| a, c (Å) | 10.8330 (12), 39.169 (5) |
| V (Å3) | 3980.8 (12) |
| Z | 6 |
| Radiation type | Cu Kα |
| μ (mm−1) | 0.65 |
| Crystal size (mm) | 0.59 × 0.34 × 0.13 |
| Data collection | |
| Diffractometer | Bruker Photon-100 CMOS |
| Absorption correction | Multi-scan (SADABS; Bruker,2014 ▸/5) |
| T min, T max | 0.669, 0.754 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 53613, 10773, 10345 |
| R int | 0.043 |
| (sin θ/λ)max (Å−1) | 0.637 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.033, 0.091, 1.05 |
| No. of reflections | 10773 |
| No. of parameters | 702 |
| No. of restraints | 1 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.19, −0.15 |
| Absolute structure | Refined as an inversion twin |
| Absolute structure parameter | 0.38 (16) |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989016009403/zl2665sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989016009403/zl2665Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989016009403/zl2665Isup3.cdx
Supporting information file. DOI: 10.1107/S2056989016009403/zl2665Isup4.cml
CCDC reference: 1484412
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
Financial support from the State University of New York for the acquisition and maintenance of X-ray diffractometer is gratefully acknowledged.
supplementary crystallographic information
Crystal data
| C34H28O4 | Dx = 1.253 Mg m−3 |
| Mr = 500.56 | Cu Kα radiation, λ = 1.54178 Å |
| Trigonal, P32 | Cell parameters from 9883 reflections |
| a = 10.8330 (12) Å | θ = 3.4–78.4° |
| c = 39.169 (5) Å | µ = 0.65 mm−1 |
| V = 3980.8 (12) Å3 | T = 173 K |
| Z = 6 | Plate, colourless |
| F(000) = 1584 | 0.59 × 0.34 × 0.13 mm |
Data collection
| Bruker Photon-100 CMOS diffractometer | 10345 reflections with I > 2σ(I) |
| Radiation source: sealedtube | Rint = 0.043 |
| φ and ω scans | θmax = 79.0°, θmin = 3.4° |
| Absorption correction: multi-scan (SADABS; Bruker,2014/5) | h = −13→12 |
| Tmin = 0.669, Tmax = 0.754 | k = −13→13 |
| 53613 measured reflections | l = −48→48 |
| 10773 independent reflections |
Refinement
| Refinement on F2 | Hydrogen site location: mixed |
| Least-squares matrix: full | H atoms treated by a mixture of independent and constrained refinement |
| R[F2 > 2σ(F2)] = 0.033 | w = 1/[σ2(Fo2) + (0.0547P)2 + 0.4031P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.091 | (Δ/σ)max < 0.001 |
| S = 1.05 | Δρmax = 0.19 e Å−3 |
| 10773 reflections | Δρmin = −0.15 e Å−3 |
| 702 parameters | Absolute structure: Refined as an inversion twin |
| 1 restraint | Absolute structure parameter: 0.38 (16) |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refined as a 2-component inversion twin.1. Twinned data refinement Scales: 0.62 (16) 0.38 (16) 2. Fixed Uiso At 1.2 times of: All C(H) groups At 1.5 times of: All C(H,H,H) groups 3.a Aromatic/amide H refined with riding coordinates: C21(H21), C34(H34), C18(H18), C30(H30), C134(H134), C24(H24), C22(H22), C12(H12), C133(H133), C16(H16), C112(H112), C130(H130), C28(H28), C124(H124), C113(H113), C119(H119), C19(H19), C131(H131), C15(H15), C118(H118), C120(H120), C31(H31), C114(H114), C116(H116), C25(H25), C27(H27), C121(H121), C122(H122), C128(H128), C13(H13), C20(H20), C132(H132), C26(H26), C14(H14), C33(H33), C32(H32), C126(H126), C127(H127), C115(H115), C125(H125) 3.b Idealised Me refined as rotating group: C8(H8A,H8B,H8C), C110(H11A,H11B,H11C), C10(H10A,H10B,H10C), C108(H10D,H10E, H10F) |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O4 | 0.79329 (17) | 0.30493 (18) | 0.54107 (4) | 0.0438 (4) | |
| O101 | 0.96274 (18) | 0.63537 (17) | 0.51401 (4) | 0.0443 (4) | |
| O103 | 1.02631 (18) | 0.4316 (2) | 0.41723 (4) | 0.0475 (4) | |
| O2 | 0.56790 (17) | 0.38169 (16) | 0.51915 (4) | 0.0434 (4) | |
| O1 | 0.3803 (2) | 0.25072 (18) | 0.48545 (4) | 0.0458 (4) | |
| O3 | 0.64592 (18) | 0.29005 (17) | 0.58294 (4) | 0.0452 (4) | |
| O102 | 1.09927 (18) | 0.5677 (2) | 0.48612 (5) | 0.0540 (4) | |
| O104 | 1.03157 (19) | 0.27494 (18) | 0.45336 (5) | 0.0508 (4) | |
| C9 | 0.6769 (2) | 0.2477 (2) | 0.55396 (5) | 0.0345 (4) | |
| C123 | 0.7593 (2) | 0.5516 (2) | 0.37918 (6) | 0.0363 (4) | |
| C29 | 0.2698 (2) | 0.2158 (2) | 0.56419 (5) | 0.0329 (4) | |
| C7 | 0.4665 (2) | 0.2687 (2) | 0.51152 (5) | 0.0355 (4) | |
| C4 | 0.4035 (2) | −0.0443 (2) | 0.58720 (5) | 0.0346 (4) | |
| C3 | 0.5035 (2) | −0.0149 (2) | 0.56313 (5) | 0.0340 (4) | |
| C23 | 0.2515 (2) | 0.0341 (2) | 0.62110 (5) | 0.0354 (4) | |
| C109 | 0.9775 (2) | 0.3424 (2) | 0.44350 (6) | 0.0356 (4) | |
| C101 | 0.8547 (2) | 0.4732 (2) | 0.46876 (5) | 0.0327 (4) | |
| H101 | 0.772 (3) | 0.452 (3) | 0.4837 (7) | 0.039* | |
| C104 | 0.6822 (2) | 0.3191 (2) | 0.41216 (5) | 0.0333 (4) | |
| C105 | 0.7663 (2) | 0.4780 (2) | 0.41074 (5) | 0.0328 (4) | |
| C107 | 0.9869 (2) | 0.5616 (2) | 0.49006 (5) | 0.0362 (4) | |
| C5 | 0.3298 (2) | 0.0406 (2) | 0.58914 (5) | 0.0333 (4) | |
| C106 | 0.8498 (2) | 0.5531 (2) | 0.43712 (5) | 0.0318 (4) | |
| C102 | 0.8425 (2) | 0.3301 (2) | 0.45915 (5) | 0.0327 (4) | |
| H102 | 0.829 (3) | 0.276 (3) | 0.4797 (7) | 0.039* | |
| C2 | 0.5490 (2) | 0.1126 (2) | 0.53935 (5) | 0.0335 (4) | |
| H2 | 0.586 (3) | 0.095 (3) | 0.5182 (7) | 0.040* | |
| C6 | 0.3366 (2) | 0.1245 (2) | 0.56296 (5) | 0.0322 (4) | |
| C129 | 0.9408 (2) | 0.7112 (2) | 0.43578 (6) | 0.0342 (4) | |
| C11 | 0.5749 (2) | −0.1004 (2) | 0.55719 (6) | 0.0371 (4) | |
| C21 | 0.4450 (3) | −0.2535 (3) | 0.65930 (7) | 0.0527 (6) | |
| H21 | 0.5199 | −0.2513 | 0.6724 | 0.063* | |
| C34 | 0.3107 (3) | 0.3209 (2) | 0.58899 (6) | 0.0397 (5) | |
| H34 | 0.3819 | 0.3349 | 0.6052 | 0.048* | |
| C18 | 0.2271 (3) | −0.2583 (2) | 0.62137 (6) | 0.0418 (5) | |
| H18 | 0.1516 | −0.2602 | 0.6086 | 0.050* | |
| C103 | 0.7127 (2) | 0.2471 (2) | 0.43591 (5) | 0.0337 (4) | |
| C111 | 0.6241 (2) | 0.0917 (2) | 0.44298 (6) | 0.0359 (4) | |
| C30 | 0.1673 (2) | 0.1994 (3) | 0.54024 (6) | 0.0434 (5) | |
| H30 | 0.1397 | 0.1294 | 0.5228 | 0.052* | |
| C134 | 1.0485 (2) | 0.7733 (2) | 0.41147 (6) | 0.0401 (5) | |
| H134 | 1.0651 | 0.7145 | 0.3963 | 0.048* | |
| C1 | 0.4199 (2) | 0.1303 (2) | 0.53089 (5) | 0.0326 (4) | |
| H1 | 0.357 (3) | 0.052 (3) | 0.5158 (7) | 0.039* | |
| C17 | 0.3680 (2) | −0.1561 (2) | 0.61388 (5) | 0.0375 (4) | |
| C24 | 0.1072 (3) | −0.0091 (2) | 0.62101 (7) | 0.0448 (5) | |
| H24 | 0.0548 | −0.0382 | 0.6003 | 0.054* | |
| C22 | 0.4763 (3) | −0.1550 (3) | 0.63322 (6) | 0.0440 (5) | |
| H22 | 0.5728 | −0.0861 | 0.6286 | 0.053* | |
| C12 | 0.4937 (3) | −0.2481 (2) | 0.55401 (6) | 0.0440 (5) | |
| H12 | 0.3930 | −0.2941 | 0.5563 | 0.053* | |
| C133 | 1.1322 (3) | 0.9209 (3) | 0.40914 (7) | 0.0529 (6) | |
| H133 | 1.2055 | 0.9623 | 0.3925 | 0.063* | |
| C117 | 0.5695 (2) | 0.2447 (2) | 0.38556 (6) | 0.0371 (4) | |
| C16 | 0.7221 (3) | −0.0358 (3) | 0.55332 (7) | 0.0479 (5) | |
| H16 | 0.7794 | 0.0649 | 0.5551 | 0.057* | |
| C112 | 0.4757 (2) | 0.0265 (2) | 0.44520 (6) | 0.0387 (4) | |
| H112 | 0.4305 | 0.0803 | 0.4403 | 0.046* | |
| C130 | 0.9202 (3) | 0.7994 (2) | 0.45808 (6) | 0.0429 (5) | |
| H130 | 0.8489 | 0.7587 | 0.4752 | 0.051* | |
| C28 | 0.3246 (3) | 0.0744 (3) | 0.65205 (6) | 0.0469 (5) | |
| H28 | 0.4232 | 0.1038 | 0.6526 | 0.056* | |
| C124 | 0.7148 (3) | 0.6522 (2) | 0.38033 (7) | 0.0473 (5) | |
| H124 | 0.6850 | 0.6725 | 0.4014 | 0.057* | |
| C113 | 0.3938 (3) | −0.1154 (3) | 0.45445 (6) | 0.0458 (5) | |
| H113 | 0.2931 | −0.1580 | 0.4557 | 0.055* | |
| C119 | 0.3685 (3) | 0.2182 (3) | 0.35267 (7) | 0.0509 (6) | |
| H119 | 0.2984 | 0.2446 | 0.3485 | 0.061* | |
| C19 | 0.1960 (3) | −0.3573 (3) | 0.64730 (7) | 0.0493 (6) | |
| H19 | 0.0997 | −0.4267 | 0.6520 | 0.059* | |
| C131 | 1.0040 (3) | 0.9472 (3) | 0.45532 (8) | 0.0554 (7) | |
| H131 | 0.9885 | 1.0071 | 0.4703 | 0.066* | |
| C15 | 0.7860 (3) | −0.1173 (3) | 0.54693 (8) | 0.0566 (7) | |
| H15 | 0.8866 | −0.0720 | 0.5445 | 0.068* | |
| C118 | 0.4674 (2) | 0.2838 (2) | 0.37867 (6) | 0.0422 (5) | |
| H118 | 0.4654 | 0.3563 | 0.3919 | 0.051* | |
| C120 | 0.3719 (3) | 0.1143 (3) | 0.33279 (7) | 0.0559 (7) | |
| H120 | 0.3049 | 0.0701 | 0.3148 | 0.067* | |
| C31 | 0.1052 (3) | 0.2850 (3) | 0.54173 (8) | 0.0554 (7) | |
| H31 | 0.0353 | 0.2732 | 0.5254 | 0.066* | |
| C114 | 0.4573 (3) | −0.1955 (3) | 0.46184 (8) | 0.0539 (6) | |
| H114 | 0.4010 | −0.2924 | 0.4685 | 0.065* | |
| C116 | 0.6871 (3) | 0.0096 (3) | 0.45039 (8) | 0.0498 (6) | |
| H116 | 0.7878 | 0.0514 | 0.4493 | 0.060* | |
| C25 | 0.0393 (3) | −0.0097 (3) | 0.65115 (9) | 0.0627 (8) | |
| H25 | −0.0591 | −0.0381 | 0.6508 | 0.075* | |
| C27 | 0.2558 (4) | 0.0723 (3) | 0.68193 (7) | 0.0617 (8) | |
| H27 | 0.3071 | 0.1000 | 0.7028 | 0.074* | |
| C121 | 0.4727 (3) | 0.0754 (3) | 0.33914 (7) | 0.0529 (6) | |
| H121 | 0.4751 | 0.0043 | 0.3254 | 0.063* | |
| C8 | 0.4058 (4) | 0.3808 (3) | 0.46891 (7) | 0.0588 (7) | |
| H8A | 0.3398 | 0.3574 | 0.4497 | 0.088* | |
| H8B | 0.5041 | 0.4326 | 0.4605 | 0.088* | |
| H8C | 0.3909 | 0.4403 | 0.4853 | 0.088* | |
| C122 | 0.5707 (3) | 0.1388 (2) | 0.36532 (6) | 0.0439 (5) | |
| H122 | 0.6391 | 0.1101 | 0.3696 | 0.053* | |
| C128 | 0.8006 (3) | 0.5232 (3) | 0.34810 (6) | 0.0479 (5) | |
| H128 | 0.8302 | 0.4543 | 0.3468 | 0.058* | |
| C13 | 0.5581 (3) | −0.3293 (3) | 0.54756 (7) | 0.0509 (6) | |
| H13 | 0.5012 | −0.4300 | 0.5456 | 0.061* | |
| C20 | 0.3049 (4) | −0.3547 (3) | 0.66614 (7) | 0.0543 (7) | |
| H20 | 0.2837 | −0.4225 | 0.6838 | 0.065* | |
| C132 | 1.1094 (3) | 1.0066 (3) | 0.43078 (8) | 0.0598 (8) | |
| H132 | 1.1662 | 1.1073 | 0.4289 | 0.072* | |
| C26 | 0.1132 (4) | 0.0303 (3) | 0.68154 (8) | 0.0677 (9) | |
| H26 | 0.0660 | 0.0290 | 0.7021 | 0.081* | |
| C14 | 0.7037 (3) | −0.2644 (3) | 0.54406 (8) | 0.0543 (6) | |
| H14 | 0.7477 | −0.3199 | 0.5397 | 0.065* | |
| C33 | 0.2479 (3) | 0.4054 (3) | 0.59017 (7) | 0.0517 (6) | |
| H33 | 0.2762 | 0.4768 | 0.6072 | 0.062* | |
| C32 | 0.1450 (3) | 0.3866 (3) | 0.56682 (8) | 0.0562 (7) | |
| H32 | 0.1015 | 0.4440 | 0.5680 | 0.067* | |
| C110 | 1.1609 (3) | 0.4582 (4) | 0.40345 (8) | 0.0621 (7) | |
| H11A | 1.1830 | 0.5166 | 0.3828 | 0.093* | |
| H11B | 1.1551 | 0.3673 | 0.3978 | 0.093* | |
| H11C | 1.2362 | 0.5087 | 0.4204 | 0.093* | |
| C10 | 0.7575 (3) | 0.4261 (3) | 0.59574 (8) | 0.0578 (7) | |
| H10A | 0.7695 | 0.5023 | 0.5802 | 0.087* | |
| H10B | 0.8472 | 0.4248 | 0.5972 | 0.087* | |
| H10C | 0.7314 | 0.4433 | 0.6185 | 0.087* | |
| C126 | 0.7558 (3) | 0.6936 (4) | 0.32010 (9) | 0.0718 (10) | |
| H126 | 0.7545 | 0.7419 | 0.3000 | 0.086* | |
| C127 | 0.7991 (3) | 0.5949 (4) | 0.31868 (7) | 0.0664 (9) | |
| H127 | 0.8284 | 0.5750 | 0.2975 | 0.080* | |
| C115 | 0.6036 (3) | −0.1331 (3) | 0.45941 (9) | 0.0614 (7) | |
| H115 | 0.6477 | −0.1882 | 0.4640 | 0.074* | |
| C108 | 1.0862 (4) | 0.7347 (3) | 0.53338 (8) | 0.0646 (8) | |
| H10D | 1.1554 | 0.8085 | 0.5181 | 0.097* | |
| H10E | 1.1296 | 0.6840 | 0.5441 | 0.097* | |
| H10F | 1.0572 | 0.7792 | 0.5511 | 0.097* | |
| C125 | 0.7142 (3) | 0.7225 (3) | 0.35077 (10) | 0.0658 (9) | |
| H125 | 0.6846 | 0.7915 | 0.3518 | 0.079* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O4 | 0.0354 (8) | 0.0405 (8) | 0.0537 (9) | 0.0178 (7) | 0.0057 (7) | 0.0050 (7) |
| O101 | 0.0495 (9) | 0.0365 (8) | 0.0416 (8) | 0.0175 (7) | −0.0063 (7) | −0.0078 (6) |
| O103 | 0.0415 (9) | 0.0614 (11) | 0.0440 (8) | 0.0289 (8) | 0.0093 (7) | 0.0128 (8) |
| O2 | 0.0400 (8) | 0.0322 (8) | 0.0563 (9) | 0.0169 (7) | 0.0008 (7) | 0.0066 (7) |
| O1 | 0.0583 (10) | 0.0419 (8) | 0.0412 (8) | 0.0280 (8) | −0.0065 (7) | 0.0010 (7) |
| O3 | 0.0464 (9) | 0.0379 (8) | 0.0429 (8) | 0.0148 (7) | 0.0022 (7) | −0.0064 (6) |
| O102 | 0.0349 (9) | 0.0540 (10) | 0.0670 (11) | 0.0177 (8) | −0.0096 (8) | −0.0159 (9) |
| O104 | 0.0415 (9) | 0.0425 (9) | 0.0731 (11) | 0.0245 (8) | 0.0025 (8) | 0.0080 (8) |
| C9 | 0.0395 (11) | 0.0308 (10) | 0.0378 (10) | 0.0210 (9) | 0.0015 (8) | 0.0038 (8) |
| C123 | 0.0285 (9) | 0.0275 (9) | 0.0447 (11) | 0.0079 (8) | −0.0063 (8) | 0.0014 (8) |
| C29 | 0.0314 (10) | 0.0299 (9) | 0.0387 (11) | 0.0162 (8) | 0.0053 (8) | 0.0043 (8) |
| C7 | 0.0397 (11) | 0.0367 (11) | 0.0360 (10) | 0.0234 (9) | 0.0049 (8) | 0.0009 (8) |
| C4 | 0.0363 (10) | 0.0277 (9) | 0.0409 (10) | 0.0169 (8) | −0.0010 (8) | −0.0003 (8) |
| C3 | 0.0368 (10) | 0.0275 (9) | 0.0407 (10) | 0.0183 (8) | 0.0017 (8) | 0.0001 (8) |
| C23 | 0.0407 (11) | 0.0270 (9) | 0.0424 (10) | 0.0199 (8) | 0.0063 (9) | 0.0054 (8) |
| C109 | 0.0333 (10) | 0.0300 (10) | 0.0405 (10) | 0.0136 (8) | −0.0048 (8) | −0.0036 (8) |
| C101 | 0.0309 (10) | 0.0283 (9) | 0.0352 (10) | 0.0120 (8) | 0.0012 (8) | −0.0001 (7) |
| C104 | 0.0298 (9) | 0.0275 (9) | 0.0386 (10) | 0.0113 (8) | −0.0011 (8) | −0.0011 (8) |
| C105 | 0.0297 (9) | 0.0271 (9) | 0.0391 (10) | 0.0123 (8) | −0.0010 (8) | 0.0006 (8) |
| C107 | 0.0366 (11) | 0.0293 (10) | 0.0372 (10) | 0.0122 (8) | −0.0012 (8) | 0.0024 (8) |
| C5 | 0.0320 (9) | 0.0275 (9) | 0.0403 (10) | 0.0148 (8) | 0.0005 (8) | −0.0008 (8) |
| C106 | 0.0285 (9) | 0.0267 (9) | 0.0390 (10) | 0.0129 (8) | 0.0015 (8) | 0.0013 (7) |
| C102 | 0.0317 (10) | 0.0280 (9) | 0.0352 (10) | 0.0127 (8) | −0.0014 (8) | 0.0018 (8) |
| C2 | 0.0366 (10) | 0.0293 (10) | 0.0371 (10) | 0.0185 (9) | 0.0029 (8) | 0.0003 (8) |
| C6 | 0.0297 (9) | 0.0285 (9) | 0.0379 (10) | 0.0141 (8) | −0.0003 (8) | −0.0017 (7) |
| C129 | 0.0310 (9) | 0.0279 (9) | 0.0407 (11) | 0.0124 (8) | −0.0076 (8) | −0.0002 (8) |
| C11 | 0.0409 (11) | 0.0331 (10) | 0.0435 (11) | 0.0231 (9) | 0.0053 (9) | 0.0023 (8) |
| C21 | 0.0789 (19) | 0.0489 (14) | 0.0456 (13) | 0.0434 (14) | −0.0060 (12) | −0.0020 (11) |
| C34 | 0.0435 (11) | 0.0334 (10) | 0.0439 (11) | 0.0206 (9) | 0.0057 (9) | 0.0020 (8) |
| C18 | 0.0512 (13) | 0.0328 (10) | 0.0444 (11) | 0.0232 (10) | 0.0078 (10) | 0.0023 (9) |
| C103 | 0.0296 (9) | 0.0273 (9) | 0.0407 (10) | 0.0117 (8) | −0.0004 (8) | −0.0021 (8) |
| C111 | 0.0333 (10) | 0.0287 (10) | 0.0399 (10) | 0.0111 (8) | −0.0013 (8) | −0.0005 (8) |
| C30 | 0.0386 (11) | 0.0473 (12) | 0.0462 (12) | 0.0230 (10) | −0.0007 (9) | 0.0023 (10) |
| C134 | 0.0330 (10) | 0.0325 (11) | 0.0479 (12) | 0.0111 (9) | −0.0018 (9) | 0.0046 (9) |
| C1 | 0.0341 (10) | 0.0282 (9) | 0.0362 (10) | 0.0162 (8) | 0.0013 (8) | 0.0001 (8) |
| C17 | 0.0475 (12) | 0.0311 (10) | 0.0413 (11) | 0.0253 (9) | 0.0055 (9) | 0.0008 (8) |
| C24 | 0.0429 (12) | 0.0338 (11) | 0.0601 (14) | 0.0211 (10) | 0.0096 (10) | 0.0063 (10) |
| C22 | 0.0540 (13) | 0.0405 (12) | 0.0479 (12) | 0.0314 (11) | −0.0010 (10) | −0.0019 (9) |
| C12 | 0.0470 (13) | 0.0328 (11) | 0.0551 (13) | 0.0221 (10) | 0.0115 (10) | 0.0026 (9) |
| C133 | 0.0401 (13) | 0.0383 (12) | 0.0644 (16) | 0.0076 (10) | −0.0075 (11) | 0.0143 (11) |
| C117 | 0.0306 (10) | 0.0291 (9) | 0.0405 (10) | 0.0065 (8) | −0.0004 (8) | 0.0038 (8) |
| C16 | 0.0426 (12) | 0.0359 (11) | 0.0693 (16) | 0.0228 (10) | 0.0041 (11) | 0.0035 (11) |
| C112 | 0.0350 (10) | 0.0323 (11) | 0.0439 (11) | 0.0132 (9) | −0.0009 (9) | 0.0015 (9) |
| C130 | 0.0483 (13) | 0.0372 (11) | 0.0466 (12) | 0.0240 (10) | −0.0086 (10) | −0.0053 (9) |
| C28 | 0.0597 (15) | 0.0412 (12) | 0.0442 (12) | 0.0284 (11) | 0.0031 (11) | 0.0043 (10) |
| C124 | 0.0358 (11) | 0.0352 (11) | 0.0671 (15) | 0.0149 (10) | −0.0128 (10) | 0.0014 (10) |
| C113 | 0.0353 (11) | 0.0368 (12) | 0.0510 (13) | 0.0072 (9) | −0.0015 (10) | 0.0045 (10) |
| C119 | 0.0336 (11) | 0.0499 (14) | 0.0525 (13) | 0.0083 (10) | −0.0045 (10) | 0.0143 (11) |
| C19 | 0.0654 (16) | 0.0325 (11) | 0.0494 (13) | 0.0240 (11) | 0.0166 (11) | 0.0035 (9) |
| C131 | 0.0707 (18) | 0.0381 (12) | 0.0644 (16) | 0.0325 (13) | −0.0281 (14) | −0.0136 (11) |
| C15 | 0.0479 (14) | 0.0536 (15) | 0.0787 (18) | 0.0331 (13) | 0.0109 (13) | 0.0082 (13) |
| C118 | 0.0325 (10) | 0.0364 (11) | 0.0482 (12) | 0.0100 (9) | −0.0028 (9) | 0.0057 (9) |
| C120 | 0.0425 (13) | 0.0533 (15) | 0.0414 (12) | 0.0010 (11) | −0.0077 (10) | 0.0037 (11) |
| C31 | 0.0486 (14) | 0.0681 (17) | 0.0632 (16) | 0.0395 (13) | 0.0062 (12) | 0.0192 (14) |
| C114 | 0.0485 (14) | 0.0295 (11) | 0.0682 (16) | 0.0079 (10) | −0.0093 (12) | 0.0084 (10) |
| C116 | 0.0372 (12) | 0.0345 (12) | 0.0738 (17) | 0.0150 (10) | −0.0039 (11) | 0.0053 (11) |
| C25 | 0.0582 (16) | 0.0410 (13) | 0.093 (2) | 0.0279 (12) | 0.0374 (16) | 0.0202 (14) |
| C27 | 0.099 (2) | 0.0475 (14) | 0.0420 (13) | 0.0394 (16) | 0.0096 (14) | 0.0059 (11) |
| C121 | 0.0499 (14) | 0.0384 (12) | 0.0451 (12) | 0.0032 (11) | 0.0017 (11) | −0.0038 (10) |
| C8 | 0.079 (2) | 0.0536 (15) | 0.0515 (14) | 0.0394 (15) | −0.0100 (13) | 0.0079 (11) |
| C122 | 0.0402 (12) | 0.0330 (11) | 0.0464 (12) | 0.0093 (9) | −0.0002 (9) | −0.0011 (9) |
| C128 | 0.0423 (12) | 0.0469 (13) | 0.0429 (12) | 0.0136 (11) | −0.0050 (9) | −0.0004 (10) |
| C13 | 0.0634 (16) | 0.0358 (12) | 0.0622 (15) | 0.0314 (12) | 0.0172 (12) | 0.0056 (11) |
| C20 | 0.090 (2) | 0.0389 (12) | 0.0417 (12) | 0.0383 (14) | 0.0087 (12) | 0.0054 (10) |
| C132 | 0.0591 (16) | 0.0268 (11) | 0.0795 (19) | 0.0111 (11) | −0.0274 (14) | 0.0048 (12) |
| C26 | 0.102 (3) | 0.0474 (15) | 0.0592 (17) | 0.0412 (16) | 0.0416 (18) | 0.0158 (13) |
| C14 | 0.0666 (17) | 0.0505 (14) | 0.0664 (16) | 0.0446 (14) | 0.0177 (13) | 0.0105 (12) |
| C33 | 0.0654 (16) | 0.0380 (12) | 0.0602 (15) | 0.0322 (12) | 0.0197 (13) | 0.0059 (11) |
| C32 | 0.0607 (16) | 0.0552 (15) | 0.0737 (17) | 0.0447 (14) | 0.0236 (14) | 0.0229 (13) |
| C110 | 0.0464 (14) | 0.082 (2) | 0.0612 (16) | 0.0350 (14) | 0.0173 (12) | 0.0150 (14) |
| C10 | 0.0629 (17) | 0.0386 (12) | 0.0589 (15) | 0.0156 (12) | −0.0056 (12) | −0.0118 (11) |
| C126 | 0.0500 (16) | 0.0654 (19) | 0.0699 (19) | 0.0064 (14) | −0.0224 (14) | 0.0262 (15) |
| C127 | 0.0517 (15) | 0.074 (2) | 0.0434 (13) | 0.0089 (15) | −0.0114 (11) | 0.0078 (13) |
| C115 | 0.0510 (15) | 0.0342 (12) | 0.096 (2) | 0.0194 (11) | −0.0111 (14) | 0.0103 (13) |
| C108 | 0.0704 (19) | 0.0515 (15) | 0.0595 (16) | 0.0213 (15) | −0.0239 (14) | −0.0203 (13) |
| C125 | 0.0434 (14) | 0.0411 (14) | 0.103 (3) | 0.0135 (12) | −0.0251 (15) | 0.0133 (14) |
Geometric parameters (Å, º)
| O4—C9 | 1.203 (3) | C133—H133 | 0.9500 |
| O101—C107 | 1.340 (3) | C133—C132 | 1.368 (5) |
| O101—C108 | 1.443 (3) | C117—C118 | 1.395 (3) |
| O103—C109 | 1.327 (3) | C117—C122 | 1.400 (3) |
| O103—C110 | 1.442 (3) | C16—H16 | 0.9500 |
| O2—C7 | 1.204 (3) | C16—C15 | 1.389 (4) |
| O1—C7 | 1.331 (3) | C112—H112 | 0.9500 |
| O1—C8 | 1.446 (3) | C112—C113 | 1.385 (3) |
| O3—C9 | 1.329 (3) | C130—H130 | 0.9500 |
| O3—C10 | 1.451 (3) | C130—C131 | 1.396 (4) |
| O102—C107 | 1.196 (3) | C28—H28 | 0.9500 |
| O104—C109 | 1.206 (3) | C28—C27 | 1.382 (4) |
| C9—C2 | 1.537 (3) | C124—H124 | 0.9500 |
| C123—C105 | 1.493 (3) | C124—C125 | 1.388 (4) |
| C123—C124 | 1.395 (3) | C113—H113 | 0.9500 |
| C123—C128 | 1.383 (3) | C113—C114 | 1.380 (4) |
| C29—C6 | 1.489 (3) | C119—H119 | 0.9500 |
| C29—C34 | 1.390 (3) | C119—C118 | 1.389 (3) |
| C29—C30 | 1.396 (3) | C119—C120 | 1.384 (5) |
| C7—C1 | 1.524 (3) | C19—H19 | 0.9500 |
| C4—C3 | 1.349 (3) | C19—C20 | 1.381 (4) |
| C4—C5 | 1.491 (3) | C131—H131 | 0.9500 |
| C4—C17 | 1.496 (3) | C131—C132 | 1.381 (5) |
| C3—C2 | 1.529 (3) | C15—H15 | 0.9500 |
| C3—C11 | 1.492 (3) | C15—C14 | 1.388 (4) |
| C23—C5 | 1.494 (3) | C118—H118 | 0.9500 |
| C23—C24 | 1.390 (3) | C120—H120 | 0.9500 |
| C23—C28 | 1.393 (3) | C120—C121 | 1.376 (4) |
| C109—C102 | 1.529 (3) | C31—H31 | 0.9500 |
| C101—H101 | 0.99 (3) | C31—C32 | 1.375 (5) |
| C101—C107 | 1.514 (3) | C114—H114 | 0.9500 |
| C101—C106 | 1.527 (3) | C114—C115 | 1.381 (4) |
| C101—C102 | 1.536 (3) | C116—H116 | 0.9500 |
| C104—C105 | 1.493 (3) | C116—C115 | 1.390 (4) |
| C104—C103 | 1.356 (3) | C25—H25 | 0.9500 |
| C104—C117 | 1.497 (3) | C25—C26 | 1.378 (5) |
| C105—C106 | 1.346 (3) | C27—H27 | 0.9500 |
| C5—C6 | 1.348 (3) | C27—C26 | 1.374 (5) |
| C106—C129 | 1.490 (3) | C121—H121 | 0.9500 |
| C102—H102 | 0.96 (3) | C121—C122 | 1.386 (4) |
| C102—C103 | 1.533 (3) | C8—H8A | 0.9800 |
| C2—H2 | 0.98 (3) | C8—H8B | 0.9800 |
| C2—C1 | 1.539 (3) | C8—H8C | 0.9800 |
| C6—C1 | 1.530 (3) | C122—H122 | 0.9500 |
| C129—C134 | 1.391 (3) | C128—H128 | 0.9500 |
| C129—C130 | 1.392 (3) | C128—C127 | 1.394 (4) |
| C11—C12 | 1.394 (3) | C13—H13 | 0.9500 |
| C11—C16 | 1.393 (3) | C13—C14 | 1.376 (4) |
| C21—H21 | 0.9500 | C20—H20 | 0.9500 |
| C21—C22 | 1.391 (4) | C132—H132 | 0.9500 |
| C21—C20 | 1.383 (4) | C26—H26 | 0.9500 |
| C34—H34 | 0.9500 | C14—H14 | 0.9500 |
| C34—C33 | 1.388 (3) | C33—H33 | 0.9500 |
| C18—H18 | 0.9500 | C33—C32 | 1.376 (4) |
| C18—C17 | 1.397 (3) | C32—H32 | 0.9500 |
| C18—C19 | 1.391 (3) | C110—H11A | 0.9800 |
| C103—C111 | 1.489 (3) | C110—H11B | 0.9800 |
| C111—C112 | 1.398 (3) | C110—H11C | 0.9800 |
| C111—C116 | 1.396 (3) | C10—H10A | 0.9800 |
| C30—H30 | 0.9500 | C10—H10B | 0.9800 |
| C30—C31 | 1.393 (4) | C10—H10C | 0.9800 |
| C134—H134 | 0.9500 | C126—H126 | 0.9500 |
| C134—C133 | 1.392 (3) | C126—C127 | 1.368 (6) |
| C1—H1 | 0.98 (3) | C126—C125 | 1.373 (6) |
| C17—C22 | 1.392 (3) | C127—H127 | 0.9500 |
| C24—H24 | 0.9500 | C115—H115 | 0.9500 |
| C24—C25 | 1.389 (4) | C108—H10D | 0.9800 |
| C22—H22 | 0.9500 | C108—H10E | 0.9800 |
| C12—H12 | 0.9500 | C108—H10F | 0.9800 |
| C12—C13 | 1.392 (3) | C125—H125 | 0.9500 |
| C107—O101—C108 | 115.4 (2) | C15—C16—C11 | 120.6 (2) |
| C109—O103—C110 | 115.50 (19) | C15—C16—H16 | 119.7 |
| C7—O1—C8 | 115.0 (2) | C111—C112—H112 | 119.5 |
| C9—O3—C10 | 114.9 (2) | C113—C112—C111 | 120.9 (2) |
| O4—C9—O3 | 124.1 (2) | C113—C112—H112 | 119.5 |
| O4—C9—C2 | 123.3 (2) | C129—C130—H130 | 119.9 |
| O3—C9—C2 | 112.58 (18) | C129—C130—C131 | 120.2 (2) |
| C124—C123—C105 | 121.3 (2) | C131—C130—H130 | 119.9 |
| C128—C123—C105 | 120.1 (2) | C23—C28—H28 | 119.5 |
| C128—C123—C124 | 118.6 (2) | C27—C28—C23 | 120.9 (3) |
| C34—C29—C6 | 120.32 (19) | C27—C28—H28 | 119.5 |
| C34—C29—C30 | 118.8 (2) | C123—C124—H124 | 120.0 |
| C30—C29—C6 | 120.9 (2) | C125—C124—C123 | 120.0 (3) |
| O2—C7—O1 | 123.8 (2) | C125—C124—H124 | 120.0 |
| O2—C7—C1 | 124.2 (2) | C112—C113—H113 | 119.7 |
| O1—C7—C1 | 112.05 (18) | C114—C113—C112 | 120.6 (2) |
| C3—C4—C5 | 120.32 (19) | C114—C113—H113 | 119.7 |
| C3—C4—C17 | 122.02 (19) | C118—C119—H119 | 119.9 |
| C5—C4—C17 | 117.58 (18) | C120—C119—H119 | 119.9 |
| C4—C3—C2 | 119.78 (18) | C120—C119—C118 | 120.1 (3) |
| C4—C3—C11 | 124.55 (19) | C18—C19—H19 | 120.0 |
| C11—C3—C2 | 115.66 (18) | C20—C19—C18 | 120.0 (3) |
| C24—C23—C5 | 122.0 (2) | C20—C19—H19 | 120.0 |
| C24—C23—C28 | 118.4 (2) | C130—C131—H131 | 120.0 |
| C28—C23—C5 | 119.6 (2) | C132—C131—C130 | 120.0 (3) |
| O103—C109—C102 | 112.96 (17) | C132—C131—H131 | 120.0 |
| O104—C109—O103 | 123.5 (2) | C16—C15—H15 | 119.8 |
| O104—C109—C102 | 123.5 (2) | C14—C15—C16 | 120.4 (3) |
| C107—C101—H101 | 106.2 (15) | C14—C15—H15 | 119.8 |
| C107—C101—C106 | 112.23 (17) | C117—C118—H118 | 119.6 |
| C107—C101—C102 | 110.49 (17) | C119—C118—C117 | 120.8 (2) |
| C106—C101—H101 | 109.1 (15) | C119—C118—H118 | 119.6 |
| C106—C101—C102 | 111.29 (17) | C119—C120—H120 | 120.2 |
| C102—C101—H101 | 107.2 (16) | C121—C120—C119 | 119.7 (2) |
| C105—C104—C117 | 117.26 (18) | C121—C120—H120 | 120.2 |
| C103—C104—C105 | 120.32 (18) | C30—C31—H31 | 120.0 |
| C103—C104—C117 | 122.36 (19) | C32—C31—C30 | 120.0 (3) |
| C104—C105—C123 | 118.82 (18) | C32—C31—H31 | 120.0 |
| C106—C105—C123 | 120.74 (18) | C113—C114—H114 | 120.4 |
| C106—C105—C104 | 120.42 (18) | C113—C114—C115 | 119.2 (2) |
| O101—C107—C101 | 110.97 (18) | C115—C114—H114 | 120.4 |
| O102—C107—O101 | 123.7 (2) | C111—C116—H116 | 119.8 |
| O102—C107—C101 | 125.4 (2) | C115—C116—C111 | 120.5 (2) |
| C4—C5—C23 | 118.71 (18) | C115—C116—H116 | 119.8 |
| C6—C5—C4 | 120.79 (19) | C24—C25—H25 | 119.7 |
| C6—C5—C23 | 120.48 (18) | C26—C25—C24 | 120.6 (3) |
| C105—C106—C101 | 118.93 (18) | C26—C25—H25 | 119.7 |
| C105—C106—C129 | 121.73 (18) | C28—C27—H27 | 119.9 |
| C129—C106—C101 | 119.31 (17) | C26—C27—C28 | 120.3 (3) |
| C109—C102—C101 | 114.49 (17) | C26—C27—H27 | 119.9 |
| C109—C102—H102 | 103.6 (16) | C120—C121—H121 | 119.6 |
| C109—C102—C103 | 111.72 (17) | C120—C121—C122 | 120.7 (3) |
| C101—C102—H102 | 108.5 (16) | C122—C121—H121 | 119.6 |
| C103—C102—C101 | 109.15 (17) | O1—C8—H8A | 109.5 |
| C103—C102—H102 | 109.1 (16) | O1—C8—H8B | 109.5 |
| C9—C2—H2 | 103.9 (16) | O1—C8—H8C | 109.5 |
| C9—C2—C1 | 113.86 (16) | H8A—C8—H8B | 109.5 |
| C3—C2—C9 | 111.29 (17) | H8A—C8—H8C | 109.5 |
| C3—C2—H2 | 108.7 (16) | H8B—C8—H8C | 109.5 |
| C3—C2—C1 | 109.97 (17) | C117—C122—H122 | 119.8 |
| C1—C2—H2 | 108.8 (16) | C121—C122—C117 | 120.4 (2) |
| C29—C6—C1 | 118.19 (17) | C121—C122—H122 | 119.8 |
| C5—C6—C29 | 122.85 (18) | C123—C128—H128 | 119.7 |
| C5—C6—C1 | 118.93 (18) | C123—C128—C127 | 120.6 (3) |
| C134—C129—C106 | 119.5 (2) | C127—C128—H128 | 119.7 |
| C134—C129—C130 | 118.7 (2) | C12—C13—H13 | 119.9 |
| C130—C129—C106 | 121.7 (2) | C14—C13—C12 | 120.3 (2) |
| C12—C11—C3 | 120.1 (2) | C14—C13—H13 | 119.9 |
| C16—C11—C3 | 121.59 (19) | C21—C20—H20 | 120.0 |
| C16—C11—C12 | 118.3 (2) | C19—C20—C21 | 120.0 (2) |
| C22—C21—H21 | 120.0 | C19—C20—H20 | 120.0 |
| C20—C21—H21 | 120.0 | C133—C132—C131 | 120.2 (2) |
| C20—C21—C22 | 120.0 (3) | C133—C132—H132 | 119.9 |
| C29—C34—H34 | 119.9 | C131—C132—H132 | 119.9 |
| C33—C34—C29 | 120.2 (2) | C25—C26—H26 | 120.2 |
| C33—C34—H34 | 119.9 | C27—C26—C25 | 119.6 (2) |
| C17—C18—H18 | 119.6 | C27—C26—H26 | 120.2 |
| C19—C18—H18 | 119.6 | C15—C14—H14 | 120.3 |
| C19—C18—C17 | 120.8 (2) | C13—C14—C15 | 119.5 (2) |
| C104—C103—C102 | 119.08 (18) | C13—C14—H14 | 120.3 |
| C104—C103—C111 | 124.94 (19) | C34—C33—H33 | 119.7 |
| C111—C103—C102 | 115.87 (18) | C32—C33—C34 | 120.6 (2) |
| C112—C111—C103 | 120.8 (2) | C32—C33—H33 | 119.7 |
| C116—C111—C103 | 120.99 (19) | C31—C32—C33 | 120.0 (2) |
| C116—C111—C112 | 118.0 (2) | C31—C32—H32 | 120.0 |
| C29—C30—H30 | 119.8 | C33—C32—H32 | 120.0 |
| C31—C30—C29 | 120.4 (2) | O103—C110—H11A | 109.5 |
| C31—C30—H30 | 119.8 | O103—C110—H11B | 109.5 |
| C129—C134—H134 | 119.8 | O103—C110—H11C | 109.5 |
| C129—C134—C133 | 120.5 (2) | H11A—C110—H11B | 109.5 |
| C133—C134—H134 | 119.8 | H11A—C110—H11C | 109.5 |
| C7—C1—C2 | 110.82 (17) | H11B—C110—H11C | 109.5 |
| C7—C1—C6 | 110.29 (16) | O3—C10—H10A | 109.5 |
| C7—C1—H1 | 107.3 (16) | O3—C10—H10B | 109.5 |
| C2—C1—H1 | 107.5 (16) | O3—C10—H10C | 109.5 |
| C6—C1—C2 | 111.88 (17) | H10A—C10—H10B | 109.5 |
| C6—C1—H1 | 108.9 (16) | H10A—C10—H10C | 109.5 |
| C18—C17—C4 | 121.7 (2) | H10B—C10—H10C | 109.5 |
| C22—C17—C4 | 119.9 (2) | C127—C126—H126 | 120.2 |
| C22—C17—C18 | 118.3 (2) | C127—C126—C125 | 119.6 (3) |
| C23—C24—H24 | 119.9 | C125—C126—H126 | 120.2 |
| C25—C24—C23 | 120.2 (3) | C128—C127—H127 | 119.8 |
| C25—C24—H24 | 119.9 | C126—C127—C128 | 120.4 (3) |
| C21—C22—C17 | 120.8 (3) | C126—C127—H127 | 119.8 |
| C21—C22—H22 | 119.6 | C114—C115—C116 | 120.8 (2) |
| C17—C22—H22 | 119.6 | C114—C115—H115 | 119.6 |
| C11—C12—H12 | 119.6 | C116—C115—H115 | 119.6 |
| C13—C12—C11 | 120.9 (2) | O101—C108—H10D | 109.5 |
| C13—C12—H12 | 119.6 | O101—C108—H10E | 109.5 |
| C134—C133—H133 | 119.9 | O101—C108—H10F | 109.5 |
| C132—C133—C134 | 120.3 (3) | H10D—C108—H10E | 109.5 |
| C132—C133—H133 | 119.9 | H10D—C108—H10F | 109.5 |
| C118—C117—C104 | 121.9 (2) | H10E—C108—H10F | 109.5 |
| C118—C117—C122 | 118.2 (2) | C124—C125—H125 | 119.6 |
| C122—C117—C104 | 119.8 (2) | C126—C125—C124 | 120.8 (3) |
| C11—C16—H16 | 119.7 | C126—C125—H125 | 119.6 |
| O4—C9—C2—C3 | 112.9 (2) | C102—C103—C111—C116 | −42.2 (3) |
| O4—C9—C2—C1 | −122.1 (2) | C2—C3—C11—C12 | −129.8 (2) |
| O103—C109—C102—C101 | −52.7 (2) | C2—C3—C11—C16 | 47.6 (3) |
| O103—C109—C102—C103 | 72.0 (2) | C6—C29—C34—C33 | −179.7 (2) |
| O2—C7—C1—C2 | −44.6 (3) | C6—C29—C30—C31 | 179.8 (2) |
| O2—C7—C1—C6 | 79.8 (3) | C129—C134—C133—C132 | 0.0 (4) |
| O1—C7—C1—C2 | 136.50 (18) | C129—C130—C131—C132 | −1.1 (4) |
| O1—C7—C1—C6 | −99.1 (2) | C11—C3—C2—C9 | −89.4 (2) |
| O3—C9—C2—C3 | −64.5 (2) | C11—C3—C2—C1 | 143.46 (19) |
| O3—C9—C2—C1 | 60.5 (2) | C11—C12—C13—C14 | −0.3 (4) |
| O104—C109—C102—C101 | 129.4 (2) | C11—C16—C15—C14 | 0.5 (4) |
| O104—C109—C102—C103 | −105.9 (2) | C34—C29—C6—C5 | 59.7 (3) |
| C9—C2—C1—C7 | 46.1 (2) | C34—C29—C6—C1 | −118.4 (2) |
| C9—C2—C1—C6 | −77.4 (2) | C34—C29—C30—C31 | −1.3 (3) |
| C123—C105—C106—C101 | −179.44 (18) | C34—C33—C32—C31 | −1.0 (4) |
| C123—C105—C106—C129 | −1.4 (3) | C18—C17—C22—C21 | 0.2 (3) |
| C123—C124—C125—C126 | 0.6 (4) | C18—C19—C20—C21 | 0.1 (4) |
| C123—C128—C127—C126 | −0.5 (4) | C103—C104—C105—C123 | 163.0 (2) |
| C29—C6—C1—C7 | 21.4 (3) | C103—C104—C105—C106 | −15.3 (3) |
| C29—C6—C1—C2 | 145.28 (18) | C103—C104—C117—C118 | 132.3 (2) |
| C29—C34—C33—C32 | −0.2 (4) | C103—C104—C117—C122 | −51.8 (3) |
| C29—C30—C31—C32 | 0.2 (4) | C103—C111—C112—C113 | −174.8 (2) |
| C4—C3—C2—C9 | 91.4 (2) | C103—C111—C116—C115 | 175.3 (3) |
| C4—C3—C2—C1 | −35.7 (3) | C111—C112—C113—C114 | 0.3 (4) |
| C4—C3—C11—C12 | 49.4 (3) | C111—C116—C115—C114 | −1.2 (5) |
| C4—C3—C11—C16 | −133.2 (3) | C30—C29—C6—C5 | −121.4 (2) |
| C4—C5—C6—C29 | −177.45 (19) | C30—C29—C6—C1 | 60.5 (3) |
| C4—C5—C6—C1 | 0.7 (3) | C30—C29—C34—C33 | 1.4 (3) |
| C4—C17—C22—C21 | 176.8 (2) | C30—C31—C32—C33 | 1.0 (4) |
| C3—C4—C5—C23 | −163.1 (2) | C134—C129—C130—C131 | 1.7 (3) |
| C3—C4—C5—C6 | 15.2 (3) | C134—C133—C132—C131 | 0.6 (4) |
| C3—C4—C17—C18 | −132.3 (2) | C17—C4—C3—C2 | −172.26 (19) |
| C3—C4—C17—C22 | 51.2 (3) | C17—C4—C3—C11 | 8.6 (3) |
| C3—C2—C1—C7 | 171.79 (17) | C17—C4—C5—C23 | 13.6 (3) |
| C3—C2—C1—C6 | 48.2 (2) | C17—C4—C5—C6 | −168.1 (2) |
| C3—C11—C12—C13 | 178.3 (2) | C17—C18—C19—C20 | 0.4 (3) |
| C3—C11—C16—C15 | −178.3 (2) | C24—C23—C5—C4 | −122.2 (2) |
| C23—C5—C6—C29 | 0.8 (3) | C24—C23—C5—C6 | 59.5 (3) |
| C23—C5—C6—C1 | 178.93 (18) | C24—C23—C28—C27 | −0.3 (3) |
| C23—C24—C25—C26 | −0.9 (4) | C24—C25—C26—C27 | 0.5 (4) |
| C23—C28—C27—C26 | −0.1 (4) | C22—C21—C20—C19 | −0.5 (4) |
| C109—C102—C103—C104 | −89.5 (2) | C12—C11—C16—C15 | −0.9 (4) |
| C109—C102—C103—C111 | 94.1 (2) | C12—C13—C14—C15 | −0.1 (4) |
| C101—C106—C129—C134 | 115.8 (2) | C117—C104—C105—C123 | −14.3 (3) |
| C101—C106—C129—C130 | −64.9 (3) | C117—C104—C105—C106 | 167.5 (2) |
| C101—C102—C103—C104 | 38.2 (3) | C117—C104—C103—C102 | 171.84 (19) |
| C101—C102—C103—C111 | −138.24 (19) | C117—C104—C103—C111 | −12.1 (3) |
| C104—C105—C106—C101 | −1.3 (3) | C16—C11—C12—C13 | 0.8 (4) |
| C104—C105—C106—C129 | 176.77 (18) | C16—C15—C14—C13 | 0.1 (4) |
| C104—C103—C111—C112 | −43.7 (3) | C112—C111—C116—C115 | 0.5 (4) |
| C104—C103—C111—C116 | 141.7 (2) | C112—C113—C114—C115 | −1.0 (4) |
| C104—C117—C118—C119 | 176.6 (2) | C130—C129—C134—C133 | −1.2 (3) |
| C104—C117—C122—C121 | −175.8 (2) | C130—C131—C132—C133 | −0.1 (4) |
| C105—C123—C124—C125 | 177.7 (2) | C28—C23—C5—C4 | 58.8 (3) |
| C105—C123—C128—C127 | −177.8 (2) | C28—C23—C5—C6 | −119.5 (2) |
| C105—C104—C103—C102 | −5.2 (3) | C28—C23—C24—C25 | 0.8 (3) |
| C105—C104—C103—C111 | 170.81 (19) | C28—C27—C26—C25 | 0.0 (4) |
| C105—C104—C117—C118 | −50.5 (3) | C124—C123—C105—C104 | 122.2 (2) |
| C105—C104—C117—C122 | 125.3 (2) | C124—C123—C105—C106 | −59.6 (3) |
| C105—C106—C129—C134 | −62.2 (3) | C124—C123—C128—C127 | 0.7 (3) |
| C105—C106—C129—C130 | 117.1 (2) | C113—C114—C115—C116 | 1.4 (5) |
| C107—C101—C106—C105 | 159.64 (19) | C119—C120—C121—C122 | 0.2 (4) |
| C107—C101—C106—C129 | −18.4 (3) | C19—C18—C17—C4 | −177.1 (2) |
| C107—C101—C102—C109 | −50.6 (2) | C19—C18—C17—C22 | −0.6 (3) |
| C107—C101—C102—C103 | −176.70 (17) | C118—C117—C122—C121 | 0.2 (3) |
| C5—C4—C3—C2 | 4.3 (3) | C118—C119—C120—C121 | 0.7 (4) |
| C5—C4—C3—C11 | −174.8 (2) | C120—C119—C118—C117 | −1.2 (4) |
| C5—C4—C17—C18 | 51.0 (3) | C120—C121—C122—C117 | −0.6 (4) |
| C5—C4—C17—C22 | −125.4 (2) | C116—C111—C112—C113 | −0.1 (3) |
| C5—C23—C24—C25 | −178.2 (2) | C8—O1—C7—O2 | −7.3 (3) |
| C5—C23—C28—C27 | 178.7 (2) | C8—O1—C7—C1 | 171.6 (2) |
| C5—C6—C1—C7 | −156.78 (19) | C122—C117—C118—C119 | 0.7 (3) |
| C5—C6—C1—C2 | −32.9 (3) | C128—C123—C105—C104 | −59.4 (3) |
| C106—C101—C107—O101 | 91.5 (2) | C128—C123—C105—C106 | 118.9 (2) |
| C106—C101—C107—O102 | −87.5 (3) | C128—C123—C124—C125 | −0.7 (3) |
| C106—C101—C102—C109 | 74.7 (2) | C20—C21—C22—C17 | 0.4 (4) |
| C106—C101—C102—C103 | −51.3 (2) | C110—O103—C109—O104 | −7.8 (3) |
| C106—C129—C134—C133 | 178.2 (2) | C110—O103—C109—C102 | 174.3 (2) |
| C106—C129—C130—C131 | −177.7 (2) | C10—O3—C9—O4 | 9.6 (3) |
| C102—C101—C107—O101 | −143.65 (18) | C10—O3—C9—C2 | −173.1 (2) |
| C102—C101—C107—O102 | 37.3 (3) | C127—C126—C125—C124 | −0.4 (4) |
| C102—C101—C106—C105 | 35.2 (3) | C108—O101—C107—O102 | 5.3 (3) |
| C102—C101—C106—C129 | −142.85 (18) | C108—O101—C107—C101 | −173.7 (2) |
| C102—C103—C111—C112 | 132.4 (2) | C125—C126—C127—C128 | 0.3 (4) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C101—H101···O2 | 0.99 (3) | 2.39 (3) | 3.384 (3) | 176 (2) |
| C102—H102···O4 | 0.96 (3) | 2.48 (3) | 3.242 (3) | 136 (2) |
| C16—H16···O4 | 0.95 | 2.59 | 3.407 (3) | 145 |
| C116—H116···O104 | 0.95 | 2.54 | 3.388 (3) | 148 |
References
- Allen, C. F. H. (1945). Chem. Rev. 37, 209–268. [DOI] [PubMed]
- Allen, C. F. H. (1962). Chem. Rev. 62, 653–664.
- Allen, C. F. H. & Sheps, L. J. (1934). Can. J. Res. 11, 171–179.
- Boeyens, J. C. A. (1978). J. Cryst. Mol. Struct. 8, 317–320.
- Bruker (2013). APEX2 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2014). SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- De Maré, G. R., Panchenko, Y. N. & Vander Auwera, J. (1997). J. Phys. Chem. A, 101, 3998–4004.
- Desiraju, G. R. & Steiner, T. (1999). The Weak Hydrogen Bond in Structural Chemistry and Biology. New York: Oxford University Press Inc.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Fuchs, B. & Yankelevich, S. (1968). Isr. J. Chem. 6, 511–515.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Pal, R., Mukherjee, S., Chandrasekhar, S. & Guru Row, T. N. (2014). J. Phys. Chem. A, 118, 3479–3489. [DOI] [PubMed]
- Rabideau, P. W. & Sygula, A. (1989). The conformational analysis of cyclohexenes, cyclohexadienes and related hydroaromatic compounds, edited by P. W. Rabideau, pp. 67–53. New York: VCH.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. A71, 3–8.
- Takahashi, T., Xi, Z., Yamazaki, A., Liu, Y., Nakajima, K. & Kotora, M. (1998). J. Am. Chem. Soc. 120, 1672–1680.
- Traetteberg, M. (1968). Acta Chem. Scand. 22, 2305–2312.
- Wang, X., Eckert, J., Liu, L. & Jacobson, A. J. (2011). Inorg. Chem. 50, 2028–2036. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989016009403/zl2665sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989016009403/zl2665Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989016009403/zl2665Isup3.cdx
Supporting information file. DOI: 10.1107/S2056989016009403/zl2665Isup4.cml
CCDC reference: 1484412
Additional supporting information: crystallographic information; 3D view; checkCIF report