Abstract
Atrazine (ATZ) was administered daily by gavage to pregnant female Sprague Dawley rats at doses of 0, 6.25, 25 or 50 mg/kg/day, either during gestation, lactation and post‐weaning (G/L/PW cohort) to F1 generation female offspring or only from postnatal day (PND 21) until five days after sexual maturation (vaginal opening) when the estrogen‐primed, luteinizing hormone (LH) surge was evaluated (PW cohort). Additional subgroups of F1 females received the vehicle or ATZ from PND 21–133 or from PND 120–133. Slight reductions in fertility and the percentage of F1 generation pups surviving to PND 21 in the gestationally exposed 50 mg/kg dose group were accompanied by decreased food intake and body weight of dams and F1 generation offspring. The onset of puberty was delayed in of the F1 generation G/L/PW females at doses of 25 and 50 mg/kg/day. F1 generation females in the PW high‐dose ATZ group also experienced a delay in the onset of puberty. ATZ had no effect on peak LH or LH AUC in ovariectomized rats 5 days after sexual maturation, irrespective of whether the F1 generation females were treated from gestation onward or only peripubertally. There was no effect of ATZ treatment on the estrous cycle, peak LH or LH AUC of F1 generation females exposed from gestation through to PND 133 or only for two weeks from PND 120–133. These results indicate that developing females exposed to ATZ are not more sensitive compared to animals exposed to ATZ as young adults
Keywords: hormones, cyclicity, risk assessment, postnatal evaluation
INTRODUCTION
The chlorotriazine herbicide atrazine (2‐chloro‐4‐[ethylamino]‐6‐[isopropyamino]‐s‐triazine) is utilized in agriculture for weed control in corn and sugarcane (Bridges, 2008). Atrazine (ATZ) inhibits electron transport in the photosynthetic pathway (photosystem II) of plants by reversibly binding to the D1 protein (Devine et al., 1993; Trebst, 2008). Oral (gavage) administration of ATZ suppressed the estrogen‐induced (Simpkins et al., 2011) or estrogen‐plus‐progesterone‐induced (McMullin et al., 2004) luteinizing hormone (LH) surge in ovariectomized adult female Sprague–Dawley (SD) (Simpkins et al., 2011), Long Evans (Cooper et al., 2000, 2007), and Wistar (McMullin et al., 2004; Foradori et al., 2009a) rats. ATZ also suppressed the endogenous LH surge that occurs on the afternoon of proestrus in intact, normally cycling female SD rats when administered as a gavage bolus dose of 50 mg/kg/day, but not when administered as a temporally distributed dose in feed (500 ppm) at equivalent daily doses (Foradori et al., 2014). Gavage doses of 50 mg ATZ/day administered to weanling female SD (Ashby et al., 2002) or Wistar rats (Laws et al., 2000) delayed the onset of sexual maturation as indicated by a prolongation in the number of days needed to achieve vaginal patency.
In the conduct of risk assessments it is often assumed, based upon scientific considerations (Dourson et al., 2002; Scheuplein et al., 2002) or legal mandate (Food Quality Protection Act [FQPA]; U.S. Food and Drug Administration, 1996), that developing and prepubertal children are more sensitive to the effects of chemicals than are adults. In this study, we investigated whether female SD rats exposed to ATZ in utero were more sensitive to the effect of ATZ on sexual maturation or the estrogen‐induced LH surge compared to rats exposed to ATZ only postweaning (PW). These data are critical for determining whether the default 10‐fold FQPA uncertainty factor should be retained in the regulation of exposure of children to ATZ.
In this study, the effect of ATZ on sexual maturation and on the LH surge was evaluated in a cohort of female offspring that were exposed during gestation, lactation, and PW (G/L/PW) compared to a cohort of females that were exposed only PW. We found that neither developing fetuses, nor animals administered ATZ PW until sexual maturation and the commencement of the estrous cycle were more sensitive to the effect of ATZ on the LH surge than were young adult, 19‐week‐old females. The no observed effect level (NOEL) based upon sexual maturation delay observed in this study was greater in F1 progeny administered ATZ only PW (NOEL = 25 mg/kg) compared to animals exposed to ATZ during gestation, lactation, and PW (NOEL = 6.5 mg/kg/day). However, these NOELs were similar to those reported by other investigators who evaluated the effects of ATZ on sexual maturation when administered either during gestation, lactation, and PW (NOEL = 20 mg/kg/day) (Davis et al., 2011) or only PW (NOEL = 10–12.5 mg/kg/day) (Laws et al., 2000; Ashby et al., 2002).
MATERIALS AND METHODS
This study assessed the effect of ATZ on sexual maturation and the estrogen‐induced LH surge in female SD rats exposed to ATZ in utero and/or PW. The in‐life portion of this study was conducted at WIL Research, LLC, according to the USEPA Good Laboratory Practices regulations (40 CFR Parts 160 and 792), the WIL Institutional Animal Care and Use Committee, and the study director‐approved protocol.
Experimental Design
The study was composed of two cohorts of animals (Fig. 1). The G/L/PW cohort (top panel, Fig. 1) was composed of groups of F0 generation females and their F1 generation offspring that were exposed to ATZ from Gestation Day 0 (GD 0) through to the end of lactation on Postnatal Day 21 (PND 21). Nested within the G/L/PW cohort were two subgroups (Groups 1 and 2), each composed of a vehicle control group and three ATZ‐treated groups. F1 generation pups in Group 1 were administered the vehicle control or ATZ by gavage PW from PND 21 until 5 days after they attained sexual maturation, whereas Group 2, F1 generation females were treated PW until PND 133.
Figure 1.
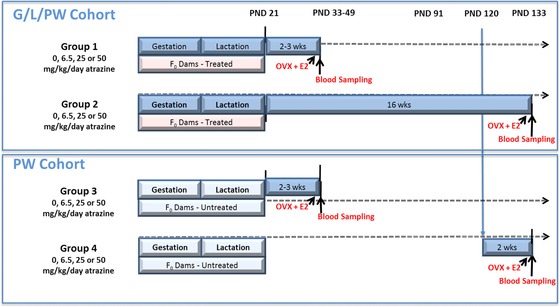
Study design for groups of F0 dams and their F1 generation progeny exposed to ATZ (blue bars) during gestation, lactation, and postweaning (G/L/PW: Groups 1 and 2) or only postweaning (PW: Groups 3 and 4).
F0 generation females in the PW cohort (bottom panel, Fig. 1) were untreated during gestation and lactation, but the F1 generation females received ATZ or the vehicle daily by gavage from PND 21 until 5 days after sexual maturation (Group 3), or from PND 120 to PND 133 (Group 4). Two additional groups of animals, one group each in the G/L/PW (Group 2R) and the PW cohort (Group 4R), were included in the study to assess recovery from the effect of treatment on LH. The LH data from Groups 2R and 4R are not provided because there was no effect of treatment on LH. However, for the purpose of statistical analyses, data on body weight, food consumption, sexual maturation, and vaginal smears, collected from control animals in these two groups, were combined with their respective controls in the G/L/PW and PW cohorts. Data from ATZ‐treated groups in Group 2 were combined with the corresponding data from Group 2R, and likewise, data for dose groups in Group 4 were combined with data from Group 4R, as shown in Table 1.
Table 1.
Allocation of F0 and F1 Animals to Groups for the Purpose of Statistical Analyses
| Atrazine dose (mg/kg/day) | |||||
|---|---|---|---|---|---|
| Cohort parameters | Data source | 0a | 6.5 | 25 | 50 |
| Cohort 1: Atrazine administered during gestation/lactation and postweaning (G/L/PW cohort) | |||||
| Number of litters produced by F0 dams | —‐ | 25 | 25 | 25 | 23 |
| Number of F1 females: Group 1, PND 21 | Figure 3, Panel B | 118 | 20 | 24 | 17 |
| Number of F1 females: Group 2, PND 21b, c | Figure 4, Panel A | 50 | 40 | 47 | 31 |
| Number of litters: VO | Figure 5 | 144 | 60 | 70 | 59 |
| Number of F0 litters: VO versus litter mean weight gain | Figure 6a | 119 | 20 | 24 | 17 |
| Number of Group 2, F1 females: Estrous cycle | Figure 9 | 48 | 20 | 23 | 20 |
| Number of Group 1, F1 females: LH surge | Figure 8 | 15 | 17 | 19 | 19 |
| Number of Group 1, F1 females: LH surge AUC | Figure 8 | 9 | 12 | 12 | 13 |
| Number of Group 2, F1 females: LH surge | Figure 8 | 19 | 20 | 19 | 18 |
| Number of Group 2, F1 females: LH surge AUC | Figure 8 | 19 | 18 | 19 | 20 |
| Cohort 2: Atrazine administered only postweaning (PW cohort) | |||||
| Number of litters | —‐ | 25 | 25 | 24 | 25 |
| Number of F1 females: Group 3, PND 21 | Figure 3, Panel D | 24 | 24 | 21 | 24 |
| Number of F1 females: Group 4, PND21b, c | Figure 4, Panel C | 47 | 48 | 42 | 49 |
| Number of litters: VO | Figure 5 | 144 | 24 | 21 | 24 |
| Number of F0 litters: VO versus litter mean weight gain | Figure 6b | 119 | 21 | 24 | 24 |
| Number of Group 4, F1 females: Estrous cycle | Figure 9 | 48 | 24 | 21 | 24 |
| Number of Group 3, F1 females: LH surge | Figure 8 | 20 | 20 | 19 | 18 |
| Number of Group 3, F1 females: LH surge AUC | Figure 8 | 17 | 13 | 16 | 15 |
| Number of Group 4, F1 females: LH surge | Figure 8 | 20 | 19 | 19 | 19 |
| Number of Group 4, F1 females: LH surge AUC | Figure 8 | 20 | 18 | 19 | 18 |
Vehicle control litters in Groups 1, 2, 2R, 3, 4, and 4R were used to create a combined control group of vehicle‐treated animals.
Data collected during the period from PND 1‐49 for F1 females assigned to recovery Groups 2R and 4R were combined with data from the corresponding ATZ treatment Groups 2 and 4 because the animals in Groups 2R and 4R were treated the same as animals in Groups 2 and 4, respectively, during this interval of time.
Data collected for the period from PND 1 to 21 for F1 females in recovery Group 4R were combined with data from the vehicle control group in Cohorts 1 and 2 because the animals in Group 4R were untreated during this period.
Animals
Two hundred and twenty‐five young adult (13 weeks of age) female SD rats (Crl:CD(SD)) were obtained from Charles River Laboratories (Raleigh, NC), and were maintained in AAALAC‐approved animal facilities at WIL (Ashland, OH). Animals were housed individually in clean, suspended wire‐mesh cages (Allentown Inc., Allentown, NJ) in an environmentally controlled room (22 ± 3°C; 50 ± 20% humidity) on a 14‐hr light/10‐hr dark photoperiod (lights‐on at 0500 h, lights‐off at 1900 h). Water and food (Rodent LabDiet, PMI Nutrition International, LLC) were available ad libitum. Twenty‐five F0 generation females were randomly assigned to control and each of three treatment groups in the G/L/PW or the PW‐exposed cohorts, based on stratified body weights on GD 0. A group of young adult males that was not treated with ATZ was obtained from Charles River and housed in the same animal room as the F0 generation females. These males were used to inseminate the F0 generation females and were sacrificed after PND 21. F1 generation female offspring (15–25 litters; 1–2 pups/litter) were assigned to their respective control or treated groups prenatally in the G/L/PW (Groups 1 and 2) cohort and postnatally in the PW (Groups 3 and 4) cohort; F1 generation male offspring were sacrificed after PND 21. The numbers of litters in the control group and in each of the three treatment groups are provided in Table 1 for Cohort 1 (Groups 1 and 2) and Cohort 2 (Groups 3 and 4), as defined in Figure 1.
Atrazine Treatment
ATZ was supplied by Syngenta Crop Protection, LLC, as an analytically certified, 98.8% pure, white powder that was stable for use during the period of the study. ATZ was prepared approximately weekly as a suspension in 1% methylcellulose and deionized water at concentrations of 1.3, 5, or 10 mg/ml. Suspensions were stored refrigerated (at 2–8°C) until use. Sample storage stability and sample homogeneity were assessed by high‐pressure liquid chromatography before study conduct and were within ±10% of target. The concentration of ATZ, which was assessed in every dose suspension by high‐pressure liquid chromatography, ranged from of 94.7 to 101% of target.
ATZ (6.5, 25, or 50 mg/kg/day) was administered daily by gavage to three groups of 25 mated, F0 generation, female SD rats, at a dose volume of 5 ml/kg approximately 7 to 8.45 hr (1200–1345 h) after lights‐on (0500 h), during gestation and lactation (G/L/PW cohort: Groups 1 and 2; Fig. 1). The PW cohort of F0 generation females (Groups 3 and 4; Fig. 1) was untreated during gestation and lactation. Control group animals received 5 ml/kg of the vehicle (1% methylcellulose in water).
F1 generation female offspring were administered either ATZ or the vehicle by gavage approximately 6 to 9 hr after lights‐on from PND 21 until 5 days after the attainment of sexual maturation (vaginal opening [VO], Group 1 and Group 3). To assess whether developmental exposure to ATZ increased the sensitivity of F1 generation females to the effects of ATZ, treatment continued for an additional 5 days and the LH surge was evaluated. The effect of ATZ on the LH surge was assessed on PND 133 in young adult F1 generation females that were exposed to ATZ from GD 0 until PND 133 (Group 2) or from PND 120 to PND 133 (Group 4).
Reproduction and Development
F0 generation females were placed in the home cage of an untreated male in the evening. If evidence of mating (i.e., the presence of a vaginal plug and or evidence of sperm in a vaginal lavage) was noted the following morning, which was then designated GD 0, the dam was returned to an individual plastic maternity cage (Allentown Inc., Allentown, NJ and LabProducts Inc., Seaford, DE) and provided with ground corn cob nesting material (Bed‐O'Cobs®; The Andersons, Cob Products Division, Maumee, OH). If there was no evidence of mating, the female remained paired until mating was confirmed. Individual maternal body weight and food consumption were measured on GD 0, 3, 6, 9, 12, 15, 18, and 21, as well as on PND 0 (when possible), 1, 4, 7, 10, 14, 17, and 21. All pregnant females were allowed to deliver their offspring and rear their litters until PND 21. The number of neonates was counted on PND 1, and individual neonates were weighed, sex was determined, and the litters were then standardized to eight pups (four males, four females) when possible. Parental animals and their offspring were examined twice daily for evidence of toxicity or effects of treatment on nursing or survival. Individual pup body weights were recorded on PND 1, 4, 7, 10, 14, 17, and 21.
Litter parameters were defined as follows:
Sex Ratio and Sexual Maturation (Vaginal Patency)
The pup's sex was determined on PND 0, 1, 4, 14, and 21 by evaluating the anogenital distance (Tyl and Marr, 2006). The day that the female rat became sexually mature was defined as the postnatal day when the vaginal lumen was first observed to be completely open (day of VO). Evidence of vaginal patency was first assessed on PND 25 according to the procedures of Adams et al. (1985).
Estrous Cycle
Vaginal lavages were performed daily for F1 females in Groups 2 and 4, beginning on PND 113 and continuing until PND 130, the day the animals were ovariectomized in preparation for LH determination. The slides were air dried, stained with Wright–Giemsa stain, and evaluated microscopically. The daily vaginal smears were examined and categorized as being in the proestrus [P], estrus [E], diestrus [D], or metestrus [M] stage of the estrous cycle, based on standard cytological characteristics (Freeman, 1988). Estrous cycle stages determined from vaginal smears are known to be highly correlated with estrous cycle stages based on histological examinations (Yener et al., 2007; Westwood, 2008).
Blood Sample Collection
Three days before the scheduled day of blood collection, each animal was anesthetized with isoflurane, and a polyurethane catheter was implanted into the femoral vein. The opposite end of the cannula was tunneled subcutaneously to exit the skin in the scapular region. The catheter was attached to a SAI Quick ConnectTM harness with an injection cap and a jacket adaptor. A bilateral ovariectomy (OVX) was performed, and a 12 to 14 mm Silastic® capsule (0.062″ ID × 0.125″ OD) containing estradiol benzoate (4 mg/ml in sesame oil) was implanted subcutaneously in the right flank region. This procedure was performed in the morning (0600–1200 h). Implanted estradiol capsules result in estradiol blood levels comparable to levels observed in intact animals on the morning of proestrus, resulting in a daily afternoon surge in plasma LH levels (Foradori et al., 2009a). Blood samples (approximately 0.5 ml/animal/interval) were collected from each rat via the femoral vein access port 3 days after surgery (ovaries removed and femoral cannula implanted). Blood samples were collected within 10 min of the target collection times at 1100, 1400, 1600, 1800, 2000, and 2300 h (i.e., 6, 9, 11, 13, 15, and 18 hr after lights‐on; lights were turned on at 0500 h and turned out at 1900 h). Samples were collected under red light conditions after 1900 h. Blood samples (0.5 ml/animal/interval) were collected into prechilled, uniquely labeled tubes containing sodium heparin as the anticoagulant. Blood samples were centrifuged, and plasma was put into two uniquely labeled polypropylene vials and stored at −70°C. All plasma samples were shipped on dry ice from WIL Research to the University of Arizona, College of Medicine—Phoenix. Upon receipt, the samples were catalogued and stored at −80°C until analysis of LH concentrations by radioimmunoassay (RIA).
RIA for LH
RIAs of LH were conducted according to good scientific practices under the direction of the investigator (R.J.H.) at the University of Arizona. Plasma LH concentration was measured using reagents provided by the National Hormone and Peptide Program (NHPP) (Harbor UCLA Medical Center, Torrance, CA). Rat LH‐RP3 was used to create a standard curve (range: 0.0049–10.0 ng). Tracer ovine LH (oLH, NHPP) was iodinated using the chloramine‐T method by the Colorado State University peptide assay core and shipped to the University of Arizona within 3 days of iodination. For assay, plasma samples were incubated overnight at 4°C with antiserum (NIDDK‐Anti‐rLH‐SII; diluted 1:300,000). Following incubation, iodinated oLH was added to each tube (∼10,000 cpm/tube) and incubated overnight at 4°C. Bound LH was separated from free LH by incubation with goat anti‐rabbit γ globin (NHPP, Harbor UCLA Medical Center, 1:1000) in a 5% polyethylene glycol (Fisher Biotech; BP233‐1) solution followed by centrifugation in a Beckman J6B centrifuge (2000 × g for 15 min). The mean intra‐assay and interassay coefficients of variation were 11.5 and 3.0%, respectively, and the average lower limit of quantitation (LLOQ) was 0.61 ng/ml. For samples that were below the LLOQ (∼8% of the 1836 samples collected), the LLOQ value was assigned and used in subsequent data analysis. Almost all the LLOQ samples were collected at 0600 or 1800 h, when basal LH level was expected to be low. In 3.4% of the samples, LH levels could not be determined because of inadequate sample volume or for other technical reasons. Any RIA sample with an LH value above the upper limit of the RIA sensitivity (15% bound in a competitive‐binding assay) was diluted appropriately and reassayed, if sample volume allowed.
Data Analyses
Statistical analyses of the in‐life date were conducted by Sielken and Associates Consulting, while the LH data were analyzed by R.J.H using Prism 5.0 software (GraphPad Software Inc., San Diego, CA). F0 and F1 generation body weight and food consumption data were analyzed using a two‐tailed Dunnett's test at a 5% significance level. F0 generation reproductive indices and F1 progeny survival indices were assessed using the trend test described by Mantel (1963). The effect of litter size was evaluated using linear regression analyses. The litter mean number of days to attain vaginal patency was statistically compared between the control and ATZ‐treated groups using a two‐tailed Dunnett's test following a one‐way analysis of variance (ANOVA). In addition, an analysis of covariance was conducted where the covariate was the litter mean pup weight gain from PND 21 to 28. Regression analyses of mean litter weight gain from PND 21 to 28 versus the average day of VO for each litter was determined for each group, and the significance of correlation between these parameters (R 2) was assessed using a two‐sided t‐test.
The mean percentages of days of the estrous cycle spent in estrus (E), diestrus (D), proestrus (P), or metestrus (M) were evaluated statistically using a one‐way ANOVA followed by a two‐tailed, Dunnett's t‐test for Groups 2 and 4 animals. The estrous cycle patterns were displayed graphically (Supporting Information Table S2) for individual animals in Group 2 from PND 113 until PND 130 and from PND 120 to PND 130 for Group 4 animals. Animals that displayed two or three successive days in D or P followed by one or two successive days in M or E were considered to have normal cycles and this block of time was unfilled in the graph (white background). Animals that exceeded the specified number of days in D or E were considered to have abnormal cycles and the background was filled with either a gray bar (D) or a red bar (E) for the period of time during the exceedance occurred.
LH data were first analyzed using a two‐way ANOVA to determine treatment, dose group, and interaction effects, and post‐hoc analyses were performed using the Bonferroni method (Dunn, 1961; 1964). When a sample fell below the LLOQ (85% bound in a competitive‐binding assay), the value of the LLOQ was inserted in the data analysis so as not to bias the analysis against low values. The LH area under the curve (AUC) for each individual animal was calculated using Prism 5.0 software (GraphPad Software Inc., San Diego, CA). Peak LH levels and AUC data were analyzed by one‐way ANOVA and post‐hoc analyses using the Dunnett's test (Dunnett, 1955; 1964). The level of statistical significance was set at p ≤ 0.05 in all statistical analyses. Numerical values are reported as the mean ± the standard error of the mean (SEM). Statistically significant differences were indicated on tabular and graphical data by footnotes.
RESULTS
Maternal Body Weight, Food Consumption, and Clinical Signs
There was no statistically significant effect of ATZ on body weight during gestation of F0 generation females at doses up to 50 mg/kg/day (Fig. 2A; Supporting Information Table S1 provides group mean, SEM, and N plotted in Figs. 2, 3, 4, 5). Food consumption in the 50 mg/kg/day F0 generation females was intermittently reduced or elevated above control levels during gestation (Fig. 2B). During lactation, group mean body weight in the 50 mg/kg ATZ group was not statistically different from controls (Fig. 2C). Food consumption was significantly less than controls in the 50 mg/kg ATZ‐treated group during lactation during the PND intervals from 1 to 4, 7 to 10, 10 to 14, 14 to 17, and 17 to 21 (Fig. 2D). There were no effects of ATZ treatment on body weight or food consumption in groups administered ATZ at doses of 25 mg/kg/day or less, either during gestation or lactation. Other than the effects noted on body weight and food consumption, there were no treatment‐related symptoms of toxicity in F0 generation females at any dose.
Figure 2.
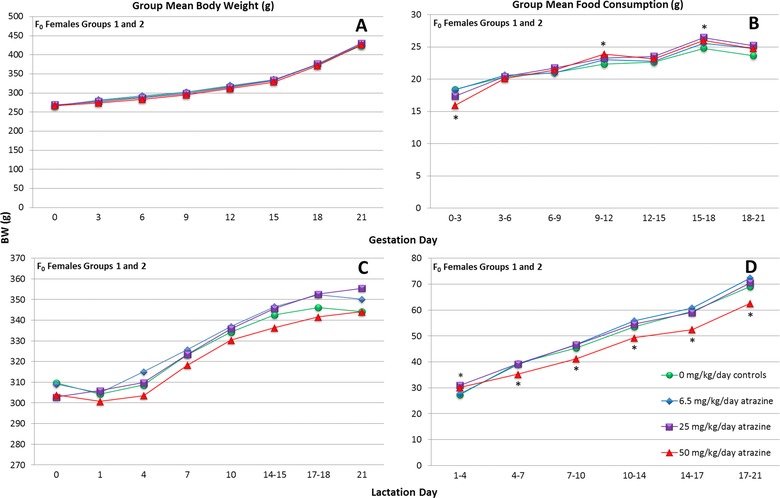
Group mean body weight (gm) and food consumption (gm/day) of ATZ‐treated, F0 generation (Groups 1 and 2 combined) female SD rats during gestation and lactation. An asterisk “*” indicates that the combined group mean is significantly difference (p < 0.05) from the control group.
Figure 3.
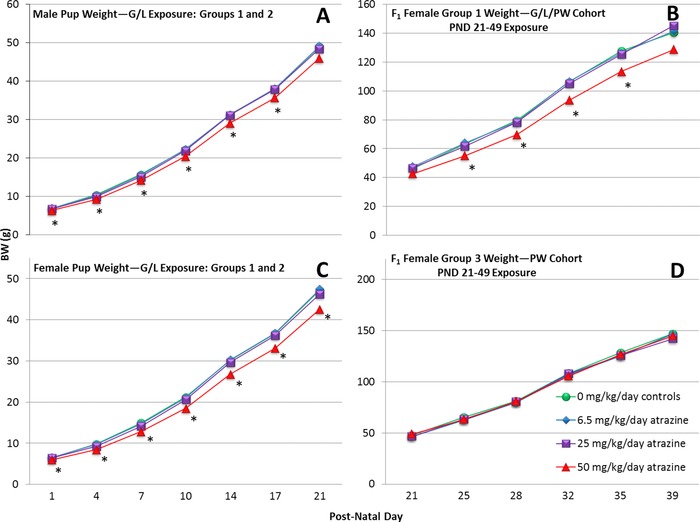
Group mean body weights (g) of ATZ‐treated F1 generation male and female offspring during lactation and F1 generation females from PND 21 until the day of VO in animals in the G/L/PW (Group 1) or the PW (Group 3) cohorts. An asterisk “*” indicates that the group mean is significantly different (p < 0.05) from the control group.
Figure 4.
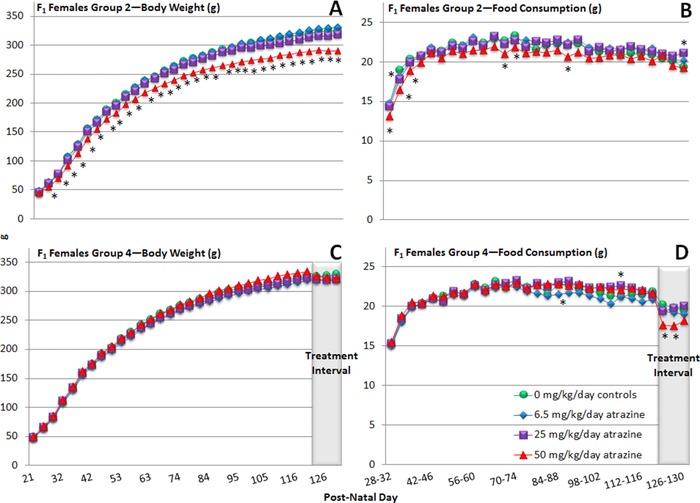
Group mean body weight (gm) and food consumption (gm/day) of F1 generation, female SD rats exposed to ATZ via their dams during gestation and lactation and by gavage from PND 21 to PND 133 (Group 2) or administered ATZ daily by gavage from PND 120 to PND 133 (Group 4). An asterisk “*” indicates that the group mean is significantly different (p < 0.05) from the control group (SEMs are not shown because they overlapped and prevented viewing the group mean symbols).
Figure 5.
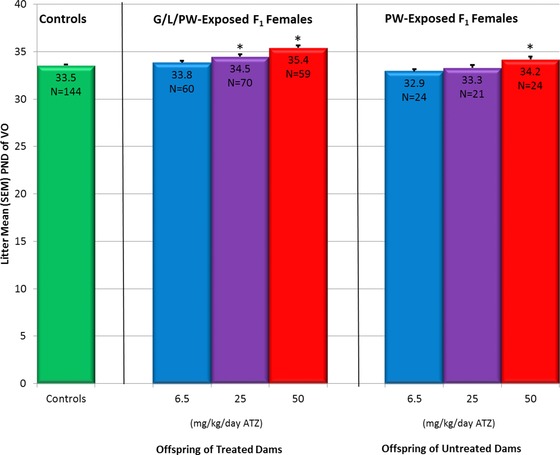
Effect of ATZ (6.5, 25, and 50 mg/kg BW) on the number of days to vaginal opening (VO) in F1 generation female SD rats exposed to ATZ via dams treated during gestation and lactation and then administered ATZ by gavage from PND 21 until 5 days after vaginal opening (Groups 1 and 2 combined, middle panel) or administered ATZ by gavage from PND 21 until 5 days after vaginal opening (Group 3, right panel). The control group mean (left panel) was statistically compared to the ATZ group means (±SEM) by using a Dunnett's test after an ANOVA test. *p < 0.05.
F0 Generation Fertility and F1 Generation Survival, Body Weight, and Food Consumption
Administration of ATZ by gavage during gestation at doses up to 50 mg/kg/day had no effect on mating, although the fertility index, the percent of sperm‐positive females that delivered live pups (gestation index), and the number of live born fetuses were significantly lower in the 50 mg/kg dose group (Group 1) compared to the controls (Table 2). There were no differences between the control and ATZ‐treated groups in the mean number of F1 generation pups born alive. However, by the end of PND 0, the mean number of pups alive per litter (i.e., live birth index) was significantly reduced in the 50 mg/kg ATZ‐treated group. The survival index, calculated on PND 21, was also significantly reduced in the high‐dose ATZ‐treated group. The sex ratio was unaffected by treatment (Table 2).
Table 2.
Fertility and Reproduction
| Dose (mg/kg/day) | 0 | 6.5 | 25 | 50 |
|---|---|---|---|---|
| F0 females | ||||
| No. cohabitated with males | 125 | 25 | 25 | 25 |
| No. with evidence (sperm positive) of mating | 124 | 25 | 25 | 25 |
| No. pregnant | 124 | 25 | 25 | 23 |
| No. with live pups | 124 | 25 | 25 | 23 |
| Mating index (%)a | 99.2 | 100 | 100 | 100 |
| Fertility index (%)b | 99.2 | 100 | 100 | 92f |
| Gestation index (%)c | 100 | 100 | 100 | 92g |
| F1 offspring | ||||
| Mean no. born with SE | 15.1 ± 0.19 | 14.7 ± 0.59 | 15.6 ± 0.51 | 15.6 ± 0.47 |
| Animals born | 1868 | 368 | 389 | 359 |
| Litters | 124 | 25 | 25 | 23 |
| Percentage males | 49.7 | 51.0 | 50.1 | 53.4 |
| Sex ratio M/F | 0.99 | 1.04 | 1.00 | 1.15 |
| Live birth index (%)d | 99.1 ± 0.25 | 97.7 ± 1.08 | 98.7 ± 0.63 | 95.4 ± 1.03g, h |
| Litters | 124 | 25 | 25 | 23 |
| Survival index (%) PND 21e | 97.6 ± 0.69 | 96.3 ± 1.84 | 95.8 ± 1.63 | 79.6 ± 6.71g, h |
| Litters | 119 | 20 | 24 | 19 |
Mating index = (number of sperm‐positive females / number of females cohabited with males) × 100.
Fertility index = (number of pregnant females / number of females cohabited with males) × 100.
Gestation index = (number of females with live born / number of sperm‐positive females) × 100.
Live birth index = mean percentage of pups per litter alive on PND 0 with SEM.
Survival index = mean percentage of live pups surviving PND 1 to PND 21 per litter with SEM.
Significantly different from control; p < 0.05.
Significantly different from control; p < 0.01.
Statistically significant dose trend (Mantel, 1963); p < 0.01.
Mean body weights of the 50 mg/kg/day, ATZ‐treated F1 generation males were significantly less than those of controls from PND 1 to PND 17 (Fig. 3A). Body weights of the F1 female offspring were reduced from PND 1 to PND 21 (Fig. 3C), and body weights of the F1 generation females were reduced in the Group 1, 50 mg/kg/day ATZ animals on PNDs 25, 28, 32, and 35 (Fig. 3B). There was no significant effect of ATZ on the body weight of F1 generation females (Group 3) administered ATZ from PND 21 until 5 days after VO (Fig. 3D).
The group mean body weight of F1 generation 50 mg/kg ATZ‐treated G/L/PW females that continued treatment until PND 133 (Group 2) was significantly less than the control group throughout the entire period (Fig. 4A). Average food consumption also tended to be less in this group, but it was intermittently significantly reduced from PND 28 to PND 130 (Fig. 4B). Although food intake in F1 generation females administered 50 mg/kg ATZ from PND 120 to PND 133 (Group 4) was significantly less than controls from PND 120 to PND 130 (Fig. 4D), body weight was not significantly affected (Fig. 4C). There were no treatment‐related clinical signs in F1 generation females during the period from PND 21 to PND 133.
Sexual Maturation
The litter mean number of days required to attain vaginal patency (sexual maturation) was 33.5 days for F1 generation, untreated females (Fig. 5, left panel). The litter mean number of days required to achieve sexual maturation was significantly longer (34.5 and 35.4 days, respectively) in the G/L/PW 25 and 50 mg/kg ATZ‐exposed groups (Fig. 5, Groups 1 and 2 combined, middle panel) and 34.2 days in the PW 50 mg/kg ATZ‐treated group (Fig. 5, Group 3, right panel).
An inverse relationship between litter mean body weight gain from PND 21 to PND 28 and the litter mean day on which VO was observed in the control group (y = −0.1475x + 38.111; R 2 = 0.19; p < 0.01; Fig. 6A) and in the 50 mg/kg ATZ‐treated, G/L/PW cohort (y = −0.2985x + 43.381; R 2 = 0.39; p < 0.01). A similar relationship existed for the high‐dose litters of the PW Cohort (y = −0.1549x + 39.11; R 2 = 0.09; p > 0.05; Fig. 6B), although R 2 was not statistically significant.
Figure 6.
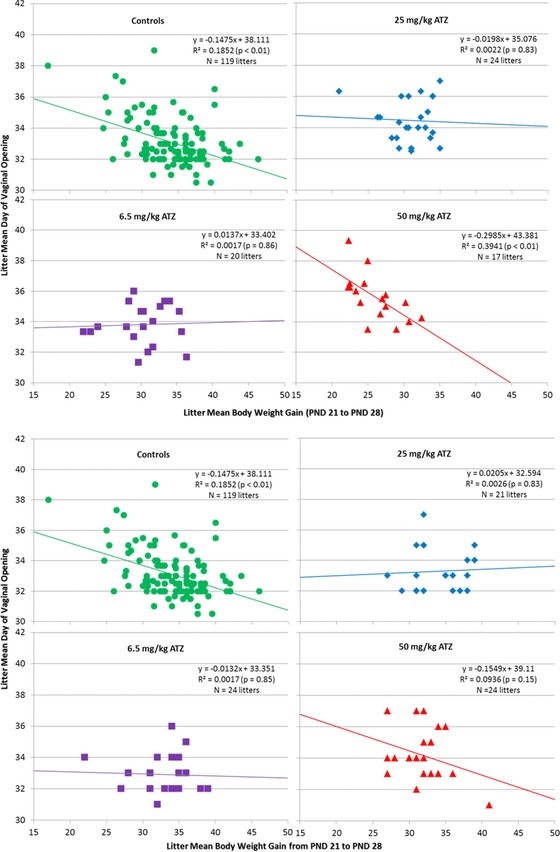
(a) Correlation between the litter mean body weight gain from PND 21 to PND 28 and litter mean day to vaginal opening in control, Group 1 and Group 2 ATZ‐treated (6.5, 50, or 100 mg/kg/day) female SD rats exposed to ATZ during gestation, lactation, and from PND 21 until VO. The deviations of the regression equations and correlation coefficients from null were statistically evaluated by two‐sided t‐tests. (b) Correlation between the litter mean body weight gain from PND 21 to PND 28 and litter mean day to vaginal opening in control, Group 1 and Group 2 ATZ‐treated (6.5, 50, or 100 mg/kg/day) female SD rats exposed to ATZ during gestation only from PND 21 until VO. The deviations of the regression equations and correlation coefficients from null were statistically evaluated by two‐sided t‐tests.
LH Surge
Exposure to ATZ at doses of 50 mg/kg/day during gestation, lactation, and for 5 days after the onset of puberty (Group 1; Fig. 7A), or only during the prepubertal period (Group 3; Fig. 7B), had no statistically significant effect on peak LH (Fig. 8, top panel) or LH AUC (Fig. 8, bottom panel). LH levels were reduced 9 hr after lights‐on in Group 1 and Group 3, but the reduction was only statistically significant in Group 1 (Fig. 7). There were no effects on any LH parameters in the G/L/PW groups exposed to ATZ at dose of ≤25 mg/kg/day.
Figure 7.
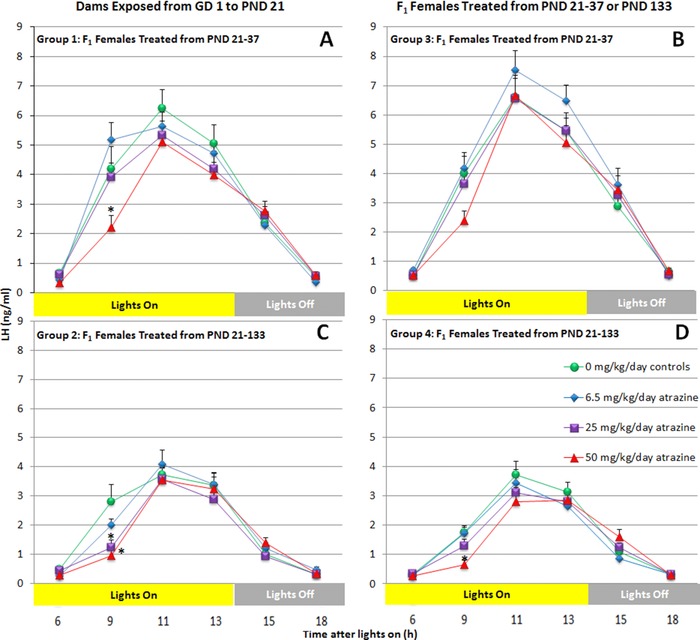
The estradiol‐induced LH surge in ovariectomized, female SD rats exposed to 0, 6.5, 25, or 50 mg/kg ATZ from GD 0 to 5 days after the day of VO (Panel B); from PND 21 to 5 days after the day of VO (Panel A); from PND 21 to PND 133 (Panel C); or PND 120 to PND 133 (Panel D). Blood samples were collected at 6, 9, 11, 13, 15, and 18 hr after lights‐on. Each point represents the mean ± SEM of 9 to 15 rats. *p < 0.05.
Figure 8.
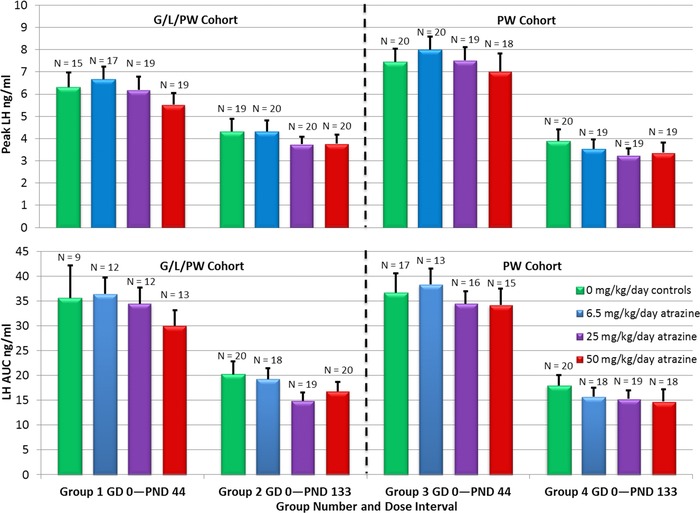
Mean (± SEM) peak LH (top panel) and LH AUC (bottom panel) in estrogen‐primed F1 generation females in the G/L/PW (Groups 1 and 2) and PW (Groups 3 and 4) cohorts.
There were no effects of ATZ on peak LH or LH AUC in F1 females exposed to ATZ from conception until PND 133 (Group 2; Figs. 7C, 8) or dosed for 2 weeks from PND 120 to PND 133 (Group 4; Figs. 7D, 8). There were statistically significant reductions in LH 0900 h in Group 2 females exposed to 25 or 50 mg/kg ATZ from gestation until 5 days after VO, and also in the 50 mg/kg Group 4 females that received ATZ from PND 120 to PND 133.
Estrous Cycle Evaluation
The percentages of days spent by F1 G/L/PW female offspring exposed to ATZ daily by gavage at doses up to 50 mg/kg/day from PND 1 to PND 130 (Group 2, Fig. 9, left panel) were comparable to those observed in controls (Group 2) and in F1 females administered ATZ from PND 120 to PND 133 (Group 4, Fig. 9, right panel). The percentage of animals that displayed at least one episode of prolonged diestrus (i.e., diestrus lasting for 4 days or more) was increased in the 25 mg/kg/day (52%), and 50 mg/kg/day (45%) Group 2 animals compared to the control group (31%). In Group 4, the corresponding values were 28.6 and 54%, respectively, compared to 33% in the control group. These differences were attributed to a few animals that displayed prolonged periods of diestrus (Supporting Information Table S2). Very few ATZ‐treated or vehicle control group animals displayed persistent estrus in either Group 2 (Supporting Information Table S2A) or Group 4 (Supporting Information Table S2B). The detections of proestrus or metestrus were infrequent and no differences among groups were apparent.
Figure 9.
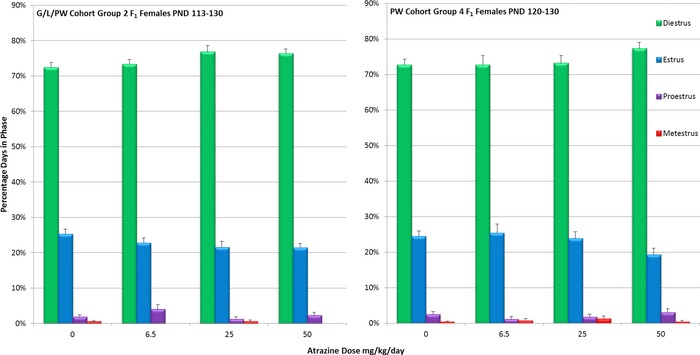
Mean percent days (± SEM) estrus (E), diestrus (D), proestrus (P), or metestrus (M) calculated from PND 113 to PND 130 in animals exposed to ATZ during gestation, lactation, and daily by gavage from PND 1 to 133 (Group 2). For animals in Group 4, the mean percent days in E, D, P, or M were calculated from the beginning of treatment on PND 120 to PND 130, the day when animals in both Groups 2 and 4 were ovariectomized in preparation for LH surge determination on PND 133.
DISCUSSION
The purpose of this study was to isolate the effects of gestational/lactational exposure to ATZ on sexual maturation (VO) and the LH surge from the effect of exposure during the peripubertal period. The effects of 50 mg/kg ATZ on body weight and food consumption, in dams exposed to ATZ during gestation (Groups 1 and 2), are consistent with the results reported by Scialli et al. (2014). The effects of 50 mg/kg ATZ on F1 progeny body weights of dams treated during lactation, compared to F1 pups that were not exposed to ATZ during this period, are consistent with results reported by Davis et al. (2011). DeSesso et al. (2014) also reported that maternal body weights and food consumption (F0 and F1 generations) were significantly reduced in the 500 ppm (38.7 mg/kg/day) group for approximately 10 weeks before mating, during gestation, and during lactation in a multigeneration reproduction feeding study. It is difficult to determine in these studies whether the effect of treatment on pup body weight was due to a direct effect on the pup, or whether it was secondary to weight gain reductions in the dams or decreased milk production by the dams via an effect of ATZ on prolactin (Stoker et al., 2000).
The effect of ATZ on fertility and decreased pup survival in Groups 1 and 2 animals administered ATZ at doses of 50 mg/kg/day are consistent with effects observed in other studies where high doses of ATZ were administered by gavage (Foradori et al., 2014; Scialli et al., 2014). Rapid absorption and elimination of ATZ and its chlorometabolites (McMullin et al., 2004) likely account for the absence of effects of ATZ on fertility and pup survival in multigeneration studies, where the daily dose of ATZ (38.7 mg/kg/day) was distributed over the day as determined by infrequent feeding bouts during the day, with most food consumption occurring after lights‐out (DeSesso et al., 2014).
The results from this study confirm previous reported findings of delayed sexual maturation in male (Stoker et al., 2000; Rosenberg et al., 2008) and female rats (Laws et al., 2000; Ashby et al., 2002) following high bolus dose administration of ATZ. The lowest observed effect level observed in F1 generation females in the present study was 25 mg/kg/day, although others have reported effects at doses as low as 12.5 mg/kg/day.
The onset of puberty in both males and females is dependent on the peripubertal initiation of GnRH pulses in rodents (Ojeda and Skinner, 2006), primates (Plant and Witchel, 2006), and humans (Grumbach, 2002). Because high doses of ATZ (200 mg/kg) reduced the frequency of LH pulses and concomitantly increased pulse amplitude in young adult females while not affecting GnRH biosynthesis (Foradori et al, 2009b), it is possible that delayed sexual maturation, observed in animals administered high doses of ATZ, may be due to an effect on the GnRH pulse generator. However, it is also known that length of time needed to reach sexual maturation is inversely proportional to body weight rather than to chronological age (Kennedy and Mitra, 1963; Odum et al., 2004). Delayed puberty in girls has been associated with reduced body fat (Kaplowitz, 2008) or small weight for gestational age at birth (Ibanez and de Zegher, 2006), whereas trends toward an increased prevalence of precocious puberty (Kaplowitz, 2006) have been associated with overnutrition (Burt Solorzano and McCartney, 2010). Early‐life nutritional effects have also been reported to alter puberty onset and reproductive function postpubertally (Chan et al., 2015).
Although analysis of covariance was used in this study to remove the effect of ATZ on body weight gain in pups from PND 21 to PND 28, this analysis was unable to fully compensate for body weight gain suppression that occurred during gestation and lactation in the mid‐ and high‐dose groups. The results suggest that caution must be taken when interpreting effects of development in toxicity studies in the presence of body weight gain reduction. Paired feeding studies conducted to parse out the effects of gavage doses of ATZ on body weight gain from effects on sexual development in males (Trentacoste et al., 2001) and females (Laws et al., 2000) have been inconclusive, possibly because forced food restriction in the pair‐fed group acts like a stressor, thereby activating the hypothalamic–pituitary–adrenal (HPA) axis and subsequently altering developmental parameters.
The LH surge data clearly indicate that female SD rats are not more sensitive to the effect of ATZ on the LH surge when exposed during gestation, lactation, and through to the onset of sexual maturity, compared to young adults exposed to ATZ beginning on PND 21 until the onset of sexual maturity, or for 14 days from PND 120 to PND 133. The estrogen‐induced peak LH (7 ng/ml) in the ovariectomized 7‐week female controls was approximately twofold greater than peak LH observed in the 20‐week females (3.5–4.0 ng/ml). This level was comparable to peak LH observed in 32‐week‐old female SD controls (Simpkins et al., 2011). It has been suggested that neuroendocrine aging of the hypothalamus–pituitary–gonadal (HPG) axis in female SD rats accounts for increased estrous cycle impairment, decreased LH titer during the LH surge, and increased vulnerability to the effect of ATZ (Simpkins et al., 2011).
It has been previously shown that high doses of ATZ accelerate reproductive aging in female SD rats, as indicated by the appearance of persistent estrus, commencing after approximately 6 to 9 months of treatment (Simpkins et al., 2011). Estrous cycle data collected in this study after 3 months of treatment indicate that there were no effects of treatment on the estrous cycle at doses up to 50 mg/kg/day; 2 weeks of ATZ treatment, beginning on PND 120, also had no effect. The slightly increased proportions of animals displaying prolonged periods of diestrus at doses of ≥25 mg/kg/day are consistent with published studies that indicate ATZ induces constant diestrus before it changes over to constant estrus (Cooper et al., 1996). It is known that the reproductive aging processes in rodents are unlike those observed in nonhuman primates and in women. Reproductive senescence in women is attributed to a depletion of ovarian follicles and not to an impairment of the hypothalamic control of the LH secretion during ovulation (Simpkins et al., 2011). The control of the LH surge in women is also mediated by mechanisms that are uniquely different from rodents (Plant, 2012).
The results from the developmental studies on SD rats also raise the possibility that ATZ may be acting simultaneously on both the HPA and the HPG axes. Thus the ATZ‐induced reduction in the frequency of pulsatile LH release depends on the presence of the adrenal gland, whereas the effect of ATZ on the preovulatory LH surge does not (Foradori et al., 2011). This separation of effects of ATZ on LH appears to be maintained in peripubertal exposed animals in that the LH surge mechanism is resistant to relatively high doses of ATZ. In contrast, these same animals displayed delayed sexual maturation, which as discussed previously, may in part be due to an effect of ATZ on pulsatile LH.
The mechanism whereby ATZ alters LH secretion is unknown (Cooper et al., 2000). The possibility exists that the acute effect of ATZ on the HPA axis could mediate the effect of ATZ on the HPG axis, resulting in a suppression of the LH surge. ATZ and deisopropylatrazine induced a rapid increase in plasma progesterone and corticosterone secretion within minutes following gavage dosing (Fraites et al., 2009) and corticosterone levels remained elevated for up to 24 hr following large bolus doses of ATZ (Foradori et al., 2011). Elevated plasma corticosterone is known to suppress the LH surge (Kamel and Kubajak, 1987; Gore et al., 2006). However, the administration of diaminochlorotriazine (DACT), a metabolite of ATZ, did not increase plasma progesterone and corticosterone levels (Fraites et al., 2009), whereas DACT suppressed the LH surge in female SD rats when administered by gavage daily for 4 days (Cooper et al., 2000), 1 month (Minnema, 2001), or 6 months in diet (Minnema, 2002). Thus, the absence of an effect of DACT on plasma progesterone and corticosterone levels is inconsistent with the hypothesis that these adrenal hormones mediate the effect of DACT on the LH surge. The rapid conversion of ATZ to its mono‐ and didealkylated metabolites also suggests that parent ATZ is unlikely to play a major role in the effect of the chlorotriazines on the LH surge.
It is known that progesterone, when delivered at the appropriate time of day, can augment the LH surge (Kempers and Ryan, 1977). However, progesterone treatment can also inhibit a daily signal that drives LH surges and thus is thought to prevent repeated LH surges in response to elevated levels of estradiol (Freeman et al., 1976; Attardi, 1984). The timing of the progesterone feedback to the HPG axis also appears to be critical to the effect of ATZ on the LH surge. Goldman et al. (2013) showed that a single dose of 100 mg/kg ATZ administered 8 hr after lights‐on enhanced peak LH in estrogen‐primed, ovariectomized female Long Evans rats, whereas the LH surge was suppressed after the fourth daily dose of ATZ. In contrast, in an unpublished study from our laboratory, female SD rats administered 100 mg/kg ATZ immediately after lights‐on in a 14:10 light/dark cycle, displayed no effect on the LH surge on Day 1, indicating that ATZ administered before the “critical window of time” was incapable of enhancing the LH surge via HPA activation. Successive daily doses of ATZ progressively suppressed the LH surge in subgroups of animals tested after two or three doses and resulted in statistically‐significantly reduced peak LH after the fourth dose. These results are consistent with Foradori et al. (2009), who showed that recovery from the effect of ATZ on the LH surge required 4 days.
Collectively, these data suggest that there may be dual and perhaps opposing processes that influence the effect of ATZ on the LH surge; a rapid, adaptable, progesterone‐mediated effect resulting from the activation of the HPA axis and a delayed, more sustained inhibitory effect mediated through a direct action on the HPG axis. This interpretation is consistent with Foradori et al. (2011), who reported that adrenalectomy did not block the effect of ATZ on the LH surge yet eliminated the effect of ATZ on pulsatile LH. Similarly, in the present study, ATZ had no effect on the LH surge in sexually mature animals, yet delayed the onset of puberty, a process that relies on the activation of the LH pulse generator.
Overall, this study indicates that high bolus doses of ATZ may differentially affect the mechanisms controlling pulsatile LH versus the mechanisms controlling rodent LH surge in developing animals because sexual maturation was delayed, yet estrogen‐primed LH surge was unaffected. The study also illustrates the difficulty of separating the effect of ATZ on sexual maturation from its effect on either maternal or fetal/peripubertal body weight development. The absence of an effect of ATZ on the LH surge in female SD rats exposed to ATZ at doses up to 50 mg/kg/day through their dams during gestation, lactation, or exposed only during the postlactation period indicates that developing rats are not more sensitive than young adult female rats. This suggests that it may be inappropriate to retain the 10‐fold “FQPA uncertainty factor” that is predicated on the default assumption that developing animals are inherently more sensitive to the effects of chemicals than are adults.
Supporting information
Supporting Tables
REFERENCES
- Adams J, Buelke‐Sam J, Kimmel CA, Nelson CJ, Reiter LW, Sobotka TJ, Tilson HA, Nelson BK. 1985. Collaborative behavioral teratology study: protocol design and testing procedures. Neurobehav Toxicol Teratol 7(6):579–586. [PubMed] [Google Scholar]
- Ashby J, Tinwell H, Stevens J, Pastoor T, Breckenridge CB. 2002. The effects of atrazine on the sexual maturation of female rats. Regul Toxicol Pharmacol 35(3):468–473. [DOI] [PubMed] [Google Scholar]
- Attardi B. 1984. Progesterone modulation of the luteinizing hormone surge: regulation of hypothalamic and pituitary progestin receptors. Endocrinology 115(6):2113–2122. [DOI] [PubMed] [Google Scholar]
- Bridges DC. 2008. Benefits of triazine herbicides in corn and sorghum production The triazine herbicides: 50 years revolutionizing agriculture. In: LeBaron HM, McFarland JE, Burnside OC, editors. San Diego: Elsevier; p 163–174. [Google Scholar]
- Burt Solorzano CM, McCartney CR. 2010. Obesity and the pubertal transition in girls and boys. Reproduction 140(3):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KA, Tsoulis MW, Sloboda DM. 2015. Early‐life nutritional effects on the female reproductive system. J Endocrinol 224(2):R45–R62. doi: 10.1530/JOE-1514-0469. Epub 2014 Oct 1527. [DOI] [PubMed] [Google Scholar]
- Cooper RL, Stoker TE, Goldman JM, Parrish MB, Tyrey L. 1996. Effect of atrazine on ovarian function in the rat. Reprod Toxicol 10(4):257–264. [DOI] [PubMed] [Google Scholar]
- Cooper RL, Stoker TE, Tyrey L, Goldman JM, McElroy WK. 2000. Atrazine disrupts the hypothalamic control of pituitary‐ovarian function. Toxicol Sci 53(2):297–307. [DOI] [PubMed] [Google Scholar]
- Cooper RL, Laws SC, Das PC, Narotsky MG, Goldman JM, Lee Tyrey E, Stoker TE. 2007. Atrazine and reproductive function: mode and mechanism of action studies. Birth Defects Res B Dev Reprod Toxicol 80(2):98–112. [DOI] [PubMed] [Google Scholar]
- Davis LK, Murr AS, Best DS, Fraites MJP, Zorrilla LM, Narotsky MG, Stoker TE, Goldman JM, Cooper RL. 2011. The effects of prenatal exposure to atrazine on pubertal and postnatal reproductive indices in the female rat. Reprod Toxicol 32(1):43–51. [DOI] [PubMed] [Google Scholar]
- DeSesso JM, Scialli AR, White TE, Breckenridge CB. 2014. Multigeneration reproduction and male developmental toxicity studies on atrazine in rats. Birth Defects Res B Dev Reprod Toxicol 101(3):237–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine M, Duke SO, Fedtke C. 1993. Herbicidal inhibition of photosynthetic electron transport Physiology of herbicide action. Englewood Cliffs, NJ: P T R Prentice Hall; p 113–140. [Google Scholar]
- Dourson M, Charnley G, Scheuplein R. 2002. Differential sensitivity of children and adults to chemical toxicity. II. Risk and regulation. Regul Toxicol Pharmacol 35(3):448–467. [DOI] [PubMed] [Google Scholar]
- Dunn OJ. 1961. Multiple comparisons among means. J Am Stat Assoc 56(293):52–64. [Google Scholar]
- Dunn OJ. 1964. Multiple comparisons using rank sums. Technometrics 6(3):241–252. [Google Scholar]
- Dunnett CW. 1955. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc 50:1096–1121. [Google Scholar]
- Dunnett CW. 1964. New tables for multiple comparisons with a control. Biometrics 20(3):482–491. [Google Scholar]
- Foradori CD, Hinds LR, Hanneman WH, Legare ME, Clay CM, Handa RJ. 2009a. Atrazine inhibits pulsatile luteinizing hormone release without altering pituitary sensitivity to a gonadotropin‐releasing hormone receptor agonist in female Wistar rats. Biol Reprod 81(1):40–45. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Hinds LR, Hanneman WH, Handa RJ. 2009b. Effects of atrazine and its withdrawal on gonadotropin‐releasing hormone neuroendocrine function in the adult female Wistar rat. Biol Reprod 81(6):1099–1105. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Hinds LR, Quihuis AM, Lacagnina AF, Breckenridge CB, Handa RJ. 2011. The differential effect of atrazine on luteinizing hormone release in adrenalectomized adult female wistar rats. Biol Reprod 85(4):684–689. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Sawhney Coder P, Tisdel M, Yi KD, Simpkins JW, Handa RJ, Breckenridge CB. 2014. The effect of atrazine administered by gavage or in diet on the LH surge and reproductive performance in intact female Sprague‐Dawley and Long Evans rats. Birth Defects Res B Dev Reprod Toxicol 101(3):262–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraites MJ, Cooper RL, Buckalew A, Jayaraman S, Mills L, Laws SC. 2009. Characterization of the hypothalamic‐pituitary‐adrenal axis response to atrazine and metabolites in the female rat. Toxicol Sci 112(1):88–99. [DOI] [PubMed] [Google Scholar]
- Freeman MC, Dupke KC, Croteau CM. 1976. Extinction of the estrogen‐induced daily signal for LH release in the rat: a role for the proestrous surge of progesterone. Endocrinology 99(1):223–229. [DOI] [PubMed] [Google Scholar]
- Freeman ME. 1988. The ovarian cycle of the rat The physiology of reproduction. In: Knobil E., Neill JD, editors. New York: Raven Press; p 1893–1928. [Google Scholar]
- Goldman J, Davis LK, Murr AS, Cooper RL. 2013. Atrazine‐induced elevation or attenuation of the LH surge in the ovariectomized, estrogen‐primed female rat: role of adrenal progesterone. Reproduction 146(4):305–314. [DOI] [PubMed] [Google Scholar]
- Gore AC, Attardi B, DeFranco DB. 2006. Glucocorticoid repression of the reproductive axis: effects on GnRH and gonadotropin subunit mRNA levels. Mol Cell Endocrinol 256(1–2):40–48. [DOI] [PubMed] [Google Scholar]
- Grumbach MM. 2002. The neuroendocrinology of human puberty revisited. Horm Res 57(Suppl 2):2–14. [DOI] [PubMed] [Google Scholar]
- Ibanez L, de Zegher F. 2006. Puberty and prenatal growth. Mol Cell Endocrinol 254–255:22–25. Epub 2006 Jun 2006. [DOI] [PubMed] [Google Scholar]
- Kamel F, Kubajak CL. 1987. Modulation of gonadotropin secretion by corticosterone: interaction with gonadal steroids and mechanism of action. Endocrinology 121(2):561–568. [DOI] [PubMed] [Google Scholar]
- Kaplowitz P. 2006. Pubertal development in girls: secular trends. Curr Opin Obstet Gynecol 18(5):487–491. [DOI] [PubMed] [Google Scholar]
- Kaplowitz PB. 2008. Link between body fat and the timing of puberty. Pediatrics 121(Suppl 3):S208–S217. doi: 210.1542/peds.2007‐1813F. [DOI] [PubMed] [Google Scholar]
- Kempers RD, Ryan RJ. 1977. Acute effects of intravenous infusion of 17beta‐estradiol and 17alpha‐hydroxyprogesterone on gonadotropin release. Fertil Steril 28(6):631–637. [DOI] [PubMed] [Google Scholar]
- Kennedy GC, Mitra J. 1963. Body weight and food intake as initiating factors for puberty in the rat. J Physiol 166:408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws SC, Ferrell JM, Stoker TE, Schmid J, Cooper RL. 2000. The effects of atrazine on female wistar rats: an evaluation of the protocol for assessing pubertal development and thyroid function. Toxicol Sci 58(2):366–376. [DOI] [PubMed] [Google Scholar]
- Mantel N. 1963. Chi‐square tests with one degree of freedom; extensions of the Mantel‐Haenszel procedure. J Am Stat Assoc 58(303):690–700. [Google Scholar]
- McMullin TS, Andersen ME, Nagahara A, Lund TD, Pak T, Handa RJ, Hanneman WH. 2004. Evidence that atrazine and diaminochlorotriazine inhibit the estrogen/progesterone induced surge of luteinizing hormone in female Sprague‐Dawley rats without changing estrogen receptor action. Toxicol Sci 79(2):278–286. [DOI] [PubMed] [Google Scholar]
- Minnema DJ. 2001. Comparison of the LH Surge in female rats administered atrazine, simazine or DACT via oral gavage for one month. Vienna, VA: Covance Laboratories Inc.; 544p. [Google Scholar]
- Minnema DJ. 2002. 52‐week toxicity study of simazine, atrazine and DACT administered in the diet to female rats. Vienna, VA: Covance Laboratories Inc; 1199p. [Google Scholar]
- Odum J, Tinwell H, Tobin G, Ashby J. 2004. Cumulative dietary energy intake determines the onset of puberty in female rats. Environ Health Perspect 112(15):1472–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda SR, Skinner MK. 2006. Chapter 38 – Puberty in the rat In: Neill JD, Plant TM, Pfaff DW, Challis JRG, de Kretser DM, Richards JS, Wassarman PM, editors. Knobil and Neill's Physiology of Reproduction. 3rd ed. St. Louis: Academic Press; 2161–2226. [Google Scholar]
- Plant TM. 2012. A comparison of the neuroendocrine mechanisms underlying the initiation of the preovulatory LH surge in the human, Old World monkey and rodent. Front Neuroendocrinol 33(2):160–168. [DOI] [PubMed] [Google Scholar]
- Plant TM, Witchel SF. 2006. Chapter 40 – Puberty in Nonhuman Primates and Humans In: Neill JD, Plant TM, Pfaff DW, Challis JRG, de Kretser DM, Richards JS, Wassarman PM, editors. Knobil and Neill's Physiology of Reproduction. 3rd ed. St. Louis: Academic Press; 2177–2230. [Google Scholar]
- Rosenberg BG, Chen H, Folmer J, Liu J, Papadopoulos V, Zirkin BR. 2008. Gestational exposure to atrazine: effects on the postnatal development of male offspring. J Androl 29(3):304–311. [DOI] [PubMed] [Google Scholar]
- Scheuplein R, Charnley G, Dourson M. 2002. Differential sensitivity of children and adults to chemical toxicity. I. Biological basis. Regul Toxicol Pharmacol 35(3):429–447. [DOI] [PubMed] [Google Scholar]
- Scialli AR, DeSesso JM, Breckenridge CB. 2014. Developmental toxicity studies with atrazine and its major metabolites in rats and rabbits. Birth Defects Res B Dev Reprod Toxicol 101(3):199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins JW, Swenberg JA, Weiss N, Brusick D, Eldridge JC, Stevens JT, Handa RJ, Hovey RC, Plant TM, Pastoor TP, Breckenridge CB. 2011. Atrazine and breast cancer: a framework assessment of the toxicological and epidemiological evidence. Toxicol Sci 123(2):441–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker TE, Laws SC, Guidici DL, Cooper RL. 2000. The effect of atrazine on puberty in male wistar rats: an evaluation in the protocol for the assessment of pubertal development and thyroid function. Toxicol Sci 58(1):50–59. [DOI] [PubMed] [Google Scholar]
- Trebst A. 2008. The Mode of action of triazine herbicides in plants The Triazine Herbicides: 50 Years Revolutionizing Agriculture. In: LeBaron HM, McFarland JE, Burnside OC, editors. San Diego: Elsevier; p 101–110. [Google Scholar]
- Trentacoste SV, Friedmann AS, Youker RT, Breckenridge CB, Zirkin BR. 2001. Atrazine effects on testosterone levels and androgen‐dependent reproductive organs in peripubertal male rats. J Androl 22(1):142–148. [PubMed] [Google Scholar]
- Tyl RW, Marr MC. 2006. Developmental toxicity–methodology Developmental and Reproductive Toxicology: A Practical Approach. 2nd ed. In: Hood RD, editor. Boca Raton, FL: CRC Press, Taylor & Francis; p 207–261. [Google Scholar]
- Westwood FR. 2008. The female rat reproductive cycle: a practical histological guide to staging. Toxicol Pathol 36(3):375–384. [DOI] [PubMed] [Google Scholar]
- Yener T, Turkkani Tunc A, Aslan H, Aytan H, Cantug Caliskan A. 2007. Determination of oestrous cycle of the rats by direct examination: how reliable? Anat Histol Embryol 36(1):75–77. [DOI] [PubMed] [Google Scholar]
- 104th Congress . 1996. Food Quality Protection Act of 1996: Title III–Data collection activities to assure the health of infants and children and other measures. Washington, DC: United States Government Printing Office. 50p. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Tables


