Abstract
F10 is a novel polymeric fluoropyrimidine drug candidate with strong anticancer activity in multiple preclinical models. F10 has strong potential for impacting cancer treatment because it displays high cytotoxicity toward proliferating malignant cells with minimal systemic toxicities thus providing an improved therapeutic window relative to traditional fluoropyrimidine drugs, such as 5-fluorouracil. F10 has a unique mechanism that involves dual targeting of thymidylate synthase and Top1. In this review, the authors provide an overview of the studies that revealed the novel aspects of F10's cytotoxic mechanism and summarize results obtained in preclinical models of acute myeloid leukemia, acute lymphocytic leukemia, glioblastoma and prostate cancer that demonstrate the strong potential of F10 to improve treatment outcomes.
KEYWORDS : acute leukemia, cancer chemotherapy, fluoropyrimidine, glioblastoma, prostate cancer, thymineless death, topoisomerase
Fluoropyrimidine drugs such as 5-fluorouracil (5-FU) have been widely used for more than 50 years [1,2], and while providing a survival benefit for some malignancies they are relatively ineffective for many others and cause serious toxicities. F10 was designed to overcome the limitations of current fluoropyrimidine drugs that result in suboptimal anticancer activity and cause toxicities. Specifically, F10 was designed to display improved anticancer activity by more effectively generating metabolites that inhibit thymidylate synthase (TS) and cause DNA damage, while generating lower levels of metabolites that cause systemic toxicities. The unique aspects of F10's cytotoxic mechanism have been revealed in a series of studies conducted over the last decade that are summarized in the following sections. Furthermore, in the last 4 years, a series of preclinical studies have confirmed F10's high activity and low toxicity using challenging and relevant cancer models. Collectively, these studies demonstrate the strong potential for F10 to simultaneously improve anticancer activity and reduce systemic toxicities relative to current therapy.
F10: definition & design concept
F10 was designed to fulfill the unmet medical need for a more effective fluoropyrimidine drug with greater anticancer activity, reduced systemic toxicities and an improved pharmacological profile relative to current fluoropyrimidines. Fluoropyrimidines are drugs in the same class as 5-FU and capecitabine and were originally designed by Heidelberger et al. [1] to interfere with nucleoside metabolism in cancer cells. They remain in widespread use for cancer treatment more than 50 years [2] since initial clinical trials and by some estimates are used to treat 2 million cancer patients each year [3]. Fluoropyrimidines remain in widespread use because they provide a survival benefit in highly prevalent malignancies, such as colon cancer [4]. Current evidence in preclinical models of acute myeloid leukemia (AML) [5], acute lymphocytic leukemia (ALL) [6], glioblastoma (GBM) [7], and prostate cancer [8] reported in the last few years indicate the design of F10 results in tangible benefits in vivo that are likely to impact cancer treatment, including the treatment of malignancies such as acute leukemia and GBM. These cancers have not historically been treated with fluoropyrimidines.
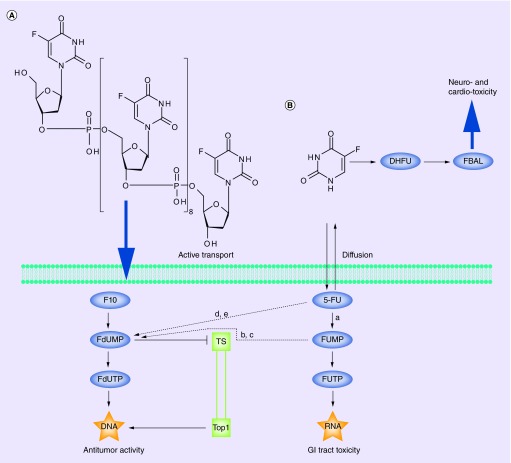
The fluoropyrimidines in current use (e.g., 5-FU) undergo both catabolic and anabolic metabolism (Figure 1); however, the DNA-directed anabolic metabolites (e.g., 5-fluoro-2′-deoxyuridine-5′-O-monophosphate [FdUMP] and 5-fluoro-2′-deoxyuridine-5′-O-triphosphate [FdUTP]) that are produced at relatively low levels are primarily responsible for the anticancer activity of the drug [9]. FdUMP inhibits TS [10], which is considered a rate-limiting enzyme in DNA synthesis. TS inhibition results in thymineless conditions that enhance incorporation of dUTP and FdUTP into DNA, and this causes massive DNA damage and, ultimately, results in thymineless death (TLD) [11]. Low TYMS expression in cancer patients is predictive of a favorable response to fluoropyrimidine treatment [12]. More than 80% of administered 5-FU is degraded, however, and individual variability in 5-FU clearance greatly affects treatment outcomes. Individuals deficient in DPD, the rate-limiting enzyme for 5-FU catabolism, are hypersensitive to 5-FU, resulting in severe toxicities [13]. While only a few percent are truly deficient in DPD, approximately one in three cancer patients develops severe 5-FU-related toxicities related to low 5-FU clearance. Furthermore, the degradation products of 5-FU catabolism (e.g., fluoro-β-alanine [FBAL]) are highly toxic (cardio- [14] and neurotoxicity [15]). While DPD deficiency and low clearance of 5-FU are predictive of severe toxicity, high 5-FU clearance is predictive of poorer survival [16] demonstrating the pharmacological challenges associated with conventional fluoropyrimidine drugs. 5-FU is administered in combination with leucovorin (LV) to enhance TS inhibition and to maximize DNA damage [17]; however, incorporation of 5-FU into RNA in vivo is at least tenfold greater than DNA [18]. Much of the gastrointestinal tract and hematologic toxicity of 5-FU has been linked to its RNA metabolites, and this has led to the recent approval of uridine triacetate by the US FDA for 5-FU overdoses. Hence, there is a need for improved fluoropyrimidine drugs, such as F10, that have improved pharmacological properties and enhanced DNA-directed effects relative to 5-FU.
Figure 1. . Structures of (A) F10 and (B) 5-fluorouracil and schematic of uptake and important metabolites.
F10 is internalized by active transport into acute lymphocytic leukemia cells (see reference [6]) and converted to FdUMP, which inhibits TS, and FdUTP which is incorporated into DNA under thymineless conditions and poisons Top1 to generate DNA double-strand breaks. 5-FU enters both malignant and nonmalignant cells by passive diffusion and is converted to FUMP (a). FUMP is inefficiently converted to FdUMP (b, c). 5-FU also can be converted to FdUMP (d, e). 5-FU is also catabolized to FBAL, which causes cardio- and neuro-toxicity. The direct conversion of F10 to DNA-directed metabolites enhances DNA damage and contributes to anticancer activity while causes mainly RNA damage that contribute to systemic toxicities.
5-FU: 5-fluorouracil; a: UMPS; b: UMPK; c: RNR; d: TP; e: TK.
F10 is a DNA polymer in which FdUMP nucleotides are serially connected [19], a design that circumvents the required metabolic activation needed to produce FdUMP from either 5-FU or capecitabine. Rather, FdUMP is released from F10 by 3′-O-exonucleases which are the predominant DNAse activities in cancer cells [20]. In principle, F10 may be entirely converted to FdUMP without any metabolic activation; however, F10 is a polyanionic compound, and cell permeability is a concern since DNases present in serum could also degrade F10 to monomeric FPs, such as 5-FU or FdU. If this occurred, then metabolic reactivation of the monomeric fluoropyrimidines produced would be required for F10 cytotoxicity in cancer cells.
To test whether extracellular degradation limited F10 cytotoxicity, we performed studies (in collaboration with Dr Peters; Amsterdam, The Netherlands) in cancer cell lines that were deficient in the anabolic activation of 5-FU and other monomeric FPs [21]. We then compared resistance factors for F10 vis-à-vis monomeric fluoropyrimidines based on cytotoxicity differences in proficient and deficient cells. First, we established that the relative cytotoxicity of F10 greatly exceeded 5-FU and other monomeric fluoropyrimidines. As we observed broadly for cancer cell lines in the NCI 60 cell line screen [22,23], we also observed F10 was much more potent toward FM3A breast cancer cells by factors that ranged from 127-fold for FdUMP to more than 14,000-fold for 5-FU [21]. These cytotoxicity data demonstrate F10 does not undergo substantial degradation to monomeric fluoropyrimidines prior to cell uptake. Further evidence that F10 does not substantially degrade prior to cell uptake was obtained from studies with FM3A/TK- cells that are deficient in thymidine kinase (TK), the enzyme that phosphorylates FdU to form FdUMP. The resistance factor for FdU in these cells relative to wild-type was 493, consistent with the important role for TK in FdU cytotoxicity. Interestingly, the resistance factor for monomeric FdUMP in this system was 945, indicating it likely undergoes dephosphorylation to FdU prior to cell uptake. By contrast, 5-FU had a resistance factor <1 in this system which is consistent with 5-FU cytotoxicity being independent of TK, which, together with a lack of dependence on TS levels in these studies, indicated 5-FU cytotoxicity occurred mainly through RNA-directed processes. The resistance factor for F10 in these TK- cells was 136, or about one-quarter that for FdU, which is consistent with limited extracellular degradation of F10 to FdU and/or FdUMP, but with cytotoxicity predominantly arising from uptake of F10 multimer.
To gain further insight into the role of F10 uptake in multimeric form for F10 cytotoxicity, we performed cell uptake studies with fluorescently labeled F10 [6]. F10 was efficiently internalized by ALL cells while normal hematopoietic stem cells (HSCs) displayed minimal uptake. Further, F10 uptake in ALL cells was both temperature- and concentration-dependent, which is consistent with F10 uptake into malignant cells occurring via active transport [24]. In studies investigating how gene expression for cell lines included in the NCI 60 cell line screen affected F10 sensitivity, high expression of clathrin and other genes associated with endocytosis were strongly correlated with F10 sensitivity [23], but not with sensitivity to monomeric fluoropyrimidines. Collectively, these studies demonstrate that F10 enters malignant cells mainly through active transport, and that FdUMP is released inside cancer cells at high levels and initiates processes that cause massive DNA damage.
F10 has unique mechanism that involves dual targeting of TS & Top1
F10 is much more potent than conventional fluoropyrimidine drugs [22,23], and while it shares certain mechanistic features such as TS inhibition and DNA damage in common with antecedent FPs (e.g., 5-FU, FdU), the increased potency appears to result from F10 also affecting additional DNA-directed processes, especially Top1 poisoning [22]. As with other fluoropyrimidines, TS inhibition and induction of a thymineless state is central to F10 cytotoxicity [11]; however, F10 inhibits TS at much lower concentrations [19] consistent with greater conversion to the TS inhibitory metabolite, FdUMP. TS inhibition by F10 is also more complete and with a longer duration [8]. Some of these differences may result from TS also being an RNA-binding protein [25] that transcriptionally regulates multiple genes, including its own expression [26]. Moderate TS inhibition, as occurs with 5-FU treatment, actually increases TS activity through a rebound effect [27] in which new TS is synthesized. By contrast, our studies show TS activity is nearly completely inhibited with the low F10 concentrations needed for cytotoxicity and this high level of inhibition is retained for sufficient time to induce cell death, reflecting sustained high FdUMP levels. Efficient TS inhibition results in accumulation of dUMP, the TS substrate and consequently to elevated dUTP levels that under thymineless conditions results in increased dUTP incorporation into DNA [28]. With F10 there is also incorporation of FdUTP into DNA under thymineless conditions [29]. The importance of TS inhibition and incorporation of dUTP and FdUTP into DNA for F10 cytotoxicity is demonstrated by experiments in which we rescued F10 cytotoxicity with exogenous thymidine [30]. Interestingly, thymidine is only effective for rescuing from F10 up until about 16 h of treatment, a time that coincides with the occurrence of DNA DSBs. Our results are consistent with F10 inducing thymineless conditions that are reversible until dUTP and FdUTP is incorporated into DNA and causes irreparable DNA damage.
An essential aspect of the DNA-directed effects of F10 that result in cytotoxic DNA damage involves Top1 poisoning [31]. The potential role of Top1 in mediating F10 cytotoxicity was first identified in a COMPARE analysis [32] of F10 cytotoxicity data in the NCI 60 cell line screen [22]. These data indicated F10 was much more potent than monomeric fluoropyrimidines (338-fold increased potency relative to 5-FU; ˜30-fold relative to FdU) which is consistent with activity via the multimer rather than degradation to monomers. The spectrum of malignant cells that were highly sensitive to F10 also differed from monomeric fluoropyrimidines, and included leukemia and CNS malignancies. Importantly, these sensitivities have been born out in vivo in our preclinical program [5–8]. The increased potency of F10 could result from the high levels of DNA-directed metabolites produced in cancer cells treated with F10 affecting additional or alternative targets. As mentioned above, we performed a COMPARE analysis of the NCI 60 cell line screen data for F10. COMPARE analysis ranks drugs tested in the NCI screen based on similarity in the response profile across the entire 60 cell line panel to identify drugs with similar mechanisms [32]. In this analysis, many of the drugs most closely correlated with F10 were known Top1 poisons, such as camptothecin (CPT). In collaboration with Yves Pommier (NCI), we undertook studies investigating the mechanism by which F10 causes Top1 poisoning [33,34]. These studies revealed that Top1 efficiently cleaved FdU-substituted DNA, but FdU interfered with the religation step of Top1 catalysis resulting in Top1 cleavage complex (Top1cc) formation [22]. The composition and stability of F10-induced Top1cc differ, however, from CPT-induced Top1cc. CPT induces noncovalent cleavage complexes and thus the complexes reverse with time, especially under high salt conditions [35]. For F10, cleavage complexes involve a covalent linkage to the drug and are expected to have increased stability. The relative timing of Top1cc and DNA DSB formation [30] suggest F10-induced Top1cc are converted to DNA double strand breaks which may occur through collision with advancing transcriptional [36] and replication [37] complexes, similar to what occurs with CPT.
The process by which inhibiting de novo thymidylate synthesis causes cytotoxicity is referred to as TLD [38], and occurs in all cell types including bacteria [39], yeast [40,41] and mammalian cells [22]. Prior studies with 5-FU/LV in colon cancer cells indicated that TLD occurred primarily by activating the apoptotic cascade [42]. Although DNA damage was the initiating lesion for inducing apoptosis, cell death was mediated primarily via the extrinsic apoptotic pathway [43]. Our studies with F10 in AML cells also indicated that F10 induced TLD, primarily via activating the extrinsic apoptotic pathway [44]. F10 cytotoxicity was markedly enhanced by agents that independently activate extrinsic apoptosis, such as agonistic Fas antibodies. A significant difference for how F10 activates the extrinsic pathway in AML relative to that reported for 5-FU/LV in colon cancer cells is that F10 treatment does not increase expression of either Fas or Fas ligand, but rather modulates their activity by promoting localization in lipid rafts [44]. Increased lipid rafts are also induced in AML cell by treatment with statins which also result in activation of the extrinsic apoptotic pathway [44]. Our studies also showed statins are synergistic with F10 through the increased activation of the extrinsic apoptotic pathway. Since statins are widely used and relatively well-tolerated, it may be possible to increase F10 activity in clinical trials by combining with statins, or other agents that activate extrinsic apoptosis.
F10 is efficacious in multiple tumor models & is well-tolerated
F10 has undergone evaluation in multiple rodent models of cancer to determine whether the therapeutic advantages observed in cancer cells could also be achieved in vivo where factors such as drug clearance, first-pass metabolism and other issues markedly increase complexity. These studies have focused on acute leukemia [5,6], GBM [7] and advanced prostate cancer [8]. These malignancies were selected for study based upon the sensitivity of these types of malignant cells to F10 the NCI 60 cell line screen and other cell-based assays [23], and also due to the unmet medical need for new therapeutics for these malignancies for which standard chemotherapy is largely ineffective. Collectively, these studies demonstrate that F10 is likely to be effective for treating human cancer, including malignancies for which fluoropyrimidine drugs are not presently used.
• F10 for the treatment of AML
We evaluated F10 in a panel of human leukemia cell lines and observed strong and uniform potency with nanomolar IC50 values in all cell lines, including when cells were cultured using human serum [5]. We not only demonstrated a very large advantage in potency for F10 relative to 5-FU, as expected based on our NCI 60 analysis, but F10 was also much more potent on a molar basis than anthracycline drugs (e.g., doxorubicin) or cytarabine (AraC), the drugs that constitute the backbone for treatment of AML with chemotherapy (Figure 2) [45]. The enhanced potency for F10 was particularly evident in cell lines that are relatively resistant to these drugs. For example, F10 was 272-fold more potent than doxorubicin to KG1a cells and was 1634-fold more potent than AraC to THP-1 cells, which is consistent with a lack of cross-resistance to F10 for cells that are relatively resistant to either anthracyclines, which may occur via drug efflux [46], or AraC, which may occur via cytidine kinase deficiency [47]. The cytotoxic mechanism of F10 in AML cells involves TS inhibition and formation of Top1cc under thymineless conditions, which results in DNA damage and induction of apoptosis [5,30,44].
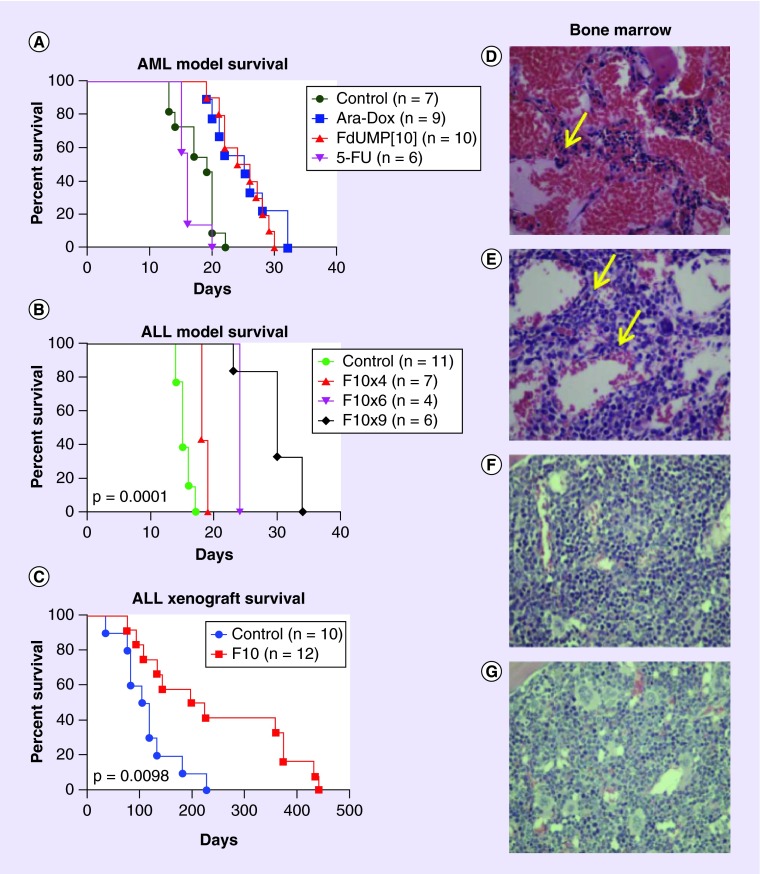
Figure 2. . F10 displays strong antileukemic activity with minimal systemic toxicities.
(A) F10 confers a survival benefit equivalent to the combination of Ara-C and doxorubicin. C57/Bl6 mice were sublethally irradiated to 4.5 Gy and injected with an MLL-ENL and Flt3 ITD syngeneic AML. Once engraftment was established by bioluminescence imaging, mice were treated with either saline (control), F10 at 300 mg/kg (FdUMP [10]), 5-FU at 121 mg/kg (5-FU) or cytarabine at 125 mg/kg plus doxorubicin at 3.75 mg/kg (Ara-Dox) on days 1, 3, 5 and 7. (B) F10 is active against multiple ALL models in vivo. Survival of C57Bl/6 mice injected with syngeneic, Ph+ B-cell ALL model and treated with saline or F10 at 300 mg/kg every other day for 4, 6 or 9 doses as indicated. (C) Survival of ALL xenograft bearing mice from time of injection treated with saline or F10 at 300 mg/kg every other day ×5 doses as indicated. All p-values were derived from log-rank tests. (D–G) F10 (F) causes much less bone marrow toxicity than 5-FU (D) or AraC-Dox (E) – regions of hypocellularity are indicated with yellow arrows. Panel (G) is vehicle-treated control. Drug dosing was the same as in panel (A).
5-FU: 5-fluorouracil; ALL: Acute lymphocytic leukemia; AML: Acute myeloid leukemia.
The prognosis for AML patients with certain molecular characteristics, including expression of the Flt3 ITD [48], MN1 [49] or with p53 deletions [50] is much poorer than patients without these features due to drug resistance. We tested F10 potency in a series of murine AML cell lines expressing these and other genetic features associated with poor prognosis for human patients and, in all cases, these factors had minimal effect on F10 response consistent with F10 being broadly applicable for AML treatment. Further evidence of the potential applicability of F10 for treating AML was obtained by demonstrating that F10 was very effective at suppressing hematopoietic colony formation from primary patient samples and inhibited engraftment of leukemic cells, consistent with F10 causing cytotoxicity to leukemia stem cells [5]. Importantly, we also demonstrated that F10 was highly selective for malignant cells and suppressed colony formation of normal HSCs only at the highest doses tested. We also established that the molecular targets of F10 (TS and Top1) are widely expressed in AML cells.
We demonstrated F10 was efficacious in multiple murine models of AML and is much less toxic than conventional therapy. These studies used syngeneic, orthotopic murine models of AML that were driven by fusion proteins generated from genomic translocations associated with human AML, as well as molecular characteristics, such as Flt3 ITD [48], which are associated with a poor prognosis. These models were previously used to evaluate chemotherapeutic efficacy for known drugs [50], and a survival benefit was demonstrated for Dox/AraC chemotherapy delivered at the maximum tolerated dose. Dose-escalation studies using the same dosing schedule used for Dox/AraC revealed that F10 was much better tolerated than 5-FU [5]. F10 treatment, as a single agent, resulted in a significant survival benefit relative to vehicle controls and comparable to the combination of Dox/AraC. By contrast, 5-FU treatment did not result in a survival benefit relative to control animals. One of the most striking findings of these studies was that not only was F10 efficacious for AML, but that the doses required for efficacy caused minimal systemic toxicity. 5-FU treatment is known to cause serious toxicities to the gastrointestinal (GI) tract [51] and bone marrow [52] and serious damage to these organs was apparent in all 5-FU-treated animals (Figure 2). Dox/AraC-treated animals also displayed serious drug-induced bone marrow hypocellularity and GI tract toxicities. In contrast, F10-treated mice displayed minimal toxicities to all normal tissues analyzed. The strong efficacy for F10 achieved with minimal toxicity may be particularly impactful for treating elderly patients for whom aggressive treatment with conventional chemotherapy may be contraindicated [53].
• F10 for treatment of ALL
F10 is highly cytotoxic to ALL cells and provides a strong survival benefit in multiple murine models of ALL (Figure 2), including models of drug-resistant disease [6]. The magnitude of the therapeutic advantages for F10 relative to 5-FU, doxorubicin and AraC in AML cells was also observed across a panel of ALL cell lines indicating that F10 is effective for treating ALL. Two ALL cell lines (DG75, SUP-B15) were tested for F10 uptake using a fluorescent F10 conjugate which was internalized by both cell lines in a temperature- and concentration-dependent manner consistent with F10 internalization via active transport [6]. ALL cells previously were shown to efficiently internalize certain oligonucleotides [24], and a strong propensity for oligonucleotide uptake may make ALL cells particularly amenable to F10 treatment. Importantly, nHSCs display minimal F10 uptake. The cytotoxic mechanism for F10 in ALL cells is similar to that determined for AML cells and involves TS inhibition and generation of Top1cc under thymineless conditions resulting in DNA damage and initiation of apoptosis [6].
F10 was strongly efficacious in syngeneic murine models of ALL that incorporate many of the characteristics that make ALL treatment challenging, including localization of leukemic cells to the bone marrow microenvironment, the presence of an intact immune system and the emergence of drug-resistant disease [6]. F10 was efficacious toward the B-cell ALL model (B6 ALL), and repeated injections over longer time periods resulted in progressively improved survival outcomes. F10 treatment also resulted in a survival advantage in a murine model of Philadelphia chromosome-positive ALL [54] in Balb/c mice. We also demonstrated strong efficacy for F10 toward human leukemic cells in vivo using ALL cell lines in xenograft models. F10 induced tumor regression and provided a survival benefit and, in several cases, complete tumor eradication was observed. F10 was also efficacious toward animal models of drug-resistant ALL. Chemotherapy with AraC and other drugs is frequently effective for inducing remission in ALL patients [55]; however, relapsed disease is chemorefractory and highly lethal [56]. F10 was highly cytotoxic toward AraC-resistant ALL cells isolated from drug-treated mice and provided a survival benefit toward leukemias generated using drug-resistant cells that were not responsive to AraC treatment demonstrating F10 is not cross-resistant with AraC in this setting, and likely will be effective in treating relapsed ALL. Mice treated with F10 displayed minimal toxicity to bone marrow, GI tract or other organs and F10 protected mice from leukemia-induced weight loss consistent with F10 being better tolerated than conventional therapy [6].
• F10 for treatment of GBM
GBM is among the most challenging malignances to treat because it is highly invasive and chemoresistance develops in malignant cells that are not amenable to removal by surgical resection [57]. The presence of GBM tumors behind the blood–brain barrier [58] poses an additional challenge for chemotherapy related to drug delivery. We demonstrated that F10 was well-tolerated upon intra-cranial (i.c.) administration via an Alzet mini-pump which circumvented the need for blood–brain barrier permeability by F10. The doses of F10 administered i.c. [7], which subsequently were shown to be highly efficacious, were much lower than those administered systemically in either our AML [5] or ALL [6] studies, which is consistent with F10 administered i.c. being retained within the brain. Our studies also showed F10 was highly cytotoxic to GBM cells, and was more than 10,000-fold more cytotoxic than 5-FU toward certain CNS malignancies that were included in the NCI 60 cell line panel [23]. F10 also efficiently inhibited TS in GBM cells; however, TS is expressed at higher levels by GBM cells relative to AML cells, therefore, somewhat higher F10 concentrations were required for complete TS inhibition. As with AML and ALL cells, F10 induced Top1cc formation under thymineless conditions which resulted in DNA damage and cell death [7].
F10 treatment nearly completely eradicated human G48a xenografts in a murine orthotopic GBM model [7]. G48a cells were derived from a GBM patient [59], and orthotopic tumors are formed by injecting these cells into immunocompromised mice. The resulting tumors are highly invasive and recapitulate this characteristic of human disease. F10 was highly effective at eradicating the predominant tumor mass that developed in the brain following i.c. injection. Perhaps of greater importance, clusters of tumor cells proximal to the leading edge of tumor growth in vehicle-treated animals were not present in mice treated with F10 indicating it effectively inhibited tumor invasiveness [7]. Importantly, F10 did not damage normal brain which is consistent with studies we performed ex vivo using normal cortical neurons removed from embryonic mice and grown in tissue culture. These studies revealed F10 did not decrease neuronal survival at concentrations sufficient to cause GBM cell death. In contrast, equivalent doses of 5-FU were toxic to neurons. These studies demonstrate the markedly different responses of neurons and normal brain tissue to F10 relative to CNS malignancies. This highly favorable therapeutic window for F10 may enable successful treatment of GBM, and contrasts with traditional fluoropyrimidine chemotherapy that causes serious neurotoxicities due to degradation metabolites such as FBAL [60], while producing lower levels of DNA-directed metabolites responsible for cytotoxicity to highly proliferative GBM cells.
• F10 for prostate cancer
While only about 15% of prostate cancer patients develop metastatic disease, survival outcomes are exceedingly poor for those that do [61]. 5-FU was evaluated in clinical trials for late stage prostate cancer but displayed minimal survival benefit and significant side effects [62]. The current standard of care for patients that no longer respond to androgen deprivation therapy is chemotherapy with docetaxel and while this provides an additional, though relatively brief, survival benefit, the disease is uniformly lethal [63]. Evaluation of F10 in the NCI 60 cell line screen demonstrated prostate cancer cells were much more sensitive to F10 than to 5-FU (600–1500-fold) [23], indicating F10 may be effective for treating late stage prostate cancer even though 5-FU was not effective in clinical studies. Since fluoropyrimidines are potent radiosensitizers [64] and radiation is also an important treatment modality for prostate cancer [65], we also evaluated the antitumor activity of F10 in combination with radiation [8]. In studies using clonogenic assays we established that F10 was a potent radiosensitizer in prostate cancer cells. F10 treatment results in highly efficient and sustained TS inhibition and, as in for other malignant cells, F10 caused DNA damage and cell death.
We evaluated F10 in a xenograft model to determine if the increased sensitivity to F10 relative to 5-FU could result in improved anticancer activity [8]. These studies used a conservative dosing regimen in which F10 was administered intravenously three-times per week for 5 weeks at 40 mg/kg. With this dosing schedule, F10 caused no weight loss and no damage was apparent to the GI tract, or any other organs, for any of the treated animals. F10 had single-agent antitumor activity and significantly decreased tumor growth rates and improved median survival in this tumor model by 18 days relative to vehicle-only control which was significant by log rank test (p < 0.001). F10 also significantly improved the antitumor activity of radiation in this model demonstrating F10 is a potent radiosensitizer in vivo in addition to its anticancer activity. Importantly, average tumor volumes did not become significantly larger for mice treated with F10+radiation consistent with the radiosensitizing properties of F10 having a sustained effect on tumor growth. In fact, histological examination of excised tumors revealed marked hypocellularity, indicating the potential for complete tumor eradication with this approach.
Conclusion
F10 displays exceptionally strong anticancer activity in multiple challenging models of human malignancies for which current fluoropyrimidine drugs are not effective and for which no chemotherapeutic options provide a long-term survival benefit. F10 also has an improved therapeutic index relative to multiple chemotherapeutic drugs in current widespread use. The improved anticancer activity of F10 appears to result from three components: endocytosis of F10 via active transport into some types of malignant cells, but not into nonmalignant cells; efficient conversion to monomeric FdUMP inside cancer cells without a requirement for anabolic metabolism resulting in more complete and sustained TS inhibition than current fluoropyrimidines; and incorporation of dUTP and FdUTP into DNA under thymineless conditions which results in trapping of Top1cc. The dual targeting of TS and Top1 results in formation of lethal DNA damage and cell death mediated via the extrinsic apoptotic pathway. F10 is highly efficacious in vivo with strong anticancer activity achieved with reduced systemic toxicities relative to conventional drugs indicating it has both a unique mechanism and appropriate pharmacological properties to be considered for further development.
Future perspective
F10 may be useful as a new agent for treatment of a range of malignancies, including several that are not adequately treated with any chemotherapeutic option and for which fluoropyrimidine drugs have not previously displayed efficacy (e.g., AML, GBM). In light of the important role 5-FU plays in colon cancer treatment and the therapeutic advantages observed for F10 relative to 5-FU, we expect F10 to also display strong activity for colon cancer treatment. The longstanding historical use of fluoropyrimidines for cancer treatment makes it likely that we will be able to achieve dosing in humans that will result in improved outcomes relative to conventional chemotherapy.
EXECUTIVE SUMMARY.
F10 design overcomes limitations in fluoropyrimidine drugs that limit efficacy
Inefficient conversion to FdUMP results in suboptimal anticancer activity for 5-fluorouracil (5-FU).
Degradation and RNA-directed metabolites cause 5-FU systemic toxicity.
F10 is internalized by some malignant cells via active transport.
Efficient conversion to FdUMP following cell uptake results in strong activity and minimal toxicity.
F10 uniquely targets both thymidylate synthase & Top1
F10 is much more potent than conventional fluoropyrimidine drugs.
Thymidylate synthase is inhibited more completely and for longer times with F10 than 5-FU.
F10 promotes FdUTP misincorporation into DNA and poisons Top1 by interfering in the religation step of Top1 catalysis.
DNA damage resulting from F10 treatment activates the extrinsic apoptotic pathway.
F10 is efficacious for treating models of acute myeloid leukemia, acute lymphocytic leukemia, glioblastoma & prostate cancer
F10 is well-tolerated in vivo with doses that result in strong anticancer activity causing minimal systemic toxicities.
F10 is cytotoxic through dual targeting of thymidylate synthase and Top1 resulting in high levels of DNA damage in proliferating malignant cells.
F10 is effective for treating drug-resistant acute leukemia and promotes long-term survival in xenograft models of acute lymphocytic leukemia.
F10 is well-tolerated upon intra-cranial administration and efficacious in xenograft models of glioblastoma that display a highly invasive phenotype.
F10 is a potent radiosensitizer and effective in models of castration-resistant prostate cancer.
Footnotes
Financial & competing interests disclosure
The authors advise Salzburg Therapeutics, which is involved in commercialization of F10 for cancer treatment and are inventors on issued patents and pending patent applications filed by Wake Innovations. The authors are grateful for support from the Wake Forest Baptist Comprehensive Cancer Center P30 CA012197. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Heidelberger C, Danenberg PV, Moran RG. Fluorinated pyrimidines and their nucleosides. Adv. Enzymol. Relat. Areas Mol. Biol. 1983;54:58–119. [PubMed] [Google Scholar]
- 2.Wilson PM, Danenberg PV, Johnston PG, Lenz HJ, Ladner RD. Standing the test of time: targeting thymidylate biosynthesis in cancer therapy. Nat. Rev. Clin. Oncol. 2014;11(5):282–298. doi: 10.1038/nrclinonc.2014.51. [DOI] [PubMed] [Google Scholar]
- 3.An Q, Robins P, Lindahl T, Barnes DE. 5-Fluorouracil incorporated into DNA is excised by the Smug1 DNA glycosylase to reduce drug cytotoxicity. Cancer Res. 2007;67(3):940–945. doi: 10.1158/0008-5472.CAN-06-2960. [DOI] [PubMed] [Google Scholar]
- 4.Bastos DA, Ribeiro SC, de Freitas D, Hoff PM. Combination therapy in high-risk stage II or stage III colon cancer: current practice and future prospects. Ther. Adv. Med. Oncol. 2010;2(4):261–272. doi: 10.1177/1758834010367905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pardee TS, Gomes E, Jennings-Gee J, Caudell D, Gmeiner WH. Unique dual targeting of thymidylate synthase and topoisomerase 1 by FdUMP[10] results in high efficacy against AML and low toxicity. Blood. 2012;119:3561–3570. doi: 10.1182/blood-2011-06-362442. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper demonstrates improved efficacy and reduced toxicity occurs for F10 relative to 5-FU in syngeneic, orthotopic models of acute myeloid leukemia, including acute myeloid leukemia with genetic factors that confer a poor prognosis.
- 6.Pardee TS, Stadelman K, Jennings-Gee J, Caudell DL, Gmeiner WH. The poison oligonucleotide F10 is highly proliferative against acure lympoblastic leukemia while sparing normal hemtopoietic cells. Oncotarget. 2014;5(12):4170–4179. doi: 10.18632/oncotarget.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Demonstrates dose–response of F10 in vivo and a long-term survival advantage in acute lymphocytic leukemia models.
- 7.Gmeiner WH, Lema-Tome C, Gibo D, Jennings-Gee J, Milligan C, Debinski W. Selective anti-tumor activity of the novel fluoropyrimidine polymer F10 towards G48a orthotopic GBM tumors. J. Neurooncol. 2014;116(3):447–454. doi: 10.1007/s11060-013-1321-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• F10 is shown to have minimal toxicity to normal cortical neurons and to display strong efficacy to orthotopic glioblastoma upon intra-cerebral administration.
- 8.Gmeiner WH, Bourland JD, Hatcher HC, Smith TL, D'Agostino RB, Jr, Blackstock W. F10 inhibits growth of PC3 xenografts and enhances the effects of radiation therapy. JSM Clin. Oncol. Res. 2014;2(4):1028. [PMC free article] [PubMed] [Google Scholar]
- 9.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer. 2003;3(5):330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 10.Carreras CW, Santi DV. The catalytic mechanism and structure of thymidylate synthase. Annu. Rev. Biochem. 1995;64:721–762. doi: 10.1146/annurev.bi.64.070195.003445. [DOI] [PubMed] [Google Scholar]
- 11.Gmeiner WH. Novel chemical strategies for thymidylate synthase inhibition. Curr. Med. Chem. 2005;12(2):191–202. doi: 10.2174/0929867053363432. [DOI] [PubMed] [Google Scholar]
- 12.Ciaparrone M, Quirino M, Schinzari G, et al. Predictive role of thymidylate synthase, dihydropyrimidine dehydrogenase and thymidine phosphorylase expression in colorectal cancer patients receiving adjuvant 5-fluorouracil. Oncology. 2006;70(5):366–377. doi: 10.1159/000098110. [DOI] [PubMed] [Google Scholar]
- 13.Diasio RB, Johnson MR. The role of pharmacogenetics and pharmacogenomics in cancer chemotherapy with 5-fluorouracil. Pharmacology. 2000;61(3):199–203. doi: 10.1159/000028401. [DOI] [PubMed] [Google Scholar]
- 14.Matsubara I, Kamiya J, Imai S. Cardiotoxic effects of 5-fluorouracil in the guinea pig. Jpn. J. Pharmacol. 1980;30(6):871–879. doi: 10.1254/jjp.30.871. [DOI] [PubMed] [Google Scholar]
- 15.Yamashita K, Yada H, Ariyoshi T. Neurotoxic effects of alpha-fluoro-beta-alanine (FBAL) and fluoroacetic acid (FA) on dogs. J. Toxicol. Sci. 2004;29(2):155–166. doi: 10.2131/jts.29.155. [DOI] [PubMed] [Google Scholar]
- 16.Gusella M, Frigo AC, Bolzonella C, et al. Predictors of survival and toxicity in patients on adjuvant therapy with 5-fluorouracil for colorectal cancer. Br. J. Cancer. 2009;100(10):1549–1557. doi: 10.1038/sj.bjc.6605052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolmark N, Rockette H, Mamounas E, et al. Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes’ B and C carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project C-04. J. Clin. Oncol. 1999;17(11):3553–3559. doi: 10.1200/JCO.1999.17.11.3553. [DOI] [PubMed] [Google Scholar]
- 18.Noordhuis P, Holwerda U, Van der Wilt CL, et al. 5-Fluorouracil incorporation into RNA and DNA in relation to thymidylate synthase inhibition of human colorectal cancers. Ann. Oncol. 2004;15(7):1025–1032. doi: 10.1093/annonc/mdh264. [DOI] [PubMed] [Google Scholar]
- 19.Gmeiner WH, Trump E, Wei C. Enhanced DNA-directed effects of FdUMP[10] compared with 5FU. Nucleosides Nucleotides Nucleic Acids. 2004;23(1–2):401–410. doi: 10.1081/ncn-120028336. [DOI] [PubMed] [Google Scholar]
- 20.Shaw JP, Kent K, Bird J, Fishback J, Froehler B. Modified deoxyoligonucleotides stable to exonuclease degradation in serum. Nucleic Acids Res. 1991;19(4):747–750. doi: 10.1093/nar/19.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bijnsdorp IV, Comijn EM, Padron JM, Gmeiner WH, Peters GJ. Mechanisms of action of FdUMP[10]: metabolite activation and thymidylate synthase inhibition. Oncol Rep. 2007;18(1):287–291. doi: 10.3892/or.18.1.287. [DOI] [PubMed] [Google Scholar]; •• This paper used cell lines deficient in metabolic activation of monomeric fluoropyrimidines to show F10 cytotoxicity did not occur predominantly as a result of extracellular degradation.
- 22.Liao ZY, Sordet O, Zhang HL, et al. A novel polypyrimidine antitumor agent FdUMP[10] induces thymineless death with topoisomerase I-DNA complexes. Cancer Res. 2005;65(11):4844–4851. doi: 10.1158/0008-5472.CAN-04-1302. [DOI] [PubMed] [Google Scholar]; •• This paper showed for the first time that F10 caused DNA damage through poisoning of Top1 by inhibiting the religation step of Top1 catalysis.
- 23.Gmeiner WH, Reinhold WC, Pommier Y. Genome-wide mRNA and microRNA profiling of the NCI 60 cell-line screen and comparison of FdUMP[10] with fluorouracil, floxuridine, and topoisomerase 1 poisons. Mol. Cancer Ther. 2010;9(12):3105–3114. doi: 10.1158/1535-7163.MCT-10-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Q, Song X, Waldschmidt T, Fisher E, Krieg AM. Oligonucleotide uptake in human hematopoietic cells is increased in leukemia and is related to cellular activation. Blood. 1996;88(5):1788–1795. [PubMed] [Google Scholar]
- 25.Chu E, Voeller D, Koeller DM, et al. Identification of an RNA binding site for human thymidylate synthase. Proc. Natl Acad. Sci. USA. 1993;90(2):517–521. doi: 10.1073/pnas.90.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Schmitz JC, Lin X, et al. Thymidylate synthase as a translational regulator of cellular gene expression. Biochim. Biophys. Acta. 2002;1587(2–3):174–182. doi: 10.1016/s0925-4439(02)00080-7. [DOI] [PubMed] [Google Scholar]
- 27.Kamm YJ, Peters GJ, Hull WE, Punt CJ, Heerschap A. Correlation between 5-fluorouracil metabolism and treatment response in two variants of C26 murine colon carcinoma. Br. J. Cancer. 2003;89(4):754–762. doi: 10.1038/sj.bjc.6601162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curtin NJ, Harris AL, Aherne GW. Mechanism of cell death following thymidylate synthase inhibition: 2′-deoxyuridine-5′-triphosphate accumulation, DNA damage, and growth inhibition following exposure to CB3717 and dipyridamole. Cancer Res. 1991;51(9):2346–2352. [PubMed] [Google Scholar]
- 29.Dusenbury CE, Davis MA, Lawrence TS, Maybaum J. Induction of megabase DNA fragments by 5-fluorodeoxyuridine in human colorectal tumor (HT29) cells. Mol. Pharmacol. 1991;39(3):285–289. [PubMed] [Google Scholar]
- 30.Jennings-Gee J, Pardee TS, Gmeiner WH. Replication-dependent irreversible topoisomerase 1 poisoning is responsible for FdUMP[10] anti-leukemic activity. Exp. Hematol. 2013;41(2):180–188. doi: 10.1016/j.exphem.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pommier Y. Drugging topoisomerases: lessons and challenges. ACS Chem. Biol. 2013;8(1):82–95. doi: 10.1021/cb300648v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shoemaker RH. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer. 2006;6(10):813–823. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- 33.Gmeiner WH, Yu S, Pon RT, Pourquier P, Pommier Y. Structural basis for topoisomerase I inhibition by nucleoside analogs. Nucleosides Nucleotides Nucleic Acids. 2003;22(5–8):653–658. doi: 10.1081/NCN-120022604. [DOI] [PubMed] [Google Scholar]
- 34.Gmeiner WH. Antimetabolite incorporation into DNA: structural and thermodynamic basis for anticancer activity. Biopolymers. 2002;65(3):180–189. doi: 10.1002/bip.10214. [DOI] [PubMed] [Google Scholar]
- 35.Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat. Rev. Cancer. 2006;6(10):789–802. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- 36.Desai SD, Zhang H, Rodriguez-Bauman A, et al. Transcription-dependent degradation of topoisomerase I-DNA covalent complexes. Mol. Cell Biol. 2003;23(7):2341–2350. doi: 10.1128/MCB.23.7.2341-2350.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin CP, Ban Y, Lyu YL, Liu LF. Proteasome-dependent processing of topoisomerase I-DNA adducts into DNA double strand breaks at arrested replication forks. J. Biol. Chem. 2009;284(41):28084–28092. doi: 10.1074/jbc.M109.030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khodursky A, Guzman EC, Hanawalt PC. Thymineless death lives on: new insights into a classic phenomenon. Annu. Rev. Microbiol. 2015;69:247–263. doi: 10.1146/annurev-micro-092412-155749. [DOI] [PubMed] [Google Scholar]
- 39.Hanawalt PC. A balanced perspective on unbalanced growth and thymineless death. Front. Microbiol. 2015;6:504. doi: 10.3389/fmicb.2015.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grogan BC, Parker JB, Guminski AF, Stivers JT. Effect of the thymidylate synthase inhibitors on dUTP and TTP pool levels and the activities of DNA repair glycosylases on uracil and 5-fluorouracil in DNA. Biochemistry. 2011;50(5):618–627. doi: 10.1021/bi102046h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seiple L, Jaruga P, Dizdaroglu M, Stivers JT. Linking uracil base excision repair and 5-fluorouracil toxicity in yeast. Nucleic Acids Res. 2006;34(1):140–151. doi: 10.1093/nar/gkj430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Houghton JA, Harwood FG, Tillman DM. Thymineless death in colon carcinoma cells is mediated via fas signaling. Proc. Natl Acad. Sci. USA. 1997;94(15):8144–8149. doi: 10.1073/pnas.94.15.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tillman DM, Petak I, Houghton JA. A Fas-dependent component in 5-fluorouracil/leucovorin-induced cytotoxicity in colon carcinoma cells. Clin. Cancer Res. 1999;5(2):425–430. [PubMed] [Google Scholar]
- 44.Gmeiner WH, Jennings-Gee J, Stuart CH, Pardee TS. Thymineless death in F10-treated AML cells occurs via lipid raft depletion and Fas/FasL co-localization in the plasma membrane with activation of the extrinsic apoptotic pathway. Leuk. Res. 2015;39(2):229–235. doi: 10.1016/j.leukres.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feldman EJ, Gergis U. Management of refractory acute myeloid leukemia: re-induction therapy or straight to transplantation? Curr. Hematol. Malig. Rep. 2012;7(1):74–77. doi: 10.1007/s11899-011-0101-2. [DOI] [PubMed] [Google Scholar]
- 46.Wielinga PR, Westerhoff HV, Lankelma J. The relative importance of passive and P-glycoprotein mediated anthracycline efflux from multidrug-resistant cells. Eur. J. Biochem. 2000;267(3):649–657. doi: 10.1046/j.1432-1327.2000.01030.x. [DOI] [PubMed] [Google Scholar]
- 47.Veuger MJ, Honders MW, Spoelder HE, Willemze R, Barge RM. Inactivation of deoxycytidine kinase and overexpression of P-glycoprotein in AraC and daunorubicin double resistant leukemic cell lines. Leuk. Res. 2003;27(5):445–453. doi: 10.1016/s0145-2126(02)00224-2. [DOI] [PubMed] [Google Scholar]
- 48.Pardee TS, Zuber J, Lowe SW. Flt3-ITD alters chemotherapy response in vitro and in vivo in a p53-dependent manner. Exp. Hematol. 2011;39(4):473–485. doi: 10.1016/j.exphem.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pardee TS. Overexpression of MN1 confers resistance to chemotherapy, accelerates leukemia onset, and suppresses p53 and Bim induction. PLoS ONE. 2012;7(8):e43185. doi: 10.1371/journal.pone.0043185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zuber J, Radtke T, Pardee TS, et al. Mouse models of human AML accurately predict chemotherapy response. Genes Dev. 2009;23(7):877–889. doi: 10.1101/gad.1771409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pritchard DM, Watson AJ, Potten CS, Jackman AL, Hickman JA. Inhibition by uridine but not thymidine of p53-dependent intestinal apoptosis initiated by 5-fluorouracil: evidence for the involvement of RNA perturbation. Proc. Natl Acad. Sci. USA. 1997;94(5):1795–1799. doi: 10.1073/pnas.94.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper linked 5-FU-induced gastrointestinal damage to RNA-mediated effects.
- 52.Lu H, Zhu S, Qian L, et al. Activated expression of the chemokine Mig after chemotherapy contributes to chemotherapy-induced bone marrow suppression and lethal toxicity. Blood. 2012;119(21):4868–4877. doi: 10.1182/blood-2011-07-367581. [DOI] [PubMed] [Google Scholar]
- 53.Krug U, Buchner T, Berdel WE, Muller-Tidow C. The treatment of elderly patients with acute myeloid leukemia. Dtsch Arztebl. Int. 2011;108(51–52):863–870. doi: 10.3238/arztebl.2011.0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ottmann OG, Pfeifer H. Management of Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) Hematology. Am. Soc. Hematol. Educ. Program. 2009:371–381. doi: 10.1182/asheducation-2009.1.371. [DOI] [PubMed] [Google Scholar]
- 55.Weiss M, Maslak P, Feldman E, et al. Cytarabine with high-dose mitoxantrone induces rapid complete remissions in adult acute lymphoblastic leukemia without the use of vincristine or prednisone. J. Clin. Oncol. 1996;14(9):2480–2485. doi: 10.1200/JCO.1996.14.9.2480. [DOI] [PubMed] [Google Scholar]
- 56.Oriol A, Vives S, Hernandez-Rivas JM, et al. Outcome after relapse of acute lymphoblastic leukemia in adult patients included in four consecutive risk-adapted trials by the PETHEMA Study Group. Haematologica. 2010;95(4):589–596. doi: 10.3324/haematol.2009.014274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Agnihotri S, Burrell KE, Wolf A, et al. Glioblastoma, a brief review of history, molecular genetics, animal models and novel therapeutic strategies. Arch. Immunol. Ther. Exp. (Warsz) 2013;61(1):25–41. doi: 10.1007/s00005-012-0203-0. [DOI] [PubMed] [Google Scholar]
- 58.van Tellingen O, Yetkin-Arik B, de Gooijer MC, Wesseling P, Wurdinger T, de Vries HE. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist. Update. 2015;19:1–12. doi: 10.1016/j.drup.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 59.Pandya H, Gibo DM, Garg S, Kridel S, Debinski W. An interleukin 13 receptor alpha 2-specific peptide homes to human glioblastoma multiforme xenografts. Neuro Oncol. 2012;14(1):6–18. doi: 10.1093/neuonc/nor141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miura K, Kinouchi M, Ishida K, et al. 5-FU metabolism in cancer and orally-administrable 5-fu drugs. Cancers (Basel) 2010;2(3):1717–1730. doi: 10.3390/cancers2031717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oudard S. Progress in emerging therapies for advanced prostate cancer. Cancer Treat. Rev. 2013;39(3):275–289. doi: 10.1016/j.ctrv.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 62.Berlin JD, Propert KJ, Trump D, et al. 5-fluorouracil and leucovorin therapy in patients with hormone refractory prostate cancer: an Eastern Cooperative Oncology Group Phase II study (E1889) Am J. Clin. Oncol. 1998;21(2):171–176. doi: 10.1097/00000421-199804000-00016. [DOI] [PubMed] [Google Scholar]
- 63.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 64.Lawrence TS, Maybaum J. Fluoropyrimidines as radiation sensitizers. Semin. Radiat. Oncol. 1993;3(1):20–28. doi: 10.1053/SRAO00300020. [DOI] [PubMed] [Google Scholar]
- 65.Martin NE, D'Amico AV. Progress and controversies: radiation therapy for prostate cancer. CA Cancer J. Clin. 2014;64(6):389–407. doi: 10.3322/caac.21250. [DOI] [PubMed] [Google Scholar]