Abstract
Co-expression of ERBB2 and ERBB4, reported in 75 % of pediatric ependymomas, correlates with worse overall survival. Lapatinib, a selective ERBB1 and ERBB2 inhibitor has produced prolonged disease stabilization in patients with ependymoma in a phase I study. Bevacizumab exposure in ependymoma xenografts leads to ablation of tumor self-renewing cells, arresting growth. Thus, we conducted an open-label, phase II study of bevacizumab and lapatinib in children with recurrent ependymomas. Patients ≤21 years of age with recurrent ependymoma received lapatinib orally twice daily (900 mg/m2/dose to the first 10 patients, and then 700 mg/ m2/dose) and bevacizumab 10 mg/kg intravenously on days 1 and 15 of a 28-day course. Lapatinib serum trough levels were analyzed prior to each course. Total and phosphorylated VEGFR2 expression was measured in peripheral blood mononuclear cells (PBMCs) before doses 1 and 2 of bevacizumab and 24–48 h following dose 2 of bevacizumab. Twenty-four patients with a median age of 10 years (range 2–21 years) were enrolled; 22 were eligible and 20 evaluable for response. Thirteen had anaplastic ependymoma. There were no objective responses; 4 patients had stable disease for ≥4 courses (range 4–14). Grade 3 toxicities included rash, elevated ALT, and diarrhea. Grade 4 toxicities included peri-tracheostomy hemorrhage (n = 1) and elevated creatinine phosphokinase (n = 1). The median lapatinib pre-dose trough concentration was 3.72 μM. Although the combination of bevacizumab and lapatinib was well tolerated in children with recurrent ependymoma, it proved ineffective.
Keywords: Children, Recurrent ependymoma, Lapatinib, Bevacizumab
Introduction
Outcome remains poor in children with relapsed ependymoma regardless of age at recurrence and therapy [1, 2]. Co-expression of ERBB2/ERBB4 has been identified in 75 % of pediatric ependymoma specimens [3]. Expression of these receptors was significantly related to tumor proliferation and was inhibited in a dose-dependent manner using an ERBB2 inhibitor [3], suggesting a role for ERBB2 receptor-targeted therapies. Furthermore, the degree of surgical resection, ERBB2/ERBB4 expression status and Ki-67 labeling index were found to have the greatest prognostic significance for overall survival in children with ependymomas [3]. Lapatinib is a selective small molecule inhibitor of ERBB1/ERBB2, inhibiting with an IC50 of 0.01 μM and inhibition of ERBB4 at lower potency [4]. A pediatric phase I study reported the recommended phase II dose (RP2D) of lapatinib in children with recurrent brain tumors as 900 mg/m2/dose twice daily (BID) with prolonged disease stabilization in ependymoma patients [5].
Over-expression of vascular endothelial growth factor (VEGF) in patients with ependymoma has been well-documented as the mediator of endothelial proliferation and tumor infiltration, resulting in poor survival [6]. Preclinical studies have demonstrated that tumor endothelial cells interact with cancer stem-like cells in the vascular niche and bevacizumab exposure in ependymoma xenografts results in ablation of tumor self-renewing cells, leading to tumor growth arrest [7, 8]. Rich et al. reported that administration of ZD6474, a tyrosine kinase inhibitor of VEGFR and EGFR, results in significant activity against ependymoma xenografts [9]. These data suggest that dual inhibition of angiogenesis and EGFR/ERBB signaling may be a strategy in the treatment of pediatric ependymomas. Clinically, a retrospective review of 8 adult patients with recurrent ependymoma treated with bevacizumab-based therapy reported a partial response (PR) in 6 patients (75 %) and stable disease for over 8 months in 1 patient [10]. A phase II study of bevacizumab and lapatinib in heavily-pretreated adults with recurrent metastatic breast cancer was well-tolerated with durable clinical responses [11]. Based on these encouraging preclinical and clinical data in addition to the non-overlapping toxicities of the two agents, the Collaborative Ependymoma Research Network (CERN) conducted an open-label, phase II study of bevacizumab and lapatinib in children with recurrent or refractory ependymomas.
Materials and methods
Study objectives
The primary objective of the study was to estimate the sustained objective response rates (defined as complete response (CR plus PR) of lapatinib 900 mg/m2/dose BID and bevacizumab 10 mg/kg IV every 2 weeks in children with recurrent or refractory ependymoma. Secondary objectives included estimation of progression free survival (PFS), determination of inhibition of the VEGFR2 phosphorylation in peripheral blood mononuclear cells (PBMC), and characterization of lapatinib steady state concentrations in the presence of bevacizumab.
Patients and methods
Patient eligibility criteria
Inclusion criteria
Eligible patients were ≤21 years with a histologically verified diagnosis of recurrent or refractory intracranial or spinal ependymoma including ependymoma (WHO grade II) or anaplastic ependymoma (WHO grade III) confirmed by the designated CERN pathologist on tissue obtained at initial presentation or recurrence. Patients must have had measurable disease (bi-dimensional measurement); any number of prior treatment regimens before or after radiotherapy; stable or improving neurological deficits for at least 1 week prior to registration; and Lansky or Karnofsky performance score ≥50. Patients were required to have recovered from the acute toxic effects of prior therapy or surgical procedures. Patients were not allowed to have received: growth factors within 7 days of study entry, myelosuppressive chemotherapy within 3 weeks (6 weeks if nitrosourea), a biologic agent within 7 days, biologic/investigational agents with a prolonged half-life within 3 weeks, craniospinal or total body irradiation within 6 months, local palliative radiotherapy within 2 weeks, or other substantial bone marrow irradiation within 6 weeks. Other requirements included adequate bone marrow (absolute neutrophil count ≥1000/μl, platelet count ≥100,000/ μl, transfusion independent hemoglobin ≥8.0 gm/dL), renal (serum creatinine ≤1.5 times upper limit of normal [ULN] for age, or GFR ≥70 ml/min/1.73 m2), and liver (total bilirubin ≤1.5 × institutional [ULN] for age, ALT ≤2.5 × institutional [ULN] for age and albumin ≥2.5 g/dL) function and stable or decreasing dose for at least 1 week.
Exclusion criteria
Patients were excluded if they received prior treatment with bevacizumab or lapatinib, had evidence of new intracranial or intratumoral hemorrhage larger than punctuate size on baseline MRI obtained within 14 days prior to study registration, were on anticoagulants or other investigational agents. If proteinuria were present on dipstick, patients had a 24-hour urine collection, and were excluded if >500 mg protein. Patients with uncontrolled infection, ≥grade 2 uncontrolled hypertension, history of stroke, myocardial infarction, or unstable angina in the previous 6 months, evidence of a bleeding diathesis, coagulopathy or PT/INR > 1.5, pre-existent coagulopathy or thrombosis, history of abdominal fistula, GI perforation, or intra-abdominal abscess within previous 6 months, non-healing wound, ulcer or bone fracture, and/or a major surgical procedure within 4 weeks prior to registration were excluded. Pregnant or lactating females and patients on enzyme-inducing anticonvulsants were excluded. Patients of childbearing or child fathering potential were required to use a medically acceptable form of birth control.
Informed consent was obtained from patients, parents or guardians, and assent was obtained as appropriate at the time of protocol enrollment. The institutional review boards of each CERN institution approved the protocol before initial patient enrollment and continuing approval was maintained throughout the study.
Drug administration
Bevacizumab, provided by Genentech, was administered intravenously every 2 weeks at 10 mg/kg/dose on day 1 and day 15 of a 28-day course. Lapatinib, supplied by GlaxoSmithKline as 250 mg tablets, was initially administered orally on an empty stomach at 900 mg/m2/dose BID to the first 10 patients [5] and then the dose changed to 700 mg/m2/dose due to concerns for toxicity. Daily dosing was rounded up or down to the nearest 125 mg. Patients could receive up to 26 courses in the absence of disease progression and unacceptable toxicity.
Dose modifications
Hematological lapatinib or bevacizumab-related dose-modifying toxicity was defined as grade 4 neutropenia for greater than 7 days or grade 4 thrombocytopenia on 2 separate occasions in a 7-day period. Non-hematological dose modifying toxicities for lapatinib and bevacizumab were defined as grade 3 or 4 toxicity with the exclusion of grade 3 or 4 electrolyte abnormality which resolves to ≤grade 2 with or without supplementation within 7 days of interrupting study drugs, grade 3 nausea/vomiting responsive to antiemetic therapy, grade 3 fever or infection, grade 3 hypertension controlled with oral medication.
Specific dose modifying lapatinib related hepatotoxicity was defined as ≥grade 3 elevation of ALT; grade 2 elevations of ALT with ≥grade 2 elevation in direct bilirubin or elevation in INR; grade 2 elevations in ALT with appearance or worsening of fatigue, nausea, vomiting, right upper quadrant pain or tenderness, fever, hypersensitivity rash or eosinophilia. Lapatinib was held for 2 weeks to allow patients to recover to ≤grade 1 and then restarted at a reduced lapatinib dose of 520 mg/m2/dose BID. Otherwise, the patient was taken off therapy. Lapatinib-related cardiac dose modifying toxicity was defined as grade III or IV left ventricular systolic dysfunction and removal from study.
For bevacizumab-related ≥grade 3 toxicities, bevacizumab was held. Patients were removed from study if therapy could not be restarted after a four-week interruption. Bevacizumab was discontinued in patients with grade 4 toxicity or with any grade arterial or venous thrombosis, cardiac ischemia or infarct, CNS ischemia (TIA, CVA), any grade CNS hemorrhage (except punctate lesions), wound dehiscence requiring medical or surgical intervention, GI perforation, GI leak or fistula, reversible posterior leukoencephalopathy syndrome.
Required observations prior to, during, and off study
Clinical examination and laboratory data including CBC, electrolytes, renal and liver function, coagulation studies, and urine were obtained at baseline, weekly during course 1, every 2 weeks during course 2, then monthly during course 3–26. Echocardiogram and radiographic imaging of the right knee were obtained at baseline, end of course 2 and every 12 weeks. If abnormalities were detected, bilateral knee on magnetic resonance imaging (MRI) was obtained for confirmation. MRI of the brain/spine and CSF cytology were obtained at baseline. An MRI of the brain/ spine was obtained at the end of course 2 and every 8 weeks thereafter. MRI of the spine and CSF cytology were repeated every 8 weeks if positive at baseline or if clinically indicated.
Evaluation of response
Patients were evaluable for response if they received at least 2 courses of therapy. Patients who received one course of therapy were not evaluable for response unless they experienced progressive disease prior to the completion of the second course. Tumor response was defined as follows: CR, disappearance of all measurable lesions on MRI; PR, ≥50 % reduction in tumor size by bi-dimensional measurement on a stable or decreasing dose of corticosteroids, accompanied by a stable or improving neurological exam and maintained for at least 8 weeks; progressive disease (PD), worsening neurologic status or>25 % increase in the bi-dimensional measurement, appearance of new lesions, or increasing corticosteroids doses; stable disease (SD), MRI response does not meet the criteria for other categories, with stable corticosteroid dose and neurologic examination sustained for at least 8 weeks.
Study design and statistical analysis
A Simon two-stage design was used. The primary endpoint of the study was objective response rate (CR + PR) sustained for at least 4 weeks. Sample size and decision criteria were chosen to reduce the expected accrual if the treatment was ineffective. The target response was at least 25 %. The therapy would be deemed ineffective if ≤2 responses were observed in at least 20 patients. More than 2 responses to the combination therapy were required to proceed to the second stage. Kaplan–Meier method was used to estimate progression free survival. All analysis was performed using SAS 9.3 (SAS Institute Inc., Cary, NC, USA) and Splus 8.2 (TIBCO Software Inc, Palo Alto, CA).
Pharmacokinetic studies
Blood samples were collected from consenting patients on day 1 of the corresponding course. Each sample, at least 2 mL of blood was collected pre-dose (at end of previous dosing interval) into a green-topped heparinized tube, centrifuged at room temperature at 7000×g for 10 min, and the plasma stored at −20 °C until analysis. Lapatinib plasma concentrations were determined using an isocratic high performance liquid chromatography assay with tandem mass spectrometry using a modified version of a method described previously [12]. The observed trough or minimum steady-state concentrations (Css,min) for all patients were summarized using descriptive statistics.
Pathology
Central review of histopathology was mandatory for all patients and involved processing of formalin-fixed paraffin-embedded (FFPE) tumor tissue.
Biologic studies
Inhibition of VEGF-R2 signaling by bevacizumab was evaluated by VEGF-R2 expression in PBMC collected prior to and following treatment with bevacizumab among consented patients. The expression of total and phospho-VEGF-R2 and the total receptor were analyzed in PBMC by immunoblotting. Primary antibody included total VEGFR2 (Abcam #10973) and pVEGFR2 (Cell Signaling #2478). Secondary antibody included Polyclonal Goat-Anti Mouse (HRP DAKO #P0447) and Polyclonal Goat-Anti Rabbit (HRP DAKO #P0448). Chemi-illuminescence was evaluated using the Clarity Western Substrate ECL Substrate BioRad (#170-5061); developed with Chemidoc technology.
Results
Patient characteristics
Between September 2009 and December 2012, 24 patients were enrolled on Stage I of the CERN08-01 study. Two patients were ineligible due to inadequate tissue for central pathology confirmation. The median time of first progression prior to the trial enrollment was 13.34 months, 95 % CI (7.51,16.49). Table 1 summarizes the characteristics of eligible patients. Two patients were evaluable for toxicity only, but not response as they received <1 course of treatment and discontinued therapy due to grade 3 toxicities.
Table 1.
Clinical characteristics of eligible patients with recurrent ependymoma
| Patient characteristics | |
| Median age at enrollment (range) | 10 years (2–21 years) |
| Male | 14 (63.6 %) |
| Female | 8 (36.4 %) |
| Histology | |
| Ependymoma | 9 (40.9 %) |
| Anaplastic ependymoma | 13 (59.1 %) |
| Location of primary ependymoma | |
| Supratentorial | 11 (50 %) |
| Infratentorial | 11 (50 %) |
| Location of recurrence | |
| Locala | 19 (86 %) |
| Distant (outside of radiation field) | 3 (13 %) |
| Prior therapy | |
| Chemotherapy | 18 (82 %) |
| One systemic therapy | 13 |
| Two systemic therapies | 3 |
| Three systemic therapies | 2 |
| Radiotherapy | 22 (100 %) |
| Surgery | 22 (100 %) |
| Courses of lapatinib and bevacizumab | |
| Median (range) | 2 (1–14) |
| Response | |
| Objective responses (CR + PR) | 0 |
| n cases stable disease for >4 months | 4 |
| n cases progressive disease | 16 |
| Median time to progression | 7.9 weeks |
Four patients had evidence of metastatic disease upon enrollment as baseline disease evaluation and the disease was within the prior radiation field.
Responses
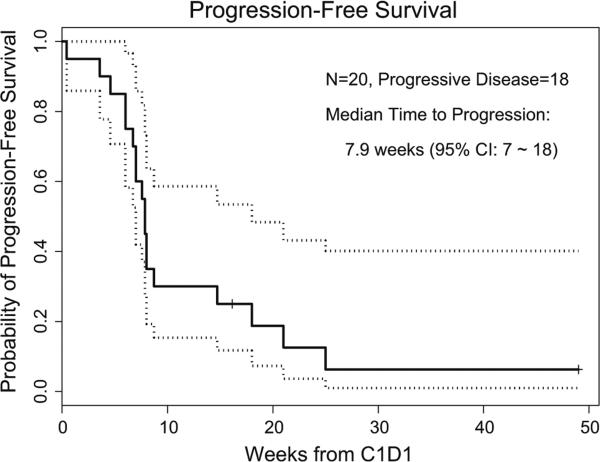
Twenty patients were evaluable for response. There were no objective responses. Prolonged SD for ≥4 courses (range 4–14 courses) was observed in 4 patients, all with anaplastic ependymoma. One of the 4 patients had local and disseminated recurrent disease. Three patients had 2 relapses and 1 patient had 1 relapse prior to enrollment. The median time to progression was 7.9 weeks (95 % confidence interval (CI): 7,18) (Fig. 1).
Fig. 1.
Kaplan–Meier survival curve showing PFS in 20 eligible patients with recurrent ependymoma. The dottedlines represent the 95 % confidence interval for the survival curve
Toxicities
All patients who received at least one dose of lapatinib and one dose of bevacizumab were evaluable for toxicity. Table 2 summarizes the number of adverse events that were ≥grade 2, attributed to therapy and observed in >10 % of evaluable patients. The most common grade 2–3 toxicities were diarrhea, rash, and elevated ALT/SGPT. Two grade 4 toxicities (peritracheal hemorrhage and elevated CPK (creatine phosphokinase) were observed. One patient presented on day 6 of course 1 with rash, diarrhea, pain and a grade 4 elevated CPK; all toxicities resolved within 1 week of holding lapatinib. The other patient had gastrostomy and tracheostomy tubes prior to starting therapy. During course 3, this patient developed a grade 4 peri-tracheostomy hemorrhage.
Table 2.
Number of Adverse Events were ≥Grade 2, attributed to therapy and observed in >10 % of evaluable patients
| Numbers of episodes for each toxicity* | Grade |
||
|---|---|---|---|
| 2 | 3 | 4 | |
| Diarrhea | 16 | 4 | 0 |
| Rash/desquamation | 6 | 3 | 0 |
| Fatigue | 3 | 0 | 0 |
| Anorexia | 2 | 0 | 0 |
| Nausea | 2 | 0 | 0 |
| Vomiting | 2 | 0 | 0 |
| ALT, SGPT (serum glutamic pyruvic) | 2 | 2 | 0 |
| AST (SGOT (serum glutamic oxaloacetic) | 1 | 0 | 0 |
| Hemorrhage, pulmonary/ Upper respiratory | 0 | 0 | 1 |
| Lymphopenia | 2 | 0 | 0 |
| Creatine phosphokinase (CPK) | 0 | 0 | 1 |
Lapatinib was initially administered orally at 900 mg/m2/dose BID in the first 10 patients [5]; however, more restrictive definitions of dose-limiting hepatotoxicity were introduced in all lapatinib trials. Three patients who were initially enrolled at 900 mg/m2/dose BID of lapatinib developed grade 3 toxicity (1 diarrhea, 2 liver dysfunction); 2 of the 3 patients were removed from therapy. The 900 mg/m2/dose BID that was the RP2D based on more liberal definitions of dose-limiting hepatotoxicity in the phase I study was deemed too toxic. The study was amended to decrease the lapatanib starting dosing to 700 mg/m2/dose BID. Three patients who had completed 1 course at 900 mg/m2/dose BID of lapatinib without evidence of toxicities had their doses for subsequent courses reduced to 700 mg/m2/dose BID. Nine patients enrolled on the amended study.
Pharmacokinetics
Twenty-three patients consented to pharmacokinetic studies, and evaluable serum samples were available from 15 patients. Seven samples from 6 patients were not evaluable due to analytical issues during sample preparation. A total of 32 steady-state trough samples were acquired and analyzed from these 15 patients with a median of 1 and a range of 1–12 determinations. Since the number of patients receiving the 700 or 900 mg/m2 dosage were near equivalent (n = 7 at 900 mg/m2 and n = 8 at 700 mg/m2), the data were combined for the analysis. Overall, the median (range) measured Css,min was 3.72 μl (0.51–7.81 μM) for all data combined from all courses. The Css,min was 4.57 μM (1.11–7.81 μM) for course 2 day 1, 2.24 μM (0.51–5.49 μM) for course 3 day 1, and 3.60 μM (1.25–4.65 μM) for all subsequent courses. In a single patient with 12 pharmacokinetic assessments, the intra-patient variability for this individual was 31.6 % with a median value of 3.60 μM.
Correlative studies
Total and phosphorylated VEGFR2 in PBMCs were evaluated in 5 consenting patients. A decrease in phosphorylated VEGFR2 was noted in 3 of 5 patient samples. Relative phosphorylation of VEGFR2 was calculated pre, immediately post and 24 h post bevacizumab infusion. A trend towards decreased phosphorylation of VEGFR2 after bevacizumab was observed; yet small sample size precludes us from making a direct correlation between bevacizumab dose and relative phosphorylation of VEGFR2.
Discussion
The combination of lapatinib and bevacizumab was well-tolerated in children with recurrent ependymoma; yet no objective responses were observed. Prolonged stabilization of disease was noted in 4 patients for ≥4 courses (range 4–14). Similar to the adult and pediatric phase I trials, the most common side effects included diarrhea, rash, and elevated ALT [5, 11].
The pediatric phase I trial reported the RP2D of lapatinib 900 mg/m2/dose BID and prolonged disease stabilization in 5 patients with recurrent ependymoma [5]. Recently, Gururangan et al. reported the lack of efficacy of bevacizumab and irinotecan in children with recurrent ependymoma [13]. Although disease stabilization was noted, the 6-month PFS was 27 %, comparable to prior studies in recurrent ependymoma [2, 13–15]. Our study's outcome is similarly disappointing. The number of prior recurrences may have contributed to the similar results given that the majority of patients enrolled in our study had at last 2 recurrences of disease prior to enrollment supporting possible chemoresistance.
The pharmacokinetic disposition of lapatinib has been well described in children with brain tumors and is similar to that found in this study [5, 16]. In the phase I study, a range of dosages was evaluated and the disposition was characterized by wide interpatient variability. The differences in systemic exposure (AUC, Cmax) between the dosages studied varied little compared with the extent of variability reported. Thus, we grouped the data in the present study from the two dosage groups for comparison with previously published data. The median (range) Css,min for pediatric patients treated on the same regimen in the prior pediatric phase I/phase II studies was similar to the median Css,min observed in the present report (Unpublished data, Dr. Clinton Stewart).
The rationale for combining these drugs for children with ependymoma included preclinical data showing efficacy of a VEGFR and EGFR inhibitor and bevacizumab's ability to arrest tumor growth in ependymoma xenograft models, the prognostic significance of ERBB2/ERBB4 expression and the non-overlapping toxicities of lapatinib and bevacizumab. Although phase I studies of both agents had been completed when we embarked on this study, the phase II studies with these agents in children with brain malignancies were ongoing. The lack of efficacy in the current study may be explained by results of a molecular biology lapatinib study that was recently published. In this study, children with recurrent medulloblastoma, ependymoma and high-grade glioma undergoing resection were stratified and randomized to pre-resection treatment with lapatinib 900 mg/m2/dose BID for 7–14 days or no treatment. Drug concentration was simultaneously assessed in tumor and plasma. Among the 8 patients enrolled on the pre-resection treatment arm (4 medulloblastomas, 4 ependymomas), no intratumoral target inhibition by lapatinib was noted. Although tumor-to-plasma ratios of lapatinib were 10–20 %, the intratumoral lapatinib concentrations were significantly lower than those required to inhibit ERBB2 [16].
The combination of lapatinib and bevacizumab proved tolerable but ineffective in children with recurrent ependymoma. This lack of efficacy may be explained by the facts that a) single agent bevacizumab demonstrated no efficacy in this patient population [17] and b) that the recently published data demonstrated that intratumoral lapatinib concentrations were below the IC50 required to inhibit EGFR and ERBB receptors [16].
Acknowledgments
Funding CERN, Glaxo Smith Kline, Genentech.
Footnotes
Conflict of interest The authors agree that they have no conflict of interest.
References
- 1.Messahel B, Ashley S, Saran F, Ellison D, Ironside J, Phipps K, Cox T, Chong WK, Robinson K, Picton S, Pinkerton CR, Mallucci C, Macarthur D, Jaspan T, Michalski A, Grundy RG, CsCLGBT Committee Relapsed intracranial ependymoma in children in the UK: patterns of relapse, survival and therapeutic outcome. Eur J Cancer. 2009;45(10):1815–1823. doi: 10.1016/j.ejca.2009.03.018. doi:10.1016/j.ejca. 2009.03.018. [DOI] [PubMed] [Google Scholar]
- 2.Zacharoulis S, Ashley S, Moreno L, Gentet JC, Massimino M, Frappaz D. Treatment and outcome of children with relapsed ependymoma: a multi-institutional retrospective analysis. Childs Nerv Syst. 2010;26(7):905–911. doi: 10.1007/s00381-009-1067-4. doi:10.1007/s00381-009-1067-4. [DOI] [PubMed] [Google Scholar]
- 3.Gilbertson RJ, Bentley L, Hernan R, Junttila TT, Frank AJ, Haapasalo H, Connelly M, Wetmore C, Curran T, Elenius K, Ellison DW. ERBB receptor signaling promotes ependymoma cell proliferation and represents a potential novel therapeutic target for this disease. Clin Cancer Res. 2002;8(10):3054–3064. [PubMed] [Google Scholar]
- 4.Rusnak DW, Lackey K, Affleck K, Wood ER, Alligood KJ, Rhodes N, Keith BR, Murray DM, Knight WB, Mullin RJ, Gilmer TM. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Molecular cancer therapeutics. 2001;1(2):85–94. [PubMed] [Google Scholar]
- 5.Fouladi M, Stewart CF, Blaney SM, Onar-Thomas A, Schaiquevich P, Packer RJ, Gajjar A, Kun LE, Boyett JM, Gilbertson RJ. Phase I trial of lapatinib in children with refractory CNS malignancies: a pediatric brain tumor consortium study. J Clin Oncol. 2010;28(27):4221–4227. doi: 10.1200/JCO.2010.28.4687. doi:10.1200/JCO.2010.28.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korshunov A, Golanov A, Timirgaz V. Immunohisto-chemical markers for prognosis of ependymal neoplasms. J Neurooncol. 2002;58(3):255–270. doi: 10.1023/a:1016222202230. [DOI] [PubMed] [Google Scholar]
- 7.Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, Jensen P, Magdaleno S, Dalton J, Calabrese C, Board J, Macdonald T, Rutka J, Guha A, Gajjar A, Curran T, Gilbertson RJ. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8(4):323–335. doi: 10.1016/j.ccr.2005.09.001. doi:10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11(1):69–82. doi: 10.1016/j.ccr.2006.11.020. doi:10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 9.Rich JN, Sathornsumetee S, Keir ST, Kieran MW, Laforme A, Kaipainen A, McLendon RE, Graner MW, Rasheed BK, Wang L, Reardon DA, Ryan AJ, Wheeler C, Dimery I, Bigner DD, Friedman HS. ZD6474, a novel tyrosine kinase inhibitor of vascular endothelial growth factor receptor and epidermal growth factor receptor, inhibits tumor growth of multiple nervous system tumors. Clin Cancer Res. 2005;11(22):8145–8157. doi: 10.1158/1078-0432.CCR-05-0319. doi:10.1158/1078-0432.CCR-05-0319. [DOI] [PubMed] [Google Scholar]
- 10.Green RM, Cloughesy TF, Stupp R, DeAngelis LM, Woyshner EA, Ney DE, Lassman AB. Bevacizumab for recurrent ependymoma. Neurology. 2009;73(20):1677–1680. doi: 10.1212/WNL.0b013e3181c1df34. doi:10.1212/WNL.0b013e3181c1df34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rugo HS, Chien AJ, Franco SX, Stopeck AT, Glencer A, Lahiri S, Arbushites MC, Scott J, Park JW, Hudis C, Nulsen B, Dickler MN. A phase II study of lapatinib and bevacizumab as treatment for HER2-overexpressing metastatic breast cancer. Breast Cancer Res Treat. 2012;134(1):13–20. doi: 10.1007/s10549-011-1918-z. doi:10.1007/s10549-011-1918-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai F, Freeman BB, Fraga CH, Fouladi M, Stewart CF. Determination of lapatinib (GW572016) in human plasma by liquid chromatography electrospray tandem mass spectrometry (LC-ESI-MS/MS). J Chromatogr B. 2006;831(1–2):169–175. doi: 10.1016/j.jchromb.2005.11.044. doi:10.1016/j.jchromb.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 13.Gururangan S, Fangusaro J, Young Poussaint T, Onar-Thomas A, Gilbertson RJ, Vajapeyam S, Gajjar A, Goldman S, Friedman HS, Packer RJ, Boyett JM, Kun LE, McLendon R. Lack of efficacy of bevacizumab ? irinotecan in cases of pediatric recurrent ependymoma—a pediatric brain tumor consortium study. Neuro Oncol. 2012;14(11):1404–1412. doi: 10.1093/neuonc/nos213. doi:10.1093/neuonc/nos213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouffet E, Capra M, Bartels U. Salvage chemotherapy for metastatic and recurrent ependymoma of childhood. Childs Nerv Syst. 2009;25(10):1293–1301. doi: 10.1007/s00381-009-0883-x. doi:10.1007/s00381-009-0883-x. [DOI] [PubMed] [Google Scholar]
- 15.Merchant TE, Boop FA, Kun LE, Sanford RA. A retrospective study of surgery and reirradiation for recurrent ependymoma. Int J Radiat Oncol Biol Phys. 2008;71(1):87–97. doi: 10.1016/j.ijrobp.2007.09.037. doi:10. 1016/j.ijrobp.2007.09.037. [DOI] [PubMed] [Google Scholar]
- 16.Fouladi M, Stewart CF, Blaney SM, Onar-Thomas A, Schaiquevich P, Packer RJ, Goldman S, Geyer JR, Gajjar A, Kun LE, Boyett JM, Gilbertson RJ. A molecular biology and phase II trial of lapatinib in children with refractory CNS malignancies: a pediatric brain tumor consortium study. J Neurooncol. 2013;114(2):173–179. doi: 10.1007/s11060-013-1166-7. doi:10.1007/s11060-013-1166-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glade Bender JL, Adamson PC, Reid JM, Xu L, Baruchel S, Shaked Y, Kerbel RS, Cooney-Qualter EM, Stempak D, Chen HX, Nelson MD, Krailo MD, Ingle AM, Blaney SM, Kandel JJ, Yamashiro DJ, Study CsOG Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: a children's oncology group study. J Clin Oncol. 2008;26(3):399–405. doi: 10.1200/JCO.2007.11.9230. doi:10.1200/JCO.2007.11.9230. [DOI] [PubMed] [Google Scholar]