Abstract
Importance
Levels of fibroblast growth factor 23 (FGF23) are elevated in chronic kidney disease (CKD) and strongly associated with left ventricular hypertrophy, heart failure, and death. Whether FGF23 is an independent risk factor for atrial fibrillation in CKD is unknown.
Objective
To investigate the association of FGF23 with atrial fibrillation in CKD.
Design, Setting, and Participants
Prospective cohort study of 3876 individuals with mild to severe CKD who enrolled in the Chronic Renal Insufficiency Cohort Study between June 19, 2003, and September 3, 2008, and were followed up through March 31, 2013.
Exposures
Baseline plasma FGF23 levels.
Main Outcomes and Measures
Prevalent and incident atrial fibrillation.
Results
The study cohort comprised 3876 participants. Their mean (SD) age was 57.7 (11.0) years, and 44.8% (1736 of 3876) were female. Elevated FGF23 levels were independently associated with increased odds of prevalent atrial fibrillation (n = 660) after adjustment for cardiovascular and CKD-specific factors (odds ratio of highest vs lowest FGF23 quartile, 2.30; 95% CI, 1.69-3.13; P < .001 for linear trend across quartiles). During a median follow-up of 7.6 years (interquartile range, 6.3-8.6 years), 247 of the 3216 participants who were at risk developed incident atrial fibrillation (11.9 events per 1000 person-years). In fully adjusted models, elevated FGF23 was independently associated with increased risk of incident atrial fibrillation after adjustment for demographic, cardiovascular, and CKD-specific factors, and other markers of mineral metabolism (hazard ratio of highest vs lowest FGF23 quartile, 1.59; 95% CI, 1.00-2.53; P = .02 for linear trend across quartiles). The results were unchanged when further adjusted for ejection fraction, but individual adjustments for left ventricular mass index, left atrial area, and interim heart failure events partially attenuated the association of elevated FGF23 with incident atrial fibrillation.
Conclusions and Relevance
Elevated FGF23 is independently associated with prevalent and incident atrial fibrillation in patients with mild to severe CKD. The effect may be partially mediated through a diastolic dysfunction pathway that includes left ventricular hypertrophy, atrial enlargement, and heart failure events.
Individuals with chronic kidney disease (CKD) are at high risk of developing heart failure, atherosclerotic disease, and arrhythmias, including high rates of atrial fibrillation.1-4 Classic risk factors for atrial fibrillation, such as older age, hypertension, diabetes, and coronary heart disease, are common in individuals with CKD. However, high rates of arterial calcification, left ventricular hypertrophy, and volume overload in CKD likely further increase risk of atrial fibrillation by exacerbating pathological cardiac remodeling and activating the autonomic nervous system.5-7 Because atrial fibrillation is an independent risk factor for heart failure, ischemic stroke, and death, it is important to identify novel mechanisms of disease that can be targeted to prevent atrial fibrillation and improve cardiovascular outcomes in CKD 8-10.
Disordered phosphate homeostasis contributes to the pathogenesis of cardiovascular disease in patients with CKD.11 Fibroblast growth factor 23 (FGF23) is an osteocyte-derived endocrine hormone that exerts its primary actions in the kidney to regulate phosphate homeostasis.12 Levels of FGF23 rise early in the course of CKD as part of the adaptive response to maintain neutral phosphate balance when renal excretory capacity declines.12 Chronic FGF23 elevation in CKD is independently associated with development of heart failure and death.13-19 As a putative underlying molecular mechanism, FGF23 may induce pathological left ventricular hypertrophy by activating fibroblast growth factor receptor (FGFR) 4 on cardiac myocytes.20,21 Pathological left ventricular hypertrophy induces diastolic dysfunction, which increases left ventricular filling pressures and left atrial size,22 and (along with volume overload) predisposes to development of atrial fibrillation. We hypothesized that elevated FGF23 is a novel risk factor for development of atrial fibrillation in patients with CKD and that this association is mediated in part by a mechanistic pathway that involves pathological cardiac remodeling.
Methods
Study Population
We analyzed the association between FGF23 levels and atrial fibrillation in the Chronic Renal Insufficiency Cohort (CRIC) Study, which is a prospective cohort investigation of individuals with mild to severe CKD that was designed to evaluate risk factors for CKD progression and cardiovascular disease.23 The CRIC investigators enrolled 3939 men and women 21 to 74 years old between June 19, 2003, and September 3, 2008, at 7 clinical centers across the United States. Individuals were eligible to participate if they met specific age-defined criteria for estimated glomerular filtration rate (eGFR) between 20 and 70 mL/min/1.73 m2. Exclusion criteria included pregnancy, New York Heart Association class III or IV heart failure, human immunodeficiency virus infection, multiple myeloma, polycystic kidney disease, renal cancer, cirrhosis, recent chemotherapy or immunosuppressive therapy, organ transplantation, previous dialysis treatment for at least 1 month, enrollment in other studies, institutionalization, or inability to provide informed consent23.
Excluding 63 participants with inadequate samples to measure baseline FGF23, the final study population included 3876 participants whom we included in analyses of prevalent atrial fibrillation, which was present in 660 participants at study entry. Our prospective analyses of incident atrial fibrillation were restricted to the 3216 participants without a self-reported history of atrial fibrillation and without atrial fibrillation on the 12-lead electrocardiogram that was performed at the baseline visit. The CRIC Study was approved by institutional review boards at participating centers. All participants provided written informed consent.
Primary Exposure
The primary exposure was baseline plasma FGF23 level, measured in duplicate using a second-generation assay (C-Terminal; Immutopics) by the CRIC Central Laboratory after a single thaw of frozen samples that were collected fasting (in 96.2% [3729 of 3876] of participants) and stored at −80°C. The mean intraassay coefficient of variation was less than 5%, the mean interassay coefficient of variation was 7.6%, and the lower limit of detection was 3 reference units (RU)/mL 20,24.
Outcomes
The primary outcomes were prevalent atrial fibrillation at enrollment and incident atrial fibrillation during longitudinal follow-up. Prevalent atrial fibrillation was ascertained at the baseline visit by electrocardiogram or participants' affirmative response to the question “Have you ever been diagnosed with or has a doctor or other health professional ever told you that you have atrial fibrillation?”25 Standardized equipment was used to obtain electrocardiograms, which were analyzed centrally at Wake Forest University (Winston-Salem, North Carolina) using the Minnesota Code Manual of Electrocardiographic Findings Standard Procedures for Measurement and Classification to identify atrial fibrillation.25,26 Incident atrial fibrillation was ascertained by adjudication of medical records of hospitalizations that were reviewed for International Classification of Diseases, Ninth Edition, code 427.31 (atrial fibrillation) or 427.32 (atrial flutter) by 2 independent physicians (J.J.S., N.B., J.H.S., J.C., A.C.R., R.D., and M.R. were adjudicators for all CRIC outcomes).
Covariates
Demographics, medication use, and clinical information were ascertained at the baseline and annual study visits. Self-reported history of cardiovascular disease was defined as a composite of prior heart failure, coronary revascularization, myocardial infarction, ischemic stroke, amputation, or peripheral artery revascularization.17 Standard protocols were used to measure resting blood pressure and body mass index, and standard assays were used to measure serum creatinine, albumin, calcium, and phosphate levels, as well as the ratio of urinary albumin to creatinine, at a central laboratory.23,27 Plasma parathyroid hormone was measured using an intact assay (Scantibodies).12 The creatinine-based Chronic Kidney Disease Epidemiology Collaboration equation was used to calculate eGFR 28.
Echocardiograms
Transthoracic echocardiograms were obtained at the year 1 visit in 85.7% (3321 of 3876) of participants using American Society of Echocardiography protocols and were reviewed at a central laboratory.17,29,30 Excluding individuals who developed incident atrial fibrillation before undergoing echocardiography, the median time between the baseline and echocardiogram visits among participants at risk for incident atrial fibrillation was 378 days (interquartile range [IQR], 343-419 days). Not all parameters were assessable in all participants.31 In the subpopulation of individuals at risk for incident atrial fibrillation, left ventricular mass index was assessable in 2372 participants, left atrial area in 2693 participants, and ejection fraction in 2661 participants. Left ventricular mass was calculated using the 5:6 ratio area-length method and indexed to height2.7 (Superscript 2.7 is used to index LV mass to height), which is preferred in CKD because it eliminates the contribution of volume overload inherent to weight-based formulas 15.
Heart Failure Events
Acute heart failure events were adjudicated by 2 independent physicians who reviewed hospitalization records.17 They classified each event as possible, probable, or definite using a combination of symptoms (orthopnea, paroxysmal nocturnal dyspnea, and dyspnea on exertion) accompanied by either physical examination findings (comprising >2 of pulmonary rales, a third heart sound [S3] gallop, jugular venous distention >5 cm, and peripheral edema), chest radiographic findings (pulmonary edema, vascular congestion, and pleural effusion), or invasive hemodynamic or echocardiographic evidence of heart failure (pulmonary capillary wedge pressure >18 mm Hg, cardiac index <2.0 L/min/m2, and left ventricular ejection fraction ≤35%) 17.
Statistical Analysis
We used standard descriptive statistics to compare demographics and clinical characteristics of the entire study population according to presence or absence of prevalent atrial fibrillation at baseline. We also summarized baseline characteristics according to FGF23 quartiles among those at risk for incident atrial fibrillation. We used logistic regression to test the association between FGF23 and prevalent atrial fibrillation overall and in the subgroup in which it was ascertained objectively by electrocardiogram.
To test the association between FGF23 and incident atrial fibrillation, we excluded the 660 individuals with prevalent atrial fibrillation at baseline and censored for death and administrative end of follow-up in 2013. We used Cox proportional hazards models to analyze time to incident atrial fibrillation according to baseline FGF23 levels expressed in quartiles and as a continuous variable using natural log-transformation. We confirmed no violation of the proportional hazards assumption using Schoenfeld residuals and visual inspection.
In multivariable analyses of prevalent and incident atrial fibrillation, we hierarchically adjusted for demographics (age, sex, and race/ethnicity), cardiovascular risk factors (prior cardiovascular disease, diabetes, systolic blood pressure, current smoking, and diuretic use as a surrogate for volume overload), CKD-specific factors (eGFR and ratio of urinary albumin to creatinine, categorized as <300 mg/dL, ≥300 mg/dL, or missing), and other markers of mineral metabolism (calcium, phosphate, and parathyroid hormone levels). We evaluated additional possible confounders, such as body mass index, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use, total number of blood pressure medications, and levels of C-reactive protein, hemoglobin, and serum albumin, by adjusting the full multivariable model individually for these factors. The maximum proportion of missing data for any covariate was less than 1.6%, and no imputation was used. All models included a study site stratification term to account for possible regional variability.
We assessed whether the association of FGF23 with incident atrial fibrillation was modified by age, sex, race/ethnicity, history of cardiovascular disease, eGFR, CKD stage, ratio of urinary albumin to creatinine, and serum phosphate level by testing the significance of the interaction terms of each of these variables with FGF23. To ensure that FGF23 testing predated development of atrial fibrillation and to minimize misclassification of participants with paroxysmal atrial fibrillation as at risk for incident atrial fibrillation, we performed a sensitivity analysis in which we excluded individuals who developed incident atrial fibrillation within 1 year of FGF23 testing. Because onset of end-stage renal disease (ESRD) likely increases risk of atrial fibrillation through different mechanisms (ie, lability in serum electrolytes), we conducted another sensitivity analysis in which we censored for onset of ESRD, defined as initiation of chronic dialysis or receipt of a kidney transplant.
To investigate potential cardiac mediators of the association of FGF23 with incident atrial fibrillation, we individually adjusted the full multivariable model for left ventricular mass index, left atrial area, ejection fraction, and definite or probable heart failure events that occurred before incident atrial fibrillation as a time-varying covariate. For analyses of echocardiography parameters, we excluded individuals who developed incident atrial fibrillation before their echocardiogramand adjusted for the number of days between the baseline and echocardiogram visits. To explore potential exposure-mediator interaction,32 we tested for interactions between FGF23 and left ventricular mass index, left atrial area, and ejection fraction.
Analyses were performed using statistical software (SAS, version 9.4; SAS Institute Inc). Two-sided P < .05 was considered statistically significant.
Results
Prevalent Atrial Fibrillation
Table 1 lists characteristics of the 3876 individuals according to presence or absence of prevalent atrial fibrillation at baseline, which was ascertained by electrocardiogram criteria in 51 individuals and by self-report in 609 individuals. Higher plasma FGF23, expressed as a continuous variable or in quartiles, was associated with significantly higher likelihood of prevalent atrial fibrillation in all univariable and multivariable-adjusted analyses. In the full model of continuous FGF23, the odds ratio was 1.46 (95% CI, 1.27-1.67) per 1-U increase in natural log FGF23 (Table 2). Categorical analyses demonstrated that these effects were driven primarily by FGF23 quartiles 3 and 4, suggesting a threshold effect above the median. Restricting the analysis to individuals with objective electrocardiographic evidence of atrial fibrillation yielded a higher multivariable-adjusted point estimate, with an odds ratio of atrial fibrillation of 2.32 (95% CI, 1.65-3.25) per 1-U increase in natural log FGF23.
Table 1. Baseline Characteristics According to Atrial Fibrillation Prevalence.
| Variable | Prevalent Atrial Fibrillation (n = 660) | No Prevalent Atrial Fibrillation (n = 3216) | P Value |
|---|---|---|---|
| Age, mean (SD), y | 60.9 (9.3) | 57.0 (11.2) | <.001 |
| Female sex, No. (%) | 306 (46.4) | 1430 (44.5) | .37 |
| Black, No. (%) | 318 (48.2) | 1302 (40.5) | <.001 |
| Hispanic, No. (%) | 52 (7.9) | 443 (13.8) | <.001 |
| Current smoking, No. (%) | 86 (13.0) | 422 (13.1) | .95 |
| BMI, mean (SD)a | 32.9 (8.1) | 31.9 (7.7) | .003 |
| Hypertension, No. (%) | 579 (87.7) | 2757 (85.7) | .18 |
| Systolic BP, mean (SD), mm Hg | 128.3 (22.4) | 128.6 (22.2) | .69 |
| Diastolic BP, mean (SD), mm Hg | 69.1 (12.6) | 72.1 (12.8) | <.001 |
| ACEI or ARB use, No./total No. (%) | 471/655 (71.9) | 2177/3193 (68.2) | .06 |
| Diuretic use, No./total No. (%) | 476/655 (72.7) | 1818/3193 (56.9) | <.001 |
| Total No. of BP medications, mean (SD) | 3.1 (1.4) | 2.5 (1.5) | <.001 |
| Diabetes, No. (%) | 341 (51.7) | 1538 (47.8) | .07 |
| Heart failure, No. (%) | 183 (27.7) | 194 (6.0) | <.001 |
| Stroke, No. (%) | 98 (14.9) | 287 (8.9) | <.001 |
| Cardiovascular disease, No. (%) | 388 (58.8) | 909 (28.3) | <.001 |
| Ratio (IQR) of urinary albumin to creatinine, mg/g | 44.4 (9.3-382.2) | 53.3 (8.4-479.7) | .11 |
| Estimated glomerular filtration rate, mean (SD), mL/min/1.73 m2 | 42.2 (14.7) | 44.7 (15.1) | <.001 |
| CRP level, median (IQR), mg/L | 3.0 (1.3-7.5) | 2.5 (1.0-6.2) | <.001 |
| Serum calcium level, mean (SD), mg/dL | 9.2 (0.5) | 9.2 (0.5) | .84 |
| Serum phosphate level, mean (SD), mg/dL | 3.7 (0.7) | 3.7 (0.7) | .50 |
| Intact parathyroid hormone level, median (IQR), pg/mL | 61.5 (38.9-97.0) | 52.3 (34.0-88.0) | <.001 |
| FGF23 level, median (IQR), RU/mL | 181.2 (113.3-307.6) | 138.6 (93.9-227.4) | <.001 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CRP, C-reactive protein; FGF23, fibroblast growth factor 23; IQR, interquartile range; RU, reference units.
SI conversion factors: To convert calcium level to millimoles per liter, multiply by 0.25; CRP level to nanomoles per liter, multiply by 9.624; parathyroid hormone level to nanograms per liter, multiply by 1.0.
Calculated as weight in kilograms divided by height in meters squared.
Table 2. Fibroblast Growth Factor 23 (FGF23) and Prevalent Atrial Fibrillationa.
| Variable | Cases/Total No. | Per 1-U Increase in Natural Log FGF23 | FGF23 Quartile, RU/mL | ||||
|---|---|---|---|---|---|---|---|
| Quartile 1 (≤95.9) | Quartile 2 (96.0-145.6) | Quartile 3 (145.7-239.2) | Quartile 4 (>239.2) | P for Trend | |||
| Prevalence, No./total No. (%) | 660 | 660/3876 (17.0) | 117/969 (12.1) | 124/969 (12.8) | 185/969 (19.1) | 234/969 (24.1) | NA |
| Odds ratio (95% CI) | |||||||
| Unadjusted | 660/3876 | 1.51 (1.36-1.68) | 1 [Reference] | 1.07 (0.82-1.40) | 1.72 (1.34-2.21) | 2.32 (1.82-2.96) | <.001 |
| Plus demographic factorsb | 660/3876 | 1.53 (1.37-1.71) | 1 [Reference] | 1.03 (0.78-1.35) | 1.66 (1.28-2.14) | 2.30 (1.80-2.95) | <.001 |
| Plus cardiovascular risk factorsc | 655/3847 | 1.36 (1.21-1.54) | 1 [Reference] | 0.95 (0.71-1.25) | 1.44 (1.10-1.88) | 1.83 (1.40-2.40) | <.001 |
| Plus CKD-specific factorsd | 655/3847 | 1.44 (1.27-1.64) | 1 [Reference] | 1.00 (0.75-1.33) | 1.62 (1.22-2.15) | 2.18 (1.62-2.93) | <.001 |
| Plus markers of mineral metabolisme | 633/3729 | 1.46 (1.27-1.67) | 1 [Reference] | 1.04 (0.78-1.39) | 1.63 (1.22-2.19) | 2.30 (1.69-3.13) | <.001 |
Abbreviations: CKD, chronic kidney disease; NA, not applicable; RU, reference units.
Continuous results are reported as odds ratios per 1-U increase in natural log-transformed FGF23.
Adjusts for age, sex, and race/ethnicity.
Adjusts for factors in model 1 and for cardiovascular disease, systolic blood pressure, diabetes, smoking, and diuretic use.
Adjusts for factors in model 2 and for estimated glomerular filtration rate and ratio of urinary albumin to creatinine.
Full multivariable model adjusts for factors in model 3 and for levels of calcium, phosphate, and parathyroid hormone.
Incident Atrial Fibrillation
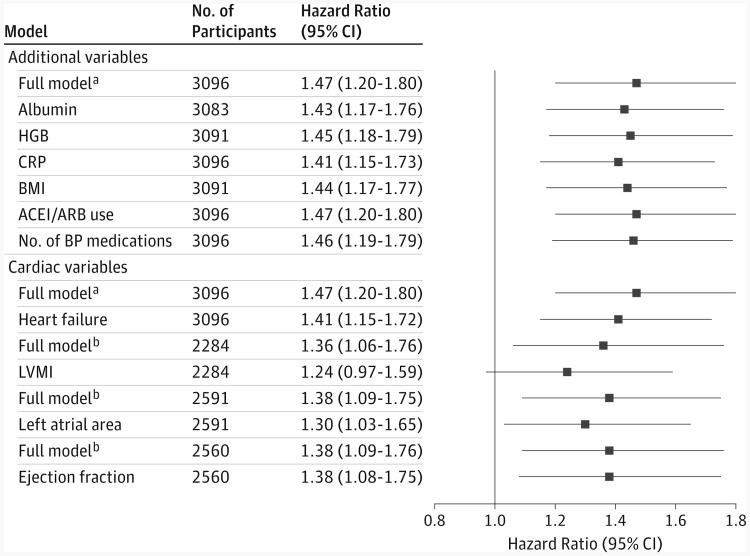
Among the 3216 participants at risk for incident atrial fibrillation, the mean (SD) eGFR was 44.7 (15.1) mL/min/1.73m2,and the median FGF23 was 138.6 RU/mL (IQR, 93.9-227.4 RU/mL). Clinical and demographic characteristics of participants at risk for incident atrial fibrillation are listed in Table 3 according to FGF23 quartiles. During a median follow-up of 7.6 years (IQR, 6.3-8.6 years), 247 incident atrial fibrillation events occurred (11.9 events per 1000 person-years), with increasing incidence across ascending FGF23 quartiles (Table 4). In unadjusted and adjusted analyses, higher baseline FGF23, expressed either as a continuous variable or in quartiles, was associated with increased risk of incident atrial fibrillation. In the full model of continuous FGF23, the hazard ratio (HR) was 1.47 (95% CI, 1.20-1.80) per 1-U increase in natural log FGF23. Consistent with the prevalent atrial fibrillation results, quartile analyses suggested that risk of atrial fibrillation increased significantly when FGF23 levels were above the median. No candidate factors significantly modified the association of higher FGF23 with incident atrial fibrillation (P ≥ .10 for all interactions). Further adjustment of the full model for body mass index, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use, total number of blood pressure medications, and levels of C-reactive protein, hemoglobin, and albumin did not substantially attenuate the point estimates for FGF23 and risk of incident atrial fibrillation (Figure).
Table 3. Baseline Characteristics of Individuals at Risk for Development of Atrial Fibrillation According to Fibroblast Growth Factor 23 (FGF23) Quartiles.
| Variable | FGF23 Quartile, RU/mL | P Value | |||
|---|---|---|---|---|---|
| Quartile 1 (≤93.9) (n = 804) | Quartile 2 (94.0-138.6) (n = 805) | Quartile 3 (138.7-227.4) (n = 803) | Quartile 4 (>227.4) (n = 804) | ||
| Age, mean (SD), y | 55.7 (11.2) | 57.7 (11.0) | 57.7 (11.1) | 56.8 (11.4) | <.001 |
| Female sex, No. (%) | 283 (35.2) | 321 (39.9) | 364 (45.3) | 462 (57.5) | <.001 |
| Black, No. (%) | 325 (40.4) | 311 (38.6) | 304 (37.9) | 362 (45.0) | .02 |
| Hispanic, No. (%) | 65 (8.1) | 113 (14.0) | 133 (16.6) | 132 (16.4) | <.001 |
| Current smoking, No. (%) | 65 (8.1) | 81 (10.1) | 106 (13.2) | 170 (21.1) | <.001 |
| BMI, mean (SD)a | 30.3 (6.5) | 31.3 (7.0) | 32.3 (7.8) | 33.8 (9.0) | <.001 |
| Hypertension, No. (%) | 600 (74.6) | 689 (85.6) | 721 (89.8) | 747 (92.9) | <.001 |
| Systolic BP, mean (SD), mm Hg | 123.6 (19.8) | 127.0 (21.5) | 131.2 (23.0) | 132.7 (23.1) | <.001 |
| Diastolic BP, mean (SD), mm Hg | 73.0 (12.3) | 72.4 (12.3) | 72.1 (13.5) | 71.1 (13.0) | .03 |
| ACEI or ARB use, No./total No. (%) | 484/797 (60.7) | 561/797 (70.4) | 574/798 (71.9) | 558/801 (69.7) | <.001 |
| Diuretic use, No./total No. (%) | 323/797 (40.5) | 411/797 (51.6) | 486/798 (60.9) | 598/801 (74.7) | <.001 |
| Total No. of BP medications, mean (SD) | 2.0 (1.5) | 2.4 (1.4) | 2.7 (1.5) | 3.0 (1.4) | <.001 |
| Diabetes, No. (%) | 242 (30.1) | 356 (44.2) | 455 (56.7) | 485 (60.3) | <.001 |
| Heart failure, No. (%) | 23 (2.9) | 28 (3.5) | 51 (6.4) | 92 (11.4) | <.001 |
| Stroke, No. (%) | 59 (7.3) | 59 (7.3) | 72 (9.0) | 97 (12.1) | .002 |
| Cardiovascular disease, No. (%) | 154 (19.2) | 211 (26.2) | 239 (29.8) | 305 (37.9) | <.001 |
| Ratio (IQR) of urinary albumin to creatinine, mg/g | 14.5 (5.0-125.6) | 32.2 (6.4-278.3) | 106.9 (15.0-753.4) | 247.9 (30.2-1443.6) | <.001 |
| Estimated glomerular filtration rate, mean (SD), mL/min/1.73 m2 | 54.9 (13.8) | 47.8 (13.4) | 40.9 (12.1) | 35.3 (13.3) | <.001 |
| CRP level, median (IQR), mg/L | 1.8 (0.9-4.6) | 2.1 (0.9-4.8) | 2.5 (1.0- 6.0) | 4.0 (1.5-8.6) | <.001 |
| Serum calcium level, mean (SD), mg/dL | 9.2 (0.4) | 9.2 (0.5) | 9.2 (0.5) | 9.1 (0.6) | <.001 |
| Serum phosphate level, mean (SD), mg/dL | 3.4 (0.5) | 3.6 (0.6) | 3.8 (0.6) | 4.1 (0.8) | <.001 |
| Intact parathyroid hormone level, median (IQR), pg/mL | 39.0 (28.7-56.0) | 47.0 (32.0-73.8) | 60.6 (38.7-101.0) | 82.5 (46.1-137.1) | <.001 |
| LVMI, mean (SD), g/m2.7b | 46.6 (12.1) | 49.0 (12.2) | 51.9 (13.5) | 56.5 (15.4) | <.001 |
| Left atrial area, mean (SD), cm2 | 22.0 (5.0) | 22.4 (4.9) | 23.2 (5.7) | 24.1 (5.7) | <.001 |
| Ejection fraction, mean (SD), % | 55.5 (7.1) | 54.7 (7.6) | 54.5 (7.8) | 54.3 (8.3) | .02 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CRP, C-reactive protein; IQR, interquartile range; LVMI, left ventricular mass index; RU, reference units.
SI conversion factors: To convert calcium level to millimoles per liter, multiply by 0.25; CRP level to nanomoles per liter, multiply by 9.624; parathyroid hormone level to nanograms per liter, multiply by 1.0.
Calculated as weight in kilograms divided by height in meters squared.
Superscript 2.7 is used to index LV mass to height.
Table 4. Incidence of Atrial Fibrillation and Its Association With Fibroblast Growth Factor 23 (FGF23)a.
| Variable | Events/Total No. | Per 1-U Increase in Natural Log FGF23 | FGF23 Quartile, RU/mL | P for Trend | |||
|---|---|---|---|---|---|---|---|
| Quartile 1 (≤93.9) | Quartile 2 (94.0-138.6) | Quartile 3 (138.7-227.4) | Quartile 4 (>227.4) | ||||
| Incidence rate (95% CI), events per 1000 person-years | 247/3216 | 11.9 (10.4-13.4) | 7.5 (5.4-10.1) | 9.6 (7.1-12.5) | 14.2 (11.1-17.8) | 17.2 (13.7-21.4) | <.001 |
| Hazard ratio (95% CI) | |||||||
| Unadjusted | 247/3216 | 1.57 (1.35-1.83) | 1 [Reference] | 1.28 (0.86-1.93) | 1.93 (1.32-2.82) | 2.40 (1.65-3.49) | <.001 |
| Plus demographic factorsb | 247/3216 | 1.78 (1.51-2.11) | 1 [Reference] | 1.19 (0.79-1.79) | 1.90 (1.29-2.79) | 2.66 (1.81-3.92) | <.001 |
| Plus cardiovascular risk factorsc | 247/3192 | 1.61 (1.35-1.92) | 1 [Reference] | 1.07 (0.71-1.62) | 1.60 (1.08-2.37) | 2.06 (1.38-3.09) | <.001 |
| Plus CKD-specific factorsd | 247/3192 | 1.48 (1.22-1.81) | 1 [Reference] | 1.00 (0.66-1.51) | 1.37 (0.91-2.07) | 1.64 (1.05-2.56) | .01 |
| Plus markers of mineral metabolisme | 237/3096 | 1.47 (1.20-1.80) | 1 [Reference] | 1.00 (0.65-1.54) | 1.34 (0.87-2.05) | 1.59 (1.00-2.53) | .02 |
Abbreviations: CKD, chronic kidney disease; RU, reference units.
Continuous results are reported as hazard ratios per 1-U increase in natural log-transformed FGF23.
Adjusts for age, sex, and race/ethnicity.
Adjusts for factors in model 1 and for cardiovascular disease, systolic blood pressure, diabetes, smoking, and diuretic use.
Adjusts for factors in model 2 and for estimated glomerular filtration rate and ratio of urinary albumin to creatinine.
Full multivariable model adjusts for factors in model 3 and for levels of calcium, phosphate, and parathyroid hormone.
Figure. Mediation Analyses of Fibroblast Growth Factor 23 and Incident Atrial Fibrillation.
ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CRP, C-reactive protein; FGF23, fibroblast growth factor 23; HGB, hemoglobin; and LVMI, left ventricular mass index. aThe full model adjusts for age, sex, race/ethnicity, cardiovascular disease, systolic blood pressure, diabetes, smoking, diuretic use, estimated glomerular filtration rate, ratio of urinary albumin to creatinine, and levels of calcium, phosphate, and parathyroid hormone. bThese models include only individuals with available echocardiographic data and are adjusted for the same variables in the full model plus each specific echocardiographic feature.
Sensitivity Analyses
The association between FGF23 and incident atrial fibrillation remained qualitatively unchanged when we repeated the main analysis excluding individuals in whom incident atrial fibrillation occurred during the first year after FGF23 testing: the HR per 1-U increase in natural log FGF23 was 1.36 (95%CI, 1.09-1.70). Censoring individuals at onset of ESRD eliminated 54 subsequent atrial fibrillation events, but elevated FGF23 remained independently associated with incident atrial fibrillation: the HR was 1.35 (95% CI, 1.06-1.73) per 1-U increase in natural log FGF23.
Mediation Analyses
The 16 individuals who developed incident atrial fibrillation before undergoing echocardiography were excluded from the analyses of altered cardiac structure and function as potential mediators of the association of elevated FGF23 with incident atrial fibrillation. Among participants who underwent echocardiography, the mean (SD) left ventricular mass index was 50.8 (13.8) g/m2.7, the mean (SD) left atrial area was 22.9 (5.4) cm2, and the mean (SD) ejection fraction was 54.8%(7.7%). When we added left ventricular mass index to the full multivariable model, the point estimate for the association between FGF23 and incident atrial fibrillation was substantially attenuated and no longer statistically significant (Figure). Inclusion of left atrial area partially attenuated the FGF23 effect, whereas inclusion of ejection fraction did not alter the association between FGF23 and incident atrial fibrillation. There was no significant interaction between FGF23 and left ventricular mass index, left atrial area, and ejection fraction (P ≥ .10 for all interactions).
Seventy-six probable or definite heart failure events occurred before onset of incident atrial fibrillation, with a median time between heart failure and atrial fibrillation of 4 months (IQR, 0-32 months). Adjusting for intercurrent heart failure events partially attenuated the association between elevated FGF23 and incident atrial fibrillation (Figure).
Discussion
Elevated FGF23 has emerged as a novel risk factor for cardiovascular disease, especially in patients with CKD. In the present study of individuals with mild to severe CKD, we demonstrate a strong association of elevated FGF23 with atrial fibrillation that was independent of demographics, classic cardiovascular risk factors, severity of kidney dysfunction and proteinuria, and other markers of mineral metabolism. In contrast, the strong association between elevated FGF23 and incident atrial fibrillation was substantially attenuated only by adjustment for left ventricular mass index and (to a lesser extent) by left atrial area and interim heart failure events. These data suggest a novel pathophysiological pathway in which elevated FGF23 levels in CKD contribute to greater left ventricular mass, diastolic dysfunction, increased left atrial diameter, and development of atrial fibrillation. This paradigm is supported by animal and in vitro data that demonstrated direct hypertrophic effects of elevated FGF23 levels on cardiac myocytes20 and by epidemiological studies17,20,33,34 that reported strong independent associations between elevated FGF23 and prevalent and incident left ventricular hypertrophy and heart failure.
Left ventricular hypertrophy predisposes to atrial fibrillation by inducing diastolic dysfunction that increases left ventricular filling pressure, which promotes left atrial enlargement and fibrosis and activates arrhythmogenic neurohormonal pathways.35,36 Similar proarryhthmogenic pathways can be activated by primary defects in left ventricular systolic function.35,36 Therefore, it is particularly notable that increased left ventricular mass index most potently attenuated the effect of FGF23 on atrial fibrillation, whereas ejection fraction had no impact. It is tempting to speculate that this contrast could derive from FGF23 promoting atrial fibrillation primarily via a pathway that involves diastolic rather than systolic dysfunction in CKD. Although adjustment for left ventricular mass index was the only variable that could render the association of elevated FGF23 with incident atrial fibrillation statistically nonsignificant, we acknowledge that adjustment for it and left atrial area did not completely attenuate FGF23-associated risk. This finding could relate to imprecision of the echocardiographic measurements, the time lag between FGF23 testing and echocardiography, and possible mediator-outcome confounding and collider bias that are inherent to mediation analyses,32 or it may suggest alternative biologic mechanisms linking elevated FGF23 to incident atrial fibrillation in CKD that we did not study.
Previous studies of FGF23 and atrial fibrillation in predominantly non-CKD populations yielded inconsistent results. Higher FGF23was associated with incident atrial fibrillation in the MultiEthnic Study of Atherosclerosis (MESA) and Cardiovascular Health Study (CHS) but not in the Atherosclerosis Risk in Communities (ARIC) Study.37,38 Although elevated left ventricular mass partially mediated the effects of FGF23 on incident atrial fibrillation in a subset of individuals with available measurements in CHS, it did not attenuate the effect in parallel analyses in MESA. Neither left atrial diameter in CHS nor intercurrent heart failure events in CHS or MESA altered the association between FGF23 and incident atrial fibrillation. Potential reasons for disparate results across these studies and ours include significant differences in the study populations' risk factor profiles that translated into differences in atrial fibrillation incidence across studies (highest in CHS, followed by CRIC, ARIC, and MESA [in descending order]) and the lower FGF23 levels in the predominantly non-CKD cohorts relative to the CRIC Study.37 Perhaps there is an arrhythmogenic threshold level of FGF23 that is more likely to be exceeded in patients with CKD, which could explain the null findings in the ARIC Study, in which participants had higher eGFR. A threshold effect is supported by our quartile analyses that demonstrated significantly increased risk of atrial fibrillation only when FGF23 levels were above the CRIC Study median (quartiles 3 and 4). In addition, the unique combination of FGF23 excess and α-Klotho deficiency in CKD may exacerbate α-Klotho–independent cardiac toxicity of FGF23 specifically in CKD by reducing FGF23 binding to FGFR–α-Klotho complexes in the kidney and thereby promoting FGF23 binding to FGFR4in cardiac myocytes that activates prohypertrophic signaling pathways.21,39 Reliable assays for α-Klotho are needed to test this hypothesis.
The present study has limitations. The results of the secondary cross-sectional analyses of prevalent atrial fibrillation cannot demonstrate temporality or causality, but their general concordance with the primary analyses of incident atrial fibrillation supports the conclusion that FGF23 is a risk factor for atrial fibrillation. We could not classify atrial fibrillation as paroxysmal, persistent, or permanent and may have misclassified individuals with subclinical episodes of paroxysmal atrial fibrillation that were not detected. Repeated measurements of FGF23 and other covariates were also not available. However, because FGF23 levels remain generally stable over time19,40,41 and the proportionality assumption was not violated, we deduce that risk of atrial fibrillation related to baseline FGF23 was constant over time, despite potential changes in FGF23 and covariate levels during longitudinal follow-up. We also did not adjust for levels of brain-type natriuretic peptide (BNP), which predicts atrial fibrillation.42 However, even if adjustment for higher BNP had attenuated the risk relationship between FGF23 and incident atrial fibrillation, we would invoke elevated BNP as another mediating factor on a causal pathway linking FGF23 to atrial fibrillation because elevated cardiac wall stress due to diastolic dysfunction increases BNP secretion.43 Furthermore, we reported that FGF23 directly induces cardiac myocyte expression of BNP as part of the pathological left ventricular hypertrophy phenotype 20,21.
Atrial fibrillation increases risk of arterial thromboembolic events, heart failure, and death,44 and these risks are magnified in patients with CKD.45,46 Successful management of atrial fibrillation in CKD also presents clinicians with unique challenges. Many newer anticoagulants are contraindicated or require dose adjustments with more rigorous monitoring in advanced CKD, and standard anticoagulation therapies are associated with heightened risk of hemorrhagic complications in patients with CKD.47,48 Furthermore, warfarin sodium therapy is independently associated with increased risk of mortality in patients with CKD undergoing hemodialysis 49.
Conclusions
Collectively, these complexities emphasize the critical importance of primary prevention of atrial fibrillation in CKD, which requires understanding of modifiable mechanisms of disease. Future studies should examine the diagnostic usefulness of repeated measurements of FGF23 as a clinical indicator of risk of atrial fibrillation and other cardiovascular events in patients with CKD. Simultaneously, additional studies should determine whether dietary or pharmacological strategies aimed at reducing elevated FGF23 levels or interrupting FGF23-associated cardiac injury by selectively blocking FGFR4 in cardiac myocytes could represent novel therapeutic strategies to ameliorate the burden of atrial fibrillation in CKD.
Key Points.
Question
Is elevated fibroblast growth factor 23 (FGF23) in chronic kidney disease (CKD) a risk factor for atrial fibrillation?
Findings
In this cohort study of 3876 individuals with mild to severe CKD, elevated FGF23 was significantly associated with incident atrial fibrillation. Adjustment for left ventricular mass index, left atrial area, and heart failure events partially attenuated the association of elevated FGF23 with incident atrial fibrillation.
Meaning
Elevated FGF23 may be a risk factor for atrial fibrillation in CKD, and the effect may be partially mediated through a diastolic dysfunction pathway that includes left ventricular hypertrophy, atrial enlargement, and heart failure events.
Acknowledgments
Funding/Support: This study was supported by grants R01DK081374 (to Dr Wolf), K24DK093723 (Dr Wolf), K23DK081673 (Dr Isakova), and K23 DK094829 (Dr Ricardo) from the National Institutes of Health; by a National Kidney Foundation of Illinois Young Investigator Grant (Dr Mehta); and by a Strategically Focused Research Network Center Grant from the American Heart Association (Dr Wolf). Funding for the Chronic Renal Insufficiency Cohort (CRIC) Study was obtained by grants U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902 under a cooperative agreement from the National Institute of Diabetes and Digestive and Kidney Diseases. In addition, this work was supported in part by the following: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award UL1TR000003 from the National Institutes of Health National Center for Advancing Translational Sciences, grant UL1 TR-000424 from The Johns Hopkins University, General Clinical Research Center M01 RR-16500 from the University of Maryland, Clinical and Translational Science Collaborative of Cleveland, grant UL1TR000439 from the National Center for Advancing Translational Sciences component of the National Institutes of Health and National Institutes of Health Roadmap for Medical Research, grant UL1TR000433 from the Michigan Institute for Clinical and Health Research, Clinical and Translational Science Award grant UL1RR029879 from the University of Illinois at Chicago, grant P30GM103337 from Tulane University Translational Research in Hypertension and Renal Biology, and Kaiser Permanente University of California San Francisco Clinical and Translational Science Institute UL1 RR-024131 from the National Institutes of Health National Center for Research Resources.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The National Institutes of Health contributed to the design, development, and steering of the Chronic Renal Insufficiency Cohort Study.
Footnotes
Author Contributions: Drs Mehta and Wolf had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Scialla, Rahman, Feldman, Go, Isakova, Wolf.
Acquisition, analysis, or interpretation of data: Mehta, Cai, Lee, Bansal, Sondheimer, Chen, Hamm, Ricardo, Navaneethan, Deo, Rahman, Go, Isakova, Wolf.
Drafting of the manuscript: Mehta, Wolf.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Mehta, Cai, Lee, Scialla, Bansal, Chen, Isakova, Wolf.
Obtained funding: Hamm, Rahman, Feldman, Go, Wolf.
Administrative, technical, or material support: Feldman, Go, Wolf.
Study supervision: Feldman, Wolf.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Scialla reported received consulting fees from Ultragenyx. Dr Navaneethan reported serving on the event adjudication committee for clinical trials sponsored by Abbvie, Bayer, and Boeringher-Ingelheim. Dr Isakova reported receiving honoraria from Bayer. Dr Wolf reported receiving research support, honoraria, or consultant fees from Amgen, Ardelyx, DiaSorin, Keryx, Lilly, Pfizer, Shire, and Ultragenyx. No other disclosures were reported.
Group Information: The Chronic Renal Insufficiency Cohort (CRIC) Study Investigators were Lawrence J. Appel, MD, MPH (Welch Center for Prevention, Epidemiology and Clinical Research, The Johns Hopkins University, Baltimore, Maryland); Jiang He, MD, PhD (Department of Epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, Louisiana); John W. Kusek, PhD (Division of Kidney, Urologic, and Hematologic Diseases, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland); James P. Lash, MD (Division of Nephrology, Department of Medicine, University of Illinois at Chicago); Akinlolu Ojo, MD, PhD (Division of Nephrology, Department of Medicine, University of Michigan Health System, Ann Arbor); and Raymond R. Townsend, MD (Division of Nephrology and Hypertension, Perlman School of Medicine, University of Pennsylvania, Philadelphia).
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Alonso A, Lopez FL, Matsushita K, et al. Chronic kidney disease is associated with the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2011;123(25):2946–2953. doi: 10.1161/CIRCULATIONAHA.111.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baber U, Howard VJ, Halperin JL, et al. Association of chronic kidney disease with atrial fibrillation among adults in the United States: Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Circ Arrhythm Electrophysiol. 2011;4(1):26–32. doi: 10.1161/CIRCEP.110.957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bansal N, Fan D, Hsu CY, Ordonez JD, Marcus GM, Go AS. Incident atrial fibrillation and risk of end-stage renal disease in adults with chronic kidney disease. Circulation. 2013;127(5):569–574. doi: 10.1161/CIRCULATIONAHA.112.123992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96(7):2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 6.Nattel S, Harada M. Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. J Am Coll Cardiol. 2014;63(22):2335–2345. doi: 10.1016/j.jacc.2014.02.555. [DOI] [PubMed] [Google Scholar]
- 7.Reinecke H, Brand E, Mesters R, et al. Dilemmas in the management of atrial fibrillation in chronic kidney disease. J Am Soc Nephrol. 2009;20(4):705–711. doi: 10.1681/ASN.2007111207. [DOI] [PubMed] [Google Scholar]
- 8.Go AS, Fang MC, Udaltsova N, et al. ATRIA Study Investigators. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. Circulation. 2009;119(10):1363–1369. doi: 10.1161/CIRCULATIONAHA.108.816082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reinecke H, Nabauer M, Gerth A, et al. AFNET Study Group. Morbidity and treatment in patients with atrial fibrillation and chronic kidney disease. Kidney Int. 2015;87(1):200–209. doi: 10.1038/ki.2014.195. [DOI] [PubMed] [Google Scholar]
- 10.Ananthapanyasut W, Napan S, Rudolph EH, et al. Prevalence of atrial fibrillation and its predictors in nondialysis patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5(2):173–181. doi: 10.2215/CJN.03170509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scialla JJ, Wolf M. Roles of phosphate and fibroblast growth factor 23 in cardiovascular disease. Nat Rev Nephrol. 2014;10(5):268–278. doi: 10.1038/nrneph.2014.49. [DOI] [PubMed] [Google Scholar]
- 12.Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isakova T, Xie H, Yang W, et al. Chronic Renal Insufficiency Cohort (CRIC) Study Group. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305(23):2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutiérrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359(6):584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutiérrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119(19):2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf M. Mineral (Mal) Adaptation to Kidney Disease: Young Investigator Award Address: American Society of Nephrology Kidney Week 2014. Clin J Am Soc Nephrol. 2015;10(10):1875–1885. doi: 10.2215/CJN.04430415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scialla JJ, Xie H, Rahman M, et al. Chronic Renal Insufficiency Cohort (CRIC) Study Investigators. Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol. 2014;25(2):349–360. doi: 10.1681/ASN.2013050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kendrick J, Cheung AK, Kaufman JS, et al. HOST Investigators. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol. 2011;22(10):1913–1922. doi: 10.1681/ASN.2010121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scialla JJ, Astor BC, Isakova T, Xie H, Appel LJ, Wolf M. Mineral metabolites and CKD progression in African Americans. J Am Soc Nephrol. 2013;24(1):125–135. doi: 10.1681/ASN.2012070713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grabner A, Amaral AP, Schramm K, et al. Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab. 2015;22(6):1020–1032. doi: 10.1016/j.cmet.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frohlich ED, Tarazi RC, Dustan HP. Clinical-physiological correlations in the development of hypertensive heart disease. Circulation. 1971;44(3):446–455. doi: 10.1161/01.cir.44.3.446. [DOI] [PubMed] [Google Scholar]
- 23.Feldman HI, Appel LJ, Chertow GM, et al. Chronic Renal Insufficiency Cohort (CRIC) Study Investigators. The Chronic Renal Insufficiency Cohort (CRIC) Study: design and methods. J Am Soc Nephrol. 2003;14(suppl 2):S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 24.Fliser D, Kollerits B, Neyer U, et al. MMKD Study Group. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18(9):2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 25.Soliman EZ, Prineas RJ, Go AS, et al. Chronic Renal Insufficiency Cohort (CRIC) Study Group. Chronic kidney disease and prevalent atrial fibrillation: the Chronic Renal Insufficiency Cohort (CRIC) Am Heart J. 2010;159(6):1102–1107. doi: 10.1016/j.ahj.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prineas R, Crow RS, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings. Boston, MA: John Wright PSG Inc; 1982. [Google Scholar]
- 27.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study Group. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4(8):1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lang RM, Bierig M, Devereux RB, et al. Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Bansal N, Keane M, Delafontaine P, et al. CRIC Study Investigators. A longitudinal study of left ventricular function and structure from CKD to ESRD: the CRIC Study. Clin J Am Soc Nephrol. 2013;8(3):355–362. doi: 10.2215/CJN.06020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park M, Hsu CY, Li Y, et al. Chronic Renal Insufficiency Cohort (CRIC) Study Group. Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol. 2012;23(10):1725–1734. doi: 10.1681/ASN.2012020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richiardi L, Bellocco R, Zugna D. Mediation analysis in epidemiology: methods, interpretation and bias. Int J Epidemiol. 2013;42(5):1511–1519. doi: 10.1093/ije/dyt127. [DOI] [PubMed] [Google Scholar]
- 33.Shibata K, Fujita S, Morita H, et al. Association between circulating fibroblast growth factor 23, α-Klotho, and the left ventricular ejection fraction and left ventricular mass in cardiology inpatients. PLoS One. 2013;8(9):e73184. doi: 10.1371/journal.pone.0073184. published corrections appear in PLoS One. 2014;9(4):e93346 and 2013;8(10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poelzl G, Trenkler C, Kliebhan J, et al. FGF23 is associated with disease severity and prognosis in chronic heart failure. Eur J Clin Invest. 2014;44(12):1150–1158. doi: 10.1111/eci.12349. [DOI] [PubMed] [Google Scholar]
- 35.Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124(20):2264–2274. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 36.Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91(6A):2D–8D. doi: 10.1016/s0002-9149(02)03373-8. [DOI] [PubMed] [Google Scholar]
- 37.Mathew JS, Sachs MC, Katz R, et al. Fibroblast growth factor-23 and incident atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis (MESA) and the Cardiovascular Health Study (CHS) Circulation. 2014;130(4):298–307. doi: 10.1161/CIRCULATIONAHA.113.005499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alonso A, Misialek JR, Eckfeldt JH, et al. Circulating fibroblast growth factor-23 and the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities Study. J Am Heart Assoc. 2014;3(5):e001082. doi: 10.1161/JAHA.114.001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu MC, Shi M, Cho HJ, et al. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J Am Soc Nephrol. 2015;26(6):1290–1302. doi: 10.1681/ASN.2014050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bouma-de Krijger A, Bots ML, Vervloet MG, et al. Time-averaged level of fibroblast growth factor-23 and clinical events in chronic kidney disease. Nephrol Dial Transplant. 2014;29(1):88–97. doi: 10.1093/ndt/gft456. [DOI] [PubMed] [Google Scholar]
- 41.Yang W, Xie D, Anderson AH, et al. CRIC Study Investigators. Association of kidney disease outcomes with risk factors for CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2014;63(2):236–243. doi: 10.1053/j.ajkd.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patton KK, Heckbert SR, Alonso A, et al. N-terminal pro-B-type natriuretic peptide as a predictor of incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis: the effects of age, sex and ethnicity. Heart. 2013;99(24):1832–1836. doi: 10.1136/heartjnl-2013-304724. [DOI] [PubMed] [Google Scholar]
- 43.Nishikimi T, Yoshihara F, Morimoto A, et al. Relationship between left ventricular geometry and natriuretic peptide levels in essential hypertension. Hypertension. 1996;28(1):22–30. doi: 10.1161/01.hyp.28.1.22. [DOI] [PubMed] [Google Scholar]
- 44.Vermond RA, Geelhoed B, Verweij N, et al. Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality: a community-based study from the Netherlands. J Am Coll Cardiol. 2015;66(9):1000–1007. doi: 10.1016/j.jacc.2015.06.1314. [DOI] [PubMed] [Google Scholar]
- 45.Olesen JB, Lip GY, Kamper AL, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367(7):625–635. doi: 10.1056/NEJMoa1105594. [DOI] [PubMed] [Google Scholar]
- 46.Bansal N, Fan D, Hsu CY, Ordonez JD, Go AS. Incident atrial fibrillation and risk of death in adults with chronic kidney disease. J Am Heart Assoc. 2014;3(5):e001303. doi: 10.1161/JAHA.114.001303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Capodanno D, Angiolillo DJ. Antithrombotic therapy in patients with chronic kidney disease. Circulation. 2012;125(21):2649–2661. doi: 10.1161/CIRCULATIONAHA.111.084996. [DOI] [PubMed] [Google Scholar]
- 48.Hart RG, Eikelboom JW, Ingram AJ, Herzog CA. Anticoagulants in atrial fibrillation patients with chronic kidney disease. Nat Rev Nephrol. 2012;8(10):569–578. doi: 10.1038/nrneph.2012.160. [DOI] [PubMed] [Google Scholar]
- 49.Chan KE, Lazarus JM, Thadhani R, Hakim RM. Anticoagulant and antiplatelet usage associates with mortality among hemodialysis patients. J Am Soc Nephrol. 2009;20(4):872–881. doi: 10.1681/ASN.2008080824. [DOI] [PMC free article] [PubMed] [Google Scholar]