Abstract
Alcohol consumption during pregnancy is a significant public health problem and may result in a wide range of adverse outcomes for the child. The developing central nervous system (CNS) is particularly susceptible to ethanol toxicity. Children with fetal alcohol spectrum disorders (FASD) have a variety of cognitive, behavioral, and neurological impairments. FASD currently represents the leading cause of mental retardation in North America ahead of Down syndrome and cerebral palsy. Ethanol exposure during development causes multiple abnormalities in the brain such as permanent loss of neurons, ectopic neurons, and alterations in synaptogenesis and myelinogenesis. These alcohol-induced structural alterations in the developing brain underlie many of the behavioral deficits observed in FASD. The cellular and molecular mechanisms of ethanol neurotoxicity, however, remain unclear. Ethanol elicits cellular stresses, including oxidative stress and endoplasmic reticulum stress. Glycogen synthase kinase 3β (GSK3β), a multifunctional serine/ threonine kinase, responds to various cellular stresses. GSK3β is particularly abundant in the developing CNS, and regulates diverse developmental events in the immature brain, such as neurogenesis and neuronal differentiation, migration, and survival. Available evidence indicates that the activity of GSK3β in the CNS is affected by ethanol. GSK3β inhibition provides protection against ethanol neurotoxicity, whereas high GSK3β activity/expression sensitizes neuronal cells to ethanol-induced damages. It appears that GSK3β is a converging signaling point that mediates some of ethanol’s neurotoxic effects.
Keywords: Alcohol, Apoptosis, Development, Fetal alcohol syndrome, Neurodegeneration
Introduction
Fetal alcohol spectrum disorders (FASD) are caused by maternal alcohol consumption during pregnancy and characterized by a spectrum of structural anomalies and neurocognitive and behavioral disabilities [1]. Fetal alcohol syndrome (FAS), the most severe form of FASD, displays the complete phenotype of characteristic intrauterine growth restriction, central nervous system (CNS) malformations, mental retardation, and craniofacial and skeletal defects. Epidemiologic studies indicate that in the USA, FAS occurs in 0.2–1.5 per 1,000 live births, whereas alcohol-related birth defects and brain developmental disorders occur in approximately nine per 1,000 live births [2, 3]. FASD currently represent the leading cause of mental retardation in North America, ahead of Down syndrome and cerebral palsy [3–5]. While it is established that heavy alcohol abuse during pregnancy can produce gross teratogenic effects in the developing CNS, moderate levels of prenatal alcohol exposure can also be harmful and impair cognitive, behavioral, and motor functions [1, 6–9]. The economic burden of FASD/FAS is high; annual cost estimates for the United States exceed four billion [10]. Prominent CNS abnormalities in FASD include: microencephaly, abnormal cortical thickness, reduced cerebral white matter volume, ventriculomegaly, and cerebellar hypoplasia [11–16]. These alcohol-induced structural alterations in the brain underlie many of the behavioral deficits observed in FASD. Experimental models have been successfully used to investigate teratogenic effects of ethanol, and CNS abnormalities in FASD are replicated in experimental models. Ethanol exposure disrupts a variety of developmental events in experimental FASD models; these include neurogenesis, neuron survival, cell migration, cell adhesion, axon outgrowth, synapse formation, and neurotransmitter function [17–29]. Despite attempts to increase public awareness of the risks involved, increasing numbers of women are drinking during pregnancy in the USA [30]. Therefore, it is important to develop strategies that can prevent or ameliorate alcohol-induced damages to the brain. To develop effective strategies, the initial step is to understand the mechanism of ethanol neurotoxicity.
Glycogen synthase kinase 3beta (GSK3β), a serine/ threonine kinase originally identified as a regulator of glycogen metabolism, is a central component of the Wnt signaling pathway important for proper axis formation during embryonic development [31]. It is now known that GSK3β plays an important role in the development of the CNS, and regulates diverse early events, such as neurogenesis, neuronal migration, cell adhesion, synapse formation, neuronal survival, and cell polarity/neurite outgrowth in an immature brain. Coincidently, these events are affected by ethanol. This review will discuss the association between GSK3β activity and ethanol neurotoxicity and potential underlying mechanisms.
GSK3β Signaling
Regulation of GSK3β
GSK3 is a multifunctional serine/threonine kinase; originally found in mammals, homologues have been found in all eukaryotes [31, 32]. GSK3 was named for its ability to phosphorylate, and thereby inactivate, glycogen synthase, a key regulatory molecule in the synthesis of glycogen. There are two highly homologous forms of GSK3 in mammals encoded by distinct genes, GSK3α (51 kDa) and GSK3β (47 kDa). It is now known that GSK3 is an important component of diverse signaling pathways involved in the regulation of cell fate, protein synthesis, glycogen metabolism, cell mobility, transformation, proliferation, and survival [31–33]. Despite a high degree of similarity and functional overlap, these isoforms are not functionally identical and redundant. Between these isoforms, GSK3β is better studied, probably due to its prominent role in the CNS. This review mainly discusses studies on GSK3β.
Unlike most protein kinases, GSK3β is constitutively active in resting cells and undergoes a rapid and transient inhibition in response to a number of external signals [31, 32]. GSK3β activity is regulated by site-specific phosphorylation. Full activity of GSK3β generally requires phosphorylation at tyrosine 216 (Tyr216), and conversely, phosphorylation at serine 9 (Ser9) inhibits GSK3β activity. Phosphorylation of Ser9 is probably the most important regulatory mechanism. Several kinases are capable of mediating this modification, including p70 S6 kinase, extracellular signal-regulated kinases (ERKs), p90Rsk (also called MAPKAP kinase-1), protein kinase B (also called Akt), certain isoforms of protein kinase C (PKC), and cyclic AMP-dependent protein kinase (protein kinase A, PKA) [32, 34]. In opposition to the inhibitory modulation of GSK3β that occurs by serine phosphorylation, tyrosine phosphorylation of GSK3β increases the enzyme’s activity. Studies of tyrosine phosphorylation are relatively sparse and information about this regulatory mechanism is fragmentary. Stimulation of pGSK3β(Tyr216) is reported to be mediated by alterations in intracellular calcium levels, a calcium-dependent tyrosine kinase, proline-rich tyrosine kinase 2 (PYK2), or Fyn, a member of the Src tyrosine family [35–38]. pGSK3β(Tyr216) is also subject to the regulation of mitogen-activated protein kinase (MEK1/2) [39]. It has been suggested that GSK3β tyrosine phosphorylation is an autophosphorylation event [40].
Although phosphorylation of GSK3β is the most important mechanism of regulation, GSK3β can be activated without apparent changes in pGSK3β(Tyr216) and pGSK3β(Ser9) [41]. Regulation of GSK3β may also be mediated by its subcellular localization. Some substrates of GSK3β, such as tau, are cytosolic, whereas others, notably several transcription factors, are nuclear. Thus, it is evident that GSK3β must be located in both compartments. Diehl et al. [42] report an increase in nuclear GSK3β during the S phase of the cell cycle. Pro-apoptotic stimuli cause the translocation of Tyr216-phosphorylated GSK3β to the nucleus [43]. A recent study reveals a nuclear localization sequence in GSK3β, shedding light on the mechanisms underlying its shuttling between cytosolic and nuclear compartments [44].
GSK3β and Wnt Signaling
The role of GSK3β in the Wnt signaling pathway is briefly discussed here because of the importance of Wnt signals in all stages of neuronal development. The Wnts are a family of secreted, cysteine-rich, and glycosylated protein ligands that signal by activating the Frizzled family of membrane-bound receptors. Wnt signal transduction causes nuclear translocation of β-catenin and ultimately results in the activation of genes regulated by the T-cell factor (TCF)/ lymphoid enhancer factor (LEF) family of transcription factors. β-catenin is the most well-known substrate of GSK3β. In the absence of Wnt signals, free cytoplasmic β-catenin is incorporated into a cytoplasmic complex that includes Axin, GSK3β, and adenmatous polyposis coli (APC). This enables GSK3β to phosphorylate β-catenin and results in ubiquitin-mediated degradation of β-catenin [31, 45]. Wnt signaling inactivates GSK3β and prevents it from phosphorylating β-catenin, thus stabilizing β-catenin in the cytoplasm. As β-catenin accumulates, it translocates into the nucleus where it binds to TCF/LEF and dramatically increases their transcriptional activity. Thus, GSK3β must be inactivated for Wnt signaling to proceed, and the presence of active GSK3β negatively regulates Wnt signaling. The Wnt signaling pathway is an integral component of embryonic development [45–47]. Many constituents of Wnt signaling pathways are expressed in the developing and mature nervous systems, and Wnt signaling has proven to be essential for neural development at various stages and across species; it is involved in morphogenesis and patterning, as well as differentiation processes and lineage decision events during both central and peripheral nervous system development. Wnt signaling controls the initial formation of the neural plate and many subsequent patterning decisions, such as formation of the neural tube in the embryonic nervous system; it continues to be important at later stages of development, for example, during the differentiation of synapses [45–47]. In the adult brain, Wnt signaling has been demonstrated to participate in degenerative processes leading to cell death during aging [46].
GSK3β Substrates
More than 40 proteins are substrates of GSK3β, and these proteins have roles in a wide spectrum of cellular processes, including glycogen metabolism, transcription, translation, cytoskeletal regulation, cell differentiation, proliferation, transformation, and apoptosis [32, 33, 48]. These substrates can be divided into three major groups of substrate proteins, namely, metabolic/signaling proteins, structural proteins, and transcription factors. The proteins belonging to the first group include acetylCoA carboxylase, amyloid precursor protein, APC tumor suppressor protein, ATP-citrate lyase, axin, cyclic AMP-dependent protein kinase, cyclin D1, translation initiation factor 2B (eIF2B), glycogen synthase, insulin receptor substrate-1, myelin basic protein, NGF receptor, protein phosphatase 1, protein phosphatase inhibitor-2, and pyruvate dehydrogenase. The structural proteins include dynamin-like protein, microtubule-associated protein 1B (MAP1B), microtubule-associated protein 2, neural cell-adhesion protein, neurofilaments, spindle-associated protein Astrin, ninein, and tau. GSK3β-targeted transcription factors are AP-1 (Jun family), β-catenin, C/EBPα, CREB, glucocorticoid receptor, HSF-1, Myc, NFAT, and NF-κB.
The Role of GSK3β in Neuronal Development
GSK3β regulates a number of developing events that are affected by prenatal ethanol exposure. Understanding the role of GSK3β in these events will shed light on the mechanism of ethanol neurotoxicity.
The Expression of GSK3β in the Developing Brain
GSK3β is widely expressed in all tissues and is particularly abundant in the central nervous system [49]. In the developing brain, GSK3β is predominantly expressed in neurons and barely detectable in astrocytes [50, 51]. Leroy and Brion [50] show the expression of GSK3β in the developing rat brain is highest from 18 days of embryonic life up to 10 days of postnatal life (PD10). The expression decreases thereafter and is lowest in the adult; strong GSK3β immunoreactivity is localized in developing neurons, but only weakly detected in layers containing neuroblasts. During development and in the adult, GSK3β is detected in the perikarya and proximal part of dendrites. In the embryo, an intense GSK3β immunoreactivity is also observed in axonal tracts. This axonal immunoreactivity has markedly decreased by PD10 and is absent at PD20 and in the adult [50]. Similarly, Takahashi et al. [51] show a high expression of GSK3β during the first 2 weeks of postnatal life; the GSK3β level peaks at PD8–11. Its expression decreases significantly at PD20, and is lowest in the adult. Coyle-Rink et al. [52] examine the expression of GSK3β in the developing mouse brain. As in the rat brain, a strong GSK3β expression is observed in the mouse brain during the first two postnatal weeks and then the level of GSK3β drastically decreases after PD18. The window of GSK3β expression corresponds to the major period of dendritic extension and synaptogenesis, and is also a period that the brain is susceptible to ethanol exposure [53, 54].
GSK3β and Cell Proliferation
Postmitotic neurons express more GSK3β than cycling neuroblasts in the developing brain [50], and neuronal overexpression of a constitutively active GSK3β induces microcephaly [55, 56]. This suggests that GSK3β activity may negatively regulate neurogenesis. Cui et al. [56] show that inhibition of GSK3β can promote the proliferation of cerebellar granule neuron progenitors in vitro [57], suggesting that activation of GSK3β may suppress the division of neuronal precursors. Also using an in vitro model of cerebellar granule neuron progenitors, Knoepfler and Kenney [58] show that GSK3β activation inhibits cell cycle progression, and the inhibitory effect of GSK3β is mediated by phosphorylating and destabilizing N-Myc. GSK3β is implicated in adult hippocampal neurogenesis [59]. Lithium, an inhibitor of GSK3 enhances the proliferation of adult dentate gyrus-derived neural precursor cells in culture [59]. The proliferation and differentiation of neural precursor cells are mutually exclusive during brain development. Fibroblast growth factor 2 (FGF2) promotes neural precursor cell proliferation and concurrently inhibits their differentiation. FGF2 inactivates GSK3β and induces β-catenin nucleus accumulation, enhancing the proliferation of neural stem/precursor cells, and concurrently inhibiting their differentiation in culture [60]. This is supported by the study of Jin et al. [61] showing that FGF2-stimulated proliferation of cortical neural progenitor cells is mediated by GSK3β inactivation. According to some studies, however, GSK3β activity appears to be necessary for cell cycle progression. Maurer et al. [62] show that inhibition of GSK3β by SB216763, a specific GSK3β inhibitor, suppresses proliferation while promoting neuronal differentiation in neural stem cells isolated from the adult rat subventricular zone. Yeste-Velasco et al. [63] show that GSK3β activation by serum and potassium withdraw induces the expression of several cell cycle regulating proteins, such as cyclin D1, cyclin E, and transcription factor E2F-1; it also phosphorylates retinoblastoma protein in cultured cerebellar granule cells. In some tumor cells, inhibition of GSK3β results in cell cycle arrest and apoptosis [33].
Multiple mechanisms are involved in GSK3β regulation of cell proliferation. First, GSK3β modulation of cell proliferation may be mediated by mitogenic transcription factors. The activity of some mitogenic transcription factors, such as AP-1 and NF-κB, is inhibited by GSK3β activation [32, 33, 64]. Second, GSK3β may inhibit cell cycle progression by interacting with cell cycle regulatory proteins, such as cyclin D1 and D2 [42, 65]. Third, GSK3β may regulate spindle microtubule assembly and accurate chromosome segregation through interactions with the spindle-associated protein Astrin, a substrate for GSK3β [66].
GSK3β and Cell Migration
During nervous system development, neuronal cells undergo directional movements to specific sites in response to extracellular signals and establish proper connections to other cells. Cell migration in the direction of extracellular cues is mediated by actin and microtubule cytoskeletons. The initial step for this process is cell polarization towards the source of the extracellular signal, as well as asymmetric distribution of actin and microtubule cytoskeletons. Microtubule orientation and organization at the leading edge are critical for directional cell migration. GSK3β regulates the dynamics of microtubule cytoskeletons and neuronal polarization in response to extracellular cues [67–70], and therefore is an important signaling component that links extracellular signals to cytoskeletal components and governs neuronal migration. MAP1B, a neuron-specific microtubule-associated protein, is a substrate of GSK3β; it is implicated in the control of the dynamic stability of microtubules and in the cross-talk between microtubules and actin filaments in neurons. González-Billault et al. [71] show that GSK3β-dependent MAP1B phosphorylation is required for Reelin-regulated neuronal migration in vivo and in vitro. In addition to directly regulating microtubule organization, GSK3β can regulate other signaling proteins involved in cell migration. For example, platelet activating factor (PAF) induces apoptosis and inhibits the migration of cerebellar granule neurons in a GSK3β-dependent manner; GSK3β inhibitors block the effect of PAF [72]. Focal adhesion kinase (FAK) is a non-receptor tyrosine kinase found in focal adhesion events and plays an important role in cell adhesion, spreading, and migration [73]. It is shown that GSK3β regulates cell spreading and migration by phosphorylating FAK at selected serine sites [74]. Aspartyl (asparaginyl)-beta-hydroxylase (AAH) regulates cell motility by catalyzing post-translational hydroxylation of proteins involved in cell migration, such as Notch and Jagged [75]. AAH is a substrate of GSK3β and GSK3β-mediated phosphorylation induces AAH degradation, resulting in retarded cell motility [76]. Microglia play a prominent role in the brain’s inflammatory response to injury or infection by migrating to affected locations and secreting inflammatory molecules. GSK3 inhibitors reduce the migration of microglia in cultured mouse hippocampal slices [76]. The regulation of GSK3β on microglia migration may underlie its role in inflammatory response to injury in the CNS.
GSK3β and Neuronal Differentiation
Neurons are highly polarized cells that contain a single long axon and multiple dendrites. Initial differentiation of neurons is to establish neuronal polarity. Polarization occurs when one of the multiple neurites emerging from the cell body initiates a phase of rapid elongation, becoming the axon; the remaining neurites will develop as dendrites. Rearrangement of the neuronal cytoskeleton provides support for the dramatic morphological changes that occur during neuronal polarization and neurite outgrowth. Several microtubule-associated proteins (MAPs) important for neuronal polarity and axonal outgrowth, such as tau and MAP1B, are substrates of GSK3β [68]. Inhibition of GSK3β provides a positive regulatory signal for cytoskeleton rearrangement involved in neuronal polarity and axon extension [77].
Neuronal over-expression of a constitutively active GSK3β causes a delayed postnatal maturation and differentiation of neurons in the mouse brain [55, 56]. In various in vitro models of neuronal development, inhibition of GSK3β by selective inhibitors or molecular approaches is shown to promote neurite outgrowth; in contrast, activation of GSK3β causes neurite retraction [38, 78–82]. In addition, inactivation of GSK3β mediates a leptin-induced increase in axonal growth cone size in developing mouse cortical neurons [83]. GSK3β also negatively regulates the differentiation of neural stem cells. GSK3β inhibitors promote neuronal differentiation in neural stem cells isolated from the adult rat subventricular zone [62]. Inactivation of GSK3β is also beneficial for axonal regeneration after a spinal cord lesion [81].
On the other hand, some reports demonstrate that GSK3β activity is necessary for neurite outgrowth and axonal extension. For example, lithium or other GSK3β inhibitors are shown to reduce neurite outgrowth and axon elongation rates in primary cultures of rat hippocampal neurons and sensory neurons [84, 85]. The complex effects of GSK3β on neuronal differentiation are highlighted by studies using PC12 cells. PC12 cells are rat pheochromocytoma cells that are extensively used to study the cellular/ molecular mechanisms of neuronal differentiation. PC12 cells differentiate into neuronal-like cells by extending neurites and expressing neurotransmitters in response to neurotrophic factors such as NGF and bFGF. There are conflicting reports on the effect of GSK3β in PC12 cell differentiation; GSK3β activity can be either negative or positive for neurite outgrowth of PC12 cells [86–91].
The effect of GSK3β on neuronal differentiation appears to be mediated by its interaction with microtubule-associated proteins, such as MAP1B, which is essential for neuronal polarization, migration, neurite outgrowth, and axon elongation [85, 92]. GSK3β phosphorylation of MAP1B acts as a molecular switch regulating the control that MAP1B exerts on microtubule dynamics in growing axons and growth cones [88]. Besides MAP1B, GSK3β may interact with other proteins that also participate in regulating neuronal differentiation. For example, E2F1 transcription factor is a key downstream target of the retinoblastoma tumor suppressor protein and is involved in neuronal differentiation. NGF-induced GSK3β/E2F1 interaction facilitates E2F1 degradation, increasing neurite outgrowth in PC12 cells [90]. NGF promotes the interaction between GSK3β and MRP2, an actin-binding protein in PC12 cells; the interaction enhances neurite outgrowth [89]. As observed in GSK3β, the effects of ethanol on neurite outgrowth and axonal extension are complex. Depending on the model system examined and the concentration of ethanol applied, ethanol can either promote or inhibit neurite outgrowth [29, 82, 93–97].
GSK3β and Neuronal Injury
GSK3β is an important modulator of apoptosis [32, 33, 98]. Lucas et al. [99] use transgenic mice that conditionally over-express GSK3β to demonstrate the link between GSK3β activity and neurodegeneration. They show that over-expression of GSK3β to specific regions of the brain in mice results in neuronal death characteristic of apoptosis in these regions [99]. The finding is supported by studies using various in vitro models, which show the overexpression of GSK3β in neurons is sufficient to trigger neuronal cell death; in contrast, expression of inhibitory GSK3β binding proteins or a dominant interfering form of GSK3β reduces neuronal apoptosis [100–103]. Interestingly, relatively low levels of GSK3β over-expression, which alone do not induce apoptosis, greatly facilitate pro-apoptotic signaling and promote apoptosis [104].
Due to its prominent role in regulation of neuronal survival, GSK3β has been linked to several key neuropathological mechanisms of neurodegenerative diseases [32, 34, 98, 105, 106]. GSK3β is shown to mediate neuronal apoptosis in various models of neurodegeneration, including Alzheimer’s disease, Parkinson’s disease, familial amyotrophic lateral sclerosis, and human immunodeficiency virus-1 encephalitis [107–115]. GSK3β is activated and induces neuronal death in response to various environmental/ cellular stresses, such as deprivation of trophic factors, oxidative stress, and endoplasmic reticulum (ER) stress [43, 102, 103, 109, 110, 116–119]. GSK3β also mediates cell death caused by some extrinsic signals. For example, Maggirwar et al. [120] report that the human immunodeficiency virus type 1 regulatory protein Tat activates GSK3β and induces apoptosis in cerebellar granule neurons. Similarly, platelet activating factor induces apoptosis and inhibits migration of cerebellar granule neurons through the activation of GSK3β [72]. GSK3β activation during neuronal death is commonly mediated by deregulation of phosphorylation at Ser9. Bhat et al. [43] report that pro-apoptotic stimuli (nerve growth factor withdrawal, ischemia, or staurosporine treatment) increase Tyr216 phosphorylation of GSK3β and induce its nuclear translocation.
Some studies suggest that GSK3β activity is necessary for neuronal survival. Gómez-Sintes et al. [121] investigate conditional over-expression of dominant-negative (DN) GSK3β in the mouse brain. Transgenic mice expressing DN GSK3β display increased neuronal apoptosis and impaired motor coordination. These results reveal the importance of intact GSK3β activity for adult neuron viability and physiology, and warn of potential neurological toxicity of GSK3β pharmacological inhibition beyond physiological levels. Lithium is shown to have an opposing effect on neuronal survival, depending on the developmental status of the neuron; it induces apoptosis in immature cerebellar granule cells, but promotes survival in mature neurons [122].
It is unclear how GSK3β executes both pro- and anti-apoptotic action. It has been suggested that GSK3β promotes cell death caused by the intrinsic apoptotic pathway, but inhibits the death receptor-mediated extrinsic apoptotic signaling pathway [98]. The intrinsic apoptotic signaling cascade can be induced by numerous stimuli that cause cell damage, such as DNA damage, oxidative stress, and endoplasmic reticulum stress. The intrinsic apoptotic signaling pathway causes the disruption of mitochondria, leading to cell destruction. The extrinsic apoptotic pathway, on the other hand, is mediated by the activation of cell-surface death domain-containing receptors and initiates apoptosis by activating caspase-8.
Several potential contributory mechanisms underlying GSK3β modulation of cell survival are evident. It is suggested that GSK3β-induced phosphorylation of tau may destabilize microtubules to contribute to cytoskeletal collapse associated with apoptosis [123], and GSK3β-mediated phosphorylation of pyruvate dehydrogenase may impair Krebs cycle activity [124]. Effects of GSK3β on neuronal survival may be mediated by modulation of transcription factors. It is shown that GSK3β activity is required for AP1-dependent expression of pro-apoptotic Bim and inhibitors of GSK3β block AP-1 activation and protect neurons from apoptosis caused by trophic factor deprivation [125]. Activation of GSK3β is shown to antagonize NF-κB-mediated neuronal survival, resulting in decreased cell viability [126]. GSK3β binds to p53 and promotes p53-induced apoptosis [127]. Mixed lineage kinase 3 (MLK3) is a mitogen-activated protein kinase kinase member that activates the c-Jun N-terminal kinase pathway. GSK3β-dependent MLK3 phosphorylation mediates neuronal death caused by NGF deprivation [128]. Transcription factor NFAT3 is identified as a key target in GSK3β-promoted apoptosis in cerebellar granule neurons [129]. Additionally, the effect of GSK3β on neuronal survival may be regulated by translational control. Translation initiation factor 2B is a substrate of GSK3β. GSK3β phosphorylates and inhibits eIF2B, resulting in translational suppression and programmed cell death [130]. Finally, GSK3β is also present in mitochondria and may directly modify the activity of pro-apoptotic proteins. GSK3β activation appears to be upstream of mitochondria apoptotic signaling, such as caspase-3/caspase-9 processing and cytochrome c release [131]. It has been demonstrated that GSK3β phosphorylates pro-apoptotic Bax and promotes its mitochondrial localization during neuronal apoptosis [132].
In addition to the direct effect on neurons, GSK3 may contribute to neuronal injury by modulating brain inflammation. Excessive neuroinflammation contributes to neurodegeneration. GSK3β activation promotes the production of inflammatory molecules and microglia migration, which together make GSK3 a powerful regulator of inflammation [133].
GSK3β in Ethanol-Induced Neuronal Injuries
Evidence of Ethanol Modulation of GSK3β Activity
We examined the effect of ethanol on GSK3β with an animal model of developmental ethanol exposure [134]. Seven-day-old C57BL/6 mouse pups were injected subcutaneously with saline or ethanol (2.5 g/kg, 20% solution in saline) twice at 0 h and 2 h. The injection causes significant apoptosis in the cerebral cortex and induces a dephosphorylation of GSK3β at Ser9, but has little effect on the phosphorylation at Tyr216 and the expression of total GSK3β. The finding is supported by our in vitro model using differentiating neuroblastoma cells (N2a). Ethanol causes a strong dephosphorylation of GSK3β at Ser9 in N2a cells without affecting p-GSK3β(Tyr216) [82]. Ethanol-induced activation of GSK3β is evident by an increase in the phosphorylation of tau, a substrate of GSK3β. Similar to our observations, de la Monte and Wands [135] demonstrate that prenatal ethanol exposure alters GSK3β activity in cultured cerebellar neurons. In their study, female rats were fed an ethanol-containing diet throughout pregnancy. Cerebellar primary neuronal cultures were generated with cerebellar tissue harvested from control or ethanol-exposed postnatal day 2 pups. Insulin-stimulated Akt and GSK3β phosphorylation (Ser9) are diminished in these cells, while the levels of non-phosphorylated GSK3β are increased; thus, the activity of GSK3β is increased. Their subsequent in vivo study shows that chronic gestational exposure to ethanol induces a dephosphorylation of GSK3β at Ser9 and increases the activity of GSK3β in the developing cerebellum [136]. This research group also shows that ethanol activates GSK3β in cultured human CNS-derived primitive neuroectodermal tumor 2 (PNET2) cells [75].
However, it appears in the adult brain and some other cell types, ethanol may inhibit GSK3β. Neznanova et al. [137] demonstrate that acute ethanol exposure, at a dose commonly regarded as reinforcing, strongly phosphorylates GSK3β at Ser9 in the medial prefrontal cortex, with corresponding increased phosphorylation of Akt in ethanol-preferring AA rats. They suggest the GSK3β pathway may be involved in high ethanol preference. In cardiac cells, ethanol increases pGSK3β(Ser9) and inactivates GSK3β [138].
So far, available evidence indicates that ethanol modulates GSK3β activity by changing pGSK3β(Ser9); alterations in pGSK3β(Tyr216) have not been reported. In addition to phosphorylation, GSK3β activity can be regulated by its localization. Since some pro-apoptotic stimuli cause nuclear accumulation of pGSK3β(Tyr216) [43], a careful evaluation of subcellular distribution of phosphorylated GSK3β is needed.
GSK3β Activity and Ethanol Neurotoxicity
Studies using selective GSK3β inhibitors or genetic approaches to modulate GSK3β activity have established an association between GSK3β activity and ethanol neurotoxicity. Lithium is a commonly used GSK3 inhibitor; it inhibits activity of both GSK3α and GSK3β and promotes inhibitory phosphorylation at Ser21 and Ser9 of GSK3α and GSK3β, respectively [139]. To evaluate the effect of lithium on ethanol neurotoxicity, we intraperitoneally injected 7-day-old-mice with lithium 30 min prior to ethanol exposure [134]. Lithium effectively blocks ethanol-induced caspase-3 activation in the developing cerebral cortex (Fig. 1). It is interesting to note that lithium administration after ethanol exposure also provides neuroprotection. Zhong et al. [140] show that an intraperitoneal injection of lithium at 15 min following ethanol exposure reduces ethanol-induced apoptosis in the striatum and superficial frontal cortex of 7-day-old mice. Lithium also prevents ethanol-induced apoptosis and activation of caspase-3 and caspase-9 in cultured cerebellar granule neurons [140]. This is confirmed by a study using the same paradigm of lithium treatment; injection of lithium at 15 min following ethanol exposure blocks ethanol-induced caspase-3 activation as well as ethanol-mediated down-regulation of p-GSK3β(Ser9), p-Akt, and p-AMPK in the forebrain of 7-day-old mice [141].
Fig. 1.
Lithium alleviates ethanol-induced caspase-3 activation in the developing brain. a Seven-day-old C57BL/6 mice were injected with LiCl (40 mg/kg body weight; 4 mg/ml) or saline 30 min prior to ethanol exposure. Mice were then injected subcutaneously with ethanol (2.5 g/kg, 20% solution in saline). At specified times after ethanol injection, the expression of active caspase-3 in the cerebral cortex was analyzed with immunohistochemistry. Bar=100 µM. b Mice were injected with ethanol with/without lithium as described above. The cerebral cortex was dissected and protein was extracted. The expression of active caspase-3 was determined with immunoblotting. From [134]
Lithium is effective in protecting ethanol-induced neurodegeneration. However, the role of GSK3β remains unclear because lithium also inhibits GSK3α and modulates the activity of other protein kinases/phosphatases, such as PKA and PP2A [139, 142, 143]. The outcomes of other selective GSK3β inhibitors are somewhat confusing. Takadera and Ohyashiki [144] show that two GSK3 inhibitors (SB216763 and alsteropaullone) completely eliminated apoptosis of primary rat cortical neurons in culture caused by ethanol exposure. SB216763 inhibits GSK3α and GSK3β in an ATP competitive manner [145]. Alsteropaullone is a potent inhibitor for GSK3β and cyclin-dependent protein kinase 5 (CDK5) [146]. Since a specific CDK5 inhibitor fails to offer protection, they conclude that GSK3β mediates ethanol neurotoxicity. According to Zhong et al. [140], however, lithium protects cerebellar granule neurons from ethanol-induced apoptosis, but SB415286 does not. SB415286 is a GSK3 inhibitor structurally distinct from SB216763 and inhibits both GSK3α and GSK3β in an ATP competitive manner [145]. They therefore suggest that GSK3 is not involved in ethanol-induced neurodegeneration.
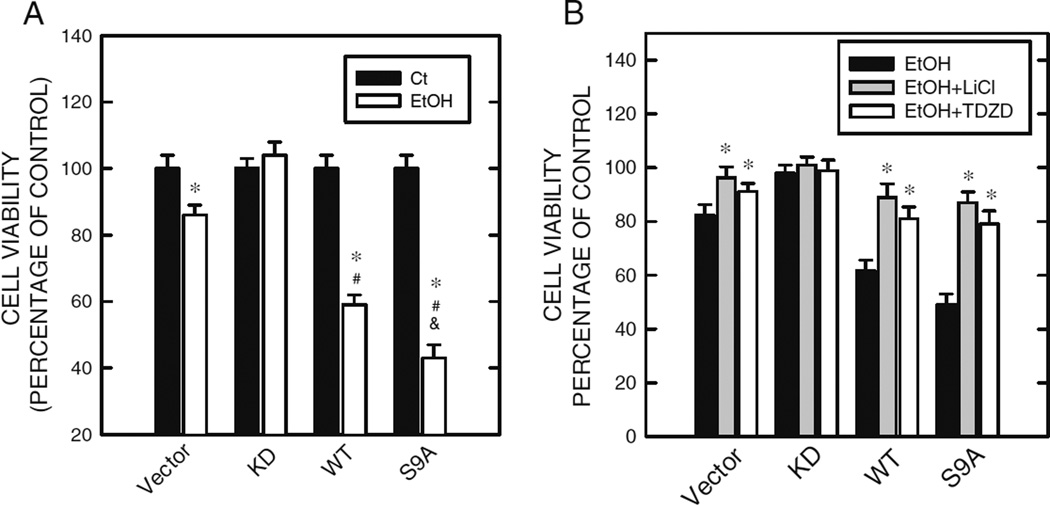
To clarify whether GSK3β contributes to ethanol neurotoxicity, we over-express wild type (WT), S9A mutant, or kinase-deficient (KD) GSK3β in SK-N-MC neuroblastoma cells, a cell line relatively insensitive to ethanol exposure. The KD and S9A GSK3β specifically inhibit and activate GSK3β, respectively. Over-expression of wild type or S9A mutant GSK3β in SK-N-MC cells does not induce cell death, but greatly promotes ethanol-induced cell death of SK-N-MC cells; whereas, over-expression of KD GSK3β confers resistance to ethanol neurotoxicity (Fig. 2a) [133]. Lithium and TDZD-8 abolish hypersensitivity to ethanol caused by over-expression of WT and S9A GSK3β (Fig. 2b). TDZD-8 is a highly selective, non-ATP competitive inhibitor of GSK3β; it binds to the active site of GSK3β [147]. The evidence supports that GSK3β activity indeed contributes to ethanol-induced neuronal death.
Fig. 2.
GSK3β expression/activity modulates ethanol’s effect on cell viability. a SK-N-MC cells stably over-expressing wide type (WT), S9A mutant (S9A), and kinase-deficient (KD) GSK3β were exposed to ethanol (0 or 400 mg/dl) for 48 h. Cell viability was determined by MTT assay and expressed as percentage of non-ethanol-treated controls. * denotes significant difference from matched, non-ethanol controls. # denotes significant difference from ethanol-treated vector cells. & denotes significant difference from ethanol-treated WT cells. b SK-N-MC cells stably over-expressing WT, S9A, and KD GSK3β were pretreated with GSK3β inhibitors (lithium, 20 mM and TDZD-8, 10 µM) for 30 min, then exposed to ethanol (0 or 400 mg/dl) for 48 h. Cell viability was determined as described above. * denotes significant difference from cells that were not treated with GSK3β inhibitors. From [134]
In addition to mediating neuronal death, GSK3β is also involved in ethanol-mediated inhibition of neuronal migration and differentiation. AAH is a substrate of GSK3β and regulates cell motility by catalyzing post-translational hydroxylation of proteins involved in cell migration, such as Notch and Jagged [75]. Ethanol induces GSK3β-dependent AAH phosphorylation, resulting in AAH degradation which impairs migration of PNET2 cells [75]. Lithium mitigates ethanol-induced AAH protein degradation and impaired motility. Inactivation of GSK3β promotes neurite outgrowth of N2a cells, while its activation causes an inhibition of neurite outgrowth. Ethanol inhibits neurite outgrowth by activating GSK3β through the dephosphorylation of GSK3β at Ser9 [82]. Inhibition of GSK3β activity by lithium or down-regulation of GSK3β by siRNA reverses ethanol-induced inhibition of neurite outgrowth. Cyanidin-3-glucoside, one of the anthocyanins rich in pigmented fruits, promotes pGSK3β(Ser9) and therefore blocks ethanol-induced inhibition of neurite outgrowth [82].
Mechanisms Underlying Ethanol Modulation of GSK3β Activity
GSK3β can be activated by various cellular stresses, such as oxidative stress and ER stress [102, 103, 109, 114, 125]; ethanol exposure is known to induce oxidative stress or ER stress [148, 149]. It is unclear whether ethanol directly affects GSK3β activity. Research using a cell-free setting would be helpful to address this question. It is known that ethanol activation of GSK3β is associated with dephosphorylation at Ser9. The decrease in pGSK3β(Ser9) may be mediated by the activation of phosphatases that dephosphorylate GSK3β, such as PP2A or the impairment of kinases that regulate GSK3β phosphorylation. It is reported that ethanol activates PP2A in rat hepatoma cells [150]. A systematic analysis of ethanol’s effect on related phosphatases in neurons is necessary.
Neurotrophic factors, such as insulin/insulin-like growth factor or brain-derived neurotrophic factor (BDNF) support neuronal survival by activating the PI3K/Akt pathway [151, 152]. Activation of the PI3K/Akt signal pathway causes GSK3β phosphorylation at Ser9 and inactivates GSK3β. Ethanol interferes with BDNF- and insulin/IGF-mediated PI3K/Akt activation in cerebellar granule neurons [135, 136, 153]. de la Monte and her associates demonstrate that ethanol causes GSK3β dephosphorylation at Ser9 by blocking insulin/IGF-mediated PI3K/Akt activation [133, 134]. Alternatively, ethanol may inhibit PI3K/Akt signaling pathways through the activation of phosphatase and tensin homolog deleted in chromosome 10 (PTEN). PTEN is a phosphatase that inactivates PI3K, and ethanol increases PTEN phosphatase activity in the developing cerebellum [136]. The activity of some other upstream kinases of GSK3β, such as ERK, PKC, and PKA is also altered by ethanol [154–156]. Further study is needed to determine whether alterations in these kinases contribute to ethanol-induced dephosphorylation of GSK3β.
Direct modifications of downstream proteins as a result of GSK3β activation induce ethanol neurotoxicity. One of these is Bax, a key pro-apoptotic protein. We demonstrate that ethanol activates Bax in a GSK3β-dependent manner in the developing mouse brain and in cultured neuronal cells [134]. Furthermore, over-expression of WT or S9A GSK3β sensitizes cells to ethanol-induced Bax activation. Contrarily, blocking GSK3β activity by a dominant-negative GSK3β mutant confers resistance to ethanol-induced Bax activation. Together, these results indicate that Bax is downstream of GSK3β and mediates ethanol neurotoxicity. AAH is a substrate of GSK3β and regulates cell motility. Carter et al. [75] demonstrate that ethanol-induced GSK3β-dependent AAH degradation impairs the motility of neuronal cells. cAMP response element binding protein (CREB) is a substrate of GSK3β and is implicated in alcohol drinking behavior [157]. Ethanol is shown to induce CREB phosphorylation in the central and medial amygdala as well as nucleus accumbens of rats [158, 159]. Acute ethanol exposure stimulates, but chronic exposure decreases CREB phosphorylation in the striatum and cerebellum of rats [160, 161]. It is unclear, however, whether GSK3β is involved in ethanol modulation of CREB phosphorylation/activity.
Conclusions and Future Research
Studies using animal models and cell culture systems demonstrate that ethanol alters GSK3β activity in the developing neurons, and some neurotoxic effects of ethanol, such as promotion of cell death and disruption of migration and differentiation, is mediated, at least in part, by GSK3β activation. Suppression of GSK3β activity by inhibitors or genetic approaches ameliorates ethanol neurotoxicity. GSK3β has become one of the most attractive therapeutic targets for the treatment of diabetes, inflammation, and multiple neurological diseases, including Alzheimer’s, stroke, and bipolar disorders [133, 162]. Since it is a converging point of many signaling pathways and plays an essential role in neuronal development and neurodegeneration, the involvement of GSK3β in ethanol neurotoxicity provides a potential target for therapeutic efforts.
Several issues regarding the relationship between GSK3β and ethanol neurotoxicity remain. First, although available evidence supports that ethanol activates GSK3β, it is unclear whether ethanol directly affects GSK3β or acts only by modulating its upstream regulatory proteins. Second, it appears that neuronal vulnerability to ethanol correlates to GSK3β activity in vitro; high activity/expression of GSK3β sensitizes neuronal cells to ethanol damage. It is well known that the vulnerability of the developing brain to ethanol is temporal- and regional-dependent. Does the profile of developmental expression of GSK3β contribute to the differential sensitivity to ethanol? Third, although excessive GSK3β activation is harmful for neurons, basal GSK3β activity is required for neuronal survival and physiology. A more effective and specific inhibition or knock-down of GSK3β in the developing brain is necessary to firmly establish its role in ethanol neurotoxicity. Fourth, despite a high degree of similarity and functional overlap, GSK3β and GSK3α are not functionally identical and redundant. It is worthwhile to evaluate the role of GSK3α in ethanol neurotoxicity as well.
Acknowledgments
I would like to thank Kimberly Bower for reading this manuscript. This research was supported by grants from the National Institutes of Health (AA015407 and AA017226).
References
- 1.Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- 2.Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM., Jr Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56:317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 3.May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome. Alcohol Res Health. 2001;25:159–167. [PMC free article] [PubMed] [Google Scholar]
- 4.Stratton K, Howe C, Battagila F, editors. Fetal alcohol syndrome: diagnosis, epidemiology, prevention, and treatment. Washington D.C: National Academy Press; 1996. [Google Scholar]
- 5.Nash K, Sheard E, Rovet J, Koren G. Understanding fetal alcohol spectrum disorders (FASDs): toward identification of a behavioral phenotype. Scientific World Journal. 2008;8:873–882. doi: 10.1100/tsw.2008.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattson SN, Schoenfeld AM, Riley EP. Teratogenic effects of alcohol on brain and behavior. Alcohol Res Health. 2001;25:185–191. [PMC free article] [PubMed] [Google Scholar]
- 7.O’Malley KD, Nanson J. Clinical implications of a link between fetal alcohol spectrum disorder and attention-deficit hyperactivity disorder. Can J Psychiatry. 2002;47:349–354. doi: 10.1177/070674370204700405. [DOI] [PubMed] [Google Scholar]
- 8.O’Callaghan FV, O’Callaghan M, Najman JM, Williams GM, Bor W. Maternal alcohol consumption during pregnancy and physical outcomes up to 5 years of age: a longitudinal study. Early Hum Dev. 2003;71:137–148. doi: 10.1016/s0378-3782(03)00003-3. [DOI] [PubMed] [Google Scholar]
- 9.O’Callaghan FV, O’Callaghan M, Najman JM, Williams GM, Bor W. Prenatal alcohol exposure and attention, learning and intellectual ability at 14 years: a prospective longitudinal study. Early Hum Dev. 2007;83:115–123. doi: 10.1016/j.earlhumdev.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Lupton C, Burd L, Harwood R. Cost of fetal alcohol spectrum disorders. Am J Med Genet C Semin Med Genet. 127C:42–50. doi: 10.1002/ajmg.c.30015. [DOI] [PubMed] [Google Scholar]
- 11.Clarren SK, Alvord EJ, Sumi SM, Streissguth AP, Smith DW. Brain malformations related to prenatal exposure to ethanol. J Pediatr. 1978;92:64–67. doi: 10.1016/s0022-3476(78)80072-9. [DOI] [PubMed] [Google Scholar]
- 12.Danis RP, Newton N, Keith L. Pregnancy and alcohol. Curr Probl Obstet Gynecol. 1981;4:2–48. [PubMed] [Google Scholar]
- 13.Swayze VW2nd, Johnson VP, Hanson JW, Piven J, Sato Y, Giedd JN, Mosnik D, Andreasen NC. Magnetic resonance imaging of brain anomalies in fetal alcohol syndrome. Pediatrics. 1997;99:232–240. doi: 10.1542/peds.99.2.232. [DOI] [PubMed] [Google Scholar]
- 14.Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001;43:148–154. [PubMed] [Google Scholar]
- 15.Bookstein FL, Streissguth AP, Connor PD, Sampson PD. Damage to the human cerebellum from prenatal alcohol exposure: the anatomy of a simple biometrical explanation. Anat Rec B New Anat. 2006;289:195–209. doi: 10.1002/ar.b.20114. [DOI] [PubMed] [Google Scholar]
- 16.Sowell ER, Mattson SN, Kan E, Thompson PM, Riley EP, Toga AW. Abnormal cortical thickness and brain-behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cereb Cortex. 2008;18:136–144. doi: 10.1093/cercor/bhm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller MW. Migration of cortical neurons is altered by gestational exposure to ethanol. Alcohol Clin Exp Res. 1993;17:304–314. doi: 10.1111/j.1530-0277.1993.tb00768.x. [DOI] [PubMed] [Google Scholar]
- 18.Swanson DJ, King MA, Walker DW, Heaton MB. Chronic prenatal ethanol exposure alters the normal ontogeny of choline acetyltransferase activity in the rat septohippocampal system. Alcohol Clin Exp Res. 1995;19:1252–1260. doi: 10.1111/j.1530-0277.1995.tb01608.x. [DOI] [PubMed] [Google Scholar]
- 19.Miller MW. Limited ethanol exposure selectively alters the proliferation of precursor cells in the cerebral cortex. Alcohol Clin Exp Res. 1996;20:139–143. doi: 10.1111/j.1530-0277.1996.tb01056.x. [DOI] [PubMed] [Google Scholar]
- 20.Luo J, Miller MW. Growth factor-mediated neural proliferation: target of ethanol toxicity. Brain Res Brain Res Rev. 1998;27:157–167. doi: 10.1016/s0165-0173(98)00009-5. [DOI] [PubMed] [Google Scholar]
- 21.Minana R, Climent E, Barettino D, Segui JM, Renau-Piqueras J, Guerri C. Alcohol exposure alters the expression pattern of neural cell adhesion molecules during brain development. J Neurochem. 2000;75:954–964. doi: 10.1046/j.1471-4159.2000.0750954.x. [DOI] [PubMed] [Google Scholar]
- 22.Olney JW, Ishimaru MJ, Bittigau P, Ikonomidou C. Ethanol-induced apoptotic neurodegeneration in the developing brain. Apoptosis. 2000;5:515–521. doi: 10.1023/a:1009685428847. [DOI] [PubMed] [Google Scholar]
- 23.Yanni PA, Lindsley TA. Ethanol inhibits development of dendrites and synapses in rat hippocampal pyramidal neuron cultures. Brain Res Dev Brain Res. 2000;120:233–243. doi: 10.1016/s0165-3806(00)00015-8. [DOI] [PubMed] [Google Scholar]
- 24.Bearer CF. L1 cell adhesion molecule signal cascades: targets for ethanol developmental neurotoxicity. Neurotoxicology. 2001;22:625–633. doi: 10.1016/s0161-813x(01)00034-1. [DOI] [PubMed] [Google Scholar]
- 25.Ikonomidou C, Bittigau P, Koch C, Genz K, Hoerster F, Felderhoff-Mueser U, Tenkova T, Dikranian K, Olney JW. Neurotransmitters and apoptosis in the developing brain. Biochem Pharmacol. 2001;62:401–405. doi: 10.1016/s0006-2952(01)00696-7. [DOI] [PubMed] [Google Scholar]
- 26.Goodlett CR, Horn KH, Zhou FC. Alcohol teratogenesis: mechanisms of damage and strategies for intervention. Exp Biol Med (Maywood) 2005;230:394–406. doi: 10.1177/15353702-0323006-07. [DOI] [PubMed] [Google Scholar]
- 27.Soscia SJ, Tong M, Xu XJ, Cohen AC, Chu J, Wands JR, de la Monte SM. Chronic gestational exposure to ethanol causes insulin and IGF resistance and impairs acetylcholine homeostasis in the brain. Cell Mol Life Sci. 2006;63:2039–2056. doi: 10.1007/s00018-006-6208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumada T, Jiang Y, Cameron DB, Komuro H. How does alcohol impair neuronal migration? J Neurosci Res. 2007;85:465–470. doi: 10.1002/jnr.21149. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman EJ, Mintz CD, Wang S, McNickle DG, Salton SR, Benson DL. Effects of ethanol on axon outgrowth and branching in developing rat cortical neurons. Neuroscience. 2008;157:556–565. doi: 10.1016/j.neuroscience.2008.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebrahim SH, Diekman ST, Floyd RL, Decoufle P. Comparison of binge drinking among pregnant and nonpregnant women, United States, 1991–1995. Am J Obstet Gynecol. 1999;180:1–7. doi: 10.1016/s0002-9378(99)70139-0. [DOI] [PubMed] [Google Scholar]
- 31.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 33.Luo J. Glycogen synthase kinase 3beta (GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett. 2009;273:194–200. doi: 10.1016/j.canlet.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaytor MD, Orr HT. The GSK3 beta signaling cascade and neurodegenerative disease. Curr Opin Neurobiol. 2002;12:275–278. doi: 10.1016/s0959-4388(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 35.Hartigan JA, Johnson GV. Transient increases in intracellular calcium result in prolonged site-selective increases in Tau phosphorylation through a glycogen synthase kinase 3beta-dependent pathway. J Biol Chem. 1999;274:21395–21401. doi: 10.1074/jbc.274.30.21395. [DOI] [PubMed] [Google Scholar]
- 36.Lesort M, Jope RS, Johnson GV. Insulin transiently increases tau phosphorylation: involvement of glycogen synthase kinase-3beta and Fyn tyrosine kinase. J Neurochem. 1999;72:576–584. doi: 10.1046/j.1471-4159.1999.0720576.x. [DOI] [PubMed] [Google Scholar]
- 37.Hartigan JA, Xiong XC, Johnson GV. Glycogen synthase kinase 3beta is tyrosine phosphorylated by PYK2. Biochem Biophys Res Commun. 2001;284:485–489. doi: 10.1006/bbrc.2001.4986. [DOI] [PubMed] [Google Scholar]
- 38.Sayas CL, Ariaens A, Ponsioen B, Moolenaar WH. GSK-3 is activated by the tyrosine kinase Pyk2 during LPA1-mediated neurite retraction. Mol Biol Cell. 2006;17:1834–1844. doi: 10.1091/mbc.E05-07-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi-Yanaga F, Shiraishi F, Hirata M, Miwa Y, Morimoto S, Sasaguri T. Glycogen synthase kinase-3beta is tyrosine-phosphorylated by MEK1 in human skin fibroblasts. Biochem Biophys Res Commun. 2004;316:411–415. doi: 10.1016/j.bbrc.2004.02.061. [DOI] [PubMed] [Google Scholar]
- 40.Cole A, Frame S, Cohen P. Further evidence that the tyrosine phosphorylation of glycogen synthase kinase-3 (GSK3) in mammalian cells is an autophosphorylation event. Biochem J. 2004;377:249–255. doi: 10.1042/BJ20031259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baltzis D, Pluquet O, Papadakis AI, Kazemi S, Qu LK, Koromilas AE. The eIF2alpha kinases PERK and PKR activate glycogen synthase kinase 3 to promote the proteasomal degradation of p53. J Biol Chem. 2007;282:31675–31687. doi: 10.1074/jbc.M704491200. [DOI] [PubMed] [Google Scholar]
- 42.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhat RV, Shanley J, Correll MP, Fieles WE, Keith RA, Scott CW, Lee CM. Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3beta in cellular and animal models of neuronal degeneration. Proc Natl Acad Sci U S A. 2000;97:11074–11079. doi: 10.1073/pnas.190297597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meares GP, Jope RS. Resolution of the nuclear localization mechanism of glycogen synthase kinase-3: functional effects in apoptosis. J Biol Chem. 2007;282:16989–17001. doi: 10.1074/jbc.M700610200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ille F, Sommer L. Wnt signaling: multiple functions in neural development. Cell Mol Life Sci. 2005;62:1100–1108. doi: 10.1007/s00018-005-4552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patapoutian A, Reichardt LF. Roles of Wnt proteins in neural development and maintenance. Curr Opin Neurobiol. 2000;10:392–399. doi: 10.1016/s0959-4388(00)00100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malaterre J, Ramsay RG, Mantamadiotis T. Wnt-Frizzled signalling and the many paths to neural development and adult brain homeostasis. Front Biosci. 2007;12:492–506. doi: 10.2741/2077. [DOI] [PubMed] [Google Scholar]
- 48.Manoukian AS, Woodgett JR. Role of glycogen synthase kinase-3 in cancer: regulation by Wnts and other signaling pathways. Adv Cancer Res. 2002;84:203–229. doi: 10.1016/s0065-230x(02)84007-6. [DOI] [PubMed] [Google Scholar]
- 49.Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990;9:2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leroy K, Brion JP. Developmental expression and localization of glycogen synthase kinase-3beta in rat brain. J Chem Neuroanat. 1999;16:279–293. doi: 10.1016/s0891-0618(99)00012-5. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi M, Tomizawa K, Ishiguro K. Distribution of tau protein kinase I/glycogen synthase kinase-3beta, phosphatases 2A and 2B, and phosphorylated tau in the developing rat brain. Brain Res. 2000;857:193–206. doi: 10.1016/s0006-8993(99)02424-5. [DOI] [PubMed] [Google Scholar]
- 52.Coyle-Rink J, Del Valle L, Sweet T, Khalili K, Amini S. Developmental expression of Wnt signaling factors in mouse brain. Cancer Biol Ther. 2002;1:640–645. doi: 10.4161/cbt.313. [DOI] [PubMed] [Google Scholar]
- 53.Heaton MB, Paiva M, Madorsky I, Shaw G. Ethanol effects on neonatal rat cortex: comparative analyses of neurotrophic factors, apoptosis-related proteins, and oxidative processes during vulnerable and resistant periods. Brain Res Dev Brain Res. 2003;145:249–262. doi: 10.1016/j.devbrainres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 54.Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol. 2003;25:447–458. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- 55.Spittaels K, Van den Haute C, Van Dorpe J, Geerts H, Mercken M, Bruynseels K, Lasrado R, Vandezande K, Laenen I, Boon T, Van Lint J, Vandenheede J, Moechars D, Loos R, Van Leuven F. Glycogen synthase kinase-3beta phosphorylates protein tau and rescues the axonopathy in the central nervous system of human four-repeat tau transgenic mice. J Biol Chem. 2000;275:41340–41349. doi: 10.1074/jbc.M006219200. [DOI] [PubMed] [Google Scholar]
- 56.Spittaels K, Van den Haute C, Van Dorpe J, Terwel D, Vandezande K, Lasrado R, Bruynseels K, Irizarry M, Verhoye M, Van Lint J, Vandenheede JR, Ashton D, Mercken M, Loos R, Hyman B, Van der Linden A, Geerts H, Van Leuven F. Neonatal neuronal overexpression of glycogen synthase kinase-3beta reduces brain size in transgenic mice. Neuroscience. 2002;113:797–808. doi: 10.1016/s0306-4522(02)00236-1. [DOI] [PubMed] [Google Scholar]
- 57.Cui H, Meng Y, Bulleit RF. Inhibition of glycogen synthase kinase 3beta activity regulates proliferation of cultured cerebellar granule cells. Brain Res Dev Brain Res. 1998;111:177–188. doi: 10.1016/s0165-3806(98)00136-9. [DOI] [PubMed] [Google Scholar]
- 58.Knoepfler PS, Kenney AM. Neural precursor cycling at sonic speed: N-Myc pedals, GSK-3 brakes. Cell Cycle. 2006;5:47–52. doi: 10.4161/cc.5.1.2292. [DOI] [PubMed] [Google Scholar]
- 59.Boku S, Nakagawa S, Masuda T, Nishikawa H, Kato A, Kitaichi Y, Inoue T, Koyama T. Glucocorticoids and lithium reciprocally regulate the proliferation of adult dentate gyrus-derived neural precursor cells through GSK-3beta and beta-catenin/TCF pathway. Neuropsychopharmacology. 2009;34:805–815. doi: 10.1038/npp.2008.198. [DOI] [PubMed] [Google Scholar]
- 60.Shimizu T, Kagawa T, Inoue T, Nonaka A, Takada S, Aburatani H, Taga T. Stabilized beta-catenin functions through TCF/ LEF proteins and the Notch/RBP-Jkappa complex to promote proliferation and suppress differentiation of neural precursor cells. Mol Cell Biol. 2008;28:7427–7441. doi: 10.1128/MCB.01962-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jin L, Hu X, Feng L. NT3 inhibits FGF2-induced neural progenitor cell proliferation via the PI3K/GSK3 pathway. J Neurochem. 2005;93:1251–1261. doi: 10.1111/j.1471-4159.2005.03118.x. [DOI] [PubMed] [Google Scholar]
- 62.Maurer MH, Brömme JO, Feldmann RE, Jr, Järve A, Sabouri F, Bürgers HF, Schelshorn DW, Krüger C, Schneider A, Kuschinsky W. Glycogen synthase kinase 3beta (GSK3beta) regulates differentiation and proliferation in neural stem cells from the rat subventricular zone. J Proteome Res. 2007;6:1198–1208. doi: 10.1021/pr0605825. [DOI] [PubMed] [Google Scholar]
- 63.Yeste-Velasco M, Folch J, Trullàs R, Abad MA, Enguita M, Pallàs M, Camins A. Glycogen synthase kinase-3 is involved in the regulation of the cell cycle in cerebellar granule cells. Neuropharmacology. 2007;53:295–307. doi: 10.1016/j.neuropharm.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 64.Ma C, Wang J, Gao Y, Gao TW, Chen G, Bower KA, Odetallah M, Ding M, Ke Z, Luo J. The role of glycogen synthase kinase 3beta in the transformation of epidermal cells. Cancer Res. 2007;67:7756–7764. doi: 10.1158/0008-5472.CAN-06-4665. [DOI] [PubMed] [Google Scholar]
- 65.Huang W, Chang HY, Fei T, Wu H, Chen YG. GSK3 beta mediates suppression of cyclin D2 expression by tumor suppressor PTEN. Oncogene. 2007;26:2471–2482. doi: 10.1038/sj.onc.1210033. [DOI] [PubMed] [Google Scholar]
- 66.Cheng TS, Hsiao YL, Lin CC, Yu CT, Hsu CM, Chang MS, Lee CI, Huang CY, Howng SL, Hong YR. Glycogen synthase kinase 3beta interacts with and phosphorylates the spindle-associated protein astrin. J Biol Chem. 2008;283:2454–2464. doi: 10.1074/jbc.M706794200. [DOI] [PubMed] [Google Scholar]
- 67.Ciani L, Salinas PC. c-Jun N-terminal kinase (JNK) cooperates with Gsk3beta to regulate dishevelled-mediated microtubule stability. BMC Cell Biol. 2007;8:27. doi: 10.1186/1471-2121-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barth AI, Caro-Gonzalez HY, Nelson WJ. Role of adenomatous polyposis coli (APC) and microtubules in directional cell migration and neuronal polarization. Semin Cell Dev Biol. 2008;19:245–251. doi: 10.1016/j.semcdb.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 70.Gärtner A, Huang X, Hall A. Neuronal polarity is regulated by glycogen synthase kinase-3 (GSK-3beta) independently of Akt/ PKB serine phosphorylation. J Cell Sci. 2006;119:3927–3934. doi: 10.1242/jcs.03159. [DOI] [PubMed] [Google Scholar]
- 71.González-Billault C, Del Río JA, Ureña JM, Jiménez-Mateos EM, Barallobre MJ, Pascual M, Pujadas L, Simó S, Torre AL, Gavin R, Wandosell F, Soriano E, Avila J. A role of MAP1B in Reelin-dependent neuronal migration. Cereb Cortex. 2005;15:1134–1145. doi: 10.1093/cercor/bhh213. [DOI] [PubMed] [Google Scholar]
- 72.Tong N, Sanchez JF, Maggirwar SB, Ramirez SH, Guo H, Dewhurst S, Gelbard HA. Activation of glycogen synthase kinase 3beta (GSK-3beta) by platelet activating factor mediates migration and cell death in cerebellar granule neurons. Eur J Neurosci. 2001;13:1913–1922. doi: 10.1046/j.0953-816x.2001.01572.x. [DOI] [PubMed] [Google Scholar]
- 73.Hanks SK, Ryzhova L, Shin NY, Brábek J. Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front Biosci. 2003;8:d982–d996. doi: 10.2741/1114. [DOI] [PubMed] [Google Scholar]
- 74.Bianchi M, De Lucchini S, Marin O, Turner DL, Hanks SK, Villa-Moruzzi E. Regulation of FAK Ser-722 phosphorylation and kinase activity by GSK3 and PP1 during cell spreading and migration. Biochem J. 2005;391:359–370. doi: 10.1042/BJ20050282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carter JJ, Tong M, Silbermann E, Lahousse SA, Ding FF, Longato L, Roper N, Wands JR, de la Monte SM. Ethanol impaired neuronal migration is associated with reduced aspartyl-asparaginyl-beta-hydroxylase expression. Acta Neuropathol. 2008;116:303–315. doi: 10.1007/s00401-008-0377-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuskaitis CJ, Jope RS. Glycogen synthase kinase-3 regulates microglial migration, inflammation, and inflammation-induced neurotoxicity. Cell Signal. 2009;21:264–273. doi: 10.1016/j.cellsig.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arévalo JC, Chao MV. Axonal growth: where neurotrophins meet Wnts. Curr Opin Cell Biol. 2005;17:112–115. doi: 10.1016/j.ceb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 78.Muñoz-Montaño JR, Lim F, Moreno FJ, Avila J, Díaz-Nido J. Glycogen synthase kinase-3 modulates neurite outgrowth in cultured neurons: possible implications for neurite pathology in Alzheimer’s disease. J Alzheimers Dis. 1999;1:361–378. doi: 10.3233/jad-1999-1602. [DOI] [PubMed] [Google Scholar]
- 79.Orme MH, Giannini AL, Vivanco MD, Kypta RM. Glycogen synthase kinase-3 and Axin function in a beta-catenin-independent pathway that regulates neurite outgrowth in neuroblastoma cells. Mol Cell Neurosci. 2003;24:673–686. doi: 10.1016/s1044-7431(03)00229-x. [DOI] [PubMed] [Google Scholar]
- 80.Castelo-Branco G, Rawal N, Arenas E. GSK-3beta inhibition/beta-catenin stabilization in ventral midbrain precursors increases differentiation into dopamine neurons. J Cell Sci. 2004;117:5731–5737. doi: 10.1242/jcs.01505. [DOI] [PubMed] [Google Scholar]
- 81.Dill J, Wang H, Zhou F, Li S. Inactivation of glycogen synthase kinase 3 promotes axonal growth and recovery in the CNS. J Neurosci. 2008;28:8914–8928. doi: 10.1523/JNEUROSCI.1178-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen G, Bower KA, Xu M, Ding M, Shi X, Ke ZJ, Luo J. Cyanidin-3-glucoside reverses ethanol-induced inhibition of neurite outgrowth: role of glycogen synthase kinase 3beta. Neurotox Res. 2009;15:321–31. doi: 10.1007/s12640-009-9036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Valerio A, Ghisi V, Dossena M, Tonello C, Giordano A, Frontini A, Ferrario M, Pizzi M, Spano P, Carruba MO, Nisoli E. Leptin increases axonal growth cone size in developing mouse cortical neurons by convergent signals inactivating glycogen synthase kinase-3beta. J Biol Chem. 2006;281:12950–12958. doi: 10.1074/jbc.M508691200. [DOI] [PubMed] [Google Scholar]
- 84.Takahashi M, Yasutake K, Tomizawa K. Lithium inhibits neurite growth and tau protein kinase I/glycogen synthase kinase-3beta-dependent phosphorylation of juvenile tau in cultured hippocampal neurons. J Neurochem. 1999;73:2073–2083. [PubMed] [Google Scholar]
- 85.Owen R, Gordon-Weeks PR. Inhibition of glycogen synthase kinase 3beta in sensory neurons in culture alters filopodia dynamics and microtubule distribution in growth cones. Mol Cell Neurosci. 2003;23:626–637. doi: 10.1016/s1044-7431(03)00095-2. [DOI] [PubMed] [Google Scholar]
- 86.Goold RG, Gordon-Weeks PR. Microtubule-associated protein 1B phosphorylation by glycogen synthase kinase 3beta is induced during PC12 cell differentiation. J Cell Sci. 2001;114:4273–4284. doi: 10.1242/jcs.114.23.4273. [DOI] [PubMed] [Google Scholar]
- 87.Goold RG, Gordon-Weeks PR. The MAP kinase pathway is upstream of the activation of GSK3beta that enables it to phosphorylate MAP1B and contributes to the stimulation of axon growth. Mol Cell Neurosci. 2005;28:524–534. doi: 10.1016/j.mcn.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 88.Trivedi N, Marsh P, Goold RG, Wood-Kaczmar A, Gordon-Weeks PR. Glycogen synthase kinase-3beta phosphorylation of MAP1B at Ser1260 and Thr1265 is spatially restricted to growing axons. J Cell Sci. 2005;118:993–1005. doi: 10.1242/jcs.01697. [DOI] [PubMed] [Google Scholar]
- 89.Seng S, Avraham HK, Jiang S, Venkatesh S, Avraham S. KLHL1/MRP2 mediates neurite outgrowth in a glycogen synthase kinase 3beta-dependent manner. Mol Cell Biol. 2006;26:8371–8384. doi: 10.1128/MCB.02167-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou F, Zhang L, Wang A, Song B, Gong K, Zhang L, Hu M, Zhang X, Zhao N, Gong Y. The association of GSK3 beta with E2F1 facilitates nerve growth factor-induced neural cell differentiation. J Biol Chem. 2008;283:14506–14515. doi: 10.1074/jbc.M706136200. [DOI] [PubMed] [Google Scholar]
- 91.Zhang W, Smith A, Liu JP, Cheung NS, Zhou S, Liu K, Li QT, Duan W. GSK3beta modulates PACAP-induced neuritogenesis in PC12 cells by acting downstream of Rap1 in a caveolae-dependent manner. Cell Signal. 2009;21:237–245. doi: 10.1016/j.cellsig.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 92.Gonzalez-Billault C, Avila J, Cáceres A. Evidence for the role of MAP1B in axon formation. Mol Biol Cell. 2001;12:2087–2098. doi: 10.1091/mbc.12.7.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Messing RO, Henteleff M, Park JJ. Ethanol enhances growth factor-induced neurite formation in PC12 cells. Brain Res. 1991;565:301–311. doi: 10.1016/0006-8993(91)91662-k. [DOI] [PubMed] [Google Scholar]
- 94.Zou J, Rabin RA, Pentney RJ. Ethanol enhances neurite outgrowth in primary cultures of rat cerebellar macroneurons. Brain Res Dev Brain Res. 1993;72:75–84. doi: 10.1016/0165-3806(93)90161-3. [DOI] [PubMed] [Google Scholar]
- 95.Saunders DE, Zajac CS, Wappler NL. Alcohol inhibits neurite extension and increases N-myc and c-myc proteins. Alcohol. 1995;12:475–483. doi: 10.1016/0741-8329(95)00034-o. [DOI] [PubMed] [Google Scholar]
- 96.Lindsley TA, Kerlin AM, Rising LJ. Time-lapse analysis of ethanol’s effects on axon growth in vitro. Brain Res Dev Brain Res. 2003;147:191–199. doi: 10.1016/j.devbrainres.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 97.Bingham SM, Mudd LM, Lopez TF, Montague JR. Effects of ethanol on cultured embryonic neurons from the cerebral cortex of the rat. Alcohol. 2004;32:129–135. doi: 10.1016/j.alcohol.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 98.Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol. 2006;79:173–189. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lucas JJ, Hernandez F, Gomez-Ramos P, Moran MA, Hen R, Avila J. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J. 2001;20:27–39. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem. 1998;273:19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- 101.Crowder RJ, Freeman RS. Glycogen synthase kinase-3beta activity is critical for neuronal death caused by inhibiting phosphatidylinositol 3-kinase or Akt but not for death caused by nerve growth factor withdrawal. J Biol Chem. 2000;275:34266–34271. doi: 10.1074/jbc.M006160200. [DOI] [PubMed] [Google Scholar]
- 102.Hetman M, Cavanaugh JE, Kimelman D, Xia Z. Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal. J Neurosci. 2000;20:2567–2574. doi: 10.1523/JNEUROSCI.20-07-02567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Song L, De Sarno P, Jope RS. Central role of glycogen synthase kinase-3beta in endoplasmic reticulum stress-induced caspase-3 activation. J Biol Chem. 2002;277:44701–44708. doi: 10.1074/jbc.M206047200. [DOI] [PubMed] [Google Scholar]
- 104.Bijur GN, De Sarno P, Jope RS. Glycogen synthase kinase-3beta facilitates staurosporine- and heat shock-induced apoptosis. Protection by lithium. J Biol Chem. 2000;275:7583–7590. doi: 10.1074/jbc.275.11.7583. [DOI] [PubMed] [Google Scholar]
- 105.Bhat RV, Budd SL. GSK3beta signalling: casting a wide net in Alzheimer’s disease. Neurosignals. 2002;11:251–261. doi: 10.1159/000067423. [DOI] [PubMed] [Google Scholar]
- 106.Aghdam SY, Barger SW. Glycogen synthase kinase-3 in neurodegeneration and neuroprotection: lessons from lithium. Curr Alzheimer Res. 2007;4:21–31. doi: 10.2174/156720507779939832. [DOI] [PubMed] [Google Scholar]
- 107.Takashima A, Yamaguchi H, Noguchi K, Michel G, Ishiguro K, Sato K, Hoshino T, Hoshi M, Imahori K. Amyloid beta peptide induces cytoplasmic accumulation of amyloid protein precursor via tau protein kinase I/glycogen synthase kinase-3beta in rat hippocampal neurons. Neurosci Lett. 1995;198:83–86. doi: 10.1016/0304-3940(95)11964-x. [DOI] [PubMed] [Google Scholar]
- 108.Everall IP, Bell C, Mallory M, Langford D, Adame A, Rockestein E, Masliah E. Lithium ameliorates HIV-gp120-mediated neurotoxicity. Mol Cell Neurosci. 2002;21:493–501. doi: 10.1006/mcne.2002.1196. [DOI] [PubMed] [Google Scholar]
- 109.Chen G, Bower KA, Ma C, Fang S, Thiele CJ, Luo J. Glycogen synthase kinase 3beta (GSK3beta) mediates 6-hydroxydopamine-induced neuronal death. FASEB J. 2004;18:1162–1164. doi: 10.1096/fj.04-1551fje. [DOI] [PubMed] [Google Scholar]
- 110.Koh SH, Lee YB, Kim KS, Kim HJ, Kim M, Lee YJ, Kim J, Lee KW, Kim SH. Role of GSK-3beta activity in motor neuronal cell death induced by G93A or A4V mutant hSOD1 gene. Eur J Neurosci. 2005;22:301–309. doi: 10.1111/j.1460-9568.2005.04191.x. [DOI] [PubMed] [Google Scholar]
- 111.Liu F, Gong X, Zhang G, Marquis K, Reinhart P, Andree TH. The inhibition of glycogen synthase kinase 3beta by a metabotropic glutamate receptor 5 mediated pathway confers neuroprotection to Abeta peptides. J Neurochem. 2005;95:1363–1372. doi: 10.1111/j.1471-4159.2005.03474.x. [DOI] [PubMed] [Google Scholar]
- 112.Noble W, Planel E, Zehr C, Olm V, Meyerson J, Suleman F, Gaynor K, Wang L, LaFrancois J, Feinstein B, Burns M, Krishnamurthy P, Wen Y, Bhat R, Lewis J, Dickson D, Duff K. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci U S A. 2005;102:6990–6995. doi: 10.1073/pnas.0500466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang W, Yang Y, Ying C, Li W, Ruan H, Zhu X, You Y, Han Y, Chen R, Wang Y, Li M. Inhibition of glycogen synthase kinase-3beta protects dopaminergic neurons from MPTP toxicity. Neuropharmacology. 2007;52:1678–1684. doi: 10.1016/j.neuropharm.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 114.Chen YY, Chen G, Fan Z, Luo J, Ke ZJ. GSK3beta and endoplasmic reticulum stress mediate rotenone-induced death of SK-N-MC neuroblastoma cells. Biochem Pharmacol. 2008;76:128–138. doi: 10.1016/j.bcp.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 115.Toiber D, Berson A, Greenberg D, Melamed-Book N, Diamant S, Soreq H. N-acetylcholinesterase-induced apoptosis in Alzheimer’s disease. PLoS ONE. 2008;3:e3108. doi: 10.1371/journal.pone.0003108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brewster JL, Linseman DA, Bouchard RJ, Loucks FA, Precht TA, Esch EA, Heidenreich KA. Endoplasmic reticulum stress and trophic factor withdrawal activate distinct signaling cascades that induce glycogen synthase kinase-3beta and a caspase-9-dependent apoptosis in cerebellar granule neurons. Mol Cell Neurosci. 2006;32:242–253. doi: 10.1016/j.mcn.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 117.Lee KY, Koh SH, Noh MY, Park KW, Lee YJ, Kim SH. Glycogen synthase kinase-3beta activity plays very important roles in determining the fate of oxidative stress-inflicted neuronal cells. Brain Res. 2007;1129:89–99. doi: 10.1016/j.brainres.2006.10.055. [DOI] [PubMed] [Google Scholar]
- 118.Eom TY, Roth KA, Jope RS. Neural precursor cells are protected from apoptosis induced by trophic factor withdrawal or genotoxic stress by inhibitors of glycogen synthase kinase 3. J Biol Chem. 2007;282:22856–22864. doi: 10.1074/jbc.M702973200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Takadera T, Fujibayashi M, Kaniyu H, Sakota N, Ohyashiki T. Caspase-dependent apoptosis induced by thapsigargin was prevented by glycogen synthase kinase-3 inhibitors in cultured rat cortical neurons. Neurochem Res. 2007;32:1336–1342. doi: 10.1007/s11064-007-9310-4. [DOI] [PubMed] [Google Scholar]
- 120.Maggirwar SB, Tong N, Ramirez S, Gelbard HA, Dewhurst S. HIV-1 Tat-mediated activation of glycogen synthase kinase-3beta contributes to Tat-mediated neurotoxicity. J Neurochem. 1999;73:578–586. doi: 10.1046/j.1471-4159.1999.0730578.x. [DOI] [PubMed] [Google Scholar]
- 121.Gómez-Sintes R, Hernández F, Bortolozzi A, Artigas F, Avila J, Zaratin P, Gotteland JP, Lucas JJ. Neuronal apoptosis and reversible motor deficit in dominant-negative GSK-3 conditional transgenic mice. EMBO J. 2007;26:2743–2754. doi: 10.1038/sj.emboj.7601725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.D’Mello SR, Anelli R, Calissano P. Lithium induces apoptosis in immature cerebellar granule cells but promotes survival of mature neurons. Exp Cell Res. 1994;211:332–338. doi: 10.1006/excr.1994.1095. [DOI] [PubMed] [Google Scholar]
- 123.Tsukane M, Yoshizaki C, Yamauchi T. Development and specific induction of apoptosis of cultured cell models overexpressing human tau during neural differentiation: implication in Alzheimer’s disease. Anal Biochem. 2007;360:114–122. doi: 10.1016/j.ab.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 124.Hoshi M, Takashima A, Noguchi K, Murayama M, Sato M, Kondo S, Saitoh Y, Ishiguro K, Hoshino T, Imahori K. Regulation of mitochondrial pyruvate dehydrogenase activity by tau protein kinase I/glycogen synthase kinase 3beta in brain. Proc Natl Acad Sci U S A. 1996;93:2719–2723. doi: 10.1073/pnas.93.7.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hongisto V, Smeds N, Brecht S, Herdegen T, Courtney MJ, Coffey ET. Lithium blocks the c-Jun stress response and protects neurons via its action on glycogen synthase kinase 3. Mol Cell Biol. 2003;23:6027–6036. doi: 10.1128/MCB.23.17.6027-6036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sui Z, Sniderhan LF, Fan S, Kazmierczak K, Reisinger E, Kovács AD, Potash MJ, Dewhurst S, Gelbard HA, Maggirwar SB. Human immunodeficiency virus-encoded Tat activates glycogen synthase kinase-3beta to antagonize nuclear factor-kappaB survival pathway in neurons. Eur J Neurosci. 2006;23:2623–2634. doi: 10.1111/j.1460-9568.2006.04813.x. [DOI] [PubMed] [Google Scholar]
- 127.Watcharasit P, Bijur GN, Song L, Zhu J, Chen X, Jope RS. Glycogen synthase kinase-3beta (GSK3beta) binds to and promotes the actions of p53. J Biol Chem. 2003;278:48872–48879. doi: 10.1074/jbc.M305870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mishra R, Barthwal MK, Sondarva G, Rana B, Wong L, Chatterjee M, Woodgett JR, Rana A. Glycogen synthase kinase-3beta induces neuronal cell death via direct phosphorylation of mixed lineage kinase 3. J Biol Chem. 2007;282:30393–30405. doi: 10.1074/jbc.M705895200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Benedito AB, Lehtinen M, Massol R, Lopes UG, Kirchhausen T, Rao A, Bonni A. The transcription factor NFAT3 mediates neuronal survival. J Biol Chem. 2005;280:2818–2825. doi: 10.1074/jbc.M408741200. [DOI] [PubMed] [Google Scholar]
- 130.Pap M, Cooper GM. Role of translation initiation factor 2B in control of cell survival by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3beta signaling pathway. Mol Cell Biol. 2002;22:578–586. doi: 10.1128/MCB.22.2.578-586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jope RS, Roh MS. Glycogen synthase kinase-3 (GSK3) in psychiatric diseases and therapeutic interventions. Curr Drug Targets. 2006;7:1421–1434. doi: 10.2174/1389450110607011421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Linseman DA, Butts BD, Precht TA, Phelps RA, Le SS, Laessig TA, Bouchard RJ, Florez-McClure ML, Heidenreich KA. Glycogen synthase kinase-3beta phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J Neurosci. 2004;24:9993–10002. doi: 10.1523/JNEUROSCI.2057-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res. 2007;32:577–595. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu Y, Chen G, Ma C, Bower KA, Xu M, Fan Z, Shi X, Ke ZJ, Luo J. Over-expression of GSK3 beta sensitizes neuronal cells to ethanol toxicity. J Neurosci Res. 2009 doi: 10.1002/jnr.22098. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.de la Monte SM, Wands JR. Chronic gestational exposure to ethanol impairs insulin-stimulated survival and mitochondrial function in cerebellar neurons. Cell Mol Life Sci. 2002;59:882–893. doi: 10.1007/s00018-002-8475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xu J, Yeon JE, Chang H, Tison G, Chen GJ, Wands J, de la Monte S. Ethanol impairs insulin-stimulated neuronal survival in the developing brain: role of PTEN phosphatase. J Biol Chem. 2003;278:26929–26937. doi: 10.1074/jbc.M300401200. [DOI] [PubMed] [Google Scholar]
- 137.Neznanova O, Björk K, Rimondini R, Hansson AC, Hyytiä P, Heilig M, Sommer WH. Acute ethanol challenge inhibits glycogen synthase kinase-3beta in the rat prefrontal cortex. Int J Neuropsychopharmacol. 2009;12:275–280. doi: 10.1017/S1461145708009620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhou K, Zhang L, Xi J, Tian W, Xu Z. Ethanol prevents oxidant-induced mitochondrial permeability transition pore opening in cardiac cells. Alcohol Alcohol. 2009;44:20–24. doi: 10.1093/alcalc/agn098. [DOI] [PubMed] [Google Scholar]
- 139.Jope RS. Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol Sci. 2003;24:441–443. doi: 10.1016/S0165-6147(03)00206-2. [DOI] [PubMed] [Google Scholar]
- 140.Zhong J, Yang X, Yao W, Lee W. Lithium protects ethanol-induced neuronal apoptosis. Biochem Biophys Res Commun. 2006;350:905–910. doi: 10.1016/j.bbrc.2006.09.138. [DOI] [PubMed] [Google Scholar]
- 141.Chakraborty G, Saito M, Mao RF, Wang R, Vadasz C, Saito M. Lithium blocks ethanol-induced modulation of protein kinases in the developing brain. Biochem Biophys Res Commun. 2008;367:597–602. doi: 10.1016/j.bbrc.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sasaki T, Han F, Shioda N, Moriguchi S, Kasahara J, Ishiguro K, Fukunaga K. Lithium-induced activation of Akt and CaM kinase II contributes to its neuroprotective action in a rat microsphere embolism model. Brain Res. 2006;1108:98–106. doi: 10.1016/j.brainres.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 143.Liang MH, Wendland JR, Chuang DM. Lithium inhibits Smad3/4 transactivation via increased CREB activity induced by enhanced PKA and AKT signaling. Mol Cell Neurosci. 2008;37:440–453. doi: 10.1016/j.mcn.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Takadera T, Ohyashiki T. Glycogen synthase kinase-3 inhibitors prevent caspase-dependent apoptosis induced by ethanol in cultured rat cortical neurons. Eur J Pharmacol. 2004;499:239–245. doi: 10.1016/j.ejphar.2004.07.115. [DOI] [PubMed] [Google Scholar]
- 145.Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee Cox L, Mills D, Brown MJ, Haigh D, Ward RW, Smith DG, Murray KJ, Reith AD, Holder JC. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 146.Leost M, Schultz C, Link A, Wu YZ, Biernat J, Mandelkow EM, Bibb JA, Snyder GL, Greengard P, Zaharevitz DW, Gussio R, Senderowicz AM, Sausville EA, Kunick C, Meijer L. Paullones are potent inhibitors of glycogen synthase kinase-3beta and cyclin-dependent kinase 5/p25. Eur J Biochem. 2000;267:5983–5994. doi: 10.1046/j.1432-1327.2000.01673.x. [DOI] [PubMed] [Google Scholar]
- 147.Martinez A, Alonso M, Castro A, Pérez C, Moreno FJ. First non-ATP competitive glycogen synthase kinase 3beta (GSK-3beta) inhibitors: thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer’s disease. J Med Chem. 2002;45:1292–1299. doi: 10.1021/jm011020u. [DOI] [PubMed] [Google Scholar]
- 148.Chen G, Ma C, Bower KA, Shi X, Ke Z, Luo J. Ethanol promotes endoplasmic reticulum stress-induced neuronal death: involvement of oxidative stress. J Neurosci Res. 2008;86:937–946. doi: 10.1002/jnr.21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liangpunsakul S, Wou SE, Zeng Y, Ross RA, Jayaram HN, Crabb DW. Effect of ethanol on hydrogen peroxide-induced AMPK phosphorylation. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1173–G1181. doi: 10.1152/ajpgi.90349.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dolcet X, Egea J, Soler RM, Martin-Zanca D, Comella JX. Activation of phosphatidylinositol 3-kinase, but not extracellular-regulated kinases, is necessary to mediate brain-derived neurotrophic factor-induced motoneuron survival. J Neurochem. 1999;73:521–531. doi: 10.1046/j.1471-4159.1999.0730521.x. [DOI] [PubMed] [Google Scholar]
- 152.Duarte AI, Santos P, Oliveira CR, Santos MS, Rego AC. Insulin neuroprotection against oxidative stress is mediated by Akt and GSK-3beta signaling pathways and changes in protein expression. Biochim Biophys Acta. 2008;1783:994–1002. doi: 10.1016/j.bbamcr.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 153.Li Z, Ding M, Thiele CJ, Luo J. Ethanol inhibits brain-derived neurotrophic factor-mediated intracellular signaling and activator protein-1 activation in cerebellar granule neurons. Neuroscience. 2004;126:149–162. doi: 10.1016/j.neuroscience.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 154.Kalluri HS, Ticku MK. Regulation of ERK phosphorylation by ethanol in fetal cortical neurons. Neurochem Res. 2003;28:765–769. doi: 10.1023/a:1022822119560. [DOI] [PubMed] [Google Scholar]
- 155.Asyyed A, Storm D, Diamond I. Ethanol activates cAMP response element-mediated gene expression in select regions of the mouse brain. Brain Res. 2006;1106:63–71. doi: 10.1016/j.brainres.2006.05.107. [DOI] [PubMed] [Google Scholar]
- 156.Wilkie MB, Besheer J, Kelley SP, Kumar S, O’Buckley TK, Morrow AL, Hodge CW. Acute ethanol administration rapidly increases phosphorylation of conventional protein kinase C in specific mammalian brain regions in vivo. Alcohol Clin Exp Res. 2007;31:1259–1267. doi: 10.1111/j.1530-0277.2007.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pandey SC. The gene transcription factor cyclic AMP-responsive element binding protein: role in positive and negative affective states of alcohol addiction. Pharmacol Ther. 2004;104:47–58. doi: 10.1016/j.pharmthera.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 158.Li J, Li YH, Yuan XR. Changes of phosphorylation of cAMP response element binding protein in rat nucleus accumbens after chronic ethanol intake: naloxone reversal. Acta Pharmacol Sin. 2003;24:930–936. [PubMed] [Google Scholar]
- 159.Pandey SC, Zhang H, Ugale R, Prakash A, Xu T, Misra K. Effector immediate-early gene arc in the amygdala plays a critical role in alcoholism. J Neurosci. 2008;28:2589–2600. doi: 10.1523/JNEUROSCI.4752-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang X, Horn K, Wand GS. Chronic ethanol exposure impairs phosphorylation of CREB and CRE-binding activity in rat striatum. Alcohol Clin Exp Res. 1998a;22:382–390. [PubMed] [Google Scholar]
- 161.Yang X, Horn K, Baraban JM, Wand GS. Chronic ethanol administration decreases phosphorylation of cyclic AMP response element-binding protein in granule cells of rat cerebellum. J Neurochem. 1998b;70:224–232. doi: 10.1046/j.1471-4159.1998.70010224.x. [DOI] [PubMed] [Google Scholar]
- 162.Woodgett JR. Physiological roles of glycogen synthase kinase-3: potential as a therapeutic target for diabetes and other disorders. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3:281–290. doi: 10.2174/1568008033340153. [DOI] [PMC free article] [PubMed] [Google Scholar]