Summary
T cells recognizing self or microbial antigens may trigger or reactivate immune-mediated diseases. Monitoring the frequency of specific T cell clonotypes to assess a possible link with the course of disease has been a difficult task with currently available technology. Our goal was to track individual candidate pathogenic T cell clones, selected on the basis of previous extensive studies from patients with immune-mediated disorders of the CNS, including multiple sclerosis, HTLV-I associated myelopathy/tropical spastic paraparesis (HAM/ TSP) and chronic Lyme neuroborreliosis. We developed and applied a highly specific and sensitive technique to track single CD4+ and CD8+ T cell clones through the detection and quantification of T cell receptor (TCR) α or β chain complementarity-determining region 3 transcripts by real-time reverse transcriptase (RT)-PCR. We examined the frequency of the candidate pathogenic T cell clones in the peripheral blood and CSF during the course of neurological disease. Using this approach, we detected variations of clonal frequencies that appeared to be related to clinical course, significant enrichment in the CSF, or both. By integrating clono-type tracking with direct visualization of antigen-specific staining, we showed that a single T cell clone contributed substantially to the overall recognition of the viral peptide/MHC complex in a patient with HAM/ TSP. T cell clonotype tracking is a powerful new technology enabling further elucidation of the dynamics of expansion of autoreactive or pathogen-specific T cells that mediate pathological or protective immune responses in neurological disorders.
Keywords: autoimmunity, HAM/TSP, Lyme disease, multiple sclerosis, T cell receptors, immunotherapy
Introduction
The activation and expansion of antigen-specific T cells are considered crucial events for the initiation of organ-specific inflammatory disorders. A possible role of pathogenic T cell responses has been extensively investigated in inflammatory diseases of the CNS such as multiple sclerosis, HTLV-I associated myelopathy/tropical spastic paraparesis (HAM/ TSP) and chronic Lyme neuroborreliosis. Multiple sclerosis, the most frequent demyelinating disease of the CNS, is characterized by focal areas of inflammation and loss of myelin in the white matter of the brain and spinal cord, together with variable extent of axonal damage, atrophy and gliosis. The most common clinical course, particularly early after onset, is relapsing-remitting (RRMS). While the causative agent of multiple sclerosis is unknown, myelin antigen-specific CD4+ T cells are thought to play a role in the inflammatory relapses that lead to myelin destruction (Martin and McFarland, 1995; Steinman, 1996; Hohlfeld and Wekerle, 2001). Myelin basic protein (MBP) has been implicated as one of the antigens recognized by pathogenic T cells (Hafler and Weiner, 1995; Bielekova et al., 2000). HAM/TSP is a slowly progressive neurological syndrome characterized by paraparesis, sensory and sphincter dysfunction. The main histopathological abnormalities are thoracic spinal cord atrophy with perivascular demyelination and axonal degeneration. In HAM/TSP, the high frequency of HTLV-I Tax-specific cytotoxic T lymphocytes (CTL) in the patients’ peripheral blood and CSF (Jacobson et al., 1990; Elovaara et al., 1993; Greten et al., 1998; Bieganowska et al., 1999), their oligoclonal expansion (Utz et al., 1996) and their proinflammatory phenotype (Biddison et al., 1997; Kubota et al., 1998; Bieganowska et al., 1999) have suggested a retrovirus-induced T-cell mediated pathogenesis. Chronic Lyme neuroborreliosis or post-treatment Lyme disease syndrome probably represents an immune-mediated organ manifestation that is triggered by Borrelia burgdorferi in genetically susceptible individuals. Our group has recently examined the antigenic recognition of CSF-derived T cells that responded to B. burgdorferi proteins and to self-antigens in a patient with chronic Lyme neuroborreliosis (Hemmer et al., 1999). These findings suggested that molecular mimicry (Wucherpfennig and Strominger, 1995) may be one of the mechanisms leading to autoimmunity in patients suffering from this disorder.
Similar to other organ-specific immune-mediated disorders, in these neurological diseases current data suggest a pathogenic role of antigen-specific T cells in the inflammatory process. However, mainly due to technical difficulties, important questions remained elusive so far. In particular, it has been problematical to follow the frequency of candidate pathogenic T cell clones over time, particularly in target organ compartments, and to assess a potential relationship of an individual clone with the course of disease. Here, we hypothesized that the different clinical, pathological and immunological features of these neurological disorders may be associated with different profiles of clonal dynamics or distribution. The alternating phases of RRMS were an appropriate context to assess a possible relationship between longitudinal variation of clonal frequency and markers of disease activity, such as clinical exacerbations or the detection of active lesions by MRI. In contrast, HAM-TSP may be considered a prototypic example of a chronic-progressive immunemediated CNS disorder initiated and sustained by a persistent viral infection. Chronic Lyme neuroborreliosis may be seen as a paradigm for a chronic-relapsing disorder in which immune-mediated inflammatory damage can continue even after the causal infectious agent has been cleared from the body. To address these questions, we have developed and applied a novel technology termed T cell clonotype tracking (TCCT) to follow single CD4+ and CD8+ T cell clones in a highly specific and sensitive manner.
In principle, we quantified TCR transcripts that originate from a single T cell clone by real-time reverse transcriptase (RT)-PCR using oligonucleotide primers that flank the clones’ TCR complementarity-determining region 3 (CDR3). Using this approach we tracked T cell clones for which the results of previous extensive studies suggested a pathogenic involvement, based on their recognition of immunodominant epitopes of disease-related antigens, their clonal expansion, and their phenotypic and functional characteristics. In a patient with multiple sclerosis we tracked T cell clone P2–10, a CD4+ T helper 1 (Thl) clone that recognized the central immunodominant epitope of MBP, MBP83–99 (Bielekova et al., 2000). In a patient with HAM/ TSP we tracked A6, a cytotoxic CD8+ T cell clone which recognized the immunodominant HLA-A2-restricted HTLV-I Tax epitope Tax11–19 (Utz et al., 1996). In a patient with chronic Lyme neuroborreliosis we tracked CSF3, a CD4+ Thl clone that recognized multiple pathogen-derived and self-antigen epitopes (Hemmer et al., 1999). As control we used a MBP111–129-specific, superantigen-responsive T cell clone (HD4-1C2) that was found clonally expanded in a healthy subject, and has also been studied comprehensively (Muraro et al., 1997). We determined the frequencies of the selected T cell clones in the peripheral blood compartment and in available CSF cell specimens and examined their relationship with clinical aspects of disease. The results illustrate the power of our new methodology and reveal important features of the kinetics of frequency and of the distribution of the candidate pathogenic T cell clones during the course of neurological disease.
Material and methods
Case reports
Patient MS502 is a 31-year-old Caucasian male diagnosed with clinically definite RRMS according to Poser’s criteria (Poser et al., 1983) 10 years ago. He has active disease by both clinical and MRI criteria, with predominance of corticospinal motor and brainstem dysfunction. His treatment history includes 15 months of cyclosporin (1991–1992; good response on MRI inflammatory activity), 18 months of interferon β-lb (1994–1995; very good clinical and MRI response, discontinued because of local reactions and depression), 5 weeks of altered peptide ligand (APL) CGP77116 based onMBP83–99 sequence (1998; discontinued because of treatment-related atypical multiple sclerosis exacerbation) (Bielekova et al., 2000) and since 1999 treatment with interferon β-la, resulting in good clinical and MRI response with current expanded disability status scale (EDSS; Kurtzke, 1983) score 1.5.
Patient HAM3 is a 69-year-old Caucasian male diagnosed with HAM-TSP 12 years ago, based on clinical presentation, MRI findings and HTLV-I positive serology. However, the characteristic clinical symptoms (persistent back pain, leg weakness, urinary problems) started 33 years ago. His treatment history consisted of Anti-TAC (Zenapax, humanized mAb against CD25) for 4 months in 1995, without significant clinical response. His clinical course showed continuous slow progression of disability, with a current EDSS 6.5.
Patient NB1 is a 34-year-old Caucasian male who presented in 1993 with meningoencephalitis and cerebral vasculitis. He was diagnosed with Lyme borreliosis based on the positive serological results, according to Centers for Disease Control criteria and high anti-borrelial intrathecal antibody levels (CSF/serum index: 2.05). His neurological symptoms resolved upon antibiotic therapy (ceftriaxone 2 g intravenously per day for 4 weeks), but recurred multiple times in the subsequent 7 years. He has persistent high titres of B. burgdorferi-specific antibodies in serum and CSF and positive intrathecal antibody index. CSF was repeatedly negative for B. burgdorferi by both PCR and culture of the organism.
Healthy blood donor HD4, a 35-year-old Afro-American male, was enrolled at the NIH Blood Bank.
All patients were followed under research protocols reviewed and approved by NIH Institutional Review Boards and written informed consent was obtained from all subjects in conformity to the Declaration of Helsinki.
T cell clones
The MBP83–99-specific CD4+ T cell clone P2–10 was isolated from peptide-stimulated peripheral blood mononuclear cells (PBMC) from a patient with multiple sclerosis (MS502) after a screening of peripheral blood T cell responses employing a panel of 15 myelin protein-derived immunodominant peptides in an interleukin (IL)-7-modified proliferation assay, as described previously (Bielekova et al., 1999, 2000). The MBP111–129-specific CD4+ T cell clone 1C2 was established from MBP-stimulated peripheral blood mononuclear cells (PBMC) from a healthy donor (HD4) by split-well cloning as described previously (Muraro et al., 1997). Clone 1C2 responds to staphylococcal enterotoxin A (SEA) at low nanomolar concentrations of the superantigen (P. A. Muraro, unpublished data). The HTLV-I Tax11–19-specific CD8+ T cell clone A6 was generated from CD8+ cells from a patient with HAM/TSP (HAM3) stimulated with peptide-pulsed PBMC as described previously (Utz et al., 1996). The B. burgdorferi-specific CD4+ T cell clone CSF3 was cloned by limiting dilution from CSF cells of patient NB1 stimulated with B. burgdorferi lysate as described previously (Hemmer et al., 1999). The phenotypic characteristics of T cell clones are summarized in Table 1. Expansion of the selected T cell clones in the peripheral blood or in the CSF of individual patients has been shown by limiting dilution analysis followed by TCR sequencing or single-strand conformational polymorphysm (Utz et al., 1996; Muraro et al., 1997; Hemmer et al., 1999; Bielekova et al., 2000).
Table 1.
T cell clones
| T cell clone | Patient | Source | Specificity | HLA restriction | Phenotype | TCR α chain | TCR β chain |
|---|---|---|---|---|---|---|---|
| P2–10 | MS502 | PBMC | MBP83–99 | DRB1*0404 | CD4+ Thl | ND | BV3S1J1S4 |
| 1C2 | HD4* | PBMC | MBP111–129 | DRB1*0401 | CD4+ Thl† | AV4S1AJ50 | BV7S2BJ2S2 |
| A6 | HAM3 | CD8 T cells | HTLV-I Tax11–19 | A2 | CD8+ CTL | AV2S3AJ24 | BVA2S3DB2S1BJ2S7 |
| CSF3 | NB1 | CSF | B. burgdorferi | DRB1*1501 | CD4+ Thl | AV13S2AJ29 | BV14BJ2S3 |
Healthy donor.
Secretes substantial amounts of IL-10 (> ng/ml).
TCR analysis and sequencing
An analysis of T cell clones TCR V gene usage was performed by PCR using a panel of 21 TCRAV or 22 TCRB V family-specific oligonucleotide primers (Utz et al., 1996). Nucleotide sequencing of TCR rearrangements was performed by either manual or automated sequencing as described previously (Muraro et al., 1997, 2000). Expression of functional TCR rearrangements on the cell surface was confirmed by FACS staining employing a panel of anti-TCRBV monoclonal antibodies as described previously (Muraro et al., 2000). TCR designations are given according to the WHO guidelines (WHO IUIS Nomenclature Sub-Committee on TCR Designation, 1995) following Arden’s nomenclature (Arden et al., 1995).
Molecular detection and quantification of T cell clonal frequency
Total RNA was isolated from clonal cells, PBMC and CSF cells using TriZol reagent (Gibco-BRL, Gaithersburg, MD, USA). RNA was reverse transcribed into cDNA using random hexamer primers and TaqMan Reverse Transcription Reagents (PE Biosystems, Foster City, CA, USA). Oligonucleotide primers and probes were designed using Primer Express software (PE Biosystems) according to criteria that aimed to obtain amplification of clonotypic sequences with maximum specificity. The most efficient primers ranged from 18 to 24 nucleotides. Probes were chosen to be 22–29 nucleotides long to achieve maximum specificity. Resulting amplicons ranged in size from 75 to 100 bp.
We excluded significant homologies between CDR3 rearrangements and genomic sequences by running extensive searches on the GenBank database. The annealing sites of selected clonotype-specific primer/probe combinations are shown in Fig. 1. In preliminary experiments we ruled out non-target amplification from control genomic DNA samples and confirmed the specific amplification of target TCR rearrangements by DNA electrophoresis and direct automated sequencing of PCR amplicons. For all experiments, real-time quantitative RT-PCR was performed on an ABI Prism 7700 Sequence Detection System (PE Biosystems) using the TaqMan Gold RT-PCR Kit (PE Biosystems) according to the manufacturer’s recommendations. Briefly, this method monitors the degradation of a dual-labelled fluorescent probe (TaqMan probe) in real time concomitant with PCR amplification (Heid et al., 1996). Input target RNA levels are correlated with the time (measured in PCR cycles) at which the reporter fluorescent emission increases beyond a threshold level. PCR conditions were: one cycle at 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s and annealing/extension at 60°C for 1 min. Oligonucleotides (PE Biosystems) were used at final concentrations of 200 nM for forward and reverse primer and 100 nM for the fluorogenic probe. We first assessed the specificity of each PCR primer/probe combination against cDNA from the target T cell clone and controls including 12 non-target clones and PBMC from six unrelated subjects. Selected primer/probe sets led to efficient amplification of target cDNA equivalent to 5 × 103 cells/reaction, with mean threshold cycle (CT) values ranging from 7 to 21 cycles (Table 2). There was some non-specific amplification of non-target cDNA from some allogeneic PBMC samples (equivalent to 105 cells/reaction) only above 31–35 PCR cycles. Thus, a difference of mean CT number from amplification of non-target and target T cell (ACT) of a minimum of 10 PCR cycles (corresponding to a >1000-fold difference in amplification product) provided a robust margin of security for a selective detection of target sequences even in presence of a large excess of non-target cells. To assess the sensitivity of TCCT, we ‘spiked’ different numbers of clonal T cells into constant numbers of allogeneic PBMC. T cell clones employed in spiking experiments were in a resting state (>12 days after last restimulation) to minimize the potential increase of number of TCR transcripts per individual T cell induced by recent in vitro activation (Kurokawa et al., 1999; Nixon et al., 1999). Compared with clonotype tracking employing conventional quantitative end-point PCR (Fig. 2A), TaqMan PCR was on average 10-fold more sensitive, resulting in a lower limit of detection of 1 target cell/106 control cells for clone 1C2 (Fig. 2B), 1 cell/106 for T cell clone P2–10,1 cell/5 × 104 for T cell clone A6 and 1 cell/ 5 × 106 for T cell clone CSF3 (Fig. 2C). Relative quantification of gene expression was calculated using the ΔΔCT method (Weiner et al., 1999). Amplification of GAPDH or 18S rRNA (for in vitro stimulated cell cultures) was used for sample normalization as described previously (Wandinger et al., 2001). To determine the absolute frequencies of target cells, 10°-106 clonal cells were spiked at 10-fold increment into either 106 or 5 × 106 allogeneic PBMC. The normalized PCR amplification signals derived from the cDNAs served as calibrators. Then, each of the normalized target values for the experimental samples was divided by the normalized value of the calibrator. All reactions were run in duplicate and results were always confirmed by at least one additional independent run.
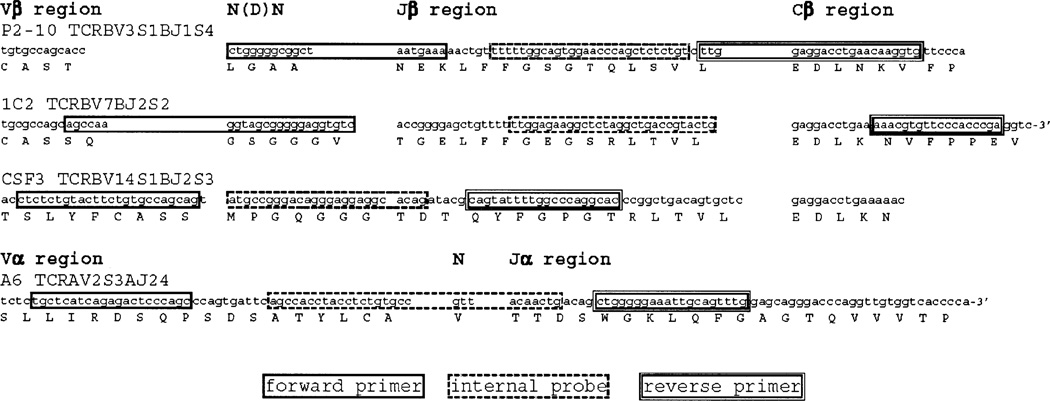
Fig. 1.
Annealing sites of clonotype-specific oligonucleotide primers and probes. The nucleotide and deduced amino acid sequences of TCR β (T cell clones, P2–10, 1C2, CSF3) or α chain (T cell clone A6) CDR3 are shown. The annealing sites of oligonucleotide primers and probes for TaqMan quantification of clonotypic TCR transcripts from each T cell clone are indicated by boxes (single line, forward primer; dashed line, internal probe; double line, reverse primer).
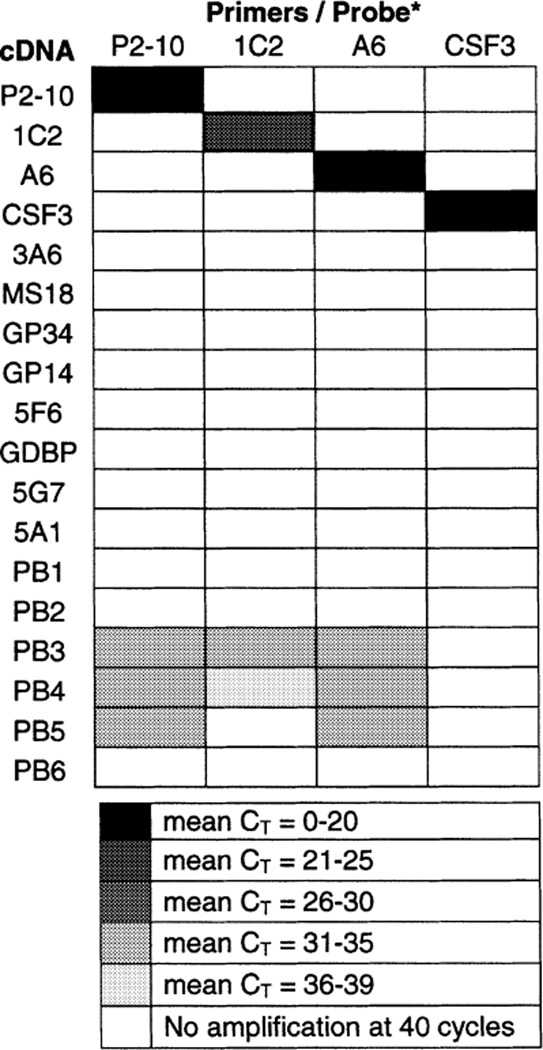
Table 2.
Specificity of primers/probe sets
To test the specificity of each clonotypic primer/probe combination (columns) we amplified cDNA (rows) from target T cell clones (P2–10, 1C2, A6, CSF3; 5 × 103 cells/reaction) and from controls that included additional non-target MBP-specific T cell clones (3A6, MS18, GP34, 5F6, GDBP, 5G7, 5A1) and allogeneic PBMC (PB1-PB6). Input cDNA was equivalent to 5 × 103 cells/reaction for target clones and 105 cells/reaction for non-target controls.
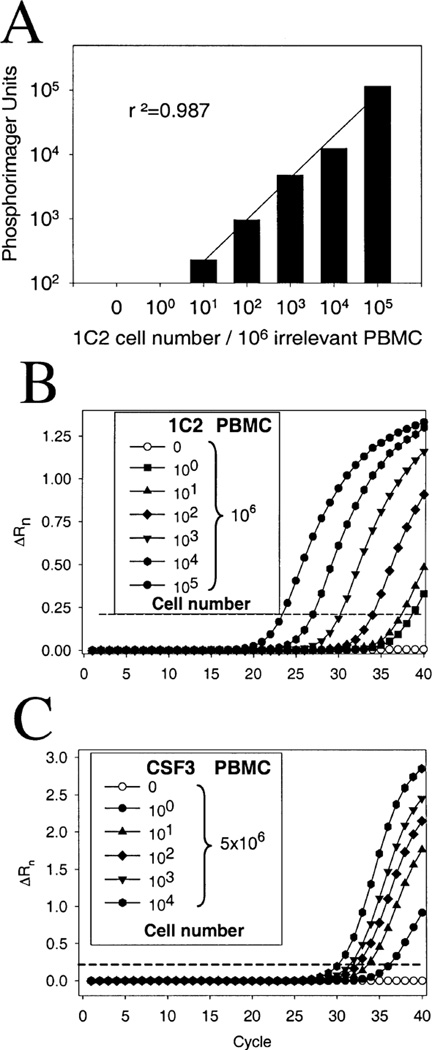
Fig. 2.
High sensitivity of TCCT. ‘Spiking’ experiments assessed the sensitivity of TCCT for the detection of clonal cells at low frequency. (A) Conventional PCR and quantification of clonotypic TCR transcripts of T cell clone 1C2 using P33-radiolabelled dCTP allowed the detection of 10 target cells/106 irrelevant cells. (B) As compared with conventional PCR, TCCT by TaqMan real-time quantitative PCR yielded a 10-fold increase in sensitivity, resulting in positive amplification of a 1 : 20 dilution of cDNA derived from of a mixture of 1 clonal cell/106 control cells. (C) The outstanding efficiency of amplification of CSF3 TCR transcripts allowed detection of 1 clonal cell in 5 × 106 control cells. High specificity of amplification was shown by the absence of any amplification of control cDNAs after up to 40 PCR cycles (Table 2).
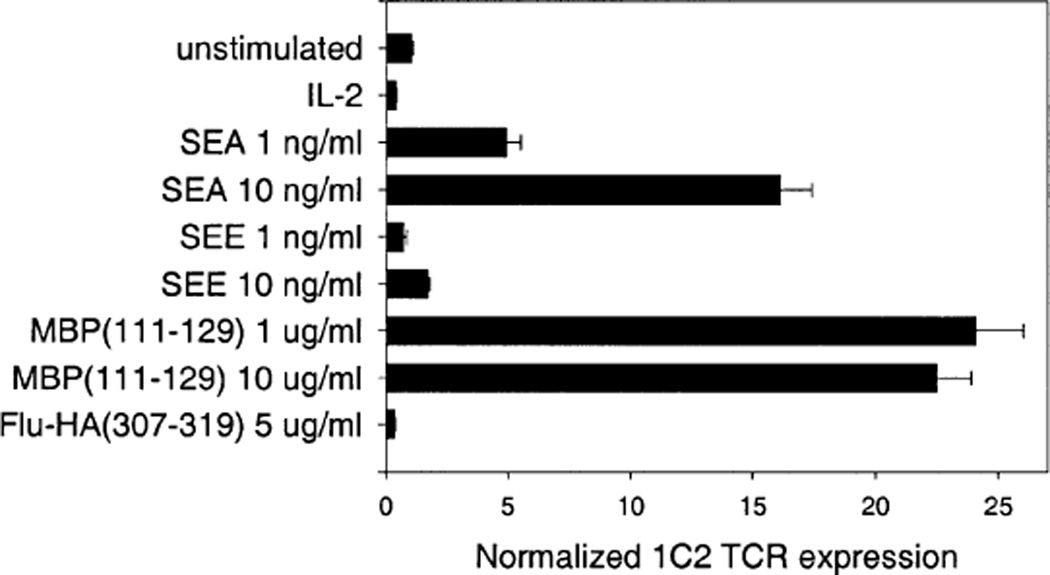
We next attempted to detect the expansion of clonal cells after in vitro stimulation of the ‘parent’ PBMC from which a clone had originally been established. For this purpose, we tracked the frequency of the MBP111–129-specific, SEA-responsive T cell clone 1C2 upon primary stimulation of the unmanipulated parent PBMC from donor HD4 with antigens and superantigens (Fig. 3). After 72 h incubation at 37° C, an expansion of the T cell clone was detected in cDNA from the PBMC cultures stimulated with MBP111–129 and SEA (Toxin Technologies, Sarasota, FL, USA), but not from those stimulated with the control antigen Flu-HA307–319, the control superantigen staphylococcal enterotoxin E (SEE) or IL-2 alone. The reliable detection of specifically activated T cells emerging from a bulk PBMC population after in vitro stimulation confirmed the potential of our approach for tracking T cell expansions ex vivo.
Fig. 3.
Detection of in vitro induced clonal expansion. Selective expansion of clone 1C2 in the unmanipulated ‘parent’ PBMC from donor HD4 was detected by TCCT as a result of stimulation with the specific antigen MBP111–129 or with the superantigen SEA, which were known from previous experiments to stimulate proliferation of the isolated clone. There was no increase of 1C2 TCR transcripts upon PBMC stimulation with control stimuli FIU-HA307-319 and SEE. Error bars indicate standard deviation.
FACS staining of antigen-specific cells with Tax11–19-A2/Ig
To enumerate Tax11–19-specific, A2-restricted T cells, we used a soluble Tax11–19/HLA-A2/Ig fusion protein for FACS staining as described previously (Greten et al., 1998). To check the specificity of Tax11–19-A2/Ig recognition, we used as control the same A2/Ig construct loaded with an HIV peptide (HIV gag77–85).
Results
Tracking of P2–10, a CD4+ T cell clone specific for the immunodominant peptide MBP83–99, in a patient with multiple sclerosis
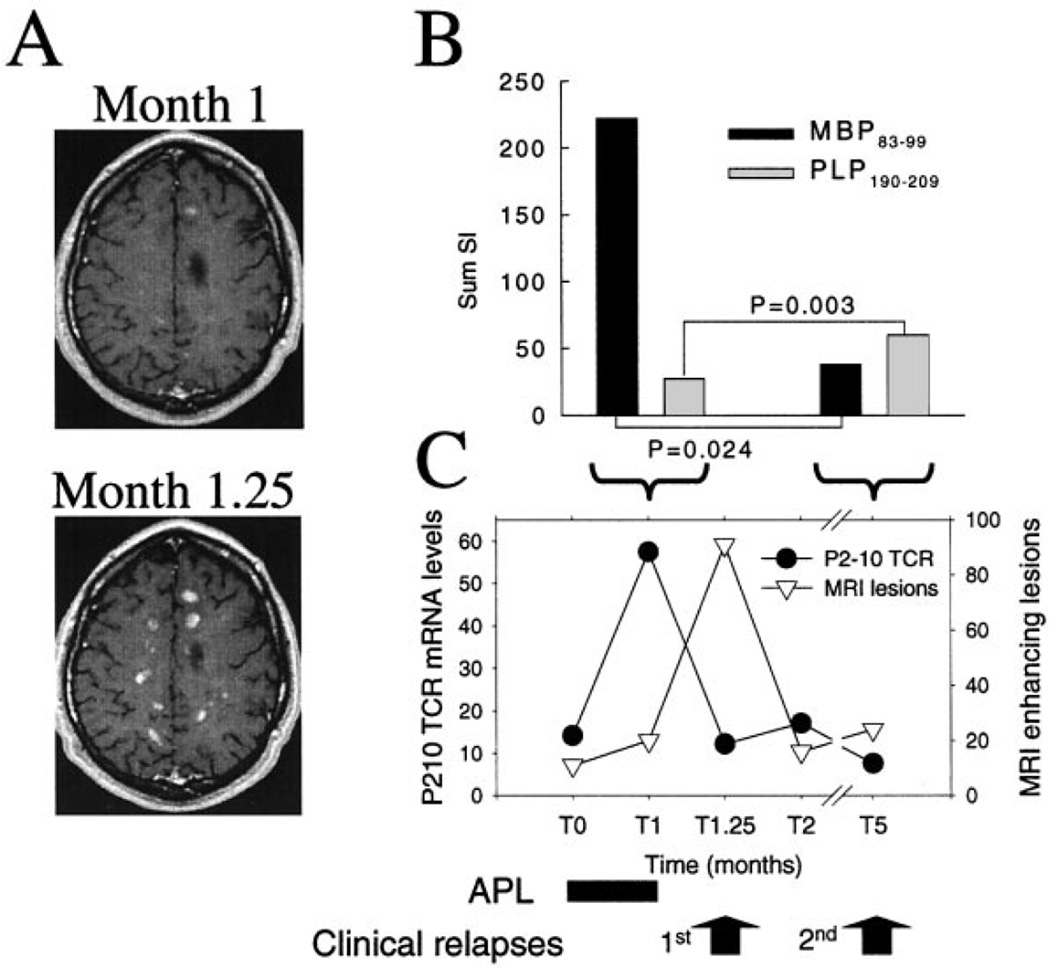
A clinical trial with an altered peptide ligand (APL) based on the immunodominant MBP83–99 peptide as a novel treatment for multiple sclerosis (Bielekova et al., 2000) gave us the opportunity to track the frequency of a potentially pathogenic T cell clone in multiple sclerosis. Five weeks after the start of APL treatment, patient MS502 presented with symptoms of a severe clinical relapse. MRI of the brain showed an impressive bout of 91 contrast-enhancing lesions (Fig. 4A, month 1.25). APL treatment was halted and intravenous steroid treatment was given, resulting in clinical and MRI remission. The patient suffered a second, milder clinical relapse 5 months after the start of APL treatment. The peripheral blood T cell response to myelin antigens was examined at two points in time by proliferation assay using a panel of 15 myelin protein-derived immunodominant peptides. One week before the onset of the first exacerbation, MBP83–99-specific T cells were found expanded in the peripheral blood (Fig. 4B). While reactivity to MBP83–99 as assessed by primary proliferation was strikingly predominant in the first relapse, the response to this epitope was markedly reduced at the time of the second relapse. In contrast, proteolipid protein (PLP)190–209-specific T cell responses increased at the time of the second relapse. The MBP83–99-specific T cell clone P2–10, isolated at the time preceding the first relapse, responded to extremely low concentrations (0.1 pg/ml) of native antigen, cross-reacted with the APL only at high doses and had a Thl phenotype (Bielekova et al., 2000; Table 1 and data not shown). These functional characteristics strongly suggested a pathogenic relevance of MBP83–99-specific T cell clone P2–10. We next assessed the possible correlation of its frequency with the course of disease. Employing TCCT, we found a >5-fold increase in the frequency of P2–10, 1 week prior to the first clinical exacerbation and the observed flare of MRI disease activity (Fig. 4C). After clinical remission, the frequency of the clone decreased to baseline levels. T cell clone P2–10 was not found expanded during the second exacerbation, which was associated with an increased cellular immune reactivity to PLP and not to MBP. The absolute frequency of P2–10 in PBMC at 1 week before the first exacerbation during the clinical trial was 5.5 cells/104 (or, 1 cell in 1818). Of note, we also detected the clone in the patient’s peripheral blood over 6 years before the APL trial, at a frequency of 2.4 P2–10 cells/ 104 (or, 1 cell in 4167) at the time of an exacerbation, a value above those observed during disease remission (1.3–1.6 P2–10 cells/104). As control, we monitored in healthy donor HD4 the circulating frequency of the MBP111–129-specific control T cell clone 1C2. Unlike the MBP-specific clone in the multiple sclerosis patient, clonal frequency of 1C2 in the peripheral blood of donor HD4 remained very stable with only minor fluctuations during a period of >4 years (data not shown). The large variation of clonal frequency in the patient with RRMS and the stability in the healthy donor appear to recapitulate at the level of single T cell clones the results of a serial study of whole peripheral blood TCR BV gene expression, which showed that skewed TCR repertoires associated to oligoclonal T cell expansions are frequently found in multiple sclerosis patients and not in healthy controls (Muraro et al., 2002).
Fig. 4.
Expansion of MBP83–99-specific T cell clone P2-10 before a multiple sclerosis exacerbation. (A) Representative post-contrast T1-weighted brain MRI scans of patient MS502 at month 1 and at month 1.25 (exacerbation) of follow-up. (B) Cellular immune reactivity of PBMC from patient MS502 in response to the prevailing epitopes (MBP83–99, black fills; and PLP190–209, grey fills) being recognized at two different time-points (T1, left pair; T5, right pair), expressed as sum of stimulation indexes (SI) of all proliferating (SI > 2) wells in an IL-7-modified proliferation assay. P values were calculated using the Mann-Whitney rank sum test. A significant expansion of MBP83–99-specific T cells was observed at month 1 of treatment with APL. In contrast, at month 5 the response to PLP was increased. The lower variance of SI for PLP190–209 accounts for the higher significance. (C) Molecular quantification of P2–10 clonal frequency in PBMC (circles) over time (expressed in months; x-axis) is shown along with disease activity assessed by MRI (triangles). SD of P210 mRNA levels were: 1.5 (TO), 2.5 (Tl), 1.2 (T1.25), 1.4 (T2) and 0.7 (T5). A 5-fold increase of P2–10 frequency (filled circles) was observed 1 week (Tl) before the onset of a clinical exacerbation associated with the appearance of a large number of new gadolinium-enhancing lesions at MRI of the brain (open triangles). No increase of clone P2–10 was found at the time of a second relapse (T5).
Tracking of A6, a T cell clone specific for the immunodominant peptide HTLV-I Tax11–19, in a patient with HAM/TSP
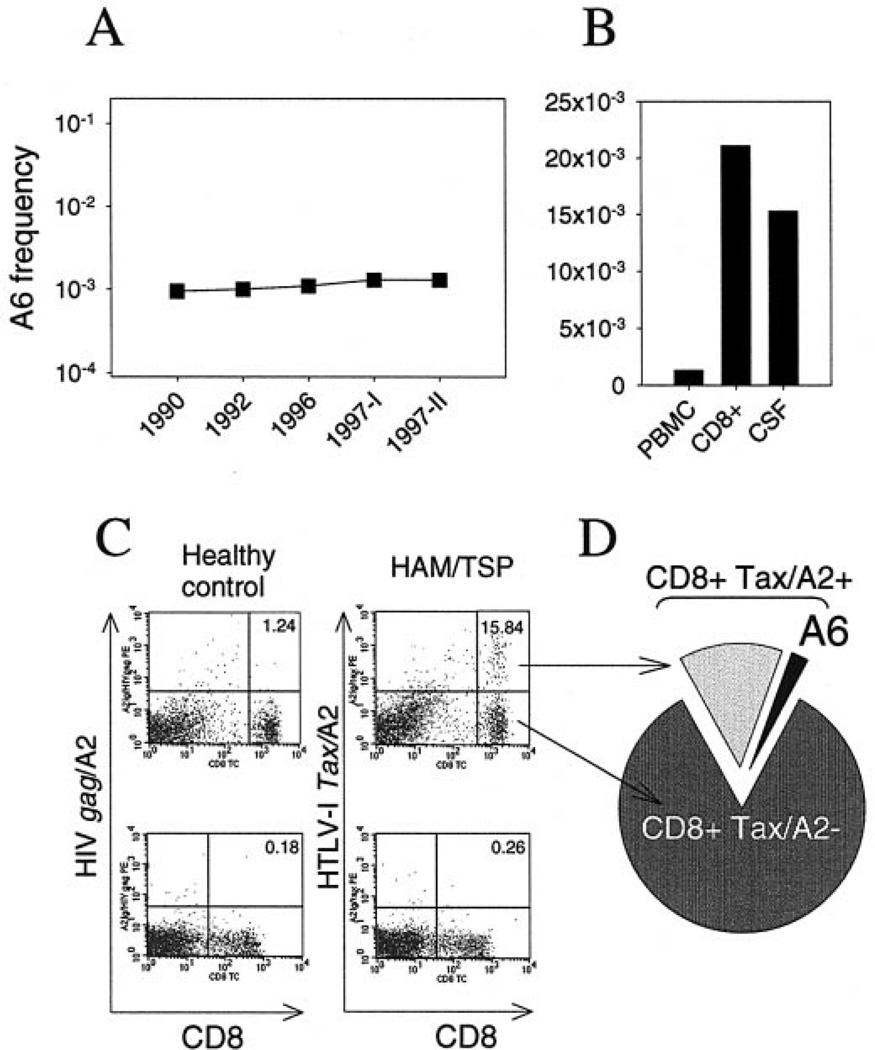
We determined the absolute frequency of the HTLV-I Tax11–19-specific CD8+ T cell clone A6 in PBMC, CD8+ subsets and CSF from patient HAM3. The frequency of the clone in PBMC, measured at five time points spanning a period of 7 years, was high and remarkably stable, ranging from 0.95 to 1.3 A6 cells/103 PBMC (mean 1.17/103, or 1 out of 850 PBMC; Fig. 5A). As expected, the clone was enriched in the CD8+ subset, with a frequency of 23.9/103 at a first time point (1996) and 21.1/103 at a second time point (1997-1), representing on average 22.5/103, or 1 out of 44 CD8+ cells (Fig. 5B). The frequency of A6 in the CSF was 15.3/103 cells (1 out of 65 cells), a striking ~12-fold enrichment with respect to the corresponding peripheral blood (1.3 A6 cells/103 PBMC; Fig. 5B). We next examined the relationship of A6 frequency with the overall recognition of the Tax epitope/A2 complex in the peripheral blood. The recognition of Tax11–19/ A2 Ig at three time-points was also stable, resulting in a staining of 15.02–16.45% of CD8+ T cells in PBMC (mean 15.85%, or 1 out of 6.3 CD8+ cells; Fig. 5C). By combining FACS staining of HTLV-I Tax/A2-specific CD8+ cells and molecular clonotype tracking, we calculated that clone A6 alone represents 14.2%, or one out of seven, of all Tax11–19- specific, HLA-A2-restricted CD8+ T cells in the peripheral blood (Fig. 5D).
Fig. 5.
Persistent expansion of T cell clone A6 and relationship with CD8+ T cell recognition of HTLV-I Tax11–19/A2 complex in a patient with HAM/TSP. (A) Stable frequency of A6 in the peripheral blood over several years of chronic progression of HAM/TSP. (B) Increased frequency of A6 in sorted CD8+ cells and in unmanipulated CSF cells (12-fold). (C) Staining of PBMC from a healthy control (left) and patient HAM3 (right) with A2 Ig loaded with HTLV-I Tax11–19 (top) or with a control peptide, HIV gag77–85 (bottom). Percentages in upper right quadrants indicate the proportion of Tax/A2 Ig positive CD8+ cells in the total CD8+ cells. (D) By staining with Tax/A2 Ig and TCCT, we determined that clone A6 alone represents 14.2% (or, one of seven) of all Tax11–19-specific, A2-restricted CD8+ T cells in the peripheral blood. This figure can be viewed in colour as Supplementary material at Brain Online.
Tracking of CSF3, a B. burgdorferi-specific T cell clone derived from the CSF of a patient with chronic Lyme neuroborreliosis
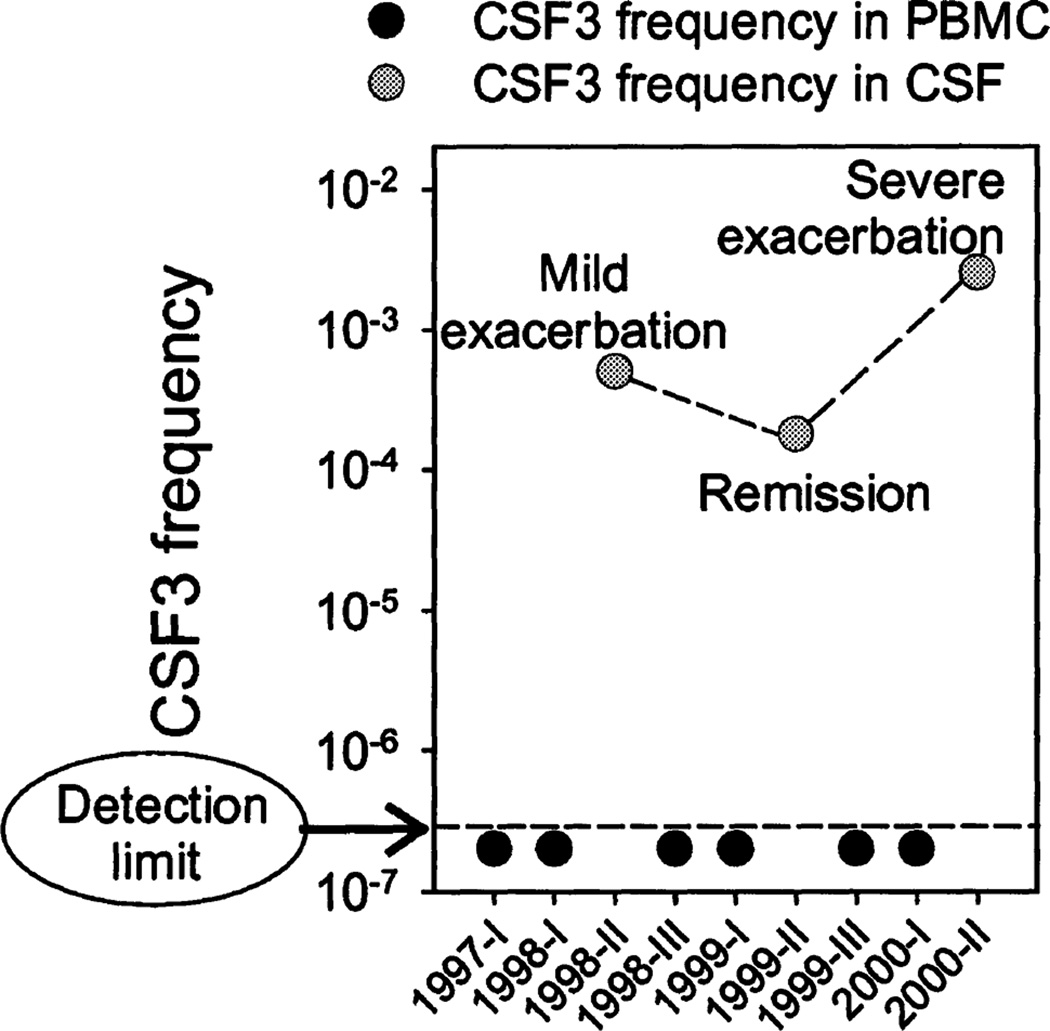
We followed the frequency of T cell clone CSF3, a clone isolated from the CSF of patient NB1, in seven PBMC and three CSF samples collected over a period of 2.5 years. The frequency of CSF3 was below our lower limit of detection of 1 cell/5 × 106 (Fig. 2C) for all PBMC samples tested. In contrast, we repeatedly detected CSF3 at a relatively high frequency in the CSF (Fig. 6). The absolute frequency of T cell clone CSF3 in the CSF during a severe clinical exacerbation (stroke-like manifestation involving the left basal ganglia and internal capsule) was increased 14-fold (25.3/104, or 1 CSF3 cell per 395 cells) compared with the frequency found during a recovery phase (1.8 CSF3 cells/ 104). An increase of 2.8-fold compared with the frequency during remission occurred during a mild clinical exacerbation (mild persistent headache, with gadolinium-enhancement of an old right caudate lesion on MRI, and normal CSF cell count and protein levels).
Fig. 6.
Frequency of T cell clone CSF3 in PBMC and CSF cells from a patient with Lyme neuroborreliosis. CSF3 TCR transcripts were undetectable in PBMC samples collected at 6 different time points. Lower limit of detection was 1 cell/5 × 106 (see Fig. 2C). In contrast, CSF3 was repeatedly found in CSF, with the highest frequency at the time of a severe exacerbation of neurological symptoms, an intermediate frequency during a milder relapse, and the lowest frequency at the time of clinical remission.
Discussion
Current evidence from studies in humans and in experimental animal models suggests that the broader T cell repertoire found following immune destruction of CNS tissue may represent the intermolecular dispersion of an initially focused, oligoclonal immune response (Lehmann et al., 1992; McRae et al., 1995; Steinman, 1996; Tuohy et al., 1997). In addition to triggering the initial immune response, minor populations of lymphocytes may play an important role in defining the immune microenvironment within established lesions (Brosnan and Raine, 1996). Limited clonal heterogeneity has been shown in lymphocytes infiltrating multiple sclerosis plaques (Oksenberg et al., 1990; Babbe et al., 2000). Here, we applied a highly specific approach to assess the frequency of selected individual T cell clonotypes in three CNS disorders with immune involvement and different causes. Indeed, our goal was not to characterize the diversity of the pathogenic effector T cell repertoire in these diseases, but rather to probe the dynamics of frequency of immunodominant autoreactive and foreign Ag-specific T cell clones that were considered representative of potentially pathogenic responses based on previous extensive studies. Rather than a primary clonotyping technique such as CDR3 spectratyping (Immunoscope), which allows the detection of clonal expansions through the analysis of length distribution in TCR transcripts (Cochet et al., 1992; Babbe et al., 2000; Goebels et al., 2000; Gorski et al., 1994), our approach required a secondary clonotyping method for the quantification of frequency of individual selected clones. We developed a highly specific molecular tracking technique that allowed a direct ex vivo determination of clonal frequency without prior in vitro expansion. Our technique provided an improved sensitivity compared with previous methods, showing limits of detection ranging from 1 clonal cell in 103 irrelevant cells to 1 in 2 × 105 (Moss et al., 1995; Bieganowska et al., 1997; Hanawa et al., 1997; Pantaleo et al., 1997; Nixon et al., 1999; Babbe et al., 2000; Kusaka et al., 2000).
First, we asked whether the frequency of a T cell clone that recognized an immunodominant myelin epitope could be linked to markers of disease activity in a phasic disease such as RRMS. We tracked P2–10, a Thl clone specific for MBP83–99 that was possibly implicated in a disease exacerbation of a patient treated with an APL. The frequency of P2–10 increased strikingly after the start of APL treatment, reaching a peak of 1 cell in ~1800 PBMC just prior to the onset of a severe clinical exacerbation. This finding suggests a possible role of the clone as a trigger, rather than as a bystander, a secondary effector, or a regulatory cell population in this exacerbation. In fact, the kinetic of clone P2–10’s frequency closely resembles that of encephalitogenic cells in animal models of multiple sclerosis. Indeed, autoimmune effector T cells that mediate EAE reach their highest frequency in the peripheral compartments before the onset of clinical disease and rapidly decrease when massive migration into the CNS is observed (Flugel et al., 2001; Targoni et al., 2001). To our knowledge, this is the first report of a human autoreactive Thl clone reproducing in a patient with multiple sclerosis the kinetics of encephalitogenic T cells in EAE. An important implication is that an increased frequency of autoreactive effector T cells might only be detected during a short period of time preceding multiple sclerosis relapses. This notion may partly explain the conflicting results of studies comparing the frequency of myelin-reactive T cells in multiple sclerosis patients and controls. An additional important finding is the detection of P2–10 in the patient’s peripheral blood over 6 years before the start of APL treatment. This observation, consistent with the results of a long-term follow-up of the MBP-specific T cell repertoire in multiple sclerosis patients (Goebels et al., 2000), shows that the clone previously existed in the peripheral circulation and the APL stimulated its expansion. MBP-specific T cells can also be raised from the peripheral blood of healthy subjects (Ota et al., 1990; Martin et al., 1990; Pette et al., 1990; Muraro et al., 1997) and can persist over prolonged periods of time (Goebels et al., 2000). Unlike the MBP-specific T cell clone from the multiple sclerosis patient, however, the frequency in the peripheral blood of the control clone 1C2 from the healthy donor remained stable for at least 4 years. These data support the concept that the dynamic instability with expansions and contractions of clonal frequency might be an important feature of a fraction of myelin-reactive T cells in RRMS (Muraro et al., 2002). Our results do not show an involvement of T cell clone P2–10 in a second exacerbation that was associated with a moderately increased proliferative response to a PLP peptide, an observation reminiscent of surges of T cell reactivity to PLP184–209 reported in multiple sclerosis patients, sometimes in association with clinical relapses (Pender et al., 2000). The high frequency determined for clone P2–10 by our molecular tracking method supports the notion that function-based assays such as limiting dilution analysis may lead to an underestimate of the precursor frequency of autoreactive T cell clones, as previously suggested (Bieganowska et al., 1997).
In HAM/TSP, we tracked T cell clone A6 which was isolated from a patient with a typical chronic-progressive disease course. A6 has been investigated extensively in previous studies, which provided remarkable insight on TCR structure and function (Garboczi et al., 1996; Ding et al., 1998, 1999; Baker et al., 2000, 2001). Therefore, it was interesting to assess the possible pathogenic involvement of the clone by monitoring its frequency over time and in the CSF versus the peripheral circulation. We quantified the frequency of A6 in the peripheral blood as being on average 1 out of 850 PBMC. This frequency appeared remarkably stable over a long period of time. In sorted CD8+ T cells, clone A6 represented 1 out of 44 cells. Again, these frequencies indicate that limiting dilution analysis may underestimate the precursor frequency of Tax11–19-specific CTL in HAM-TSP patients, which had previously been calculated in the range of 1 out of 75 to 1 out of 320 cells in the CD8+ subpopulation (1 out of 300 CD8+ cells in the very same patient) (Elovaara et al., 1993). A similar conclusion had been suggested by studies in which Ag-specific cells were enumerated using a Tax11–19-loaded HLA-A2 Ig fusion protein (Greten et al., 1998) or a HLA-A2/Tax11–19 tetramer (Bieganowska et al., 1999). Our results showed that clone A6 alone represents 14.2%, or one out of seven, of all Tax11–19-specific, HLA-A2-restricted CD8+ T cells in the peripheral blood. To our knowledge, this is the first direct measurement (i.e. without prior in vitro expansion) of the proportion of an individual T cell clone within the repertoire of T cells that recognize an MHC/Ag complex related to a neurological disease. The high degree of representation of this single clone suggests a possible involvement of the clone in the disease process, particularly in view of the degenerate TCR recognition of antigenic ligands (Mason, 1998). The most compelling support to this notion, however, comes from the fact that the frequency of T cell clone A6 in the CSF, determined by TCCT as being 1 out of 65 cells, is increased ~12-fold with respect to that in peripheral blood leukocytes.
In the patient with chronic Lyme neuroborreliosis, the enrichment of clone CSF3 in the CSF as compared with the peripheral blood was even more striking. In fact, despite the exquisite sensitivity of the primer/probe combination, no CSF3 clonotypic transcripts were amplified from any of six PBMC samples obtained from the patient over a period of 2.5 years. In contrast, CSF3 was consistently detectable in the CSF, and its frequency ranged from 1 out of 5555 to 1 out of 395 CSF cells, i.e. at least 900- to 12 000-fold greater than in the peripheral blood. The highest frequency was found at the time of a severe clinical exacerbation, while the lowest coincided with recovery, and an intermediate value was found during a mild exacerbation. The extreme bias of the frequency of CSF3 toward the site of inflammation and the correlation of clonal expansions with the clinical course supports the hypothesis of a pathogenic link between this clone and the disease process. We are in the process of extending these observations by examining matching PBMC and CSF samples collected during the most recent follow-up of the patient.
Taken together, our results suggest a relationship between the dynamics of frequency of functionally relevant T cell clones and the course of neurological disease. In a patient with RRMS we found that a putative pathogenic T cell clone that was previously detectable at lower frequency in the peripheral circulation became greatly expanded in close association with an inflammatory flare. In a patient with a chronic progressive disorder such as HAM/TSP, a candidate pathogenic clone had persistently high frequencies in the peripheral blood, was enriched several-fold in the CNS compartment, and accounted for a sizeable proportion of the overall T cell recognition of the target Ag/MHC complex. In the patient with Lyme neuroborreliosis, we found evidence of a substantial sequestration of autoreactive T cells within the target organ compartment. Our observations have implications for the pathogenesis of neurological immune disorders and may be important for designing successful therapeutic interventions. T cell clonotype tracking has great potential for further studies on immune-mediated diseases and in clinical trials.
Acknowledgments
We wish to thank Drs M. Nagai and Y. Yamano for help with FACS analysis, Dr S. Leitman and her staff at the Department of Transfusion Medicine, CC, NIH, for providing leuka-phereses, T. Simonis and her staff at the HLA-Laboratory, CC, NIH, for HLA typing and Dr M. Pender for critical reading of the manuscript. K.-P.W. was supported by a grant of the Deutsche Forschungsgemeinschaft (Wa 1343/1-1).
Abbreviations
- APL
altered peptide ligand
- CDR3
complementarity-determining region 3
- CT
threshold cycle
- Flu-HA
influenza haemagglutinin
- HAM/TSP
HTLV-I associated myelopathy/tropical spastic paraparesis
- MBP
myelin basic protein
- MRI
magnetic resonance imaging
- PBMC
peripheral blood mononuclear cells
- PLP
proteolipid protein
- RRMS
relapsing-remitting multiple sclerosis
- RT-PCR
reverse transcriptase-PCR
- SEA
staphylococcal enterotoxin A
- SEE
staphylococcal enterotoxin E
- SI
stimulation index
- TCCT
T cell clonotype tracking
- TCR
T cell receptor
Footnotes
This work was presented in part at the 54th American Academy of Neurology Annual Meeting, April 2002, Denver, CO, USA.
References
- Arden B, Clark SP, Kabelitz D, Mak TW. Human T-cell receptor variable gene segment families [Review] Immunogenetics. 1995;42:455–500. doi: 10.1007/BF00172176. [DOI] [PubMed] [Google Scholar]
- Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, Hohlfeld R, et al. Clonal expansions of CD8+ T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. 1 Exp Med. 2000;192:393–404. doi: 10.1084/jem.192.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BM, Gagnon SI, Biddison WE, Wiley DC. Conversion of a T cell antagonist into an agonist by repairing a defect in the TCR/ peptide/MHC interface: implications for TCR signaling. Immunity. 2000;13:475–484. doi: 10.1016/s1074-7613(00)00047-9. [DOI] [PubMed] [Google Scholar]
- Baker BM, Turner RV, Gagnon SI, Wiley DC, Biddison WE. Identification of a crucial energetic footprint on the alphal helix of human histocompatibility leukocyte antigen (HLA)-A2 that provides functional interactions for recognition by tax peptide/ HLA-A2-specific T cell receptors. 1 Exp Med. 2001;5:551–562. doi: 10.1084/jem.193.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddison WE, Kubota R, Kawanishi T, Taub DD, Cruikshank WW, Center DM, et al. Human T cell leukemia virus type I (HTLV-I)-specific CD8+ CTL clones from patients with HTLV-I-associated neurologic disease secrete proinflammatory cytokines, chemokines, and matrix metalloproteinase. 1 Immunol. 1997;159:2018–2025. [PubMed] [Google Scholar]
- Bieganowska KD, Ausubel LI, Modabber Y, Slovik E, Messersmith W, Hafler DA. Direct ex vivo analysis of activated, Fas-sensitive autoreactive T cells in human autoimmune disease. 1 Exp Med. 1997;185:1585–1594. doi: 10.1084/jem.185.9.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieganowska KD, Hollsberg P, Buckle GJ, Lim D-G, Greten TF, Schneck IP, et al. Direct analysis of viral-specific CD8+ T cells with soluble HLA-A2/Tax11-19 tetramer complexes in patients with human T cell lymphotropic virus-associated myelopathy. 1 Immunol. 1999;162:1765–1771. [PubMed] [Google Scholar]
- Bielekova B, Muraro PA, Golestaneh L, Pascal J, McFarland HF, Martin R. Preferential expansion of autoreactive T lymphocytes from the memory T-cell pool by IL-7. 1 Neuroimmunol. 1999;100:115–123. doi: 10.1016/s0165-5728(99)00200-3. [DOI] [PubMed] [Google Scholar]
- Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G, et al. Encephalitogenic potential of myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med. 2000;6:1167–1175. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- Brosnan CF, Raine CS. Mechanisms of immune injury in multiple sclerosis [Review] Brain Pathol. 1996;6:243–257. doi: 10.1111/j.1750-3639.1996.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Cochet M, Pannetier C, Regnault A, Darche S, Leclerc C, Kourilsky P. Molecular detection and in vivo analysis of the specific T cell response to a protein antigen. Eur J Immunol. 1992;22:2639–2637. doi: 10.1002/eji.1830221025. [DOI] [PubMed] [Google Scholar]
- Ding YH, Smith KJ, Garboczi DN, Utz U, Biddison WE, Wiley DC. Two human T cell receptors bind in a similar diagonal mode to the HLA-A2/Tax peptide complex using different TCR amino acids. Immunity. 1998;8:403–411. doi: 10.1016/s1074-7613(00)80546-4. [DOI] [PubMed] [Google Scholar]
- Ding YH, Baker BM, Garboczi DN, Biddison WE, Wiley DC. Four A6-TCR/peptide/HLA-A2 structures that generate very different T cell signals are nearly identical. Immunity. 1999;11:45–56. doi: 10.1016/s1074-7613(00)80080-1. [DOI] [PubMed] [Google Scholar]
- Elovaara I, Koenig S, Brewah AY, Woods RM, Lehky T, Jacobson S. High human T cell lymphotropic virus type 1 (HTLV-l)-specific precursor cytotoxic T lymphocyte frequencies in patients with HTLV-1-associated neurological disease. J Exp Med. 1993;177:1567–1573. doi: 10.1084/jem.177.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügel A, Berkowicz T, Ritter T, Labeur M, Jenne DE, Li Z, et al. Migratory activity and functional changes of green fluorescent effector cells before and during experimental autoimmune encephalomyelitis. Immunity. 2001;14:547–560. doi: 10.1016/s1074-7613(01)00143-1. [DOI] [PubMed] [Google Scholar]
- Garboczi DN, Ghosh P, Utz U, Fan QR, Biddison WE, Wiley DC. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature. 1996;384:134–141. doi: 10.1038/384134a0. [DOI] [PubMed] [Google Scholar]
- Goebels N, Hofstetter H, Schmidt S, Brunner C, Wekerle H, Hohlfeld R. Repertoire dynamics of autoreactive T cells in multiple sclerosis patients and healthy subjects: epitope spreading versus clonal persistence. Brain. 2000;123:508–518. doi: 10.1093/brain/123.3.508. [DOI] [PubMed] [Google Scholar]
- Gorski J, Yassai M, Zhu X, Kissela B, Keever C, Flomenberg N. Circulating T cell repertoire complexity in normal individuals and bone marrow recipients analyzed by CDR3 spectratyping: correlation with immune status. J Immunol. 1994;152:5109–5119. [PubMed] [Google Scholar]
- Greten TF, Slansky JE, Kubota R, Soldan SS, Jaffee EM, Leist TP, et al. Direct visualization of antigen-specific T cells: HTLV-1 Tax11-19-specific CD8+ T cells are activated in peripheral blood and accumulate in cerebrospinal fluid from HAM/TSP patients. Proc Natl Acad Sci USA. 1998;95:7568–7573. doi: 10.1073/pnas.95.13.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafler DA, Weiner HL. Immunologic mechanisms and therapy in multiple sclerosis. [Review] Immunol Rev. 1995;144:75–107. doi: 10.1111/j.1600-065x.1995.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Hanawa H, Koike T, Sakaue M, Kishi K, Shibata A, Abo T. Novel analysis of minimal residual disease in leukemia with TCR beta rearrangement - detection of monoclonality by single strand conformation polymorphism and PCR using a clonotype primer of leukemic T cell receptor beta-chain RNA. Leuk Res. 1997;21:201–210. doi: 10.1016/s0145-2126(96)00113-0. [DOI] [PubMed] [Google Scholar]
- Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- Hemmer B, Gran B, Zhao Y, Marques A, Pascal J, Tzou A, et al. Identification of candidate T-cell epitopes and molecular mimics in chronic Lyme disease. Nat Med. 1999;5:1375–1382. doi: 10.1038/70946. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R, Wekerle H. Immunological update on multiple sclerosis [Review] Curr Opin Neurol. 2001;14:299–304. doi: 10.1097/00019052-200106000-00006. [DOI] [PubMed] [Google Scholar]
- Jacobson S, Shida H, McFarlin DE, Fauci AS, Koenig S. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature. 1990;348:245–248. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- Kubota R, Kawanishi T, Matsubara H, Manns A, Jacobson S. Demonstration of human T lymphotropic virus type I (HTLV-I) tax-specific CD8+ lymphocytes directly in peripheral blood of HTLV-I-associated myelopathy/tropical spastic paraparesis patients by intracellular cytokine detection. J Immunol. 1998;161:482–488. [PubMed] [Google Scholar]
- Kurokawa M, Furukawa H, Yabe T, Matzui T, Toda M, Hamada C, et al. Frequency of clonally expanded T cells evaluated by PCR from a single cell. J Immunol Methods. 1999;224:203–208. doi: 10.1016/s0022-1759(99)00022-8. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Kusaka S, Grailer AP, Fechner JH, Jr, Jankowska-Gan E, Oberley T, Sollinger WJ, et al. Clonotype analysis of human alloreactive T cells: a novel approach to studying peripheral tolerance in a transplant recipient. J Immunol. 2000;164:2240–2247. doi: 10.4049/jimmunol.164.4.2240. [DOI] [PubMed] [Google Scholar]
- Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- Martin R, McFarland HF. Immunological aspects of experimental allergic encephalomyelitis and multiple sclerosis [Review] Crit Rev Clin Lab Sci. 1995;32:121–182. doi: 10.3109/10408369509084683. [DOI] [PubMed] [Google Scholar]
- Martin R, Jaraquemada D, Flerlage M, Richert JR, Whitaker JN, Long EO, et al. Fine specificity and HLA restriction of myelin basic protein-specific cytotoxic T cell lines from multiple sclerosis patients and healthy individuals. J Immunol. 1990;145:540–548. [PubMed] [Google Scholar]
- Mason D. A very high level of crossreactivity is an essential feature of the T-cell receptor [Review] Immunol Today. 1998;19:395–404. doi: 10.1016/s0167-5699(98)01299-7. [DOI] [PubMed] [Google Scholar]
- McRae BL, Vanderlugt CL, Dal Canto MC, Miller SD. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J Exp Med. 1995;182:75–85. doi: 10.1084/jem.182.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss PA, Rowland-Jones SL, Frodsham PM, McAdam S, Giangrande P, McMichael AJ, et al. Persistent high frequency of human immunodeficiency virus-specific cytotoxic T cells in peripheral blood of infected donors. Proc Natl Acad Sci USA. 1995;92:5773–5777. doi: 10.1073/pnas.92.13.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraro PA, Vergelli M, Kalbus M, Banks D, Nagle JW, Tranquill LR, et al. Immunodominance of a low-affinity major histocompatibility complex-binding myelin basic protein epitope (residues 111–129) in HLA-DR4 (Bl*0401) subjects is associated with a restricted T cell receptor repertoire. J Clin Invest. 1997;100:339–349. doi: 10.1172/JCI119539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraro PA, Jacobsen M, Necker A, Nagle JW, Gaber R, Sommer N, et al. Rapid identification of local T cell expansion in inflammatory organ diseases by flow cytometric T cell receptor Vbeta analysis. J Immunol Methods. 2000;246:131–143. doi: 10.1016/s0022-1759(00)00309-4. [DOI] [PubMed] [Google Scholar]
- Muraro PA, Bonanni L, Mazzanti B, Pantalone A, Traggiai E, Massacesi L, et al. Short-term dynamics of circulating T cell receptor V beta repertoire in relapsing-remitting MS. J Neuroimmunol. 2002;127:149–159. doi: 10.1016/s0165-5728(02)00105-4. [DOI] [PubMed] [Google Scholar]
- Nixon DF, Douek D, Kuebler PJ, Jin X, Vesanen M, Bonhoeffer S, et al. Molecular tracking of an human immunodeficiency virus nef specific cytotoxic T-cell clone shows persistence of clone-specific T-cell receptor DNA but not mRNA following early combination antiretroviral therapy. Immunol Lett. 1999;66:219–228. doi: 10.1016/s0165-2478(98)00162-x. [DOI] [PubMed] [Google Scholar]
- Oksenberg JR, Stuart S, Begovich AB, Bell RB, Erlich HA, Steinman L, et al. Limited heterogeneity of rearranged T-cell receptor V alpha transcripts in brains of multiple sclerosis patients. Nature. 1990;345:344–346. doi: 10.1038/345344a0. [DOI] [PubMed] [Google Scholar]
- Ota K, Matsui M, Milford EL, Mackin GA, Weiner HL, Hafler DA. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990;346:183–187. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- Pantaleo G, Soudeyns H, Demarest J, Vaccarezza M, Graziosi C, Paolucci S, et al. Evidence for rapid disappearance of initially expanded HIV-specific CD8+ T cell clones during primary HIV infection. Proc Natl Acad Sci USA. 1997;94:9848–9853. doi: 10.1073/pnas.94.18.9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pender MP, Csurhes PA, Greer JM, Mowat PD, Henderson RD, Cameron KD, et al. Surges of increased T cell reactivity to an encephalitogenic region of myelin proteolipid protein occur more often in patients with multiple sclerosis than in healthy subjects. J Immunol. 2000;165:5322–5331. doi: 10.4049/jimmunol.165.9.5322. [DOI] [PubMed] [Google Scholar]
- Pette M, Fujita K, Kitze B, Whitaker JN, Albert E, Kappos L, et al. Myelin basic protein-specific T lymphocyte lines from MS patients and healthy individuals. Neurology. 1990;40:1770–1776. doi: 10.1212/wnl.40.11.1770. [DOI] [PubMed] [Google Scholar]
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- Steinman L. multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. [Review] Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- Targoni OS, Baus J, Hofstetter HH, Hesse MD, Karulin AY, Boehm BO, et al. Frequencies of neuroantigen-specific T cells in the central nervous system versus the immune periphery during the course of experimental allergic encephalomyelitis. J Immunol. 2001;166:4757–4764. doi: 10.4049/jimmunol.166.7.4757. [DOI] [PubMed] [Google Scholar]
- Tuohy VK, Yu M, Weinstock-Guttman B, Kinkel RP. Diversity and plasticity of self recognition during the development of multiple sclerosis. J Clin Invest. 1997;99:1682–1690. doi: 10.1172/JCI119331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utz U, Banks D, Jacobson S, Biddison WE. Analysis of the T-cell receptor repertoire of human T-cell leukemia virus type 1 (HTLV-1) tax-specific CD8+ cytotoxic T lymphocytes from patients with HTLV-1-associated disease: evidence for oligoclonal expansions. J Virol. 1996;70:843–851. doi: 10.1128/jvi.70.2.843-851.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandinger KP, Sturzebecher CS, Bielekova B, Detore G, Rosenwald A, Staudt LM, et al. Complex immunomodulatory effects of interferon-beta in multiple sclerosis include the upregulation of T helper 1-associated marker genes. Ann Neurol. 2001;50:349–357. doi: 10.1002/ana.1096. [DOI] [PubMed] [Google Scholar]
- Weiner J, Jung CK, Shakel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270:41–49. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- WHO IUIS Nomenclature Sub-Committee on TCR Designation. Nomenclature for T-cell receptor (TCR) gene segments of the immune system. Immunogenetics. 1995;42:451–453. doi: 10.1007/BF00172175. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]