Abstract
Accumulating data suggest that bone-seeking radiopharmaceuticals can be used to treat prostate cancer bone metastasis and improve the clinical outcome of patients with advanced prostate cancer. It remains to be elucidated whether radiopharmaceuticals enhance the disruption of the onco-niche or the eradication of micrometastatic cells in the bone marrow. The purpose of this review is to investigate the role of bone-targeted radioisotope therapy in the setting of multimodality therapy for advanced prostate cancer. We examine available data and evaluate whether dose escalation, newer generations, or repeated dosing of radiopharmaceuticals enhance their antitumor effects and whether their combination with hormone ablative therapy, chemotherapy, or novel targeted therapy can improve clinical efficacy.
Keywords: advanced prostate cancer, bone metastases, onco-niche, radiopharmaceuticals
Introduction
A hallmark of metastatic prostate cancer is the development of osteoblastic bone metastasis. Almost all patients with advanced prostate cancer eventually develop skeletal metastasis. In most patients with prostate cancer, bone is the only site of clinical metastasis. Not surprisingly, many established prognostic factors for advanced prostate cancer (eg, performance status, alkaline phosphatase level, hemoglobin level) highlight the clinical consequences of osseous metastasis. Hence, patients who develop widespread, progressive, or early bone metastasis tend to suffer more from their symptoms and fare worse with their prostate cancer. Conversely, patients who develop limited, stable, or delayed bone metastasis tend to experience less morbidity and have a better clinical outcome.
Increasingly, advanced prostate cancer is considered to be a treatable although not curable disease. Patients with prostate cancer and bone metastases may experience substantial palliative benefit and even significant survival advantage from the use of hormone ablative therapy or chemotherapy. There is promise that one can gain even more from such treatments by combining them with bone-targeted agents (eg, calcitriol, atrasentan) to improve control of bone metastases. Bone-seeking radiopharmaceutical is another therapeutic option for this very purpose.
Here, we review the underlying physical characteristics of various bone-seeking radiopharmaceuticals. We also discuss their clinical efficacy and how they may be used to overcome the putative biologic properties of prostate cancer bone metastasis. Finally, we examine ways in which the use of radiopharmaceuticals can be optimized in the setting of multimodality therapy; primarily, how they can be combined with hormone ablative therapy, chemotherapy, or targeted therapy for the treatment of prostate cancer bone metastases.
Tumor-Host Cell Interactions
For the longest time, we have focused on metastatic tumor cells in the study and treatment of metastasis. However, we now know that host cells and the microenvironment are also implicated in the metastatic process. Whether an immigrating metastatic tumor cell becomes established in a foreign tissue largely depends on favorable interactions between the metastatic tumor cell and host cells.1–2
The concept of tumor-host cell interactions is compatible with the concept of an onco-niche in which cancer cells interact with host cells and the microenvironment.
The Onco-niche
The osteoblast is probably the most important host cell in prostate cancer bone metastasis, which has the unique feature of being predominantly osteoblastic. It is hypothesized that prostate cancer initially stimulates an osteoblastic response by causing proliferation and differentiation of osteoblasts. In the bone marrow, the osteoblastic niche provides a microenvironment that supports and sustains hematopoetic stem cells.3 It remains to be elucidated whether this osteoblastic niche also provides a favorable onco-niche for prostate cancer stem cells. If the sequence of events culminating in bone metastasis starts with prostate cancer-induced osteoblast overactivity, then therapeutic strategies targeting osteoblasts are logical and appropriate for the treatment of prostate cancer bone metastasis.
The concept of an onco-niche is intimately linked to that of cancer stem cells. One cannot help but notice that the prowess that allows a metastatic malignant cell to migrate, extravasate, invade, and thrive at distant sites is already ingrained within the stem cell from which it is derived. We previously postulate that the nature of the involved stem cell determines both the resultant malignant cell’s predilection to metastasize and its pattern of metastasis.4 Just as a stem-cell niche supports a normal stem cell, an onco-niche sustains a cancer stem cell. If one could manipulate the onco-niche and render it more akin to the stem-cell niche, then one might be able to keep cancer cells in check, making them more indolent, if not dormant.
Therefore, the idea of an onco-niche is important because it has therapeutic implications. For a growing pool of prostate cancer stem cells, the corresponding onco-niche in the bone also needs to expand to maintain them as cancer stem cells. Thus, one way to treat prostate cancer would be to eliminate the prostate cancer stem cells. Another way would be to induce them to behave like normal stem cells or differentiate into more benign or indolent entities by modulating the onco-niche. In principle, one could modulate the onco-niche by restricting its expansion (eg, by limiting osteoblatic proliferation) or by converting it back into a quasi-stem cell niche (eg, by mitigating the effects of inflammation, oxidation, angiogenesis, and/or hypoxia in the bone marrow). The modulation of the onco-niche is especially relevant if the eradication of cancer stem cells is unlikely or impossible. The time will come when modulation of the onco-niche becomes an integral aspect of cancer therapy: it would be like managing the soil so that even if a malignant seed remains, it does not germinate or grow like a weed.
Targeting the Onco-niche
Interestingly, it has been observed that irradiated bone no longer provides a favorable niche for metastasis. Figure 1 shows the bone-scan image of a patient with metastatic prostate cancer, who had previously received external beam radiation to the cervical spine. This patient was subsequently spared from bone metastasis at the irradiated site when the prostate cancer relapsed and progressed to the skeleton. Similarly, Jacobsson and Näslund reported that previously irradiated bone (to 5,000 Gy) appeared to be protected from future metastasis.5 We postulate that using radiopharmaceuticals to radiate multiple bone metastases in a systemic manner may similarly improve control of the bone onco-niche and treatment of bone metastases.
Figure 1.
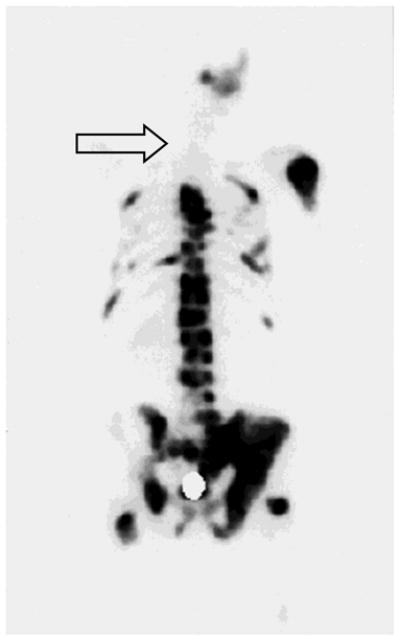
A bone-scan image showing a previously irradiated bone in the cervical spine (arrow) that no longer provides a favorable microenvironment (onco-niche) for subsequent prostate cancer osseous metastasis.
Therefore, when treating prostate cancer bone metastasis, it is preferable to use a treatment that not only eliminates or reduces the burden of metastatic prostate cancer cells in the bone but also alters the onco-niche by disrupting the osteoblasts, endothelial cells, and other stromal cells or factors. In principle, one can further enhance the effects of radiation therapy by combining it with hormone ablative therapy, chemotherapy, or targeted therapy that destroys the malignant cancers as well as the onco-niche. This is the basis of multimodality therapy: treating the various components of a complex disease using various therapies.
Radiopharmaceuticals
Clinical Data
Bone-seeking radiopharmaceuticals are like magic bullets or smart bombs that target bone metastases by preferential deposition at sites of increased osteoblastic activity and bone matrix synthesis. Table 1 summarizes the unique physical properties of various radiopharmaceuticals. These agents are ideally suited for the treatment of patients with multifocal osteoblastic metastases and predominant or only bone metastases. Another potential benefit of bone-seeking radiopharmaceuticals is that they can be used repeatedly for palliation of bone pain. Table 2 summarizes the clinical characteristics of various radiopharmaceuticals.
Table 1.
Physical Characteristics of Various Bone-Seeking Radiopharmaceuticals
| Agent | Carrier ligand | Half-life (days) | Maximum energy (MeV)* | γ-emission (MeV) | Maximum range (mm) |
|---|---|---|---|---|---|
| 32P | _ | 14.3 | 1.71 (β) | None | 8.5 |
| 89Sr | Cl2 | 50.5 | 1.46 (β) | None | 7 |
| 153Sm | EDTMP | 1.9 | 0.81 (β) | 0.103 | 2.5 |
| 186 Re | HEDP | 3.7 | 1.07 (β) | 0.137 | 5 |
| 188 Re | HEDP | 0.7 | 2.12 (β) | 0.155 | 10 |
| 117mSn | DTPA | 13.6 | 0.13 and 0.16 (conversion ē) | 0.159 | <0.001 |
| 223Ra | Cl2 | 11.4 | 5.78 (α) average | 0.154 | <0.01 |
Derived from type of radiation particles emitted (in parenthesis)
Table 2.
Clinical Characteristics of Various Bone-Seeking Radiopharmaceuticals
| Agent (dose) | Response pain (%) | Response onset (days) | Response duration (mos) | Peak effect (wks) | Recovery (wks) | Response PSA <50%* | Pain flare | Low WBC gr 3–4 | Low platelets gr 3–4 |
|---|---|---|---|---|---|---|---|---|---|
| 32P | |||||||||
| 444 mBq7 | 77 | 5–14 | 3 | 4–5 | 6–7 | – | – | 0 | 11% |
|
| |||||||||
| 89Sr | |||||||||
| 150 mBq23 | 35 | 10–20 | 4.6 | 4–8 | 12 | 13% | 18% | 0 | 0 |
| 400 mBq24 | 70 | 50% | 12% | 33% | |||||
|
| |||||||||
| 153Sm | |||||||||
| 1 mCi/kg49 | 65 | 5–10 | 4 | 3–4 | 8 | 9% | 6% | 5% | 3% |
|
| |||||||||
| 186Re | |||||||||
| 1.1 mCi/kg50 | 73 | 2–7 | 2.1 | 4 | 8 | <50%** | 20% | 0 | 7% |
|
| |||||||||
| 188Re | |||||||||
| 1.1 mCi/kg14 | 60 | 2–7 | 2.6 | 4 | 8 | 7% | 10% | 0 | 0 |
| Repeat ×1 | 92 | 5.7 | 39% | 7% | |||||
|
| |||||||||
| 223Ra | |||||||||
| 50 kBq/kg16 | 60 | <10 | – | 2–4 | – | 29% | 18% | 3% | 0 |
| Repeat ×4 | |||||||||
PSA response is defined as greater than 50% decrease from baseline
PSA response occurred in highest treatment levels but were not generally sustained.
PSA, prostate-specific antigen; WBC, white blood cells
Phosphorus-32 (32P) has a propensity to deposit in bone: about 85% of total body phosphorous is bound as inorganic phosphate to hydroxyapatite in the skeleton. Animal studies have shown that 3–5 times more 32P is absorbed at the site of bone metastasis than in normal bone.6 Although 32P is rarely used for palliation of bone pain in the Western world, it is advantageous over other radiopharmaceuticals with respect to lower cost and convenience of (ie, oral) administration. A study comparing a single oral dose of 32P with intravenous (i.v.) strontium-89 (89Sr) demonstrated similar efficacy and toxicity.7
89Sr is a calcium analogue. About 10 times more 89Sr is absorbed at the site of bone metastasis than in the normal bone marrow.8 Bone dosimetry studies suggest that light skeletal metastases (≤5 lesions) absorb about 4,000 Gy of radiation, moderate metastases (5–10 lesions) 8,000 Gy, and diffuse metastases 1,000 Gy.9 In a randomized phase II study, Tu and colleagues showed that consolidation therapy using one dose of 89Sr (55 μCi/kg) increased progression-free and overall survival time of patients who had responded to induction chemotherapy.10 An update of this study indicated that combining chemotherapy with 89Sr was safe and feasible in selected patients.11
Samarium-153 (153Sm) lexidronam is composed of radioactive samarium and a tetraphosphonate chelator, ethylenediaminetetramethylene phosphonic acid (EDTMP). 153Sm emits both beta and gamma radiation. 153Sm-EDTMP collects in areas of bone turnover in association with hydroxyapatite and is rapidly taken up at sites of osteoblastic bone metastases. Compared with normal bone surfaces, osteoblastic lesions can accumulate 4–7 times as much 153Sm-EDTMP.12
Rhenium-186 and rhenium-188 (186Re and 188Re) emit beta particles. Maxon and colleagues estimated the lesion-to-marrow absorbed dose ratio of 186Re-1-1-hydroethylidene diphosphate (HEDP) to be between 20:1 and 30:1.13 In a randomized phase II study, Palmedo and colleagues showed that repeated (double-injection) 188Re-HEDP therapy enhanced pain palliation and improved progression-free and overall survival time of patients with hormone-refractory prostate cancer, compared with single-injection 188Re-HEDP therapy.14
Radium-223 (223Ra) is another bone-seeking radiopharmaceutical being investigated for the treatment of bone metastasis in clinical trials. 223Ra emits alpha particles, which have a higher energy and travel a shorter distance than beta particles. Limited studies indicate that 223Ra has a tumor-to-marrow absorbed dose ratio of 30:1.15 Preclinical and pilot phase I studies have not found any limiting toxicity. In a randomized, placebo-controlled phase II study, Nilsson and colleagues demonstrated that 4 injections of 223Ra (50 kBq/kg) given every 4 weeks significantly reduced bone-specific alkaline phosphatase levels and delayed time-to-prostate–specific antigen (PSA) progression in patients with hormone-refractory prostate cancer.16 Their finding that patients who received 223Ra had an overall survival advantage suggests that 223Ra therapy produces genuine antitumor and bone-targeted effects.
Selection and Utility
The clinical effects and toxicity profiles of radiopharmaceuticals depend on their half-life and radiation energy. Because all bone-seeking radiopharmaceuticals either directly deposit at or possess carrier ligands that bind to the bone matrix, they act near, rather than on, the cancer cells. Because they settle near the site of active bone formation, these agents are suitable for the treatment of osteoblastic metastases. Theoretically, bone-seeking radiopharmaceuticals that emit a higher energy have a longer range and will thus provide a greater palliative benefit because of their increased antitumor potential. However, bone-marrow toxicity also increases proportionally with energy emitted. Therefore, when using radiopharmaceuticals, one needs to strike a balance between their efficacy and their toxicity. The goals of treatment for prostate cancer bone metastasis include improved palliation of pain, decreased intake of analgesics, delayed use of or decreased need for chemotherapy/radiation therapy, enhanced quality of life, and prolonged time of disease-free or perhaps overall survival. Contraindications for the use of radiopharmaceuticals include thrombocytopenia (<100 × 109/L), leucopenia (<3 × 109/L), impending spinal-cord compression, acute renal insufficiency, and pregnancy. Like radiation therapy and chemotherapy, radiopharmaceuticals alone may not satisfactorily palliate pain due to vertebral collapse, degenerative/disc disease, nerve-root impingement, skeletal fracture, or derived from visceral origins.
Although no difference appears to exist in the response rate or palliative efficacy of various radiopharmaceuticals, differences do exist in their onset and duration of response as well as in their intensity and duration of toxicity (Table 2). In general, the onset of response is rapid (usually 2–3 days) after treatment with short-lived radioisotopes (eg, 153Sm-EDTMP). In contrast, the response onset is delayed (to a few weeks) after treatment with long-lived radioisotopes (eg, 89Sr). Furthermore, the duration of response is longer for the long-lived than the short-lived radioisotopes. Unfortunately, long-lived radioisotopes tend to cause more myelosuppression for a longer period of time than short-lived radioisotopes because of their greater energy of radiation and longer range of effect in bone (Table 1).
Although an ideal radiopharmaceutical does not exist, one can select an appropriate agent and make the best tradeoff between efficacy and toxicity in accordance with a patient’s clinical presentation. Important criteria for selecting the optimal radiopharmaceutical include time to response, response duration, and bone marrow reserve. Patients with advanced metastases and severe pain tend to have a limited bone marrow reserve and require immediate pain relief. Hence, they may benefit from the use of short-lived bone-seeking radiopharmaceuticals such as 153Sm-EDTMP and 186Re-HEDP. These types of radiopharmaceuticals are especially useful for patients for whom hormone ablative therapy, chemotherapy, and other therapeutic options are no longer available or effective. If necessary, these patients may benefit from repeated or multiple use of such agents at relatively short time intervals (eg, every 2–3 months). However, patients with early metastases, favorable prognosis (ie, life expectancy greater than 6 months), adequate pain control (using conventional analgesics), and sufficient bone marrow reserve may benefit more from the use of long-lived radiopharmaceuticals such as 89Sr. Importantly, when patients with progressive disease and severe pain respond to induction or frontline therapies, such as hormone ablative therapy or chemotherapy, their reduced tumor burden and improved clinical condition may render multimodality therapy using long-lived, high-energy, and increased-range bone-seeking radiopharmaceuticals more tenable and practical.
Dose Response and Intensity
It remains to be determined if radiopharmaceuticals provide a dose response in antitumor activity rather than mere pain relief. Breen and colleagues reported that when a dose of 150 MBq 89Sr is given, about 3,000 cGy at 28 days and up to 30,000 cGy at infinite time would be delivered to a particular bone metastasis.17 Perhaps this is the reason that 89Sr has not been shown to provide a dose response for pain relief. Indeed, pooled results from several small studies suggest that 89Sr is ineffective at doses less than 30 μCi/kg and that its activity reaches a plateau between 40–80 μCi/kg.18 However, Mertens and colleagues showed that increased 89Sr dosage correlated with complete pain relief.19
Interestingly, 89Sr studies have demonstrated that increased 89Sr dosage versus placebo improved pain relief and survival time (Table 3).20,21 In addition, increased 89Sr dosage versus focal radiation therapy delayed new pains or need for treatment of these new pains.22,23 Furthermore, increased 89Sr dose (400 vs 150 MBq) adjuvant to focal radiation therapy versus placebo prolonged time to new pains and to radiation therapy for these pains.24,25 Finally, increased 89Sr (400 vs 150 MBq) and 153Sm-EDTMP (2.5 vs 1.0 mCi/kg)26 (Table 4) dosages provided superior antitumor effects (such as PSA responses and overall survival time) beyond mere enhanced pain relief of patients with castrate-resistant prostate cancer.
Table 3.
Dose Intensity* and Response for 89Sr Therapy
| Study, yr (phase) | Treatment (n) | Response | Survival | Comments |
|---|---|---|---|---|
| Lewington et al., 21 1991 (RII) | 89Sr 150 MBq (36) | 28 % (pain relief) | – | – |
| Placebo (26) | 15%, P<.03 | |||
|
| ||||
| Buchali et al.,20 1988 (III) | 89Sr 225 MBq (25) | 37% (pain relief) | 46% (2 yrs) | – |
| Placebo (24) | 50% | 4%, P<.05 | ||
|
| ||||
| Oosterhof et al.,23 2003 (III) | 89Sr 150 MBq (101) | 35% (pain relief) | 7.2 mos | OS, P=.05 |
| Local XRT (102) | 33% | 11.0 mos | ||
|
| ||||
| Quilty et al.,22 1994 (III) | 89Sr 200 MBq (76) | 65% (pain relief) | 7.7 mos | 64% (no new pains) |
| Local XRT (72) | 67% | 6.5 mos | 42%, P<.05 | |
|
| ||||
| Smeland et al.,25 2003 (III) | 89Sr 150 MBq (30) | 30% (pain relief) | 12 mos | Time to XRT/new pain, NS |
| Placebo (34) (Adj to local XRT) | 20% | |||
|
| ||||
| Porter et al.,24 1993 (III) | 89Sr 400 MBq (68) | 40% (pain free) | 7.1 mos | Time to XRT/new pain, P<.002 |
| Placebo (58) (Adj to local XRT) | 23%, P<.05 | |||
Highlighted in red
RII, randomized phase II; adj, adjuvant; XRT, external beam radiotherapy; OS, overall survival; NS, not significant
Table 4.
Dose Intensity* and Response for 153Sm-EDTMP, 188Re-HEDP, and 223Ra Therapy
| Study, yr (phase) | Treatment (n) | Response | Survival (mos) | Comments |
|---|---|---|---|---|
| Sartor et al.,49 2004 (III) | 1.0 mCi/kg 153Sm (101) | −37% (opiate use) | 7.0 | – |
| Placebo (51) | +26%, P<.05 | |||
|
| ||||
| Resche et al.,51 1997 (RII) | 1.0 mCi/kg 153Sm (54) | 70% (pain relief) | 4.5 | OS better in breast cancer |
| 0.5 mCi/kg (49) | 67% | 7.5 | ||
|
| ||||
| Serafini et al.,52 1998 (III) | 1.0 mCi/kg 153Sm (39) | 31% (pain free) | – | – |
| 0.5 mCi/kg (40) | 28% | |||
| Placebo (37) | 14%, P<.016 | |||
|
| ||||
| Collins et al.,26 1993 (RII) | 2.5 mCi/kg 153Sm (20) | 42% (PSA<25%) | 9 | – |
| 1.0 mCi/kg (20) | 7% (8 wks) | 6, P=.03 | ||
|
| ||||
| Turner and Claringbold,27 1991 (RII) | Repeat 153Sm (15) | 87% (pain relief) | 9 | 24 wks |
| Single dose (23) | 61% | 4, P<.05 | 8 wks, P<.05 (pain relief) | |
| Fixed 2 Gy to BM | ||||
|
| ||||
| Palmedo et al.,14 2003 (RII) | Repeat 188Re (28) | 39% (PSA<50%) | 12.7 | 7.0 mos (TTP) |
| Single dose (30) | 7% (8 wks) | 7.0, P=.04 | 2.3 mos, P=.001 | |
|
| ||||
| Nilsson et al.,16 2007 (RII) | Repeat ×4 223Ra (33) | −66% (ΔBSAP) | 15.1 | 26 wks (TTP) |
| Single dose (31) | +9%, P<.0001 | 10.7 | 8 wks, P<.05 | |
Highlighted in red
RII, randomized phase II; PSA, prostate-specific antigen; BSAP, bone-specific alkaline phosphatase; OS, overall survival; TTP, time-to-progression
Another way to intensify treatment besides increasing the dose is to repeat the treatment. Radiopharmaceuticals are particularly amenable to this dose-escalating approach because of the feasibility and safety of their repeated administration (especially for the short-acting 153Sm-EDTMP, 188Re-HEDP, and 223Ra). For example, Turner and Claringbold found that repeated 153Sm-EDTMP treatment improved the quality of pain relief and prolonged the survival time of patients with castrate-resistant prostate cancer, compared with one-time 153Sm-EDTMP treatment.27 Palmedo and colleagues also demonstrated that repeated (double-injection) 188Re-HEDP therapy improved the PSA response rate, time to progression, and survival time, compared with single-dose 188Re-HEDP therapy.14 More recently, Nilsson and colleagues reported that repeated treatments using 223Ra after focal radiation therapy provided a disease-modifying effect by delaying time-to-PSA progression and prolonging overall survival time (Table 4).16
These results suggest that dose intensity, if not dose response, may be appropriate and beneficial using the right agents (eg, short-acting radiopharmaceuticals like 153Sm-EDTMP, 188Re-HEDP, and 223Ra) under the right circumstances (eg, advanced prostate cancer with symptomatic bone metastases). Clearly, additional studies are needed to confirm these results. A double-blind, randomized phase III trial (ALSYMPCA) combining 6 injections of 223Ra 4 weeks apart with the best standard of care (eg, docetaxel) for patients with symptomatic, castrate-resistant prostate cancer is currently under way.
Multimodality Therapy
Multimodality therapy for bone metastasis involves targeting the epithelial, stromal, and endothelial components in the bone using various agents. For example, cytotoxic agents are used to eliminate the malignant epithelial cell, stromal antagonists to target the mesenchymal element, and vascular inhibitors to target the endothelial component. Hence, when treating bone metastasis, one must treat the malignant epithelial cell that has spread to the bone and the osteoblasts, osteoclasts, and endothelial cells in the bone that support and sustain it.
There are many ways to improve the therapeutic benefits of radiopharmaceuticals. However, it remains unknown whether targeting both the tumor and bone compartments would improve their therapeutic efficacy. It also remains to be established whether giving certain radiopharmaceuticals repeatedly or in combination with other treatment modalities (eg, hormone ablative therapy, chemotherapy) is safe, feasible, and advantageous over their one-time administration or their administration alone. More studies are also needed to determine the optimal sequence and schedule of these combination treatments using radiopharmaceuticals.
In general, patients with advanced widespread bone metastases may benefit from an initial response to systemic treatment (such as hormone ablative therapy) followed by consolidation bone-targeted therapy using radiopharmaceuticals. Although patients with unfavorable prognostic features, such as severe bone marrow suppression (eg, severe thrombocytopenia), proximal long bone involvement, or superscan may not be able to tolerate or benefit from treatment using radiopharmaceuticals from the outset or alone, they could withstand the myelosuppressive effects of radiopharmaceuticals much better when their bone marrow reserve improves after response to systemic treatment.
For the same reasons, patients with advanced castrate-resistant prostate cancer and compromised bone marrow reserve may respond to secondary hormone ablative therapy (eg, ketoconazole, diethylstibesterol, low-dose dexamethasone) or even chemotherapeutic agents or regimens (eg, cyclophosphamide, vincristine, and dexamethasone [CVD]28 for patients with severe thrombocytopenia) that render consolidation therapy using radiopharmaceuticals safer and more feasible. This consideration, along with evidence that radiopharmaceuticals provide the best results in patients with a moderate tumor burden in bone18,29 and that treatment delays the development of new bone pain in preexisting, clinically silent sites22,24, suggests that earlier intervention using radiopharmaceuticals for the treatment of bone metastases may be warranted.
Hormone Ablative Therapy
There is ample evidence that radiation therapy synergizes with hormonal ablation in their antitumor effect for the treatment of primary prostate cancer.30,31 It is of interest to know if this synergistic antitumor effect also applies to radiopharmaceuticals for the systemic treatment of prostate cancer bone metastasis. After hormone ablative therapy, a flare reaction predicts therapeutic response and indicates rapid bone repair and increased osteoblastic activity within the affected bone metastasis.32,33 Indeed, Bushnell and colleagues demonstrated an increased uptake of bone-seeking radiopharmaceuticals 4 weeks to 3 months following the start of hormone ablative therapy.34
Therefore, one way to enhance the therapeutic efficacy of bone-seeking radiopharmaceuticals is to increase their tumor-absorbed dose and to deliver it at the time of a flare reaction in the bone metastases. Whether this therapeutic strategy provides improved clinical outcome remains to be determined. A randomized phase II trial (2003-922) using this strategy (chemohormonal therapy with or without one dose of 89Sr) for patients with androgen-dependent prostate cancer has been completed at The University of Texas M. D. Anderson Cancer Center.
Chemotherapy
Radiosensitization is a well-recognized and widely used modality for improving the overall efficacy of radiation therapy and perhaps also that of bone-seeking radiopharmaceuticals. The cytotoxic effect of chemotherapy may render cancer cells more vulnerable to radiation damage. Geldof and colleagues demonstrated this synergism in vitro when they studied the effect of 186Re-HEDP combined with cisplatin on prostate cancer cells.35 Mertens and colleagues conducted clinical studies that suggest a synergy between chemotherapy and radiopharmaceuticals.36
It is important to point out that certain chemotherapeutic agents, such as taxanes, anthracyclines, and platins, inherently possess antitumor as well as radiosensitizing properties. Consequently, use of these cytotoxic agents is beneficial not only because they enhance the antitumor effects of radiopharmaceuticals (Table 5) but also because they are efficacious by themselves for the treatment of prostate cancer bone metastases.
Table 5.
Chemoradiation Results Using Bone-Seeking Radiopharmaceuticals
| Study, yr (Phase, n) | Treatment | Response | Survival (mos) |
|---|---|---|---|
| Pagliaro et al.,40 2003 (I/II, 15) | 89Sr 55 mCi/kg + | 13% (PSA≤50%) | 8 |
| Gemcitabine 800 mg/m2 ×6 | |||
|
| |||
| Lam et al.,39 2009 (I, 12) | 188Re 37 MBq/kg + | NR | NR |
| Capecitabine ≤2,500 mg/m2/d | |||
|
| |||
| Sciuto et al.,53 1996 (RII, 30) | 89Sr 148 MBq + | 58% (pain relief) | 5.7 |
| Carboplatin 100 mg/m2 d1, 21 | 87%, P=.025 | 8.1 | |
|
| |||
| Mertens et al.,36 1992 (II, 17) | 89Sr 148 MBq + | 8+ | |
| Cisplatin 35 mg/m2 | 55% (pain relief) | ||
|
| |||
| Sciuto et al.,54 2002 (RII, 70) | 89Sr 148 MBq + | 63% (pain relief) | 6 |
| Cisplatin 50 mg/m2 | 91%, P<.01 | 9 | |
|
| |||
| Tu et al.,55 1996 (I/II, 25) | 89Sr 55 μCi/kg ≤3 + | 32% (PSA≤75%) | 15 |
| Doxorubicin 20 mg/m2 ≤×20 | |||
|
| |||
| Tu et al.,10 2001 (RII, 72) | 89Sr 55 μCi/kg, s/p KAVE + | 7 mo (TTP) | 17 |
| Doxorubicin 20 mg/m2 ×6 | 14 mo, P<.001 | 28, P=.001 | |
|
| |||
| Akerley et al.,56 2002 (II, 44) | 89Sr 2.2 MBq/kg + | 48% (PSA≤50%) | 13 |
| Vinblastine 4 mg/m2/estram ×8 | |||
|
| |||
| Amato et al.,44 2008 (II, 29) | 89Sr 148 MBq + | 78% (PSA≤50%) | 23 |
| KATE | |||
|
| |||
| Fizazi et al.,45 2009 (II, 43) | 153Sm-EDTMP 37 MBq/kg, s/p | 77% (PSA≤50%) | 29 |
| Docetaxel/estram + | |||
| Docetaxel 20 mg/m2 ×6 | |||
|
| |||
| Tu et al.,57 2009 (I, 18) | 153Sm-EDTMP 1 mCi/kg ×2 + | 28% (PSA≤50%) | NR |
| Docetaxel ≤35 mg/m2 d1, 8, 15 | |||
|
| |||
| Morris et al.,58 2009 (I, 28) | 153Sm-EDTMP 1 mCi/kg ≤×6 + | 54% (PSA≤50%) | NR |
| Docetaxel ≤75 mg/m2 ≤×13 | |||
RII, randomized phase II; PSA, prostate-specific antigen; KATE, ketoconazole combined with doxorubicin alternating with estramustine combined with paclitaxel;
KAVE, ketoconazole combined with doxorubicin alternating with estramustine combined with vinblastine; EDTMP, ethylenediaminetetramethylene phosphonic acid; TTP, time-to-progression; NR, not reported
A flare reaction may also occur after chemotherapy for the treatment of prostate cancer.32 The fact that a flare reaction represents bone healing or increased bone formation within the osseous metastasis has important implications for the optimal timing of radiopharmaceutical delivery after a response to chemotherapy.
A caveat about the selection of chemotherapeutic agents when combined with bone-seeking radiopharmaceuticals for the treatment of prostate cancer: they ought to possess antitumor activity, have radiosensitizing properties, and be minimally myelosuppressive. Therefore, it may not be prudent to use capecitabine37 or gemcitabine38, which possess some radiosensitizing properties but minimal antitumor effects, in combination with radiopharmaceuticals.39,40 When radiopharmaceuticals are combined with chemotherapeutic agents, such as gemcitabine, that provide minimal antitumor activity but cause substantial myelosuppression in the face of diffuse tumor infiltration in the bone marrow, the benefit-risk ratio is likely to be unfavorable (Table 5).40 Also, because the clinical efficacy of carboplatin and etoposide41 is not guaranteed and because their hematologic toxic effects are potentially prohibitive, these chemotherapeutic agents are also not ideal agents for combination with radiopharmaceuticals. We propose that the following (modified) chemotherapeutic regimens (after prior docetaxel treatment) for the treatment of prostate cancer before the administration of radiopharmaceuticals: doxorubicin 20 mg/m2 i.v. on days 1, 8, and 15 q 28 days combined with maintenance ketoconazole;42 cyclophosphamide 150 mg p.o. on days 1–21 q 28 days combined with vincristine 1 mg i.v. weekly and maintenance dexamethasone (CVD);28 and paclitaxel 100 mg/m2 i.v. on days 1, 8, and 15 combined with maintenance diethylstibesterol.43 These agents or regimens are efficacious; doxorubicin and paclitaxel have radiosensitizing activity; a low-dose weekly schedule and CVD are minimally myelosuppressive.
Another important aspect of multimodality therapy that needs to be addressed is the optimal sequence for combining chemotherapy with radiopharmaceuticals. Because not all patients will respond to either treatment alone and because any clinical benefits or toxic effects could be additive, if not synergistic, it is important to personalize care and select only patients who respond to the induction chemotherapy for consolidation bone-targeted therapy using pharmaceuticals. In this manner, the therapeutic benefit could be enhanced and the potential toxic effects reduced. It remains to be determined how to combine which chemotherapeutic agents with what radiopharmaceuticals. For example, would it be advantageous to add a radiopharmaceutical as soon as a response is achieved (ie, after one cycle of chemotherapy) or after a maximum response (ie, after 4–6 cycles of chemotherapy)? Tu and colleagues,10 Amato and colleagues,44 and Fizazi and colleagues45 have explored this therapeutic strategy using KAVE or KATE with 89Sr and docetaxel with 153Sm-EDTMP, respectively. Randomized phase III trials designed to confirm these preliminary results (Table 5) are currently being conducted by the Cancer Treatment Support Unit (CTSU) of the National Cancer Institute (MDA-3410) and the Cancer Research UK (Trapeze).
Selected Targeted Agents
There is scant evidence supporting the combination of selected targeted agents with bone-seeking radiopharmaceuticals. Theoretically, any agents with radiosensitizing properties and antitumor effects may be used to enhance the therapeutic efficacy of radiopharmaceuticals. For example, certain selected targeted agents, such as abiraterone, tyrosine kinase inhibitors, or COX-2 inhibitors, could affect the onco-niche in such a manner that patients with advanced prostate cancer would experience prolonged remission or increased survival time even though they still harbor viable cancer cells after treatment using these agents in combination with radiopharmaceuticals or in a maintenance fashion after radiopharmaceuticals.
It is important to point out that in Tu and colleagues’ randomized phase II trial, which showed an overall survival improvement, only patients who had benefited from chemotherapy (namely KAVE) were randomized to receive 89Sr. Furthermore, patients continued maintenance ketoconazole until disease progression.10 Considering how well some patients responded to ketoconazole and how ketoconazole might have affected the onco-niche by inhibiting osteoblast proliferation/differentiation (unpublished data), we believe that it is entirely plausible that an effective maintenance drug such as ketoconazole could have contributed to the improved clinical efficacy and outcome of this particular multimodality program. It is of interest whether a novel compound related to ketoconazole, namely abiraterone,46 would provide similar, if not superior, results in a combination regimen or maintenance fashion.
Finally, it remains to be determined whether certain tyrosine kinase inhibitors, such as sunitinib and imatinib, 47,48 that have radiosensitizing properties but possess only marginal antitumor activity against prostate cancer will improve the clinical efficacy of radiopharmaceuticals. It is unclear whether antiangiogenesis agents by promoting hypoxia will counteract the effect of radiation, which induces the expression of proangiogenic factors. Similarly, it is unknown whether agents that target the PI3k/Akt/PTEN pathway will attenuate the effect of radiation, which mediates transient increased mTOR function. It remains to be clarified whether other radiosensitizing agents with some putative antitumor activity, such as COX-2 inhibitors, soy isoflavones, and curcumin, can be used to enhance the effects of radiopharmaceuticals or in a maintenance setting after radiopharmaceuticals for the treatment of prostate cancer bone metastases.
Conclusions
Accumulating data suggest that bone-seeking radiopharmaceuticals can be used to control bone metastasis and improve the clinical outcome of patients with prostate cancer and bone metastases. These agents target both cancer cells and the onco-niche. They should be considered as another therapeutic option in our armamentarium against bone metastases. Additional studies need to be performed to determine whether newer generations or repeated dosing of radiopharmaceuticals will accentuate their antitumor effects and whether combining radiopharmaceuticals with hormone ablative therapy, chemotherapy, or targeted therapy will their enhance clinical efficacy.
Acknowledgments
This work was supported in part by the Cancer Therapy Evaluation Program (CTEP), Division of Cancer Treatment and Diagnosis, National Cancer Institute (to S.-M.T.), SPORE P50 CA 90270, the National Institutes of Health (CA 86342), and the Association for the Cure of Cancer of the Prostate (CaPCURE) (to S.-H.L. and C.J.L.). We thank Lionel Santibañez for editorial assistance.
Glossary and abbreviations
- 40 μCi
1.48 MBq
- 20 cGy
1 MBq
- CVD
cyclophosphamide, vincristine, and dexamethasone
- DTPA
diethylenetriaminepentaacetic acid
- EDTMP
ethylenediaminetetramethylene phosphonic acid
- HEDP
1-1-hydroethylidene diphosphate
- KATE
ketoconazole combined with doxorubicin alternating with estramustine combined with paclitaxel
- KAVE
ketoconazole combined with doxorubicin alternating with estramustine combined with vinblastine
- MDP
methyl diphosphonate
References
- 1.Park YG, Zhao X, Lesueur F, et al. Sipa 1 is a candidate for underlying the metastasis efficiency modifier locus Mtes 1. Nat Genet. 2005;37:1055–1062. doi: 10.1038/ng1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grum-Schwensen B, Klingelhofer J, Berg CH, et al. Suppression of tumor development and metastasis formation in mice lacking the S100A4(mts1) gene. Cancer Res. 2005;65:3772–3780. doi: 10.1158/0008-5472.CAN-04-4510. [DOI] [PubMed] [Google Scholar]
- 3.Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333–337. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- 4.Tu SM, Lin SH, Logothetis CJ. Stem-cell origin of metastasis and heterogeneity in solid tumours. Lancet Oncol. 2002;3:508–513. doi: 10.1016/s1470-2045(02)00820-3. [DOI] [PubMed] [Google Scholar]
- 5.Jacobsson H, Näslund I. Reduced incidence of bone metastases in irradiated areas after external radiation therapy of prostatic carcinoma. Int J Radiat Oncol Biol Phys. 1991;20:1297–1303. doi: 10.1016/0360-3016(91)90241-u. [DOI] [PubMed] [Google Scholar]
- 6.Silberstein EB, Elgazzar AH, Kapilivsky A. Phosphorus-32 radiopharmaceuticals for the treatment of painful osseous metastases. Sem Nucl Med. 1992;22:17–27. doi: 10.1016/s0001-2998(05)80153-9. [DOI] [PubMed] [Google Scholar]
- 7.Nair N. Relative efficacy of 32P and 89Sr in palliation of skeletal metastases. J Nucl Med. 1999;40:256–261. [PubMed] [Google Scholar]
- 8.Robinson RG, Blake GM, Preston DF, et al. Strontium-89: treatment results and kinetics in patients with painful metastatic prostate and breast cancer in bone. Radiographics. 1989;9:271–281. doi: 10.1148/radiographics.9.2.2467331. [DOI] [PubMed] [Google Scholar]
- 9.Blake GM, Zivanovic MA, Blaquiere RM, et al. Strontium-89 therapy: measurement of absorbed dose to skeletal metastases. J Nucl Med. 1988;29:549–557. [PubMed] [Google Scholar]
- 10.Tu SM, Millikan RE, Mengistu B, et al. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet. 2001;357:336–341. doi: 10.1016/S0140-6736(00)03639-4. [DOI] [PubMed] [Google Scholar]
- 11.Tu SM, Kim J, Pagliaro LC, et al. Therapy tolerance in selected patients with androgen-independent prostate cancer following strontium-89 combined with chemotherapy. J Clin Oncol. 2005;23:7904–7910. doi: 10.1200/JCO.2005.01.2310. [DOI] [PubMed] [Google Scholar]
- 12.Ahonen A, Joensuu H, Hiltunen J, et al. Samarium-153-EDTMP in bone metastases. J Nucl Biol Med. 1994;38(4 suppl 1):123–127. [PubMed] [Google Scholar]
- 13.Maxon HR, Deutsch EA, Thomas SR, et al. Re-186(Sn) HEDP for treatment of multiple metastatic foci in bone: human biodistribution and dosimetric studies. Radiology. 1988;166:501–507. doi: 10.1148/radiology.166.2.3122267. [DOI] [PubMed] [Google Scholar]
- 14.Palmedo H, Manka-Waluch A, Albers P, et al. Repeated bone-targeted therapy for hormone-refractory prostate carcinoma: randomized phase II trial with the new, high-energy radiopharmaceutical rhenium-188 hydroxyethylidenediphosphonate. J Clin Oncol. 2003;21:2869–2875. doi: 10.1200/JCO.2003.12.060. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson S, Larsen RH, Fosså SD, et al. First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin Cancer Res. 2005;11:4451–4459. doi: 10.1158/1078-0432.CCR-04-2244. [DOI] [PubMed] [Google Scholar]
- 16.Nilsson S, Franzén L, Parker C, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007;8:587–594. doi: 10.1016/S1470-2045(07)70147-X. [DOI] [PubMed] [Google Scholar]
- 17.Breen SL, Powe JE, Porter AT. Dose estimation in strontium-89 radiotherapy of metastatic prostatic carcinoma. J Nucl Med. 1992;33:1316–1323. [PubMed] [Google Scholar]
- 18.Laing AH, Ackery DM, Bayly RJ, et al. Strontium-89 chloride for pain palliation in prostatic skeletal malignancy. Br J Radiol. 1991;64:816–822. doi: 10.1259/0007-1285-64-765-816. [DOI] [PubMed] [Google Scholar]
- 19.Mertens WC, Stitt L, Porter AT. Strontium 89 therapy and relief of pain in patients with prostatic carcinoma metastatic to bone: a dose response relationship? Am J Clin Oncol. 1993;16:238–242. doi: 10.1097/00000421-199306000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Buchali K, Correns HJ, Schuerer M, et al. Results of a double blind study of 89-strontium therapy of skeletal metastases of prostatic carcinoma. Eur J Nucl Med. 1988;14:349–351. doi: 10.1007/BF00254382. [DOI] [PubMed] [Google Scholar]
- 21.Lewington VJ, McEwan AJ, Ackery DM, et al. A prospective randomized double-blind crossover study to examine the efficacy of strontium-89 in pain palliation in patients with advanced prostate cancer metastatic to bone. Eur J Cancer. 1991;27:954–958. doi: 10.1016/0277-5379(91)90257-e. [DOI] [PubMed] [Google Scholar]
- 22.Quilty PM, Kirk D, Bolger JJ, et al. A comparison of the palliative effects of strontium-89 and external beam radiotherapy in metastatic prostate cancer. Radiother Oncol. 1994;31:33–40. doi: 10.1016/0167-8140(94)90411-1. [DOI] [PubMed] [Google Scholar]
- 23.Oosterhof GO, Roberts JT, de Reijke TM, et al. Strontium(89) chloride versus palliative local field radiotherapy in patients with hormonal escaped prostate cancer: a phase II study of the European Organisation for Research and Treatment of Cancer, Genitourinary Group. Eur Urol. 2003;44:519–526. doi: 10.1016/s0302-2838(03)00364-6. [DOI] [PubMed] [Google Scholar]
- 24.Porter AT, McEwan AJ, Powe JE, et al. Results of a randomized phase-III trial to evaluate the efficacy of strontium-89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. Int J Radiat Oncol Biol Phys. 1993;25:805–813. doi: 10.1016/0360-3016(93)90309-j. [DOI] [PubMed] [Google Scholar]
- 25.Smeland S, Erikstein B, Aas M, et al. Role of strontium-89 as adjuvant to palliative external beam radiotherapy is questionable: results of a double-blind randomized study. Int J Radiat Oncol Biol Phys. 2003;56:1397–1404. doi: 10.1016/s0360-3016(03)00274-8. [DOI] [PubMed] [Google Scholar]
- 26.Collins C, Eary JF, Donaldson G, et al. Samarium-153-EDTMP in bone metastases of hormone refractory prostate carcinoma: a phase I/II trial. J Nucl Med. 1993;34:1839–1844. [PubMed] [Google Scholar]
- 27.Turner JH, Claringbold PG. A phase II study of treatment of painful multifocal skeletal metastases with single and repeated dose samarium-153 ethylenediaminetetramethylene phosphonate. Eur J Cancer. 1991;27:1084–1086. doi: 10.1016/0277-5379(91)90297-q. [DOI] [PubMed] [Google Scholar]
- 28.Daliani DD, Assikis V, Tu SM, et al. Phase II trial of cyclophosphamide, vincristine, and dexamethasone in the treatment of androgen-independent prostate cancer. Cancer. 2003;97:561–567. doi: 10.1002/cncr.11078. [DOI] [PubMed] [Google Scholar]
- 29.de Klerk JM, Zonnenberg BA, van Het Schip AD, et al. Can bone marrow scintigraphy predict platelet toxicity after treatment with 186Re-HEDP? Nucl Med Commun. 1999;20:833–836. doi: 10.1097/00006231-199909000-00009. [DOI] [PubMed] [Google Scholar]
- 30.D’Amico AV, Chen MH, Renshaw AA, et al. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008;299:289–295. doi: 10.1001/jama.299.3.289. [DOI] [PubMed] [Google Scholar]
- 31.Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–2527. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 32.Pollen JJ, Shlaer WJ. Osteoblastic response to successful treatment of metastatic cancer of the prostate. Am J Roentgenol. 1979;132:927–931. doi: 10.2214/ajr.132.6.927. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu N, Masuda H, Yamanaka H, et al. Fluorodeoxyglucose positron emission topography scan of prostate cancer bone metastases with flare reaction after endocrine therapy. J Urol. 1999;161:608–609. [PubMed] [Google Scholar]
- 34.Bushnell DL, Madsen M, Kahn D, et al. Enhanced uptake of 99Tcm-MDP in skeletal metastases from prostate cancer following initiation of hormone treatment: potential for increasing delivery of therapeutic agents. Nucl Med Commun. 1999;20:875–881. doi: 10.1097/00006231-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Geldof AA, de Rooij L, Versteegh RT, et al. Combination 186Re-HEDP and cisplatin supra-additive treatment effects in prostate cancer cells. J Nucl Med. 1999;40:667–671. [PubMed] [Google Scholar]
- 36.Mertens WC, Porter AT, Reid RH, et al. Strontium-89 and low-dose infusion cisplatin for patients with hormone refractory prostate carcinoma metastatic to bone: a preliminary report. J Nucl Med. 1992;33:1437–1443. [PubMed] [Google Scholar]
- 37.Spicer J, Plunkett T, Somaiah N, et al. Phase II study of oral capecitabine in patients with hormone-refractory prostate cancer. Prostate Cancer Prostatic Dis. 2005;8:364–368. doi: 10.1038/sj.pcan.4500821. [DOI] [PubMed] [Google Scholar]
- 38.Morant R, Bernhard J, Maibach R, et al. Response and palliation in a phase II trial of gemcitabine in hormone-refractory metastatic prostatic carcinoma. Swiss Group for Clinical Cancer research (SACC) Ann Oncol. 2000;11:183–188. doi: 10.1023/a:1008332724977. [DOI] [PubMed] [Google Scholar]
- 39.Lam MG, Bosma TB, van Rijk PP, et al. (188)Re-HEDP combined with capecitabine in hormone-refractory prostate cancer patients with bone metastases: a phase I safety and toxicity study. Eur J Nucl Med Mol Imaging. 2009;36:1425–1433. doi: 10.1007/s00259-009-1119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pagliaro LC, Delpassand ES, Williams D, et al. A phase I/II study of strontium-89 combined with gemcitabine in the treatment of patients with androgen-independent prostate carcinoma and bone metastases. Cancer. 2003;97:2988–2994. doi: 10.1002/cncr.11412. [DOI] [PubMed] [Google Scholar]
- 41.Oliver I, Keefe D, Myers M. Phase II study of prolonged ambulatory infusion carboplatin and oral etoposide for patients progressing through hormonal therapy for prostate cancer. Intern Med J. 2005;35:405–408. doi: 10.1111/j.1445-5994.2005.00864.x. [DOI] [PubMed] [Google Scholar]
- 42.Sella A, Kilbourn R, Amato R, et al. Phase II study of ketoconazole combine with weekly doxorubicin in patients with androgen-independent prostate cancer. J Clin Oncol. 1994;12:683–688. doi: 10.1200/JCO.1994.12.4.683. [DOI] [PubMed] [Google Scholar]
- 43.Kelly WK, Curley T, Slovin S, et al. Paclitaxel, estramustine phosphate, and carboplatin in patients with advanced prostate cancer. J Clin Oncol. 2001;19:44–53. doi: 10.1200/JCO.2001.19.1.44. [DOI] [PubMed] [Google Scholar]
- 44.Amato RJ, Hernandez-McClain J, Henary H. Bone-targeted therapy: phase II study of strontium-89 in combination with alternating weekly chemohormonal therapies for patients with advanced androgen-independent prostate cancer. Am J Clin Oncol. 2008;31:532–538. doi: 10.1097/COC.0b013e318172aa92. [DOI] [PubMed] [Google Scholar]
- 45.Fizazi K, Beuzeboc P, Lumbroso J, et al. Phase II trial of consolidation docetaxel and samarium-153 in patients with bone metastases from castration-resistant prostate cancer. J Clin Oncol. 2009;27:2429–2435. doi: 10.1200/JCO.2008.18.9811. [DOI] [PubMed] [Google Scholar]
- 46.Attard G, Reid AH, A’Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–3748. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dror Michaelson M, Regan MM, Oh WK, et al. Phase II study of sunitinib in men with advanced prostate cancer. Ann Oncol. 2009;20:913–920. doi: 10.1093/annonc/mdp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mathew P, Thall PF, Bucana CD, et al. Platelet-derived growth factor receptor inhibition and chemotherapy for castration-resistant prostate cancer with bone metastases. Clin Cancer Res. 2007;13:5816–5824. doi: 10.1158/1078-0432.CCR-07-1269. [DOI] [PubMed] [Google Scholar]
- 49.Sartor O, Reid RH, Hoskin PJ, et al. Samarium-153-Lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology. 2004;63:940–945. doi: 10.1016/j.urology.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 50.Lam MG, de Klerk JM, van Rijk PP, et al. Bone seeking radiopharmaceuticals for palliation of pain in cancer patients with osseous metastases. Anticancer Agents Med Chem. 2007;7:381–397. doi: 10.2174/187152007781058596. [DOI] [PubMed] [Google Scholar]
- 51.Resche I, Chatal JF, Pecking A, et al. A dose-controlled study of 153Sm-ethylenediaminetetramethylenephosphonate (EDTMP) in the treatment of patients with painful bone metastases. Eur J Cancer. 1997;33:1583–1591. doi: 10.1016/s0959-8049(97)00155-x. [DOI] [PubMed] [Google Scholar]
- 52.Serafini AN, Houston SJ, Resche I, et al. Palliation of pain associated with metastatic bone cancer using samarium-153 lexidronam: a double-blind placebo-controlled clinical trial. J Clin Oncol. 1998;16:1574–1581. doi: 10.1200/JCO.1998.16.4.1574. [DOI] [PubMed] [Google Scholar]
- 53.Sciuto R, Maini CL, Tofani A, et al. Radiosensitization with low-dose carboplatin enhances pain palliation in radioisotope therapy with strontium-89. Nucl Med Commun. 1996;17:799–804. doi: 10.1097/00006231-199609000-00011. [DOI] [PubMed] [Google Scholar]
- 54.Sciuto R, Festa A, Rea S, et al. Effects of low-dose cisplatin on 89Sr therapy for painful bone metastases from prostate cancer: a randomized clinical trial. J Nucl Med. 2002;43:79–86. [PubMed] [Google Scholar]
- 55.Tu SM, Delpassand ES, Jones D, et al. Strontium-89 combined with doxorubicin in the treatment of patients with androgen-independent prostate cancer. Urol Oncol. 1996;2:191–197. doi: 10.1016/s1078-1439(97)00013-6. [DOI] [PubMed] [Google Scholar]
- 56.Akerley W, Butera J, Wehbe T, et al. A multiinstitutional, concurrent chemoradiation trial of strontium-89, estramustine, and vinblastine for hormone refractory prostate carcinoma involving bone. Cancer. 2002;94:1654–1660. doi: 10.1002/cncr.10437. [DOI] [PubMed] [Google Scholar]
- 57.Tu SM, Mathew P, Wong FC, et al. Phase I study of concurrent weekly docetaxel and samarium-153 lexidronam in patients with castration-resistant metastatic prostate cancer. J Clin Oncol. 2009;27:3319–3324. doi: 10.1200/JCO.2008.20.5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morris MJ, Pandit-Taskar N, Carrasquillo J, et al. Phase I study of samarium-153 lexidronam with docetaxel in castration-resistant metastatic prostate cancer. J Clin Oncol. 2009;27:2417–2418. doi: 10.1200/JCO.2008.20.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]


