Abstract
The ubiquitously expressed plasma membrane Na+–H+ exchanger NHE1 is a 12 transmembrane-spanning protein that directs important cell functions such as homeostatic intracellular volume and pH control. The 315 amino acid cytosolic tail of NHE1 binds plasma membrane phospholipids and multiple proteins that regulate additional, ion-translocation independent functions. This review focuses on NHE1 structure/function relationships, as well as the role of NHE1 in kidney proximal tubule functions, including pH regulation, vectorial Na+ transport, cell volume control and cell survival. The implications of these functions are particularly critical in the setting of progressive, albuminuric kidney diseases, where the accumulation of reabsorbed fatty acids leads to disruption of NHE1-membrane phospholipid interactions and tubular atrophy, which is a poor prognostic factor for progression to end stage renal disease. This review amplifies the vital role of the proximal tubule NHE1 Na+–H+ exchanger as a kidney cell survival factor.
Keywords: Na+–H+ exchange, Ion transport, Apoptosis, Cell survival, Tubular atrophy, Chronic kidney disease
Introduction
Discovery of Na+–H+ exchange
In 1966 Mitchell hypothesized the existence of an electroneutral transport mechanism that couples the exchange, or antiport, of cations and protons across the mitochondrial inner membrane [1]. Subsequent studies of bacterial membrane transport processes provided the first evidence of an electroneutral Na+–H+ exchanger [2, 3], and Na+–H+ exchanger activity was described shortly thereafter in mammals; specifically in apical membrane preparations from the small intestines and renal proximal tubules of rats [4].
The SLC9A family
The first mammalian Na+–H+ exchanger gene to be cloned, now known as SLC9A1, encodes the ubiquitously expressed, amiloride-sensitive Na+–H+ exchanger NHE1 (sometimes notated as NHE-1) [5]. SLC9A1 maps to human chromosome 1p36.11 and to chromosome 4D2.3 in mice. We now know that the SLC9A gene family includes nine members encoding NHE1-NHE9,1 all of which are bona fide Na+–H+ exchangers. NHE1-5 localize primarily to the plasma membrane, in contrast to NHE6-9 that reside in organelle membrane compartments [6, 7]. Of the plasma membrane NHEs, NHE1 and NHE2 are expressed in multiple tissues, whereas NHE3 is restricted primarily to kidney and intestine [8], NHE4 mainly to stomach and kidney [9] and NHE5 predominantly to brain, testis and spleen [10, 11]. Following the cloning of numerous Na+–H+ exchanger genes from multiple species, it is now appreciated that mammalian NHE proteins share no substantial amino acid sequence identity with their bacterial, fungal, or plantal counterparts. However, three-dimensional modeling predictions based on the crystal structure of a bacterial electrogenic Na+–2H+ antiporter (NhaA) [12] indicate that all NHEs are likely to adopt a similar three-dimensional conformation and thus may share common ancestry and transport mechanisms [13, 14].
The SLC9B and SLC9C families
The wider mammalian SLC9 superfamily includes two other, smaller gene families: SLC9B and SLC9C. Each family includes two members, neither of which exhibit substantial sequence homology to NHEs of the SLC9A family. However, the two SLC9B family members do exhibit sequence similarity to cation/proton exchangers from lower organisms [15]. No functional data is available for the testes-expressed SLC9B1 product, aka the Na+–H+ exchanger domain-containing protein NHEDC1 [16]. The SLC9B2 product NHEDC2, also known as NHA2, exhibits a broader expression pattern and appears capable of NHE activity inasmuch as it promotes Na+ tolerance at acidic extracellular pH when heterologously expressed in yeast [17]. In the kidney, NHA2 localizes to the distal convoluted tubule, where it has been speculated to play a role in blood pressure control [6, 17]. The SLC9C1 product ‘NHE10’ is expressed in osteoclasts and sperm [18]. Although innate NHE10-regulated Na+–H+ activity has been difficult to demonstrate, when expressed as a chimeric protein that includes the first transmembrane span of NHE1, trafficking to the plasma membrane was enhanced and Na+–H+ exchange was detectable [19]. The function of SLC9C2 is yet to be determined.
NHE1 action
Substrates and inhibitors
NHE1, in common with NHE2-5, mediates the electroneutral (1:1 stoichiometry) exchange of Na+ and H+ across the plasma membrane of cells, typically exploiting the inwardly directed Na+ gradient established by the Na+–K+ ATPase to extrude H+, especially when intracellular pH is acidic. NHE1 is quiescent in resting cells [20, 21], but can be activated by a variety of stimuli, as discussed later. The K m for extracellular Na+ is ~6–10 mM, and the pK i (reporting the K m for intracellular H+ in pH units) is ~6.6–6.8 [22, 23]. 2 It has also been demonstrated that NHE1 is capable of operating in reverse mode [24], and also as a Na+–Li+ exchanger [25, 26]. NHE1, like NHE2-5, is inhibited by amiloride and its derivatives [e.g., 5-(N-ethyl-N-isopropyl)amiloride EIPA] [27], enabling the pharmacological distinction of NHEs from amiloride-insensitive Na+/HCO3 − co-transporters (NBCs), one of which (NBCe1-A) sits alongside NHE1 and regulates intracellular Na+ and pH in the kidney proximal tubule [28]. Furthermore, NHE1 can be pharmacologically distinguished from other NHEs by virtue of differences in inhibitory constants for amiloride. For example, EIPA is at least one order of magnitude more selective for NHE1 (K i ~ 0.02 µM) [29, 30] compared to any of the other plasma membrane expressed NHEs (K i ~ 0.5–500 µM) [23, 29–33]; benzoyl guanidine derivatives, such as cariporide (aka HOE692) exhibit even greater NHE1 specificity (reviewed in Ref. [27]). The precise mode of action of these drugs is unknown, but mutagenesis studies reveal that hydrophobic residues in the vicinity of the fourth transmembrane-spanning domain (TM4, NHE1 residues 160–180)3 of NHE1 are major, albeit not exclusive, determinants of amiloride and cariporide sensitivity [34–39]. It is interesting to note that a non-canonical splice variant of NHE1 from reticulocytes, which lacks this TM region, is thought to contribute to the amiloride-insensitive Na+–Li+ exchange activity evident in erythrocytes [26].
Mode of action
NHE1 is hypothesized to operate via an alternating access model whereby Na+ binding to an extracellular NHE1 site triggers a conformational change that translocates Na+ across the membrane. The NHE1 structural alteration simultaneously exposes a cytoplasmic substrate-binding site for intracellular H+, permitting H+ binding, reversal of the conformational change, and export of H+ [40]. NHE1 forms homodimers with inter-monomer interfaces between adjacent TM domains and between adjacent cytosolic domains [41–43]. Component monomers within a dimer are capable of acting independently; inactivating mutations in one monomer do not exert a dominant negative effect on NHE1 activity, at least at acidic cytosolic pH [41, 44]. However, dimers may co-operate to perform coupled exchange (i.e., 2Na+–2H+ exchange) at neutral or alkaline pH [44, 45], a mode of action that is supported by the crystal structures of the distantly related bacterial Na+–H+ antiporter NhaA [46]. NHE1 can also functionally couple with other transporters, influencing their actions and producing novel net transport functions. Examples of transporters influenced or functionally linked to NHE1 including Na+–Ca2+ exchangers (NHE1 loads intracellular Na+, thereby promoting Ca2+ influx) [47, 48], Cl−–HCO3 − exchangers (extruded H+ and HCO3 − titrate each other, resulting in net NaCl influx) [49], and the H+-coupled peptide transporter PEPT2 (NHE1 disposes of intracellular H+, promoting peptide influx) [50].
NHE1 distribution
The number of cell types that express NHE1 is so diverse that the transporter is often described as being ubiquitous. Reports of mammalian cell types that lack NHE1 (e.g., some dopaminergic neurons and microglia) [51, 52] are an exception, making immortalized cell lines that do not express NHE1 a valuable commodity for heterologous NHE1 expression studies [53]. Within the kidney, NHE1 exhibits the broadest distribution of the plasma membrane expressed NHEs, with documented expression in all nephron segments with the exception of the macula densa and intercalated cells of the distal nephron [54, 55]. In some cell types, NHE1 expression coincides with that of other NHEs. For example, proximal tubules express basolateral NHE1 and apical NHE2 and NHE3, while renal thick ascending limb epithelia express basolateral NHE1 and NHE4, as well as apical NHE2 and NHE3 [54, 56–58]. The half-life of plasma membrane NHE1 is relatively long (~24 h) and, unlike NHE3, NHE1 surface expression is not significantly regulated by trafficking or recycling [59–61]. However, a recent report demonstrated NHE1 expression may be regulated to some extent by ubiquitination and proteasomal degradation [62]. Although typically located in the basolateral membrane of diverse epithelia, NHE1 is found in the apical membrane of choroid plexus epithelia [63]. Besides diverse epithelia, other notable NHE1-expressing cell types include neurons, astrocytes [64], peripheral blood cells [65], myocytes [66], and sperm [67].
NHE1 structure–function
Domain structure
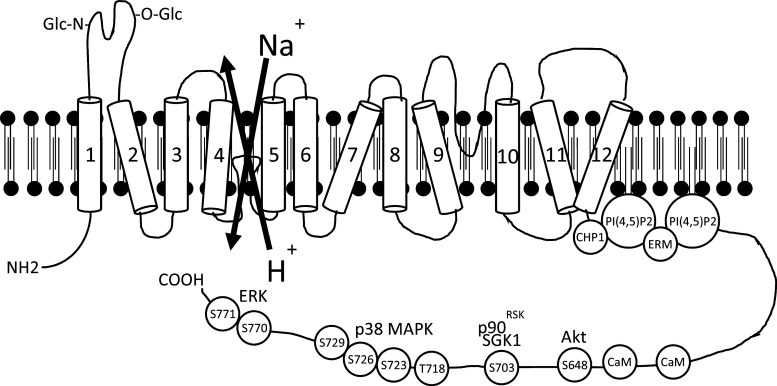
No high-resolution crystal structures exist for any SLC9 family members, but circular dichroism, electron paramagnetic resonance, nuclear magnetic resonance and mutagenesis studies have yielded some insights [68]. The biggest potential breakthrough has arisen from solving the structures of the bacterial Na+–H+ exchangers NhaA [12] and NapA [46]. Although sequence homology between bacterial Na+–H+ exchangers and mammalian NHEs at the amino acid level is very low, there are significant structural similarities, and efforts are underway to create a model NHE1 structure extrapolated from NhaA, which can then be reconciled with the wealth of NHE1 structure–function studies [13, 40, 69]. Extrapolation from NhaA confirms common features such as a short N-terminal cytosolic tail, an ion-translocating domain with 12 TMs, and a relatively long carboxy-terminal cytosolic domain that serves a regulatory function. The features of the NHE1 transport and regulatory domains are considered below and shown in Fig. 1.
Fig. 1.
Schematic diagram of NHE1 Na+–H+ exchanger structure. Numbers represent predicted transmembrane domains. Phosphorylation sites are depicted by the amino acid abbreviation and residue numbers. CaM calmodulin, CHP1 calcineurin-homologous protein 1, ERK extracellular signal-related kinase, ERM ezrin/radixin/moesin, MAPK mitogen-activated protein kinase, p90 RSK p90 ribosomal S6 kinase, PI(4,5)P2 phosphatidylinositol 4,5-bisphosphate, SGK1 serum and glucocorticoid-regulated kinase 1
N-terminal tail
This short 15 amino acid sequence, which extends into the cytosol, has no known role other than presumably to anchor TM1 in the membrane.
Transmembrane-spanning ion-translocation domain
This 485 amino acid sequence is composed of 12 TMs joined by short loops as well as a long re-entrant loop that dips into the plane of the membrane between TM9 and TM10. The first extracellular loop that joins TM1 to TM2 contains both N- and O-linked glycosylation sites [70, 71], with N-glycosylation assisting in the targeting of mature NHE1 to the basolateral membrane of polarized epithelial cells [59]. Once at the plasma membrane, the extracellular loops joining TM1 to TM2 and TM3 to TM4 can be proteolytically cleaved without any obvious detriment to Na+–H+ exchange activity [71]. However, it has not been determined whether it is the entire region encompassed by TM1-TM3, or just the integrity of the extracellular loops in that region, that is dispensable for basal NHE1 activity.
Interpretations of current homology models indicate that TM4, in addition to being a major determinant of inhibitor sensitivity (see above), contributes residues that line the intracellular and extracellular substrate access pathways [13, 40]. Both models also predict that disordered regions in the middle of TM11 and one other span (either TM4 or TM6, depending on the model) come together to form the transport ‘gate’/catalytic core that occludes the extracellular and intracellular substrate access pathways [13, 40]. However, the identity of the other spans that contributes to the access pathways of specific residues that form the substrate-binding sites remain controversial and are not readily reconciled by structure–function studies [69]. In addition to a substrate–H+ binding site, residues in the vicinity of the cytoplasmic loops that join TM2 to TM3 and TM10 to TM11 contribute to allosteric regulation of NHE1 by pHi (activation by H+), either by directly supporting the action of an allosteric H+-modifier site or by influencing the interaction of the membrane domain with the carboxy-terminal regulatory domain, which itself can influence the set-point of the modifier site [22, 72].
Carboxy-terminal regulatory domain
The 315 amino acid cytosolic carboxy-terminal domain of NHE1 is the site of numerous regulatory events that can activate NHE1. The initial juxtamembrane portion of the carboxy terminus contains two polybasic motifs (at residues 513–520 and 556–564 of NHE1) that bind inner leaflet phosphoinositides such as phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] [73–77], consistent with NHE1 compartmentalization to lipid rafts [78–80], which may be enriched for phosphoinositides [81]. The cytoskeletal adaptor proteins ezrin/radixin/moesin (ERM) bind the same NHE1 residues [82–84], but this interaction, as well as downstream ramifications, appear to be independent of Na+–H+ translocation, whereas PI(4,5)P2 binding to the same sites is required for Na+–H+ exchange [73, 82, 83]. The 4.1 adaptor protein family shares sequence homology to ERM, and multiple splice variants are expressed in kidney, including 4.1B in the proximal tubule and 4.1R in the thick ascending limb of Henle’s loop [85]. The 4.1R isoform interacts with the same C-terminal NHE1 residues as ERM and PI(4,5)P2, with K D = 100–200 nM [86]. Calmodulin binding to NHE1 (see below) lowers the affinity of the 4.1R-NHE1 interaction, which permits PI(4,5)P2 binding and NHE1 activation. Experiments using NHE1 constructs with K/A and R/A mutations at the 513–520 and 556–564 sites, as well as ATP depletion to modulate PI(4,5)P2 levels, indicate that PI(4,5)P2 binding elicits an alkaline shift in NHE1 K m for intracellular H+, thereby causing an alkaline shift in pHi [73]. Although the activating NHE1–PI(4,5)P2 interaction has been confirmed by many labs, the selectivity of NHE1 binding to other phospholipids remains unclear. One report, using co-sedimentation methods, demonstrated promiscuous binding between an NHE1 cytosolic domain polypeptide and PI(4,5)P2, phosphatidylinositol, phosphatidylserine, phosphatidylglycerol and phosphatidic acid [74]. Another report, using membrane overlay assays and surface plasmon resonance, identified specific interactions between the NHE1 cytosolic domain and phosphatidylinositol polyphosphates, with negligible binding between NHE1 and other membrane phospholipids [75]. In addition to differences in assay methods, the NHE1 polypeptide sequences used for the binding studies differed—mouse peptide, residues 546–602 [74]; rat peptide, residues 501–815 [75]— which may account for some discrepancy. The NHE1-phosphoinositide interactions are low affinity [5.2 × 10−5 M for PI(4,5)P2; 2.5 × 10−5 M for PI(3,4,5)P3] [75], which is likely to be relevant in vivo, by facilitating rapid on–off binding, as opposed to higher affinity interactions, which tend to be irreversible.
The Ca2+-stimulated binding of either calcineurin-homologous protein CHP1 (to residues 515–530 of NHE1; [87–89]) or calmodulin (to residues 636–656; [90–93]) also enhances NHE1 activity, by eliciting an alkaline shift in K m for intracellular H+. In the case of calmodulin, this occurs by the masking of an autoinhibitory domain in the cytosolic tail that, when unoccupied, promotes an acidic shift in the pH-dependent K m for NHE1 [91, 92]. A truncated NHE1 that lacks the distal cytosolic tail also exhibits a substantially more acidic K m for intracellular H+ than the intact protein [94, 95], perhaps due to loss of the influence of several phosphorylatable serine and threonine residues. These sites are constitutively phosphorylated in quiescent cells [96], but can then be further phosphorylated in response to many extracellular stimuli, including growth factors, hormones, extracellular matrix–integrin interactions, and sustained intracellular acidosis (Fig. 1) [97]. NHE1 phosphorylation by MAP kinases [98–100], p90RSK [101], NIK [102], ROCK1 [103], JAK2 [93], Akt [104, 105] and SGK1 [106] regulate many cell phenotypes, such as proliferation [107–109], differentiation [110, 111], adhesion [112] and migration [113, 114]. An exception is NHE1 activation by extracellular hypertonic stimuli, which does not require NHE1 phosphorylation [115, 116]. Many of the phosphorylation and binding partner studies have previously been extensively reviewed [47, 117, 118].
Cellular mechanisms of NHE1 function
pH regulation
NHE1 harnesses the inwardly directed Na+ gradient to remove H+ from the intracellular milieu, thereby resisting acidosis. Virtually all physiological processes are pH sensitive and the importance of NHE1 in the protection of these processes is reflected in its near ubiquitous expression and frequent reference to the role of NHE1 as an important housekeeping protein. A critical NHE1 function, which is discussed below, is resistance to proximal tubule apoptotic stress in the context of chronic kidney diseases (CKDs), such as diabetic nephropathy. A universal feature of apoptosis is the activation of endonucleases and executioner caspases, which catalyze degradation of DNA and intracellular proteins, respectively. Many of these enzymes are maximally catalyzed at pH values that are encountered in apoptosis, but well below physiologic intracellular pH range, which serves as a safeguard to prevent accidental activation, with lethal consequences [119–122]. By perpetually extruding H+, in exchange for Na+, NHE1 plays an important housekeeping role by maintaining homeostatic intracellular pH that is sufficiently high to allow optimal function of most enzymes and structural proteins, while preventing activation of endonucleases and caspases.
Additional examples of the impact of NHE1 action on pH are maintenance of neuronal excitability by influence upon pH-sensitive ion channels and receptors (reviewed in [123]), support of the inflammatory response by disposing of the intracellular acid load that accompanies the respiratory burst in immune cells (reviewed in [124]), and promotion of cell migration by influencing pH-dependent protein–protein interactions within focal adhesion complexes that link the cytoskeleton to the extracellular matrix. In the case of migration, NHE1 is recruited to focal adhesion complexes, and an interaction with integrins stimulates NHE1 action, thereby creating pH nanodomains around these structures [125, 126]. The NHE1 action generates an intracellular alkaline nanodomain that could influence actin remodeling and promote focal adhesion turnover at the leading edge of migrating cells [127, 128], but also causes a complementary extracellular acidic nanodomain that strengthens the interaction between integrins and their extracellular matrix ligands [129]. NHE1 activity also facilitates migration by promoting the degradation of matrix proteins via enhancement of the expression and activity of pericellular matrix metalloproteinases [130, 131].
Vectorial Na+ transport
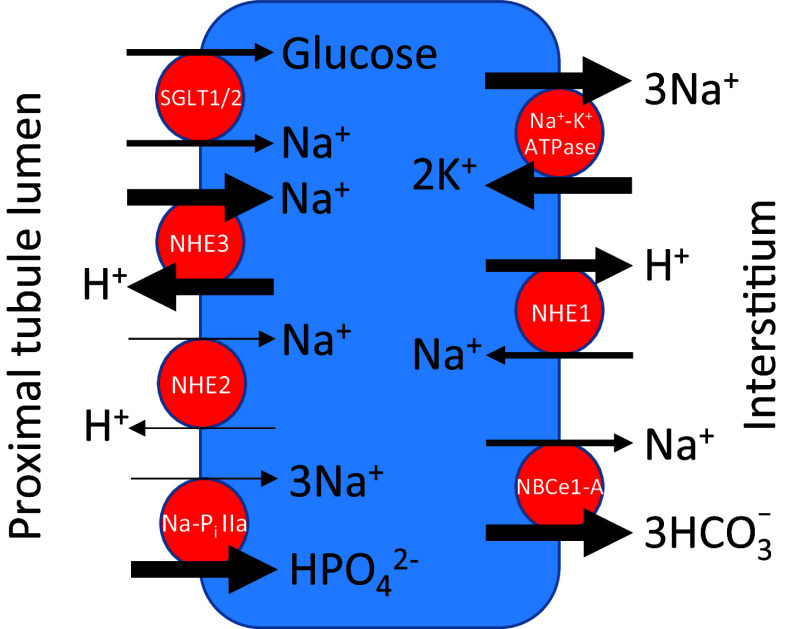
A major function of the kidney is maintenance of extracellular fluid volume, and this is mediated predominantly through proximal tubule isosmotic reabsorption of 50–90 % of filtered Na+. Because of the large amounts of solute and water transported by the proximal tubule, considerable coordination is required between multiple apical and basolateral transporters to maintain cell volume and pH (see Fig. 2).
Fig. 2.
Major proximal tubule Na+ transporters. For simplicity, “pure” anion transporters, paracellular pathways, and distinction between S1, S2 and S3 proximal tubule segments have been omitted. The thickness of the arrows is intended to reflect the relative, quantitative transport of the indicated ion. NHE Na+–H+ exchanger, NaP i IIa renal Na+-phosphate co-transporter, NBCe1-A electrogenic Na+/HCO3 − co-transporter
Of the luminal Na+ transporters NHE3 is responsible for the greatest quantitative uptake of Na+ from ultrafiltrate, with most reabsorption occurring within the initial S1 segment [132, 133]. NHE2 is also expressed in the proximal tubule brush border, but in comparative studies with microperfused proximal tubules derived from NHE2 and NHE3 knockout mice, relatively little Na+–H+ translocation was mediated by NHE2 [134]. The Na+–glucose transporters, SGLT1 and SGLT2 are expressed within proximal tubule brush border, and also contribute to luminal Na+ reabsorption. SLGT1 is a high affinity, low capacity transporter, with a 2:1 stoichiometry for Na+ and glucose. SGLT2 is low affinity, high capacity, and transports Na+ and glucose in a 1:1 ratio. The NaPi-IIa Na+-phosphate co-transporter is a minor contributor to luminal Na+ uptake, but a major effector of inorganic phosphate reabsorption, with approximately 80 % of filtered phosphate reabsorbed by proximal tubule NaPi-IIa, which is localized primarily in the S1 segment brush border [135].
The low cytosolic Na+ and high K+ relative to plasma concentrations is largely attributable to Na+–K+ ATPase activity, which is localized exclusively to the basolateral proximal tubule membrane. As shown in Fig. 2, the Na+ pump is the major basolateral Na+ transporter, and is highly regulated by intracellular Na+ concentration [136], implying that luminal Na+ uptake represents the rate-limiting step in net proximal tubule Na+ reabsorption. The Na+/HCO3 − co-transporter NBCe1-A, which localizes to the basolateral membrane, is also a source of proximal tubule Na+ reabsorption, and the major pathway for HCO3 − exit across the peritubular membrane [137]. The relatively small contribution of NHE1 to basolateral Na+ transport highlights the importance of its biological functions that are mediated by ion transport-independent mechanisms. However, in contrast to proximal tubule NHE3 and Na+–K+ ATPase, which are responsible for large transcellular Na+ fluxes to maintain extracellular fluid volume, relatively small changes in NHE1-regulated Na+ flux can have a large impact on other physiologic functions, such as maintenance of cell volume (discussed later).
Although the direction of NHE1-dependent Na+ flux is opposite to Na+–K+ ATPase and NBCe1-A mediated Na+ translocation, NHE1 may play a regulatory role in proximal tubule vectorial Na+ transport through functional interaction with these transporters. Stimulation of proximal tubule Na+–K+ ATPase concomitantly increased NHE1 expression and activity, as well as physical association of NHE1 with the Na+–K+ ATPase α1 subunit [138]. NHE1 was required for Na+–K+ ATPase activity, as well as α1 subunit phosphorylation and Na+ pump trafficking to the plasma membrane. The mechanism by which NHE1 enhances proximal tubule Na+–K+ ATPase was not identified, but the authors noted that it was unlikely to be related to Na+ entry across the basolateral membrane, and speculated that dual phosphorylation by common kinases could foster protein–protein interactions, or that non-transport NHE1 scaffolding properties could be responsible [138].
Despite co-localization to the basolateral membrane, and common teleology for regulating intracellular Na+ and pH, we are unaware of functional interactions between NHE1 and NBCe1-A in the proximal tubule. However, cooperation between NHE1 and NBCe1 has been described in cardiac tissue. The two transporters are spatially separate in the heart (NHE1 is expressed at the intercalated disc and gap junctions; NBCe1 and NBCn1 are expressed in transverse tubules), but they nevertheless coordinately facilitate Na+ influx, intracellular alkalinization, and ultimately Ca2+ loading of the sarcoplasmic reticulum for excitation–contraction coupling [139]. In studies of NHE1-deficient MDCK cells, which more closely resemble distal, rather than proximal tubule cells, NBCe1 expression was upregulated, which compensated for defects in intracellular volume and pH regulation [140]. However, a cell migration phenotype was not rescued, indicating that not all NHE1 and NBCe1 functions are redundant.
Cell volume regulation
NHE1 contributes to a cell volume control mechanism—regulatory volume increase (RVI)—under hypertonic stress NHE1 promotes the net uptake of osmolytes, and thence water, thereby tending to counter cell shrinkage (reviewed in [141]). Other transporters have been implicated in RVI, such as NKCC1 Na+/K+/2Cl− and the AE2 Cl−–HCO3 − exchangers [142, 143], but neither is expressed in the proximal tubule [144, 145], thereby rendering NHE1 as a major regulator of proximal tubule RVI. Interestingly, in proximal tubular epithelial cells, as well as fibroblasts, acute cell shrinkage causes opposite effects on NHE1 (activation) and NHE3 (inhibition) [78, 146, 147]. In the case of NHE1, extracellular hypertonicity increases tyrosine kinase activity in challenged cells that signal via a number of intermediates, such as JAK2 and calmodulin, to activate NHE1 via the C-terminal portion of its regulatory cytosolic domain [95, 148]. The extrusion of H+ results in an increase in intracellular HCO3 − concentration that then stimulates Cl−–HCO3 − exchange in AE2-expressing cell types. Because changes in intracellular pH and HCO3 − are buffered, the counter-transported Na+ and Cl− produce a net increase in intracellular osmolality that promotes RVI.
The in vivo relevance of RVI to the proximal tubule has been difficult to discern, though it is plausible that constant fine tuning of cell volume is important for multiple intracellular processes, such as regulation of cell membrane curvature and proper approximation between interacting intracellular proteins. As discussed in the next section, RVI may also be critical for counteracting multiple cell volume perturbations, including apoptotic shrinkage [143, 149]. NHE1 has been implicated in pathophysiology of cell hypertrophy, particularly involving the cardiomyocyte, in the context of congestive heart failure [118]. In circulating leukocytes, studies that originated primarily from Sergio Grinstein’s lab indicated a role for NHE1, both in cell volume and intracellular pH changes associated with phagocytosis [148, 150, 151]. Moreover, phagocytic cup formation is mediated by dynamic changes in plasma membrane morphology and cell volume, which require electrostatic interactions between anionic membrane phospholipids and poly-cationic intracellular proteins [152]. Although NHE1 has not specifically been shown to be a phospholipid binding partner for phagocytosis, we speculate that it is a plausible candidate, considering its known interaction with inner leaflet membrane phospholipids [74, 75].
NHE1 function at the systemic level
Genetic studies
In 1997 Cox et al. [153] characterized mice with an ataxia phenotype that was caused by a spontaneous NHE1 mutation of residue 441, between TM11 and TM12, which resulted in the insertion of a premature stop codon and unstable mRNA encoding a transporter without the C-terminal tail. These mice die within a few weeks of birth due to lethal seizures. Electroencephalography revealed a “slow wave epilepsy” pattern, hence the moniker Swe/Swe for these mice. Shortly thereafter Bell et al. [154] reported a virtually identical phenotype in mice with global NHE1 gene deletion. In contrast to NHE3 knockout mice or humans with loss of function NBCe1 mutations, which result in proximal tubule salt and HCO3 − wasting [155, 156], neither Swe/Swe nor NHE1 knockout mice demonstrate a renal phenotype.
Human NHE1 mutations had not been identified until recently, when a non-synonymous Gly305Arg substitution was shown to cause Lichtenstein-Knorr syndrome, a rare, autosomal recessive disorder characterized by ataxia and neurosensory deafness, with onset of symptoms typically by 1–2 years of age [157]. The mutation removes a critical NHE1 glycosylation site, resulting in loss of NHE1 targeting to the plasma membrane and a consequent absence of NHE1 Na+–H+ exchange activity.
A recent report describes that gene deletions of either NHE1 or huntingtin, the gene mutated in Huntington’s disease, resulted in a similar chemotaxis phenotype in Dictyostelium amoebae in response to extracellular K+-regulated cAMP and Ca2+ stimuli [158]. Although this is consistent with the well-established role of NHE1 in cell migration [113], the novel finding is that in huntingtin-null organisms, actin filaments were disorganized, resulting in defective NHE1 trafficking to the plasma membrane [158]. Instead of normal expression at the leading edge of migrating cells, NHE1 was mislocalized to a perinuclear region in huntingtin knockout cells. Taken together, these data imply that there is a functional relationship between huntingtin and NHE1. However, human NHE1 mutations in Huntington’s disease have not been described.
The reason for predominant brain phenotypes in NHE1-deficient, despite ubiquitous expression, is unclear. We speculate that the threshold may be lower for neuron dysfunction when some of the previously mentioned NHE1 functions, such as homeostatic intracellular pH or intracellular volume control are aberrant, perhaps due to less redundancy with NHE1-regulated pathways.
Kidney proximal tubule NHE1 function in animal models
Although neither the NHE1-deficient mice nor humans with loss of function NHE1 mutations display an overt renal phenotype, multiple groups have utilized animal models to test the effects of a stressor “second hit” to unmask NHE1 functions in vivo. In studies designed to identify the role of NHE1 in tubular atrophy, a critical pathologic predictor of chronic, progressive kidney diseases [159–161], which is regulated by apoptosis [162], Wu et al. [149] showed that Swe/Swe mice receiving tail vein injections of adriamycin, a chemotherapeutic agent that is toxic to murine glomerular epithelial cells, developed an augmented renal phenotype that included proximal tubular epithelial cell apoptosis. Swe/Swe mice injected with streptozotocin to induce diabetes, developed hallmarks of diabetic nephropathy, including albuminuria, azotemia, and tubular epithelial cell apoptosis [84]. Importantly, the Swe/Swe mice were bred onto a C57BL/6 genetic background, which is resistant to adriamycin and streptozotocin toxicity, indicating that the “two hit” combination of NHE1 loss of function and glomerular injury was sufficient to overcome the protective C57BL/6 background. In a rat model of obstructive nephropathy, the resulting tubular epithelial cell apoptosis was associated with diminished NHE1 expression, and pharmacologic inhibition of NHE1 enhanced apoptosis [163]. Recent in vitro studies suggest that one mechanism for NHE1 suppression is through mechanical stretch-induced RhoA and MAP kinase activation [164]. Taken together, multiple studies in models of CKD suggest that tubular epithelial cell NHE1 is cytoprotective [47].
NHE1-regulated mechanisms of proximal tubule epithelial cell survival
Further support of NHE1 in the defense against apoptosis includes multiple in vitro studies demonstrating that apoptotic stress activates NHE1 [47, 83, 84, 149]. An invariant feature of apoptosis is cell volume decrease, and as previously mentioned, NHE1 might relieve apoptotic stress through activation of RVI pathways [143, 149]. Apoptotic cells also undergo cytosol acidification, which catalyzes pro-apoptotic enzymes [120, 121, 165, 166], suggesting that NHE1-regulated Na+–H+ exchange, which is regulated by the N-terminal, transmembrane domain of NHE1, might defend against renal tubular epithelial cell apoptosis by alkalinizing cytosolic pH, as well as expanding cell volume.
As mentioned previously, two polybasic juxtamembrane domains anchor NHE1 to the plasma membrane inner leaflet through binding to PI(4,5)P2 [73–75, 78] and ERM adaptor proteins [82, 83]. Both ERM and PI(4,5)P2 are substrates for PI-3 kinase, and the PI(3,4,5)P3 product docks the pro-survival kinase Akt, leading to its activation and phosphorylation of downstream targets that block apoptosis. This scaffolding function of NHE1, which is independent of Na+–H+ exchange activity, represents one mechanism of proximal tubule defense against apoptotic stress and tubular atrophy [75]. NHE1 activation of PI-3 kinase and Akt has also been implicated in glomerular epithelial cell (podocyte) survival, although in this case, the downstream effects of Akt activation are augmentation of autophagy and reduction in endoplasmic reticulum stress [167].
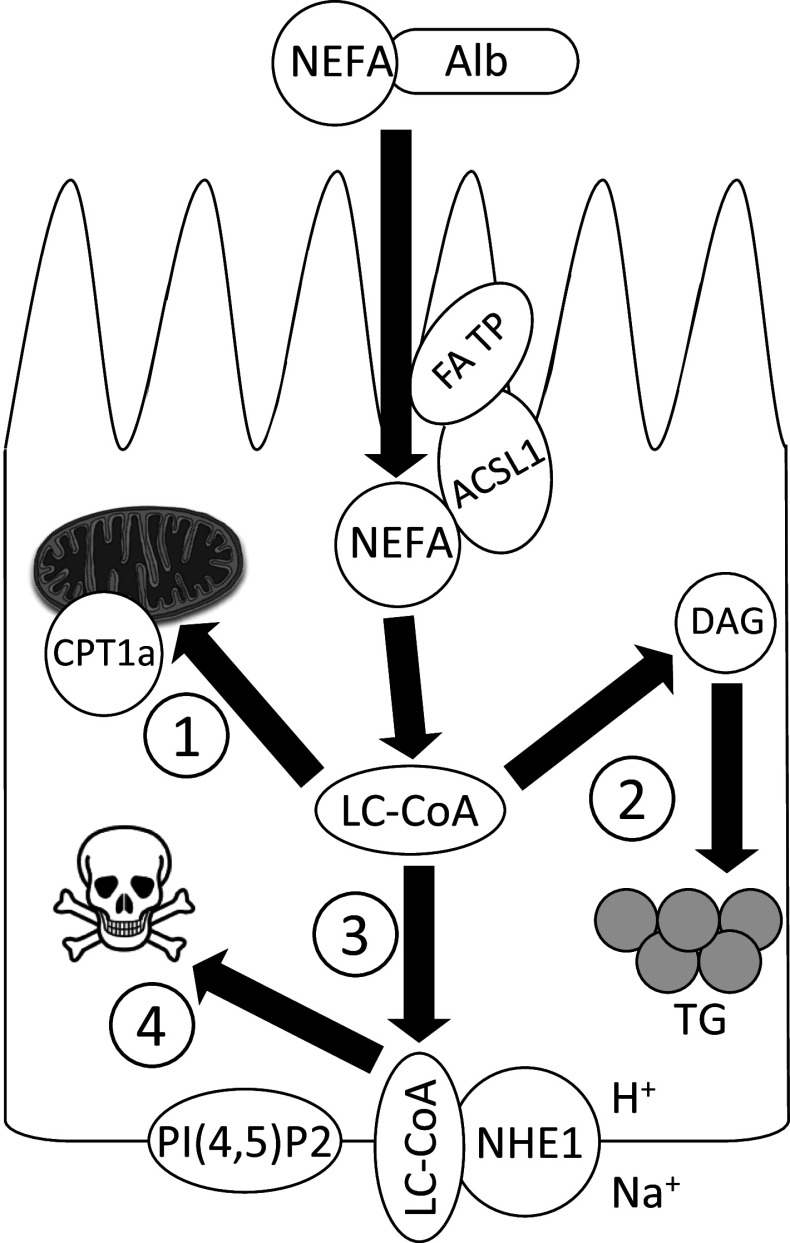
Consistent with the notion that NHE1 acts as a proximal tubule cell survival factor, multiple studies have demonstrated that tubular epithelial cell NHE1 becomes inactivated during apoptosis [83, 149, 163, 164]. However, the mechanisms of inactivation have been elusive until recently. Initial studies indicated that the NHE1 cytosolic tail undergoes caspase-3-dependent degradation [149, 168], but there are no consensus caspase cleavage sequences within the cytosolic tail, and relevant non-consensus sites have not been mapped. Decreased NHE1 mRNA and protein expression was noted in ureteral obstruction models [163, 164]. More recently, using animal models of kidney diseases characterized by glomerular damage and albuminuria, Khan et al. [76] have postulated that the aberrantly filtered albumin-bound fatty acids are reabsorbed by the proximal tubule. The intracellular fatty acids are then rapidly catalyzed to long chain acyl-CoA (LC-CoA), in preparation for β-oxidation and ATP generation (Fig. 3). However, the rate-limiting enzyme for LC-CoA transport into mitochondria, carnitine palmitoyl transferase-1a (CPT1a) becomes saturated by the large LC-CoA flux, resulting in extensive lipid droplet deposition within the proximal tubule cytoplasm [76, 169, 170]. Although the lipid droplets represent a non-toxic intracellular depot, the storage capacity in non-adipocytes is limited. The overflow LC-CoA, which bear structural similarity to PI(4,5)P2 bind the NHE1 cytosolic tail with greater affinity compared to PI(4,5)P2 [76]. If LC-CoA reach sufficiently high (low-mid µM) intracellular concentration, they compete with PI(4,5)P2 for binding to NHE1, and uncoupling of the NHE1-PI(4,5)P2 interaction leads to loss of NHE1 activity [76] (Fig. 3). In this scheme, proximal tubule NHE1 fulfills a unique role by serving as a metabolic sensor for lipotoxicity.
Fig. 3.
Proposed sequence of events following aberrant fatty acid reabsorption at the apical surface of the proximal tubule in the setting of nephrotic syndrome. The numbers signify the order of metabolic pathway activation. ACSL1 long chain acyl-CoA synthetase-1, Alb albumin, CPT1a carnitine palmitoyl transferase-1a, DAG diacylglycerol, FA TP fatty acid transport protein, LC-CoA long chain fatty acyl-CoA, NEFA non-esterified fatty acid, NHE1 Na+–H+ exchanger-1, PI(4,5)P2 phosphatidylinositol 4,5-bisphosphate
Acute kidney injury
The role of proximal tubule NHE1 appears to be quite different in the pathophysiology of acute kidney injury (AKI), which is most commonly induced by ischemia (reviewed in [47]). In particular, the S3 proximal tubule segment, which delicately balances high O2 demand and low basal O2 tension in the cortico-medullary region, is the nephron portion most vulnerable to ischemia. The divergent mechanisms of NHE1 in the pathophysiology of CKD and AKI are illustrated by reports that NHE1 inhibitors improve renal blood flow and ameliorate the clinical course of ischemic AKI [171, 172], whereas NHE1 inhibition exacerbates apoptosis in the context of CKD [75, 76, 83, 149, 163]. A plausible explanation for the discrepancy is that in CKD the stimulus for NHE1 activation is likely cell volume shrinkage due to an apoptotic stimulus, whereas in ischemic conditions anaerobic metabolism causes intracellular acidosis, which triggers NHE1 activity. While Na+ influx in shrunken cells may restore cell volume and function (in CKD), Na+ and H2O movement into proximal tubule cells with normal volume leads to swelling, which is a cardinal feature of necrosis (in AKI). In ischemic cells that express the NCX1 Na+–Ca2+ exchanger, NCX1 is activated in the reverse mode, to extrude excess intracellular Na+, which can then perpetuate Ca2+-dependent necrosis and apoptosis pathways (reviewed in [47]).
Conclusions
NHE1 is a ubiquitously expressed ion exchanger, which regulates electroneutral Na+–H+ translocation that is critical for many cell functions, most notably maintenance of intracellular pH and cell volume. NHE1 was the initially discovered Na+–H+ exchanger over 25 years ago, and is commonly and perhaps pejoratively referred to as a “housekeeping protein”, implying that it is uninteresting or unworthy of scientific inquiry. However, extensive mapping and functional studies involving the regulatory NHE1 cytosolic domain have revealed that multiple protein and lipid binding partners direct an expanding list of NHE1 housekeeping chores. Among these is relief of proximal tubule apoptotic stress and CKD progression, which is accomplished by a novel mechanism, whereby NHE1 serves as a metabolic sensor for aberrantly accumulated fatty acid metabolites. We are optimistic that NHE1-regulated pathways may be exploited for further investigation of the pathophysiology of tubular atrophy, since it is a strong predictor of CKD progression, for which there are currently no specific diagnostic tests or therapies.
Abbreviations
- AE2
Anion exchange protein 2
- AKI
Acute kidney injury
- CHP1
Calcineurin-homologous protein 1
- CKD
Chronic kidney disease
- ERM
Ezrin/radixin/moesin
- ESRD
End stage renal disease
- JAK2
Janus kinase 2
- LC-CoA
Long chain fatty acyl-CoA
- MDCK
Madin-Darby canine kidney cell line
- NaPi-IIa
Na+-phosphate co-transporter
- NBC
Na+/HCO3 − co-transporter
- NCX1
Na+–Ca2+ exchanger 1
- NHE
Na+–H+ exchanger
- NIK
Nck (non-catalytic region of tyrosine kinase adaptor protein 1)-interacting kinase
- NKCC1
Na+/K+/Cl− co-transporter 1
- pHi
Intracellular pH
- PI(4,5)P2
Phosphatidylinositol 4,5-bisphosphate
- ROCK1
Rho-associated, coiled-coil-containing protein kinase 1
- RVI
Regulatory volume increase
- SGK1
Serum and glucocorticoid-regulated kinase 1
- SGLT
Sodium-glucose co-transporter
- TM
Transmembrane-spanning domain
Footnotes
NHE3 is the transporter responsible for the activity originally detected in the apical membranes preparations from rat proximal tubules.
The K m for intracellular H+ in pH units is sometimes referred to as pK i. Note that ‘pK i’ is not defined as the protonation state of titratable groups in the protein.
Another determinant, Gly346 at the extracellular end of TM9, is hypothesized to be in three-dimensional proximity to the clustered determinants in TM4 [24].
References
- 1.Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966;41:445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- 2.Harold FM, Papineau D. Cation transport and electrogenesis by Streptococcus faecalis. II. Proton and sodium extrusion. J Membr Biol. 1972;8:45–62. doi: 10.1007/BF01868094. [DOI] [PubMed] [Google Scholar]
- 3.West IC, Mitchell P. Proton/sodium ion antiport in Escherichia coli . Biochem J. 1974;144:87–90. doi: 10.1042/bj1440087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murer H, Hopfer U, Kinne R. Sodium/proton antiport in brush-border-membrane vesicles isolated from rat small intestine and kidney. Biochem J. 1976;154:597–604. [PMC free article] [PubMed] [Google Scholar]
- 5.Sardet C, Franchi A, Pouyssegur J. Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell. 1989;56:271–280. doi: 10.1016/0092-8674(89)90901-x. [DOI] [PubMed] [Google Scholar]
- 6.Fuster DG, Alexander RT. Traditional and emerging roles for the SLC9 Na+/H+ exchangers. Pflugers Arch. 2014;466:61–76. doi: 10.1007/s00424-013-1408-8. [DOI] [PubMed] [Google Scholar]
- 7.Donowitz M, Ming TC, Fuster D. SLC9/NHE gene family, a plasma membrane and organellar family of Na(+)/H(+) exchangers. Mol Aspects Med. 2013;34:236–251. doi: 10.1016/j.mam.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tse CM, Brant SR, Walker MS, Pouyssegur J, Donowitz M. Cloning and sequencing of a rabbit cDNA encoding an intestinal and kidney-specific Na+/H+ exchanger isoform (NHE-3) J Biol Chem. 1992;267:9340–9346. [PubMed] [Google Scholar]
- 9.Pizzonia JH, Biemesderfer D, Abu-Alfa AK, Wu MS, Exner M, Isenring P, Igarashi P, Aronson PS. Immunochemical characterization of Na+/H+ exchanger isoform NHE4. Am J Physiol. 1998;275:F510–F517. doi: 10.1152/ajprenal.1998.275.4.F510. [DOI] [PubMed] [Google Scholar]
- 10.Klanke CA, Su YR, Callen DF, Wang Z, Meneton P, Baird N, Kandasamy RA, Orlowski J, Otterud BE, Leppert M, et al. Molecular cloning and physical and genetic mapping of a novel human Na+/H+ exchanger (NHE5/SLC9A5) to chromosome 16q22.1. Genomics. 1995;25:615–622. doi: 10.1016/0888-7543(95)80002-4. [DOI] [PubMed] [Google Scholar]
- 11.Baird NR, Orlowski J, Szabo EZ, Zaun HC, Schultheis PJ, Menon AG, Shull GE. Molecular cloning, genomic organization, and functional expression of Na+/H+ exchanger isoform 5 (NHE5) from human brain. J Biol Chem. 1999;274:4377–4382. doi: 10.1074/jbc.274.7.4377. [DOI] [PubMed] [Google Scholar]
- 12.Hunte C, Screpanti E, Venturi M, Rimon A, Padan E, Michel H. Structure of a Na+/H+ antiporter and insights into mechanism of action and regulation by pH. Nature. 2005;435:1197–1202. doi: 10.1038/nature03692. [DOI] [PubMed] [Google Scholar]
- 13.Nygaard EB, Lagerstedt JO, Bjerre GP, Shi B, Budamagunta M, Poulsen KA, Meinild S, Rigor RR, Voss JC, Cala PM, Pedersen SF. Structural modeling and electron paramagnetic resonance spectroscopy of the human Na+/H+ exchanger isoform 1, NHE1. J Biol Chem. 2010;286:634–648. doi: 10.1074/jbc.M110.159202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padan E, Kozachkov L, Herz K, Rimon A. NhaA crystal structure: functional-structural insights. J Exp Biol. 2009;212:1593–1603. doi: 10.1242/jeb.026708. [DOI] [PubMed] [Google Scholar]
- 15.Brett CL, Donowitz M, Rao R. Evolutionary origins of eukaryotic sodium/proton exchangers. Am J Physiol Cell Physiol. 2005;288:C223–C239. doi: 10.1152/ajpcell.00360.2004. [DOI] [PubMed] [Google Scholar]
- 16.Ye G, Chen C, Han D, Xiong X, Kong Y, Wan B, Yu L. Cloning of a novel human NHEDC1 (Na+/H+ exchanger like domain containing 1) gene expressed specifically in testis. Mol Biol Rep. 2006;33:175–180. doi: 10.1007/s11033-006-0010-y. [DOI] [PubMed] [Google Scholar]
- 17.Fuster DG, Zhang J, Shi M, Bobulescu IA, Andersson S, Moe OW. Characterization of the sodium/hydrogen exchanger NHA2. JASN. 2008;19:1547–1556. doi: 10.1681/ASN.2007111245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SH, Kim T, Park ES, Yang S, Jeong D, Choi Y, Rho J. NHE10, an osteoclast-specific member of the Na+/H+ exchanger family, regulates osteoclast differentiation and survival [corrected] Biochem Biophys Res Commun. 2008;369:320–326. doi: 10.1016/j.bbrc.2008.01.168. [DOI] [PubMed] [Google Scholar]
- 19.Wang D, Hu J, Bobulescu IA, Quill TA, McLeroy P, Moe OW, Garbers DL. A sperm-specific Na+/H+ exchanger (sNHE) is critical for expression and in vivo bicarbonate regulation of the soluble adenylyl cyclase (sAC) Proc Natl Acad Sci USA. 2007;104:9325–9330. doi: 10.1073/pnas.0611296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barber DL. Mechanisms of receptor-mediated regulation of Na-H exchange. Cell Signal. 1991;3:387–397. doi: 10.1016/0898-6568(91)90069-7. [DOI] [PubMed] [Google Scholar]
- 21.Wakabayashi S, Shigekawa M, Pouyssegur J. Molecular physiology of vertebrate Na+/H+ exchangers. Physiol Rev. 1997;77:51–74. doi: 10.1152/physrev.1997.77.1.51. [DOI] [PubMed] [Google Scholar]
- 22.Hisamitsu T, Yamada K, Nakamura TY, Wakabayashi S. Functional importance of charged residues within the putative intracellular loops in pH regulation by Na+/H+ exchanger NHE1. FEBS J. 2007;274:4326–4335. doi: 10.1111/j.1742-4658.2007.05962.x. [DOI] [PubMed] [Google Scholar]
- 23.Yu FH, Shull GE, Orlowski J. Functional properties of the rat Na/H exchanger NHE-2 isoform expressed in Na/H exchanger-deficient Chinese hamster ovary cells. J Biol Chem. 1993;268:25536–25541. [PubMed] [Google Scholar]
- 24.Wakabayashi S, Hisamitsu T, Pang TX, Shigekawa M. Kinetic dissection of two distinct proton binding sites in Na+/H+ exchangers by measurement of reverse mode reaction. J Biol Chem. 2003;278:43580–43585. doi: 10.1074/jbc.M306690200. [DOI] [PubMed] [Google Scholar]
- 25.Busch S, Burckhardt BC, Siffert W. Expression of the human sodium/proton exchanger NHE-1 in Xenopus laevis oocytes enhances sodium/proton exchange activity and establishes sodium/lithium countertransport. Pflugers Arch. 1995;429:859–869. doi: 10.1007/BF00374811. [DOI] [PubMed] [Google Scholar]
- 26.Zerbini G, Maestroni A, Breviario D, Mangili R, Casari G. Alternative splicing of NHE-1 mediates Na-Li countertransport and associates with activity rate. Diabetes. 2003;52:1511–1518. doi: 10.2337/diabetes.52.6.1511. [DOI] [PubMed] [Google Scholar]
- 27.Masereel B, Pochet L, Laeckmann D. An overview of inhibitors of Na+/H+ exchanger. Eur J Med Chem. 2003;38:547–554. doi: 10.1016/s0223-5234(03)00100-4. [DOI] [PubMed] [Google Scholar]
- 28.Parker MD, Musa-Aziz R, Rojas JD, Choi I, Daly CM, Boron WF. Characterization of human SLC4A10 as an electroneutral Na/HCO3 cotransporter (NBCn2) with Cl- self-exchange activity. J Biol Chem. 2008;283:12777–12788. doi: 10.1074/jbc.M707829200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orlowski J. Heterologous expression and functional properties of amiloride high affinity (NHE-1) and low affinity (NHE-3) isoforms of the rat Na/H exchanger. J Biol Chem. 1993;268:16369–16377. [PubMed] [Google Scholar]
- 30.Tse CM, Levine SA, Yun CH, Montrose MH, Little PJ, Pouyssegur J, Donowitz M. Cloning and expression of a rabbit cDNA encoding a serum-activated ethylisopropylamiloride-resistant epithelial Na+/H+ exchanger isoform (NHE-2) J Biol Chem. 1993;268:11917–11924. [PubMed] [Google Scholar]
- 31.Bookstein C, Musch MW, DePaoli A, Xie Y, Rabenau K, Villereal M, Rao MC, Chang EB. Characterization of the rat Na+/H+ exchanger isoform NHE4 and localization in rat hippocampus. Am J Physiol. 1996;271:C1629–C1638. doi: 10.1152/ajpcell.1996.271.5.C1629. [DOI] [PubMed] [Google Scholar]
- 32.Szabo EZ, Numata M, Shull GE, Orlowski J. Kinetic and pharmacological properties of human brain Na(+)/H(+) exchanger isoform 5 stably expressed in Chinese hamster ovary cells. J Biol Chem. 2000;275:6302–6307. doi: 10.1074/jbc.275.9.6302. [DOI] [PubMed] [Google Scholar]
- 33.Attaphitaya S, Park K, Melvin JE. Molecular cloning and functional expression of a rat Na+/H+ exchanger (NHE5) highly expressed in brain. J Biol Chem. 1999;274:4383–4388. doi: 10.1074/jbc.274.7.4383. [DOI] [PubMed] [Google Scholar]
- 34.Pedersen SF, King SA, Nygaard EB, Rigor RR, Cala PM. NHE1 inhibition by amiloride- and benzoyl guanidine-type compounds: inhibitor binding loci deduced from chimeras of NHE1 homologues with endogenous differences in inhibitor sensitivity. J Biol Chem. 2007;282:19716–19727. doi: 10.1074/jbc.M701637200. [DOI] [PubMed] [Google Scholar]
- 35.Touret N, Poujeol P, Counillon L. Second-site revertants of a low-sodium-affinity mutant of the Na+/H+ exchanger reveal the participation of TM4 into a highly constrained sodium-binding site. Biochemistry. 2001;40:5095–5101. doi: 10.1021/bi0025464. [DOI] [PubMed] [Google Scholar]
- 36.Counillon L, Franchi A, Pouyssegur J. A point mutation of the Na+/H+ exchanger gene (NHE1) and amplification of the mutated allele confer amiloride resistance upon chronic acidosis. Proc Natl Acad Sci USA. 1993;90:4508–4512. doi: 10.1073/pnas.90.10.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Counillon L, Noel J, Reithmeier RA, Pouyssegur J. Random mutagenesis reveals a novel site involved in inhibitor interaction within the fourth transmembrane segment of the Na+/H+ exchanger-1. Biochemistry. 1997;36:2951–2959. doi: 10.1021/bi9615405. [DOI] [PubMed] [Google Scholar]
- 38.Noël J, Germain D, Vadnais J. Glutamate 346 of human Na+–-H+ exchanger NHE1 is crucial for modulating both the affinity for Na+ and the interaction with amiloride derivatives. Biochemistry. 2003;42:15361–15368. doi: 10.1021/bi035296a. [DOI] [PubMed] [Google Scholar]
- 39.Khadilkar A, Iannuzzi P, Orlowski J. Identification of sites in the second exomembrane loop and ninth transmembrane helix of the mammalian Na+/H+ exchanger important for drug recognition and cation translocation. J Biol Chem. 2001;276:43792–43800. doi: 10.1074/jbc.M106659200. [DOI] [PubMed] [Google Scholar]
- 40.Landau M, Herz K, Padan E, Ben-Tal N. Model structure of the Na+/H+ exchanger 1 (NHE1): functional and clinical implications. J Biol Chem. 2007;282:37854–37863. doi: 10.1074/jbc.M705460200. [DOI] [PubMed] [Google Scholar]
- 41.Fafournoux P, Noel J, Pouyssegur J. Evidence that Na+/H+ exchanger isoforms NHE1 and NHE3 exist as stable dimers in membranes with a high degree of specificity for homodimers. J Biol Chem. 1994;269:2589–2596. [PubMed] [Google Scholar]
- 42.Hisamitsu T, Pang T, Shigekawa M, Wakabayashi S. Dimeric interaction between the cytoplasmic domains of the Na+/H+ exchanger NHE1 revealed by symmetrical intermolecular cross-linking and selective co-immunoprecipitation. Biochemistry. 2004;43:11135–11143. doi: 10.1021/bi049367x. [DOI] [PubMed] [Google Scholar]
- 43.Moncoq K, Kemp G, Li X, Fliegel L, Young HS. Dimeric structure of human Na+/H+ exchanger isoform 1 over-produced in saccharomyces cerevisiae. J Biol Chem. 2007;283:4145–4154. doi: 10.1074/jbc.M704844200. [DOI] [PubMed] [Google Scholar]
- 44.Hisamitsu T, Ammar YB, Nakamura TY, Wakabayashi S. Dimerization is crucial for the function of the Na+/H+ exchanger NHE1. Biochemistry. 2006;45:13346–13355. doi: 10.1021/bi0608616. [DOI] [PubMed] [Google Scholar]
- 45.Fuster D, Moe OW, Hilgemann DW. Steady-state function of the ubiquitous mammalian Na/H exchanger (NHE1) in relation to dimer coupling models with 2Na/2H stoichiometry. J Gen Physiol. 2008;132:465–480. doi: 10.1085/jgp.200810016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee C, Kang HJ, von BC, Newstead S, Uzdavinys P, Dotson DL, Iwata S, Beckstein O, Cameron AD, Drew D (2013) A two-domain elevator mechanism for sodium/proton antiport. Nature 501:573–577 [DOI] [PMC free article] [PubMed]
- 47.Schelling JR, Abu Jawdeh BG. Regulation of cell survival by Na+/H+ exchanger-1 (NHE1) Am J Physiol Renal Physiol. 2008;295:F625–F632. doi: 10.1152/ajprenal.90212.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yi YH, Ho PY, Chen TW, Lin WJ, Gukassyan V, Tsai TH, Wang DW, Lew TS, Tang CY, Lo SJ, Chen TY, Kao FJ, Lin CH. Membrane targeting and coupling of NHE1-integrin αIIbβ3-NCX1 by lipid rafts following integrin-ligand interactions trigger Ca2+ oscillations. J Biol Chem. 2009;284:3855–3864. doi: 10.1074/jbc.M804334200. [DOI] [PubMed] [Google Scholar]
- 49.Mason MJ, Smith JD, Garcia-Soto JJ, Grinstein S. Internal pH-sensitive site couples Cl−(-)HCO3 − exchange to Na+ -H+ antiport in lymphocytes. Am J Physiol. 1989;256:C428–C433. doi: 10.1152/ajpcell.1989.256.2.C428. [DOI] [PubMed] [Google Scholar]
- 50.Wada M, Miyakawa S, Shimada A, Okada N, Yamamoto A, Fujita T. Functional linkage of H+/peptide transporter PEPT2 and Na+/H+ exchanger in primary cultures of astrocytes from mouse cerebral cortex. Brain Res. 2005;1044:33–41. doi: 10.1016/j.brainres.2005.02.064. [DOI] [PubMed] [Google Scholar]
- 51.Rocha MA, Crockett DP, Wong LY, Richardson JR, Sonsalla PK. Na(+)/H(+) exchanger inhibition modifies dopamine neurotransmission during normal and metabolic stress conditions. J Neurochem. 2008;106:231–243. doi: 10.1111/j.1471-4159.2008.05355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hwang IK, Yoo KY, An SJ, Li H, Lee CH, Choi JH, Lee JY, Lee BH, Kim YM, Kwon YG, Won MH. Late expression of Na(+)/H(+) exchanger 1 (NHE1) and neuroprotective effects of NHE inhibitor in the gerbil hippocampal CA1 region induced by transient ischemia. Exp Neurol. 2008;212:314–323. doi: 10.1016/j.expneurol.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 53.Franchi A, Perucca-Lostanlen D, Pouyssegur J. Functional expression of a human Na+/H+ antiporter gene transfected into antiporter-deficient mouse L cells. Proc Natl Acad Sci USA. 1986;83:9388–9392. doi: 10.1073/pnas.83.24.9388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biemesderfer D, Reilly RF, Exner M, Igarashi P, Aronson PS. Immunocytochemical characterization of Na+–-H+ exchanger isoform NHE-1 in rabbit kidney. Am J Physiol. 1992;263:F833–F840. doi: 10.1152/ajprenal.1992.263.5.F833. [DOI] [PubMed] [Google Scholar]
- 55.Peti-Peterdi J, Chambrey R, Bebok Z, Biemesderfer D, St John PL, Abrahamson DR, Warnock DG, Bell PD. Macula densa Na(+)/H(+) exchange activities mediated by apical NHE2 and basolateral NHE4 isoforms. Am J Physiol Renal Physiol. 2000;278:F452–F463. doi: 10.1152/ajprenal.2000.278.3.F452. [DOI] [PubMed] [Google Scholar]
- 56.Amemiya M, Loffing J, Lotscher M, Kaissling B, Alpern RJ, Moe OW. Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney Int. 1995;48:1206–1215. doi: 10.1038/ki.1995.404. [DOI] [PubMed] [Google Scholar]
- 57.Chambrey R, St John PL, Eladari D, Quentin F, Warnock DG, Abrahamson DR, Podevin RA, Paillard M. Localization and functional characterization of Na+/H+ exchanger isoform NHE4 in rat thick ascending limbs. Am J Physiol. 2001;281:F707–F717. doi: 10.1152/ajprenal.2001.281.4.F707. [DOI] [PubMed] [Google Scholar]
- 58.Chambrey R, Warnock DG, Podevin RA, Bruneval P, Mandet C, Belair MF, Bariety J, Paillard M. Immunolocalization of the Na+/H+ exchanger isoform NHE2 in rat kidney. Am J Physiol. 1998;275:F379–F386. doi: 10.1152/ajprenal.1998.275.3.F379. [DOI] [PubMed] [Google Scholar]
- 59.Coupaye-Gerard B, Bookstein C, Duncan P, Chen XY, Smith PR, Musch M, Ernst SA, Chang EB, Kleyman TR. Biosynthesis and cell surface delivery of the NHE1 isoform of Na+/H+ exchanger in A6 cells. Am J Physiol. 1996;271:C1639–C1645. doi: 10.1152/ajpcell.1996.271.5.C1639. [DOI] [PubMed] [Google Scholar]
- 60.Cavet ME, Akhter S, Murtazina R, de Medina FS, Tse CM, Donowitz M. Half-lives of plasma membrane Na+/H+ exchangers NHE1-3: plasma membrane NHE2 has a rapid rate of degradation. Am J Physiol. 2001;281:C2039–C2048. doi: 10.1152/ajpcell.2001.281.6.C2039. [DOI] [PubMed] [Google Scholar]
- 61.Su X, Pang TX, Wakabayashi S, Shigekawa M. Evidence for involvement of the putative first extracellular loop in differential volume sensitivity of the Na+/H+ exchangers NHE1 and NHE2. Biochemistry. 2003;42:1086–1094. doi: 10.1021/bi020427d. [DOI] [PubMed] [Google Scholar]
- 62.Simonin A, Fuster D. Nedd4-1 and beta-arrestin-1 are key regulators of Na+/H+ exchanger 1 ubiquitylation, endocytosis, and function. J Biol Chem. 2010;285:38293–38303. doi: 10.1074/jbc.M110.115089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Damkier HH, Prasad V, Hubner CA, Praetorius J. Nhe1 is a luminal Na+/H+ exchanger in mouse choroid plexus and is targeted to the basolateral membrane in Ncbe/Nbcn2-null mice. Am J Physiol Cell Physiol. 2009;296:C1291–C1300. doi: 10.1152/ajpcell.00062.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pizzonia JH, Ransom BR, Pappas CA. Characterization of Na+/H+ exchange activity in cultured rat hippocampal astrocytes. J Neurosci Res. 1996;44:191–198. doi: 10.1002/(SICI)1097-4547(19960415)44:2<191::AID-JNR12>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 65.Rutherford PA, Pizzonia JH, Biemesderfer D, Abu-Alfa A, Reilly R, Aronson PS. Expression of Na+–-H+ exchanger isoforms NHE1 and NHE3 in kidney and blood cells of rabbit and rat. Exp Nephrol. 1997;5:490–497. [PubMed] [Google Scholar]
- 66.Fliegel L, Dyck JR, Wang H, Fong C, Haworth RS. Cloning and analysis of the human myocardial Na+/H+ exchanger. Mol Cell Biochem. 1993;125:137–143. doi: 10.1007/BF00936442. [DOI] [PubMed] [Google Scholar]
- 67.Woo AL, James PF, Lingrel JB. Roles of the Na, K-ATPase alpha4 isoform and the Na+/H+ exchanger in sperm motility. Mol Reprod Dev. 2002;62:348–356. doi: 10.1002/mrd.90002. [DOI] [PubMed] [Google Scholar]
- 68.Lee BL, Sykes BD, Fliegel L. Structural analysis of the Na+/H+ exchanger isoform 1 (NHE1) using the divide and conquer approach. Biochem Cell Biol. 2011;89:189–199. doi: 10.1139/o10-140. [DOI] [PubMed] [Google Scholar]
- 69.Schushan M, Landau M, Padan E, Ben-Tal N (2011) Two conflicting NHE1 model structures: compatibility with experimental data and implications for the transport mechanism. J Biol Chem 286:le9 [DOI] [PMC free article] [PubMed]
- 70.Counillon L, Pouyssegur J, Reithmeier RA. The Na+/H+ exchanger NHE-1 possesses N- and O-linked glycosylation restricted to the first N-terminal extracellular domain. Biochemistry. 1994;33:10463–10469. doi: 10.1021/bi00200a030. [DOI] [PubMed] [Google Scholar]
- 71.Shrode LD, Gan BS, D’Souza SJ, Orlowski J, Grinstein S. Topological analysis of NHE1, the ubiquitous Na+/H+ exchanger using chymotryptic cleavage. Am J Physiol. 1998;275:C431–C439. doi: 10.1152/ajpcell.1998.275.2.C431. [DOI] [PubMed] [Google Scholar]
- 72.Wakabayashi S, Hisamitsu T, Pang TX, Shigekawa M. Mutations of Arg440 and Gly455/Gly456 oppositely change pH sensing of Na+/H+ exchanger 1. J Biol Chem. 2003;278:11828–11835. doi: 10.1074/jbc.M213243200. [DOI] [PubMed] [Google Scholar]
- 73.Aharonovitz O, Zaun HC, Balla T, York JD, Orlowski J, Grinstein S. Intracellular pH regulation by Na+/H+ exchange requires phosphatidylinositol 4,5-bisphosphate. J Cell Biol. 2000;150:213–224. doi: 10.1083/jcb.150.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wakabayashi S, Nakamura TY, Kobayashi S, Hisamitsu T. Novel phorbol ester-binding motif mediates hormonal activation of Na+/H+ exchanger. J Biol Chem. 2010;285:26652–26661. doi: 10.1074/jbc.M110.130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abu Jawdeh BG, Khan S, Deschênes I, Hoshi M, Goel M, Lock JT, Shinlapawittayatorn K, Babcock G, Lakhe-Reddy S, DeCaro G, Yadav SP, Mohan ML, Naga Prasad SV, Schilling WP, Ficker E, Schelling JR. Phosphoinositide binding differentially regulates NHE1 Na+/H+ exchanger-dependent proximal tubule cell survival. J Biol Chem. 2011;286:42435–42445. doi: 10.1074/jbc.M110.212845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khan S, Abu Jawdeh BG, Goel M, Schilling WP, Parker MD, Puchowicz MA, Yadav SP, Harris RC, El-Meanawy A, Hoshi M, Shinlapawittayatorn K, Deschenes I, Ficker E, Schelling JR. Lipotoxic disruption of NHE1 interaction with PI(4,5)P2 expedites proximal tubule apoptosis. J Clin Invest. 2014;124:1057–1068. doi: 10.1172/JCI71863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shimada-Shimizu N, Hisamitsu T, Nakamura TY, Hirayama N, Wakabayashi S. Na+/H+ exchanger 1 is regulated via its lipid-interacting domain, which functions as a molecular switch: a pharmacological approach using indolocarbazole compounds. Mol Pharmacol. 2014;85:18–28. doi: 10.1124/mol.113.089268. [DOI] [PubMed] [Google Scholar]
- 78.Fuster D, Moe OW, Hilgemann DW. Lipid- and mechanosensitivities of sodium/hydrogen exchangers analyzed by electrical methods. Proc Natl Acad Sci USA. 2004;101:10482–10487. doi: 10.1073/pnas.0403930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bullis BL, Li XJ, Rieder CV, Singh DN, Berthiaume LG, Fliegel L. Properties of the Na+/H+ exchanger protein—detergent-resistant aggregation and membrane microdistribution. Eur J Biochem. 2002;269:4887–4895. doi: 10.1046/j.1432-1033.2002.03202.x. [DOI] [PubMed] [Google Scholar]
- 80.Bourguignon LYW, Singleton PA, Diedrich F, Stern R, Gilad E. CD44 interaction with Na+–-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. J Biol Chem. 2004;279:26991–27007. doi: 10.1074/jbc.M311838200. [DOI] [PubMed] [Google Scholar]
- 81.Fujita A, Cheng J, Tauchi-Sato K, Takenawa T, Fujimoto T. A distinct pool of phosphatidylinositol 4,5-bisphosphate in caveolae revealed by a nanoscale labeling technique. Proc Natl Acad Sci USA. 2009;106:9256–9261. doi: 10.1073/pnas.0900216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Denker SP, Huang DC, Orlowski J, Furthmayr H, Barber DL. Direct binding of the Na-H exchanger NHE1 to ERM proteins regulates the cortical cytoskeleton and cell shape independently of H+ translocation. Mol Cell. 2000;6:1425–1436. doi: 10.1016/s1097-2765(00)00139-8. [DOI] [PubMed] [Google Scholar]
- 83.Wu KL, Khan S, Lakhe-Reddy S, Jarad G, Mukherjee A, Obejero-Paz CA, Konieczkowski M, Sedor JR, Schelling JR. The NHE1 Na+/H+ exchanger recruits ezrin/radixin/moesin proteins to regulate Akt-dependent cell survival. J Biol Chem. 2004;279:26280–26286. doi: 10.1074/jbc.M400814200. [DOI] [PubMed] [Google Scholar]
- 84.Khan S, Wu KL, Sedor JR, Abu Jawdeh BG, Schelling JR. The NHE1 Na+/H+ exchanger regulates cell survival by activating and targeting ezrin to specific plasma membrane domains. Cell Mol Biol. 2006;52:115–121. [PubMed] [Google Scholar]
- 85.Ramez M, Blot-Chabaud M, Cluzeaud F, Chanan S, Patterson M, Walensky LD, Marfatia S, Baines AJ, Chasis JA, Conboy JG, Mohandas N, Gascard P. Distinct distribution of specific members of protein 4.1 gene family in the mouse nephron. Kidney Int. 2003;63:1321–1337. doi: 10.1046/j.1523-1755.2003.00870.x. [DOI] [PubMed] [Google Scholar]
- 86.Nunomura W, Denker SP, Barber DL, Takakuwa Y, Gascard P. Characterization of cytoskeletal protein 4.1R interaction with NHE1 (Na(+)/H(+) exchanger isoform 1) Biochem J. 2012;446:427–435. doi: 10.1042/BJ20120535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin X, Barber DL. A calcineurin homologous protein inhibits GTPase-stimulated Na-H exchange. Proc Natl Acad Sci USA. 1996;93:12631–12636. doi: 10.1073/pnas.93.22.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pang T, Su X, Wakabayashi S, Shigekawa M. Calcineurin homologous protein as an essential cofactor for Na+/H+ exchangers. J Biol Chem. 2001;276:17367–17372. doi: 10.1074/jbc.M100296200. [DOI] [PubMed] [Google Scholar]
- 89.Pang TX, Hisamitsu T, Mori H, Shigekawa M, Wakabayashi S. Role of calcineurin B homologous protein in pH regulation by the Na+/H+ exchanger 1: tightly bound Ca2+ ions as important structural elements. Biochemistry. 2004;43:3628–3636. doi: 10.1021/bi0360004. [DOI] [PubMed] [Google Scholar]
- 90.Bertrand B, Wakabayashi S, Ikeda T, Pouyssegur J, Shigekawa M. The Na+/H+ exchanger isoform 1 (NHE1) is a novel member of the calmodulin-binding proteins. Identification and characterization of calmodulin-binding sites. J Biol Chem. 1994;269:13703–13709. [PubMed] [Google Scholar]
- 91.Wakabayashi S, Bertrand B, Ikeda T, Pouyssegur J, Shigekawa M. Mutation of calmodulin-binding site renders the Na+/H+ exchanger (NHE1) highly H(+)-sensitive and Ca2+ regulation-defective. J Biol Chem. 1994;269:13710–13715. [PubMed] [Google Scholar]
- 92.Wakabayashi S, Ikeda T, Iwamoto T, Pouyssegur J, Shigekawa M. Calmodulin-binding autoinhibitory domain controls “pH-sensing” in the Na+/H+ exchanger NHE1 through sequence-specific interaction. Biochemistry. 1997;36:12854–12861. doi: 10.1021/bi9715472. [DOI] [PubMed] [Google Scholar]
- 93.Garnovskaya MN, Mukhin YV, Vlasova TM, Raymond JR. Hypertonicity activates Na+/H+ exchange through Janus kinase 2 and calmodulin. J Biol Chem. 2003;278:16908–16915. doi: 10.1074/jbc.M209883200. [DOI] [PubMed] [Google Scholar]
- 94.Wakabayashi S, Fafournoux P, Sardet C, Pouyssegur J. The Na+/H+ antiporter cytoplasmic domain mediates growth factor signals and controls “H+-sensing”. Proc Natl Acad Sci USA. 1992;89:2424–2428. doi: 10.1073/pnas.89.6.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bianchini L, Kapus A, Lukacs G, Wasan S, Wakabayashi S, Pouyssegur J, Yu FH, Orlowski J, Grinstein S. Responsiveness of mutants of NHE1 isoform of Na+/H+ antiport to osmotic stress. Am J Physiol. 1995;269:C998–C1007. doi: 10.1152/ajpcell.1995.269.4.C998. [DOI] [PubMed] [Google Scholar]
- 96.Sardet C, Fafournoux P, Pouyssegur J. α-thrombin, epidermal growth factor, and okadaic acid activate the Na+/H+ exchanger, NHE-1, by phosphorylating a set of common sites. J Biol Chem. 1991;266:19166–19171. [PubMed] [Google Scholar]
- 97.Odunewu A, Fliegel L. Acidosis mediated regulation of the NHE1 isoform of the Na+/H+ exchanger in renal cells. Am J Physiol Renal Physiol. 2013;305:F370–F381. doi: 10.1152/ajprenal.00598.2012. [DOI] [PubMed] [Google Scholar]
- 98.Bianchini L, L’Allemain G, Pouyssegur J. The p42/p44 mitogen-activated protein kinase cascade is determinant in mediating activation of the Na+/H+ exchanger (NHE1 isoform) in response to growth factors. J Biol Chem. 1997;272:271–279. doi: 10.1074/jbc.272.1.271. [DOI] [PubMed] [Google Scholar]
- 99.Wang H, Silva NL, Lucchesi PA, Haworth R, Wang K, Michalak M, Pelech S, Fliegel L. Phosphorylation and regulation of the Na+/H+ exchanger through mitogen-activated protein kinase. Biochemistry. 1997;36:9151–9158. doi: 10.1021/bi970802f. [DOI] [PubMed] [Google Scholar]
- 100.Pedersen SF, Darborg BV, Rentsch ML, Rasmussen M. Regulation of mitogen-activated protein kinase pathways by the plasma membrane Na(+)/H(+) exchanger, NHE1. Arch Biochem Biophys. 2006;462:195–201. doi: 10.1016/j.abb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 101.Takahashi E, Abe J, Gallis B, Aebersold R, Spring DJ, Krebs EG, Berk BC. p90(RSK) is a serum-stimulated Na+/H+ exchanger isoform-1 kinase. Regulatory phosphorylation of serine 703 of Na+/H+ exchanger isoform-1. J Biol Chem. 1999;274:20206–20214. doi: 10.1074/jbc.274.29.20206. [DOI] [PubMed] [Google Scholar]
- 102.Yan WH, Nehrke K, Choi J, Barber DL. The Nck-interacting kinase (NIK) phosphorylates the Na+–-H+ exchanger NHE1 and regulates NHE1 activation by platelet-derived growth factor. J Biol Chem. 2001;276:31349–31356. doi: 10.1074/jbc.M102679200. [DOI] [PubMed] [Google Scholar]
- 103.Tominaga T, Ishizaki T, Narumiya S, Barber DL. p160ROCK mediates RhoA activation of Na-H exchange. EMBO J. 1998;17:4712–4722. doi: 10.1093/emboj/17.16.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Snabaitis AK, Cuello F, Avkiran M. Protein kinase B/Akt phosphorylates and inhibits the cardiac Na+/H+ exchanger NHE1. Circ Res. 2008;103:881–890. doi: 10.1161/CIRCRESAHA.108.175877. [DOI] [PubMed] [Google Scholar]
- 105.Meima ME, Webb BA, Witkowska HE, Barber DL. The sodium-hydrogen exchanger NHE1 is an Akt substrate necessary for actin filament reorganization by growth factors. J Biol Chem. 2009;284:26666–26675. doi: 10.1074/jbc.M109.019448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Voelkl J, Pasham V, Ahmed MS, Walker B, Szteyn K, Kuhl D, Metzler B, Alesutan I, Lang F. Sgk1-dependent stimulation of cardiac Na/H exchanger Nhe1 by dexamethasone. Cell Physiol Biochem. 2013;32:25–38. doi: 10.1159/000350120. [DOI] [PubMed] [Google Scholar]
- 107.Moolenaar WH, Tsien RY, van der Saag PT, de Laat SW. Na+/H+ exchange and cytoplasmic pH in the action of growth factors in human fibroblasts. Nature. 1983;304:645–648. doi: 10.1038/304645a0. [DOI] [PubMed] [Google Scholar]
- 108.Besson P, Fernandez-Rachubinski F, Yang W, Fliegel L. Regulation of Na+/H+ exchanger gene expression: mitogenic stimulation increases NHE1 promoter activity. Am J Physiol. 1998;274:C831–C839. doi: 10.1152/ajpcell.1998.274.3.C831. [DOI] [PubMed] [Google Scholar]
- 109.Putney LK, Barber DL. Na-H exchange-dependent increase in intracellular pH times G2/M entry and transition. J Biol Chem. 2003;278:44645–44649. doi: 10.1074/jbc.M308099200. [DOI] [PubMed] [Google Scholar]
- 110.Rao GN, Sardet C, Pouyssegur J, Berk BC. Na+/H+ antiporter gene expression increases during retinoic acid-induced granulocytic differentiation of HL60 cells. J Cell Physiol. 1992;151:361–366. doi: 10.1002/jcp.1041510217. [DOI] [PubMed] [Google Scholar]
- 111.Wang H, Singh D, Fliegel L. The Na+/H+ antiporter potentiates growth and retinoic acid-induced differentiation of P19 embryonal carcinoma cells. J Biol Chem. 1997;272:26545–26549. doi: 10.1074/jbc.272.42.26545. [DOI] [PubMed] [Google Scholar]
- 112.Tominaga T, Barber DL. Na-H exchange acts downstream of RhoA to regulate integrin-induced cell adhesion and spreading. Mol Biol Cell. 1998;9:2287–2303. doi: 10.1091/mbc.9.8.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Denker SP, Barber DL. Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J Cell Biol. 2002;159:1087–1096. doi: 10.1083/jcb.200208050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schwartz MA, Lechene C, Ingber DE. Insoluble fibronectin activates the Na/H antiporter by clustering and immobilizing integrin α5β1, independent of cell shape. Proc Natl Acad Sci USA. 1991;88:7849–7853. doi: 10.1073/pnas.88.17.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grinstein S, Woodside M, Sardet C, Pouyssegur J, Rotin D. Activation of the Na+/H+ antiporter during cell volume regulation. Evidence for a phosphorylation-independent mechanism. J Biol Chem. 1992;267:23823–23828. [PubMed] [Google Scholar]
- 116.McSwine RL, Li J, Villereal ML. Examination of the role for Ca2+ in regulation and phosphorylation of the Na+/H+ antiporter NHE1 via mitogen and hypertonic stimulation. J Cell Physiol. 1996;168:8–17. doi: 10.1002/(SICI)1097-4652(199607)168:1<8::AID-JCP2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 117.Baumgartner M, Patel H, Barber DL. Na+/H+ exchanger NHE1 as plasma membrane scaffold in the assembly of signaling complexes. Am J Physiol Cell Physiol. 2004;287:C844–C850. doi: 10.1152/ajpcell.00094.2004. [DOI] [PubMed] [Google Scholar]
- 118.Odunewu-Aderibigbe A, Fliegel L. The Na(+)/H(+) exchanger and pH regulation in the heart. IUBMB Life. 2014;66:679–685. doi: 10.1002/iub.1323. [DOI] [PubMed] [Google Scholar]
- 119.Gottlieb RA, Nordberg J, Skowronski E, Babior BM. Apoptosis induced in Jurkat cells by several agents is preceded by intracellular acidification. Proc Natl Acad Sci USA. 1996;93:654–658. doi: 10.1073/pnas.93.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Matsuyama S, Llopis J, Deveraux QL, Tsien RY, Reed JC. Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat Cell Biol. 2000;2:318–325. doi: 10.1038/35014006. [DOI] [PubMed] [Google Scholar]
- 121.Segal MS, Beem E. Effect of pH, ionic charge, and osmolality on cytochrome c-mediated caspase-3 activity. Am J Physiol. 2001;281:C1196–C1204. doi: 10.1152/ajpcell.2001.281.4.C1196. [DOI] [PubMed] [Google Scholar]
- 122.Counis MF, Torriglia A. Acid DNases and their interest among apoptotic endonucleases. Biochimie. 2006;88:1851–1858. doi: 10.1016/j.biochi.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 123.Ruffin VA, Salameh AI, Boron WF, Parker MD. Intracellular pH regulation by acid-base transporters in mammalian neurons. Front Physiol. 2014;5:43. doi: 10.3389/fphys.2014.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shi Y, Kim D, Caldwell M, Sun D. The role of Na(+)/H(+) exchanger isoform 1 in inflammatory responses: maintaining H(+) homeostasis of immune cells. Adv Exp Med Biol. 2013;961:411–418. doi: 10.1007/978-1-4614-4756-6_35. [DOI] [PubMed] [Google Scholar]
- 125.Plopper GE, McNamee HP, Dike LE, Bojanowski K, Ingber DE. Convergence of integrin and growth factor receptor signaling pathways within the focal adhesion complex. Mol Biol Cell. 1995;6:1349–1365. doi: 10.1091/mbc.6.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ludwig FT, Schwab A, Stock C. The Na(+)/H(+)-exchanger (NHE1) generates pH nanodomains at focal adhesions. J Cell Physiol. 2013;228:1351–1358. doi: 10.1002/jcp.24293. [DOI] [PubMed] [Google Scholar]
- 127.Srivastava J, Barreiro G, Groscurth S, Gingras AR, Goult BT, Critchley DR, Kelly MJ, Jacobson MP, Barber DL. Structural model and functional significance of pH-dependent talin-actin binding for focal adhesion remodeling. Proc Natl Acad Sci USA. 2008;105:14436–14441. doi: 10.1073/pnas.0805163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Frantz C, Barreiro G, Dominguez L, Chen X, Eddy R, Condeelis J, Kelly MJ, Jacobson MP, Barber DL. Cofilin is a pH sensor for actin free barbed end formation: role of phosphoinositide binding. J Cell Biol. 2008;183:865–879. doi: 10.1083/jcb.200804161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stock C, Gassner B, Hauck CR, Arnold H, Mally S, Eble JA, Dieterich P, Schwab A. Migration of human melanoma cells depends on extracellular pH and Na+/H+ exchange. J Physiol. 2005;567:225–238. doi: 10.1113/jphysiol.2005.088344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Greco MR, Antelmi E, Busco G, Guerra L, Rubino R, Casavola V, Reshkin SJ, Cardone RA. Protease activity at invadopodial focal digestive areas is dependent on NHE1-driven acidic pHe. Oncol Rep. 2014;31:940–946. doi: 10.3892/or.2013.2923. [DOI] [PubMed] [Google Scholar]
- 131.Lin Y, Wang J, Jin W, Wang L, Li H, Ma L, Li Q, Pang T. NHE1 mediates migration and invasion of HeLa cells via regulating the expression and localization of MT1-MMP. Cell Biochem Funct. 2012;30:41–46. doi: 10.1002/cbf.1815. [DOI] [PubMed] [Google Scholar]
- 132.Jacobsen C, Kragh-Hansen U, Sheikh MI. Na+ -H+ exchange in luminal-membrane vesicles from rabbit proximal convoluted and straight tubules in response to metabolic acidosis. Biochem J. 1986;239:411–416. doi: 10.1042/bj2390411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Baum M. Axial heterogeneity of rabbit proximal tubule luminal H+ and basolateral HCO3 − transport. Am J Physiol. 1989;256:F335–F341. doi: 10.1152/ajprenal.1989.256.2.F335. [DOI] [PubMed] [Google Scholar]
- 134.Choi JY, Shah M, Lee MG, Schultheis PJ, Shull GE, Muallem S, Baum M. Novel amiloride-sensitive sodium-dependent proton secretion in the mouse proximal convoluted tubule. J Clin Invest. 2000;105:1141–1146. doi: 10.1172/JCI9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Forster IC, Hernando N, Biber J, Murer H. Phosphate transporters of the SLC20 and SLC34 families. Mol Aspects Med. 2013;34:386–395. doi: 10.1016/j.mam.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 136.Spring KR, Giebisch G. Kinetics of Na+ transport in Necturus proximal tubule. J Gen Physiol. 1977;70:307–328. doi: 10.1085/jgp.70.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Romero MF, Hediger MA, Boulpaep EL, Boron WF. Expression cloning and characterization of a renal electrogenic Na+/HCO3 − cotransporter. Nature. 1997;387:409–413. doi: 10.1038/387409a0. [DOI] [PubMed] [Google Scholar]
- 138.Holthouser K, Mandal A, Merchant ML, Schelling JR, Delamere NA, Valdes RR, Jr, Tyagi SC, Lederer ED, Khundmiri SJ. Ouabain stimulates Na-K ATPase through sodium hydrogen exchanger-1 (NHE-1) dependent mechanism in human kidney proximal tubule cells. Am J Physiol Renal Physiol. 2010;299:F77–F90. doi: 10.1152/ajprenal.00581.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Garciarena CD, Ma YL, Swietach P, Huc L, Vaughan-Jones RD. Sarcolemmal localisation of Na+/H+ exchange and Na+ -HCO3− co-transport influences the spatial regulation of intracellular pH in rat ventricular myocytes. J Physiol. 2013;591:2287–2306. doi: 10.1113/jphysiol.2012.249664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schwab A, Rossmann H, Klein M, Dieterich P, Gassner B, Neff C, Stock C, Seidler U. Functional role of Na+ -HCO3− cotransport in migration of transformed renal epithelial cells. J Physiol. 2005;568:445–458. doi: 10.1113/jphysiol.2005.092957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Alexander RT, Grinstein S. Na/H exchangers and the regulation of volume. Acta Physiol (Oxf) 2006;187:159–167. doi: 10.1111/j.1748-1716.2006.01558.x. [DOI] [PubMed] [Google Scholar]
- 142.Russell JM. Sodium-potassium-chloride cotransport. Physiol Rev. 2000;80:211–276. doi: 10.1152/physrev.2000.80.1.211. [DOI] [PubMed] [Google Scholar]
- 143.Okada Y, Maeno E, Shimizu T, Dezaki K, Wang J, Morishima S. Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD) J Physiol. 2001;532:3–16. doi: 10.1111/j.1469-7793.2001.0003g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kaplan MR, Plotkin MD, Lee WS, Xu ZC, Lytton J, Hebert SC. Apical localization of the Na-K-Cl cotransporter, rBSC1, on rat thick ascending limbs. Kidney Int. 1996;49:40–47. doi: 10.1038/ki.1996.6. [DOI] [PubMed] [Google Scholar]
- 145.Stuart-Tilley AK, Shmukler BE, Brown D, Alper SL. Immunolocalization and tissue-specific splicing of AE2 anion exchanger in mouse kidney. JASN. 1998;9:946–959. doi: 10.1681/ASN.V96946. [DOI] [PubMed] [Google Scholar]
- 146.Kapus A, Grinstein S, Wasan S, Kandasamy R, Orlowski J. Functional characterization of three isoforms of the Na+/H+ exchanger stably expressed in Chinese hamster ovary cells. ATP dependence, osmotic sensitivity, and role in cell proliferation. J Biol Chem. 1994;269:23544–23552. [PubMed] [Google Scholar]
- 147.Soleimani M, Watts BA, III, Singh G, Good DW. Effect of long-term hyperosmolality on the Na+/H+ exchanger isoform NHE-3 in LLC-PK1 cells. Kidney Int. 1998;53:423–431. doi: 10.1046/j.1523-1755.1998.00771.x. [DOI] [PubMed] [Google Scholar]
- 148.Krump E, Nikitas K, Grinstein S. Induction of tyrosine phosphorylation and Na+/H+ exchanger activation during shrinkage of human neutrophils. J Biol Chem. 1997;272:17303–17311. doi: 10.1074/jbc.272.28.17303. [DOI] [PubMed] [Google Scholar]
- 149.Wu KL, Khan S, Lakhe-Reddy S, Wang LM, Jarad G, Miller RT, Konieczkowski M, Brown AM, Sedor JR, Schelling JR. Renal tubular epithelial cell apoptosis is associated with caspase cleavage of the NHE1 Na+/H+ exchanger. Am J Physiol Renal Physiol. 2003;284:F829–F839. doi: 10.1152/ajprenal.00314.2002. [DOI] [PubMed] [Google Scholar]
- 150.Grinstein S, Clarke CA, Rothstein A. Activation of Na+/H+ exchange in lymphocytes by osmotically induced volume changes and by cytoplasmic acidification. J Gen Physiol. 1983;82:619–638. doi: 10.1085/jgp.82.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fukushima T, Waddell TK, Grinstein S, Goss GG, Orlowski J, Downey GP. Na+/H+ exchange activity during phagocytosis in human neutrophils: role of Fcgamma receptors and tyrosine kinases. J Cell Biol. 1996;132:1037–1052. doi: 10.1083/jcb.132.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yeung T, Terebiznik M, Yu L, Silvius J, Abidi WM, Philips M, Levine T, Kapus A, Grinstein S. Receptor activation alters inner surface potential during phagocytosis. Science. 2006;313:347–351. doi: 10.1126/science.1129551. [DOI] [PubMed] [Google Scholar]
- 153.Cox GA, Lutz CM, Yang CL, Biemesderfer D, Bronson RT, Fu A, Aronson PS, Noebels JL, Frankel WN. Sodium/hydrogen exchanger gene defect in slow-wave epilepsy mutant mice. Cell. 1997;91:139–148. doi: 10.1016/s0092-8674(01)80016-7. [DOI] [PubMed] [Google Scholar]
- 154.Bell SM, Schreiner CM, Schultheis PJ, Miller ML, Evans RL, Vorhees CV, Shull GE, Scott WJ. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am J Physiol. 1999;276:C788–C795. doi: 10.1152/ajpcell.1999.276.4.C788. [DOI] [PubMed] [Google Scholar]
- 155.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet. 1998;19:282–285. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- 156.Igarashi T, Inatomi J, Sekine T, Cha SH, Kanai Y, Kunimi M, Tsukamoto K, Satoh H, Shimadzu M, Tozawa F, Mori T, Shiobara M, Seki G, Endou H. Mutations in SLC4A4 cause permanent isolated proximal renal tubular acidosis with ocular abnormalities. Nat Genet. 1999;23:264–266. doi: 10.1038/15440. [DOI] [PubMed] [Google Scholar]
- 157.Guissart C, Li X, Leheup B, Drouot N, Montaut-Verient B, Raffo E, Jonveaux P, Roux AF, Claustres M, Fliegel L, Koenig M. Mutation of SLC9A1, encoding the major Na+/H+ exchanger, causes ataxia-deafness Lichtenstein-Knorr syndrome. Hum Mol Genet. 2015;24:463–470. doi: 10.1093/hmg/ddu461. [DOI] [PubMed] [Google Scholar]
- 158.Wessels D, Lusche DF, Scherer A, Kuhl S, Myre MA, Soll DR. Huntingtin regulates Ca chemotaxis and K-facilitated cAMP chemotaxis, in conjuction with the monovalent cation/H exchanger Nhe1, in a model developmental system: insights into its possible role in Huntingtons disease. Dev Biol. 2014;394:24–38. doi: 10.1016/j.ydbio.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 159.Risdon RA, Sloper JC, De Wardener HE. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet. 1968;2:363–366. doi: 10.1016/s0140-6736(68)90589-8. [DOI] [PubMed] [Google Scholar]
- 160.Schainuck LI, Striker GE, Cutler RE, Benditt EP. Structural-functional correlations in renal disease. Hum Pathol. 1970;1:631–641. doi: 10.1016/s0046-8177(70)80061-2. [DOI] [PubMed] [Google Scholar]
- 161.Bohle A, Mackensen-Haen S, von Gise H. Significance of tubulointerstitial changes in the renal cortex for the excretory function and concentration ability of the kidney: a morphometric contribution. Am J Nephrol. 1987;7:421–433. doi: 10.1159/000167514. [DOI] [PubMed] [Google Scholar]
- 162.Schelling JR, Nkemere N, Kopp JB, Cleveland RP. Fas-dependent fratricidal apoptosis is a mechanism of tubular epithelial cell deletion in chronic renal failure. Lab Invest. 1998;78:813–824. [PubMed] [Google Scholar]
- 163.Manucha W, Carrizo L, Ruete C, Valles PG (2007) Apoptosis induction is associated with decreased NHE1 expression in neonatal unilateral ureteric obstruction. BJU Int 100:191–198 [DOI] [PubMed]
- 164.Bocanegra V, Gil Lorenzo AF, Cacciamani V, Benardon ME, Costantino VV, Valles PG. RhoA and MAPKinase signal transduction pathways regulate NHE1 Na+/H+ exchanger-dependent proximal tubule cell apoptosis following mechanical stretch. Am J Physiol Renal Physiol. 2014;307:F881–F889. doi: 10.1152/ajprenal.00232.2014. [DOI] [PubMed] [Google Scholar]
- 165.Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, Maundrell K, Gambale F, Sadoul R, Martinou JC. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 166.Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 167.Feng Z, Tang L, Wu L, Cui S, Hong Q, Cai G, Wu D, Fu B, Wei R, Chen X. Na/H exchanger-1 reduces podocyte injury caused by endoplasmic reticulum stress via autophagy activation. Lab Invest. 2014;94:439–454. doi: 10.1038/labinvest.2014.4. [DOI] [PubMed] [Google Scholar]
- 168.Lupescu A, Geiger C, Zahir N, Aberle S, Lang PA, Kramer S, Wesselborg S, Kandolf R, Foller M, Lang F, Bock CT. Inhibition of Na+/H+ exchanger activity by parvovirus B19 protein NS1. Cell Physiol Biochem. 2009;23:211–220. doi: 10.1159/000204110. [DOI] [PubMed] [Google Scholar]
- 169.Chander PN, Gealekman O, Brodsky SV, Elitok S, Tojo A, Crabtree M, Gross SS, Goligorsky MS. Nephropathy in Zucker diabetic fat rat is associated with oxidative and nitrosative stress: prevention by chronic therapy with a peroxynitrite scavenger ebselen. J Am Soc Nephrol. 2004;15:2391–2403. doi: 10.1097/01.ASN.0000135971.88164.2C. [DOI] [PubMed] [Google Scholar]
- 170.Wang Z, Jiang T, Li J, Proctor G, McManaman JL, Lucia S, Chua S, Levi M. Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes. 2005;54:2328–2335. doi: 10.2337/diabetes.54.8.2328. [DOI] [PubMed] [Google Scholar]
- 171.Yamashita J, Ohkita M, Takaoka M, Kaneshiro Y, Matsuo T, Kaneko K, Matsumura Y. Role of Na+/H+ exchanger in the pathogenesis of ischemic acute renal failure in mice. J Cardiovasc Pharmacol. 2007;49:154–160. doi: 10.1097/FJC.0b013e318030c2c9. [DOI] [PubMed] [Google Scholar]
- 172.Wu D, Russano K, Kouz I, Abraham WM. NHE1 inhibition improves tissue perfusion and resuscitation outcome after severe hemorrhage. J Surg Res. 2013;181:e75–e81. doi: 10.1016/j.jss.2012.07.026. [DOI] [PubMed] [Google Scholar]