Abstract
Objective
OCEANS is a randomized, placebo (PL)-controlled, phase 3 trial evaluating the efficacy and safety of bevacizumab combined with gemcitabine + carboplatin (GC) for patients with platinum-sensitive recurrent ovarian cancer (ROC). The study met its primary endpoint, demonstrating improved progression-free survival with GC + bevacizumab compared with GC + PL. Herein, we describe results of final overall survival (OS) and updated safety.
Methods
Patients with recurrent platinum-sensitive ROC (recurring ≥6 months after first-line platinum-based therapy) and measurable disease at baseline were randomized to receive GC + bevacizumab or GC + PL for 6–10 cycles; PL or bevacizumab was then continued until disease progression. In this updated analysis, a Cox proportional hazards model was used to compare OS between the 2 treatment arms.
Results
At the data cutoff date (July 19, 2013), 353 patients (72.9%) had died. Median follow-up for OS was 58.2 months in the experimental arm and 56.4 months in the control arm. Consistent with interim analyses, median OS was comparable between arms (GC + bevacizumab: 33.6 months; GC + PL: 32.9 months; hazard ratio = 0.95; log-rank p = 0.65), and was consistent across all examined patient subgroups. The frequency and severity of adverse events were consistent with previous analyses; no new safety concerns were identified.
Conclusions
Results from final OS analysis of the phase 3 OCEANS study showed no significant difference in OS for patients treated with GC + bevacizumab compared with GC + PL.
Introduction
Ovarian cancer, the fifth leading cause of cancer deaths among women, has the highest mortality rate among all gynecologic cancers [1]. Due to the biology of the disease and a lack of specific symptoms, the majority of cases are usually detected at an advanced stage [2]. While first-line therapy, which includes cytoreductive surgery and platinum- and taxane-based chemotherapy, usually elicits a good initial response, most patients eventually relapse [3–5]. Current treatment options for patients upon platinum-sensitive disease recurrence consist of re-treatment with a platinum doublet [5]. Almost all patients will subsequently go on to develop additional recurrences, requiring repeated courses of chemotherapy. Therefore, more effective treatments for recurrent ovarian cancer are needed.
Bevacizumab is currently approved in the United States for the treatment of a variety of solid tumors [6], and has been evaluated in ovarian cancer in both newly diagnosed and relapsed disease [7–10]. The Ovarian Cancer Study Comparing Efficacy and Safety of Chemotherapy and Anti-angiogenic Therapy in Platinum-Sensitive Recurrent Disease (OCEANS) was initiated to study the addition of bevacizumab to gemcitabine and carboplatin (GC) combination treatment as a second-line therapy for patients with epithelial ovarian, primary peritoneal, or fallopian tube cancer [9]. The study met its primary endpoint, demonstrating that the addition of bevacizumab to GC conferred a significant improvement in progression-free survival (PFS) for patients compared with GC + placebo (PL) (median: 12.4 months for GC + bevacizumab vs. 8.4 months for GC + PL; hazard ratio [HR] = 0.484; 95% confidence interval [CI]: 0.388–0.605; log-rank p-value <0.0001). Furthermore, GC + bevacizumab was associated with a higher objective response rate (78.5% vs. 57.4%, respectively; p < 0.001) and prolonged the duration of response (10.4 months vs. 7.4 months, respectively; HR = 0.534; 95% CI: 0.408–0.698) compared with GC + PL [9, 11]. Patients in the bevacizumab arm compared with those in the placebo arm experienced a higher incidence of grade ≥3 hypertension (17.4% vs. <1%, respectively) and proteinuria (8.5% vs. <1%, respectively), while the rate of neutropenia and febrile neutropenia was similar in both arms [9]. No gastrointestinal (GI) perforations occurred during the study treatment or safety assessment period, but 2 patients in the bevacizumab arm experienced GI perforations after discontinuation of study treatment [9].
The OCEANS study was not powered to detect a difference in overall survival (OS) between the arms, which was 1 of the secondary endpoints. At the time of the final PFS analysis, the interim OS analysis showed no difference between treatment groups (HR: 1.03; 95% CI: 0.79–1.33) [9]. The final OS analysis was pre-specified at 353 events, and these results are described herein.
Methods
Patients and study design
The patient eligibility criteria and study design for OCEANS (NCT00434642) have been previously described [9]. Briefly, all patients were required to have histologically confirmed recurrent ovarian cancer (ie, epithelial ovarian, fallopian tube, or primary peritoneal carcinoma), disease progression after ≥6 months after completion of front-line platinum-based chemotherapy, and measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0. Patients were excluded if they had prior chemotherapy in the recurrent setting; previous treatment with systemic bevacizumab or other vascular endothelial growth factor pathway agents; or history of abdominal fistula, GI perforations, or intra-abdominal abscess.
Patients were randomized 1:1 to receive GC + bevacizumab or GC + PL, with platinum-free interval (6–12 vs. >12 months) and cytoreductive surgery for recurrent disease (yes vs. no) as stratification factors. Gemcitabine (1000 mg/m2 on days 1 and 8) and carboplatin (area under the curve 4 mg/mL/min on day 1) and either bevacizumab (15 mg/kg on day 1) or PL were administered to patients for 6–10 cycles of 21 days. After 6–10 cycles, bevacizumab or PL were administered until disease progression or unacceptable toxicity.
Treatment unblinding was permitted only after disease progression, at the time of the final PFS analysis, or for medically emergent situations. Patients still receiving bevacizumab at the time of unblinding were given the option to continue with bevacizumab treatment in the open-label phase of the study. Patients who were unblinded due to disease progression were not restricted in their post-progression therapy options.
Updated OS analyses
The study protocol stipulated that a final analysis of OS be conducted at 353 deaths (72.9%). This allows 60% power, assuming a median OS of 18 months in the GC + PL arm and a decrease in the death rate in the GC + bevacizumab arm by 21% (ie, HR = 0.79). OS was assessed for all patients who enrolled in the study and were randomized to 1 of the treatment groups (ie, the intention-to-treat [ITT] population). Patients were monitored for survival every 3 months, until death, withdrawal of consent, loss to follow-up, or study termination. Duration of OS was defined as the time from randomization to death by any cause in the ITT population. The analysis of OS utilized a 2-sided stratified (using the aforementioned stratification factors) and unstratified log-rank test comparing the 2 treatment arms (GC + bevacizumab, GC + PL) with hypothesis testing performed at the α = 0.048 level. OS in each treatment arm was estimated via Kaplan–Meier methods, and 95% CIs for median OS were constructed with the Brookmeyer–Crowley methodology [12]. Stratified and unstratified HRs were estimated with a Cox regression model. Exploratory analyses examined OS according to subgroups defined by patient characteristics at baseline, including age, primary tumor site, time from last platinum therapy to recurrence, size of largest lesion, and baseline CA-125 level.
Safety
Safety analyses were performed on the safety patient population (ie, patients who received any partial or full dose of study treatment) as described previously [9]. The analysis included only treatment-emergent events that occurred within 30 days of the last study drug administration on or before the cutoff date of July 19, 2013. Patients who discontinued treatment were followed for 30 days for adverse events at a treatment-termination visit. Patients, who at that time had an ongoing serious adverse event or adverse event leading to study drug treatment discontinuation, were followed every 2 months until either the event resolved, the investigator assessed the event as stable, or the patient was lost to follow-up.
All adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 3.0 and coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 13.1. Adverse events were documented in MedDRA system organ class and preferred toxicity term categories according to the maximum reported severity.
Of note, at the time of the primary PFS analysis, the MedDRA defined GI perforations to include NCI CTCAE grade 1 intra-abdominal abscess and fistula (excluding evidence of abdominal free air attributable to other causes, such as paracentesis or recent surgical procedure) or grade ≥2 GI perforations (including intra-abdominal abscess and fistula). However, at the time of the final OS analysis, the MedDRA reported abscess/fistula separate from GI perforations. Thrombocytopenia (grade ≥4) was not included in the analysis of adverse events of special interest but is described as a preferred term in adverse events of grades 3–5. Due to these MedDRA coding definition changes, the number of patients with adverse events of special interest may have changed from the number of patients previously reported [9].
RESULTS
Patient disposition and characteristics
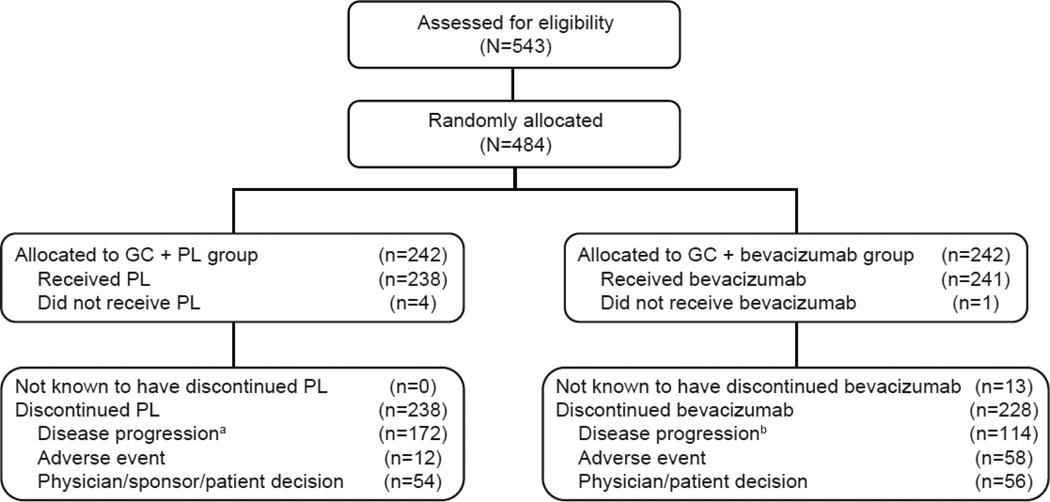
Between April 2007 and January 2010, the OCEANS study enrolled a total of 484 patients with recurrent ovarian cancer (Fig. 1). As previously described, baseline patient and disease characteristics were similar between patients in each treatment group [9].
Fig. 1. CONSORT diagram of all randomly assigned patients.
aIncludes 168 patients with disease progression per RECIST and 4 patients with clinical disease progression.
bIncludes 109 patients with disease progression per RECIST and 5 patients with clinical disease progression.
cFive patients who were randomly assigned to the GC + PL arm received 1 or 2 doses of bevacizumab in error and were assigned to the GC + bevacizumab arm for all safety analyses.
dFour patients who were randomly assigned to the GC + PL arm did not receive any protocol treatment and thus were excluded from the safety analysis.
GC, gemcitabine + carboplatin; PL, placebo.
All patients had completed treatment by the data cutoff date of July 19, 2013. Patients in the GC + bevacizumab arm received a wider range of treatment cycles than those in the GC + PL arm (GC +bevacizumab: 1–88; GC + PL: 1–40), as well as a higher median number of cycles (GC +bevacizumab: 12; GC + PL: 10) (Table 1). The median duration of treatment for the GC + bevacizumab arm was likewise higher (GC +bevacizumab: 37.4 weeks; GC + PL: 32.1 weeks), while the median dose intensity (92.3%) was the same in both arms (see Table 1).
Table 1.
Duration of survival and safety follow-up and bevacizumab/placebo exposure.
| GC + PL | GC + bevacizumab |
All patients | |
|---|---|---|---|
| Safety | |||
| Duration of safety follow-up,a months | n=233 | n=247 | |
| Median (range) | 8.4 (1.0–34.1) | 9.6 (1.0–62.1) | - |
| Bevacizumab/PL exposure | n=233 | n=246 | |
| Cycles, n | |||
| Median (range) | 10.0 (1.0–40.0) | 12.0 (1.0–88.0) | - |
| Estimated dose intensity,b % | |||
| Median (range) | 92.3 (60.0–108.7) | 92.3 (60.0–100.6) | - |
| Duration of bevacizumab/PL,c weeks | |||
| Median (range) | 32.1 (0.1–144.1) | 37.4 (0.1–265.9) | - |
| Survival | n=242 | n=242 | N=484 |
| Duration of survival follow-up, months | |||
| Median (range) | 56.4 (0.3–72.6) | 58.2 (3.9d–73.9) | 57.5 (0.3–73.9) |
Time from the first dose of study drug or chemotherapy until 30 days after the last dose of study drug or chemotherapy during treatment period.
The actual dose received divided by the intended dose × 100.
The time from start of bevacizumab/PL to the date of last bevacizumab/PL.
Censored observation.
GC, gemcitabine + carboplatin; PL, placebo.
Overall survival
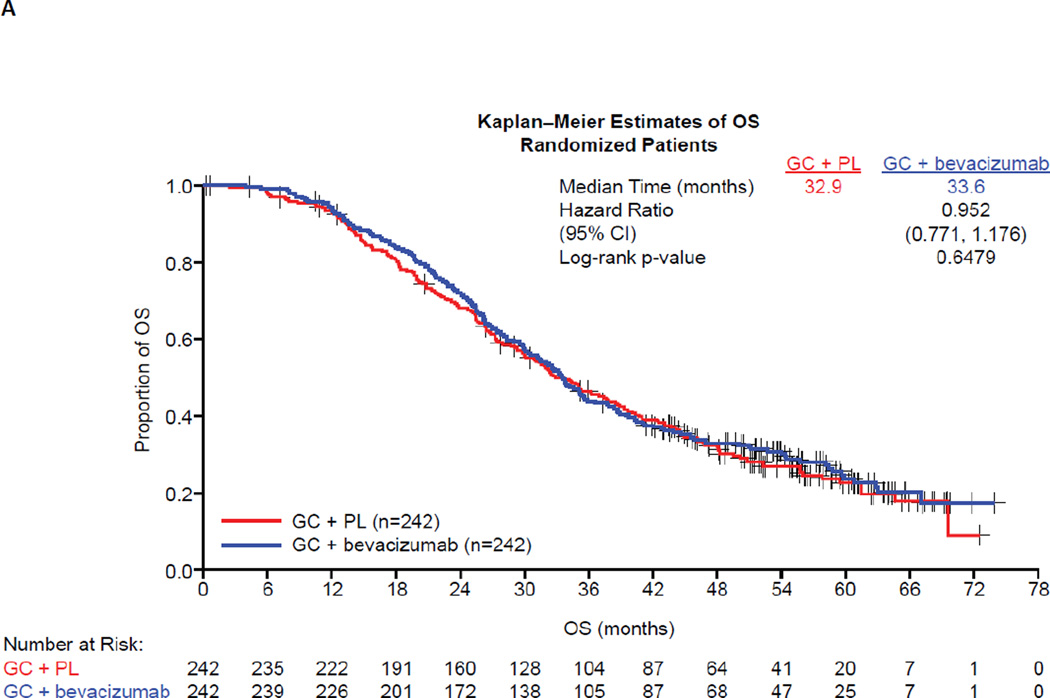
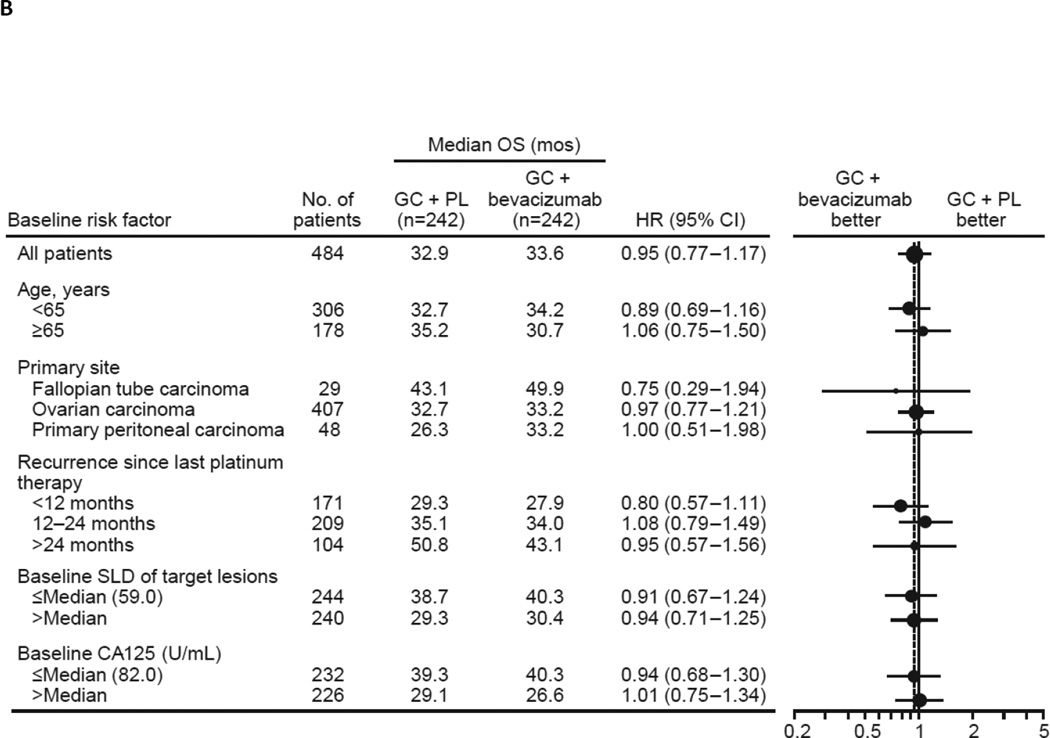
In the final OS analysis, a total of 353 patients (72.9%) had died while 131 (27.1%) were still in survival follow-up, with a similar distribution between treatment groups (see Table 1). The median follow-up time was 58.2 months in the GC + bevacizumab arm and 56.4 months in the GC + PL arm (see Table 1). The overall median OS was similar in the ITT population between treatment groups (GC + bevacizumab: 33.6 months; GC + PL: 32.9 months) (Fig. 2a). The stratified HR for OS was 0.95 (95% CI: 0.77–1.18; log-rank p value = 0.65) (see Fig. 2a). OS was also evaluated in clinically relevant patient subgroups defined by baseline risk factors. In all subgroups evaluated, no statistically significant differences were observed between treatment groups with respect to OS (Fig. 2b). In subgroup analyses, HRs for OS ranged from 0.75 to 1.49.
Fig. 2.
Updated overall survival (A) in the total study population and (B) according to patient subgroups. CI, confidence interval; GC, gemcitabine + carboplatin; HR, hazard ratio; OS, overall survival; PL, placebo.
Subsequent anti-cancer therapy
After study treatment, the majority of patients in both arms (GC + bevacizumab: 88.8%; GC + PL: 91.3%) received ≥1 additional line of anti-cancer therapy and the median number of subsequent therapies was 5 (range 1–14) in each arm (Table 2). Most patients (88.2%) received subsequent cytotoxic chemotherapy consisting predominantly of liposomal doxorubicin or paclitaxel. Patients in both arms received subsequent treatment with biologics/small molecules, including bevacizumab, but this was higher for patients in the GC + bevacizumab arm at 38.0% (n = 92) compared with 23.1% (n = 56) in the GC + PL arm. Other, non-bevacizumab anti-cancer therapies used in subsequent lines of treatment were well balanced between the 2 arms.
Table 2.
Subsequent anti-cancer therapy received after study treatment for randomized patients.
| GC + PL (n=242) |
GC + bevacizumab (n=242) |
All patients (N=484) |
|
|---|---|---|---|
| Any anti-cancer therapy, n (%) | 221 (91.3) | 215 (88.8) | 436 (90.1) |
| Chemotherapy | 217 (89.7) | 210 (86.8) | 427 (88.2) |
| Liposomal doxorubicin | 163 (67.4) | 155 (64.0) | 318 (65.7) |
| Paclitaxel* | 121 (50.0) | 118 (48.8) | 239 (49.4) |
| Platinum therapy | 96 (39.7) | 104 (43.0) | 200 (41.3) |
| Experimental chemotherapy | 13 (5.4) | 16 (6.6) | 29 (6.0) |
| Gemcitabine + platinum | 9 (3.7) | 10 (4.1) | 19 (3.9) |
| Paclitaxel + platinum | 5 (2.1) | 7 (2.9) | 12 (2.5) |
| Other | 145 (59.9) | 132 (54.5) | 277 (57.2) |
| Biologics/small molecules | 97 (40.1) | 64 (26.4) | 161 (33.3) |
| Bevacizumab | 92 (38.0) | 56 (23.1) | 148 (30.6) |
| Experimental biologic | 13 (5.4) | 10 (4.1) | 23 (4.8) |
| PARP inhibitors | 10 (4.1) | 4 (1.7) | 14 (2.9) |
| Experimental/other small molecule inhibitor |
11 (4.5) | 2 (0.8) | 13 (2.7) |
| AMG-386 | 6 (2.5) | 3 (1.2) | 9 (1.9) |
| Small molecule VEGFR inhibitors (ie, pazopanib, sorafenib) |
5 (2.1) | 2 (0.8) | 7 (1.4) |
| Hormonal therapy | 33 (13.6) | 28 (11.6) | 61 (12.6) |
| Radiation | 27 (11.2) | 33 (13.6) | 60 (12.4) |
| Surgery | 23 (9.5) | 18 (7.4) | 41 (8.5) |
| Other | 13 (5.4) | 7 (2.9) | 20 (4.1) |
| Number of lines of anti-cancer therapya | |||
| Median (range) | 5 (1–14) | 5 (2–14) | 5 (1–14) |
| Patients receiving the following number of lines of therapy, n (%) |
|||
| ≥3 | 221 (91.3) | 213 (88.0) | 434 (89.6) |
| ≥5 | 148 (61.2) | 135 (55.8) | 283 (58.5) |
| ≥7 | 77 (31.8) | 57 (23.6) | 134 (27.7) |
| ≥9 | 24 (9.9) | 21 (8.7) | 45 (9.3) |
| ≥11 | 6 (2.5) | 4 (1.7) | 10 (2.1) |
The frontline and the OCEANS study regimen as the second line are included.
Data was not collected as to whether or not paclitaxel was administered on a weekly regimen.
GC, gemcitabine + carboplatin; PARP, poly ADP ribose polymerase; PL, placebo; VEGFR, vascular endothelial growth factor receptor.
Safety
The safety population comprised 247 patients in the GC + bevacizumab arm and 233 patients in the GC + PL arm (see Fig. 1). Five patients who were randomized to receive GC + PL but received 1 or 2 doses of bevacizumab were assigned to the GC + bevacizumab for all safety analyses (see Fig. 1). While the median duration of follow-up for safety analyses was the same as was reported at the time of the primary PFS analysis, the maximum duration of follow-up increased in both treatment groups (GC + bevacizumab: from 33.5 to 62.1 months; GC + PL: from 29.3 to 34.1 months) (see Table 1).
All patients experienced at least 1 adverse event of any grade (Table 3). The events for which there was a greatest difference in frequency between the GC + bevacizumab and GC + PL arms included hypertension (42.1% vs. 8.6%, respectively), epistaxis (54.7% vs. 14.2%, respectively), headache (48.6% vs. 30.0%, respectively), and proteinuria (19.8% vs. 3.4%, respectively) (Table 4).
Table 3.
Overview of adverse events.
| GC + PL (n=233) |
GC + bevacizumab (n=247) |
|
|---|---|---|
| n (%) | ||
| Any adverse event | 233 (100) | 247 (100) |
| Grade 3–5 adverse event | 192 (82.4) | 223 (90.3) |
| Grade 5 adverse event | 1 (0.4) | 1 (0.4) |
| Serious adverse event | 59 (25.3) | 90 (36.4) |
| Serious adverse event (grade 3–5) | 47 (20.2) | 76 (30.8) |
| Adverse event leading to study drug (bevacizumab/PL) discontinuation |
11 (4.7) | 55 (22.3) |
| Adverse events of special interest | ||
| Arterial thromboembolic event (any grade) | 1 (0.4) | 6 (2.4) |
| Bleeding (CNS) (any grade) | 2 (0.9) | 2 (0.8) |
| Bleeding (non-CNS) (grade ≥3) | 2 (0.9) | 14 (5.7) |
| LV systolic dysfunction/CHF (grade ≥3) | 2 (0.9) | 2 (0.8) |
| Febrile neutropenia (any grade) | 4 (1.7) | 4 (1.6) |
| Fistula/abscess (any grade)a | 0 | 2 (0.8) |
| GI perforation (any grade) | 1 (0.4) | 2 (0.8) |
| Hypertension (grade ≥3) | 2 (0.9) | 45 (18.2) |
| Neutropenia (grade ≥4) | 51 (21.9) | 52 (21.1) |
| Proteinuria (grade ≥3) | 2 (0.8) | 27 (10.9) |
| PRES (any grade) | 0 | 2 (0.8) |
| Wound healing complication | 0 | 2 (0.8) |
| Venous thromboembolic event (grade ≥3) | 6 (2.6) | 11 (4.5) |
Includes all fistula/abscess events: anal fistula, female genital tract fistula, pelvic abscess, perirectal abscess, rectal abscess (narratives provided only for GI-related events (anal fistula, perirectal abscess, and rectal abscess).
CHF, congestive heart failure; CNS, central nervous system; GC, gemcitabine + carboplatin; GI, gastrointestinal; LV, left ventricular; PRES, posterior reversible encephalopathy syndrome; PL, placebo.
Table 4.
Treatment-emergent adverse eventsaof any grade with a ≥5% difference or grade 3–5 adverse events with a ≥2% difference between treatment groups.
| MedDRA preferred term | GC + PL (n=233) |
GC + bevacizumab (n=247) |
||
|---|---|---|---|---|
| n (%) | ||||
| All grades | Grade 3–5 | All grades | Grade 3–5 | |
| Blood and lymphatic system disorders | ||||
| Thrombocytopenia | 119 (51.1) | 79 (33.9) | 143 (57.9) | 99 (40.1) |
| Gastrointestinal disorders | ||||
| Diarrhea | 68 (29.2) | 4 (1.7) | 95 (38.5) | 7 (2.8) |
| Gingival bleeding | 1 (0.4) | 0 | 17 (6.9) | 0 |
| Hemorrhoids | 6 (2.6) | 0 | 19 (7.7) | 1 (0.4) |
| Nausea | 153 (65.7) | 3 (1.3) | 179 (72.5) | 11 (4.5) |
| Stomatitis | 16 (6.9) | 0 | 38 (15.4) | 0 |
| General disorders and administration site conditions | ||||
| Fatigue | 175 (75.1) | 10 (4.3) | 202 (81.8) | 16 (6.5) |
| Mucosal inflammation | 23 (9.9) | 0 | 38 (15.4) | 0 |
| Infections and infestations | ||||
| Sinusitis | 21 (9.0) | 1 (0.4) | 36 (14.6) | 1 (0.4) |
| Injury, poisoning, and procedural complications | ||||
| Contusion | 21 (9.0) | 0 | 43 (17.4) | 0 |
| Musculoskeletal and connective tissue disorders | ||||
| Arthralgia | 44 (18.9) | 2 (0.9) | 69 (27.9) | 5 (2.0) |
| Back pain | 31 (13.3) | 1 (0.4) | 51 (20.6) | 2 (0.8) |
| Nervous system disorders | ||||
| Dizziness | 39 (16.7) | 2 (0.9) | 57 (23.1) | 1 (0.4) |
| Headache | 70 (30.0) | 2 (0.9) | 120 (48.6) | 9 (3.6) |
| Psychiatric disorders | ||||
| Insomnia | 36 (15.5) | 0 | 51 (20.6) | 0 |
| Renal and urinary disorders | ||||
| Proteinuria | 8 (3.4) | 1 (0.4) | 49 (19.8) | 24 (9.7) |
| Respiratory, thoracic, and mediastinal disorders | ||||
| Cough | 43 (18.5) | 0 | 64 (25.9) | 0 |
| Dysphonia | 8 (3.4) | 0 | 33 (13.4) | 0 |
| Dyspnea | 56 (24.0) | 4 (1.7) | 74 (30.0) | 11 (4.5) |
| Epistaxis | 33 (14.2) | 1 (0.4) | 135 (54.7) | 12 (4.9) |
| Oropharyngeal pain | 23 (9.9) | 0 | 40 (16.2) | 0 |
| Rhinorrhea | 9 (3.9) | 0 | 25 (10.1) | 0 |
| Sinus congestion | 4 (1.7) | 0 | 19 (7.7) | 0 |
| Vascular disorders | ||||
| Hypertension | 20 (8.6) | 2 (0.9) | 104 (42.1) | 42 (17.0) |
All reported events were included, regardless of relationship to study drug; maximum severity is selected for each event for each patient; only those events that occurred within 30 days after the last study drug and on or before the cutoff date (July 15, 2013) were included in this analysis.
GC, gemcitabine + carboplatin; MedDRA, Medical Dictionary for Regulatory Activities; PL, placebo.
A higher frequency of grades 3–5 adverse events occurred in patients in the GC + bevacizumab arm versus the GC + PL arm (90.3% vs. 82.4%, respectively) (see Table 3). While the grade 3 adverse events were comparable between the 2 treatment arms (GC + bevacizumab: 42.1%; GC + PL: 41.6%), grade 4 adverse events had a higher occurrence among patients in the GC + bevacizumab arm compared with the GC + PL arm (GC + bevacizumab: 47.8%; GC + PL: 40.3%). Among these, thrombocytopenia (GC + bevacizumab: 28.3%; GC + PL: 18.9%) and proteinuria (GC + bevacizumab: 9.3%; GC + PL: 0.4%) had the greatest differences in frequency between treatment arms.
Among the patients evaluated for safety, a comparable frequency of deaths occurred in both study arms (GC + bevacizumab: 73.3%; GC + PL: 73.4%) (Table 4). The majority were attributed to disease progression (GC + bevacizumab: 70.9%; GC + PL: 72.1%). One patient in each treatment group experienced a grade 5 adverse event (GC + bevacizumab: grade 5 intracranial hemorrhage; GC + PL: grade 5 acute myocardial infarction) within the specified safety evaluation period.
Adverse events leading to discontinuation
Patients in the GC + bevacizumab arm discontinued the study drug due to an adverse event of any grade at a greater frequency than in the GC + PL arm (GC + bevacizumab: 22.3%; GC + PL: 4.7%). The hematologic adverse events of neutropenia and thrombocytopenia each led to the discontinuation of 4 patients (1.6%) in the GC + bevacizumab arm, while hypertension (10 patients, 4.0%), proteinuria (9 patients, 3.6%), and epistaxis (3 patients, 1.2%) were the most common non-hematologic causes.
Adverse events of special interest to bevacizumab
As expected, adverse events of special interest to bevacizumab, for which there was the greatest difference in frequency between the proportion of patients in the GC + bevacizumab arm and the GC + PL arm for all grades, were hypertension (43.7% vs. 8.6%, respectively), proteinuria (21.5% vs. 4.3%, respectively), and non-CNS bleeding (68.0% vs. 32.6%, respectively).
Discussion
Results of the final OS analyses from OCEANS were consistent with those previously reported [9, 13]. The current analysis, performed following 353 deaths (72.9%), showed a median OS of 33.6 months for patients in the GC + bevacizumab arm compared with 32.9 months for those in the GC + PL arm. Notably, the median OS of 33.6 months in the experimental arm in the current study reflects a trend of increasing survival of patients with ovarian cancer concomitant with the development of novel treatment combinations. In the AGO-OVAR study reported by Pfisterer et al in 2006, the median OS was 18 months for patients receiving GC compared with 17.3 months for those receiving carboplatin alone [14]; whereas in the CALYPSO trial [15], which was published in 2012, the median OS was 33.0 months and 30.7 months for patients on carboplatin + pegylated liposomal doxorubicin or carboplatin + paclitaxel, respectively. Most recently, the GOG-213 study [16] reported a median OS of 42.2 versus 37.3 months for patients receiving paclitaxel + carboplatin in combination with bevacizumab followed by bevacizumab maintenance compared with paclitaxel + carboplatin alone. The 5-year survival rate for women with ovarian cancer in the United States has improved significantly (p < 0.05) from 36% between 1975–1997 to 38% between 1987–1989 and 45% between 2004–2010 [17]. These increasing survival rates likely represent the cumulative benefit of improving PFS along multiple lines of therapy and improving surgical management and supportive care for patients with ovarian cancer. In the current study, the majority of patients (GC + bevacizumab: 89%; GC + PL: 90%) were treated with additional anti-cancer therapy, with more than half (GC + bevacizumab: 56%; GC + PL: 61%) receiving a total of ≥5 lines of therapy and a significant proportion of patients in the GC + PL arm (38%) receiving subsequent bevacizumab. The multiple lines of therapy and long post-progression survival have made it increasingly difficult to demonstrate a statistically significant improvement in OS in individual trials in ovarian cancer [18, 19]. However, the improving duration of survival in patients with this disease represents the impact of cumulative benefit of treatment regimens and paradigms over time. Thus, the importance of improved PFS as a primary endpoint for clinical trials and its long-term impact on duration of survival should not be underestimated.
The OCEANS study is the first phase 3 study in ovarian cancer that incorporated treatment with bevacizumab until progression of disease. The current analysis provides a final safety assessment based on a longer maximum duration of follow-up than previous analyses. At the time of the primary PFS analysis [9], more patients in the GC + PL arm compared with the GC + bevacizumab arm had discontinued study treatment, and the longer median PFS experienced by patients in the latter treatment group allowed for a more prolonged exposure to the study drug. Therefore, since the previous safety analysis, the maximum duration of follow-up increased by a greater extent for the bevacizumab arm compared with the control arm (GC + bevacizumab: from 33.5 to 62.1 months; GC + PL: from 29.3 to 34.1 months). No new safety signals arose during this additional follow-up period, and the safety data of the current analysis are therefore in accordance with the overall adverse event profile of bevacizumab in patients with various solid tumors [20].
One limitation of the present analysis is that the OCEANS study was not powered to determine differences in OS, due to the fact that the study was specifically designed to evaluate PFS as its single primary endpoint. Furthermore, maintenance of treatment blinding after disease progression was not allowed in the OCEANS protocol. This had been contested in another ovarian cancer study, for which the initial protocol stipulation of treatment blinding even after disease progression was challenged by investigators and patients and was therefore deemed infeasible; the primary endpoint of that trial was subsequently changed from OS to PFS [7].
In conclusion, results of these analyses of final OS and safety from the phase 3 OCEANS study of GC ± bevacizumab represent the final planned report of patient data from the study. Consistent with previously reported data, no statistically significant difference in OS outcomes was observed between treatment groups, and no new safety signals were identified following prolonged exposure to bevacizumab. The data, however, continue to demonstrate the longer duration of survival over time for patients with recurrent ovarian cancer.
Highlights.
Median follow-up for OS was 57.5 months in the updated analyses.
Results of the updated OS analyses were consistent with interim analyses.
Bevacizumab did not improve OS when added to GC therapy in this patient population.
Acknowledgments
Third-party medical writing support was provided by Ann Tang and assistance funded by Genentech, Inc. The study sponsor provided support for the conduct of the study and participated in the design of the study; in the collection, analysis, and interpretation of data; and in writing and reviewing the manuscript. All authors, including those employed by the sponsor, participated in the collection, analysis, and interpretation of data; participated in writing and reviewing the manuscript; had full access to study data; and provided final approval to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: A.H. and Y.V.W. are employees of Genentech, Inc. A.H. also has a patent pending with Genentech, Inc. S.V.B. has served on an advisory board for and received fees from Genentech, Inc.
References
- 1.American Cancer Society. Ovarian cancer. 2014 Available at: http://www.cancer.org/acs/groups/cid/documents/webcontent/003130-pdf.pdf. Last Medical Review: 08/05/2014; Last Revised: 12/23/2014. [Google Scholar]
- 2.Baldwin LA, Huang B, Miller RW, Tucker T, Goodrich ST, Podzielinski I, et al. Ten-year relative survival for epithelial ovarian cancer. Obstet Gynecol. 2012;120:612–618. doi: 10.1097/AOG.0b013e318264f794. [DOI] [PubMed] [Google Scholar]
- 3.McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with Stage III and Stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 4.Piccart MJ, Bertelsen K, James K, Cassidy J, Mangioni C, Simonsen E, et al. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J Natl Cancer Inst. 2000;92:699–708. doi: 10.1093/jnci/92.9.699. [DOI] [PubMed] [Google Scholar]
- 5.Coleman RL, Monk BJ, Sood AK, Herzog TJ. Latest research and treatment of advanced-stage epithelial ovarian cancer. Nat Rev Clin Oncol. 2013;10:211–224. doi: 10.1038/nrclinonc.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keating GM. Bevacizumab: a review of its use in advanced cancer. Drugs. 2014;74:1891–1925. doi: 10.1007/s40265-014-0302-9. [DOI] [PubMed] [Google Scholar]
- 7.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 8.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 9.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039–2045. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 11.Aghajanian C, Goff B, Nycum LR, Wang Y, Husain A, Blank S. Independent radiologic review: bevacizumab in combination with gemcitabine and carboplatin in recurrent ovarian cancer. Gynecol Oncol. 2014;133:105–110. doi: 10.1016/j.ygyno.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Brookmeyer R, Crowley J. A confidence interval for median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 13.Aghajanian C, Nycum LR, Goff B, Nguyen H, Husain A, Blank SV. Updated overall survival analysis in OCEANS, a randomized phase 3 trial of gemcitabine (G) + carboplatin (C) and bevacizumab (BV) or placebo (PL) followed by BV or PL in platinum-sensitive recurrent epithelial ovarian (ROC), primary peritoneal (PPC), or fallopian tube cancer (FTC) Ann Oncol. 2012;23(suppl 9):ix319–ix333. [Google Scholar]
- 14.Pfisterer J, Plante M, Vergote I, du Bois A, Hirte H, Lacave AJ, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24:4699–4707. doi: 10.1200/JCO.2006.06.0913. [DOI] [PubMed] [Google Scholar]
- 15.Wagner U, Marth C, Largillier R, Kaern J, Brown C, Heywood M, et al. Final overall survival results of phase III GCIG CALYPSO trial of pegylated liposomal doxorubicin and carboplatin vs paclitaxel and carboplatin in platinum-sensitive ovarian cancer patients. Br J Cancer. 2012;107:588–591. doi: 10.1038/bjc.2012.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coleman RL, Brady MF, Herzog TJ, Sabbatini P, Armstrong DK, Walker JL, et al. A phase III randomized controlled clinical trial of carboplatin and paclitaxel alone or in combination with bevacizumab followed by bevacizumab and secondary cytoreductive surgery in platinum-sensitive, recurrent ovarian, peritoneal primary and fallopian. Gynecol Oncol. 2015;137(Suppl. 1):1–210. [Google Scholar]
- 17.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 18.Tate Thigpen J. Contemporary phase III clinical trial endpoints in advanced ovarian cancer: assessing the pros and cons of objective response rate, progression-free survival, and overall survival. Gynecol Oncol. 2015;136:121–129. doi: 10.1016/j.ygyno.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Matulonis UA, Oza AM, Ho TW, Ledermann JA. Intermediate clinical endpoints: A bridge between progression-free survival and overall survival in ovarian cancer trials. Cancer. 2015;121:1737–1746. doi: 10.1002/cncr.29082. [DOI] [PubMed] [Google Scholar]
- 20.Randall LM, Monk BJ. Bevacizumab toxicities and their management in ovarian cancer. Gynecol Oncol. 2010;117:497–504. doi: 10.1016/j.ygyno.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]