Abstract
Background
Individualized prediction of outcomes may help with therapy decisions for patients with non-small cell lung cancer (NSCLC). We developed a nomogram by analyzing 17 clinical factors and outcomes from a randomized study of sublobar resection for NSCLC in high-risk operable patients. The study compared sublobar resection alone, to sublobar resection with brachytherapy. There were no differences in primary and secondary outcomes between the study arms, and were thus combined for this analysis.
Methods
The clinical factors of interest (considered as continuous variables) were assessed in a univariate Cox Proportional Hazards model for significance at the 0.10 level for their impact on overall survival (OS), local recurrence free survival (LRFS) and any recurrence free survival (RFS). The final multivariable model was developed using a stepwise model selection.
Results
173 of 212 patients had complete data on all 17 risk factors. Median (range) follow-up was 4.94 (0.04–6.22) years. The 5-year OS, LRFS and RFS were 58.4%, 53.2% and 47.4% respectively. Age, baseline DLCO % and maximum tumor diameter were significant predictors for OS, LRFS and RFS in the multivariable model. Nomograms were subsequently developed for predicting 5-year OS, LFRS and RFS.
Conclusions
Age, baseline DLCO% and maximum tumor diameter significantly predicted outcomes after sublobar resection. Such nomograms may be helpful for treatment planning in early stage NSCLC and to guide future studies.
Keywords: pulmonary function, lung cancer surgery, lung cancer clinical trials, lobectomy, segmentectomy, wedge resection, statistics (clinical trial)
Patients with early stage lung cancer and limited pulmonary reserve present significant challenges in management. The merits of surgical resection versus ablative techniques such as radiofrequency ablation (RFA) or stereotactic radiosurgery (SRS) are highly contentious1,2,3,4,5. For patients who undergo resection, the appropriateness of wedge resection versus segmentectomy is also an area of active debate6,7,8. In addition to the specifics of treatment, physicians caring for these patients must also recognize that the survival of these patients is determined to a large degree by the severity of their comorbidities. Consequently, the development of tools to predict the overall survival of these patients based on comorbidities and tumor characteristics could potentially allow for more individualized treatment decisions.
The present study was undertaken to develop a nomogram to predict survival of patients with early stage lung cancer who underwent sublobar resection, utilizing data from a prospective, randomized study (ACOSOG Z4032). The American College of Surgeons Surgical Oncology Group Z4032 trial was a multi-center, randomized study designed to compare outcomes following sublobar resection with brachytherapy to sublobar resection alone. Patients with clinical stage I non-small lung cancer were eligible for enrollment if they were considered high-risk for lobectomy. The study completed accrual in January of 2010 with enrollment of 224 patients.
The primary endpoint of the study was local recurrence, and results were published in 20149. There was no statistically significant difference in the time to local recurrence, the overall local recurrence rates, patterns of recurrence (local, regional or distant), or overall survival between sublobar resection with or without brachytherapy. The overall 5-year survival rate was 61.4% in the sublobar group and 55.6% in the sublobar plus brachytherapy group (p=0.38). For the present study, therefore, data from both arms were combined.
MATERIAL AND METHODS
Data source
This was a secondary analysis of data from ACOSOG Z4032, which was a randomized study evaluating the role of intraoperative brachytherapy in patients who were considered high-risk for lobectomy. Eligible patients were required to have a biopsy-proven stage I lung cancer and were considered to be high-risk on the basis of one major criterion or two minor criteria (Table 1).
Table 1.
Entry criteria for enrollment in ACOSOG Z4032. Patients must have either one major or two minor criteria.
| Major Criteria | SR** (N=114) |
SRB** (N=108) |
|---|---|---|
| 1. FEV1 ≤ 50% predicted | 67 (58.8%) | 49 (45.4%) |
| 2. DLCO ≤ 50% predicted | 72 (63.2%) | 74 (68.5%) |
| Minor Criteria | ||
| 1. Age ≥75 | 43 (37.7%) | 42 (38.9%) |
| 2. FEV1 51–60% predicted | 18 (15.8%) | 25 (23.1%) |
| 3. DLCO 51–60% predicted | 19 (16.7%) | 19 (17.6%) |
| 4. Pulmonary hypertension (defined as a pulmonary artery systolic pressure greater than 40mmHg) as estimated by echocardiography or right heart catheterization |
4 (3.5%) | 1 (0.9%) |
| 5. Poor left ventricular function (defined as an ejection fraction of 40% or less) |
9 (7.9%) | 3 (2.8%) |
| 6. Resting or Exercise Arterial pO2 ≤ 55 mm Hg or SpO2 ≤ 88% |
5 (4.4%) | 6 (5.6%) |
| 7. pCO2 > 45 mm Hg | 3 (2.6%) | 3 (2.8%) |
| 8. Modified Medical Research Council (MMRC) Dyspnea Scale ≥ 3. |
31 (27.2%) | 17 (15.7%) |
One patient may have multiple criteria; all randomized non-excluded patients
Data for the present study was obtained from review of the de-identified operative and pathology reports that were prospectively submitted to ACOSOG along with the required case-report forms10. Study sites were not queried for new information, and all information was available as part of the original data collection by ACOSOG (now part of the Alliance for Clinical Trials in Oncology). All patients provided written informed consent before trial enrollment in accordance with applicable guidelines. At each participating site, institutional review board approval was obtained in accord with an assurance filed with and approved by the US Department of Health and Human Services.
Statistical analysis
A total of 17 baseline clinical and patient related factors were included in this exploratory analysis. A complete case analysis including only patients with no missing data for any of the 17 factors was used. Local recurrence was defined as recurrence within the primary tumor lobe at the staple line (local progression), recurrence within the primary tumor lobe away from the staple line (involved lobe failure) or recurrence within hilar lymph nodes. Regional recurrence was defined as recurrence within another lobe on the same side as the resection, or within ipsilateral mediastinal of subcarinal lymph nodes. Distant recurrence was defined as recurrence within contralateral, mediastinal or hilar lymph nodes, or distant metastatic disease. Recurrence free survival was defined as the time from randomization to the earlier of any recurrence (local, regional or distant) or death from any cause.
Each factor was assessed in a univariable Cox proportional hazards model for overall survival (OS), local recurrence free survival (LRFS) and recurrence free survival (RFS) outcomes. Factors that were significant at the 0.10 level from the univariable models were then included in an initial multivariable model for each outcome. Race and histology were included in all of the initial multivariable models regardless of significance in the univariable models. The final multivariable model for developing the nomogram utilized a stepwise model selection approach (p ≤ 0.1 for entering the model, and p ≤ 0.05 for staying in the model).
Prior to developing a nomogram, the linearity assumption was tested using splines for all continuous factors in the final multivariable model for each outcome11. A nomogram based on the final multivariable model for each outcome was developed to predict for the 5-year OS, 5 year RFS and 5 year LRFS. Concordance index (optimism-corrected) and calibration plots were obtained for each model using internal bootstrapping to guard against over fitting. Statistical analyses were conducted by the Alliance Statistics and Data Center. SAS version 9.3 and R 3.0.3 were used for the analyses.
Nomogram Interpretation
A nomogram provides a graphical representation of a statistical predictive model, and allows for a numerical prediction of a clinical event (e.g. overall survival). The variable with the largest effect on the outcome will be assigned a maximum of 100 points. Other variables will be assigned a lower maximum value proportional to their effect size12. To predict the outcome, the total number of points is calculated, and a vertical line is drawn from the total points axis to the outcome axis.
The predictive ability of the nomogram is measured by the concordance-index (C-index), which ranges in value from 0.0 to 1.0. This index reflects the probability that when two random patients are selected, the patient with the higher predictive score will experience the measured outcome. The index ranges from 0.0 to 1.0 where 0.5 indicates a random prediction, 1.0 indicates a perfect prediction, and values <0.5 indicate prediction in the opposite direction.
RESULTS
Baseline Demographics
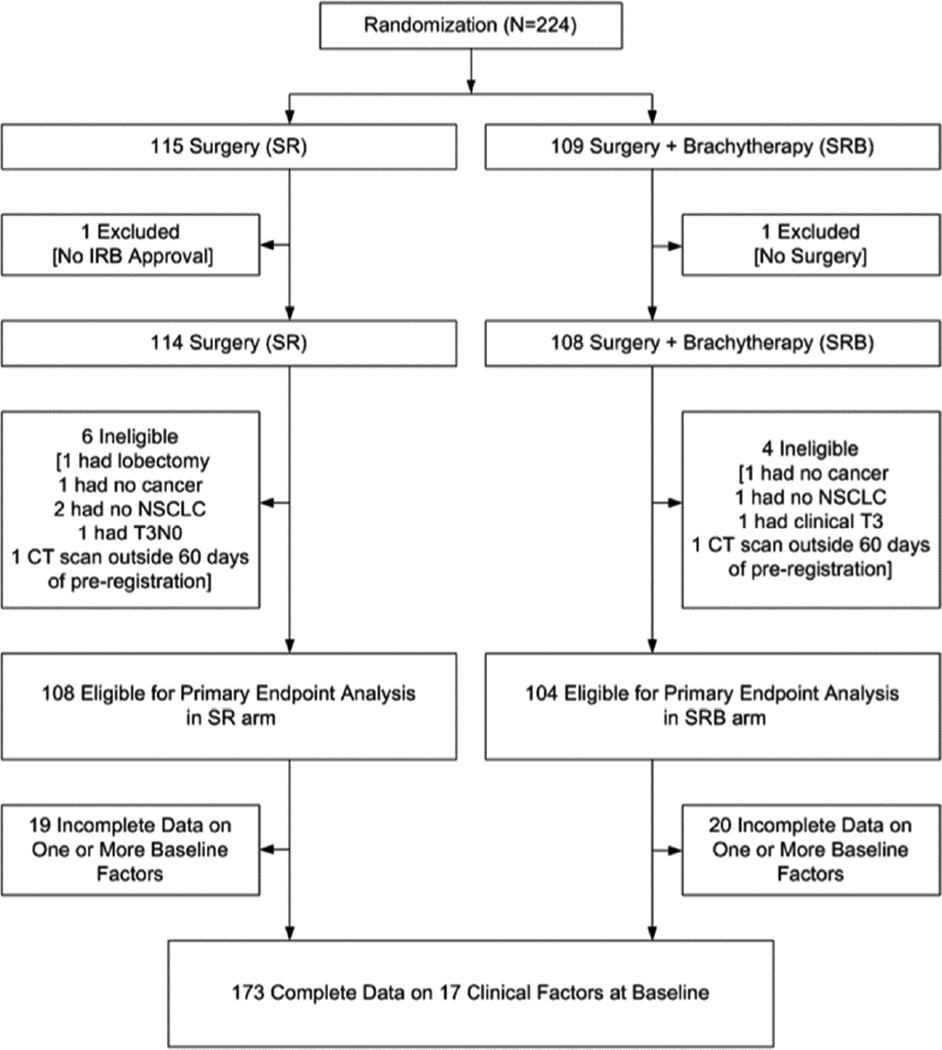
Data was frozen for this analysis on April 24, 2014. A total of 224 patients were registered. Two patients were excluded from all analyses: one patient from the SR arm had the intervention at a hospital that was not institutional review board approved; one patient on the SRB arm did not have surgery. An additional 10 registered patients (six in the SR arm and four in the SRB arm) were found to be ineligible. Furthermore, 39 patients were excluded due to incomplete data on one or more baseline factors. Thus, 173 randomized patients were included in this analysis (Figure 1). No imputation approaches for missing data were employed in this analysis.
Figure 1.
Patient CONSORT Diagram
Table 2 summarizes the 17 clinical variables for the 173 patients with complete data. Median age was 70 years, with a mean DLCO of 45% predicted. The majority of patients underwent a video-assisted thoracoscopic surgery (VATS) procedure (n=115, 66.5%) and a wedge resection was performed more often than a segmentectomy (74.6% vs. 25.4%). No lymph nodes were sampled in 61 (35.3%) patients. Squamous cell carcinoma (n=81, 46.8%) and adenocarcinoma (n=92, 53.2%) were equally common. Overall 58.4% of the 173 patients in this analysis were alive at 5 years.
Table 2.
Baseline Characteristics
| Factors | N (%) (n=173) |
|---|---|
| Arm | |
| Sublobar resection (SR) | 89 (51.4%) |
| SR+Brachytherapy | 84 (48.6%) |
| Age in years (median, range) | 70.0 (49.0 – 87.0) |
| BMI (median, range) | 27.3 (15.6 – 75.7) |
| Baseline Performance Status | |
| 0 | 37 (21.4%) |
| 1 | 96 (55.5%) |
| 2 | 40 (23.1%) |
| Race | |
| White | 162 (93.6%) |
| Black or African American | 10 (5.8%) |
| Unknown | 1 (0.6%) |
| Method of payment | |
| Uninsured/Medicaid | 20 (11.6%) |
| Private and/or Medicare | 146 (84.4%) |
| Military and/or Veterans Sponsored | 3 (1.7%) |
| Other Means of Payment | 4 (2.3%) |
| ASA class on surgery day | |
| I/II | 20 (11.6%) |
| III/IV | 153 (88.4%) |
| Baseline DLCO% (median, range) | 45.0 (8.0 – 97.0) |
| Baseline FEV1% (median, range) | 5.0 (22.0 – 110.0) |
| Surgery Approach | |
| Thoracotomy | 58 (33.5%) |
| VATS | 115 (66.5%) |
| Type of Resection | |
| Segmentectomy | 44 (25.4%) |
| Wedge Resection | 129 (74.6%) |
| Lymph Node Evaluation | |
| None | 61 (35.3%) |
| MLND/Sampling | 112 (64.7%) |
| Histology Type | |
| Adenocarcinoma | 92 (53.2%) |
| Squamous Cell Carcinoma | 81 (46.8%) |
| Clinical Nodule Size | |
| ≤2 cm | 107 (61.8%) |
| >2 cm | 66 (38.2%) |
| Actual Margin Size (cm) (median, range) | 1.0 (0.0 – 3.6) |
| Maximum Tumor Diameter (cm) (median, range) | 1.8 (0.4 – 6.5) |
| Margin Tumor Ratio (median, range) | 0.5 (0.0 – 4.0) |
Overall Survival
The results of the univariable and multivariable models for overall survival are provided in Table 3. Age, baseline DLCO%, margin-tumor ratio, maximum tumor diameter and histology were significant predictors of overall survival in the univariable analysis. Age, baseline DLCO% and maximum tumor diameter retained significance in the final multivariable model after stepwise selection. All continuous variables passed the formal test for linearity assumption.
Table 3.
Univariable and Multivariable Cox Proportional Hazards Model Results for Overall Survival (n=173, events=72).
| Factors | Univariable Model | Initial Multivariable Model |
||
|---|---|---|---|---|
| Hazard Ratio (95% CI) |
P-value | Hazard Ratio (95% CI) |
P-value | |
| Arm: SRB vs. SR | 1.17 (0.73, 1.86) |
0.51 | - | - |
| Age | 1.03 (1.00, 1.06) |
0.06 | 1.03 (1.01, 1.06) |
0.04 |
| BMI | 1.00 (0.97, 1.03) |
0.87 | - | - |
| Baseline Performance Status | 0.59 | - | - | |
| Baseline Performance Status : 2 vs. 0 | 1.02 (0.53, 1.96) |
0.96 | - | - |
| Baseline Performance Status: 1 vs. 0 | 0.79 (0.45, 1.40) |
0.42 | - | - |
| Race: White vs. Others1, 3 | 2.09 (0.66, 6.64) |
0.21 | 1.51 (0.46, 4.94) |
0.50 |
| Method of payment: Uninsured/Medicaid vs. Others |
1.05 (0.50, 2.18) |
0.91 | - | - |
| ASA class: III/IV vs. I/II | 1.51 (0.69, 3.28) |
0.30 | - | - |
| Baseline DLCO% | 0.97 (0.96, 0.99) |
<0.01 | 0.97 (0.95, 0.99) |
<0.01 |
| Baseline FEV1% | 1.00 (0.99, 1.02) |
0.61 | - | - |
| Surgery Approach: VATS vs. Thoracotomy |
1.16 (0.70, 1.90) |
0.56 | - | - |
| Type of Resection: Wedge Resection vs. Segmentectomy |
0.72 (0.44, 1.20) |
0.21 | - | - |
| Clinical Nodule Size: >2 cm vs. ≤2 cm | 1.33 (0.83, 2.12) |
0.24 | - | - |
| Actual Margin Size (cm) | 0.81 (0.61, 1.08) |
0.15 | - | - |
| Maximum Tumor Diameter (cm) | 1.37 (1.06, 1.78) |
0.02 | 1.29 (1.00, 1.68) |
0.05 |
| Margin Tumor Ratio 2,3 | 0.66 (0.44, 0.98) |
0.04 | - | - |
| Lymph Node Evaluation: MLND/Sampling vs. None |
0.93 (0.57, 1.49) |
0.75 | - | - |
| Histology Type: Squamous cell carcinoma vs. Adenocarcinoma1,3 |
1.63 (1.02, 2.60) |
0.04 | 1.24 (0.76, 2.02) |
0.39 |
Race and histology were included in the initial multivariable model regardless of significance in the univariable model, due to clinical consideration and a noticeable trend across outcomes.
Factors with p-value < 0.1 were included in the initial multivariable model. A stepwise model selection was utilized to get the final multivariate model.
The stepwise selection eliminated this factor from the final multivariable model used for developing the nomogram.
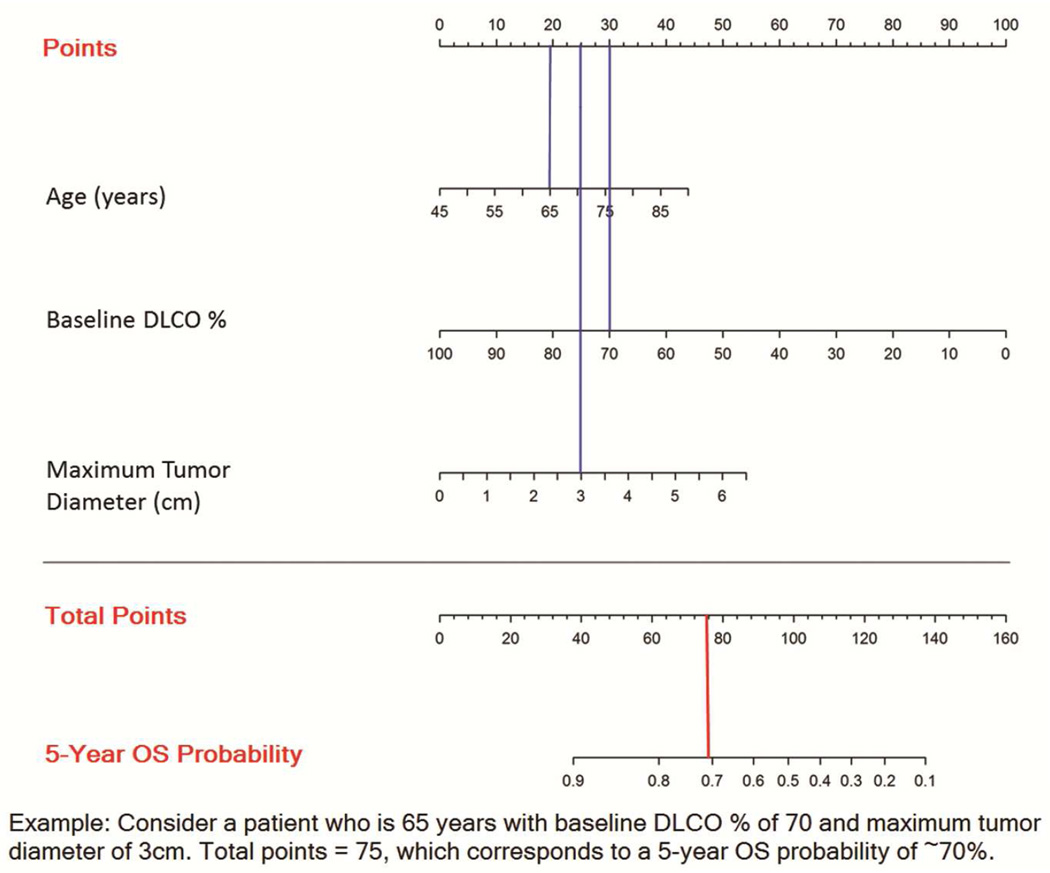
Figure 2 shows the nomogram for predicting overall survival for patients undergoing sublobar resection for stage I lung cancer enrolled in ACOSOG Z4032. The C-index (optimism corrected) for 5-year OS is 0.622.
Figure 2.
Nomogram for predicting 5-year overall survival
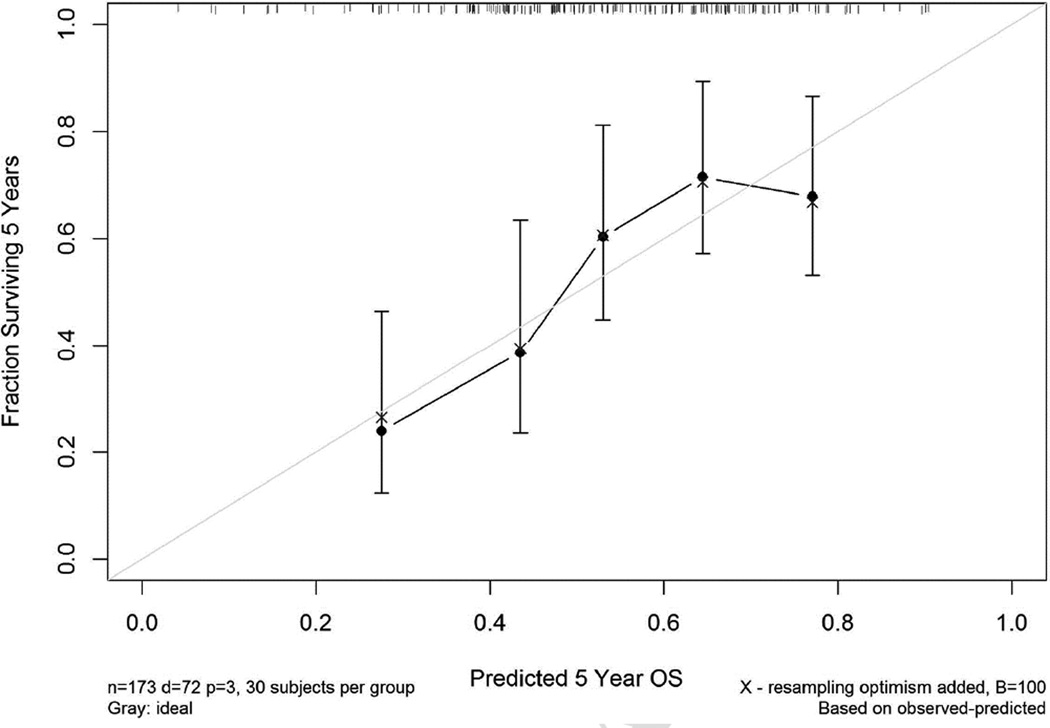
Figure 3 shows the calibration plot, demonstrating good agreement between the predicted and observed overall survival.
Figure 3.
Calibration plot for the 5-year overall survival nomogram
Recurrence-free survival
The results for the univariable and multivariable models for local recurrence-free and any recurrence-free survival are presented in Appendix Tables 1 and 2. Similar to overall survival outcome, age, baseline DLCO% and maximum tumor diameter were significant predictors in the final multivariable model for these outcomes. The C-index (optimism corrected) for 5-year LRFS and RFS were 0.606 and 0.591, respectively.
COMMENT
Nomograms are useful and accepted tools to predict the survival of cancer patients13,14,15. Nomograms provide a graphical display of the variables that are found to be statistically significant and their relative importance. Furthermore, the ability to incorporate multiple variables in a single model may allow nomograms to predict survival more accurately than standard TNM staging systems16,17. For lung cancer specifically, nomograms have been used to predict the response to tyrosine kinase inhibitors and the development of brain metastases following curative surgery18,19.
To our knowledge only three studies, all published in 2015, have developed nomograms to predict survival following surgical resection of lung tumors. The first study investigated the predictors of survival following resection of synchronous lung cancer in multiple lobes20. This was a pooled analysis of six previously published datasets. In that study, adenocarcinoma histology, female gender, N0 status, tumor size less than 3 cm and age less than 70 years were predictive of improved survival. A similar study developed a nomogram to predict survival following resection of typical carcinoid tumors, using data from a multi-center European registry21.
Most relevant to the present study was the publication by Liang et al of a nomogram to predict survival following surgical resection of stages I–IIIA lung cancer22. Data to construct the nomogram was pooled from over 5,000 patients from seven cardiothoracic centers in China. The nomogram was externally validated using a separate cohort of patients from the International Association for the Study of Lung Cancer dataset. The final nomogram demonstrated that gender, age, histology, number of sampled lymph nodes, and T and N stage were independent predictors for survival.
In the present analysis only three baseline factors (age, tumor size and DLCO%) were predictive of overall survival, local recurrence-free survival and any recurrence-free survival. It is important to note that only 173 patients with complete data for all 17 factors were included in this analysis, thus this is a relatively small study. No imputation approaches for missing data were employed.
To put these findings into the context of previous publications, it is important to emphasize the unique characteristics of patients enrolled in ACOSOG Z4032. First, it should be noted that only patients with clinical stage I disease were enrolled in the trial. In addition, only high-risk surgical patients on the basis of advanced age and cardiopulmonary function were eligible for this study. As a result of significant comorbidities, many of the deaths in this study were from causes other than cancer. Specifically, the overall 5-year survival was 61.4% in the sublobar resection group and 55.6% sublobar resection plus brachytherapy group. Among those who died, only 41% of deaths were from cancer. The remaining deaths were either from co-morbid disease (50%) or from unknown causes (8%).
The nomogram created in the present study should be considered exploratory and not necessarily applicable to standard-risk patients undergoing surgical resection (usually lobectomy) for lung cancer. The predictive model in our study is derived from data on high-risk surgical patients, who in most centers would be considered for sublobar resection and increasingly for alternative therapies such as stereotactic radiosurgery or radiofrequency ablation.
In the study from Liang et al discussed above, sampled lymph nodes, and T and N stage were found to be significant predictors of survival. However, in that study patients with stages I–IIIA were included, as opposed to ACOSOG Z4032, in which only patients with clinical stage I disease who were enrolled. The exclusion of advanced stage patients in the Z4032 trial likely explains why the degree of lymph node evaluation was not predictive of survival. In addition, we should note that results of pulmonary function testing were not even considered in the Liang study.
It may also be surprising that factors that surgeons traditionally associated with appropriate oncologic surgery, such as the degree of lymph node sampling, margin status, and the performance of a segmentectomy compared to a wedge resection were not significant predictors of survival in this dataset. However, this may be related to competing risk factors for mortality in these patients, as evidenced by 50% of deaths being unrelated to cancer in our series.
In our study diffusion capacity was the most important factor in predicting overall survival. Diffusion capacity was also found to be an important predictor of perioperative complications in an earlier analysis of data from Z403223. Other authors have also identified pulmonary function as a strong predictor of long-term survival following surgical resection of lung cancer, independent of perioperative mortality24,25. Significant reduction in diffusion capacity is not only a marker of advanced emphysema; it is also associated with pulmonary hypertension and cardiac disease, both of which may have a significant impact on long-term survival.
There are important limitations to this study. First, the high-risk population of patients with operable stage I cancer used to develop the nomogram should be re-emphasized. In addition, a relatively small number of patients were utilized to develop the nomogram. It is possible that a larger dataset may have shown that other factors, such as margin status and degree of lymph node sampling do impact survival as shown previously26. We should also note that the tumor histology in Z4032 did not incorporate the recent updates to adenocarcinoma classification27. It is likely that adenocarcinoma-in-situ or minimally invasive adenocarcinoma subtypes would be relevant in a survival nomogram, however this information was not available in our dataset. Similarly, PET scan data (specifically SUVmax) was not routinely collected in the case-report forms of Z4032, and was therefore not incorporated into the survival model.
Most importantly, the nomogram which we developed has not been validated using an external dataset. This is difficult given the unique characteristics of the patients enrolled in Z4032. A dataset of relatively healthy patients undergoing lobectomy for early-stage lung cancer (e.g. the IASCLC database) would not provide an appropriate comparison. However, we anticipate that a study comparing SRS to sublobar resection for high-risk patients (formerly ACOSOG Z4099, now the Stablemates study) will soon be open for accrual28. The study population in this upcoming study uses the same inclusion/exclusion criteria, and so a secondary objective of that study will be to externally validate these nomograms from Z4032.
The results of this analysis raise several questions when considering the optimal therapy for high-risk patients with NSCLC. Specifically, as traditional surgical quality measures do not appear to impact survival, is it reasonable to consider alternative approaches for these patients? In addition, are these nomograms valid for patients treated with non-operative therapy? Hopefully external validation of the nomograms developed in this study will address some of these questions.
In summary, we found that age, diffusion capacity and the maximum tumor diameter were the three factors associated with survival in high-risk operable patients with early-stage lung cancer. These nomograms may be useful in selecting individualized treatment planning for these patients and will need to be validated in future studies.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The Appendix can be viewed in the online version of this article [INSERT article doi] on http://www.annalsthoracicsurgery.org.
Presented at the Ninety-fifth Annual Meeting of the American Association for Thoracic Surgery, Seattle, WA, April 25–29, 2015.
REFERENCES
- 1.Hamaji M, Chen F, Matsuo Y, et al. Video-assisted thoracoscopic lobectomy versus stereotactic radiotherapy for stage I lung cancer. Ann Thorac Surg. 2015;99:1122–1129. doi: 10.1016/j.athoracsur.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Yu JB, Soulos PR, Cramer LD, et al. Comparative effectiveness of surgery and radiosurgery for stage I non-small cell lung cancer. Cancer. 2015 doi: 10.1002/cncr.29359. published online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambrogie MC, Fanucchi O, Dini P, et al. Wedge resection and radiofrequency ablation for stage I nonsmall cell lung cancer. Eur Respir J. 2015;45:1089–1097. doi: 10.1183/09031936.00188014. [DOI] [PubMed] [Google Scholar]
- 5.Crabtree T, Puri V, Timmerman R, et al. Treatment of stage I lung cancer in high-risk and inoperable patients: Comparison of prospective clinical trials using stereotactic body radiotherapy (RTOG 0236), sublobar resection (ACOSOG Z4032), and radiofrequency ablation (ACOSOG Z4033) J Thorac Cardiovasc Surg. 2013;145:692–699. doi: 10.1016/j.jtcvs.2012.10.038. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Sun Y, Wang R, et al. Meta-analysis of lobectomy, segmentectomy, and wedge resection for stage I non-small cell lung cancer. J Surg Oncol. 2015;111:334–340. doi: 10.1002/jso.23800. [DOI] [PubMed] [Google Scholar]
- 7.Schuchert M, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg. 2007;84:926–933. doi: 10.1016/j.athoracsur.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Altorki NA, Yip R, Hanaoka T, et al. Sublobar resection is equivalent to lobectomy for clinical stage IA lung cancer in solid nodules. J Thorac Cardiovasc Surg. 2014;147:754–764. doi: 10.1016/j.jtcvs.2013.09.065. [DOI] [PubMed] [Google Scholar]
- 9.Fernando HC, Landreneau R, Mandrekar S. Impact of brachytherapy on local recurrence rates after sublobar resection: results from ACOSOG Z4032 (Alliance), a Phase III randomized trial for high-risk operable non–small-cell lung cancer. J Clin Oncol. 2014;32:2456–2462. doi: 10.1200/JCO.2013.53.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kent M, Landreneau R, Mandrekar S, et al. Segmentectomy versus wedge resection for non-small cell lung cancer in high-risk operable patients. Ann Thorac Surg. 2013;96:1747–1755. doi: 10.1016/j.athoracsur.2013.05.104. [DOI] [PubMed] [Google Scholar]
- 11.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis, Springer Series in Statistics. 2001 [Google Scholar]
- 12.Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26:1364–1370. doi: 10.1200/JCO.2007.12.9791. [DOI] [PubMed] [Google Scholar]
- 13.Valentini V, van Stiphout RG, Lammering G, et al. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol. 2011;29:3163–3172. doi: 10.1200/JCO.2010.33.1595. [DOI] [PubMed] [Google Scholar]
- 14.Han DS, Suh YS, Kong SH, et al. Nomogram predicting long-term survival after D2 gastrectomy for gastric cancer. J Clin Oncol. 2012;30:3834–3840. doi: 10.1200/JCO.2012.41.8343. [DOI] [PubMed] [Google Scholar]
- 15.Karakiewicz PI, Briganti A, Chun FK, et al. Multi-institutional validation of a new renal cancer specific survival nomogram. J Clin Oncol. 2007;25:1316–1322. doi: 10.1200/JCO.2006.06.1218. [DOI] [PubMed] [Google Scholar]
- 16.Zaak D, Burger M, Otto W, et al. Predicting individual outcomes after radical cystectomy: An external validation of current nomograms. BJU Int. 2010;106:342–348. doi: 10.1111/j.1464-410X.2009.09138.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188–1195. doi: 10.1200/JCO.2012.41.5984. [DOI] [PubMed] [Google Scholar]
- 18.Keam B, Kim DW, Park JH, et al. Nomogram predicting clinical outcomes in non-small cell lung cancer patients treated with epidermal growth factor receptor tyrosine kinase inhibitors. Cancer Res Treat. 2014;46:323–330. doi: 10.4143/crt.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Won YW, Joo J, Yun T, et al. A nomogram to predict brain metastasis as the first relapse in curatively resected non-small cell lung cancer patients. Lung Cancer. 2015;88:201–207. doi: 10.1016/j.lungcan.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Tanvetyanon T, Finley DJ, Fabian T, et al. Prognostic nomogram to predict survival after surgery for synchronous multiple lung cancers in multiple lobes. Journal of Thoracic Oncology. 2015;10:338–345. doi: 10.1097/JTO.0000000000000400. [DOI] [PubMed] [Google Scholar]
- 21.Filosso PL, Guerrera F, Evangelista A, et al. Prognostic model of survival for typical bronchial carcinoid tumours: analysis of 1109 patients on behalf of the European Society of Thoracic Surgeons (ESTS) Neuroendocrine Tumours Working Group. Eur J Cardiothorac Surg. 2015;48:441–447. doi: 10.1093/ejcts/ezu495. [DOI] [PubMed] [Google Scholar]
- 22.Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol. 2015;33:861–869. doi: 10.1200/JCO.2014.56.6661. [DOI] [PubMed] [Google Scholar]
- 23.Fernando H, Landreneau R, Mandrekar S, et al. Thirty- and ninety-day outcomes after sublobar resection with and without brachytherapy for non-small cell lung cancer: results from a multicenter phase III study. J Thor Cardiovasc Surg. 2011;142:1143–1151. doi: 10.1016/j.jtcvs.2011.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferguson MK, Dignam JJ, Siddique J, et al. Diffusing capacity predicts long-term survival after lung resection for cancer. Eur J Cardiothorac Surg. 2012;41:e81–e86. doi: 10.1093/ejcts/ezs049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferguson MK, Watson S, Johnson E, Vigneswaran WT. Predicted postoperative lung function is associated with all-cause long-term mortality after major lung resection for cancer. Eur J Cardiothorac Surg. 2014;45:660–664. doi: 10.1093/ejcts/ezt462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guerrera F, Errico L, Evangelista A, et al. Exploring stage I non-small-cell lung cancer: development of a prognostic model predicting 5-year survival after surgical resection. Eur J Cardiothorac Surg. 2015;47:1037–1043. doi: 10.1093/ejcts/ezu410. [DOI] [PubMed] [Google Scholar]
- 27.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernando HC, Timmerman R. American College of Surgeons Oncology Group Z4099/Radiation Therapy Oncology Group 1021: a randomized study of sublobar resection compared with stereotactic body radiotherapy for high-risk stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2012;144:S35–S38. doi: 10.1016/j.jtcvs.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.