Abstract
Continued seeking and drinking of alcohol despite adverse legal, health, economic, and societal consequences is a central hallmark of human alcohol use disorders. This compulsive drive for alcohol, defined by resistance to adverse and deleterious consequences, represents a major challenge when attempting to treat alcoholism clinically. Thus, there has long been interest in developing pre-clinical rodent models for the compulsive drug use that characterizes drug addiction. Here, we review recent studies that have attempted to model compulsive aspects of alcohol and cocaine intake in rodents, and consider technical and conceptual issues that need to be addressed when trying to recapitulate compulsive aspects of human addiction. Aversion-resistant alcohol intake has been examined by pairing intake or seeking with the bitter tastant quinine or with footshock, and exciting recent work has used these models to identify neuroadaptations in the amygdala, cortex, and striatal regions that promote compulsive intake. Thus, rodent models do seem to reflect important aspects of compulsive drives that sustain human addiction, and will likely provide critical insights into the molecular and circuit underpinnings of aversion-resistant intake as well as novel therapeutic interventions for compulsive aspects of addiction.
Keywords: compulsion, addiction, aversion, punishment, alcohol, striatum, accumbens, central amygdala, cortex, adaptation, ion channel, glutamate receptor, intracellular signaling, circuit
The need for animal models of compulsive alcohol intake
Continued seeking and drinking of alcohol despite adverse legal, health, economic, and societal consequences is a central hallmark of human alcohol use disorders (AUDs) (Ahmed, 2012; Koob & Volkow, 2010; Larimer, Palmer, & Marlatt, 1999; Lesscher & Vanderschuren, 2012; Naqvi & Bechara, 2010; Sanchis-Segura & Spanagel, 2006; Spanagel, 2009; Tiffany & Conklin, 2000). This compulsive drive for alcohol, defined by resistance to adverse and deleterious consequences, represents a major challenge when attempting to treat alcoholism clinically (Anton, 2000; Koob & Volkow, 2010; Larimer et al., 1999; Naqvi & Bechara, 2010; Sanchis-Segura & Spanagel, 2006; Spanagel, 2009; Tiffany & Conklin, 2000). Thus, there has long been interest in developing pre-clinical rodent models for the compulsive drug use that characterizes drug addiction. Indeed, rodents have been shown to develop persistent drug or alcohol consumption where intake persists despite overt pairing with aversive consequences. This aversion-resistant intake is considered to model some aspects of human compulsive drives observed in addiction (Deroche-Gamonet, Belin, & Piazza, 2004; Hopf, Chang, Sparta, Bowers, & Bonci, 2010; Lesscher, van Kerkhof, & Vanderschuren, 2010; Lesscher & Vanderschuren, 2012; Seif et al., 2013; Spanagel & Hölter, 1999; Spanagel, Hölter, Allingham, Landgraf, & Zieglgänsberger, 1996; Vanderschuren & Everitt, 2004; Vengeliene, Celerier, Chaskiel, Penzo, & Spanagel, 2009; Wolffgramm, Galli, Thimm, & Heyne, 2000; Wolffgramm & Heyne, 1991). Simple behavioral models of aversion-resistant intake in rodents would greatly facilitate the identification of circuit and molecular mechanisms that promote this pathological drinking, and could assist in the development of behavioral and pharmacological therapies to target the compulsive drives which remain a nearly intractable aspect of human addiction.
There has been considerable theorizing about the brain circuits that underlie the development of addiction and the transition from recreational drug use to habits to compulsion. Several influential groups (Everitt et al., 2008; Koob & Volkow, 2010; Pierce & Vanderschuren, 2010) have considered that self-administration is initially driven by mesolimbic regions including the ventral striatum (mediating the reinforcing effects of drugs of abuse) and prefrontal cortical areas (mediating goal-directed activities focusing on drug acquisition and intake). Habitual processes become more important with repeated intake, and habit-based theories of addiction (Everitt & Robbins, 2005; Pierce & Vanderschuren, 2010; Tiffany, 1990) have inspired work examining the role of the dorsal striatum in addiction. Repeated intake and withdrawal also likely lead to the development of negative reinforcement, where intake occurs to reduce negative aspects of withdrawal (Koob, 2009), effects that persist into protracted abstinence (Heilig, Egli, Crabbe, & Becker, 2010). Thus, a number of possible mechanisms could contribute to development of addiction, including conditioning processes whereby drug-related cues can promote seeking and intake. Compulsive responding for drugs of abuse may be driven in part by habitual processes, and in part by recruitment of cortical circuits that are sufficiently strong to overcome anticipation of aversive consequences (see below).
We include a glossary that describes how concepts such as habit, compulsion, aversion resistance, and other terms used for compulsive-like intake may be related to each other. We also want to clarify that we explicitly speculate that many drinking models (dependence, longer-term intermittent binge drinking, longer-term 4-bottle choice, shorter-term mouse intake) likely lead to or involve a similar set of long-term adaptive changes and neural circuits. The way these different adaptations drive a particular alcohol-related behavior may have as much to do with the cognitive requirements and context of the behavior as it does with the method by which rats came to drink alcohol. This provides a working hypothesis, and any exceptions would be as interesting as the common principles.
Although compulsion is a critical aspect of human addiction, it is also important to note that addiction can be driven by processes included in the DSM-IV that do not necessarily reflect compulsion (although they may interact with it). These include increased motivation for drugs, cortical dysfunction that reduces control over drug seeking and taking, and a negative emotional state that develops with repeated withdrawal and may promote intake through something more akin to anxiety and depression than compulsion (see Ahmed, 2012 for further discussion). When attempting to validate rodent models for addiction, it may be beneficial if multiple aspects are captured by the models that together can be considered to recapitulate different aspects of human addiction. For example, footshock-resistant cocaine seeking is present in a subpopulation of rats which also exhibit greater motivation for cocaine, greater responding even without reinforcer delivery, and greater reinstatement (Belin, Berson, Balado, Piazza, & Deroche-Gamonet, 2011; Deroche-Gamonet et al., 2004). The co-association of these behaviors with resistance to punishment is useful in establishing the potential validity of the model for humans, although it does not necessarily imply that these other behaviors reflect compulsion per se.
Are compulsion, habit, and other terms for compulsive-like drinking similar or different constructs?
In order to understand whether a common circuit might mediate habits versus compulsions, we must first define these terms. There are a number of semantic as well as procedural differences and uncertainties when defining constructs used to describe compulsive-like drinking (Belin, Belin-Rauscent, Murray, & Everitt, 2013; Hopf et al., 2010; Lesscher et al., 2010; Spanagel & Hölter, 1999; Spanagel, Hölter, et al., 1996; Spanagel, Putzke, Stefferl, Schöbitz, & Zieglgänsberger, 1996; Turyakibahika-Thyen & Wolffgramm, 2006; Vendruscolo et al., 2012; Vengeliene et al., 2009; Wolffgramm et al., 2000; Wolffgramm & Heyne, 1991), even in human addicts (Belin et al., 2013; Moeller et al., 2010). As addressed in depth in the Glossary, compulsion and habit are similar in the sense of automaticity, where habits and compulsive drives seem to compel behavior without the ability to exercise control. However, we believe compulsions differ from habits in one key aspect: the cost associated with the alcohol drinking. We prefer the term aversion resistance since this operationally and experimentally defines compulsive-like drinking as the willingness to overcome an adverse consequence in order to get alcohol. Thus, although the respective circuitries that underlie habitual and compulsive responding are likely to largely overlap, there are also theoretical and recent experimental reasons to believe that they are not identical.
In this regard, prefrontal cortical areas are thought to promote compulsive behavior in humans, since craving and relapse correlate with prefrontal activity (Breese, Sinha, & Heilig, 2011; Koob & Volkow, 2010; Naqvi & Bechara, 2010; Tiffany & Conklin, 2000). Importantly, several groups (Naqvi & Bechara, 2010; Tiffany & Conklin, 2000) have suggested that cortical areas play a particular role in compulsive intake because of the presence of conflict during compulsion (i.e., continued intake despite the possibility of adverse consequences) and the role of some cortical areas in processing conflict (Koob & Volkow, 2010; Naqvi & Bechara, 2010; Roberts & Hall, 2008; Tiffany & Conklin, 2000). In contrast, this theory suggests that cortical areas contribute much less to habitual intake in the absence of conflict. Thus, cortical areas that process conflict would be selectively recruited in the face of challenge, but not recruited in the absence of challenge. In contrast, habit-related areas would support alcohol drinking with or without challenge. In support of a selective cortical recruitment with compulsion, our recent studies (Seif et al., 2013, discussed in detail in “Cortical circuits and compulsive alcohol use”), demonstrate that aversion-resistant alcohol intake requires cortical projections to the nucleus accumbens (NAcb) core and alcohol-related enhanced NMDA receptor function under these inputs, and that alcohol intake in the absence of aversive challenge does not (Seif et al., 2013). Thus, cortico-NAcb inputs differentially sustain alcohol intake depending on the level of conflict during consumption. By contrast, recent evidence suggests that striatal areas (which are innervated by PFC areas dorsal to cortico-accumbens areas) can mediate both habitual (Everitt et al., 1999; Everitt & Robbins, 2005; Pierce & Vanderschuren, 2010; Tiffany, 1990) and compulsive drug use. However, a recent study by Jonkman, Pelloux, & Everitt (2012) shows that the most dorsal part of dorsolateral striatum (DLS) selectively promotes punishment-resistant cocaine seeking, indicative of compulsive cocaine seeking, but does not alter cocaine seeking without punishment (see the section “The striatum and compulsive alcohol use” for further discussion).
Thus, it may be that compulsions and habits are both driven by the neural circuitry of automaticity, but with a high or low cost associated with the action, respectively. In fact, there are a range of rodent paradigms considered to model aversion resistance and habits which could reflect this gradient of high to low cost paired with responding. On the high end is responding that persists despite acute pairing with an adverse consequence such as shock. Less costly than direct shock but still aversive would be persistent responding despite previous pairing of the reinforcer with a negative consequence such as lithium chloride sickness (Dickinson, Wood, & Smith, 2002) or presentation of a cue paired with a negative reinforcer (such as a footshock-paired cue) (Johnson & Kenny, 2010; Vanderschuren & Everitt, 2004). Further, three behavioral models might reflect habits under the least intense condition: 1) resistance to pre-feeding, where responding persists despite “pre-feeding” with the particular reinforcer to decrease the value of the reinforcer (if behavior is goal-directed, pre-feeding would reduce responding since it is regulated by acute feedback about the outcome), 2) extinction, where responding persists even when the reinforcer is no longer delivered, and 3) variable interval schedules, which may promote habit learning because of the weak link between an action (lever press) and the outcome (reward delivery) (e.g., Hay, Jennings, Zitzman, Hodge, & Robinson, 2013). Importantly, a recent study found that variable interval schedules lead to habitual alcohol intake, in that responding is not reduced by pairing alcohol with lithium chloride (Mangieri, Cofresi, & Gonzales, 2012); it would be interesting to contrast the role of cortical areas in this model versus ethanol-prefeeding-devaluation models. Thus, pairing higher costs with responding seems necessary to truly recapitulate aversion resistance and conflict in rodents.
The terms maladaptive and pathological are particularly good examples of the perils of semantics. Although they may accurately describe the state of the human condition, they reflect more of a value judgment and thus may have little relevance at all in rodents. One could imagine that there is relatively little cost for a rodent to drink to binge levels by human standards, and in fact, the anxiolysis from alcohol may relieve stress (e.g., related to single housing, Wallace et al., 2009) and in fact have a positive net effect for the rodent. It has also been noted that, for resistance-to-devaluation experiments to test habit, there is little cost for a rodent to continue working since it generally has nothing else to do but sit in the home cage (see Ahmed, 2012). In humans, this interaction is only considered maladaptive and pathological in relation to the inability to maintain a “normal” life during repeated intoxication; one could imagine a different time where heavy drinking (lying drunk at the edge of the town) was not of as great a consequence (the village would care for you) as we experience in our fast-paced and complex modern world. Thus, we suggest avoiding terms like maladaptive and pathological for rodent studies.
In addition to aversion resistance, compulsive drinking in humans is also characterized by inflexibility, the inability to change behavior even when one wants to resist urges for drugs, in addition to lack of consideration about negative consequences, and loss of control, where a person cannot control the urge to drink and thus consumes much more than intended. An important question in preclinical alcoholism research is whether aversion-resistant alcohol drinking truly models these aspects of compulsive alcohol use, i.e., inflexibility or loss of control seen in human alcoholics (see Glossary for definition of these terms). Loss of control in rodent studies is generally used in relation to aversion resistance but is also often used to describe very high levels of intake. However, since we cannot know whether the rats drank more than intended, this term may be problematic. Inflexibility and aversion resistance both imply the willingness to persevere in responding despite the cost. Therefore, inflexibility is a semantically attractive term to describe at least one aspect of compulsive alcohol use in rodents. However, true determination of inflexibility may require the presentation of an alternate choice, examples of which will be discussed in the following paragraph. Interesting studies in this regard showed that compulsive rats given a choice between several different alcohol concentrations drink equivalent total amounts of alcohol when adulterated with quinine, but switch preference from a lower concentration to higher concentrations of adulterated alcohol (Turyakibahika-Thyen & Wolffgramm, 2006; Wolffgramm et al., 2000). These studies demonstrate that aversion-resistant rats may, depending on the experimental conditions and alternatives provided to the animals, develop adaptive behavior where the preferred total amount of alcohol is still consumed and the aversive consequence is tolerated but minimized. Interestingly, some aspects of motivation for drinking in humans may not necessarily represent loss of control, but rather could reflect a cognitive yet maladaptive process that allows continued alcohol intake (e.g., see discussion of “apparently irrelevant decisions” in Larimer et al., 1999). Barnea-Ygael, Yadid, Yaka, Ben-Shahar, & Zangen (2012) also have an interesting discussion about studies of cocaine addicts seeking therapy, in particular where patients often seek treatment to reduce the negative consequences of intake rather than reduce actual intake.
As noted by Ahmed (2012), loss of control is difficult to assess and confirm directly in rodents because it is difficult to distinguish, for example, from increased motivation or reinforcer value, which could promote intake in the face of negative consequences. In fact, even demonstrating punishment-resistant intake does not conclusively establish loss of control or inflexibility, in part because there is generally no alternate choice to taking the drug. Mormede, Colas, & Jones (2004) addressed this question by providing rats with a history of alcohol use with a choice between alcohol and a saccharin-sweetened water solution. They found that providing rats with a sweet-tasting alternative to alcohol reduced alcohol intake. In agreement with these findings, Ahmed and colleagues more recently demonstrated continued preference for sweetened water over cocaine in the vast majority of rats that exhibit other pathological aspects of intake (escalation, greater motivation, memory dysfunction). These findings do not necessarily support the possibility of loss of control. Given these caveats, the term “aversion-resistant alcohol intake” at least avoids semantic and conceptual uncertainties while perhaps capturing essential features of compulsive aspects of human alcohol intake. Lesscher and Vanderschuren (2010) also provided animals with an alternate choice in an alcohol consumption task. Here, the mice were provided with a choice between aversive and non-aversive alcohol. After 2 months of daily alcohol consumption, the animals failed to discriminate between the two solutions, indicative of indifferent alcohol drinking that may reflect loss of control over alcohol use.
Quinine resistance in rats as a model for compulsive alcohol intake
Continued intake despite adulteration of alcohol with the bitter tastant quinine has long been used to model aversion-resistant intake in rats (Hopf et al., 2010; Loi et al., 2010; Spanagel & Hölter, 1999; Spanagel, Hölter, et al., 1996; Spanagel, Putzke, et al., 1996; Turyakibahika-Thyen & Wolffgramm, 2006; Vengeliene et al., 2009; Wolffgramm et al., 2000; Wolffgramm & Heyne, 1991) and mice (Fachin-Scheit, Frozino Ribeiro, Pigatto, Oliveira Goeldner, & Boerngen de Lacerda et al., 2006; Lesscher et al., 2010; Lesscher, Houthuijzen, Groot Koerkamp, Holstege, & Vanderschuren, 2012; Villas Boas et al., 2012). Many earlier studies in rats utilized a 4-bottle intake model (Spanagel & Hölter, 1999; Spanagel, Hölter, et al., 1996; Spanagel, Putzke, et al., 1996; Turyakibahika-Thyen & Wolffgramm, 2006; Vengeliene et al., 2009; Wolffgramm et al., 2000; Wolffgramm & Heyne, 1991), where rats had access to 3 different concentrations of alcohol and a bottle of water. After very long-term (8 months or more) alcohol intake and protracted withdrawal, quinine-resistant alcohol intake can be observed in all rats (Spanagel, Hölter, et al., 1996; Wolffgramm & Heyne, 1991) or in a subpopulation of rats (Turyakibahika-Thyen & Wolffgramm, 2006; Wolffgramm et al., 2000). Although these very long-term intake models are very useful and robust, they are somewhat impractical and expensive.
We recently found that alcohol drinking in rats with intermittent access to alcohol (IAA, access to 20% alcohol 3 of the 7 days per week with at least 1 day between each 24-h alcohol-access session; Simms et al., 2008; Wise, 1973) leads to development of quinine-resistant intake after only 3 months (Hopf et al., 2010). Quinine resistance is not observed after 1.5 months of IAA intake, even though alcohol intake levels are similar after 1.5 and 3 months of IAA, suggesting a slowly developing process. In addition, sucrose intake remains quinine-sensitive after 3 months of IAA, suggesting a specific effect on alcohol drinking. Furthermore, rats with continuous access to alcohol (CAA), which drink about the same amount of alcohol per week but less on each drinking day relative to IAA (Simms et al., 2008), do not develop the quinine resistance observed in IAA rats. A similar quinine resistance in IAA versus CAA rats is also observed in an alcohol-preferring rat strain after 1 month of intake (Loi et al., 2010), although some quinine sensitivity was still observed in IAA rats at this time. One disadvantage of the IAA model in outbred rats is that plateau intake yields blood alcohol concentrations of about 50 mg% after 30 min of intake (Simms et al., 2008). These intake levels are greater than those observed in previous studies of quinine resistance using continuous access (Simms et al., 2008; Spanagel & Hölter, 1999; Spanagel, Hölter, et al., 1996; Wolffgramm et al., 2000; Wolffgramm & Heyne, 1991), and are more similar to drinking levels seen in alcohol-preferring rat strains (Bell, Rodd, Lumeng, Murphy, & McBride, 2006), suggesting that limited access to alcohol combined with repeated withdrawal results in high levels of alcohol intake. Moreover, the IAA rats reach 50 mg% blood alcohol concentration (the European level of legal intoxication), although they do not reach the 80 mg% considered to reflect binge intake.
Another interesting paradigm that was recently reported to exhibit aversion-resistant alcohol drinking involves dependence-induced increases in alcohol intake (see Vendruscolo et al., 2012). In this model, protracted operant responding for alcohol is combined with repeated periods of exposure to alcohol vapor, which produces very high blood alcohol levels and induces alcohol dependence in both rats and mice (for review, see Crabbe, Harris, & Koob, 2011; Koob, 2003; Lopez & Becker, 2005; Roberts, Heyser, Cole, Griffin, & Koob, 2000). Dependent rats show greatly increased alcohol intake relative to non-dependent rats. This is especially the case after a period of alcohol deprivation, an example of the so-called alcohol deprivation effect (ADE), which is considered a model for human relapse (Sanchis-Segura & Spanagel, 2006). A recent study showed that dependent rats show quinine-resistant intake and increased responding under a progressive ratio (Vendruscolo et al., 2012), suggesting that this also represents a useful model to study the circuitry of aversion-resistant intake.
Quinine resistance in mice as a model for compulsive alcohol intake
We recently also reported on aversion resistance to quinine in mice using a limited-access choice paradigm inspired by the drinking-in-the-dark model, in which mice consume high amounts of alcohol when given daily 2–4 h access to alcohol early in the dark cycle (Rhodes, Best, Belknap, Finn, & Crabbe, 2005). We extended these studies to a limited-access choice paradigm (Lesscher, Kas, van der Elst, van Lith, & Vanderschuren, 2009; Lesscher, Wallace, et al., 2009) and found that mice with access to alcohol for 2 h/day (with water always available) readily demonstrate escalation of alcohol intake. C57BL/6J mice consume much higher levels of alcohol than rats, making them a relevant and attractive model for human AUDs. In fact, C57BL/6J mice also rapidly develop resistance to quinine adulteration (Lesscher et al., 2010). While quinine adulteration does reduce alcohol intake on the first exposure to alcohol, remarkably, we observed that alcohol intake becomes insensitive to quinine adulteration after only 2 weeks of alcohol experience (when the devalued solution was the only source of alcohol for the mice, i.e., when the mice could choose between quinine-adulterated alcohol and water). Moreover, after another 6 weeks of alcohol consumption in the limited-access choice paradigm, the mice now displayed indifference to quinine, in that they now consumed the bitter-tasting alcohol even though a non-adulterated alcohol solution was simultaneously available. In other words, these mice failed to discriminate between adulterated and non-adulterated alcohol. This indifferent alcohol consumption by mice shows intriguing parallels to the behavior of human alcoholics, who drink non-beverage alcohol (e.g., eau de colognes and mouthwash) with a very aversive taste but a high alcohol content (Leon et al., 2007; Soo Hoo, Hinds, Dinovo, & Renner, 2003), which is illustrative of the persistent craving for and intake of alcohol in alcoholics despite negative consequences.
Do quinine-resistance models in rodents reflect human AUDs?
The quinine model demonstrates aversion resistance that has face validity for human alcoholism. As mentioned in the previous section, alcoholics, after alcohol dependence is established, will drink non-beverage alcohol despite the bad taste and the presence of toxic components, e.g., eau de colognes and mouthwash (Leon et al., 2007; Soo Hoo et al., 2003), and also cheap and bad tasting liquor. Moreover, most alcohol abuse causes gastrointestinal problems, including diarrhea and intestinal bleeding (e.g., Bode & Bode, 2003), and alcohol consumption causes actual pain. These aversive signs clearly demonstrate alcohol intake despite actual negative consequences, which is captured in our quinine-resistance models. In addition to this, the quinine-resistant alcohol intake models also encompass other features which are considered to recapitulate aspects of human alcohol addiction. For example, these quinine-resistance drinking paradigms can also encompass escalation of alcohol intake, reduced intake by compounds that can reduce human drinking, and development of dependence.
Escalation of intake is an important characteristic of addiction and is one of the earliest signs of the development of addiction in humans (Koob & Volkow, 2010; Uhart & Wand, 2009), perhaps indicating a progressive loss of control across repeated intake sessions. Indeed, escalated intake in rodents is often cited as evidence of relevance of a model for human addiction. Rats drinking under an IAA schedule escalate their alcohol intake across the first month of alcohol access (Simms et al., 2008). Similarly, C57BL/6 mice drinking under a limited-access paradigm show escalation of intake across the first weeks of intake (Lesscher, Houthuijzen, et al., 2012; Lesscher, van Kerkhof, et al., 2010). Escalation of alcohol intake has been observed in mice of diverse genotypes although the extent of escalation is genotype-dependent (Lesscher, Kas, et al., 2009; Rosenwasser, Fixaris, Crabbe, Brooks, & Ascheid, 2013). Alcohol-dependent rats and mice show greater intake after vapor exposure relative to before vapor exposure (Crabbe et al., 2011; Koob, 2003; Lopez & Becker, 2005; Roberts et al., 2000; Vendruscolo et al., 2012). However, is escalation a proxy for compulsivity? Rats in the IAA schedule escalate their alcohol intake to a greater extent than rats in a continuous-alcohol access (CAA) schedule, and IAA rats develop quinine resistance while CAA rats do not (Hopf et al., 2010). Similar parallels are apparent from the cocaine literature. For example, escalation of cocaine use has been associated with increased responding for cocaine under a progressive ratio (Orio, Edwards, George, Parsons, & Koob, 2009), which may be considered a measure of compulsivity as animals continue to respond despite the aversive nature of not being rewarded, as well as the increased work requirement. Moreover, escalation of drug use is paralleled by footshock-resistant cocaine seeking in a subpopulation of rats (Chen et al., 2013; Jonkman et al., 2012). Furthermore, long-term access to palatable food leads to subsequent aversion-resistant food intake (Johnson & Kenny, 2010), and an interesting recent study (Puhl, Cason, Wojnicki, Corwin, & Grigson, 2011) found that an escalating, intermittent-access, binge intake of fat led to subsequent development of addiction-like cocaine responding (increased progressive ratio and extinction responding). However, there is also evidence to suggest that escalation of drug use may not be a proxy for compulsivity, as was demonstrated by Ahmed and colleagues using a paradigm where rats can choose between receiving a cocaine infusion or sweetened water (for review, see Ahmed, 2012). The vast majority of rats with long access to cocaine (that show escalation of intake across time) still prefer sweetened water over cocaine, although, in agreement with the footshock studies described above, a small sub-population of rats do prefer cocaine over sweetened water and can perhaps be considered addicted. Moreover, in support of a dissociation of compulsive and escalated seeking at the neurobiological level, a recent study (Jonkman et al., 2012) showed that the dorsolateral striatum contributes to punished responding but not unpunished responding after escalated intake for cocaine. This work clearly indicates that escalated intake (developed by the majority of rats) does not per se indicate development of compulsion (developed by ~15–20% of rats for cocaine).
Pharmacologically, we may consider a similar pharmacological sensitivity to compounds that can reduce alcohol intake in human alcoholics, such as naltrexone, acamprosate, and veranicline (McKee et al., 2009; Spanagel, 2009), as another useful indicator of the potential validity of rodent models to human alcoholism. Acamprosate reduces 4-bottle intake (Spanagel, Hölter, et al., 1996), while naltrexone, acamprosate, and veranicline exhibit a greater effect on alcohol drinking under IAA compared to CAA (Simms et al., 2008; Steensland, Simms, Holgate, Richards, & Bartlett, 2007). In apparent contradiction, naltrexone is less effective in reducing drinking under variable-interval schedules related to habit development (Hay et al., 2013), and naltrexone reduces heavy drinking (Lesscher, Wallace, et al., 2009) but not quinine-resistant intake in mice (Fachin-Scheit et al., 2006). Whether these divergent results reflect differences in rodent strains, methodology, or other factors remains to be determined.
The quinine-resistance models capture other traits that have also been cited as evidence of relevance to human intake. For example, quinine-resistant intake under 4-bottle choice is associated with disrupted circadian patterns of intake and increased preference for higher concentrations of alcohol (Spanagel & Hölter, 1999; Vengeliene, Noori, & Spanagel, 2013; Wolffgramm et al., 2000). Further, IAA-drinking rats do not show an alcohol-deprivation effect, although CAA rats do (Simms et al., 2008); we speculate that lack of an ADE indicates that IAA-drinking rats may already be at maximum in terms of expression of pathological drinking. Finally, a recent study found moderate dependence in IAA rats (Li, Bian, Dave, & Ye, 2011); this concurs with a study showing that more moderate alcohol intake can lead to development of withdrawal (Spanagel, Putzke, et al., 1996).
Taken together, these results further support the relevance of these quinine-resistance alcohol-drinking models for studying pathological human alcohol intake including aversion resistance, with both face and predictive validity, although further refinements would be preferable in some cases (such as even greater alcohol intake under IAA).
Quinine versus footshock as an aversive consequence paired with alcohol intake
As discussed above, rodent models which exhibit quinine-resistant alcohol intake also demonstrate a number of other features (escalation, preference for higher concentrations of alcohol, sensitivity to compounds that can reduce human alcohol intake, etc.) which are considered to recapitulate aspects of human AUDs. Thus, there is a reasonable argument that quinine resistance can be useful for investigating the mechanisms of aversion-resistant intake. However, quinine-resistance models have been criticized for several reasons, some with greater scientific merit (level of aversiveness of quinine, timing of aversion with respect to intake, discussed below) and some with less (that quinine plus alcohol is like a vodka tonic, although tonic water has significant amounts of sugar compared to quinine).
Studies of aversion-resistant alcohol intake would be greatly strengthened by the development of a footshock-resistant alcohol model. Alcohol drinking in relation to footshock has been studied, where footshock reduced subsequent alcohol intake (e.g., Logrip & Zorrilla, 2012) in a context-dependent manner (Marchant, Khuc, Pickens, Bonci, & Shaham, 2013). We recently developed a paradigm which demonstrates footshock-resistant intake in outbred Wistar rats (Seif et al., 2013), where 1 in 8 FR3 responses during a 20-min operant session are paired with a moderate footshock (0.2 mA, 0.5 msec). All rats show reduced responding in the first footshock sessions, but by the 5th or 6th footshock session about half of rats exhibit responding near pre-shock baseline. This is, to our knowledge, the first demonstration of a footshock-resistant alcohol intake model. Importantly, we found that a similar cortical-NAcb circuit sustains both quinine-resistant and footshock-resistant alcohol intake, with no role in alcohol intake in the absence of punishment (see below in the section “Cortical circuits and compulsive alcohol use”). In addition to providing important new information about the circuitry of compulsive alcohol intake, and supporting the theory that cortical inputs are preferentially involved in aversion-resistant intake (Naqvi & Bechara, 2010; Tiffany & Conklin, 2000), these results also validate the use of the technically simple quinine/2-bottle choice intake for measuring aversion resistance compared to the more time consuming and technically challenging operant footshock resistance.
Drug or alcohol intake that persists despite pairing intake with footshock provides a dramatic demonstration of an animal’s pathological motivation. Nonetheless, there are also technical limitations to the use of footshock. One critical parameter is that it is difficult to fine-tune the foot-shock parameters, for example applied mA and duration of shock. Thus, investigators attempting to study footshock-induced reinstatement of alcohol seeking have found this to be a quite variable phenomenon, and in fact, the relationship between different stressors and alcohol seeking is mixed and complex (Hopf, Sparta, & Bonci, 2011). It is also difficult to know what each rat is experiencing during the footshock and how much this varies between animals. One encouraging study found that rats exhibiting footshock-resistant and footshock-sensitive cocaine seeking show no differences in shock thresholds during fear learning (Vanderschuren & Everitt, 2004), suggesting that differences in compulsivity (willingness to self-administer cocaine despite pairing with footshock) do not reflect differences in basic shock sensitivity. However, footshock involves pain, and it is difficult to rule out that any circuitry that mediates footshock-resistant drug or alcohol intake is recruited because the aversive stimulus is acute pain or a cue paired with the pain of shock. Seif et al. (2013) found that a similar cortical circuit (mPFC, insula) promotes both footshock- and quinine-resistant alcohol intake, supporting the possibility that this circuit mediates aversion-resistant intake with aversive stimuli of different sensory modalities and thus is more likely to reflect a common circuit that promotes compulsive intake whatever the aversive consequence might be. Nonetheless, additional studies are required to assure that circuits that promote footshock-resistant intake are not recruited because of the sensory modality of the aversive stimulus. Rodent aversion-resistance models have been criticized because adverse consequences associated with human drinking often do not explicitly occur at the same time as the alcohol intake. In other words, a loss of employment, relationship, or legal freedom likely occurs hours, days, or longer after a drinking episode is initiated, while for rodents the bad taste of quinine is present during each sip of alcohol. In this argument, more valid models would pair alcohol intake with the anticipation of aversive consequences. In our studies, 1 in 8 FR3 responses for alcohol is paired with footshock, and inhibiting cortico-NAcb inputs greatly reduces responding so that rats often receive no shocks in this session (Seif et al., 2013). This suggests that anticipation of footshock represents a primary obstacle to further intake. This has also been addressed by Vanderschuren and Everitt (2004), who demonstrated suppression of cocaine seeking by cues paired with footshock after shorter- but not longer-term cocaine intake (Vanderschuren & Everitt, 2004), suggesting that anticipation of aversive consequences is sufficient to deter further intake except in compulsive rats.
Are there subpopulations of compulsive rats?
A number of studies have found that a sub-population of rats exhibits aversion-resistant intake, which is interesting because only a subset of humans using alcohol or cocaine develop addiction. For long-term cocaine intake, most studies find that about 20% of rats develop footshock-resistant cocaine seeking after long-term intake. Interestingly, individual rats that develop compulsive cocaine seeking also exhibit high basal impulsivity and novelty seeking (Belin et al., 2011; Belin & Deroche-Gamonet, 2012; Belin & Everitt, 2008), suggesting that “impulsivity” may be a trait that confers greater likelihood of ultimately engaging in compulsive alcohol use. Other groups have also emphasized the importance of individual differences in cortical function in the development of addiction (George & Koob, 2010). Interesting in this respect is the recent work by Whelan and colleagues (2012), showing that hypofunction of the orbitofrontal network is associated with inhibitory control deficits and increased likelihood of initiating alcohol and substance use.
Some studies with alcohol have observed a subpopulation of quinine-resistant intake in rats (Turyakibahika-Thyen & Wolffgramm, 2006; Wolffgramm et al., 2000) and mice (Fachin-Scheit et al., 2006). However, it may be that all alcohol-drinking rats can develop compulsive intake, but with different degrees of aversion resistance depending on the intensity of the aversive consequence. We found that nearly all rats under IAA intake demonstrate aversion resistance with 30 mg/L quinine (Hopf et al., 2010; Seif et al., 2013), but some rats show aversion sensitivity with 100 mg/L quinine (Hopf et al., 2010). Nearly all mice display aversion resistance with ~100 mg/L quinine (Lesscher et al., 2010). Also, only ~50% of rats exhibit footshock-resistant alcohol intake when 1 in 8 FR3 responses is paired with footshock (Seif et al., 2013), while all rats reduce responding when 1 in 3 FR3 responses are paired with footshock (S. J. Chung and F. W. Hopf, unpublished). This is similar to what is observed with footshock-resistant cocaine intake, where enough shocks c reduce responding in all rats (Xue, Steketee, & Sun, 2012), and even completely eliminate responding (i.e., a self-induced abstinence; Barnea-Ygael et al., 2012; Cooper, Barnea-Ygael, Levy, Shaham, & Zangen, 2007). In addition, after short-term intake, all rats show reduced cocaine intake by Pavlovian-conditioned aversive stimuli, while after long-term intake all rats are resistant to suppression of intake by these cues (Vanderschuren & Everitt, 2004). Thus, it would be very useful in future studies to parametrically vary the level of aversion and determine the relationship between aversive intensity and alcohol intake. Among other things, this might provide further evidence of whether alcohol and cocaine addiction are mediated through similar or different circuitry.
Molecular and circuit mechanisms that promote aversion-resistant alcohol intake
Except for a few recent studies, very little concrete information is known about the molecular and circuit mechanisms that promote aversion-resistant alcohol intake. Here, we will discuss the involvement of the amygdala, cortical regions, and striatum in alcohol and cocaine intake, since more is known about compulsive cocaine seeking. In addition, we will include studies that address intake after long-term exposure, escalation, or development of dependence. Although these studies have generally not explicitly examined the mechanisms underlying compulsive aspects of intake, the fact that these models also exhibit aversion-resistant intake is encouraging, in that mechanisms that promote basal intake after dependence or escalation may also sustain compulsive intake.
The amygdala and compulsive alcohol use
Although the contribution of the amygdala to fear, anxiety, and emotional processing has been widely studied, clinical and preclinical evidence also suggests a central role for the amygdala in drug addiction. Human cocaine addicts and alcoholics show reduced amygdala volume (Makris, Gasic, et al., 2004; Makris, Oscar-Berman, et al., 2008), which is correlated with greater craving and propensity for relapse (Wrase et al., 2008). In fMRI studies, drug-associated cues elicit stronger amygdalar activation in drug addicts when compared with control subjects (e.g., Childress et al., 1999; Schneider et al., 2001). In rodents, chronic-intermittent alcohol exposure with repeated withdrawal leads to increased alcohol intake in both rats and mice (for review see Crabbe et al., 2011; Koob, 2003; Lopez & Becker, 2005; Roberts et al., 2000). This greater intake is paralleled by many changes within the central amygdala (CeA), including increased levels of corticotropin releasing factor (CRF), increased phosphorylated ERK, and enhanced GABAergic neurotransmission and release, as well as reduced levels of neuropeptide Y (NPY), brain-derived neurotrophic factor, and phosphorylated CREB, among others (Merlo Pich et al., 1995; Pandey et al., 2008; Roberto, Madamba, Stouffer, Parsons, & Siggins, 2004; Roy & Pandey, 2002; Sanna, Simpson, Lutjens, & Koob, 2002). Dependence-induced increases in alcohol intake are abolished by increasing NPY levels or blocking CRF receptors in the CeA (Funk, O’Dell, Crawford, & Koob, 2006; Gilpin, Misra, & Koob, 2008; Gilpin, Stewart, & Badia-Elder, 2008; Thorsell et al., 2007). Alcohol-dependent rats also show increased glucocorticoid receptor (GR) mRNA in limbic brain regions including the CeA and NAcb, and the increased intake and progressive ratio responding are decreased by GR blockade (Vendruscolo et al., 2012).
Recent studies have extended the evidence for the role of the amygdala in the escalation of drug use and have also demonstrated its involvement in compulsive alcohol drinking. Using a limited-access choice paradigm in mice, Lesscher, Wallace, and colleagues (2009) showed that local knockdown of the PKC isozyme PKCepsilon in the CeA completely abolished escalation of alcohol intake. More recently, escalation of alcohol intake in this paradigm was shown to be paralleled by gene expression changes in the CeA, with the greatest changes observed at the transition from low to high levels of alcohol intake (Lesscher et al., 2012). Moreover, this study identified a novel candidate gene for CeA control over ethanol consumption: the adapter protein 14-3-3ζ. CeA expression of 14-3-3ζ was enhanced during escalation to high ethanol intake. Subsequent local knockdown of 14-3-3ζ in the CeA dramatically increased ethanol intake, suggesting that 14-3-3ζ may perhaps serve to counteract the escalation of ethanol intake. Moreover, CeA 14-3-3ζ was shown to contribute to the development of alcoholism-like behavior. As described above, in the limited-access choice paradigm, mice rapidly develop quinine insensitivity in that they fail to adjust their alcohol intake upon quinine adulteration (Lesscher et al., 2010). In addition to enhanced ethanol intake, local knockdown of 14-3-3ζ in the CeA also led to a persistent high preference for the quinine-adulterated alcohol solution, indicative of inflexible alcohol drinking (Lesscher et al., 2012). Thus, elevated 14-3-3ζ in the CeA after escalated alcohol intake may serve to buffer against further escalation of intake and also against aversion-resistant aspects of intake.
Taken together, these results indicate that the amygdala regulates both alcohol intake and aversion-resistant intake, which is perhaps secondary to prolonged and enhanced exposure to alcohol. It has been suggested that, for drug use to become compulsive, prolonged exposure to a certain level of alcohol or other drug of abuse is a prerequisite. Mechanisms within the CeA may determine whether exposure to sufficient levels of alcohol will allow resistance to punishment to emerge. A second possible explanation for CeA control over compulsive drug use may lie in the well-described CeA control of sensitivity to negative emotional stimuli and punishment (Ciocchi et al., 2010; Maren & Quirk, 2004; Phelps & LeDoux, 2005; Sehlmeyer et al., 2009). Indeed, CeA lesions reduce conditioned suppression, i.e., the suppression of ongoing operant behavior by a conditioned fear stimulus (Killcross, Robbins, & Everitt, 1997), and reduce direct suppression of behavior by footshocks (Xue et al., 2012). Taken together, there is ample reason to suggest the importance of the amygdala in both escalation of alcohol intake and the development of compulsive drug use. Further studies are required to determine the molecular and behavioral mechanisms through which the amygdala controls the development and expression of addiction.
Cortical circuits and compulsive alcohol use
A number of researchers have considered prefrontal cortical areas to be critical contributors to compulsive aspects of addiction, since cue-induced craving and relapse can correlate with prefrontal activity, and cortical dysfunction may reduce behavioral control (Breese et al., 2011; Koob & Volkow, 2010; Naqvi & Bechara, 2010; Tiffany & Conklin, 2000). Our recent study is the first to attempt to delineate the mechanism of aversion-resistant intake in rats (Seif et al., 2013). Interestingly, optogenetic inhibition of either insular or medial prefrontal (mPFC) cortical inputs to the NAcb core dramatically reduces both quinine-resistant and footshock-resistant alcohol intake. In addition, alcohol intake in the absence of explicit pairing with an aversive challenge does not require these cortical inputs. We also used in vitro electrophysiology in combination with channelrhodopsin expression in specific pathways to identify a molecular adaptation in alcohol-drinking rats, specifically hyperpolarization-active NMDA receptors under cortical inputs to the NAcb core. These NMDAR receptors sustain quinine-resistant alcohol intake but do not regulate quinine-free intake.
These results are important for a number of reasons. First, they suggest that a common circuit mediates aversion resistance using aversive consequences of different sensory modalities (taste for quinine, somatosensory for footshock) and intake modes (2-bottle for quinine, operant for footshock), making it likely that this circuit is more generally applicable to different forms of aversion-resistant intake. Second, they indicate that NMDA receptor adaptations are present in all rats after a sufficient period of alcohol intake, but that they differentially promote alcohol intake depending on the level of conflict during the consumption. Thus, our work is the first experimental demonstration we are aware of that supports the previous suggestion (Naqvi & Bechara, 2010; Tiffany & Conklin, 2000) that cortical areas preferentially contribute to compulsive aspects of addiction, where there is conflict or challenge associated with intake. Furthermore, since the level of challenge in humans is somewhat subjective and could change from moment to moment (see also Feldstein Ewing, Filbey, Sabbineni, Chandler, & Hutchison, 2011), behavioral interventions that change the mind-set of human drinkers might significantly modulate the impact of pharmacological interventions for AUDs, and specific combinations of behavioral and pharmacological therapies might be particularly effective in reducing compulsive drives for alcohol.
Other work also implicates the cortex in compulsive cocaine and alcohol intake. The mPFC shows profound hypoactivity in rats that compulsively consume cocaine, and mPFC activation inhibits compulsive cocaine seeking while mPFC inhibition promotes compulsive cocaine seeking in otherwise non-compulsive rats (Chen et al., 2013). This is in contrast to what is seen with alcohol, where mPFC-NAcb core inputs promote compulsive drinking (Seif et al., 2013). However, in agreement, alcohol-dependent rats show cortical dysfunction (George & Koob, 2010; Richardson et al., 2009). Compulsive-cocaine rats also have decreased forebrain serotonin, and depleting forebrain serotonin (or application of 5HT2C antagonists) induces compulsive behavior in rats that had only limited cocaine intake (Pelloux, Dilleen, Economidou, Theobald, & Everitt, 2012). In the prelimbic mPFC, long-term depression of mGluR2/3 is inhibited in compulsive-cocaine rats (Kasanetz et al., 2012, see also Brown, Flynn, Smith, & Dayas, 2011), and mGluR2/3 agonists decrease cocaine reinstatement (Cannella et al., 2013). In addition, alcohol-dependent rats show decreased mGluR2 levels, and restoring mGluR2 expression in the infralimbic mPFC depresses alcohol intake (Meinhardt et al., 2013). Interestingly, the anterior cingulate cortex of human alcoholics also shows reduced mGluR2 levels (Meinhardt et al., 2013), suggesting that this mPFC-mGluR mechanism is relevant to human AUDs.
The striatum and compulsive alcohol use
The striatum, including ventral regions such as the NAcb, is probably the most studied brain region in relation to drug use and addiction. Dopamine levels increase in the NAcb in response to many different drugs that are abused by humans (Di Chiara & Imperato, 1988), and a number of pharmacological and molecular studies have confirmed the importance of the NAcb in self-administration of different classes of abused drugs (Everitt & Robbins, 2005; Ikemoto & Wise, 2004; Pierce & Kumaresan, 2006). More recently, habit-based theories of addiction (Everitt et al., 1999; Everitt & Robbins, 2005; Gerdeman, Partridge, Lupica, & Lovinger, 2003; Pierce & Vanderschuren, 2010; Tiffany, 1990) have focused on the involvement of dorsal striatal regions in cocaine and alcohol addiction. A large body of literature has demonstrated that the dorsal striatum, especially the DLS, is a critical region for expression of habits; interestingly, the NAcb core acts in concert with dopamine receptor activation within the dorsal striatum to promote expression of habitual cocaine seeking (Belin & Everitt, 2008). In addition, dopamine receptors within the NAcb sustain footshock-resistant cocaine seeking (Saunders, Yager, & Robinson, 2013). Also, as described above, cortical inputs to the NAcb core promote aversion-resistant alcohol intake with no effect on alcohol intake when not explicitly paired with aversive consequences (Seif et al., 2013).
Although little is known about the neurobiological underpinnings of compulsive alcohol seeking in rodents, a recent study found that the DLS contributes to habitual alcohol seeking in rats (Corbit, Nie, & Janak, 2012). Alcohol was devalued by pre-exposing rats to alcohol, and rats were then tested for responding for alcohol during extinction. Within 4 weeks of operant alcohol self-administration, intake became insensitive to devaluation, indicating that the behavior had become habitual. DLS inactivation restored the sensitivity to devaluation of alcohol seeking (Corbit et al., 2012). This concurs with recent evidence that the DLS contributes to habitual and aversion-resistant cocaine seeking (Jonkman et al., 2012; Zapata, Minney, & Shippenberg, 2010). After extended cocaine intake under a seeking-taking schedule, drug seeking persists during extinction of the taking link, indicating habitual responding under these conditions. However, after DLS inactivation, cocaine taking was decreased during extinction and thus had become goal-directed (Zapata et al., 2010). Jonkman and colleagues (2012) found that DLS inactivation reduces cocaine seeking when paired with probabilistic footshock, but does not reduce responding under unpunished conditions, in contrast to the findings of Zapata et al. (2010). Furthermore, changes in microRNAs in the dorsal striatum mediate escalated cocaine intake in LgA rats (Jonkman & Kenny, 2013), and a decrease in striatal D2 receptors promotes compulsive eating (Johnson & Kenny, 2010). Finally, human fMRI studies find that, compared to light alcohol drinkers, heavy drinkers and alcohol-dependent subjects show greater dorsal striatal activation to alcohol-associated cues, and that cue-induced striatal activation is positively correlated with an individual’s rating on an OCD scale (Vollstädt-Klein et al., 2010). Together, these studies indicate that striatal mechanisms contribute to escalation of drug use as well as compulsive alcohol and cocaine seeking.
Conclusion
Compulsive drives for drugs or alcohol, characterized in particular by persistent responding despite negative consequences, remain a critical obstacle for clinical treatment of human addiction. A number of animal models have been developed to examine the willingness of rodents to continue alcohol intake even when paired with aversive stimuli. They raise the hope that it will now be possible to begin to uncover the molecular and neurocircuit mechanisms that promote compulsive alcohol seeking and drinking in humans.
Recent studies using models of quinine aversion-resistance have identified neuro-adaptations in the amygdala, cortex, and NAcb, which sustain aversion-resistant intake; the findings also implicate the DLS in both habitual and aversion-resistant drug and alcohol use. Importantly, the models that capture compulsive alcohol intake often also show other features considered to recapitulate human addiction (e.g., escalation of intake, increased motivation for alcohol, increased relapse and prefrontal deficits), rendering them as highly relevant models for alcoholism. Moreover, there is reasonable evidence that these other features can be mechanistically dissociated from aversion resistance, thus emphasizing the complexity of the disorder. We have also addressed the relationship between compulsions and habits. In particular, aversion resistance may be supported and sustained by the same neural mechanisms that mediate habits, perhaps a particularly pernicious example of where addictive drives co-opt basic learning and memory mechanisms. In addition, unlike habits, compulsive intake may recruit cortical areas because of the presence of conflict during punished responding. It may in fact be that compulsions are one step beyond habits, where the compulsion is the push to step beyond a clearly bad habit, until the push itself becomes the habit. Thus, additional mechanisms, in addition to those that support habits, would be required to exert directed effort to overcome anticipated negative outcomes.
Taken together, the field has made considerable progress in developing models to elucidate the mechanisms of habitual and compulsive responding. Careful delineation of the mechanisms for the different aspects of pathological intake, i.e., increased motivation, increased responding without reinforcer delivery, –intake despite adverse consequences, etc., will greatly clarify not only the common and different mechanisms that support these different behaviors, but also the relevance of each pathological aspect of intake to human addictions.
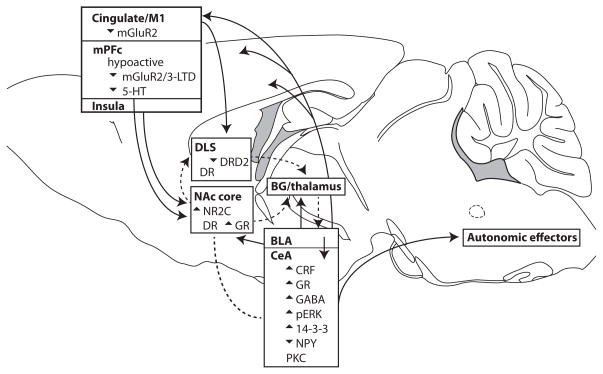
Fig. 1. Putative brain circuits (Belin et al., 2013; Childress et al., 1999; Everitt & Robbins, 2005; Koob, 2009; Lesscher & Vanderschuren, 2012; Naqvi & Bechara, 2010) and molecules, and neuroadaptations that promote “compulsive” addiction for alcohol or cocaine.
Several independent yet critical neuroadaptations (e.g., decreased DRD2 in DLS, increased NR2C in NAcore, 14-3-3ζ, and neuropeptides in CeA) likely all sustain activity in what could be called the “compulsive network”. Many regions in this circuit overlap with those proposed for motivation/action networks, including mPFC areas, basolateral and central amygdala, NAcb, and DLS. This circuit would in part be activated because of conflict during ethanol intake (wanting ethanol versus avoiding aversion); this would be considered strong top-down activation, since it would originate from cortical areas central for processing the conflict. Although the cortical regions and DLS were directly implicated in compulsivity, the CeA promotes compulsive alcohol drinking, but the mechanism remains unresolved. One possibility is that cortical activation of the CeA (through intermediary regions) during conflict generates physical/hormonal sensations which are then sensed as feedback meant to invigorate action, in this case ethanol intake or seeking in the face of challenge; this is essentially the Somatic Marker Hypothesis of Damasio (Naqvi & Bechara, 2010). The line without arrow between NAcore and CeA indicates interesting co-activity (e.g., Hall, Parkinson, Connor, Dickinson, & Everitt, 2001), although the intermediary regions and directions of interaction are uncertain. Much remains to be discovered about how the parts of the circuit shown interact to drive pathological ethanol or drug intake. See main text for further details. BG: basal ganglia (direct and indirect pathway); DR: dopamine receptor; GR: glucocorticoid receptor.
Glossary
Terminologies used to describe human compulsive drives for alcohol have attempted to capture different aspects of the repetitive, automatic, and seemingly maladaptive intake, as well as other mechanisms that motivate behavior. Here, we attempt to define and contrast different terms used to describe compulsive-like behavior in rodents and humans. Many of these constructs are complex and difficult to fully define even in clinical situations. We suggest “aversion-resistant” for pre-clinical work since it is more operationally definable and measurable, and does not require inferring the underlying motivational or experiential state of the rodent (where terms such as loss-of-control can be especially problematic). “Inflexible” also captures the essence of being willing to procure reward at all costs.
One other key source of semantic confusion may be that addiction is likely a progression across states. One begins with being goal-directed, which with time becomes habitual. Then, riding along with the habitual intake, compulsive drives develop and become established. Depending on the duration of intake and training conditions, transitions between these stages could occur at different rates. We note this only to indicate possible complexities when comparing results across models, but also to point out that compulsive- and habit-like alcohol drinking are probably mediated by common circuits.
- Aversion-resistant Intake
Drug or alcohol self-administration that persists despite explicit pairing with an aversive consequence (e.g., bitter taste, footshock, LiCl sickness in rodents). This is considered to reflect some aspects of human addiction where pursuit of alcohol (or another addictant) continues despite evidence of aversive consequences such as disruption or loss of job, relationship, health, and/or freedom. “Aversion resistance” has the advantage that it describes rodent compulsive-like intake in experimentally tractable hypotheses. We believe these intake-despite-aversion models in rodents reflect key aspects of pathological and compulsive motivation in humans with alcohol use disorders, and these models have allowed us and others to make many important discoveries about the mechanisms of compulsive-like motivation for alcohol.
- Compulsion
-
In human psychiatric conditions, this can be thought of as part of an obsession-compulsion cycle, where obsession is the buildup of emotional tension or need (often experienced as distressing) and compulsion describes the action that relieves the built-up tension and distress. Compulsions may also be actions strengthened by negative reinforcement, such as observed in obsessive-compulsive disorder. A compulsion is perhaps an urge to act that seems automatic or initiated without one’s control, and is not always welcome but ultimately always prevails. One hallmark of compulsive action is that it persists despite adverse consequences. Thus, some pre-clinical studies use “compulsive” when implying aversion resistance as defined above (continued seeking and intake despite adverse consequences). Practically speaking, we note that compulsive acts may serve to relieve built-up tension, but they often come with the cost of generating other distress (distress at weakness in self-control and having to undergo the ritual, physical dysphoria, or mental anguish related to situational disruption).
See below under Habit for comparison of habit with compulsion.
- Cost
A key concept in decision making, in which a given action requires expenditure of non-infinite resources to obtain a given reward or outcome. Thus, one must deliberate whether the gain, the value of the reward/outcome, is worth the cost. We address cost only to note that compulsive action by definition persists despite significant cost, which can be actual or anticipated distress. We note that addiction-related costs for humans reflect both shorter-term impacts (general malaise/hangover) that in turn lead to bigger issues (difficulty concentrating, getting to work on time, social inappropriateness leading to loss of job) and ultimately severe consequences. However, because the consequences are less immediate relative to rodent studies, it is easier for humans to discount the adverse impacts; this aspect of human addiction, especially denial, will be very challenging if not impossible to model in rodents.
- Habit
-
Colloquially, habit describes a set of similar but not fully overlapping concepts related to behaviors that seem automatic or occurring without volition or intention. This is adaptive since automatic behavior can be more rapid and efficient, but comes at the cost of flexibility in response choice. Habits can be defined operationally as actions that persist despite changes in the value of the reward that an action is directed toward. For example, after prefeeding with a given reward, a goal-directed animal will no longer lever-press for the reward, while a habit-directed animal will keep pressing, exhibiting evidence of resistance-to-devaluation.
Compulsion may differ from habit/automaticity in one key way: compulsion involves conflict while habit does not. Clinical and preclinical work concur in suggesting that this conflict during alcohol addiction recruits cortico-accumbal circuits, which are critical (when acting in concert with habit-related and amygdalar circuits) to drive aversion-resistant alcohol intake. Thus, the level of compulsion could be operationally related to the level of conflict. Tolerating a strong cost is more clearly aversion-resistant and perhaps inflexible. With less cost (prefeeding devaluation), then this would be habitual but not aversion-resistant. In this regard, pairing LiCl sickness with reward is very potent, and a strong cortical circuit may be needed to overcome this resistance to devaluation. This would be mechanistically different from resistance to devaluation by prefeeding, where there is likely no strong aversion (still get the reward before the session).
- Inflexible
A pattern of responding that does not vary even when the relative value of the reward changes, including when it is made very aversive. This term is somewhat problematic, since resistance-to-devaluation that defines habit is also described as inflexible, and thus the term inflexible would apply to willingness to respond with both lower (habit) and higher (aversion resistance) costs. While semantically attractive, because it captures the willingness to persevere regardless of the cost which the term “habit” lacks, inflexibility is likely really only demonstrated when the animal has an alternate choice for a valued reward, but still chooses alcohol; as yet this has been performed in very few studies.
- Loss-of-control
In the rodent literature, this term is used to reflect aversion resistance but also very high levels of voluntary alcohol intake; some rodent models exhibit intake twice the level for binge drinking, and binge drinking itself is already a gold standard for pathological excessive intake. This is considered to capture the “drinking more than intended” criterion for AUDs as described in the DSM, reflecting an individual’s inability to control the urge to drink. In that respect, loss-of-control may be a problematic term in rodent studies, since we do not know the rodent’s intent. Also, rodents are willing to undergo aversive consequences to receive alcohol or cocaine, but the amount of intake remains the same as without punishment, and thus is not uncontrolled – it merely persists despite aversion.
- Progressive Ratio
PR procedures could be considered compulsive, since they exhibit persistence in responding despite the perhaps aversive nature of not being rewarded as frequently, as well as increased work requirement for reward. It remains speculative whether PR responding leads to this internal aversive or distressed state in a rodent, but is a very interesting and perhaps testable possibility. In agreement, aversion-resistant cocaine intake is correlated with greatly increased PR responding for cocaine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
F. Woodward Hopf, Email: woody@gallo.ucsf.edu.
Heidi M.B. Lesscher, Email: h.m.b.lesscher@uu.nl.
References
- Ahmed SH. The science of making drug-addicted animals. Neuroscience. 2012;211:107–125. doi: 10.1016/j.neuroscience.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Anton RF. Obsessive-compulsive aspects of craving: development of the Obsessive Compulsive Drinking Scale. Addiction. 2000;95(Suppl 2):S211–217. doi: 10.1080/09652140050111771. [DOI] [PubMed] [Google Scholar]
- Barnea-Ygael N, Yadid G, Yaka R, Ben-Shahar O, Zangen A. Cue-induced reinstatement of cocaine seeking in the rat “conflict model”: effect of prolonged home-cage confinement. Psychopharmacology. 2012;219:875–883. doi: 10.1007/s00213-011-2416-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Belin-Rauscent A, Murray JE, Everitt BJ. Addiction: failure of control over maladaptive incentive habits. Current Opinion in Neurobiology. 2013;23:564–572. doi: 10.1016/j.conb.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V. High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology. 2011;36:569–579. doi: 10.1038/npp.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Deroche-Gamonet V. Responses to novelty and vulnerability to cocaine addiction: contribution of a multi-symptomatic animal model. Cold Spring Harbor Perspectives in Medicine. 2012;2:1–20. doi: 10.1101/cshperspect.a011940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addiction Biology. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Bode C, Bode JC. Effect of alcohol consumption on the gut. Best Practice & Research Clinical Gastroenterology. 2003;17:575–592. doi: 10.1016/s1521-6918(03)00034-9. [DOI] [PubMed] [Google Scholar]
- Breese GR, Sinha R, Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacology & Therapeutics. 2011;129:149–171. doi: 10.1016/j.pharmthera.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AL, Flynn JR, Smith DW, Dayas CV. Down-regulated striatal gene expression for synaptic plasticity-associated proteins in addiction and relapse vulnerable animals. The International Journal of Neuropsychopharmacology. 2011;14:1099–1110. doi: 10.1017/S1461145710001367. [DOI] [PubMed] [Google Scholar]
- Cannella N, Halbout B, Uhrig S, Evrard L, Corsi M, Corti C, et al. The mGluR2/3 agonist LY379268 induced anti-reinstatement effects in rats exhibiting addiction-like behavior. Neuropsychopharmacology. 2013;38:2048–2056. doi: 10.1038/npp.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, et al. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–362. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. The American Journal of Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Cooper A, Barnea-Ygael N, Levy D, Shaham Y, Zangen A. A conflict rat model of cue-induced relapse to cocaine seeking. Psychopharmacology. 2007;194:117–25. doi: 10.1007/s00213-007-0827-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH. Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum. Biological Psychiatry. 2012;72:389–395. doi: 10.1016/j.biopsych.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Harris RA, Koob GF. Preclinical studies of alcohol binge drinking. Annals of the New York Academy of Sciences. 2011;1216:24–40. doi: 10.1111/j.1749-6632.2010.05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, Wood N, Smith JW. Alcohol seeking by rats: action or habit? The Quarterly Journal of Experimental Psychology B, Comparative and Physiological Psychology. 2002;55:331–348. doi: 10.1080/0272499024400016. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Annals of the New York Academy of Sciences. 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fachin-Scheit DJ, Frozino Ribeiro A, Pigatto G, Oliveira Goeldner F, Boerngen de Lacerda R. Development of a mouse model of ethanol addiction: naltrexone efficacy in reducing consumption but not craving. Journal of Neural Transmission. 2006;113:1305–1321. doi: 10.1007/s00702-005-0416-z. [DOI] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Filbey FM, Sabbineni A, Chandler LD, Hutchison KE. How psychosocial alcohol interventions work: a preliminary look at what FMRI can tell us. Alcoholism: Clinical and Experimental Research. 2011;35:643–651. doi: 10.1111/j.1530-0277.2010.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. The Journal of Neuroscience. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neuroscience and Biobehavioral Reviews. 2010;35:232–247. doi: 10.1016/j.neubiorev.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM. It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends in Neurosciences. 2003;26:184–192. doi: 10.1016/S0166-2236(03)00065-1. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Koob GF. Neuropeptide Y in the central nucleus of the amygdala suppresses dependence-induced increases in alcohol drinking. Pharmacology, Biochemistry, and Behavior. 2008;90:475–480. doi: 10.1016/j.pbb.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Badia-Elder NE. Neuropeptide Y administration into the amygdala suppresses ethanol drinking in alcohol-preferring (P) rats following multiple deprivations. Pharmacology, Biochemistry, and Behavior. 2008;90:470–474. doi: 10.1016/j.pbb.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. The European Journal of Neuroscience. 2001;13:1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- Hay RA, Jennings JH, Zitzman DL, Hodge CW, Robinson DL. Specific and nonspecific effects of naltrexone on goal-directed and habitual models of alcohol seeking and drinking. Alcoholism: Clinical and Experimental Research. 2013;37:1100–1110. doi: 10.1111/acer.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addiction Biology. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Chang SJ, Sparta DR, Bowers MS, Bonci A. Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcoholism: Clinical and Experimental Research. 2010;34:1565–1573. doi: 10.1111/j.1530-0277.2010.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Sparta DR, Bonci A. Translational models of interactions between stress and alcohol consumption: strengths and limitations. International Laboratory Animal Research Journal. 2011;52:239–250. doi: 10.1093/ilar.52.3.239. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Wise RA. Mapping of chemical trigger zones for reward. Neuropharmacology. 2004;47(Suppl 1):190–201. doi: 10.1016/j.neuropharm.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature Neuroscience. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman S, Kenny PJ. Molecular, cellular, and structural mechanisms of cocaine addiction: a key role for microRNAs. Neuropsychopharmacology. 2013;38:198–211. doi: 10.1038/npp.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman S, Pelloux Y, Everitt BJ. Differential roles of the dorsolateral and midlateral striatum in punished cocaine seeking. The Journal of Neuroscience. 2012;32:4645–4650. doi: 10.1523/JNEUROSCI.0348-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasanetz F, Lafourcade M, Deroche-Gamonet V, Revest JM, Berson N, Balado E, et al. Prefrontal synaptic markers of cocaine addiction-like behavior in rats. Molecular Psychiatry. 2012;18:729–737. doi: 10.1038/mp.2012.59. [DOI] [PubMed] [Google Scholar]
- Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcoholism: Clinical and Experimental Research. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56(Suppl 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larimer ME, Palmer RS, Marlatt GA. Relapse prevention. An overview of Marlatt’s cognitive-behavioral model. Alcohol Research & Health. 1999;23:151–160. [PMC free article] [PubMed] [Google Scholar]
- Leon DA, Saburova L, Tomkins S, Andreev E, Kiryanov N, McKee M, et al. Hazardous alcohol drinking and premature mortality in Russia: a population based case-control study. Lancet. 2007;369:2001–2009. doi: 10.1016/S0140-6736(07)60941-6. [DOI] [PubMed] [Google Scholar]
- Lesscher HM, Houthuijzen JM, Groot Koerkamp MJ, Holstege FC, Vanderschuren LJ. Amygdala 14-3-3ζ as a novel modulator of escalating alcohol intake in mice. PLoS One. 2012;7:e37999. doi: 10.1371/journal.pone.0037999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesscher HM, Kas MJ, van der Elst S, van Lith HA, Vanderschuren LJ. A grandparent-influenced locus for alcohol preference on mouse chromosome 2. Pharmacogenetics and Genomics. 2009;19:719–729. doi: 10.1097/FPC.0b013e3283311320. [DOI] [PubMed] [Google Scholar]
- Lesscher HM, van Kerkhof LW, Vanderschuren LJ. Inflexible and indifferent alcohol drinking in male mice. Alcoholism: Clinical and Experimental Research. 2010;34:1219–1225. doi: 10.1111/j.1530-0277.2010.01199.x. [DOI] [PubMed] [Google Scholar]
- Lesscher HM, Vanderschuren LJ. Compulsive drug use and its neural substrates. Reviews in the Neurosciences. 2012;23:731–745. doi: 10.1515/revneuro-2012-0066. [DOI] [PubMed] [Google Scholar]
- Lesscher HM, Wallace MJ, Zeng L, Wang V, Deitchman JK, McMahon T, et al. Amygdala protein kinase C epsilon controls alcohol consumption. Genes, Brain, and Behavior. 2009;8:493–499. doi: 10.1111/j.1601-183X.2009.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Bian W, Dave V, Ye JH. Blockade of GABA(A) receptors in the paraventricular nucleus of the hypothalamus attenuates voluntary ethanol intake and activates the hypothalamic-pituitary-adrenocortical axis. Addiction Biology. 2011;16:600–614. doi: 10.1111/j.1369-1600.2011.00344.x. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Zorrilla EP. Stress history increases alcohol intake in relapse: relation to phosphodiesterase 10A. Addiction Biology. 2012;17:920–933. doi: 10.1111/j.1369-1600.2012.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi B, Lobina C, Maccioni P, Fantini N, Carai MA, Gessa GL, et al. Increase in alcohol intake, reduced flexibility of alcohol drinking, and evidence of signs of alcohol intoxication in Sardinian alcohol-preferring rats exposed to intermittent access to 20% alcohol. Alcoholism: Clinical and Experimental Research. 2010;34:2147–2154. doi: 10.1111/j.1530-0277.2010.01311.x. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology. 2005;181:688–696. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Makris N, Gasic GP, Seidman LJ, Goldstein JM, Gastfriend DR, Elman I, et al. Decreased absolute amygdala volume in cocaine addicts. Neuron. 2004;44:729–740. doi: 10.1016/j.neuron.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, et al. Decreased volume of the brain reward system in alcoholism. Biological Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangieri RA, Cofresi RU, Gonzales RA. Ethanol seeking by Long Evans rats is not always a goal-directed behavior. PLoS One. 2012;7:e42886. doi: 10.1371/journal.pone.0042886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Khuc TN, Pickens CL, Bonci A, Shaham Y. Context-induced relapse to alcohol seeking after punishment in a rat model. Biological Psychiatry. 2013;73:256–262. doi: 10.1016/j.biopsych.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nature Reviews Neuroscience. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, et al. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biological Psychiatry. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt MW, Hansson AC, Perreau-Lenz S, Bauder-Wenz C, Stählin O, Heilig M, et al. Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. The Journal of Neuroscience. 2013;33:2794–2806. doi: 10.1523/JNEUROSCI.4062-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, et al. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. The Journal of Neuroscience. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Maloney T, Parvaz MA, Alia-Klein N, Woicik PA, Telang F, et al. Impaired insight in cocaine addiction: laboratory evidence and effects on cocaine-seeking behaviour. Brain. 2010;133:1484–1493. doi: 10.1093/brain/awq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormede P, Colas A, Jones BC. High ethanol preferring rats fail to show dependence following short- or long-term ethanol exposure. Alcohol and Alcoholism. 2004;39:183–189. doi: 10.1093/alcalc/agh037. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Structure & Function. 2010;214:435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orio L, Edwards S, George O, Parsons LH, Koob GF. A role for the endocannabinoid system in the increased motivation for cocaine in extended-access conditions. The Journal of Neuroscience. 2009;29:4846–4857. doi: 10.1523/JNEUROSCI.0563-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Ugale R, Prakash A, Xu T, Misra K. Effector immediate-early gene arc in the amygdala plays a critical role in alcoholism. The Journal of Neuroscience. 2008;28:2589–2600. doi: 10.1523/JNEUROSCI.4752-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux Y, Dilleen R, Economidou D, Theobald D, Everitt BJ. Reduced forebrain serotonin transmission is causally involved in the development of compulsive cocaine seeking in rats. Neuropsychopharmacology. 2012;37:2505–2514. doi: 10.1038/npp.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neuroscience and Biobehavioral Reviews. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Vanderschuren LJ. Kicking the habit: the neural basis of ingrained behaviors in cocaine addiction. Neuroscience and Biobehavioral Reviews. 2010;35:212–219. doi: 10.1016/j.neubiorev.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl MD, Cason AM, Wojnicki FH, Corwin RL, Grigson PS. A history of bingeing on fat enhances cocaine seeking and taking. Behavioral Neuroscience. 2011;125:930–942. doi: 10.1037/a0025759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiology & Behavior. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Chan SH, Crawford EF, Lee YK, Funk CK, Koob GF, et al. Permanent impairment of birth and survival of cortical and hippocampal proliferating cells following excessive drinking during alcohol dependence. Neurobiology of Disease. 2009;36:1–10. doi: 10.1016/j.nbd.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. The Journal of Neuroscience. 2004;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Roberts KL, Hall DA. Examining a supramodal network for conflict processing: a systematic review and novel functional magnetic resonance imaging data for related visual and auditory stroop tasks. Journal of Cognitive Neuroscience. 2008;20:1063–1078. doi: 10.1162/jocn.2008.20074. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Fixaris MC, Crabbe JC, Brooks PC, Ascheid S. Escalation of intake under intermittent ethanol access in diverse mouse genotypes. Addiction Biology. 2013;18:496–507. doi: 10.1111/j.1369-1600.2012.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Pandey SC. The decreased cellular expression of neuropeptide Y protein in rat brain structures during ethanol withdrawal after chronic ethanol exposure. Alcoholism: Clinical and Experimental Research. 2002;26:796–803. [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addiction Biology. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Sanna PP, Simpson C, Lutjens R, Koob G. ERK regulation in chronic ethanol exposure and withdrawal. Brain Research. 2002;948:186–191. doi: 10.1016/s0006-8993(02)03191-8. [DOI] [PubMed] [Google Scholar]
- Saunders BT, Yager LM, Robinson TE. Cue-evoked cocaine “craving”: role of dopamine in the accumbens core. The Journal of Neuroscience. 2013;33:13989–14000. doi: 10.1523/JNEUROSCI.0450-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, et al. Subcortical correlates of craving in recently abstinent alcoholic patients. The American Journal of Psychiatry. 2001;158:1075–1083. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C, Schöning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, et al. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS One. 2009;4:e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Chang SJ, Simms JA, Gibbs SL, Dadgar J, Chen BT, et al. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nature Neuroscience. 2013;16:1094–1100. doi: 10.1038/nn.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, et al. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcoholism: Clinical and Experimental Research. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo Hoo GW, Hinds RL, Dinovo E, Renner SW. Fatal large-volume mouthwash ingestion in an adult: a review and the possible role of phenolic compound toxicity. Journal of Intensive Care Medicine. 2003;18:150–155. doi: 10.1177/0885066602250783. [DOI] [PubMed] [Google Scholar]
- Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiological Reviews. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Hölter SM. Long-term alcohol self-administration with repeated alcohol deprivation phases: an animal model of alcoholism? Alcohol and Alcoholism. 1999;34:231–243. doi: 10.1093/alcalc/34.2.231. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Hölter SM, Allingham K, Landgraf R, Zieglgänsberger W. Acamprosate and alcohol: I. Effects on alcohol intake following alcohol deprivation in the rat. European Journal of Pharmacology. 1996;305:39–44. doi: 10.1016/0014-2999(96)00174-4. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Putzke J, Stefferl A, Schöbitz B, Zieglgänsberger W. Acamprosate and alcohol: II. Effects on alcohol withdrawal in the rat. European Journal of Pharmacology. 1996;305:45–50. doi: 10.1016/0014-2999(96)00175-6. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell A, Repunte-Canonigo V, O’Dell LE, Chen SA, King AR, Lekic D, et al. Viral vector-induced amygdala NPY overexpression reverses increased alcohol intake caused by repeated deprivations in Wistar rats. Brain. 2007;130:1330–1337. doi: 10.1093/brain/awm033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Conklin CA. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction. 2000;95(Suppl 2):S145–153. doi: 10.1080/09652140050111717. [DOI] [PubMed] [Google Scholar]
- Turyabahika-Thyen K, Wolffgramm J. Loss of flexibility in alcohol-taking rats: promoting factors. European Addiction Research. 2006;12:210–221. doi: 10.1159/000094423. [DOI] [PubMed] [Google Scholar]
- Uhart M, Wand GS. Stress, alcohol and drug interaction: an update of human research. Addiction Biology. 2009;14:43–64. doi: 10.1111/j.1369-1600.2008.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr, Logrip ML, et al. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. The Journal of Neuroscience. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Celerier E, Chaskiel L, Penzo F, Spanagel R. Compulsive alcohol drinking in rodents. Addiction Biology. 2009;14:384–396. doi: 10.1111/j.1369-1600.2009.00177.x. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Noori HR, Spanagel R. The use of a novel drinkometer system for assessing pharmacological treatment effects on ethanol consumption in rats. Alcoholism: Clinical and Experimental Research. 2013;37(Suppl 1):E322–328. doi: 10.1111/j.1530-0277.2012.01874.x. [DOI] [PubMed] [Google Scholar]
- Villas Boas GR, Zamboni CG, Peretti MC, Correia D, Rueda AV, Camarini R, et al. GABA(B) receptor agonist only reduces ethanol drinking in light-drinking mice. Pharmacology, Biochemistry, and Behavior. 2012;102:233–240. doi: 10.1016/j.pbb.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Wichert S, Rabinstein J, Bühler M, Klein O, Ende G, et al. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction. 2010;105:1741–1749. doi: 10.1111/j.1360-0443.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iñiguez SD, et al. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nature Neuroscience. 2009;12:200–209. doi: 10.1038/nn.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R, Conrod PJ, Poline JB, Lourdusamy A, Banaschewski T, Barker GJ, et al. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nature Neuroscience. 2012;15:920–925. doi: 10.1038/nn.3092. [DOI] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J, Galli G, Thimm F, Heyne A. Animal models of addiction: models for therapeutic strategies? Journal of Neural Transmission. 2000;107:649–668. doi: 10.1007/s007020070067. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J, Heyne A. Social behavior, dominance, and social deprivation of rats determine drug choice. Pharmacology, Biochemistry, and Behavior. 1991;38:389–399. doi: 10.1016/0091-3057(91)90297-f. [DOI] [PubMed] [Google Scholar]
- Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, et al. Amygdala volume associated with alcohol abuse relapse and craving. The American Journal of Psychiatry. 2008;165:1179–1184. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
- Xue Y, Steketee JD, Sun W. Inactivation of the central nucleus of the amygdala reduces the effect of punishment on cocaine self-administration in rats. The European Journal of Neuroscience. 2012;35:775–783. doi: 10.1111/j.1460-9568.2012.08000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata A, Minney VL, Shippenberg TS. Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. The Journal of Neuroscience. 2010;30:15457–15463. doi: 10.1523/JNEUROSCI.4072-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]