Abstract
Background
Sublobar resection (SR) in high-risk operable patients may result in long-term decrease in pulmonary function. We previously reported 3-month pulmonary function outcomes from a randomized phase III study comparing SR alone to SR with brachytherapy (SRB) in patients with non-small cell lung cancer. We now report on long-term pulmonary function after SR.
Methods
Pulmonary function was measured at baseline, and at 3, 12 and 24 months. A ≥10% decline from baseline in FEV1% or DLCO% was considered clinically meaningful. The impact of study arm, tumor location, size, approach (VATS vs. thoracotomy), and SR type (wedge vs. segmentectomy) on pulmonary function was assessed using a Wilcoxon rank sum test. A generalized estimating equation model was used to assess the impact of each factor on longitudinal data including all 4 time-points.
Results
Complete pulmonary function data at all time-points was available in 69 patients. No significant differences were observed in pulmonary function between SR and SRB, thus the study arms were combined for all analyses. A ≥10% decline (p=0.02) in FEV1% was demonstrated for lower lobe resections at 3 months, but was not seen at 12 or 24 months. A ≥10% decline (p=0.05) in DLCO% was seen for thoracotomy at 3 months but was not seen at 12 or 24 months.
Conclusions
Clinically meaningful declines in pulmonary function occurred after lower lobe resection and after thoracotomy at 3 months, but subsequently recovered. This study suggests that SR does not result in sustained decreased pulmonary function in high-risk operable patients.
Keywords: pulmonary function, lung cancer surgery, lung cancer clinical trials, lobectomy, segmentectomy, wedge resection, statistics (clinical trial)
Sublobar resection (SR) is usually offered for patients with clinical stage IA lung cancer who have limited pulmonary reserve or significant medical comorbidities but are still considered candidates for surgery. However, the premise that sublobar resection preserves pulmonary function more than lobectomy remains controversial. Some retrospective series have shown greater reduction in pulmonary function in patients undergoing lobectomy compared to SR [1, 2]. However, the Lung Cancer Study Group reported no significant difference in pulmonary function among patients randomized to either lobectomy or sublobar resection for clinical stage I lung cancer [3].
The American College of Surgeons Oncology Group (ACOSOG) Z4032 was a randomized trial undertaken to compare SR alone to SR with brachytherapy (SRB) for high-risk operable patients with early-stage non–small cell lung cancer. The primary endpoint of this trial was time to local recurrence. No significant difference was observed in local recurrence rates and this has been reported elsewhere [4]. ACOSOG is now part of the Alliance for Clinical Trials in Oncology.
Patients enrolled in ACOSOG Z4032 underwent pulmonary function testing (PFTs) at baseline and at 3, 12 and 24 months. The impact of SR on pulmonary function at 3 months has been previously reported [5]. In the present analysis we report on the long-term impact of sublobar resection on pulmonary function among the high-risk operable patients enrolled in ACOSOG Z4032.
Patients and Methods
ACOSOG Z4032 was open to patients with clinical stage IA or 1B lung cancer, who were considered to be high-risk for lobectomy [6]. Enrolled patients were randomized to undergo either sublobar resection alone (wedge or segmentectomy) or sublobar resection with intraoperative brachytherapy. The surgical approach (VATS versus open and wedge resection versus segmentectomy) was at the discretion of the operating surgeon. Each participant signed an IRB-approved, protocol-specific informed consent in accordance with federal and institutional guidelines.
The primary endpoint of the trial was time to local recurrence. No difference in recurrence-free survival, overall survival or locoregional recurrence was observed between study arms [4].
Secondary endpoints of this trial included describing the impact of treatment on quality of life and pulmonary function [7]. Consequently, patients enrolled in ACOSOG Z4032 underwent pulmonary function testing, including diffusion capacity for carbon monoxide (DLCO) pre-operatively, as well as 3, 12 and 24 months following surgery.
Statistical analysis
Pulmonary function tests included percentage predicted forced expiratory volume in 1 second (FEV1%) and percentage predicted carbon monoxide diffusing capacity of the lung (DLCO%), both of which were measured preoperatively and at 3, 12 and 24 months after intervention. Chi-squared tests for categorical variables and Wilcoxon rank sum tests for continuous variables were used to compare the baseline patient characteristics between the SR and SRB arms among patients with complete and incomplete PFT data. The impact of study arm, tumor location (lower lobe versus upper/middle lobe), pathological tumor size (≤ or > 2cm), surgical approach (VATS vs. thoracotomy), and sublobar resection type (wedge vs. segmentectomy) on PFTs was assessed. Specifically, the median percentage changes in the DLCO%, and FEV1% from baseline to months 3, 12 and 24 were compared between the different subgroups using a Wilcoxon signed rank test. Additionally, a ≥10% decline from baseline in FEV1% or DLCO% was considered clinically meaningful, and compared between the different subgroups using a Fisher’s exact or Chi-squared test at each time point. A generalized estimating equation (GEE) model was subsequently used to assess the impact of each factor on longitudinal PFT data across all 4 time-points [8,9]. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center.
Results
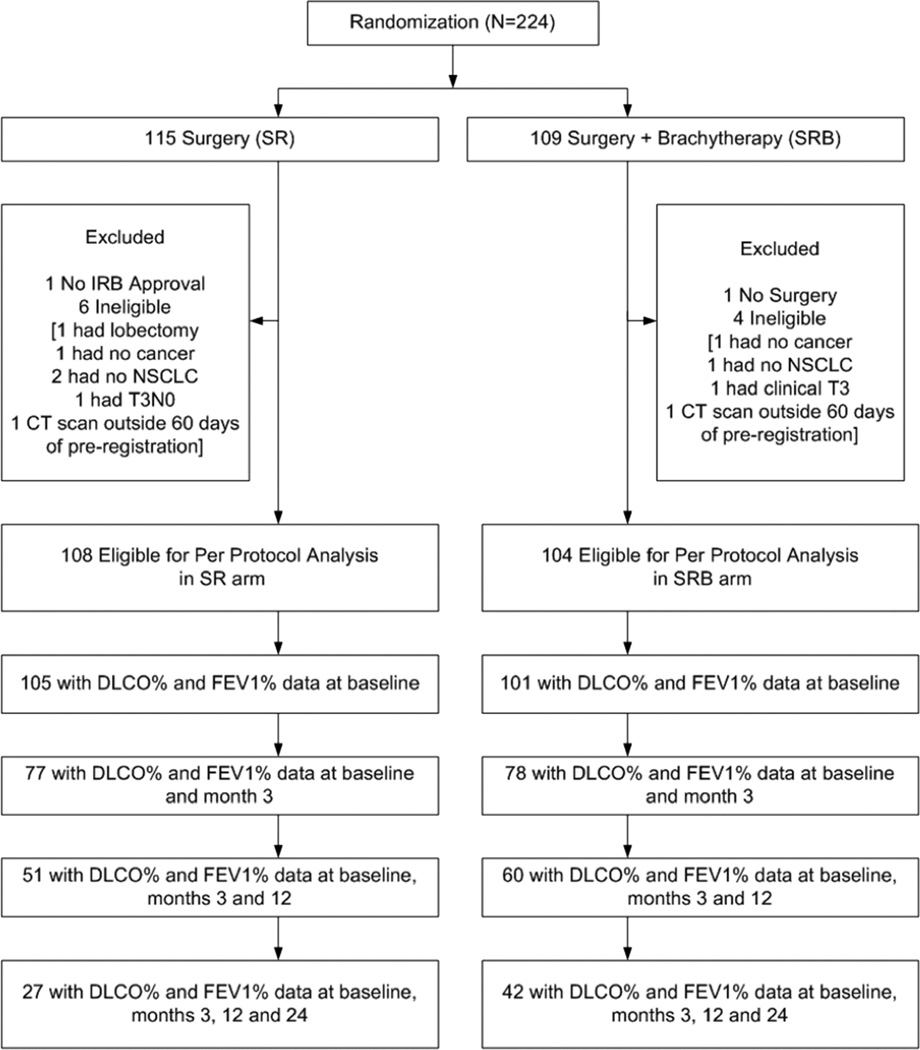
Data were frozen for this analysis on July 15, 2013. A total of 224 patients were randomized to the Z4032 trial, twelve of whom were deemed ineligible. Among the 212 evaluable patients, 155 completed PFTs at 3 months, 111 patients completed PFTs at 3 and 12 months, and 69 patients completed PFTs at all three time points (3, 12 and 24 months). Therefore, the present analysis included 69 patients for whom PFT (DLCO% and FEV1%) data was complete at all time-points (Figure 1). The specific reasons for missing PFT data are listed in Table 1.
Figure 1.
Patient CONSORT Diagram. IRB, institutional review board; DLCO%, percentage predicted diffusing capacity of the lung for carbon monoxide; FEV1%, percentage predicted forced expiratory volume in 1 second.
Table 1.
Reasons for missing PFT data at baseline, 3, 12 and 24 months
| Reason* | Baseline | 3 months | 12 months | 24 months | Total |
|---|---|---|---|---|---|
| Death | 0 | 24 (24%) | 52 (32%) | 94 (38%) | 170 (33%) |
| Early Termination of follow-up |
0 | 4 (4%) | 20 (12%) | 24 (10%) | 48 (9%) |
| Not done | 6 (100%) | 45 (44%) | 62 (38%) | 96 (39%) | 209 (41%) |
| Missed visit | 0 | 28 (28%) | 26 16%) | 18 (7%) | 72 14%) |
| Missing | 0 | 0 (0%) | 2 (1%) | 16 (6%) | 18 (3%) |
| Total | 6 | 101 | 162 | 248 | 517 |
Study arms combined, data presented as aggregate for missing DLCO% and FEV1% rather than individual patients
Baseline characteristics of these 69 patients are shown in Table 2. Sublobar resection was performed in 27 patients; the remaining 42 patients underwent sublobar resection plus brachytherapy. There were no differences in baseline characteristics and operative factors analyzed between study arms. In addition, there were no differences in baseline characteristics and operative factors analyzed between patients with complete and incomplete PFT data The characteristics of the complete PFT cohort in each study arm (27 SR arm, 42 in SRB arm) was also compared with the cohort of patients with complete data for either DLCO% or FEV1% at each time point (baseline, 3, 12 and 24 months) with no statistically significant differences (data not shown).
Table 2.
Patient characteristics: comparison of complete PFTs (DLCO % and FEV1% at baseline, 3, 12 and 24 months) vs. incomplete PFT cohorts
| SR [Incomplete PFT] (N=81) |
SR [Complete PFT] (N=27) |
p value | SRB [Incomplete PFT] (N=62) |
SRB [Complete PFT] (N=42) |
p value | Complete PFT (SR vs. SRB) p value |
|
|---|---|---|---|---|---|---|---|
| Age (in years) | 0.331 | 0.741 | 0.601 | ||||
| Median | 70.0 | 70.0 | 72.0 | 69.5 | |||
| Range | 49.0–85.0 | 58.0–82.0 | 50.0–87.0 | 53.0–87.0 | |||
| Sex | 0.312 | 0.432 | 0.352 | ||||
| Female | 48 (59.3%) | 13 (48.1%) | 32 (51.6%) | 25 (59.5%) | |||
| Ethnicity | 0.072 | 0.052 | 0.362 | ||||
| Hispanic/Latino | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.4%) | |||
| Not Hispanic/Latino | 72 (88.9%) | 27 (100.0%) | 50 (80.6%) | 39 (92.9%) | |||
| Unknown | 9 (11.1%) | 0 (0.0%) | 12 (19.4%) | 2 (4.8%) | |||
| Race | 0.822 | 0.572 | 0.322 | ||||
| White | 76 (93.8%) | 25 (92.6%) | 58 (93.5%) | 41 (97.6%) | |||
| Black/African American | 5 (6.2%) | 2 (7.4%) | 3 (4.8%) | 1 (2.4%) | |||
| Unknown | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 0 (0.0%) | |||
| Performance Status | 0.402 | 0.592 | 0.532 | ||||
| 0 | 13 (16.0%) | 6 (22.2%) | 12 (19.4%) | 11 (26.2%) | |||
| 1 | 46 (56.8%) | 17 (63.0%) | 37 (59.7%) | 21 (50.0%) | |||
| 2 | 22 (27.2%) | 4 (14.8%) | 13 (21.0%) | 10 (23.8%) | |||
| Baseline DLCO % | 0.871 | 0.341 | 0.901 | ||||
| Median | 46.5 | 46.0 | 43.0 | 46.0 | |||
| Range | 18.0–94.0 | 18.0–97.0 | 8.0–83.0 | 10.0–83.0 | |||
| Baseline FEV1 % | 0.831 | 0.461 | 0.481 | ||||
| Median | 47.0 | 49.0 | 51.0 | 54.0 | |||
| Range | 26.0–117.0 | 22.0–108.0 | 25.0–96.0 | 25.0–110.0 | |||
| Baseline FVC % | 0.131 | 0.861 | 0.901 | ||||
| Median | 70.0 | 77.0 | 77.0 | 77.0 | |||
| Range | 33.0–110.0 | 40.0–104.0 | 38.0–109.0 | 34.0–124.0 | |||
| Tumor Location | 0.912 | 0.792 | 0.692 | ||||
| Middle/Upper Lobe | 55 (67.9%) | 18 (66.7%) | 40 (64.5%) | 26 (61.9%) | |||
| Lower Lobe | 26 (32.1%) | 9 (33.3%) | 22 (35.5%) | 16 (38.1%) | |||
| Surgery Performed | 0.402 | 0.342 | 0.912 | ||||
| Thoracotomy | 23 (28.4%) | 10 (37.0%) | 28 (45.2%) | 15 (35.7%) | |||
| VATS | 58 (71.6%) | 17 (63.0%) | 34 (54.8%) | 27 (64.3%) | |||
| Resection Type | 0.472 | 0.112 | 0.232 | ||||
| Segmentectomy | 27 (33.3%) | 7 (25.9%) | 17 (27.4%) | 6 (14.3%) | |||
| Wedge Resection | 54 (66.7%) | 20 (74.1%) | 45 (72.6%) | 36 (85.7%) | |||
| Pathological Tumor Size | 0.242 | 0.932 | 0.152 | ||||
| <=2 cm | 50 (61.7%) | 20 (74.1%) | 36 (58.1%) | 24 (57.1%) | |||
| >2 cm | 31 (38.3%) | 7 (25.9%) | 26 (41.9%) | 18 (42.9%) |
Wilcoxon
Chi-Square
Abbreviations: SR, sublobar resection; SRB, sublobar resection plus brachytherapy; PFT, pulmonary function test; DLCO%, percentage predicted diffusing capacity of the lung for carbon monoxide; FEV1%, percentage predicted forced expiratory volume in 1 second; FVC%, percentage predicted forced vital capacity; VATS, video-assisted thoracoscopic surgery.
Longitudinal Pulmonary Function Data
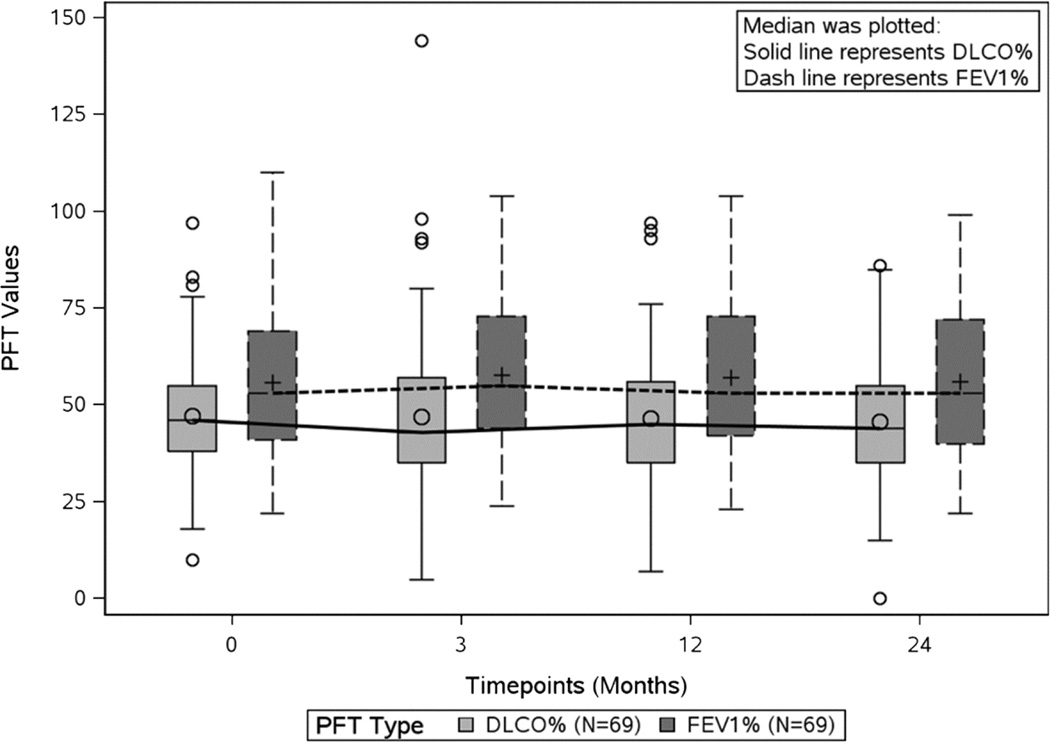
The baseline median FEV1% and median DLCO% was 46% and 53% predicted, respectively. The median change from baseline for FEV1% was +2%, +1% and +1% at 3, 12 and 24 months respectively and for DLCO% was −1%, −2% and −2% at 3, 12 and 24 months respectively. No statistically significant changes in median FEV1% or DLCO% were observed between baseline values and those measured at 3, 12 or 24 months (Figure 2).
Figure 2.
Changes in median FEV1% and DLCO% over 24 months. DLCO%, percentage predicted diffusing capacity of the lung for carbon monoxide; FEV1%, percentage predicted forced expiratory volume in 1 second.
The proportion of patients who experienced a clinically significant decline in pulmonary function (≥10% decline from baseline in FEV1% or DLCO%) is shown in Table 3. Overall 24.6% and 14.5% of patients were observed to have a long-term reduction in DLCO% and FEV1% at 24 months.
Table 3.
Summary of 10% Decline in DLCO% and FEV1% from baseline to month 3, 12 and 24
| Change from baseline to: | DLCO% No. of Patients (%) |
FEV1% No. of Patients (%) |
|---|---|---|
| Month 3 | ||
| No 10% Decline | 51 (73.9%) | 59 (85.5%) |
| >=10% Decline | 18 (26.1%) | 10 (14.5%) |
| Month 12 | ||
| No 10% Decline | 54 (78.3%) | 58 (84.1%) |
| >=10% Decline | 15 (21.7%) | 11 (15.9%) |
| Month 24 | ||
| No 10% Decline | 52 (75.4%) | 59 (85.5%) |
| >=10% Decline | 17 (24.6%) | 10 (14.5%) |
Abbreviations: DLCO%, percentage predicted diffusing capacity of the lung for carbon monoxide; FEV1%, percentage predicted forced expiratory volume in 1 second.
Tumor location
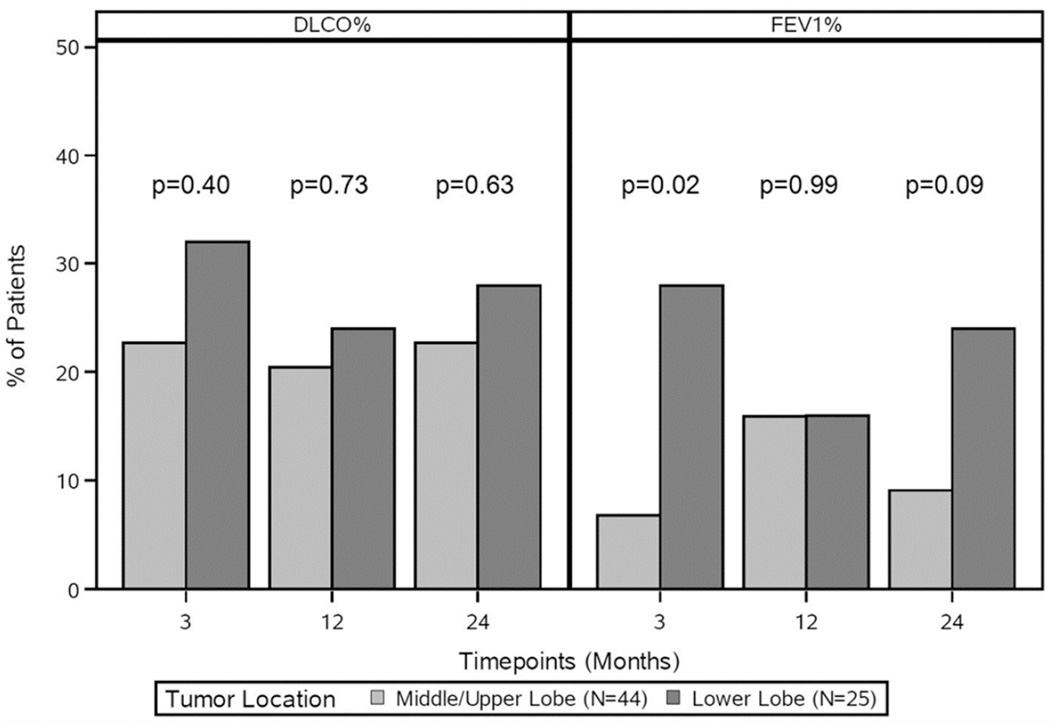
No differences were observed in baseline median FEV1% (49.5% vs. 55%, p = 0.06) or median DLCO% (47.5% vs. 42%, p = 0.23) between upper/middle and lower lobe resections. Although median values of FEV1% and DLCO% were comparable at all time-points, patients with lower lobe resections were more likely to have a ≥10% decline in FEV1% compared to those with middle/upper lobe resections at 3 months (28% vs. 6.8%, p = .02). This difference was not observed at 12 or 24 months (Figure 3).
Figure 3.
Changes in DLCO% and FEV1% by tumor location over 24 months. DLCO%, percentage predicted diffusing capacity of the lung for carbon monoxide; FEV1%, percentage predicted forced expiratory volume in 1 second.
Surgical Technique
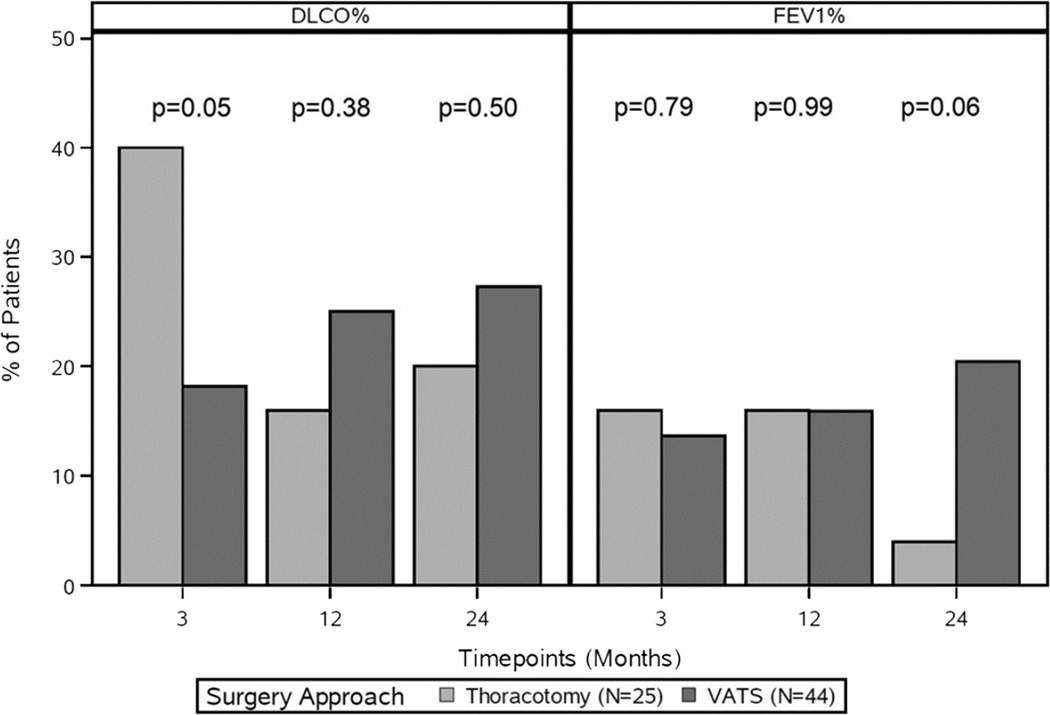
No differences were observed in baseline median FEV1% (49% vs. 54%, p = 0.29) or median DLCO% (46% vs. 47%, p = 0.62) between those who underwent a thoracotomy vs. VATS. Although median values of FEV1% and DLCO% were comparable at all time points, patients who underwent a thoracotomy were more likely to have a ≥10% decline in DLCO% compared to those treated by VATS at 3 months (40% vs. 18.2%, p = .05). No differences were observed at 12 or 24 months (Figure 4). The type of sublobar resection (wedge vs. segmentectomy) did not have a significant impact on pulmonary function at any time point.
Figure 4.
Changes in DLCO% and in FEV1% by surgery approach over 24 months. DLCO%, percentage predicted diffusing capacity of the lung for carbon monoxide; FEV1%, percentage predicted forced expiratory volume in 1 second; VATS, video-assisted thoracoscopic surgery.
Tumor size
A statistically significant relationship was found between pathologic tumor size (≤ or > 2cm) in the median DLCO% at 24 months (47% vs. 38%, p = .02). However, no difference in pulmonary function was observed at baseline or at other time points for FEV1% and DLCO%.
Longitudinal analysis: Generalized Estimating Equation
Results of the GEE model incorporating data from all 4 time points are shown in Table 4. None of the analyzed factors (study arm, tumor location, surgical technique or pathologic tumor size) were found to have an impact on the longitudinal measures of FEV1% or DLCO%.
Table 4.
Results from the multivariable GEE models for DLCO% and FEV1%
| Predictors | DLCO% | FEV1% | ||
|---|---|---|---|---|
| Estimate | P-value* | Estimate | P-value* | |
| Arm: SRB vs. SR | −4.96 | 0.20 | 1.58 | 0.74 |
| Time: Baseline vs. Month 24 | 1.32 | 0.85 | −0.23 | 0.44 |
| Month 3 vs. Month 24 | 1.13 | 1.73 | ||
| Month 12 vs. Month 24 | 0.80 | 1.16 | ||
| Tumor Location: Lower Lobe vs. Middle/Upper Lobe | −2.77 | 0.45 | 6.42 | 0.20 |
| Time: Baseline vs. Month 24 | 1.32 | 0.85 | −0.23 | 0.44 |
| Month 3 vs. Month 24 | 1.13 | 1.73 | ||
| Month 12 vs. Month 24 | 0.80 | 1.16 | ||
| Surgery Approach (VATS vs. Thoracotomy) | 2.46 | 0.46 | 4.77 | 0.32 |
| Time: Baseline vs. Month 24 | 1.32 | 0.85 | −0.23 | 0.44 |
| Month 3 vs. Month 24 | 1.13 | 1.73 | ||
| Month 12 vs. Month 24 | 0.80 | 1.16 | ||
| Resection Type: Wedge vs. Segment | 2.69 | 0.53 | 5.24 | 0.34 |
| Time: Baseline vs. Month 24 | 1.32 | 0.85 | −0.23 | 0.44 |
| Month 3 vs. Month 24 | 1.13 | 1.73 | ||
| Month 12 vs. Month 24 | 0.80 | 1.16 | ||
| Pathological Tumor Size: >2 cm vs. ≤2 cm | −5.58 | 0.12 | 2.57 | 0.61 |
| Time: Baseline vs. Month 24 | 1.32 | 0.85 | −0.23 | 0.44 |
| Month 3 vs. Month 24 | 1.13 | 1.73 | ||
| Month 12 vs. Month 24 | 0.80 | 1.16 | ||
Wald test p-value
Abbreviations: GEE, generalized estimating equation; DLCO%, percentage predicted diffusing capacity of the lung for carbon monoxide; FEV1%, percentage predicted forced expiratory volume in 1 second; VATS, video-assisted thoracoscopic surgery.
Comment
The assessment of risk for patients undergoing pulmonary resection is predicated on an accurate prediction of postoperative pulmonary function [10]. Several tools have been developed to determine this, including quantitative CT scans, perfusion scans, and most commonly, the segment-counting method [11,12,13]. However, longitudinal studies have demonstrated that the measured postoperative pulmonary function does not always correlate with predicted values. Specifically, pulmonary function has been shown to improve over time following surgery, and may return to preoperative values even after lobectomy [14,15].
The Lung Cancer Study Group performed the landmark study evaluating pulmonary function after lung cancer resection [3]. In that publication, the authors observed no significant difference in forced vital capacity (FVC) between patients undergoing lobectomy versus limited resection, and concluded that sublobar resection offered no functional benefit over lobectomy. However, the reduction in FEV1 was significantly greater in the lobectomy group versus the sublobar resection group at both 6 months and 12–18 months.
Subsequent studies have challenged the claim that lobectomy and sublobar resection are equivalent in regards to preservation of pulmonary function [16,17]. As an example, a single center review of 83 patients from Japan demonstrated a positive correlation between the number of segments removed and reduction in FEV1 and FVC, and this was most pronounced in lobectomy patients [2]. Similar studies have been published from centers in the United States [1].
Common to all of these reports is the exclusion of high-risk patients. Consequently, patients who underwent segmentectomy in these other studies were potential candidates for lobectomy on the basis of preserved cardiopulmonary function. In contrast, patients enrolled in ACOSOG Z4032 were considered to be at high-risk for lobectomy, and would likely be referred for non-operative ablative therapy if sublobar resection was not performed.
The principal finding of this analysis is that sublobar resection did not lead to a clinically significant reduction (≥10% decline from baseline in FEV1% or DLCO%) in a cohort of high-risk patients. While a thoracotomy and lower lobe resections were associated with a reduction in pulmonary function at 3 months, these effects were transient. Importantly, pulmonary function at 24 months following surgery was equivalent to baseline measurements.
We believe that this observation has important implications for treatment recommendations in high-risk patients. Non-operative therapies, such as radiofrequency ablation and stereotactic radiosurgery, are increasingly being considered for these patients [18]. A potential advantage of non-operative therapy is the preservation of lung parenchyma, in contrast to surgical resection, which mandates the loss of functional lung tissue [19, 20].
However, reductions in pulmonary function have also been observed in patients undergoing non-operative therapy. For instance, a retrospective study of stereotactic radiosurgery demonstrated a significant reduction in DLCO from a baseline of 61.5% to 44.8% at 12 months [21]. A similar study of 20 high-risk patients undergoing stereotactic radiosurgery showed that while FEV1 did not change at 12 months following treatment, a significant decline of 11% was observed for DLCO [22]. The largest study of pulmonary function following SBRT was an analysis of RTOG 0236, a prospective phase 2 trial that enrolled medically inoperable patients with early-stage lung cancer [23]. A total of 55 patients were evaluable, however only 24 patients completed pulmonary function testing at 2 years following treatment. A reduction in FEV1% and DLCO% was noted at 2 years (−5.8% and −6.3% respectively), however neither was statistically significant. In the present analysis, patients enrolled in ACOSG Z4032 were observed to have a 2% decrease in DLCO at 2 years, and an increase in FEV1 of 1%, neither of which was statistically significant.
This study has important limitations. Among 212 patients eligible for analysis, only 69 (32%) completed pulmonary function testing at all planned time points. While we found no statistically significant difference in either baseline or subsequent pulmonary function in those with complete follow-up and those without, we acknowledge that this is a potential bias. Nonetheless, it is possible that patients with a more favorable postoperative course may have been more likely to undergo pulmonary testing, which would impact the conclusions of this analysis. In addition, we note that a direct comparison between pulmonary function data from this trial and single-arm studies evaluating ablative therapies may be influenced by differences in trial design and inclusion criteria.
In summary, we found that sublobar resection performed in a high-risk operable patient population did not lead to a clinically or statistically significant reduction in pulmonary function with long-term follow-up. Although a direct comparison of surgical resection versus ablative therapy awaits appropriately powered randomized trials, this observation should be considered when treatment recommendations are developed for this cohort of patients.
Supplementary Material
Acknowledgments
Set acknowledgement: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 to the Alliance for Clinical Trials in Oncology, as well as CA076001 to the legacy American College of Surgeons Oncology Group (ACOSOG); and the following grants: U10CA180790, U10CA180791, U10CA180833, and U10CA180844. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Southern Thoracic Surgical Association 62nd Annual Meeting; Orlando, FL, November 4–7, 2015
Set conflict box: Dr Meyers discloses a financial relationship with Ethicon and Varian; Dr Putnam with GlaxoSmithKline; Dr Fernando with Galil and CSA Medical.
REFERENCES
- 1.Keenan RJ, Landreneau RJ, Maley RH, Jr, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg. 2004;78:228–233. doi: 10.1016/j.athoracsur.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 2.Harada H, Okada M, Sakamoto T, Matsuoka H, Tsubota N. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg. 2005;80:2041–2045. doi: 10.1016/j.athoracsur.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–623. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]
- 4.Fernando HC, Landreneau RJ, Mandrekar SJ, et al. The impact of brachytherapy on local recurrence rates after sublobar resection: Results from ACOSOG Z4032 (Alliance), a phase III randomized trial for high-risk operable non-small cell lung cancer (NSCLC) J Clin Oncol. 2014;32:2456–2462. doi: 10.1200/JCO.2013.53.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernando HC, Landreneau RJ, Mandrekar SJ, et al. The impact of adjuvant brachytherapy with sublobar resection on pulmonary function and dyspnea in high-risk patients with operable disease: Preliminary results from the American College of Surgeons Oncology Group Z4032 Trial. J Thorac Cardiovasc Surg. 2011;142:554–562. doi: 10.1016/j.jtcvs.2010.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Thirty and ninety-day outcomes after sublobar resection with and without brachytherapy for non-small cell lung cancer: results from a multicenter phase III study. J Thorac Cardiovasc Surg. 2011;142:1143–1151. doi: 10.1016/j.jtcvs.2011.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernando HC, Landreneau RJ, Madrekar SJ, et al. Analysis of longitudinal quality-of-life data in high-risk patients with lung cancer: results from ACOSOG Z4032 (Alliance) multicenter randomized trial. J Thorac Cardiovasc Surg. 2015;149:718–726. doi: 10.1016/j.jtcvs.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York: Springer-Verlag; 2000. [Google Scholar]
- 9.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS® System for Mixed Models. Cary, NC: SAS Institute Inc; 2006. [Google Scholar]
- 10.Brunelli A, Kim AW, Berger KI, Addrizzo-Harris D. Physiologic evaluation of patients with lung cancer being considered for resectional surgery. Chest. 2013;143:e166S–e190S. doi: 10.1378/chest.12-2395. [DOI] [PubMed] [Google Scholar]
- 11.Wu MT, Chang JM, Chiang AA, et al. Use of quantitative CT to predict postoperative lung function in patients with lung cancer. Radiology. 1994;191:257–262. doi: 10.1148/radiology.191.1.8134584. [DOI] [PubMed] [Google Scholar]
- 12.Bolliger CT, Wyser C, Roser H, et al. Lung scanning and exercise testing for the prediction of postoperative performance in lung resection candidates at increased risk for complications. Chest. 1995;108:341–348. doi: 10.1378/chest.108.2.341. [DOI] [PubMed] [Google Scholar]
- 13.Zeiher BG, Gross TJ, Kern JA, et al. Predicting postoperative pulmonary function in patients undergoing lung resection. Chest. 1995;108:68–72. doi: 10.1378/chest.108.1.68. [DOI] [PubMed] [Google Scholar]
- 14.Brunelli A, Refai M, Salati M, Xiume F, Sabbatici A. Predicted versus observed FEV1 and DLCO after major lung resection: a prospective evaluation at different time periods. Ann Thorac Surg. 2007;83:1134–1139. doi: 10.1016/j.athoracsur.2006.11.062. [DOI] [PubMed] [Google Scholar]
- 15.Kim SJ, Lee YJ, Park JS, et al. Changes in pulmonary function in lung cancer patients after video-assisted thoracic surgery. Ann Thorac Surg. 2015;99:210–217. doi: 10.1016/j.athoracsur.2014.07.066. [DOI] [PubMed] [Google Scholar]
- 16.Yoshikawa K, Tsubota N, Kodama K, et al. Prospective study of extended segmentectomy for small lung tumors: the final report. Ann Thorac Surg. 2002;73:1055–1059. doi: 10.1016/s0003-4975(01)03466-x. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimoto K, Nomori H, Mori T, Kobayashi H, Ohba Y, Shibata H, et al. Quantification of the impact of segmentectomy on pulmonary function by perfusion single-photon-emission computed tomography and multidetector computed tomography. J Thorac Cardiovasc Surg. 2009;137:1200–1205. doi: 10.1016/j.jtcvs.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16:630–637. doi: 10.1016/S1470-2045(15)70168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lencioni R, Crocetti L, Cioni R, Suh R, Glenn D, Regge D, et al. Response to radiofrequency ablation of pulmonary tumors: a prospective, intent-to treat, multicenter clinical trial (the RAPTURE study) Lancet Oncol. 2008;9:621–628. doi: 10.1016/S1470-2045(08)70155-4. [DOI] [PubMed] [Google Scholar]
- 20.Collins BT, Vahdat S, Erickson K, Collins SP, Suy S, Yu X, et al. Radical cyber- knife radiosurgery with tumor tracking: an effective treatment for inoperable small peripheral stage I non–small cell lung cancer. J Hematol Oncol. 2009;2:1–9. doi: 10.1186/1756-8722-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Videtic GM, Chandana AR, Sorenson L. A postoperative study of quality of life including fatigue and pulmonary function after stereotactic body radiotherapy for medically inoperable early-stage lung cancer. Support Care Cancer. 2013;21:211–218. doi: 10.1007/s00520-012-1513-9. [DOI] [PubMed] [Google Scholar]
- 22.Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic radiation therapy for early-stage non–small cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75:677–682. doi: 10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 23.Static S, Paulus R, Timmerman R, et al. No clinically significant changes in pulmonary function following stereotactic body radiation therapy for early-stage peripheral non-small cell lung cancer: an analysis of RTOG 0236. Int J Rad Oncol Biol Phys. 2014;88:1092–1099. doi: 10.1016/j.ijrobp.2013.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.