Abstract
Background:
There is no conclusive evidence on the effect of orthodontic force application on dental pulp tissue. The aim of this study was to compare early and delayed histological effects of intrusive forces on dental pulp of adolescents and adults.
Materials and Methods:
Patients referred to the Department of Orthodontics of Mashhad University of Medical Sciences participated in this study. They were assigned to adult (25–32-year-old) and adolescent (13–18-year-old) Groups. A cantilever spring made of 16 × 22 steel wire was used to apply intrusive force to upper first premolars (11 teeth in adolescents and 11 teeth in adults) and the opposing teeth were considered as control group. In each group, 6 pairs of teeth were extracted after one week, and the remaining 5 pairs were extracted after one month of intrusion. Histologic changes were compared between the control and intrusive groups and also between the adults and adolescents after 7 days and 1 month. Statistical analysis was performed using Statistical Package for the Social Sciences and Wilcoxon and Mann–Whitney U-tests. P ≤ 0.05 was set as statistically significant.
Results:
Significant difference was not found in any histological parameters between intrusive and control groups 1 week and 1 month after intrusion in adolescents and adults (P > 0.05). One month after intrusion, inflammatory cell response intensity (P = 0.032) and frequency of chronic inflammation (P = 0.032) were significantly higher in adults compared to adolescents.
Conclusion:
Mild intrusive force in closed apex teeth causes no significant histologic changes in adolescents and adults. However, it seems that inflammatory-related histologic pulpal changes are more severe in adults after one month of intrusion.
Key Words: Dental pulp, histology, intrusion
INTRODUCTION
The relation between orthodontic force application and dental pulp tissue has been the subject of studies in the recent years.[1,2,3] However, there is no conclusive evidence on the effect of orthodontic forces on pulpal tissue,[4] and therefore, the issue has been studied for many years in human. Proffit et al.[1] reported that light continuous forces have little or no effect on dental pulp. On the other hand, the reaction of dental pulp to orthodontic forces has been reported to vary from mild hyperemia to complete necrosis in the literature.[5,6] Type of the force application, duration and dimension of the force, age of the patients, and size of the apical foramen are among the contributory factors.[7] More pulpal changes have been observed in response to intrusive orthodontic forces.[5,8,9,10,11,12] Furthermore, higher incidence of irreversible pulpal reactions is usually expected in teeth with complete root formation.
The aim of the present study was to evaluate histologic changes of dental pulp of human premolars in response to intrusive forces and to compare pulpal changes in patients of two different age ranges (adults vs. adolescents). Furthermore, early (after 7 days) and delayed (after 30 days) response of the pulp is assessed.
MATERIALS AND METHODS
This study was approved by the Regional Ethics Committee on Research of Mashhad University University of Medical Sciences. Patients referred to Orthodontic Department of Mashhad University Dental School participated in this study. All patients required maxillary first premolar extraction for orthodontic purposes. A written informed consent form was signed by all participants before start of the study. Patients with a history of systemic disease, probing depth of more than 3 mm in upper premolars, marginal bone resorption, and anti-inflammatory drug application during the last month was excluded from the study. Furthermore, premolars with incomplete apical root formation, caries, restorations, and endodontic treatment were left out. The patients were classified into two groups according to their age: Adolescent of 13–18-year-old (n = 11) and adults of 25–32-year-old (n = 11).
For each subject, one randomly selected maxillary premolar was loaded with intrusive force. The contralateral premolar was not subjected to mechanical loading and served as control tooth. Orthodontic bands with triple buccal tubes were cemented onto the maxillary first molars. To avoid tipping and extrusion of molars, first molars were connected with a 1mm stainless steel palatal bar into a rigid unit. Orthodontic brackets (0.018 × 0.022 standard edgewise; Dentaurum, Germany) were bonded in the center of the buccal surface of the first and second upper premolar of the test side. A stainless steel wire (0.016 × 0.22, Dentaurum, Germany) was inserted into the molar tube and second premolar bracket to improve the anchorage. A cantilever spring (0.16 × 0.22 stainless steel, Dent aurum, Germany) was fabricated for each patient to generate intrusive force. The spring was positioned 3 mm above the slot of the bracket of the first premolar in the passive state and was ligated to the bracket of the test tooth, with the use of wire ligature [Figure 1].
Figure 1.
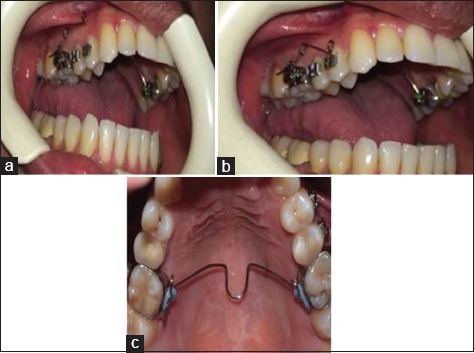
Intraoral view of the cantilever spring for application of intrusive force to upper first premolar. (a) Passive, (b) active position, (c) transpalatal arch cemented to avoid extrusion and tipping of the first molar.
The magnitude of the intrusive force for every experimental tooth was approximately 15 g. In both groups of adults and adolescents, 6 pairs of teeth were extracted after 7 days, whereas the other 5 pairs were extracted after 30 days. All the teeth were extracted by an expert dentist with minimum trauma.
After extraction, the tip of each root was cut with a bur to facilitate fixation. The teeth were fixed in 10% formalin for one week and were then immersed in decalcifying solution including 5 ml pure nitric acid, 5 ml pure formalin, and 90 ml 95% alcohol for 7–10 days immediately after removal. Then, the specimens were decalcified and embedded in paraffin. Serial sections (4–5µm thick) were cut longitudinally and stained with hematoxylin and eosin dye. The specimens were examined by an expert pathologist under a light microscope (Leica, Exwave HAD, Model Number: SSC-DC58AP). The studied histological changes were as follows:[7]
Degree of inflammation was determined by counting inflammatory cells by high magnification field (×400). Each high magnification field corresponds to 0.1 mm2 None = 0–1 cell per field, mild = 2–5 cells per field, moderate = 6–15 cells per field, severe = more than 15 cells per field[7]
Type of inflammation: Acute-chronic-none
Fibrous tissue formation (mild to severe)
Necrosis (none, partial, complete)
Disruption of odontoblastic layer
Odontoblastic aspiration into dentinal tubules
Vascular dilation
Resorption of cementum or dentin.
Statistical analysis was performed using Statistical Package for the Social Sciences (version 16.0, SPSS Inc, Chicago, ILL, USA). Kruskal–Wallis and Mann-Whitney U-tests were used for comparison. P < 0.05 was set as statistically significant.
RESULTS
Frequency distribution of the histologic parameters in the studied groups is shown in Tables 1–3 and Figures 1, 2. There was not a significant difference between the test and control teeth during the 7 days and 1 month test period in adolescents and adults. Furthermore, there was not a significant difference between the test teeth after 7 days compared to 1 month in the adolescents and adults. However, Man–Whitney U-test revealed that there was a significant difference between adolescents and adults after 1 month regarding degree of inflammation (P = 0.032), and type of inflammation (P = 0.032) in the test teeth.
Table 1.
Frequency distribution according to degree of inflammation, fibrous tissue formation, vascular dilation, and odontoblastic layer disruption in the studied groups in adolescents
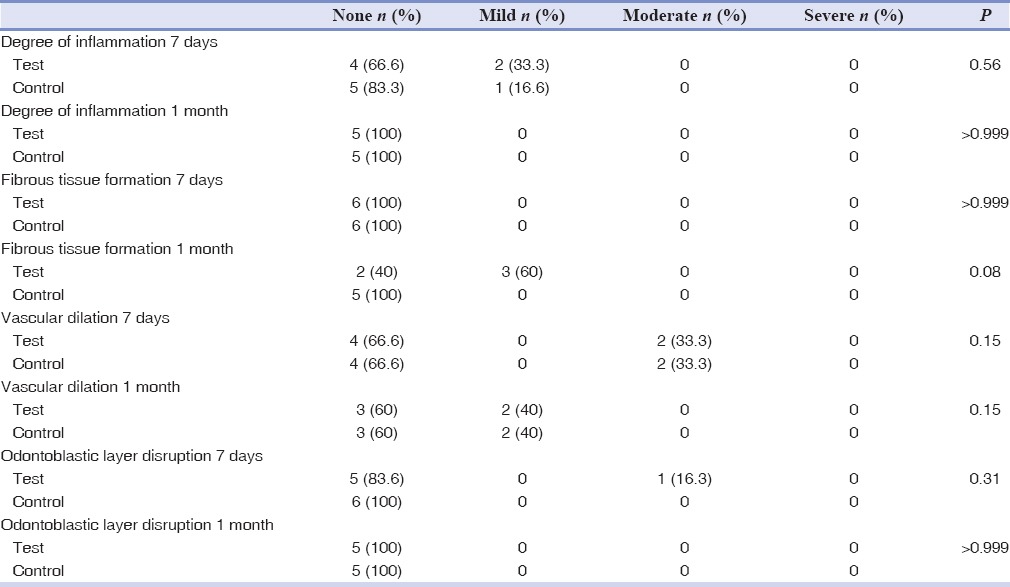
Table 3.
Frequency distribution of aspiration of odontoblasts into dentinal tubules, as well as resorption of dentin or cement in adolescents and adult patients
Figure 2.
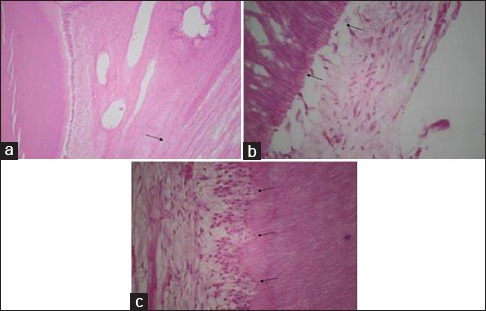
(a) Fibrous tissue formation in the dental pulp. (b) Disruption of odontoblastic layer. (c) Aspiration of odontoblasts into dentinal tubules.
Table 2.
Frequency distribution according to degree of inflammation, fibrous tissue formation, vascular dilation, and odontoblastic layer disruption in the studied groups in adult patients
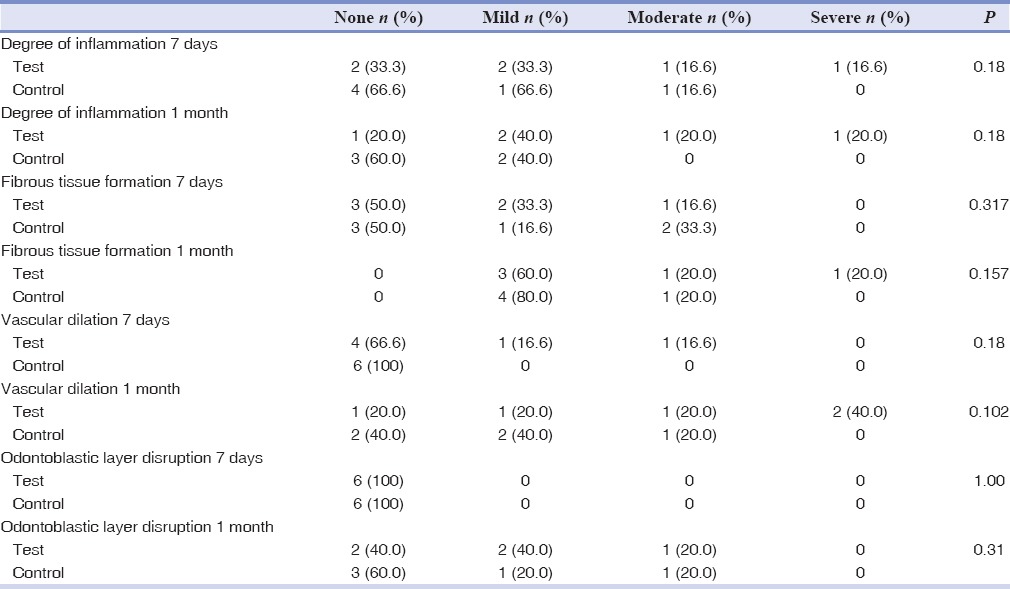
There was not an acute inflammation in the studied groups during the study. In addition, no chronic inflammation was observed in adolescents after 1 month. However, 2 teeth (33.3%) in the test group and also 1 tooth (16.6%) in the control group showed chronic inflammation after 7 days in the adolescents. In the adult group, chronic inflammation was observed in 4 teeth (66.6%) in the intrusive group and 2 teeth (40%) in the control group after 7 days. Similarly, after 1 month, 4 teeth (80%) and 2 teeth (40%) showed signs of chronic inflammation in the intrusive and control group, respectively.
DISCUSSION
Some studies have reported significant pulpal reactions to orthodontic tooth movement, yet there is not conclusive evidence on the relation between orthodontic forces and pulpal alterations.[4]
Some studies have demonstrated that most of the pulpal changes are associated with intrusive forces compared to other orthodontic movements.[5,12,13]
In the present study, pulpal reactions to orthodontic forces were investigated 7 days and 1 month after force application to study both the immediate and the late pulpal responses. Some pulpal reactions to intrusive forces have been reported to occur as early as 3 days after force application in Ramzanzadeh's study.[14]
In present study, mild inflammation was observed in adolescents only after 1 month of force application. In contrast, cases with moderate and severe inflammation were found among the adult patients after 7 days. Lazzaretti et al.[4] reported no inflammatory cell reactions 3 weeks after intrusion. The study group included 12–19-year-old adolescents. This result is in line with our findings. On the other hand, we found a significant difference in the amount of chronic inflammation between the adolescents and adults. This emphasizes the importance of applying lighter forces with suitable intervals in the adult patients.
Some studies have demonstrated that hemodynamic changes are the first observable signs after orthodontic movements.[15,16] However, there is a large controversy in this issue. Some studies have reported a considerable decrease of pulpal blood flow[3,17,18,19] after intrusion, whereas Kvinnsland et al. have demonstrated an increase or no change.[20] In the present study, we did not find a significant difference in vascular dilation between adolescents and adults after 7 days and 1 month of intrusion. In addition, no significant difference was observed between the control and intrusive groups. This can be due to the light force application in our study. These results are in line with the Ramazanzadeh et al.'s[14] study.
We found no fibrous tissue formation after 7 days and 1 month of intrusion in adolescents. However, there were some cases with mild (33.2%) and moderate (16.6%) fibrous tissue after 1 week. Furthermore, we observed one case with severe formation of fibrous tissue. Nevertheless, the difference between the adolescents and adults and also between the control and intrusive groups was not significant. Distribution of collagen fiber changes according to the age, fibers gradually change into fibrotic bundles. These changes usually occur in response to external stimuli such as caries or enamel attrition.[5] Since the studied teeth in the present study was completely intact, it seems that the observed difference between the adolescents and adults is related to the age of patients, albeit not statistically significant. Similarly, Ramazanzadeh et al.[14] reported no significant difference between their intrusive and control group regarding fibrous tissue formation.
Aspiration of odontoblasts into dentinal tubules has been demonstrated as pulpal reactions to external stimuli in several studies.[14,16,21] Ramazanzadeh et al.[14] found odontoblasts aspiration in 1 case (10%) after 3 days and also 3 weeks of intrusion. They associated their finding to the probable trauma of the forceps during tooth extraction. We also found 2 cases of odontoblasts aspiration only in the adult group. Probable trauma to the teeth during extraction or before the study in the adult group can be mentioned as possible causes of this finding.
In the current study, we did not find a significant difference in disruption of odontoblastic layer between the studied groups. This can be the result of applying light force in our study. In contrast, Ramazanzadeh et al.[14] reported more cases with odontoblastic layer disruption in the intrusive group compared to controls. Mostafa et al.[21] reported that disruption of odontoblastic layer is associated with pulpal blood flow changes.
Similar to the results of Ramazanzadeh's study,[14] we did not find a significant difference in resorption of dentin or cement between the studied groups.
It seems that some reactions of pulpal tissue including disruption of odontoblasts, aspiration of odontoblasts into dentinal tubules, and resorption of dentin or cement occur with a considerable delay in adults compared to the adolescent group. According to Graber et al.,[22] lag phase before tooth movement takes usually longer time in adults compared to younger patients. The delayed reactions of pulp in the adult group in comparison to adults might be associated with longer lag phase in these patients.
The 1-month period used in this experiment corresponds to the usual interval between consultations of orthodontic treatment and is sufficient for a tooth to move and complete its cycle of bone formation and resorption provided that the orthodontic forces are optimal. On the other hand, immediate response to orthodontic forced has been shown to occur as early as 3 days.[3] Furthermore, some studies have found some pulpal changes 7 days after orthodontic force application.[23,24,25,26] Thus, we decided to investigate immediate pulpal reactions to force application after 7 days.
The results of the present study should be interpreted with caution due to the limited number of cases in each group. Further studies with larger sample size are required.
CONCLUSION
Mild orthodontic intrusive force application in closed apex teeth causes no significant histological changes in adolescents and adults. However, it seems that inflammatory-related histological pulpal changes are more severe in adults after one month of intrusion. This emphasizes the importance of applying mild orthodontic forces with adequate rest intervals in older individuals.
Financial support and sponsorship
Mashhad University of Medical Sciences for financial support of this project.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or nonfinancial in this article.
ACKNOWLEDGMENTS
The authors would like to thank vice-chancellor of research of Mashhad University of Medical Sciences for financial support of this project.
The results mentioned in this manuscript are derived from a doctorate thesis (No: 2714).
REFERENCES
- 1.Proffit WR, Fields HW, Sarver DM. Contemporary Orthodontics. 4th ed. St. Louis: Mosby Co.; 2007. pp. 94pp. 331–48. [Google Scholar]
- 2.Andreasen JO, Andreasen L. Textbook and Atlas of Traumatic Injuries to the Teeth. 4th ed. Oxford: Blackwell Munksgaard; 2007. pp. 848–50. [Google Scholar]
- 3.Guevara MJ, McClugage SG., Jr Effects of intrusive forces upon the microvasculature of the dental pulp. Angle Orthod. 1980;50:129–34. doi: 10.1043/0003-3219(1980)050<0129:EOIFUT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 4.Lazzaretti DN, Bortoluzzi GS, Torres Fernandes LF, Rodriguez R, Grehs RA, Martins Hartmann MS. Histologic evaluation of human pulp tissue after orthodontic intrusion. J Endod. 2014;40:1537–40. doi: 10.1016/j.joen.2013.10.039. [DOI] [PubMed] [Google Scholar]
- 5.Stenvik A, Mjör IA. Pulp and dentine reactions to experimental tooth intrusion. A histologic study of the initial changes. Am J Orthod. 1970;57:370–85. doi: 10.1016/s0002-9416(70)90219-8. [DOI] [PubMed] [Google Scholar]
- 6.Reitan K. Tissue behavior during orthodontic tooth movement. Am J Orthod. 1986;89:453–68. doi: 10.1016/0002-9416(86)90001-1. [DOI] [PubMed] [Google Scholar]
- 7.Seltzer S, Bender IB. The Dental Pulp, Biologic Considerations in Dental Procedures. 3rd ed. Philadelphia: JB Lippincott Co.; 1984. pp. 295–318. [Google Scholar]
- 8.Ersahan S, Sabuncuoglu FA. Effects of magnitude of intrusive force on pulpal blood flow in maxillary molars. Am J Orthod Dentofacial Orthop. 2015;148:83–9. doi: 10.1016/j.ajodo.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Sabuncuoglu FA, Ersahan S. Changes in maxillary molar pulp blood flow during orthodontic intrusion. Aust Orthod J. 2014;30:152–60. [PubMed] [Google Scholar]
- 10.Choi YJ, Kim KH, Lee KJ, Chung CJ, Park YC. Histomorphometric evaluation of maxillary molar roots and surrounding periodontium following molar intrusion in rats. Orthod Craniofac Res. 2015;18:12–20. doi: 10.1111/ocr.12054. [DOI] [PubMed] [Google Scholar]
- 11.Dermaut LR, De Munck A. Apical root resorption of upper incisors caused by intrusive tooth movement: A radiographic study. Am J Orthod Dentofacial Orthop. 1986;90:321–6. doi: 10.1016/0889-5406(86)90088-0. [DOI] [PubMed] [Google Scholar]
- 12.Butcher EO, Taylor AC. The vascularity of the incisor pulp of the monkey and its alteration by tooth retraction. J Dent Res. 1952;31:239–47. doi: 10.1177/00220345520310020901. [DOI] [PubMed] [Google Scholar]
- 13.Villa PA, Oberti G, Moncada CA, Vasseur O, Jaramillo A, Tobón D, et al. Pulp-dentine complex changes and root resorption during intrusive orthodontic tooth movement in patients prescribed nabumetone. J Endod. 2005;31:61–6. doi: 10.1097/01.don.0000134212.20525.74. [DOI] [PubMed] [Google Scholar]
- 14.Ramazanzadeh BA, Sahhafian AA, Mohtasham N, Hassanzadeh N, Jahanbin A, Shakeri MT. Histological changes in human dental pulp following application of intrusive and extrusive orthodontic forces. J Oral Sci. 2009;51:109–15. doi: 10.2334/josnusd.51.109. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi M, Kasai K. The effects of orthodontic mechanics on the dental pulp. Semi Orthod. 2007;13:272–80. [Google Scholar]
- 16.Nixon CE, Saviano JA, King GJ, Keeling SD. Histomorphometric study of dental pulp during orthodontic tooth movement. J Endod. 1993;19:13–6. doi: 10.1016/S0099-2399(06)81034-4. [DOI] [PubMed] [Google Scholar]
- 17.Barwick PJ, Ramsay DS. Effect of brief intrusive force on human pulpal blood flow. Am J Orthod Dentofacial Orthop. 1996;110:273–9. doi: 10.1016/s0889-5406(96)80011-4. [DOI] [PubMed] [Google Scholar]
- 18.Sano Y, Ikawa M, Sugawara J, Horiuchi H, Mitani H. The effect of continuous intrusive force on human pulpal blood flow. Eur J Orthod. 2002;24:159–66. doi: 10.1093/ejo/24.2.159. [DOI] [PubMed] [Google Scholar]
- 19.McDonald F, Pitt Ford TR. Blood flow changes in permanent maxillary canines during retraction. Eur J Orthod. 1994;16:1–9. doi: 10.1093/ejo/16.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Kvinnsland S, Heyeraas K, Ofjord ES. Effect of experimental tooth movement on periodontal and pulpal blood flow. Eur J Orthod. 1989;11:200–5. doi: 10.1093/oxfordjournals.ejo.a035986. [DOI] [PubMed] [Google Scholar]
- 21.Mostafa YA, Iskander KG, El-Mangoury NH. Iatrogenic pulpal reactions to orthodontic extrusion. Am J Orthod Dentofacial Orthop. 1991;99:30–4. doi: 10.1016/S0889-5406(05)81677-4. [DOI] [PubMed] [Google Scholar]
- 22.Graber TM, Rakosi T, Petrovic AG. Dentofacial Orthopedics with Functional Appliances. 1st ed. St. Louis: Mosby Co.; 1997. pp. 268–98. [Google Scholar]
- 23.Veberiene R, Smailiene D, Baseviciene N, Toleikis A, Machiulskiene V. Change in dental pulp parameters in response to different modes of orthodontic force application. Angle Orthod. 2010;80:1018–22. doi: 10.2319/111309-641.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han G, Hu M, Zhang Y, Jiang H. Pulp vitality and histologic changes in human dental pulp after the application of moderate and severe intrusive orthodontic forces. Am J Orthod Dentofacial Orthop. 2013;144:518–22. doi: 10.1016/j.ajodo.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Perinetti G, Varvara G, Salini L, Tetè S. Alkaline phosphatase activity in dental pulp of orthodontically treated teeth. Am J Orthod Dentofacial Orthop. 2005;128:492–6. doi: 10.1016/j.ajodo.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 26.Veberiene R, Smailiene D, Danielyte J, Toleikis A, Dagys A, Machiulskiene V. Effects of intrusive force on selected determinants of pulp vitality. Angle Orthod. 2009;79:1114–8. doi: 10.2319/110408-563R.1. [DOI] [PubMed] [Google Scholar]


